Abstract
The β-lactam resistance gene mecA of Staphylococcus aureus is carried by a novel mobile genetic element, designated staphylococcal cassette chromosome mec (SCCmec), identified in the chromosome of a Japanese methicillin-resistant S. aureus (MRSA) strain. We now report identification of two additional types of mecA-carrying genetic elements found in the MRSA strains isolated in other countries of the world. There were substantial differences in the size and nucleotide sequences between the elements and the SCCmec. However, new elements shared the chromosomal integration site with the SCCmec. Structural analysis of the new elements revealed that they possessed all of the salient features of the SCCmec: conserved terminal inverted repeats and direct repeats at the integration junction points, conserved genetic organization around the mecA gene, and the presence of cassette chromosome recombinase (ccr) genes responsible for the movements of SCCmec. The elements, therefore, were considered to comprise the SCCmec family of staphylococcal mobile genetic elements together with the previously identified SCCmec. Among 38 epidemic MRSA strains isolated in 20 countries, 34 were shown to possess one of the three typical SCCmec elements on the chromosome. Our findings indicated that there are at least three distinct MRSA clones in the world with different types of SCCmec in their chromosome.
β-Lactam resistance of methicillin-resistant Staphylococcus aureus (MRSA) is determined by the function of penicillin-binding protein 2′ (PBP2′) encoded by the methicillin resistance gene mecA (20, 29). PBP2′ binds to β-lactam antibiotics with much lower affinity than the intrinsic set of PBPs of S. aureus do (7, 23, 35). By nucleotide sequence determination of an MRSA-specific chromosomal region of strain N315 (isolated in Japan in 1982), we have found that the mecA gene is carried by a novel genetic element, designated staphylococcal cassette chromosome mec (SCCmec), inserted into the chromosome (14, 17).
SCCmec is a mobile genetic element characterized by the presence of terminal inverted and direct repeats, a set of site-specific recombinase genes (ccrA and ccrB), and the mecA gene complex (14, 17). The element is precisely excised from the chromosome of N315 and integrates site and orientation specifically into an S. aureus chromosome through the function of a unique set of recombinase genes, ccrA and ccrB. SCCmec was distributed widely in Japanese MRSA strains isolated in the 1990s (11). However, most of the MRSA strains isolated in other countries did not possess SCCmec, as judged by dot-blot hybridization of extracted chromosomal DNA with probes covering various parts of the SCCmec of N315. By cloning and nucleotide sequence determination of the DNA region surrounding the mecA gene from two representative MRSA strains, NCTC 10442 (the first MRSA isolate in England in 1961) and 85/2082 (the 1985 isolate in New Zealand), we found two novel genetic elements that shared similar structural features of SCCmec. We designated them type I (NCTC 10442) and type III (85/2082) SCCmec, and we designated that of N315 type II SCCmec in the order of the year of isolation of the strains. Here, we report a detailed structural comparison of the three types of SCCmec.
MATERIALS AND METHODS
Bacterial strains and media.
All of the MRSA or pre-MRSA strains used in this study are listed in Table 1. Pre-MRSA, represented by N315, is an S. aureus strain that has a mecA gene, but is susceptible to methicillin because of a strong repression of mecA gene transcription exerted by mecI-encoded repressor function (18). Two strains, ATCC 25923 isolated in 1945 and NCTC 8325 (a kind gift from B. Berger-Bachi), were used as methicillin-susceptible S. aureus (MSSA) standard strains.
TABLE 1.
Characteristics of 38 MRSA strains isolated worldwide
| MRSA straina | Country of isolation | Yr of isolation | Coagulase isotypeb | Designation of probe(s) with positive hybridization
|
PCR result for localization of representative gene or structured
|
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCTC 10442 | N315 | 85/2082 | ccr gene typee | IS1272 | mecI | mecR1 PB/MS | mecA | MREP typingf | |||||
| NCTC 10442 | United Kingdom | 1961 | 3 | 1–6 | 2, 3, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 61/6219 | United Kingdom | 1961 | 3 | 1–6 | 3, 4, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 64/3846 | United Kingdom | 1964 | 3 | 1–6 | 2–4, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 64/4176 | United Kingdom | 1964 | 3 | 1–6 | 1–3, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 86/4372(DNH) | United Kingdom | 1986 | 3 | 1 | 1–3, 10 | 2, 3, 5 | − | + | − | −/+ | + | ii | 32 |
| KL3 | Malaysia | 1987 | 3 | 1–6 | 2–4, 10 | 2–5 | 1 | + | − | −/+ | + | i | 38 |
| KL50 | Malaysia | 1989 | 4 | 1, 5 | 1–3 | 1–7 | 3 | − | + | +/+ | + | iii | 38 |
| 86/961 | United Kingdom | 1986 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 86/560 | United Kingdom | 1986 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/1340 | Yugoslavia | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/1762 | Hungary | 1985 | 4 | 1, 5 | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/2082 | New Zealand | 1985 | 4 | 1, 5 | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/2111 | Norway | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/1836 | Germany | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/2147 | Hong Kong | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/3907 | Germany | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 86/2652 | United Kingdom | 1986 | 4 | 1, 2, 4 | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/5495 | Saudi Arabia | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/5328 | Portugal | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | − | 32 |
| 85/3619 | Austria | 1985 | 4 | 5 | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 85/3566 | Holland | 1985 | 4 | None | 1–4 | 1–7 | 3 | − | + | +/+ | + | iii | 32 |
| 82/20-1 | Japan | 1982 | 2 | 1, 2, 5 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 33 |
| 85/2235 | United States | 1985 | 2 | 5 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| 86/JO60 | Japan | 1985 | 2 | 1, 5 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| 86/BB5918 | Japan | 1986 | 2 | 1 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| 87/27 | Japan | 1987 | 2 | 1, 2, 5 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| N315 | Japan | 1982 | 2 | None | 1–10 | 2 | 2 | − | + | +/+ | + | ii | 10 |
| 87/20 | Japan | 1987 | 2 | 1, 2, 5 | 1–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| 87/25 (DNH) | Japan | 1987 | 2 | 1, 5 | 1–4, 6–10 | 1, 2, 4, 5 | 2 | − | + | +/+ | + | ii | 32 |
| 84/9580 | South Africa | 1984 | 2 | 1–6 | 1–4, 10 | 2–5, 7 | 1 | + | − | −/+ | + | i | 32 |
| 86/9302 | United Kingdom | 1986 | 2 | 1–6 | 1–4, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 85/1774 | Italy | 1985 | 2 | 1–6 | 1–4, 10 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 85/1940 | France | 1985 | 2 | 1–6 | 2, 3, 5 | 2–5 | 1 | + | − | −/+ | + | i | 32 |
| 85/4231 | Canada | 1985 | 4 | None | 1–10 | 2–5 | 2 | − | + | +/+ | + | ii | 32 |
| 85/2232 | United States | 1985 | 4 | None | 1–10 | 2–5 | 2 | − | + | +/+ | + | ii | 32 |
| 81/108 (DNH) | Japan | 1981 | 4 | 1, 5 | 2–4 | 2–5 | 2 | + | − | −/+ | + | ii | 12 |
| 93/H44 | Japan | 1993 | 4 | 1, 5 | 1–3 | 1–7 | 3 | − | + | +/+ | + | i | 11 |
| 85/4547 (DNH) | Israel | 1985 | 7 | 1, 3 | 3, 4 | 2, 5 | 2 | + | − | −/+ | + | ii | 32 |
DNH, strain whose chromosomal DNA did not typically hybridize to any of the three sets of the typing probes.
See the article by Ushioda et al. (34) for details of coagulase isotyping.
See Fig. 1 for the locations of the probes.
Localizations of the essential genes in SCCmec were estimated by PCR. The mecA gene and its regulator genes mecI and mecRI of both the penicillin-binding region (PB) and membrane spanning region (MS) are identified by using the primers described by Suzuki et al. (31). Localization of IS1272 in SCCmec was identified with the set of primers mA2, corresponding to the nucleotide sequence of the mecA gene, and iS-4, corresponding to the nucleotide sequence of IS1272 (2). A minus sign indicates that no DNA fragment was amplified by the set of primers described above.
The type of ccr complex was identified with PCR by combining primer β2, which was common to three ccrB genes, and three primers specific for each ccrA gene, α2(ccrA1), α3 (ccrA2), and α4 (ccrA3).
MREP typing is a method to amplify the right extremity region of SCCmec by using the primer sets bracketing the right SCCmec-chromosome junction point. The right PCR primer was cR4, and the left primers for each type of SCCmec were mR2 (types I and II) and mN16 (type III). For the locations of primers, see Fig. 1.
Escherichia coli strain XL1-Blue MRA(P2) was used for the propagation of phage libraries. BamHI-cleaved arms of lambda Dash II (Stratagene, La Jolla, Calif.) were used for the construction of phage libraries. L broth and L agar, used for cultivation of E. coli, and NZY broth, NZY agar, and NZY soft agar, used for the propagation of bacteriophage λ, were prepared according to the method of Sambrook et al. (25). Heart infusion agar, heart infusion broth, brain heart infusion (BHI) broth, and BHI agar (Eiken Kagaku, Co., Ltd., Japan) were used for cultivation of S. aureus.
The following antibiotics were freshly prepared and used at the indicated concentrations: ampicillin (Meiji Seika Co., Tokyo, Japan), 100 μg/ml; tetracycline (Sigma Co., St. Louis, Mo.), 10 μg/ml; latamoxef (Shionogi Pharmacy Co., Osaka, Japan), 15 μg/ml; ceftizoxime (Fujisawa Pharmacy Co., Osaka, Japan), 25 μg/ml.
Cloning and determination of the nucleotide sequence of the SCCmec of NCTC 10442.
Phage libraries were prepared from S. aureus strain NCTC 10442 with partial Sau3A1 digests of chromosomal DNA of NCTC 10442 and lambda Dash II arms cleaved with BamHI (Stratagene) as described previously (14). Phages were propagated to produce plaques on E. coli XL1-Blue MRA(P2) and were lifted onto a nylon filter (Biodyne A; Pall BioSupport, East Hills, N.Y.). Plaque hybridization was performed with digoxigenin-labeled probes (Boehringer, Mannheim, Germany) as described previously (25). Chromosomal DNA fragments containing the left and right boundaries of SCCmec were cloned with the probes 11A and cR, respectively. The preparation of the two probes was described previously (14). With probe 11A, which corresponded to the chromosomal region flanking the left extremity of type II SCCmec or the chromosomal region upstream of attBscc of methicillin-susceptible strain NCTC 8325, lambda clone LO2 containing the left boundary of SCCmec was obtained. With probe cR, which corresponded to the chromosomal region downstream of attBscc of strain NCTC 8325, lambda clone LO21 containing the right boundary of SCCmec was obtained. With probe MA, which was prepared by PCR amplification with a set of primers (mA1 and mA2) based on the nucleotide sequence of the mecA gene (29), lambda clone LO5 containing ccr genes and the mecA gene was obtained.
Lambda clone LO7 containing the region upstream of the ccr genes was cloned by using an XbaI fragment 4.3 kb in size located at the left end of LO5 as a probe. The region downstream of mecA was sequenced with a long-range PCR-amplified DNA fragment 6.6 kb in size amplified by using mA3 and cR2 primers and chromosomal DNA as a template. Similarly, the region between the right end of LO2 and the left end of LO7 was identified by using a 7.5-kb DNA fragment amplified by long-range PCR with primers mE1 (based on the nucleotide sequence of LO2) and mE2 (based on the nucleotide sequence of LO7). The nucleotide sequences of the primers used in these experiments are listed in Table 3, except for the primers described in the previous reports (14, 17). The nucleotide sequence of the entire SCCmec of NCTC 10442 was determined by using the DNA fragments of lambda clones LO2, LO21, LO5, and LO7 and DNA fragments amplified by PCR.
TABLE 3.
Primers used in this study
| Primer | Nucleotide sequence |
|---|---|
| Localization of IS1272 | |
| mA2 | 5′-AACGTTGTAACCACCCCAAGA-3′ |
| iS-4 | 5′-ACAATCTGTATTCTCAGGTCGT-3′ |
| ccr gene type | |
| β2 | 5′-ATTGCCTTGATAATAGCCITCT-3′ |
| α2 | 5′-AACCTATATCATCAATCAGTACGT-3′ |
| α3 | 5′-TAAAGGCATCAATGCACAAACACT-3′ |
| α4 | 5′-AGCTCAAAAGCAAGCAATAGAAT-3′ |
| MREP typing | |
| cR4 | 5′-GTTCAAGCCCAGAAGCGATGT-3′ |
| mR2 | 5′-GATAGACTAATTATCTTCATC-3′ |
| mN16 | 5′-ATATTCTAGATCATCAATAGTTG-3′ |
| Amplification of DNA fragments for nucleotide sequences | |
| NCTC10442 | |
| mE1 | 5′-AACTTCACTGTTGACGACTTA-3′ |
| mE2 | 5′-AACAGCATTAAGAGCAGCTGCACAA-3′ |
| 85/2082 | |
| cLt1 | 5′-AGAAGCGTATCATAAGTAGCGGA-3′ |
| cLt2 | 5′-ATCTTCTGAAGGATAATTCGCA-3′ |
| cLt3 | 5′-TCCGCTACTTATGATACGCTTCTG-3′ |
| cLt4 | 5′-CAATTGGCATGACACTAAATGGCA-3′ |
| mN1 | 5′-TGGAGAATATGAAGATTACATTCA-3′ |
| mN2 | 5′-TTTTGACGATGAAGGTCTTCA-3′ |
| mN3 | 5′-AGTAACGCAACGGGTATGATTA-3′ |
| Tn554(171–146) | 5′-TACGGCTTATTCTCCACTTCTATCCT-3′ |
| Tn554R | 5′-AAGCTGTGGCTTTGAAAAGTTGA-3′ |
| TnpA1016 | 5′-TGTGATGTAAATTCTATTCCAGT-3′ |
| TnpA636 | 5′-TGAGATCAAAGGAAGTTAAGCAAATTATTGATG-3′ |
| cad1 | 5′-TGTAATTGGCGGATATTCACTAT-3′ |
| mN4 | 5′-TAGCAACATAATAGTCATATTTGCT-3′ |
| mN5 | 5′-TTGCTTCGGGACTTACCTCTAGT-3′ |
| mN6 | 5′-ACCTCTAACGTTAACAATATTC-3′ |
| mN7 | 5′-TAATCAATACAAATCTATCGACTTCT-3′ |
| tetK1 | 5′-TTCGATAGGAACAGCAGTATAT-3′ |
| tetK4 | 5′-ATATTACTATACACTCCAGAAGA-3′ |
| merA1 | 5′-AGGCTAAGCAAAATATTTCGGCA-3′ |
| merA2 | 5′-TCTTCACAGCCTGTGCATGTCATGCCT-3′ |
| merN | 5′-ACGGATTGCTGTACGCCTCCAGA-3′ |
| mN8 | 5′-ACTCTGTACCTCATCCACAGTTTGA-3′ |
| mN9 | 5′-ACCAGACCGTCCTTTCGATTTAACAA-3′ |
| mN10 | 5′-GACAACATGATTTAGAAGTAGAGGT-3′ |
| mN11 | 5′-ATCGTCCGAGGACTTGTATCGAGTTCTA-3′ |
| mN12 | 5′-TACCATTCTTAGCTCCACCATAT-3′ |
| mN13 | 5′-ACAACTTGCGAATTATGACGA-3′ |
Amplification of DNA fragments by PCR and determination of the entire nucleotide sequence of the SCCmec of 85/2082.
We have cloned the regions containing both boundaries of SCCmec of MRSA 85/3907 (11) and determined their nucleotide sequences (DDBJ/EMBL/GenBank accession no. AB047088 and AB047089). The regions containing both boundaries of the SCCmec of 85/2082 were similar to those of 85/3907, and the surrounding region of the mecA gene was similar to that of MRSA strain ANS46 described previously by Dubin's group (4, 6). Taking advantage of this similarity, we could successfully amplify DNA fragments covering the entire SCCmec of 85/2082 by long-range PCR with several sets of primers. The DNA fragment corresponding to the chromosomal region upstream to the left extremity of SCCmec was amplified by long-range PCR with a set of primers, cLt1 and cLt4. The region spanning from the left extremity of SCCmec to transposon ΨTn554 was amplified with two sets of primers (cLt2 and mN2; mN1 and Tn554[171–146]). By using nine sets of primers, we could amplify the region spanning from transposon ΨTn554 located upstream of mecA to transposon Tn554 located downstream of mecA. The sets of primers used were as follows: mN3 and mN5 (the region in and around ΨTn554); cad1 and mN6 plus mN4 and mN7 (the region from ΨTn554 to downstream of mecA gene); mA3 and tetK1 (the region from mecA to plasmid pT181); tetK4 and merA1 (the region from pT181 to the mercury operon); is-1 and merN plus merA2 and mN9 (the mercury operon flanked by a pair of IS431 sites); mN8 and Tn554R; and mN11 and TnpA636 (the region spanning from IS431 to transposase A of Tn554).
The region spanning from Tn554 to the right extremity of SCCmec was amplified by long-range PCR with two sets of primers, TnpA1016 and mN13 and mN12 and cR1.
By using these DNA fragments amplified by long-range PCR, the nucleotide sequence of the entire SCCmec of 85/2082 was determined.
The primer cLt4 was designed based on the nucleotide sequence of pSJ9 cloned from 85/3907 (11). Primers cLt1, cLt2, cLt3, mN1, and mN2 were designed on the basis of the nucleotide sequence of the left extremity of SCCmec and its flanking chromosomal region of 85/3907 (DDBJ/EMBL/GenBank accession no. AB047088). The primers mN4, mN5, mN6, and mN7 were designed on the basis of the type II SCCmec sequence (DDBJ/EMBL/GenBank accession no. D86934). The following primers were designed on the basis of the previously reported sequences: Tn554(171–146), Tn554R, TnpA636, and TnpA1016, Tn554 (DDBJ/EMBL/GenBank accession no. X03216); cad 1, ΨTn554 (DDBJ/EMBL/GenBank accession no. L10909); is-1, IS431mec (DDBJ/EMBL/GenBank accession no. X53818); tetK1 and tetK4, plasmid pT181 (DDBJ/EMBL/GenBank accession no. JO1764); and merA2 and merN, mer operon of pI258 (DDBJ/EMBL/GenBank accession no. L29436).
The primers mN12 and mN13 were designed on the basis of the nucleotide sequence of the right extremity of SCCmec of 85/3907 (DDBJ/EMBL/GenBank accession no. AB047089).
DNA manipulation.
Colony hybridization and plaque hybridization were performed by using cellular DNA extracted from MRSA strains and digoxigenin-labeled probes (Boehringer Mannheim Biochemica, Mannheim, Germany) as described previously (31).
Large-scale and small-scale preparation of plasmid DNA, purification of phage plaques, extraction of DNA from purified phage particles, and subcloning of DNA fragments into plasmid vector pUC118 or pUC119 were performed by standard techniques (25). All of the enzymes for DNA manipulation were purchased from Takara Shuzo Co., Ltd., Kyoto, Japan, except for Taq DNA polymerase for PCR, which was purchased from Perkin-Elmer, Foster City, Calif.
Nucleotide sequence determination was performed as described previously (10), with a Dye Terminator Cycle Sequencing FS Ready Reaction kit (Perkin-Elmer). A description of primers synthesized specifically for the primer extension sequence determination was omitted from the text.
Isolation of SCCmec-excised strains.
The method of obtaining SCCmec-excised strain N315ex from N315 has been described previously (17). The type I SCCmec-excised strain 85/1940ex was obtained by the method used for the preparation of N315ex, with a slight modification. Briefly, the recombinant plasmid, pSR, which carries ccrA and ccrB genes, was introduced into 85/1940 cells by electroporation. After overnight cultivation of the transformant strain 85/1940(pSR) on BHI agar containing tetracycline (10 μg/ml), cells were resuspended in saline and plated onto BHI agar containing tetracycline (10 μg/ml) for replica plating. The loss of methicillin resistance was examined first by replica plating on the agar with and without latamoxef (15 μg/ml). Latamoxef-susceptible colonies were selected and examined further to determine whether precise excision of type I SCCmec occurred by PCR with the primer set cR2 and cL1. Plasmid pSR was eliminated by serial cultivation in drug-free BHI broth at 43°C to obtain 85/1940ex.
The SCCmec-excised strain 85/2082ex was obtained by spontaneous excision of SCCmec from 85/2082. Cells were cultivated in BHI broth at 37°C for 2 to 3 days, and then a 0.1-ml portion of the cell suspension was inoculated into new BHI broth. After 38 days of serial passages, cells were plated onto BHI agar. The strains that had lost β-lactam resistance were identified by replica plating onto BHI agar containing ceftizoxime (25 μg/ml), and precise excision of SCCmec of these strains was confirmed by PCR with the primer set cLt3 and cR2 (Fig. 1).
FIG. 1.
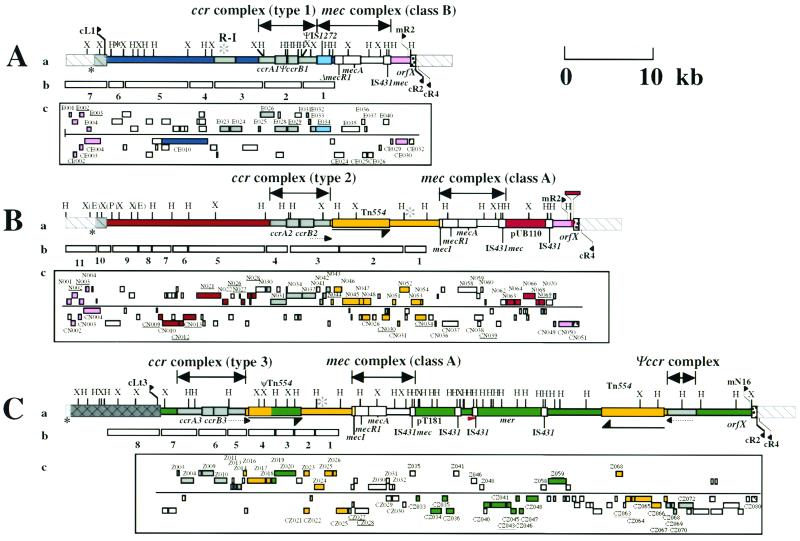
Structures of three types of SCCmec. The structures of type I SCCmec (A), type II SCCmec (B), and type III SCCmec (C) are illustrated based on the nucleotide sequences deposited in the DDBJ/EMBL/GenBank databases under accession no. AB033763 (type I), D86934 (type II), and AB037681 (type III). (a) Essential structure of SCCmec. Locations of the essential genes are illustrated. The restriction sites of HindIII and XbaI are indicated. Only the EcoRI (E) and PstI (P) restriction sites, which are inevitable for the description of probes of type II SCCmec, are indicated (in parentheses). The essential genes as well as other regions are colored based on the color of the ORFs described in panel C. The regions common to MSSA are shown in white with gray-striped bars. The regions found in S. aureus NCTC 8325 and not found in ATCC 25923 are shown in gray with striped bars. The regions common to S. aureus ATCC 25923 and not found in NCTC 8325 are shown in dark gray with cross-hatched bars. Small black asterisks signify the locus common to all three strains. Large gray asterisks signify the region common to three SCCmecs. An arrowhead signifies the location of a 15-bp sequence similar to that of N315 found between the second and the third IS431 copies of type III SCCmec. Arrows indicate the direction of Tn554 or ΨTn554. Dotted arrows indicate the transcription of direction of the ORFs located downstream of ccrB or CZ072. (b) Probes used for dot-blot hybridization. (c) ORFs in and around SCCmec. The ORFs >200 bases in size in six possible reading frames are indicated by squares. Those above the line are the ORFs that have transcription directed to the right, and those below the line have transcription directed to the left. Colored squares are the ORFs the extant gene homologues of which were found in the databases, although many of them were considered incomplete (underlined). The color corresponds to the difference in the conservation of the ORFs (or regions) in three types of SCCmec white, ORFs in three types of SCCmec with identity of more than 99%: gray, conserved in three types of SCCmec with on identity score of 47 to 92%; magenta, ORFs common to type II and type III SCCmec; yellow, ORFs common to type II and type III SCCmec; blue, ORFs unique in type I SCCmec; red, ORFs unique in type II SCCmec; green, ORFs unique in type III SCCmec. ORFX is indicated in a stippled box.
PCR amplification.
PCR was performed essentially as described previously (31) with a 50-μl reaction volume and with thermal cycler Gene Amp 9600 (Perkin-Elmer Cetus Instruments, Emeryville, Calif.). Long-range PCR was performed with Expand Taq (Boehringer Mannheim Biochemica, Mannheim, Germany) according to the procedure recommended by the manufacturer. PCR products were purified with High Pure PCR product purification kit (Boehringer Mannheim Biochemica). The nucleotide sequences of the primers used in this experiment are described in Table 1 or were reported previously (14, 17).
Computer analysis of nucleotide and protein sequences.
All of the analyses were carried out with programs in the Wisconsin Package (version 9.0; Genetics Computer Group, Madison, Wis.). A homology search was performed with the BLAST and TFastA programs for the EMBL (release no. 55.0) and GenBank (release no. 107.0) databases, and the FastA program for the SWISS-PROT database (release no. 35.0). Tree View software was obtained from the web site http://taxonomy.zoology.gla.ac.uk/rod/treview.html.
RESULTS
Two mecA-carrying elements are new members of the SCCmec family.
Initially, we tested whether the previously identified SCCmec on the chromosome of Japanese S. aureus strain N315 was also distributed in MRSA strains around the world. Thirty-eight representative epidemic strains listed in Table 1 were analyzed by dot-blot hybridization with 10 probes prepared in the N315 SCCmec. (See Fig. 1 for the location of the probes). A typical positive hybridization pattern was observed with the DNAs extracted from 9 of the 38 MRSA strains, but with others, only a few probes reacted positively, or the hybridization signal intensities were weak even if they reacted positively (Table 1). Since all of the listed strains possessed the mecA gene, the observation indicated that there were other types of genetic elements carrying the mecA gene on their chromosome.
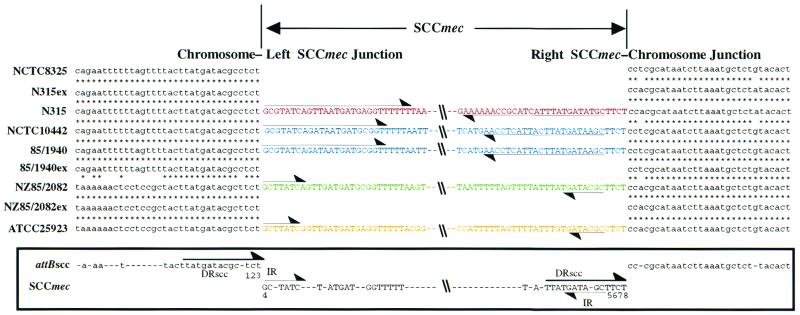
From the strains with an atypical hybridization pattern to N315 SCCmec probes, two strains, NCTC 10442 and 85/2082, which represented two different (incomplete) hybridization patterns to N315 SCCmec probes, were chosen for cloning of the DNA region surrounding the mecA gene. With a strategy of cloning described in Materials and Methods, we identified from these strains new genetic elements that were inserted at the attB site of SCCmec (14). The boundaries of the element of NCTC 10442 were identified by comparing its nucleotide sequence with that of mecA-negative S. aureus type strain NCTC 8325 (Fig. 2). The boundaries of the element of 85/2082 were determined by comparing its nucleotide sequence with that of strain 85/2082ex, a spontaneous SCCmec-excised strain (Fig. 2). (We have previously reported that some MRSA strains spontaneously generate SCCmec excisant strains when cultivated in drug-free medium [14, 17]. Strain 85/2082 was one of the strains generating spontaneous excisants.)
FIG. 2.
Boundaries of SCCmec. Nucleotide sequences around the left and right boundaries of SCCmecs of N315, NCTC 10442, 85/1940, and 85/2082 (attL and attR respectively) are aligned with the sequence around the presumptive integration site, attB, on the chromosome of MSSA strains (NCTC 8325, ATCC 25923, N315ex, 85/1940ex, and 85/2082ex). The boundary sequences of an inserted element, IE25923 (DDBJ/EMBL/GenBank accession no. AB047239), identified in an MSSA type strain, ATCC 25923, is also shown in comparison with those of SCCmec. Inverted complementary repeats IR-L and IR-R at both extremities of SCCmecs are indicated by thin arrows. Direct repeats are indicated by thick arrows. Asterisks indicate identical nucleotides in the two sequences. Consensus sequences of attBscc and inverted repeats of SCCmec are indicated below. The left- and rightmost nucleotides were each inferred to be one of the four nucleotides numbered 1 to 4 and 5 to 8, respectively, in the figure. The entire lengths of three SCCmecs were 34, 364, 53,017, and 66,896 bp. We have revised the nucleotide sequence of type II SCCmec (17). We found that 931- and 344-bp deletions had occurred in the cloned DNA fragment and a sequencing error. The 931 bp segment was deleted upstream of ORF N026, and 344 bp was deleted downstream of ORF N028 (Fig. 1). Consequently, the size of type II SCCmec changed from 51,669 bp to 53,017 bp.
The two elements were found integrated at exactly the same nucleotide position in open reading frame (ORF) X gene orfX as that N315 SCCmec utilizes for integration (17). Both elements had characteristic 15-bp direct repeat sequence (DRscc-R) at the right extremity and its counterpart (DRscc-L) in the chromosomal region abutting the left terminus of the element (Fig. 2), although direct repeat sequences of 15 bp were incomplete (with 13 identical bases) in the case of the SCCmec of NCTC 10442. Curiously, another copy of 15-bp sequence similar to that of N315 SCCmec (Fig. 2) was found on the 85/2082 element between the second and the third IS431 copies (shown by an arrowhead in Fig. 1). Degenerate inverted repeats characteristic of SCCmec were also found in the extremities of all three elements.
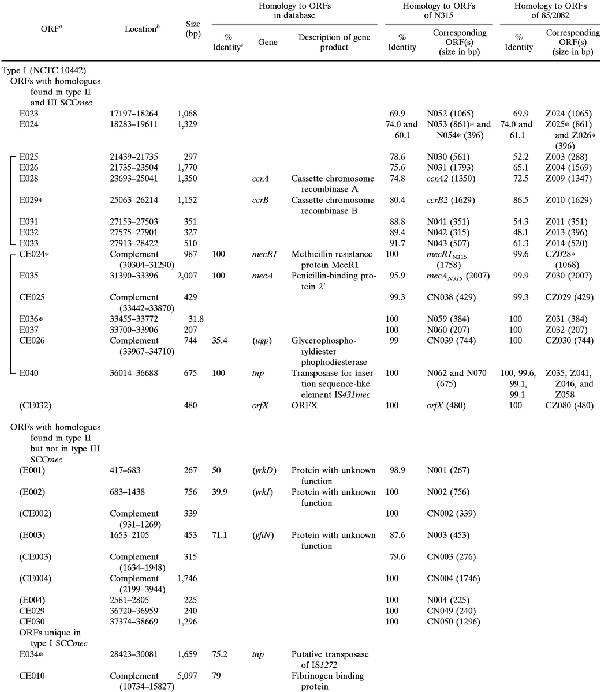
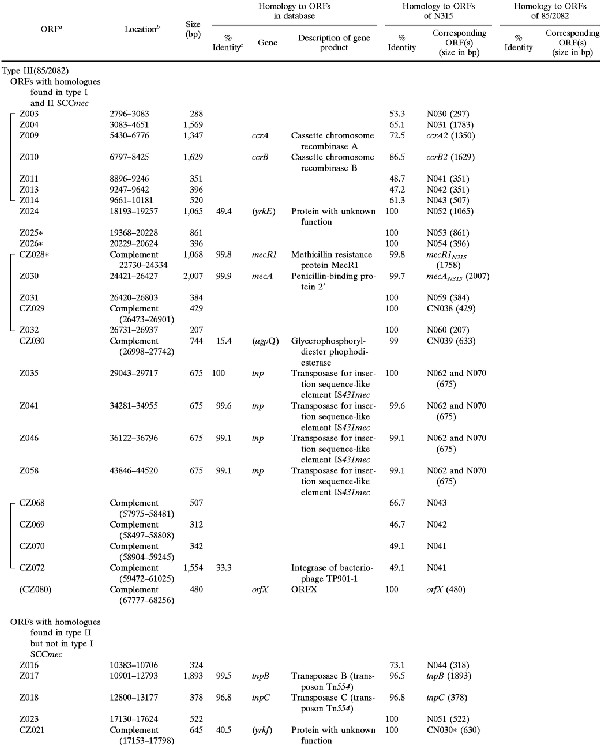
Shown in Fig. 1 are the genomic organizations of the two novel elements identified from NCTC 10442 and 85/2082 in comparison with that of N315 SCCmec. The regions conserved in all three elements are illustrated in white (amino acid identities among the corresponding ORFs were equal to or greater than 99%) and in gray (identities equal to or greater than 47%). Two classes of mec gene complex, either with a complete structure (mecI-mecR1-mecA-IS431 [class A mec complex]) or with a deletion and integration of an insertion sequence (IS1272-ΔmecR1-mecA-IS431 [class B mec complex]), were created with the entire SCCmec. Another common structure was a ccr complex (composed of ccrA, ccrB homologue genes, and surrounding ORFs) (Table 2 and Fig. 1). The ccrA and ccrB genes encoding putative site-specific recombinases of SCCmec are known to be responsible for the movement (excision and integration) of N315 SCCmec from and into the S. aureus chromosome (17). The corresponding ORFs found in the two other elements had a substantial homology to the ccr genes of N315, although the ccrB gene of NCTC 10442 had a frameshift mutation (Table 2). Based on the structural similarities described above, these elements were considered to be new members of the SCCmec family. Accordingly, the two elements found in NCTC 10442 and 85/2082 were designated type I and type III SCCmec, respectively, and that of N315 was designated type II SCCmec. The ccr gene homologues found in each SCCmec were designated ccrA and ccrB genes with an Arabic numeral suffix to show the type of SCCmec with which they were associated. It was noted that the ORFs adjacent to each type of ccr gene were also conserved (amino acid identities of the corresponding ORFs were equal to or greater than 47%) among the three types of SCCmec; thus, they were unified as a ccr complex together with the ccr genes.
TABLE 2.
ORFs in and around type I SCCmec (NCTC 10442) and type III SCCmec (85/2082) with deduced products showing similarities to extant protein sequences
 |
 |
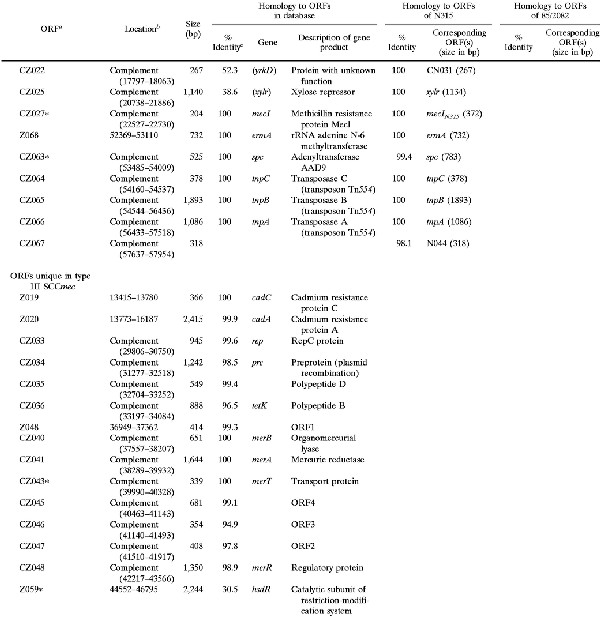 |
ORFs shown in parentheses were located outside of SCCmec. Asterisks signify incomplete ORFs that are potentially defective genes or peseudogenes containing either a deletion, nonsense mutation, or frameshift mutation.
Nucleotide position in the nucleotide sequence deposited under DDBJ/EMBL/GenBank accession no AB033763 (type I) and AB0376701 (type III).
Identity to the amino acid sequence of the best match revealed in the homology search of the GenBank and EMBL databases with TFastA.
Genes shown in parentheses had a deduced ORF with less than 70% amino acid identity to the ORF indicated.
Another region, called R-I, 3.5 kb in size was identified in type I SCCmec that had a substantial similarity to the intervening region between Tn554 (or ΨTn554) and mecI of type II and type III SCCmec (indicated by large asterisks in Fig. 1). Two ORFs of unknown function were contained in the R-I and corresponding regions of type II and type III SCCmec, the amino acid identities of the deduced polypeptides of which were greater than 52% (Table 2).
Structure outside the integration site of SCCmec.
Nucleotide sequencing of the regions around the left and right chromosome-SCCmec junctions revealed that the orfX genes of three MRSA strains, NCTC 10442, N315, and 85/2082, were extremely well conserved. All of the orfX genes were composed of 480 nucleotides with greater than 99% identity, and their encoded polypeptides were identical (Table 2). Thus, the nucleotide sequences of the chromosomal regions abutting the right junction point of SCCmec were extremely well conserved. In contrast, the nucleotide sequences abutting the left boundary of type III SCCmec differed substantially from those abutting type I and type II SCCmec.
Other genetic components of SCCmec.
The size of type I SCCmec (NCTC10442) was 34,364 bp, and that of type III SCCmec (85/2082) was 66,896 bp. In contrast, the size of type II SCCmec (N315) was 53,017 bp. These differences in size were due to the presence of a type-specific DNA region in addition to the essential structures of SCCmec. The regions commonly shared by type I and type II SCCmec are shown in magenta; they were located at the right extremities of the elements between the rightmost IS431 copy and the right junction point (Fig. 1). Two ORFs of unknown function were contained in the region, and they were identical between the two types of SCCmec (Fig. 1). The nucleotide sequence of the region was also extremely well conserved between the two types (only 1 base substitution in 2, 120 bases), but type II SCCmec possessed an additional 102 bases of unique sequence in the very end of SCCmec (shown in red in Fig. 1).
The regions common to type II and type III SCCmec are shown in yellow; these regions were located between the ccr and mec complexes (Fig. 1). In the case of type III SCCmec, another copy of Tn554 was found downstream (to the right) of the mec complex, which was also associated with a ccr-complex-like structure (designated the Ψccr complex in Fig. 1), just as Tn554 in the mid-part of the element was associated with ccr complex. The Ψccr complex was composed of a ccrB homologue and three adjacent ORFs whose deduced amino acid sequences had greater than 30% identity to the corresponding ORFs in the type II ccr complex (Fig. 1 and Table 2).
The regions unique to each type of SCCmec are illustrated in Fig. 1 in either blue (type I), red (type II), or green (type III). No antibiotic resistance gene except for mecA was found in type I SCCmec. In contrast, type III SCCmec contained multiple antibiotic resistance genes. They were transposon ΨTn554 encoding cadmium resistance inserted between ccr and mec complexes, an integrated copy of plasmid pT181 encoding tetracycline and mercury resistance, and another transposon, Tn554, encoding erythromycin and spectinomycin resistance. The latter three were found downstream (to the right) of the mec complex, and pT181 and the mer operon were found bracketed by a pair of IS431 copies.
The ORFs contained in the left half of type I SCCmec were type specific (shown in blue in Fig. 1), but mostly unknown with regard to their function, except for one. The CE010 ORF potentially encoded a polypeptide belonging to the Shine-Dalgarno repeat multigene family (16), which was nearly identical to a large surface protein of S. aureus designated plasmin-sensitive surface protein (Pls) (8, 26). It was a repeat-rich protein characteristically having unusual dipeptide repeats composed of serine and aspartate residues. Another unique ORF of type III SCCmec was Z059, the deduced amino acid sequence of which showed a high similarity to HsdR of Klebsiella pneumoniae and Salmonella enterica, which was flanked by IS431 (Fig. 1). HsdR is a catalytic subunit of the restriction-modification system (19). However, the N-terminal portion of the ORF Z059 appeared to be deleted by approximately 300 amino acid residues compared to the intact HsdR.
Distribution of the three types of SCCmec in clinical MRSA strains around the world.
The 38 MRSA strains listed in Table 1 were reanalyzed with dot-blot hybridization with the probe sets prepared in each of the three types of SCCmec (see Fig. 1 for the locations of probes). Now, 34 strains showed typical hybridization patterns to either one of the three sets of probes (Table 1). In the 34 strains, additional PCR typing experiments of various genes and right extremity polymorphism (REP; see below) listed in Table 1 agreed with the dot-blot hybridization results. For example, all of the strains with type I SCCmec reacted positively to PCR detection of IS1272 and negatively to PCR detection of the mecI gene (Table 1). The results of ccr complex typing also agreed with the SCCmec typing results (Table 1).
We also used the PCR typing method designated mec right extremity polymorphism (MREP) typing (11). MREP typing is a quick SCCmec typing method that takes advantage of the polymorphism among the three types of SCCmec in the right extremity: type III had a unique nucleotide sequence, and type II SCCmec had additional 102-bp nucleotides to the right terminus of type I SCCmec (Fig. 1). PCR primers were prepared to bracket the right SCCmec-chromosome junction point to detect the polymorphism of the three types of SCCmec. MREP typing results of the 34 strains also agreed with those of SCCmec typing, but with two exceptions: strain 85/5328 did not react positively to any set of primers, and strain 93/H44, having type III SCCmec by dot-blot hybridization, was judged as having type I MREP (typically associated with type I SCCmec of NCTC 10442) (Table 1). Four strains, 81/108, 85/4547, 86/4372, and 87/25, did not hybridize typically to any of the three sets of SCCmec probes. However, strain 87/25 hybridized with all of the probes of type II SCCmec, except for probe 5. The other three strains appeared to carry the class B mec complex (IS1272-ΔmecR1-mecA-IS431) characteristically found in type I SCCmec. However, strains 81/108 and 85/4547 responded to the primer set of the type II ccr complex. In the case of strain 86/4372, a PCR using the three sets of ccr primers did not amplify any DNA fragment (see Discussion).
Experimental precise excision of type I SCCmec with type 2 ccr genes.
Spontaneous precise excision of type II SCCmec in the culture of MRSA strains can be detected by the PCR amplification method by using the extracted DNA from the strain as a template and by using a primer set of cR2 and cL1 bracketing the integration site for SCCmec (designated attB PCR) (see Fig. 1 for the location of primers, and see Table 3 for their sequences) (14, 17). In this study, a set of primers, cR2 and cLt3 (see Fig. 1 for the location of primers), was prepared to detect spontaneous excision of type III SCCmec. DNA extracted from an overnight culture of strain 85/2082 was positive with the set of primers, which coincided with the fact that the spontaneously excised strain 85/2082ex was obtained after serial passages of the strain in drug-free medium. In contrast, the set of primers cR2 and cL1, which theoretically detect excision of type I as well as type II SCCmec, could not amplify DNA from the culture of NCTC 10442. This was in agreement with the fact that ccrB1 of NCTC 10442 had a frameshift mutation (Table 2). Moreover, all nine strains with type I SCCmec were negative with the attB PCR, and the mutation found in the ccrB1 of NCTC 10442 was commonly found in all nine of the ccrB1 genes. Therefore, we could not obtain a spontaneous excisant strain from any of the strains having type I SCCmec. To reconfirm the boundary of type I SCCmec, therefore, we introduced type 2 ccr genes into the type I SCCmec-carrying strain 85/1940. The strain was used instead of NCTC 10442, because the latter strain was resistant to tetracycline; tetracycline resistance was used as a marker for the selection of the transformants. The culture of a transformant strain 85/1940(pSR) generated β-lactam-susceptible cells with a high frequency. The nucleotide sequences around the attB region of the excisant strain 85/1940ex thus obtained are shown in Fig. 2. The type I SCCmec integration site on the chromosome of 85/1940 coincided with that inferred by comparing the corresponding regions of NCTC 10442 and NCTC 8325 strains (Fig. 2).
Molecular evolutionary relationship of members of the ccr gene family and mecA genes.
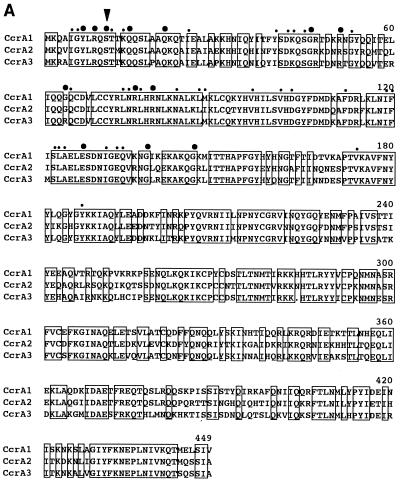
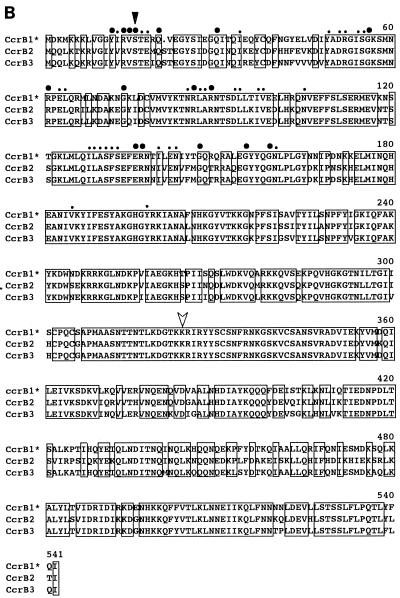
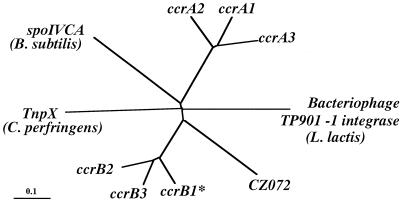
Comparisons of the deduced amino acid sequences of ccrA and ccrB genes of three types of SCCmec are shown in Fig. 3. Since ccrB1 seemed to be disrupted by a deletion of a single base, we have reconstituted a potential ccrB1 gene (ccrB1∗) by an addition of adenine at the position where it seemed to be deleted and used its deduced product for the comparison. All of the Ccr proteins were highly basic, with pI values of 10.07 to 10.49, and shared the motifs of the site-specific recombinases of the invertase-resolvase family in their N-terminal domains (27). The catalytic serine residue of the recombination active site was also conserved in all Ccr proteins. Figure 4 illustrates the phylogenetic relationship among the ccrA and ccrB genes of three types of SCCmec. We also compared them with the CcrB-like product of ORF CZ072 found in type III SCCmec and with several site-specific recombinases of gram-positive bacteria as well. The latter were the site-specific integrase of bacteriophage TP901-1 of Lactococcus lactis; a site-specific recombinase (5), spoIVCA, of Bacillus subtilis (30); and a transposase, tnpX, of Clostridium perfringens (3), which had comparable sizes and substantial amino acid similarities to ccr genes. The phylogenetic tree showed that ccrA and ccrB genes form separate subfamilies. The CZ072 ORF was more closely related to the ccr genes of subfamily B than those of subfamily A. The three site-specific recombinases of other bacterial species were distantly related to either one of the two ccr subfamilies (Fig. 4).
FIG. 3.
Deduced amino acid sequences of ccr genes. (A) Deduced amino acid sequences of ccrA1, ccrA2, and ccrA3. (B) Deduced amino acid sequences of ccrB1∗, ccrB2, and ccrB3. Amino acid sequences of ccr genes were aligned by using the Pile-Up program with a GCG default scoring matrix in the Wisconsin Package (version 9.0; Genetics Computer Group). Amino acids shared by the three peptides are boxed. Large bullets indicate amino acid residues of Ccr proteins shared by site-specific recombinases of the invertase-resolvase family. Small bullets indicate amino acid substitution within the same class of amino acids. A black arrowhead indicates the presumptive serine involved in the phosphoseryl linkage of the recombinase of DNA conserved in the NH2-terminal catalytic domain of site-specific recombinases of the invertase-resolvase family (27). A white arrowhead indicates the locus of the first amino acid residue changed by an addition of adenine in the nucleotide sequence of type I SCCmec as described in the text.
FIG. 4.
Phylogenetic relationships among ccrA genes, ccrB genes the ORF CZ072, and three site-specific recombinases. Three site-specific recombinases that showed a high similarity to ccr genes were selected to investigate phylogenetic relationships. They were the integrase (int) of bacteriophage TP901-1 found in L. lactis (1,458 bp; DDBJ/EMBL/GenBank accession no. X85213), the site-specific recombinase (spoIVCA) found in B. subtilis (1,503 bp; DDBJ/EMBL/GenBank accession no. D32216), and the transposase (tnpX) found in the conjugative transposon Tn4451 of C. perfringens (2,124 bp; DDBJ/EMBL/GenBank accession no. U15027). The nucleotide sequences of the ccrA genes (ccrA1, ccrA2, and ccrA3), ccrB genes (ccrB1∗, ccrB2, and ccrB3), ORF CZ072, int, spoIVCA, and tnpX were aligned by using the Pile-Up program with a GCG default scoring matrix. Phylogenetic relationships were developed with the Paupsearch program by the neighbor-joining method. The tree was visualized with Tree View software. The branch length indicates the distance, which is expressed as the number of substitutions per 100 bases.
The phylogenetic relationships among the mecA genes were also compared by using the nucleotide sequences of the mecA genes in three types of SCCmec and previously reported ones in coagulase-negative staphylococcal species S. epidermidis WT55 (DDBJ/EMBL/GenBank accession no. X59592) (24) and S. sciuri K8 (DDBJ/EMBL/GenBank accession no. Y13069) (37) (data not shown). All five mecA genes were 2,007 bp in size. The mecA gene of N315 was identical to that of S. epidermidis WT55, whereas it differed from those of S. aureus NCTC 10442 and S. sciuri K8 by 1 bp and from that of S. aureus 85/2082 by 2 bases. Therefore, they were very well conserved, in contrast to the ccrA and ccrB genes.
DISCUSSION
We have shown that there are at least three distinct types of SCCmec in the chromosome of MRSA worldwide. SCCmec was defined as the DNA element on the MRSA chromosome demarcated by a pair of direct repeats and inverted repeats, having ccr genes required for its movement and carrying the mecA gene (14, 17). As far as we could judge from the structure of the two elements newly identified in this study, they seem to constitute a family of SCCmec together with N315-type SCCmec.
The mecA gene is considered to have originated in some coagulase-negative staphylococcus species (36) and was then transferred into S. aureus to generate MRSA (1, 13, 32). It is likely that SCCmec serves as the carrier of the mecA gene moving across staphylococcal species, since mecA genes in other staphylococcal species have never been found without the accompaniment of SCCmec-like structure (T. Ito and Y. Katayama, unpublished observation). Since both ccrA and ccrB genes are required for the integration event, we considered that the ccrB1 gene must have been intact when SCCmec was introduced into the recipient S. aureus cell to produce NCTC 10442 or its precedent strain (17). However, so far, our search for the intact ccrB1 in MRSA as well as in methicillin-resistant coagulase-negative staphylococcus (MRC-NS) strains isolated in Japan in 1980s and 1990s have not been successful (K. Tsutsumimoto, unpublished observation). Successful excision of type I SCCmec by type II ccr genes raised another related question of whether the type 1 and type 2 ccr genes were present as distinct types before or even soon after the integration of SCCmec into the S. aureus chromosome. We cannot rule out the possibility that the type 1 ccr genes were derived from type 2 ccr genes after establishment of type II SCCmec in the MRSA chromosome as a result of sequential accumulation of mutations. To finally clarify the question, it would be necessary to find intact type 1 ccr genes retaining recombination function or, alternatively, to test the functional integrity of ccrA1 in combination with the ccrB1 gene artificially reconstructed from ΨccrB1 by eliminating the frameshift mutation. Study in this direction is under way.
It is also noteworthy that type III SCCmec contained another unit of ccr complex (Ψccr complex). Moreover, the 15-bp direct repeat sequence present at the right end of SCCmec was also found between the two IS431 copies in type III SCCmec, as illustrated in Fig. 1. These findings indicate that the type III SCCmec is composed of two separate SCCmec or SCC (cassette chromosome without mec complex) elements that were sequentially integrated in the chromosome of 85/2082. We have observed that an experimental SCC plasmid carrying type 2 ccr genes, attSCC, and tetL as a selective marker can integrate into N315 chromosome side-by-side with the type II SCCmec (H. Yuzawa, unpublished data). In light of this, the putative right-part element of type III SCCmec may be an SCC carrying a transposon, Tn554, the erythromycin and spectinomycin resistance of which serves as a selective marker. Alternatively, it may be another copy of SCCmec in which the mec complex had been deleted together with the ccrA gene after integration into the chromosome.
In contrast to the old strain, NCTC 10442, recent MRSA isolates are resistant to many antibiotics besides β-lactam antibiotics (9). Such a multiple resistance of MRSA is attained by the activity of IS431 copies downstream of mec complex (Fig. 1). IS431 is known to serve as a chromosomal deposit site for multiple resistance genes (21). The integrated copies of pUB110 (in type II SCCmec) and pT181 (in type III SCCmec) were flanked by two copies of IS431 (6, 22). Direct repeats were present at both ends of these integrated plasmids. This suggests that these plasmids were accumulated by homologous recombination events across two copies of IS431: one present on the chromosome and the other present on the plasmid (28).
We referred to the nucleotide sequences of two other MRSA strains by using the BLAST search program of The Institute for Genomic Research (TIGR) (http://www.tigr.org /cgi-bin/BlastSearch/blast.cgi?organism=s_aureus) and the Sanger Centre (http://www.sanger.ac.uk/Projects/S_aureus/). When we compared the nucleotide sequence of type I SCCmec, we found that strain COL, which is being sequenced by the genome project of TIGR, carried a DNA region corresponding to the element. The overall nucleotide identity of the region corresponding to type I SCCmec was 98% over 34,364 bases of alignment. The minor difference was found in the CE010 ORF potentially encoding pls, a polypeptide belonging to the SD repeat multigene family. On the other hand, strain 252 (EMRSA-16), which is being sequenced by the Sanger Centre, carried DNA regions divided into three contigs, which corresponded to type II SCCmec. There was a greater than 95% nucleotide identity between the two elements.
Thirty-four of 38 MRSA strains carried one of the three types of SCCmec. However, four strains reacted atypically to any of the three sets of probes. One of them, 85/25, may be interpreted simply as a carrier of type II SCCmec with a minor deletion in it: the chromosomal DNA of the strain hybridized with 9 of 10 type II probes positively and served as a positive template for the PCR detecting type 2 ccr genes (Table 1). The other three strains, however, were much more complex. The results of a PCR experiment suggested that they carried type II ccr complex (except for 85/4372), and the structure of the type ii right extremity (MREP type corresponding to type II SCCmec). However, the strains possessed the class B mec complex typically carried by type I SCCmec. There are several possible explanations for these cases. (i) The class B mec complex was introduced into a type II SCC (presumptive primordial element without a mec complex) located in a staphylococcus chromosome. (ii) Two SCC (or SCCmec) elements of type I and type II were cointegrated in the strains at the attB site, and, subsequently, homologous recombination between the two elements took place, leaving a chimeric SCCmec. In either case, the negative hybridization reaction of these strains with most probes of type I or type II SCCmec should be explained by the deletion of the region upstream of the ccr complex subsequent to their integration in the chromosome. If this was not the case, and there were unique DNA regions not hybridizable with either set of SCCmec probes, the elements carried by the strains may be referred to as other types of SCCmec.
With regard to strain 85/4372, no DNA fragment was amplified by PCR with a set of primers used for the ccr typing or those detecting the common nucleotide sequences of the three types of ccrA and ccrB genes (T. Ito, unpublished observation). Therefore, the ccr genes of this strain might have either been deleted or composed of a different nucleotide sequence from those of the three ccr genes described in this paper. Further experiments are required to clarify the complex structure of SCCmec of these strains.
There is no reason to limit the putative SCC to being only the conveyer of methicillin resistance alone. It might be serving as a vehicle for exchange of useful genes for the better survival of staphylococci in various environments. For example, plasmin-sensitive surface protein is found in type I SCCmec, and the Kdp operon, encoding potassium-dependent ATPase and its regulators, is carried by type II SCCmec. They, although mostly found as pseudogenes, might have been useful for the host cells to survive in their particular environment. Our proposal that SCC is a general genetic information exchange system of staphylococci and is not confined to antibiotic resistance also comes from our finding with an MSSA strain, ATCC 25923. The S. aureus type strain, isolated in 1945, long before the first isolation of MRSA (in 1961), carried a DNA fragment 5,877 bp in size (designated IE25923) inserted at exactly the same nucleotide position in orfX as that utilized by three SCCmecs for their integration. (The entire sequence of IE25923 is available under DDBJ/EMBL/GenBank accession no. AB047239.) Moreover, it had similar structural characteristics of SCCmecs at both ends, i.e., incomplete inverted repeats and direct repeats of 15 bp (Fig. 2). The nucleotides of both the left and right extremities showed high similarity to those of type III SCCmec: 92.6% (25 identical bases in 27 nucleotides at the left extremity) and 93.1% (27 identical bases in 29 nucleotides at the right extremity) (Fig. 2). However, the other region of IE25923 did not show any significant similarity to three types of SCCmec. No drug resistance gene was found in it. Unfortunately, no ORFs with inferable function based on the search of extant gene products or ccr genes were found in it. Therefore, IE25923 seems to be a remnant of SCC or SCCmec that was integrated in its complete form and then afterwards deleted with ccr as well as with mec complexes. Substantiation of our proposal will await finding a complete form of SCC carrying ccr genes, but with no mec complex on it.
In conclusion, we have found that the mec complex, conferring methicillin resistance to S. aureus, was conveyed by a novel family of the mobile genetic element SCCmec. SCCmec is defined by its characteristic structures at the extremities and by carriage of ccr as well as mec complexes. At least three distinct members make up the family. SCCmec may have evolved from a primordial mobile element, SCC, into which the mec complex was inserted. Exploration of staphylococcal genomes of more strains will find more diversified members of SCCmec as well as presumptive SCC, which will enable us to understand how staphylococcal species are exchanging genetic information to cope with antibiotics as well as the physiological selective pressure of the environment.
ACKNOWLEDGMENTS
We thank B. Berger-Bachi for the kind gift of NCTC 8325 and J. Takahashi for excellent technical assistance.
This work was supported by the Core University Program under Japan Society for the Promotion of Science (JSPS), coordinated by the University of Tokyo, Graduate School of Medicine and Universiti Sains Malaysia, School of Medical Sciences; by grant 11670272 and the Specially Designated Research Promotion from the Japanese Ministry of Education; and by Grant for International Health Cooperation Research 11C-4 from the Japanese Ministry of Health and Welfare.
REFERENCES
- 1.Archer G L, Niemeyer D M, Thanassi J A, Pucci M J. Dissemination among staphylococci of DNA sequences associated with methicillin resistance. Antimicrob Agents Chemother. 1994;38:447–454. doi: 10.1128/aac.38.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer G L, Thanassi J A, Niemeyer D M, Pucci M J. Characterization of IS1272, an insertion sequence-like element from Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1996;40:924–929. doi: 10.1128/aac.40.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannam T L, Crellin P K, Rood J I. Molecular genetics of the chloramphenicol-resistance transposon Tn4451 from Clostridium perfringens: the TnpX site-specific recombinase excises a circular transposon molecule. Mol Microbiol. 1995;16:535–551. doi: 10.1111/j.1365-2958.1995.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 4.Chikramane S G, Matthews P R, Noble W C, Stewart P R, Dubin D T. Tn554 inserts in methicillin-resistant Staphylococcus aureus from Australia and England. J Gen Microbiol. 1991;137:1303–1311. doi: 10.1099/00221287-137-6-1303. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen B, Brøndsted L, Vogensen F K, Hammer K. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901–1. J Bacteriol. 1996;178:5164–5173. doi: 10.1128/jb.178.17.5164-5173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubin D T, Matthews P R, Chikramane S G, Stewart P R. Physical mapping of the mec region of an American methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1991;35:1661–1665. doi: 10.1128/aac.35.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman B J, Tomasz A. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilden P, Savolainen K, Tyynela J, Vuento M, Kuusela P. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. Eur J Biochem. 1996;236:904–910. doi: 10.1111/j.1432-1033.1996.00904.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiramatsu K. Vancomycin resistance in staphylococci. Drug Resist Updates. 1998;1:135–150. doi: 10.1016/s1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu K, Asada K, Suzuki E, Okonogi K, Yokota T. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA) FEBS Lett. 1991;298:133–136. doi: 10.1016/0014-5793(92)80039-j. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu K, Kondo N, Ito T. Genetic basis for molecular epidemiology of MRSA. J Infect Chemother. 1996;2:117–129. doi: 10.3412/jsb.52.417. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Suzuki E, Takayama H, Katayama Y, Yokota T. Role of penicillinase plasmids in the stability of the mecA gene in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:600–604. doi: 10.1128/aac.34.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hürlimann-Dalei R L, Ryffel C, Kayser F H, Berger-Bächi B. Survey of the methicillin resistance-associated genes mecA, mecR1-mecI, and femA-femB in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:2617–2621. doi: 10.1128/aac.36.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jevons M P. “Celbenin”-resistant staphylococci. Br Med J. 1961;124:124–125. [Google Scholar]
- 16.Josefsson E, McCrea K W, Eidhin D N, O'Connell D, Cox J, Hook M, Foster T J. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 17.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwahara-Arai, K., N. Kondo, S. Hori, E. Tateda-Suzuki, and K. Hiramatsu. 1996. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP2′ production. 40:2680–2685. [DOI] [PMC free article] [PubMed]
- 19.Lee N S, Rutebuka O, Arakawa T, Bickle T A, Ryu J. KpnAI, a new type I restriction-modification system in Klebsiella pneumoniae. J Mol Biol. 1997;271:342–348. doi: 10.1006/jmbi.1997.1202. [DOI] [PubMed] [Google Scholar]
- 20.Matsuhashi M, Song M D, Ishino F, Wachi M, Doi M, Inoue M, Ubukata K, Yamashita N, Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to β-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986;167:975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews P, Tomasz A. Insertional inactivation of the mec gene in a transposon mutant of a methicillin-resistant clinical isolate of Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:1777–1779. doi: 10.1128/aac.34.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie T, Hoshino T, Tanaka T, Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds P E, Brown D F J. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. FEBS Lett. 1985;192:28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- 24.Ryffel C, Tesch W, Birch-Machin I, Reynolds P E, Barberis-Maino L, Kayser F H, Berger-Bachi B. Sequence comparison of mecA gene isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94:137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Savolainen K, Korhonen T K, Kuusela P. PLS, a large surface protein encoded by mec DNA of methicillin resistant staphylococcus aureus, prevents bacterial adhesion in vitro. 2000. Ninth International Symposium on Staphylococci and Staphylococcal Infections Kolding, Denmark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherratt D. Tn3 and related transposable elements: site-specific recombination and transposition. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 163–184. [Google Scholar]
- 28.Skinner S, Ingli B, Matthews P R, Stewart P R. Mercury and tetracycline resistance genes and flanking repeats assocated with methicillin resistance on the chromosome of Staphylococcus aureus. Mol Microbiol. 1988;2:289–298. doi: 10.1111/j.1365-2958.1988.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 29.Song M D, Wachi M, Doi M, Ishino F, Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987;221:167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- 30.Stragier P, Kunkel B, Kroos L, Losick R. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science. 1989;243:507–512. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Hiramatsu K, Yokota T. Survey of methicillin-resistant clinical strains of coagulase-negative staphylococci for mecA gene distribution. Antimicrob Agents Chemother. 1992;36:429–434. doi: 10.1128/aac.36.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki E, Kuwahara-Arai K, Richardson J F, Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains. Antimicrob Agents Chemother. 1993;37:1219–1226. doi: 10.1128/aac.37.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T, Okuzumi K, Iwamoto A, Hiramatsu K. A retrospective study on methicillin-resistant Staphylococcus aureus clinical strains in Tokyo University Hospital. J Infect Chemother. 1995;1:40–49. [Google Scholar]
- 34.Ushioda H, Terayama T, Sakai S, Zen-Yoji H, Nishiwaki M, Hidano A. Coagulase typing of Staphylococcus aureus and its application in routine work. In: Gustav J J, editor. Staphylococci and staphylococcus infections. Stuttgart, Germany: Fischer Verlag; 1981. pp. 77–83. [Google Scholar]
- 35.Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985;28:397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Piscitelli C, Lencastre H, Tomasz A. Tracking and evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist. 1996;2:435–441. doi: 10.1089/mdr.1996.2.435. [DOI] [PubMed] [Google Scholar]
- 37.Wu S, de Lencastre H, Tomasz A. Genetic organization of the mecA region in methicillin-susceptible and methicillin-resistant strains of Staphylococcus scuiri. J Bacteriol. 1998;180:236–242. doi: 10.1128/jb.180.2.236-242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida T, Kondo N, Hanifah Y A, Hiramatsu K. Combined use of ribotyping, PFGE typing and IS431 typing in the discrimination of nosocomial strains of methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 1997;41:687–695. doi: 10.1111/j.1348-0421.1997.tb01912.x. [DOI] [PubMed] [Google Scholar]