Abstract
Background
Thermogenic brown and beige adipocytes are recognized for their unique capacity to consume extraordinary levels of metabolites and lipids from the blood to fuel heat-producing catabolic processes [[1], [2], [3], [4], [5], [6], [7]]. In humans, the functions of thermogenic adipocytes are associated with cardiometabolic protection and improved glycemic control [[8], [9], [10], [11], [12], [13]]. Consequently, engaging these macronutrient-consuming and energy-dissipating activities has gained attention as a promising therapeutic strategy for counteracting metabolic diseases, such as obesity and diabetes.
Scope of review
In this review, we highlight new advances in our understanding of the physiological role of G protein-coupled receptors (GPCRs) in controlling thermogenic adipocyte biology. We further extend our discussion to the opportunities and challenges posed by pharmacologically targeting different elements of GPCR signaling in these highly specialized fat cells.
Major conclusions
GPCRs represent appealing candidates through which to harness adipose thermogenesis. Yet safely and effectively targeting these druggable receptors on brown and beige adipocytes has thus far proven challenging. Therefore, continued interrogation across the GPCR landscape is necessary for future leaps within the field of thermogenic fat biology to unlock the therapeutic potential of adipocyte catabolism.
Keywords: Brown adipose tissue, G protein-coupled receptor, Cell signaling, Energy expenditure, Obesity, Diabetes
Highlights
-
•
Brown and beige thermogenic adipocytes robustly consume and catabolize macronutrients.
-
•
The catabolic activity of thermogenic adipocytes promotes organismal energy balance.
-
•
Thermogenic adipocyte functions are tightly controlled by G protein-coupled receptors (GPCRs).
-
•
GPCRs can be potentially targeted at multiple levels to therapeutically harness thermogenic activity.
Brown and beige thermogenic adipocytes are catabolic fat cells capable of taking up a range of metabolites and lipids from the blood to fuel energy-dissipating processes [[1], [2], [3], [4], [5],7,[15], [16], [17], [18], [19], [20]]. Adipose thermogenesis is physiologically activated by cold temperature and is critical for defending body temperature in lower mammals and human infants. In adult humans, thermogenic adipocytes appear to act more as an energy-expending metabolic sink to buffer against potentially harmful levels of macronutrients [6], thus improving systemic insulin sensitivity and lipid control [[9], [10], [11], [12], [13]]. As a result of these functions, human brown adipose tissue (BAT) activity is associated with protection from cardiometabolic diseases [8] and excessive weight gain [21]. However, estimates vary greatly as to how much BAT activity adults possess or can accumulate through physiological stimulation. Magnitudes of BAT energy expenditure range from 7 to 211 kcal/day depending on temperature stimulation, the choice of utilized radioisotopes, and assumptions on BAT mass used in the calculations [22]. This last variable is critical as recent evidence suggests that there may be significantly more ‘recruitable’ energy-dissipating adipose depots [23] than what has been previously proposed. Moreover, radiolabeled glucose uptake, the widely used method for measuring BAT activity in humans, likely underestimates the total amount of thermogenic capacity given the relative contributions of glucose versus other substrates, such as lipids, and futile cycles to adipose energy expenditure [22]. Consistent with this point, Chondronikola and colleagues found that cold-induced BAT thermogenesis was fueled more by circulating free fatty acids (70%) than glucose (30%) [9]. Given the overall preponderance of clinical evidence linking thermogenic adipose to metabolic health, significant efforts have been placed into identifying means of leveraging this energy-expending tissue for the treatment of obesity, diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD).
Targeting G protein-coupled receptor (GPCR) signaling represents a promising strategy for clinically exploiting BAT. This family of cell surface receptors are the most druggable proteins in biology [14,24,25], accounting for approximately 20–30% of FDA-approved pharmaceutical compounds [25]. The extraordinary druggability stems, in large part, from their favorable targeting features such as, cell surface accessibility and cell type specificity. GPCRs share a conserved molecular structure comprising seven membrane-spanning α-helixes. Intracellularly, the receptors couple to a heterotrimeric transducer complex (G-protein) consisting of subunits: α, β, and γ. Binding of an activating ligand to the receptors trigger a conformational change in the α-subunit, promoting the exchange of a bound GDP for GTP. Once GTP-bound, the α-subunit is active and the receptor-transducer-complex disassembles to initiate downstream signaling cascades [26,27].
GPCRs are integrally involved in the physiological control of BAT metabolism. In fact, sympathetic tone and the β-adrenergic receptors are the canonical drivers of adipose thermogenesis in mice [1] and humans [28]. Adipocytes express all three subtypes of β-adrenergic receptors [29], ADRB1, ADRB2, and ADRB3, which are endogenously activated by the hormone and neurotransmitter, noradrenaline. In rodents, ADRB3 is the predominant subtype [1] in BAT, as demonstrated extensively through both selective pharmacological compounds [30,31] and genetic engineering [[32], [33], [34], [35]]. Whereas the β-adrenergic receptor landscape in humans is less clear. Cypess and colleagues reported that acute treatment of the β3-adrenergic agonist, mirabegron, activated BAT and increased resting metabolic rate by an average of 200 kcal/day [36] in human subjects. Moreover, prolonged administration significantly improved the whole-body lipid profile and increased insulin sensitivity by 36% [37]. These clinical studies were followed up by in vitro experiments reporting ADRB3-dependent UCP1 expression as well as lipolytic activity [38]. However, in 2021, Blondin and colleagues found that the dose of mirabegron used previously was capable of activating all three ADRBs, and subsequently proposed that ADRB2 was the critical receptor for β-adrenergic stimulation of human brown adipocytes [39]. Meanwhile, Riis-Vestergaard et al. proposed that ADRB1 may be the primary driver of human brown fat thermogenesis based on the high expression of ADRB1 in human brown adipose tissue biopsies and ADRB1-dependent UCP1 expression in an immortalized human brown adipocyte cell line [40]. Regardless, attempts to optimize the dosing of mirabegron to target BAT energy expenditure without increasing heart rate or blood pressure [37,41] revealed a narrow safety window of β-adrenergic agonism, thus, necessitating alternative therapeutic strategies.
Perhaps the most straightforward approach to non-adrenergically harness BAT activity would be to leverage the same downstream cAMP signaling through other Gs-coupled receptors. The potential of this approach was conceptually exemplified by using mice expressing designer Gs-coupled GPCRs (i.e. Gs DREADD) selectively in adipocytes [42,43]. Similar to β-adrenergic agonism, chronic activation of adipocyte Gs-signaling in the DREADD mice reduced fat mass and improved glucose control. Numerous Gs-coupled GPCRs have now been shown to stimulate BAT activity in a β-adrenergic-independent manner, including receptors for adenosine [44,45], secretin [46,47], glucagon [48], glucose-dependent insulinotropic polypeptide [49], adrenocorticotropic hormone [50], as well as the blue light-sensing, Opsin 3 receptor [51], and the orphan, constitutively active receptor, GPR3 [52]. The omega-3 lipid 12-HEPE also stimulates thermogenic activity through Gs-coupling [53], however, the GPCR responsible for this signaling has yet to be identified. However, the maximal therapeutic potential of non-adrenergic, Gs modulators likely remains to be determined. For example, in humans, infusion of secretin increased BAT glucose uptake by 57% and whole-body energy expenditure by 2% [47]. Yet, notably, secretin has a short half-life between 2 and 4 min [54]. A parallel could be made to the development of glucagon-like peptide 1 receptor (GLP-1R) agonists for obesity. Whereas native GLP-1 is similarly short-lived to secretin and provides modest effects on bodyweight, protraction and pharmacological optimization have now produced GLP-1-based molecules that can be administered once weekly and achieve substantial weight loss [55].
Given the critical importance of cAMP production to BAT thermogenic capacity, Gi-coupled receptors, which inhibit adenylyl cyclase activity, would be predicted to impinge on adipose thermogenesis. However, DREADD-based stimulation of global Gi-signaling in adipocytes did not impact β-adrenergic activation of BAT function [43]. Nevertheless, Gi-coupled receptors comprise the largest percentage of GPCRs expressed in brown adipocytes [56]. Thus, the possibility remains that receptors in this class may play a role in BAT metabolism in contexts not yet resolved.
The third major class of GPCR signaling is through Gq/11 alpha subunits. GTP-bound Gq proteins increase both diacylglycerol (DAG), which activates protein kinase C (PKC), and inositol-3-phosphate (IP3), which stimulates the release of calcium into the cytosol. The role of Gq-signaling in brown and beige adipocytes appears to be multi-faceted and has been linked to both inhibition and activation of thermogenic competence. Pharmacological or genetic approaches that directly increase Gq protein signaling suppressed brown and beige adipogenesis and decreased UCP1 expression in mature adipocytes [56]. Moreover, RGS2, a modulator of G protein signaling, was found to promote the development of thermogenic adipocytes by impeding Gq function [57]. These findings on Gq-signaling were further supported by more recent work showing that acetate-mediated GPR43 activity compromised the energy-expending capacity of human thermogenic adipocytes [58]. Additionally, the Gq-coupled neurotensin receptor 2 (NTSR2) suppresses brown adipocyte thermogenesis following binding and activation by neurotensin which is locally produced by lymphatic endothelial cells [59]. Conversely, activation of Gq through GPR120, by either polyunsaturated fatty acids [60] or a small molecule agonist [61], boosted lipid oxidation and mitochondrial respiration in brown adipocytes. Additionally, chronic stimulation of GPR35-dependent Gq-signaling by kynurenic acid was found to enhance β-adrenergic Gs-signaling through RGS14 [62], underscoring the complex manner in which Gq-coupling impacts thermogenic control.
The final major class of Gα subunits is comprised of the G12 and G13 proteins and is the least-studied family. Future efforts will be needed to evaluate a role in thermogenic adipocyte biology.
Two major factors potentially contributing to the complexities and discrepancies within brown and beige fat GPCR research may be the immense heterogeneity present in mouse and human thermogenic adipose tissue and technical challenges with assessing Gpcr expression [63]. While adipose heterogeneity has been successfully mined by recent advances in single-cell and nuclei sequencing to unearth a deeper biological understanding of specific cell populations within the tissue [58], these technologies are currently not capable of adequately surveying the full landscape of GPCRs, as these are typically among the lowest-expressed transcripts in a cell. This complication even applies, to a lesser extent, to more traditional, bulk RNA-sequencing techniques. Currently, the most accurate method to evaluate spatial and quantitative expression of GPCRs with cellular resolution is fluorescence in situ hybridization (FISH). However, this technique is low-throughput and lacks the ability to simultaneously profile the 300+ non-odorant GPCRs in a given cell culture or tissue. Conversely, GPCR qPCR arrays enable deep resolution of the GPCR-ome but do not provide expression data for sub-populations of cells within a sample. Future efforts are needed to profile the GPCR-ome of activated thermogenic adipose tissue and apply multi-plexed FISH to determine distribution at the cellular level.
1. Targetable strategies for GPCRs
The versality of GPCRs as drug candidates extends beyond their plasma membrane accessibility and cell type specificity. In the following section, we explore different features of GPCR biology that we envision as being advantageous for clinically harnessing thermogenic adipocytes.
-
i.
Ligand binding
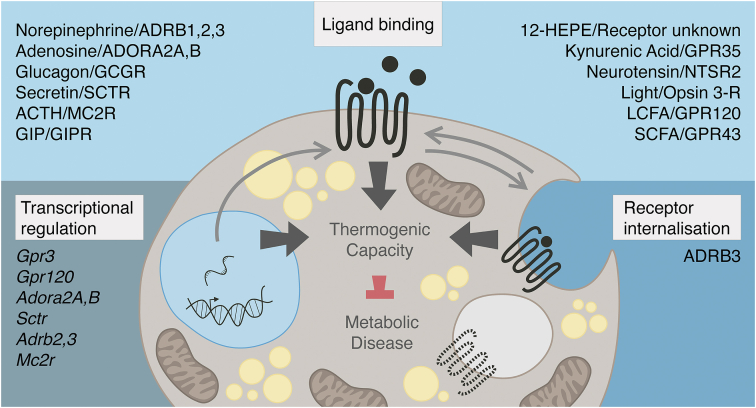
By far the largest exploited form of GPCR control is through manipulation of ligand binding, thereby directly altering downstream signaling. Pharmacotherapies across disease indications have been developed that enhance or impede ligand binding or are, themselves, engineered as more biochemically favorable versions of the naturally occurring ligand. In thermogenic adipocytes, known GPCR ligands represent a diverse range of neurotransmitters [29], hormones [[46], [47], [48], [49], [50]], metabolites [44,58,62,64], and lipids [53,60]. Building a drug using the endogenous ligand as a foundation is the most straightforward way to pharmaceutically modulate signaling given that evolution has already fine-tuned that ligand-receptor pairing. However, as underscored by β-adrenergic agonism [36], engineered hormones or neurotransmitters that reach the bloodstream will signal throughout the body on both desirable (e.g. thermogenic adipocytes) and undesirable (e.g. cardiomyocytes) cell types that express a respective GPCR. Notably, newer studies in humans with systemic administration of secretin, suggest that thermogenic activation is possible without eliciting adverse cardiovascular effects [47]. In addition to hormonal regulation, metabolite and lipid-sensing GPCRs, such as GPR120 and GPR43, have become increasingly recognized for their importance in sampling local nutrient environments within thermogenic adipose depots and toggling bioenergetic and inflammatory responses [58,60,61,64]. This class of receptors offers yet another avenue for harnessing the energy-dissipating power of thermogenic adipose tissue. However, given that the endogenous ligands for metabolite and lipid receptors often also serve as catabolic substrates for intermediary metabolism, nonmetabolizable agonists, such as those available for GPR120 [61], will likely be needed for therapeutic exploitation. A summary of ligand-activated receptors impacting thermogenic biology is presented in Figure 1.
-
ii.
Receptor internalization
Figure 1.
GPCR control of adipose thermogenesis. The capacity of brown and beige adipocytes to consume and catabolize macronutrients to generate heat is under the tight control of GPCRs. GPCR signaling in thermogenic adipocytes is influenced at the level of transcriptional regulation, ligand binding, and receptor internalization.
While ligand-binding stands as a clear first approach when targeting GPCRs, modulating the dynamics of receptor internalization poses an additional strategy through which to selectively manipulate GPCR activity. Canonical regulation of GPCRs at the cell surface is mediated by GPCR kinases (GRKs) that phosphorylate the intracellular face of receptors to recruit β-arrestins 1 or 2 (BARR), which then sterically prohibit G protein coupling [65]. BARRs further engage clathrin and adaptor protein-2 to promote endocytosis of the receptor and terminate extracellular activation. Therefore, it is tempting to speculate that compounds engineered to modulate cell surface occupancy of a specific receptor by biasing for or against interaction with BARR could be appealing therapeutic tools [66]. This potential impact of altering receptor internalization is exemplified by the GIP and GLP-1 dual agonist, tirzepatide, a powerful diabetes and obesity drug candidate [67]. Tirzepatide exhibits sustained efficacy in part due to a signaling bias that reduces GLP-1R BARR recruitment and subsequent internalization [68].
As a proof-of-concept in adipose depots, deletion of Barr2 protected mice from metabolic dysfunction during HFD challenge [69]. BARR2 is a strong negative regulator of ADRB3 and adipocyte Barr2 deficiency led to enhanced ADRB3 signaling and beneficial outcomes on systemic energy homeostasis. The extent to which other adipocyte receptors were affected by Barr2 knockdown remains unknown. Another broad means of modulating cell surface occupancy is at the level of GRKs that phosphorylate GPCRs and prime them for internalization [70,71]. This concept was evaluated by Vila-Bedmar et al. who reported that reducing GRK2 levels boosted thermogenic gene expression in BAT and energy expenditure in the animals [72,73]. However, given the whole-body nature of the constitutive [72] and inducible [73] Grk2 loss-of-function models, the cell autonomous contribution of Grk2 in thermogenic adipocytes to the organismal phenotype could not be resolved.
Finally, targeting strategies designed to modulate receptor internalization will have to account for the unique characteristics of each GPCR. Removal from the cell surface does not automatically ensure termination of signaling capacity. Even after internalization, certain GPCRs can still signal from various intracellular compartments [74] and, in some cases, this mode of signaling is actually required for the full downstream response to receptor activation [75]. Most importantly, receptor internalization dynamics remain a largely unexplored element of GPCR control, especially in thermogenic biology. Thus, future investigation will be required to determine the bona fide potential of targeting this regulatory paradigm for the therapeutic exploitation of brown and beige adipocytes. Receptors in brown and beige adipocytes that are known to be regulated by internalization are summarized in Figure 1. However, toggling receptor cell surface occupancy is not the only way to affect GPCR signaling activity beyond ligand-binding itself.
-
iii.
Transcriptional regulation
Each cell type has a unique expression signature of GPCRs that dictates the particular set of ligand-mediated signals which the cell can receive [[76], [77], [78]]. Classically, investigation into GPCR transcription has focused on mapping the more static, basal receptor profiles across tissues and cell types [56,77,79], whereas dynamic regulation of GPCR mRNA levels, and, consequently, cell surface expression, is less appreciated. Yet the signaling capacity stands to be dramatically affected by acute physiological or pathological alterations of a cell's GPCR-ome, regardless of ligand availability. Downregulation of receptor expression would conceivably act as a more sustained regulatory parallel to receptor internalization and might serve to better protect the cell against overstimulation. Indeed, activation of thermogenic adipose by exposure to cold temperature significantly decreased the expression of GIP receptor, secretin receptor, and β-adrenergic receptors 2 and 3 for periods of time ranging from 3 h up to 3 weeks [52]. In humans, SCTR expression in BAT is negatively correlated with body weight in fasted individuals and trends towards a decrease in the fed state [47]. Finally, ADORA2B and GPR3 are both down regulated in thermogenic adipose depots of overweight and obese individuals. On the other hand, acute transcriptional induction of a GPCR would potentially hypersensitize a given cell type to a ligand, even in the absence of changed ligand levels. For example, cold exposure significantly and rapidly increased the expression of the polyunsaturated fatty acid sensor, Gpr120 [60], and the adenosine receptor, ADORA2A [44], leading to elevated thermogenic capacity.
However, the necessity to understand transcriptional control of receptors is perhaps best exemplified by the GPCRs that possess some degree of intrinsic signaling activity, independent of an external ligand. Compared to purely ligand-driven GPCRs, the mRNA levels of these receptors are strongly correlated to signaling activity [80]. Despite nearly a third of GPCRs exhibiting some degree of intrinsic signaling capacity [81], including the ghrelin [80] and melanocortin 4 receptors [82], we know surprisingly little about this broadly relevant mode of signaling control. GPR3, GPR6, and GPR12 are particularly unique because they are fully constitutively active (e.g. GPR3 signals through Gs proteins upon reaching the cell surface without the need for an externally added ligand [83]). For these receptors with complete constitutive activity, transcriptional control and receptor internalization are the primary opportunities for regulation. Recently, a significant role for the constitutively active receptor, GPR3, was shown in mouse and human brown and beige adipocytes [52]. Gpr3 is transcriptionally induced in brown adipocytes by a cold-dependent lipolytic signal, and the receptor is sufficient to activate the thermogenic program and counteract organismal metabolic dysfunction. A summary of GPCRs known to be regulated at the transcriptional level in thermogenic adipocytes is included in Figure 1.
In contrast to using endogenous ligands as biochemical scaffolds for developing drug candidates, modulating GPCR activity through targeting receptor expression requires the identification and pharmacological manipulation of the endogenous transcriptional machinery. This strategy carries more technical challenges compared to ligand-based approaches. However, the possibility of identifying cell type specific transcription factors that control a desired GPCR, carries the potential to achieve more selective target engagement within an organism. Additionally, transcriptional regulation of GPCRs in a cell type-specific manner may be the only way to effectively harness receptors with significant constitutive activity.
Finally, another strategy to manipulate GPCR expression in thermogenic adipocytes would be virally delivered gene transfer [84]. This area is rapidly developing and, in 2019, the US Food and Drug Administration released a report predicting that 10–20 new cell and gene therapy products will be approved per year by 2025 [85]. The improvements in cell specific viral serotypes and customized promoters provide an even greater degree of selectivity. In thermogenic adipocytes, Sveidahl Johansen et al., showed that a one-time delivery of either adeno-associated or lentiviral particles expressing Gpr3 directly into thermogenic fat depots was sufficient to significantly boost energy dissipating capacity in mice [52]. In an earlier study, delivery of viral particles expressing the adenosine receptor, ADORA2A, into subcutaneous white adipose depots was effective at promoting the formation of energy-expending beige adipocytes [44].
2. Future perspectives
From a therapeutic consideration, BAT activity is often decreased in the target population of older, obese adults, thus raising the question as to whether human BAT activity can be leveraged to counteract metabolic diseases. However, multiple studies have indicated that BAT activity can be stimulated even in aged or metabolically diseased individuals. Hanssen et al., were able to recruit BAT in obese subjects upon short-term cold acclimation [12] and Jespersen et al. detected UCP1 positive adipose depots in subjects up to 84 years of age with BMIs up to 31 [86,87]. Moreover, in a comprehensive retrospective analysis of 134,529 PET-CT scans, Becher et al. found that the metabolic benefit of BAT was more pronounced in individuals who were overweight or obese compared to individuals with a normal weight BMI [8]. Even with the presence of recruitable thermogenic adipose in older, obese adults, significant challenges remain with regard to safely engaging this catabolic tissue. This not only includes avoiding increases in heart rate or blood pressure but also body temperature to a degree that negatively impacts sleep quality and overall quality-of-life. The 7–211 kcal/day range of BAT activity may actually be an ideal window through which to avoid dangerous increases in cardiac output and body temperature while still providing enough energy-expending capacity to meaningfully provide cardiometabolic protection, glucose and lipid control, and prevent weight gain (based on the 30 kJ/day estimate from Hall et al. [88]). Importantly, until there is a protracted, safe, and selective activator of thermogenic adipose, we won't know the maximal degree of energy expenditure and glucose and lipid uptake that can be achieved by sustained pharmacological stimulation over the current estimates attained by physiological cold exposure or administration of mirabegron or secretin. Nevertheless, considering the advances made in appetite-suppressing therapeutics [55] and the energy difference necessary to exert substantive weight loss [88], we believe it is unlikely that any amount of BAT activation would represent a standalone therapeutic to reverse obesity without a food intake modulating component. Therefore, from an obesity standpoint, we speculate that BAT-stimulating drugs would instead make a more profound impact in combination with appetite suppressants and used as means of off-setting the homeostatic decrease in energy expenditure often associated with reduced food intake and weight loss.
Yet there is still a lack of robust pharmacological tools to safely exploit these catabolic fat cells. From the array of autocrine, paracrine, and endocrine factors that physiologically activate adipocyte macronutrient uptake and energy expenditure [[89], [90], [91]], we have focused on the highly druggable GPCR family given their successful application in numerous disease indications, including obesity. While significant leaps have been made in understanding brown and beige adipocyte GPCRs, several mechanistic and translational knowledge gaps remain that prevent the true assessment of the therapeutic potential of these cells. First, thermogenesis-inducing Gs-coupled receptors must be more thoroughly examined for viable nonadrenergic alternatives to safely mimic the potent energy-expending power of sympathetic activation. Additionally, roles for receptors signaling through the other G proteins, Gi and Gq, need to be clearly resolved. Finally, focusing on more untapped elements of GPCR biology beyond ligand-binding, including receptor internalization and transcriptional control, may offer complementary approaches that boost specificity and efficacy of small molecule or peptide drug candidates. Collectively, we believe that these continued explorations across the GPCR landscape in thermogenic adipocytes will reveal key insights for novel treatment strategies to mitigate the global challenges of metabolic disease.
Acknowledgements
The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (http://cbmr.ku.dk/).
Conflict of interest
Z.G.H. is co-founder of Embark Biotech ApS, a company developing therapeutics for the treatment of diabetes and obesity.
References
- 1.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., Kang Q., Yoneshiro T., Camporez J.P., Maki H., Homma M., et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine. 2017;23(12):1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mottillo E.P., Balasubramanian P., Lee Y.-H., Weng C., Kershaw E.E., Granneman J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic 3-adrenergic receptor activation. The Journal of Lipid Research. 2014;55(11):2276–2286. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P., Kajimura S. The cellular and functional complexity of thermogenic fat. Nature Reviews Molecular Cell Biology. 2021;22(6):393–409. doi: 10.1038/s41580-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfrum C., Gerhart-Hines Z. Fueling the fire of adipose thermogenesis. Science (New York, N.Y.) 2022;375(6586):1229–1231. doi: 10.1126/science.abl7108. [DOI] [PubMed] [Google Scholar]
- 8.Becher T., Palanisamy S., Kramer D.J., Eljalby M., Marx S.J., Wibmer A.G., et al. Brown adipose tissue is associated with cardiometabolic health. Nature Medicine. 2021;27(1):58–65. doi: 10.1038/s41591-020-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chondronikola M., Volpi E., Borsheim E., Porter C., Annamalai P., Enerback S., et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chondronikola M., Volpi E., Børsheim E., Porter C., Saraf M.K., Annamalai P., et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metabolism. 2016;23(6):1200–1206. doi: 10.1016/J.CMET.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanssen M.J.W., Hoeks J., Brans B., van der Lans A.A.J.J., Schaart G., van den Driessche J.J., et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nature Medicine. 2015;21(8):863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen M.J.W., van der Lans A.A.J.J., Brans B., Hoeks J., Jardon K.M.C., Schaart G., et al. Short-term cold acclimation recruits Brown adipose tissue in obese humans. Diabetes. 2016;65(5):1179–1189. doi: 10.2337/DB15-1372. [DOI] [PubMed] [Google Scholar]
- 13.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of Clinical Investigation. 2015;125(2):478. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., et al. A comprehensive map of molecular drug targets. Nature Reviews Drug Discovery. 2017;16(1):19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P., et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163(3):643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Boström P., Sparks L.M., Ye L., Choi J.H., Giang A.-H., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabalina I.G., Petrovic N., de Jong J.M.A., Kalinovich A.V., Cannon B., Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 2013;5(5):1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Mills E.L., Pierce K.A., Jedrychowski M.P., Garrity R., Winther S., Vidoni S., et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560(7716):102–106. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneshiro T., Wang Q., Tajima K., Matsushita M., Maki H., Igarashi K., et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572(7771):614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., et al. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 21.Hollstein T., Vinales K., Chen K.Y., Cypess A.M., Basolo A., Schlögl M., et al. Reduced brown adipose tissue activity during cold exposure is a metabolic feature of the human thrifty phenotype. Metabolism Clinical and Experimental. 2021;117:154709. doi: 10.1016/j.metabol.2021.154709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpentier A.C., Blondin D.P., Virtanen K.A., Richard D., Haman F., Turcotte É.E. Brown adipose tissue energy metabolism in humans. Frontiers in Endocrinology. 2018;9:447. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitner B.P., Huang S., Brychta R.J., Duckworth C.J., Baskin A.S., McGehee S., et al. Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences. 2017;114(32):8649–8654. doi: 10.1073/pnas.1705287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriram K., Insel P.A. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Molecular Pharmacology. 2018;93(4):251. doi: 10.1124/MOL.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser A.S., Attwood M.M., Rask-Andersen M., Schiöth H.B., Gloriam D.E. Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobilka B.K. G protein coupled receptor structure and activation. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768(4):794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiological Reviews. 2005;85(4):1159–1204. doi: 10.1152/PHYSREV.00003.2005. [DOI] [PubMed] [Google Scholar]
- 28.Søndergaard E., Gormsen L.C., Christensen M.H., Pedersen S.B., Christiansen P., Nielsen S., et al. Chronic adrenergic stimulation induces brown adipose tissue differentiation in visceral adipose tissue. Diabetic Medicine. 2015;32(2):e4–e8. doi: 10.1111/dme.12595. [DOI] [PubMed] [Google Scholar]
- 29.Collins S. β-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Frontiers in Endocrinology. 2011;2(JAN) doi: 10.3389/FENDO.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao C., Goldgof M., Gavrilova O., Reitman M.L. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring, Md. 2015;23(7):1450. doi: 10.1002/OBY.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. AJP Endocrinology and Metabolism. 2010;298(6):1244–1253. doi: 10.1152/AJPENDO.00600.2009. [DOI] [PubMed] [Google Scholar]
- 32.Bachman E.S., Dhillon H., Zhang C.-Y., Cinti S., Bianco A.C., Kobilka B.K., et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845. doi: 10.1126/SCIENCE.1073160. [DOI] [PubMed] [Google Scholar]
- 33.Susulic V.S., Frederich R.C., Lawitts J., Tozzo E., Kahn B.B., Harper M.-E., et al. Targeted disruption of the β3-adrenergic receptor gene. Journal of Biological Chemistry. 1995;270(49):29483–29492. doi: 10.1074/JBC.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 34.Ueta C.B., Fernandes G.W., Capelo L.P., Fonseca T.L., Maculan F.D., Gouveia C.H.A., et al. β(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. Journal of Endocrinology. 2012;214(3):359–365. doi: 10.1530/JOE-12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soloveva V., Graves R.A., Rasenick M.M., Spiegelman B.M., Ross S.R. Transgenic mice overexpressing the β1-adrenergic receptor in adipose tissue are resistant to obesity. Molecular Endocrinology. 1997;11(1):27–38. doi: 10.1210/mend.11.1.9870. [DOI] [PubMed] [Google Scholar]
- 36.Cypess A.M., Weiner L.S., Roberts-Toler C., Elía E.F., Kessler S.H., Kahn P.A., et al. Activation of human Brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metabolism. 2015;21(1):33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Mara A.E., Johnson J.W., Linderman J.D., Brychta R.J., McGehee S., Fletcher L.A., et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. The Journal of Clinical Investigation. 2020;130(5):2209–2219. doi: 10.1172/JCI131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cero C., Lea H.J., Zhu K.Y., Shamsi F., Tseng Y.-H., Cypess A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight. 2021;6(11) doi: 10.1172/jci.insight.139160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blondin D.P., Nielsen S., Kuipers E.N., Severinsen M.C., Jensen V.H., Miard S., et al. Human Brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metabolism. 2020;32(2):287–300. doi: 10.1016/J.CMET.2020.07.005. e7. [DOI] [PubMed] [Google Scholar]
- 40.Riis-Vestergaard M.J., Richelsen B., Bruun J.M., Li W., Hansen J.B., Pedersen S.B. Beta-1 and not beta-3 adrenergic receptors may Be the primary regulator of human Brown adipocyte metabolism. Journal of Clinical Endocrinology & Metabolism. 2020;105(4):e994–e1005. doi: 10.1210/CLINEM/DGZ298. [DOI] [PubMed] [Google Scholar]
- 41.Loh R.K.C., Formosa M.F., Gerche A.L., Reutens A.T., Kingwell B.A., Carey A.L. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes, Obesity and Metabolism. 2019;21(2):276–284. doi: 10.1111/DOM.13516. [DOI] [PubMed] [Google Scholar]
- 42.Wang L., Pydi S.P., Cui Y., Zhu L., Meister J., Gavrilova O., et al. Selective activation of Gs signaling in adipocytes causes striking metabolic improvements in mice. Molecular Metabolism. 2019;27:83–91. doi: 10.1016/J.MOLMET.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caron A., Reynolds R.P., Castorena C.M., Michael N.J., Lee C.E., Lee S., et al. Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Molecular Metabolism. 2019;27:11. doi: 10.1016/J.MOLMET.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gnad T., Scheibler S., von Kügelgen I., Scheele C., Kilić A., Glöde A., et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A 2A receptors. Nature. 2014;516(7531):395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 45.Gnad T., Navarro G., Lahesmaa M., Reverte-Salisa L., Copperi F., Cordomi A., et al. Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metabolism. 2020;32(1):56–70. doi: 10.1016/j.cmet.2020.06.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y., Schnabl K., Gabler S.-M., Willershäuser M., Reber J., Karlas A., et al. Secretin-activated Brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018;175(6):1561–1574. doi: 10.1016/j.cell.2018.10.016. e12. [DOI] [PubMed] [Google Scholar]
- 47.Laurila S., Sun L., Lahesmaa M., Schnabl K., Laitinen K., Klén R., et al. Secretin activates brown fat and induces satiation. Nature Metabolism. 2021;3(6):798–809. doi: 10.1038/s42255-021-00409-4. [DOI] [PubMed] [Google Scholar]
- 48.Beaudry J.L., Kaur K.D., Varin E.M., Baggio L.L., Cao X., Mulvihill E.E., et al. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Molecular Metabolism. 2019;22:37–48. doi: 10.1016/j.molmet.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaudry J.L., Kaur K.D., Varin E.M., Baggio L.L., Cao X., Mulvihill E.E., et al. Physiological roles of the GIP receptor in murine brown adipose tissue. Molecular Metabolism. 2019 doi: 10.1016/j.molmet.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnabl K., Westermeier J., Li Y., Klingenspor M. Opposing actions of adrenocorticotropic hormone and glucocorticoids on UCP1-mediated respiration in Brown adipocytes. Frontiers in Physiology. 2019;9 doi: 10.3389/fphys.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato M., Tsuji T., Yang K., Ren X., Dreyfuss J.M., Huang T.L., et al. Cell-autonomous light sensitivity via Opsin3 regulates fuel utilization in brown adipocytes. PLoS Biology. 2020;18(2) doi: 10.1371/journal.pbio.3000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sveidahl Johansen O., Ma T., Hansen J.B., Markussen L.K., Schreiber R., Reverte-Salisa L., et al. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell. 2021 doi: 10.1016/j.cell.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leiria L.O., Wang C.-H., Lynes M.D., Yang K., Shamsi F., Sato M., et al. 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from Brown fat. Cell Metabolism. 2019;30(4):768–783. doi: 10.1016/j.cmet.2019.07.001. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laurila S., Rebelos E., Honka M.-J., Nuutila P. Pleiotropic effects of secretin: a potential drug candidate in the treatment of obesity? Frontiers in Endocrinology. 2021;12:737686. doi: 10.3389/fendo.2021.737686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Gaal L.F.V., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021;384(11):989–1002. doi: 10.1056/NEJMOA2032183. [DOI] [PubMed] [Google Scholar]
- 56.Klepac K., Kilić A., Gnad T., Brown L.M., Herrmann B., Wilderman A., et al. The G q signalling pathway inhibits brown and beige adipose tissue. Nature Communications. 2016;7(1):1–10. doi: 10.1038/ncomms10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klepac K., Yang J., Hildebrand S., Pfeifer A. RGS2: a multifunctional signaling hub that balances brown adipose tissue function and differentiation. Molecular Metabolism. 2019;30:173–183. doi: 10.1016/j.molmet.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun W., Dong H., Balaz M., Slyper M., Drokhlyansky E., Colleluori G., et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587(7832):98–102. doi: 10.1038/s41586-020-2856-x. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Li E., Czepielewski R.S., Chi J., Guo X., Han Y.-H., et al. Neurotensin is an anti-thermogenic peptide produced by lymphatic endothelial cells. Cell Metabolism. 2021;33(7):1449–1465. doi: 10.1016/j.cmet.2021.04.019. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quesada-López T., Cereijo R., Turatsinze J.-V., Planavila A., Cairó M., Gavaldà-Navarro A., et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nature Communications. 2016;7(1):1–17. doi: 10.1038/ncomms13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schilperoort M., van Dam A.D., Hoeke G., Shabalina I.G., Okolo A., Hanyaloglu A.C., et al. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Molecular Medicine. 2018;10(3) doi: 10.15252/emmm.201708047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agudelo L.Z., Ferreira D.M.S., Cervenka I., Bryzgalova G., Dadvar S., Jannig P.R., et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metabolism. 2018;27(2):378–392. doi: 10.1016/j.cmet.2018.01.004. e5. [DOI] [PubMed] [Google Scholar]
- 63.Sun W., Modica S., Dong H., Wolfrum C. Plasticity and heterogeneity of thermogenic adipose tissue. Nature Metabolism. 2021;3(6):751–761. doi: 10.1038/s42255-021-00417-4. [DOI] [PubMed] [Google Scholar]
- 64.Husted A.S., Trauelsen M., Rudenko O., Hjorth S.A., Schwartz T.W. GPCR-mediated signaling of metabolites. Cell Metabolism. 2017;25(4):777–796. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nature Reviews Molecular Cell Biology. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 66.Smith J.S., Lefkowitz R.J., Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nature Reviews Drug Discovery. 2018;17(4):243–260. doi: 10.1038/nrd.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frías J.P., Davies M.J., Rosenstock J., Pérez Manghi F.C., Fernández Landó L., Bergman B.K., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine. 2021;385(6):503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 68.Willard F.S., Douros J.D., Gabe M.B.N., Showalter A.D., Wainscott D.B., Suter T.M., et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pydi S.P., Jain S., Tung W., Cui Y., Zhu L., Sakamoto W., et al. Adipocyte β-arrestin-2 is essential for maintaining whole body glucose and energy homeostasis. Nature Communications. 2019;10(1):2936. doi: 10.1038/s41467-019-11003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribas C., Penela P., Murga C., Salcedo A., García-Hoz C., Jurado-Pueyo M., et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768(4):913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Whalen E.J., Foster M.W., Matsumoto A., Ozawa K., Violin J.D., Que L.G., et al. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129(3):511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 72.Vila-Bedmar R., Garcia-Guerra L., Nieto-Vazquez I., Mayor F., Lorenzo M., Murga C., et al. GRK2 contribution to the regulation of energy expenditure and brown fat function. The FASEB Journal. 2012;26(8):3503–3514. doi: 10.1096/FJ.11-202267. [DOI] [PubMed] [Google Scholar]
- 73.Vila-Bedmar R., Cruces-Sande M., Lucas E., Willemen H.L.D.M., Heijnen C.J., Kavelaars A., et al. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Science Signaling. 2015;8(386) doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calebiro D., Nikolaev V.O., Gagliani M.C., de Filippis T., Dees C., Tacchetti C., et al. Persistent cAMP-signals triggered by internalized G-protein–coupled receptors. PLoS Biology. 2009;7(8) doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsvetanova N.G., von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nature Chemical Biology. 2014;10(12):1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Insel P.A., Snead A., Murray F., Zhang L., Yokouchi H., Katakia T., et al. GPCR expression in tissues and cells: are the optimal receptors being used as drug targets? British Journal of Pharmacology. 2012;165(6):1613–1616. doi: 10.1111/j.1476-5381.2011.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Regard J.B., Sato I.T., Coughlin S.R. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marti-Solano M., Crilly S.E., Malinverni D., Munk C., Harris M., Pearce A., et al. Combinatorial expression of GPCR isoforms affects signalling and drug responses. Nature. 2020;587(7835):650–656. doi: 10.1038/s41586-020-2888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engelstoft M.S., Park W.-M., Sakata I., Kristensen L.V., Husted A.S., Osborne-Lawrence S., et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Molecular Metabolism. 2013;2(4):376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holst B., Schwartz T.W. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends in Pharmacological Sciences. 2004;25(3):113–117. doi: 10.1016/J.TIPS.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Seifert R., Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;366(5):381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 82.Srinivasan S., Lubrano-Berthelier C., Govaerts C., Picard F., Santiago P., Conklin B.R., et al. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. The Journal of Clinical Investigation. 2004;114(8):1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eggerickx D., Denef J.F., Labbe O., Hayashi Y., Refetoff S., Vassart G., et al. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochemical Journal. 1995;309(Pt 3):837–843. doi: 10.1042/bj3090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balkow A., Hoffmann L.S., Klepac K., Glöde A., Gnad T., Zimmermann K., et al. Direct lentivirus injection for fast and efficient gene transfer into brown and beige adipose tissue. Journal of Biological Methods. 2016;3(3):48. doi: 10.14440/jbm.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nature Reviews Genetics. 2020;21(4):255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 86.Jespersen N.Z., Larsen T.J., Peijs L., Daugaard S., Homøe P., Loft A., et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metabolism. 2013;17(5):798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 87.Jespersen N.Z., Andersen M.W., Jensen V.H., Stærkær T.W., Severinsen M.C.K., Peijs L., et al. Thermogenic genes are blunted whereas brown adipose tissue identity is preserved in human obesity. Cell Biology. 2020 [Google Scholar]
- 88.Hall K.D., Sacks G., Chandramohan D., Chow C.C., Wang Y.C., Gortmaker S.L., et al. Quantification of the effect of energy imbalance on bodyweight. Lancet (London, England) 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villarroya F., Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metabolism. 2013;17(5):638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 90.Villarroya F., Cereijo R., Villarroya J., Giralt M. Brown adipose tissue as a secretory organ. Nature Reviews Endocrinology. 2016;13(1):26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 91.Scheele C., Wolfrum C. Brown adipose crosstalk in tissue plasticity and human metabolism. Endocrine Reviews. 2020;41(1):53–65. doi: 10.1210/endrev/bnz007. [DOI] [PMC free article] [PubMed] [Google Scholar]