Abstract
Objective
This post-authorization safety study (PASS) was conducted to evaluate the long-term safety and effectiveness of insulin degludec in patients with diabetes mellitus (DM) requiring insulin therapy in routine clinical practice in India.
Methods
Data on glycated hemoglobin (HbA1c) and adverse events (AEs) were collected up to 12 months after insulin degludec initiation.
Results
A total of 1057 adult patients with DM were enrolled, including 60.07% males with the mean duration of 22.2 ± 21.90 years with type 1 DM and 10.1 ± 7.37 years with type 2 DM and the mean HbA1c of 9.6 ± 1.9%. Insulin degludec was prescribed to improve HbA1c and fasting plasma glucose (FPG). Insulin degludec daily dose was increased from 14.8 ± 8.0 U to 18.0 ± 9.46 U over 12 months resulting in a significant decrease of HbA1c by 1.8 ± 1.68% compared with baseline. There were 84 events of confirmed hypoglycemia in 51 patients during the 12-month follow-up period, and 44 AEs were reported in 2.6% of patients, of which 2 AEs were serious and unrelated to the drug.
Conclusion
Insulin degludec is well tolerated in patients with DM. It improves glycemic control with reduced HbA1c, FPG, and postprandial glucose, with a low risk of hypoglycemia.
Keywords: Insulin degludec, Diabetes mellitus, Safety, Post-authorization safety study, Effectiveness, Real-world evidence
1. Introduction
Diabetes mellitus (DM) is a group of metabolic disorders caused by impaired glucose tolerance, resulting in complications such as neuropathy, nephropathy, retinopathy, and other macrovascular complications, with an increased risk of cardiovascular events. According to the International Diabetes Federation (IDF) Diabetes Atlas 10th edition, in 2021, out of 537 million adults living with diabetes, 89 million were from India. By 2045, these figures are expected to increase to 784 million globally and up to 115.4 million in India [1]. According to the American Diabetes Association, insulin therapy is recommended for patients with type 2 diabetes mellitus (T2DM) when symptomatic and/or with glycated hemoglobin (HbA1c) ≥10% and/or blood glucose levels ≥300 mg/dL [2]. Owing to the progressive nature of the disease, many people with T2DM require insulin therapy. Timely insulin therapy provides better glycemic control in people with T2DM that is uncontrolled on multiple oral antidiabetic drugs (OADs), but it must be balanced with the risk of hypoglycemia [[3], [4], [5]].
Insulin degludec is an ultra-long-acting analog of basal insulin developed for once-daily dosing; it has a unique mode of protraction that provides a longer duration of action, exceeding 42 h. Insulin degludec forms soluble depots of multi-hexamers upon subcutaneous administration, which release monomers at a consistent rate that are then gradually absorbed into the circulation [6,7]. Additionally, 100 U/mL insulin degludec had a low within-day variability and a four-fold low pharmacodynamic day-to-day variability in glucose-lowering effect compared with insulin glargine (IGlar) U100 under steady-state conditions [8]. Lower within-day and day-to-day variations with insulin degludec were also observed in another crossover study when compared it with IGlar U300 [9].
Several phase 3 randomized controlled studies (RCTs) have shown the efficacy and tolerability of insulin degludec in patients with diabetes [[10], [11], [12], [13], [14], [15], [16]]. It is well known that RCTs are conducted on a limited set of patients under controlled conditions. Because of close monitoring of patients during the study period, there is lower generalizability of results in routine clinical practice [17]. A post-authorization safety study (PASS) is therefore mandated by some regulatory authorities to evaluate the safety of newly marketed drugs in the real-life setting. This PASS acts as real-world evidence (RWE) that complements the findings from RCTs to provide a “totality of data.”
This 1-year, multicenter, prospective, single-arm, non-interventional, PASS was aimed at evaluating the long-term safety and effectiveness of insulin degludec (Tresiba®) in Indian people with DM.
2. Materials and methods
The study protocol was approved by the regulatory authorities and the ethics committees/institutional review boards of all the participating centers. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice guidelines [18,19]. All the patients provided written informed consent prior to their study participation.
2.1. Study population
Patients with DM requiring insulin therapy and qualified for starting insulin degludec based on the clinical judgment of their physician and those who agreed to provide past data (3 months prior to baseline) for HbA1c, fasting plasma glucose (FPG), and hypoglycemic events before starting insulin degludec therapy were enrolled in the study. Patients with a known or suspected allergy to insulin degludec, with a history of previous insulin degludec use, participating in any other clinical study, with mental incapability, showing unwillingness or having language barrier preventing adequate understanding or cooperation for the study, or who were pregnant/breastfeeding/planning a pregnancy during the study were excluded.
2.2. Study design and treatment
This was a multicenter, prospective, open-label, non-interventional study conducted at 51 centers across India between July 2015 and April 2017. Patients were prescribed insulin degludec (100 U/mL) as part of routine clinical care, which was independent of the decision to include the patient in the present study. The treating physician determined the starting dose of insulin degludec and the need for further dose titrations in accordance with the clinical response and tolerability. The daily dose of degludec was increased from a starting dose of 14.8 ± 8.0 U to 18.0 ± 9.46 U over 12 months. Among the 1057 patients recruited in this study, for 349 insulin-experienced patients, the mean starting dose of degludec was 18.44 U, and for the 708 insulin-naïve patients, the mean starting dose was 12.99 U. The dose was adjusted per the discretion of the treating investigator. Protocol-defined data were collected over four visits: at baseline, 3 months, 6 months, and 1 year (end of the study). Adverse events (AEs)/serious adverse events (SAEs)/adverse drug reactions (ADRs)/serious adverse drug reactions (SADRs) and hypoglycemic events were collected for safety evaluation of degludec. Confirmed hypoglycemic episodes were defined as episodes that were severe (i.e., an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions) and/or biochemically confirmed by a plasma glucose value of <56 mg/dL, with or without symptoms consistent with hypoglycemia. Records of the most recent glycemic values and blood/plasma glucose levels (both fasting and post-meal) were documented at each visit.
2.3. Study endpoints and assessments
2.3.1. Statistical analysis
A sample size of 1000 patients, assuming 20% dropouts who have been exposed to insulin degludec during the treatment, would provide a probability of 80% of detecting at least 1 event that occurs with an incidence of 2 in 1000 patients or approximately 6 events with an incidence of 1 in 100 patients. For an unobserved event, with the above sample size, the upper limit of 95% confidence interval (CI) of the rate would be 0.375 per year. The safety analysis set included all patients who had received at least one dose of insulin degludec, and the efficacy analysis set included all patients from the safety analysis set who had at least one post-baseline measurement related to HbA1c, fasting blood glucose (FBG)/FPG, or confirmed hyperglycemic event(s). The AEs/ADRs were coded by system organ class (SOC) and preferred term (PT) using version 20.0 of the Medical Dictionary for Regulatory Activities (MedDRA). The AEs/ADRs were collected, evaluated, and tabulated by causality, seriousness, severity, action taken, AE resolution (outcome), SOC, and PT. SAEs were summarized by SOC and PT. Descriptive statistics for AEs/ADRs/SAEs/SADRs by SOC and PT were presented by the number of patients with event and the number of events. The effectiveness parameters (HbA1c, FBG/FPG, and confirmed hypoglycemic events) were evaluated by means of descriptive statistics. The effectiveness parameters were also evaluated for subgroup of patients per the therapy at baseline (previously on OADs or treated with insulin). Last observation carried forward was used to handle the missing data. Paired t-test was used to evaluate the changes in HbA1c, FBG/FPG and confirmed hypoglycemic events on each visit; the test was carried out as two sided on a 5% level of significance. Statistical analyses were performed using Statistical Analysis Software (version 9.4).
3. Results
3.1. Demographics and patient disposition
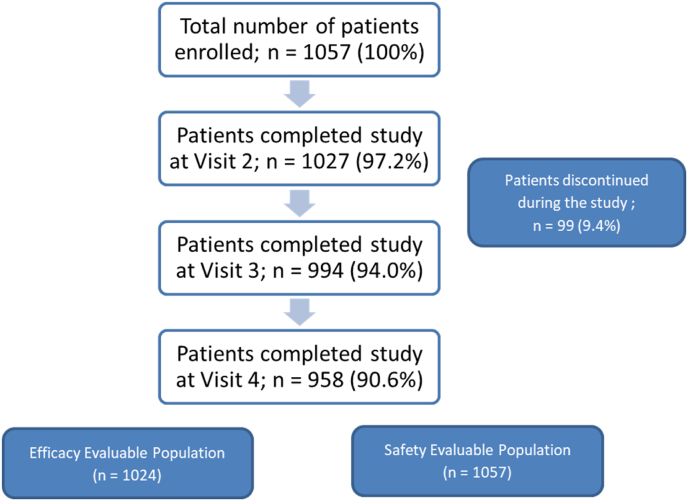
A total of 1057 patients were enrolled in the study, of which 958 (90.63%) patients completed all four scheduled visits, and the remaining 99 (9.37%) patients discontinued prematurely (Fig. 1). The safety and efficacy analysis sets included 1057 and 1024 patients, respectively. The mean age of patients was 55.0 ± 11.35 years; most (60.07%) of them were males. The mean duration of diabetes in patients with type 1 diabetes mellitus (T1DM) was 22.2 ± 21.90 years, and that in patients with T2DM was 10.1 ± 7.37 years. The mean HbA1c at baseline was 9.6% ± 1.92%. Majority of patients (n = 1052, 99.52%) had T2DM. Nephropathy, retinopathy, and autonomic neuropathy were reported in 8.7%, 7.2%, and 6.2% of patients, respectively. Coronary heart disease was reported in 8.5% of patients. The concomitant medications included lipid-modifying agents (50.2%), agents acting on the renin–angiotensin system (38.1%), antithrombotic agents (12.8%), beta-blocking agents (11.2%), and antithyroid agents (10.6%). Telmisartan was the most frequently used agent acting on the renin-angiotensin system (13.2% of patients), and atorvastatin and rosuvastatin were the most frequently used lipid-modifying agents (for 21.9% and 16.9% of patients, respectively).
Fig. 1.
Disposition of patients.
At baseline, 73.6% and 57.0% of patients were on metformin and sulfonylurea, respectively. Prior to the initiation of insulin degludec, 15.5%, 14.3%, and 11.2% of patients were on basal, bolus, and premix insulin, respectively. At baseline, 50 patients had a history of 122 confirmed hypoglycemic events. To improve HbA1c and FBG control (29.0% and 22.1%, respectively, as derived from the total number of reasons [n = 3037] and 83.8% and 63.7%, respectively, as derived from the total number of patients [n = 1057]) was the most common reason to initiate degludec. In 25.8% of patients, degludec was started to reduce the risk of hypoglycemia. Table 1 shows the baseline demographic and clinical characteristics of the enrolled patients.
Table 1.
Baseline demographic and clinical characteristics (safety analysis set).
| Parameters | Number of patients (%) N = 1057 |
|---|---|
| Age (mean ± SD, years) | 55.0 ± 11.35 |
| Gender (%) | |
| Male | 635 (60.07) |
| Female | 422 (39.92) |
| Weight (mean ± SD, kg) | 71.7 ± 13.43 |
| Waist circumference (mean ± SD, cm) | 94.2 ± 11.57 |
| Hip circumference (mean ± SD, cm) | 97.5 ± 11.68 |
| Type of diabetes (%) | |
| Type 1 | 5 (0.47) |
| Type 2 | 1052 (99.52) |
| Duration of disease (mean ± SD, years) | |
| Type 1 | 22.2 ± 21.90 |
| Type 2 | 10.1 ± 7.37 |
| Previous antidiabetic drugs, n (%) | |
| No treatment | 117 (11.07%) |
| 1 OAD | 155 (14.66%) |
| 2 OADs | 305 (28.86%) |
| >2 OADs | 288 (27.25%) |
| Basal insulin ± OAD | 164 (15.52%) |
| Bolus/Premix Insulin | 28 (2.65%) |
Abbreviations: OAD, oral antidiabetic drug; SD, standard deviation.
3.2. Safety and tolerability
A total of 44 AEs were reported in 28 (2.6%) patients during the study period (Table 2). All the AEs were either mild (36 AEs) or moderate (8 AEs) in severity. Most of these AEs (38 of 44; 86.4%) were not related to the study treatment. Most AEs (41 of 44; 93.2%) resolved during the study period. One AE of peripheral swelling resolved with sequelae, while two AEs of musculoskeletal pain and nocturia continued until the end of the study. Six ADRs were reported in five patients, of which five were mild and one was moderate in severity; none of the ADRs were serious. ADRs of hyperglycemia, gastroenteritis, and urinary tract infection were reported in one patient each. All the ADRs resolved within the study period. Insulin degludec dose was reduced in two patients and increased in one patient to manage ADRs, whereas no change was required in the remaining two patients. Two SAEs of hyponatremia and macular edema were reported in one patient each. The SAEs were moderate in severity and were neither related to the study drug nor did they require any change to the dosage regimen.
Table 2.
Summary of adverse events reported in ≥2 patients in the safety analysis set.
| Adverse Events | Number of Adverse Events (Number of Patients) |
|---|---|
| Total number of adverse events | 44 (28) |
| Asthenia | 3 (3) |
| Peripheral swelling | 3 (3) |
| Gastroenteritis | 2 (2) |
| Dizziness | 2 (2) |
| Hypoesthesia | 2 (2) |
| Pain in extremity | 2 (2) |
| Pyrexia | 2 (2) |
The incidence of confirmed hypoglycemic events was 122 events in 50 patients at baseline and 84 events in 51 (5%) patients during the study. There were no episodes of severe hypoglycemia (Table 3).
Table 3.
Summary of effectiveness endpoints at 3, 6, and 12 months.
| Parameters | Time period |
|||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| HbA1c (%) | ||||
| Overall efficacy population | 9.6 ± 1.92 | 8.3 ± 1.61 | 8.1 ± 1.39 | 7.8 ± 1.18 |
| Change from BL in overall efficacy population | −0.8 ± 1.23 | −1.4 ± 1.50 | −1.8 ± 1.68 | |
| Previous medication OAD subgroup | 9.5 ± 1.83 | 8.2 ± 1.52 | 7.9 ± 1.26 | 7.5 ± 0.99 |
| Change from BL in OAD subgroup | −0.9 ± 1.16 | −1.4 ± 1.43 | −1.9 ± 1.59 | |
| Previous medication insulin subgroup | 9.8 ± 2.08 | 8.8 ± 1.73 | 8.5 ± 1.60 | 8.2 ± 1.39 |
| Change from BL in insulin subgroup | −0.5 ± 1.32 | −1.3 ± 1.66 | −1.6 ± 1.87 | |
| FPG (mg/dL) | ||||
| Overall efficacy population | 190.7 ± 69.02 | 145.0 ± 49.77 | 132.5 ± 37.58 | 125.4 ± 31.86 |
| Change from BL in overall efficacy population | −45.1 ± 62.97 | −57.2 ± 69.84 | −64.7 ± 72.84 | |
| Previous medication OAD subgroup | 185.3 ± 62.21 | 143.0 ± 45.64 | 131.7 ± 36.17 | 123.0 ± 27.16 |
| Change from BL in OAD subgroup | −43.3 ± 51.85 | −52.2 ± 59.83 | −62.3 ± 63.96 | |
| Previous medication insulin subgroup | 202.9 ± 81.17 | 149.8 ± 58.36 | 134.4 ± 40.63 | 130.2 ± 39.26 |
| Change from BL in insulin subgroup | −49.6 ± 85.09 | −69.6 ± 89.27 | −70.4 ± 90.41 | |
| PPG, post-breakfast (mg/dL) | ||||
| Overall efficacy population | 277.4 ± 81.69 | 212.0 ± 60.14 | 199.8 ± 52.33 | 187.1 ± 48.33 |
| Change from BL in overall efficacy population | −60.3 ± 79.30 | −72.1 ± 87.10 | −86.1 ± 94.80 | |
| Previous medication OAD subgroup | 276.5 ± 75.70 | 212.0 ± 58.91 | 198.1 ± 49.63 | 181.2 ± 39.66 |
| Change from BL in OAD subgroup | −62.7 ± 70.76 | −77.9 ± 82.76 | −94.1 ± 83.65 | |
| Previous medication insulin subgroup | 279.1 ± 93.23 | 211.9 ± 62.73 | 202.9 ± 56.84 | 196.8 ± 58.78 |
| Change from BL in insulin subgroup | −55.0 ± 95.57 | −60.6 ± 94.43 | −70.2 ± 112.4 | |
| PPG, post-lunch (mg/dL) | ||||
| Overall efficacy population | 273.3 ± 109.40 | 201.6 ± 58.33 | 191.2 ± 49.19 | 174.3 ± 34.63 |
| Change from BL in overall efficacy population | −55.0 ± 74.46 | −69.3 ± 86.70 | −87.8 ± 100.2 | |
| Previous medication OAD subgroup | 267.8 ± 95.70 | 192.9 ± 51.33 | 186.1 ± 41.42 | 171.6 ± 33.91 |
| Change from BL in OAD subgroup | −58.5 ± 72.90 | −70.4 ± 84.62 | −85.7 ± 97.62 | |
| Previous medication insulin subgroup | 286.0 ± 135.6 | 220.3 ± 67.78 | 203.7 ± 63.15 | 179.2 ± 35.59 |
| Change from BL in insulin subgroup | −45.9 ± 78.32 | −66.5 ± 93.15 | −94.8 ± 109.1 | |
| Confirmed hypoglycemic eventsa | ||||
| Overall (N = 1024) | 50 (122) | 13 (24) | 18 (23) | 27 (37) |
| Patients receiving previous insulin (N = 681) | – | 7 (9) | 6 (9) | 11 (17) |
| Patients receiving previous OAD (N = 343) | – | 6 (15) | 12 (14) | 16 (20) |
Abbreviations: BL, baseline; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; N: number of patients; OAD, oral antidiabetic drugs; PPG, postprandial plasma glucose.
Change from baseline significant at all-time points for all values (p < 0.0001).
Values expressed at mean ± standard deviation.
Values calculated at last observation carried forward for missing patients.
Confirmed hypoglycemic events presented as number of patients (number of events).
No significant changes were observed in any laboratory parameters (cholesterol, triglycerides, serum creatinine, and albumin) during the study period.
3.3. Effectiveness
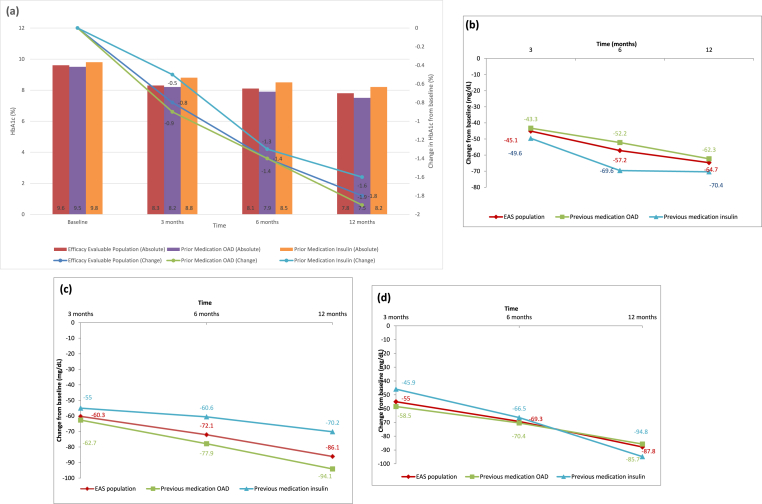
Treatment with insulin degludec showed a statistically significant reduction in the mean HbA1c from baseline at 1 year (1.8% ± 1.68%, p < 0.0001). The average HbA1c was 9.6% at baseline, 8.3% at 3 months, 8.1% at 6 months, and 7.8% at the end of the study. The mean reduction in HbA1c from baseline to 1 year was comparable between insulin-naïve and insulin-experienced patients stratified by a prior OAD use (9.5% ± 1.83%–7.5% ± 0.99%, p < 0.0001) and those receiving prior insulin therapy (9.8% ± 2.08%–8.2% ± 1.39%, p < 0.0001). The decrease in the mean HbA1c was numerically greater in insulin-naïve patients than in insulin-experienced stratified by a prior OAD use (1.9% ± 1.59% vs 1.6% ± 1.87%).
The mean FBG/FPG reduced from 190.7 ± 69.02 mg/dL at baseline to 125.4 ± 31.86 mg/dL at 1 year. The decline in the FPG values observed at all-time points was statistically significant (3 months: 45.1 ± 62.97 mg/dL, 6 months: 57.2 ± 69.84 mg/dL, and 1 year: 64.7 ± 72.84 mg/dL; p < 0.0001). The mean reduction in FBG/FPG from baseline at 1 year was comparable between insulin-naïve and insulin-experienced patients receiving prior OADs (185.3 ± 62.21 mg/dL to 123.0 ± 27.16 mg/dL; p < 0.0001) and those receiving prior insulin therapy (202.9 ± 81.17 mg/dL to 130.2 ± 39.26 mg/dL; p < 0.0001). The reduction in FBG/FPG was numerically greater in insulin-experienced patients than in insulin-naïve patients (70.4 ± 90.41 mg/dL vs 62.3 ± 63.96 mg/dL).
At 1 year, the mean postprandial glucose (PPG) (breakfast) reduced from 277.4 ± 81.69 mg/dL to 187.1 ± 48.33 mg/dL, PPG (lunch) reduced from 273.3 ± 109.40 mg/dL to 174.3 ± 34.63 mg/dL, and PPG (dinner) reduced from 242.0 ± 103.4 mg/dL to 168.0 ± 29.18 mg/dL. The mean reduction in PPG (breakfast) from baseline to 1 year was comparable between insulin-naïve (276.5 ± 75.70 mg/dL to 181.2 ± 39.66 mg/dL; difference (Δ): 94.1 ± 83.65 mg/dL; p < 0.0001) and insulin-experienced patients (279.1 ± 93.23 mg/dL to 196.8 ± 58.78 mg/dL; Δ: 70.2 ± 112.4 mg/dL; p < 0.0001). Similar significant improvements were observed for PPG (lunch) in insulin-naïve patients and insulin-experienced patients at 1 year (p < 0.0001). The mean change in glycemic parameters at each follow-up visit is shown in Fig. 2 and Table 3.
Fig. 2.
Change in glycemic parameters in patients receiving insulin degludec over 12 months. (a) Glycated hemoglobin (HbA1c). (b) Fasting plasma glucose. (c) Postprandial plasma glucose (post-breakfast). (d) Postprandial plasma glucose (post-lunch). EAS: effectiveness analysis set; OAD: oral antidiabetic drugs.
4. Discussion
This multicenter, prospective, open-label, non-interventional, PASS evaluated the long-term safety and effectiveness of insulin degludec in a large population from the real-world setting. The 1-year observation period was expected to be enough to capture any safety concerns likely to be associated with the use of insulin degludec. This study showed that switching to or starting insulin degludec in routine clinical practice in Indian patients is associated with a low risk of hypoglycemic events and a significant improvement in glycemic status. Overall, insulin degludec was well tolerated.
The AEs that occurred in about 4% (44) of patients were mild to moderate in nature, and most AEs (86.4%) were not related to the study drug. None of the AEs led to the study drug withdrawal. Most commonly observed (in ≥2 patients) AEs were asthenia, peripheral swelling, gastroenteritis, dizziness, hypoesthesia, and pain in extremity. The two SAEs of hyponatremia and macular edema were unrelated to the study drug and resolved without dose modification. The safety of insulin degludec was evaluated in 1102 patients with T1DM and in 2713 patients with T2DM in 9 clinical trials lasting 6–12 months [20]. The common AEs reported in these studies were nasopharyngitis, upper respiratory tract infection, headache, sinusitis, diarrhea, and gastroenteritis. In DEVOTE study comparing cardiovascular safety of insulin degludec and IGlar U100, there was no significant difference in the incidence of AEs in the insulin degludec (39.0%) and IGlar U100 (40.0%) groups [21]. In two randomized, double-blind, crossover studies (SWITCH-1 and SWITCH-2) comparing the rate of hypoglycemia associated with 100 U/mL insulin degludec and 100 U/mL IGlar in patients with T1DM and T2DM, the common AEs reported in both groups were nasopharyngitis, respiratory tract infections, and hypoglycemia [22,23]. Hypoglycemia and fear of hypoglycemia are known limiting factors for effective management of diabetes. Risk of hypoglycemia poses a challenge to the willingness of physicians and patients to increase the insulin dose and thus leads to ineffective glycemic control [5]. There were 84 events of confirmed hypoglycemia but no episodes of severe hypoglycemia in the present study. A decrease in the hypoglycemic events was observed with insulin degludec compared with IGlar U100 during various clinical studies. In the SWITCH-1 study, 100 U/mL insulin degludec resulted in a decreased risk of nocturnal symptomatic hypoglycemia in patients with T1DM compared with IGlar (rate ratio [RR] 0.64 [95% CI: 0.56–0.73; p < 0.001]) in the 16-week maintenance period. In this double-blind, treat-to-target, crossover trial, insulin degludec resulted in lower rates of overall symptomatic hypoglycemic episodes than did IGlar U100 [22]. Similar results were observed in the SWITCH-2 study as well in the overall symptomatic hypoglycemia with RR of 0.70 (95% CI: 0.61–0.80]; p < 0.001) [23]. In the DEVOTE study, the rate of severe hypoglycemia was high in the IGlar group compared with insulin degludec group (6.25 events/100 patient-years vs 3.70 events/100 patient-years; RR 0.60 [95% CI: 0.48–0.76] p < 0.001) [21]. In a 12-month European multicenter, prospective, observational study (ReFLeCT), a significant decrease in HbA1c, FPG, and basal insulin dosage was observed at end of the study. The risk of hypoglycemia had reduced in both patients with T1DM and those with T2DM treated with insulin degludec [24]. The overall relative risk in patients with T1DM and T2DM was reported to be 0.80 ([95% CI: 0.74–0.88]; p < 0.001) and 0.46 ([95% CI: 0.38–0.56]; p < 0.001), respectively. The risk of severe, non-severe, and nocturnal hypoglycemia had also significantly reduced in both patients with T1DM and those with T2DM treated with insulin degludec (p < 0.001). In a pooled analysis of seven pivotal studies, comparing the RR of hypoglycemic events with insulin degludec to that with IGlar U100, the RR for confirmed hypoglycemic events in patients with T2DM (five studies) was 0.83 (95% CI: 0.74–0.94) and that in patients with T1DM (two studies) was 0.75 (95% CI: 0.60–0.94). Also, compared with IGlar U100, a significantly low rate of nocturnal hypoglycemia was associated with insulin degludec administration at similar levels of glycemic control (RR: 0.68, 95% CI: 0.57–0.82) [11]. In a 6-month retrospective real-world comparative data from India conducted in insulin-naïve patients, patients on insulin degludec experienced significantly less hypoglycemic episodes as compared with IGlar U100 (12 vs 40) [25].
Significant improvements in HbA1c, FPG, and PPG were observed until month 12 irrespective of any prior therapy with OADs or insulin. The decrease in HbA1c was high (1.8% ± 1.68%) in this study compared with that (1–1.5%) in other clinical studies on insulin degludec [12,14,15,[26], [27], [28]]. Several real-world studies have also evaluated improvement in glycemic profile of patients after initiating insulin degludec. In the ReFLeCT study, switching from other basal insulin preparations to insulin degludec resulted in 0.3% and 0.4% reduction in HbA1c in patients with T1DM and those with T2DM, respectively [24]. In a European real-world study (EU-TREAT), after 6 months of insulin degludec administration, a reduction of 0.2% in HbA1c was observed in patients with T1DM and that of 0.5% was observed in patients with T2DM [29]. In another study of insulin-naïve patients withT2DM, data of 4056 patients were analyzed. After 180 days of follow-up, it was observed that degludec was associated with a larger reduction in HbA1c (estimated treatment difference, −0.27%; p = 0.03) and greater reductions in change in rate (RR: 0.70; P < 0.05) and the likelihood of hypoglycemia (odds ratio, 0.64; P < 0.01]) than glargine U300 [30]. In another real-world cohort analysis (DELIVER-D+), the mean decrease in HbA1c was similar with insulin degludec and IGlar U300 (0.58% ± 1.6% and 0.63% ± 1.7%) [31]. In another 1-year observational study in Japanese patients, insulin degludec was associated with a significant reduction in HbA1c (0.3% for patients with T1DM and 0.5% for patients with T2DM) [32]. Insulin degludec is a novel formulation of insulin with an ultra-long duration of action. It has relatively flat and stable glucose-lowering profile because of continuous, uniform delivery of insulin from the subcutaneous depot [7,33]. It was first approved in the European market in 2013 and subsequently in the United States in 2015 [34,35]. It has been available in India since 2013. This PASS in the Indian population demonstrates that starting or switching to insulin degludec is well tolerated over a 1-year study period and is associated with a low risk of hypoglycemia, with improvement in glycemic control.
The strength of this study is that the safety and effectiveness of insulin degludec was evaluated in a large number of people with diabetes over 1 year under routine clinical practice conditions. A total of 90.63% of patients completed the study. A limitation of this study is that the AEs and hypoglycemic events reported here were lower than expected. This could be because of patient-recall-related under-reporting of the events during the study follow-up visits. Unlike RCTs, this was a regulatory authority-mandated non-interventional study; thus, patients were not mandated to maintain a dairy for AEs or provided with glucometers to monitor hypoglycemia. This is one of the limitations of safety reporting in RWE studies. However, the importance of reporting AEs and hypoglycemic events was emphasized during investigators’ meeting, site initiation, and follow-up visits.
5. Conclusions
This was the first, long-term, RWE study in Indian patients with diabetes receiving insulin degludec. Insulin degludec was well tolerated, with no new safety signals. At 1 year, patients who initiated degludec treatment had reduced HbA1c, fasting glucose, and PPG, with a low risk of hypoglycemia.
CRediT authorship contribution statement
Jothydev Kesavadev: were involved in, Investigation, Writing – review & editing, Visualization. L.Sreenivasa Murthy: were involved in, Investigation, Writing – review & editing, Visualization. Tirthankar Chaudhury: were involved in, Investigation, Writing – review & editing, Visualization. Sadasiva Rao Yalamanchi: were involved in, Investigation, Writing – review & editing, Visualization. J. Giri: were involved in, Investigation, Writing – review & editing, Visualization. Sunil Gupta: were involved in, Investigation, Writing – review & editing, Visualization. Sanjeev Phatak: were involved in, Investigation, Writing – review & editing, Visualization. K.D. Modi: were involved in, Investigation, Writing – review & editing, Visualization. Sanjay Chatterjee: were involved in, Investigation, Writing – review & editing, Visualization. Aparna Manjunath: Writing – review & editing, Validation, Resources. Manjunatha Revanna: Visualization, Supervision, Writing – review & editing, Validation, Resources. Arpandev Bhattacharya: involved in, Conceptualization, and, Methodology.
Declaration of competing interest
There was no conflict of interest.
Acknowledgment
The authors would like to thank APCER Life Sciences, India for editorial support during the submission of the manuscript.
The study was funded by Novo Nordisk India Pvt Ltd., the manufacturer of insulin degludec.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2022.100184.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.International Diabetes Federation. IDF diabetes Atlas 2021. Available at: https://www. https://diabetesatlas.org/. Accessed on January 31, 2022.
- 2.American Diabetes Association Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(1):S1–S155. doi: 10.2337/cd17-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84:183–190. [PubMed] [Google Scholar]
- 4.Jellinger P.S., Davidson J.A., Blonde L., et al. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract. 2007;13(3):260–268. doi: 10.4158/EP.13.3.260. [DOI] [PubMed] [Google Scholar]
- 5.Cryer P.E. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 6.Haahr H., Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53:787–800. doi: 10.1007/s40262-014-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonassen I., Havelund S., Hoeg-Jensen T. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res (N Y) 2012;29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heise T., Hermanski L., Nosek L. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metabol. 2012;14:859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 9.Heise T., Nørskov M., Nosek L. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metabol. 2017;19(7):1032–1039. doi: 10.1111/dom.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu C., Hollander P., Miranda-Palma B., et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–1162. doi: 10.1210/jc.2012-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratner R.E., Gough S.C., Mathieu C., et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metabol. 2013;15:175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meneghini L., Atkin S.L., Gough S.C., et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–864. doi: 10.2337/dc12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller S., Buse J., Fisher M., et al. Insulin degludec, an ultra-long-acting basal insulin, versus insulin glargine in basal–bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal–Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 14.Garber A.J., King A.B., Del Prato S., et al. Insulin degludec, an ultra-long-acting basal insulin, versus insulin glargine in basal–bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal–Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–1507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 15.Onishi Y., Iwamoto Y., Yoo S.J. Insulin degludec compared with insulin glargine in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, Pan‐Asian, treat‐to‐target trial. J Diabetes Investig. 2013;4:605–612. doi: 10.1111/jdi.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinman B., Philis-Tsimikas A., Cariou B., et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN once Long) Diabetes Care. 2012;35:2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.E6(R2) Good clinical practice: integrated addendum to ICH E6(R1) guidance for industry. March 2018. https://www.fda.gov/media/93884/download Available at:
- 20.Tresiba (insulin degludec injection) [prescribing information] Novo Nordisk; Plainsboro, NJ: 2015. [Google Scholar]
- 21.Marso S.P., McGuire D.K., Zinman B., et al. DEVOTE Study Group Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017 Aug 24;377(8):723–732. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane W., Bailey T.S., Gerety G., et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 Randomized Clinical Trial. JAMA. 2017;318(1):33–44. doi: 10.1001/jama.2017.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wysham C., Bhargava A., Chaykin L., et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 Randomized Clinical Trial. JAMA. 2017 Jul 4;318(1):45–56. doi: 10.1001/jama.2017.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini G.P., Feher M., Hansen T.K., et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: a prospective study. J Clin Endocrinol Metab. 2019;104(12):5977–5990. doi: 10.1210/jc.2019-01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosal S., Sinha B., Gangopadhyay K.K. Insulin glargine versus insulin degludec in patients failing on oral therapy in type 2 diabetes: a retrospective real world comparative data from India. Diabetes Metabol Syndr. 2016;10(3):161–165. doi: 10.1016/j.dsx.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Gough S.C., Bhargava A., Jain R. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naive patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Diabetes Care. 2013;36(9):2536–2542. doi: 10.2337/dc12-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vora J., Christensen T., Rana A., Bain S.C. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes mellitus: a meta-analysis of endpoints in phase 3a trials. Diabetes Ther. 2014;5(2):435–446. doi: 10.1007/s13300-014-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insulin degludec for diabetes mellitus. Drug Therapeut Bull. 2013;51(7):78–81. doi: 10.1136/dtb.2013.7.0190. [DOI] [PubMed] [Google Scholar]
- 29.Siegmund T., Tentolouris N., Knudsen S.T., et al. A European, multicentre, retrospective, non-interventional study (EU-TREAT) of the effectiveness of insulin degludec after switching basal insulin in a population with type 1 or type 2 diabetes. Diabetes Obes Metabol. 2017;20(3):689–697. doi: 10.1111/dom.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tibaldi J., Hadley-Brown M., Liebl A., et al. A comparative effectiveness study of degludec and insulin glargine 300 U/mL in insulin-naïve patients with type 2 diabetes. Diabetes Obes Metabol. 2019;21:1001–1009. doi: 10.1111/dom.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan S.D., Bailey T.S., Roussel R., et al. Clinical outcomes in real-world patients with type 2 diabetes switching from first- to second-generation basal insulin analogues: comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study. Diabetes Obes Metabol. 2018;20(9):2148–2158. doi: 10.1111/dom.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoda S., Sato M., Sekigami T., et al. A 1-year, prospective, observational study of Japanese outpatients with type 1 and type 2 diabetes switching from insulin glargine or detemir to insulin degludec in basal–bolus insulin therapy (Kumamoto Insulin Degludec Observational study) J Diabetes Investig. 2016;7(5):703–710. doi: 10.1111/jdi.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heise T., Nosek L., Bøttcher S.G., Hastrup H., Haahr H. Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metabol. 2012;14:944–950. doi: 10.1111/j.1463-1326.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 34.Tresiba (insulin degludec); injection for subcutaneous administration. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203314lbl.pdf USPI, Available at:
- 35.Tresiba (Insulin Degludec), injection for subcutaneous administration, SmPC. Available at: https://www.ema.europa.eu/en/documents/product-information/tresiba-epar-product-information_en.pdf. Accessed on Feb 01, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.