Abstract
The purpose of this study is to investigate the development of a new and inexpensive adsorbent by immobilization synthesized silver nanoparticles (AgNPs) onto banana leaves powder (BLP), and the prepared composite (BLP)/(AgNPs) was used as an adsorbent for Zn(II), Pb(II), and Fe(III) ion removal from aqueous solutions under the influence of various reaction conditions. (BLP)/(AgNPs) demonstrated remarkable sensitivity toward Zn (II), Pb (II), and Fe (III) ions; metal ions eliminations increased with increasing contact time, agitation speed, adsorbent dose, and temperature, yielding adequate selectivity and ideal removal efficiency of 79%, 88%, and 91% for Zn (II), Pb (II), and Fe (III) ions, respectively, at pH = 5 for Zn(II) and pH = 6 for Pb(II), and Fe(III). The equilibrium contact time for elimination of Zn (II), Pb (II), and Fe (III) ions was reaches at 40 min. Langmuir, Freundlich, and Temkin equations were used to test the obtained experimental data. Langmuir isotherm model was found to be more accurate in representing the data of Zn(II), Pb(II), and Fe(III) ions adsorption onto (BLP)/(AgNPs), with a regression coefficient (R2 = 0.999) and maximum adsorption capacities of 190, 244, and 228 mg/g for Zn(II), Pb(II), and Fe(III) ions, respectively. The thermodynamic parameters proved that adsorption of metal ions is spontaneous, feasible, and endothermic, whereas Kinetic studies revealed that the process was best described by a pseudo second order kinetics.
Keywords: Agriculture waste, Banana leaves, Heavy metals, Ions removal, Silver nanoparticles, Adsorption kinetics, Adsorption isotherm
Highlights
-
•
By reduction reaction, silver nanoparticles were impregnated in banana leaves homogeneous powder and used as an adsorbent.
-
•
The fabricated composites are used as adsorbent for the removal of Zn (II), Pb (II), and Fe (III) ions from aqueous solutions.
-
•
The new adsorbent exhibited high adsorption capacity with three metal ions and followed the order Pb (II)> Fe (III) >Zn (II) ions.
-
•
The metal ions vanished from the solution within approximately 40 min.
Agriculture waste, Banana leaves, Heavy metals, Ions removal, Silver nanoparticles, Adsorption kinetics, Adsorption isotherm.
1. Introduction
Clean water is the aspiration and basic requirement of people all over the world. Clean, safe water resources are in short supply and this crisis is caused by the rapid growth of the world population, global warming, and the natural, rapid decline of water resources. Therefore, the economical use of water resources, the use of treated wastewater for agriculture and industry, and the use of low-cost and environmentally friendly technologies are effective ways to conserve limited freshwater resources and successfully contribute to the world's clean water [1, 2]. Heavy metals' presence in water resources is a global problem, as most of them are toxic to organisms, are not biodegradable, and tend to accumulate in biota, compromise the immune system, cause cancer, and threaten life on earth [3]. The coexistence of pollutants such as heavy metals in water sources may be due to common geological sources or increased wastewater discharge from different industries, such as: metallurgical, tanning, plating, mining, fertilizer, metal finishing, welding alloy manufacturing plants, fertilizers, chemicals, etc. [4, 5]. Trivalent, and divalent heavy metal ions such as, Fe+3,Zn+2 and Pb+2, are irreplaceable in all walks of life, and even small concentrations can pose a serious threat to human health since they can be effective in the food chain and may threaten all forms of life [6]. The high threat of heavy metal ions propels researchers to look for changed strategies to safeguard the environment and individuals through different remediation processes [7]. Traditional methods similar to chemical precipitation, reduction, solvent extraction, electrochemical deposition, electrolysis, filtration, and ion exchange have been used, but the majority of these methods are either economically unsuitable or inefficient, particularly for a lower concentration of heavy metal ions [8]. The adsorption method is one of the most widely used ideals, productive, and cost-effective methods for purifying water and wastewater [9, 10]. Different adsorbents such as clays, agriculture waste, carbon nanotubes, silica, polymers, and activated carbons have been studied to purify wastewater from heavy metal ions [11, 12, 13]. Adsorbents derived from agriculture waste are regarded as widely abundant, environmentally friendly, and fitting for varied environmental conditions due to their efficiency over wide pH ranges, but slow adsorption and feeble affinity for heavy metals motivate researchers to investigate chemical modification of agriculture waste adsorbents [14]. Some crops are common and grown all year round, such as bananas which are grown in more than 130 countries in the world, and the world production of bananas every year is estimated by millions tons [15]; Therefore, banana agricultural waste may be a source of adsorbents, and the extracted sieved banana leaves homogeneous powder (BLP) can be used as adsorbents to purify wastewater. Nanomaterials have been used as adsorbents in wastewater treatment and have received considerable attention because of their higher efficiency and lower cost compared to ancient materials [16]. The high surface area, high surface free energy, and great density of active sites per unit mass, result in enhanced surface reactivity of nanomaterials, making them good adsorbents [17, 18]. Due to their favorable chemical and physical properties, silver nanoparticles (AgNPs) have been investigated as suitable adsorbents due to their good performance, their high surface area, high catalytic activity, excellent biocompatibility, relatively low cost, and high adsorption capacity [19, 20]. There are different studies in the literature using silver nanoparticle composites as adsorbents to eliminate heavy metal ions and organic pollutants in aqueous solutions, showing good applicability and high efficiency [21, 22]. But, the applicability of NPs in wastewater purification may be restricted due to their low stability and tendency to aggregate [2]. Therefore, mechanical support such as banana leaves powder (BLP) is used to hold AgNPs for possible applications in wastewater treatment. (BLP) is considered an abundant and environmentally friendly agricultural waste that can be used as an adsorbent [14]. To maximize the adsorption efficiency, take advantage of both (BLP) and (AgNPs), overcome AgNPs aggregation and avoid release into solution, silver ions were impregnated into the (BLP) by taking advantage of their inherent porosity, followed by reduction of silver ions into silver nanoparticles. (BLP) was used to hold (AgNPs) because of its high oxygen density, which acts as a binding site for AgNPs [23]. The objective of this work is to fabricate (BLP)/(AgNPs) composite with the advantages of both (BLP) and (AgNPs) for removing Fe (III), Pb (II), and Zn (II) from aqueous solutions. Several reaction conditions that influence heavy metal ion uptake were investigated, including initial metal ion concentration, adsorbent dose, temperature, agitation speed, and solution pH.
2. Experimental
2.1. Materials
Silver nitrate was supplied by GAMMA laboratory chemicals. Glucose was purchased from El-Nasr Chemicals Co. (ADWIC, Egypt). Anhydrous ferric sulfate, Lead nitrate, and Zinc Nitrate Hexahydrate were purchased from Research-Lab Fine Chemical industries. All chemicals were of high grade quality, and all solutions were prepared in demineralized water following the standard procedures.
2.2. Preparation of metal solution
The stock solutions of Zn(II), Pb (II), and Fe (III) were prepared by dissolving the desired amount of metal salts in double-distilled water. The working solutions (50–250 mg/L) of the examined metal ions were adjusted by appropriate dilution of the stock solutions with double-distilled water.
2.3. Banana leaves powder preparation
The collected banana leaves were washed with hot distilled water to separate the impurities, and dried for 24 h, before being used as mechanical support to hold AgNPs in the reduction reaction.
2.4. Synthesis of silver nanoparticles impregnated in banana leaves powder
Silver nanoparticles (AgNPs) were trapped on the given banana leaves powder via reductions of Ag+ ions in aqueous solution. In a reaction mixture, 1 g of BLP was soaked with 50 ml of water, stirred with AgNO3, and followed by the addition of glucose solution. The total volume of reaction mixture was 100 ml. The concentration of AgNO3 was in the range of 0.005–0.07 mol/L, while the glucose concentration was 0.05 mol/L. The mixture was then stirred for 45 min at 75 °C. The surface of BLP appeared coated with a dark brown precipitate, referring to the formation of AgNPs. The resultant material was filtered, washed with distilled water, and dried at 60 °C for 2 h. The generation of AgNPs was assured by obtaining the absorbance of the reaction mixture filtrate spectrophotometrically at λmax = 420 nm. The intense band that appeared around 420 nm is indicated to be a surface plasmon resonance band that appeared due to the excitation of the free electrons in AgNPs [22].
2.5. Characterization techniques
The crystalline phases were determined using X-ray diffraction (XRD, GNR APD-2000 PRO X-ray). Infrared transmittance measurements of (BLP) and (BLP)/(AgNPs) were performed on a PerkinElmer 1430 spectrometer using KBr pellets. The morphology of BLP/AgNPs was examined using a scanning electron microscope (SEM), Joel-JSM-6510 TV.
2.6. Adsorption measurements
Batch adsorption experiments were used for the removal of Zn (II), Pb (II), and Fe (III) from synthetic solutions by (BLP)/(AgNPs) composite. A digital magnetic stirrer MS-H-Pro with a temperature sensor PT 1000 and a Teflon coated magnetic stir bar of 2 cm length is used to stir the heavy metal ion solution with (BLP)/(AgNPs) composite. A predetermined volume of heavy metal ions solution with a predetermined initial concentration is stirred with a predetermined amount of (BLP)/(AgNPs) composite for a predetermined time at a fixed agitation rate, temperature, and pH of the solution, which was modified by the addition of NaOH (0.1 mol/L) or HCl (0.1 mol/L) and measured using a digital pH meter (Model pH System-361, India). For one analysis, 1 ml samples were taken at regular intervals and filtered out of the (BLP)/(AgNPs)/heavy metal ions solution chemical reaction. The atomic absorption spectrophotometer, AAS, (Model, AA55; Varian Inc., USA), was used to measure the concentration of heavy metal ions in the filtrate. The amount of heavy metal ions adsorbed per unit mass of adsorbent (mg/g) at time t, (qt) was determined by Eq. (1) and the removal percentage (R %) was determined by Eq. (2),
| (1) |
| (2) |
where, the initial concentration of heavy metal ions represented by Co, Ct determines the heavy metal ions concentration at time t (mg/l), V is the solution volume (L), and m is the mass per gram of adsorbent.
3. Results and discussion
3.1. Characterization
3.1.1. X-ray diffraction pattern
XRD is characterized as a good fingerprint fitting characterization technique, which is used to explore phase and crystal structure [24]. The results of the X-ray are shown in (Figure 1). The detected peak for BLP is due to the cellulose group crystal plane [25]. The values obtained for the intense peak at 22.55 are attributed to the presence of native cellulose as the prime component, which may be crystalline or amorphous [26]. The new composite produced from (AgNPs) impregnated (BLP) can be crystallographically characterized by peaks of AgNPs around 2θ = 38.2, 45.15, and 64.36, characterize the diffraction planes of (1 1 1), (2 0 0), and (2 2 0) respectively, which relate to the face centered cubic crystal structure of silver, Generally, the broad and small peaks in the X-ray diffraction pattern are related to the small particle size, and the peaks of AgNPs around 2θ = 45.15, and 64.36, suggest that the smaller AgNPs are embedded in the BLP. This obviously confirms the fabrication of stable Ag nanoparticles on BLP [27].
Figure 1.
XRD spectra of BLP and (BLP)/(AgNPs) composite.
3.1.2. FT-IR spectra
The FTIR spectroscopy was used to identify the functional groups of (BLP) and to prove that silver nanoparticles are formed. It helps in studying the chemical composition of fabricated (BLP)/(AgNPs) composites, hence feasible interactions between its functional groups with heavy metal ions [28]. The FT-IR spectra of (BLP) and (BLP)/(AgNPs) are shown in Figure 2 As indicated, the strong adsorption peaks at 3420.54 cm−1 confirms the presence of O–H stretch (alcohol, phenol, carboxylic acid) group [29]. The peaks observed at 2920.39 cm−1 and 2852 cm−1 are assigned to asymmetric C–H and symmetric C–H groups stretching [30]. The peaks at 1732.58 cm−1 are characteristics of the bending of the C=O group [31]. The peak at 1518.25 cm−1 was assigned to C=C stretching vibration of the aromatic ring, and that at 1380.73 cm−1 was assigned to Aliphatic C–H stretching in CH3,C–O stretching, CH bending [32]. The band at 1322.59 cm−1 represented C–C and C–O ring stretching vibrations [33], whereas those at 1246.23 cm−1 could be assigned to the C–C, C–O and C=O stretching [34]. In addition, the peaks at 897 cm−1 and 665 cm−1 are attributed to the C–H rocking vibrations of cellulose and C–O–H group,respectively [33, 34, 35]. Thus, the disappearance of some peaks, and shifts in the intensity of some bands, after impregnation with AgNPs indicate that AgNPs were successfully loaded onto the (BLP). The presence of these functional groups can facilitate the adsorption of heavy metal ions.
Figure 2.
FTIR spectra of BLP and BLP/AgNPs composite.
3.1.3. Morphology of composite
The SEM image characterization is technique used to obtain the surface morphology of the created adsorbent (BLP/AgNPs). As shown in Figure 3, it is clear that the AgNPs (white spots) have a semispherical form and a uniform size distribution. Due to the strong affinity of silver for oxygen and nitrogen in the functional group of BLP, AgNPs were well adhered to the BLP surface [22].
Figure 3.

SEM image of BLP/AgNPs composite.
3.2. Adsorption of heavy metal ions and effect of reaction parameters
The adsorption experiments were conducted by examining the (BLP)/(AgNPs) composite for Zn (II), Pb(II), and Fe(III) ions elimination from aqueous solutions. The adsorption process was carried out by varying contact time, initial heavy metal ion concentration, temperature, adsorbents dose, agitation speed, and pH of the solution. The specific effect of each parameter is examined and methodically studied. The experimental findings demonstrated the expected sensitivity to Zn (II), Pb (II), and Fe (III) ions in aqueous solutions and exhibited priority for each metal ion at a particular pH. The following optimum experimental conditions: adsorbent mass of 0.1 g, metal ion concentration of 100 ppm, agitation speed of 300 rpm, and 0.1 L of aqueous solutions at 30 °C, pH = 5, for Zn (II), and pH = 6 for Pb (II) and Fe (III) ions, were adjusted barring otherwise specified.
3.2.1. Effect of contact time
The effect of contact time on (50–250) ppm of Zn (II), Pb (II), and Fe (III) ions adsorption onto BLP/AgNPs was studied, while all other variables were kept constant. Figure 4 indicates the effect of contact time on the removal percentage of initial concentration of 50 ppm of Zn (II), Pb (II), and Fe (III). The removal percentage of Zn (II), Pb (II), and Fe (III) ions removed at time t, increased sharply over time and reached a stage of stability after ∼35 min. Almost 70%, 70%, and 75% of Zn (II), Pb (II), and Fe(III) ions, respectively removed with 35 min of stirring. Increasing the contact time raises the metal ions uptake efficiency. The equilibrium contact time for Zn (II), Pb (II), and Fe(III) ions removal was estimated by 35 min, because of the sorption efficiency is approximately close to 35 min contacted time as increasing contact time up to 120 min. The adsorption of Zn (II), Pb (II), and Fe (III) ions onto the BLP/AgNPs adsorbent mainly follows the intraparticle diffusion model and adsorption complexation mechanisms [36]. The percentage of Zn (II), Pb (II), and Fe(III) ions adsorption increased rapidly during the initial stage, This is because the active sites on the (BLP)/(AgNPs) surface were available. Subsequently, the adsorption of heavy metal ions decreased as the availability of the vacant sites for adsorption reduced over time.
Figure 4.
Effect of contact time on the removal percentage of (50 ppm) Zn (II), Pb (II), and Fe (III) ions on BLP/AgNPs.
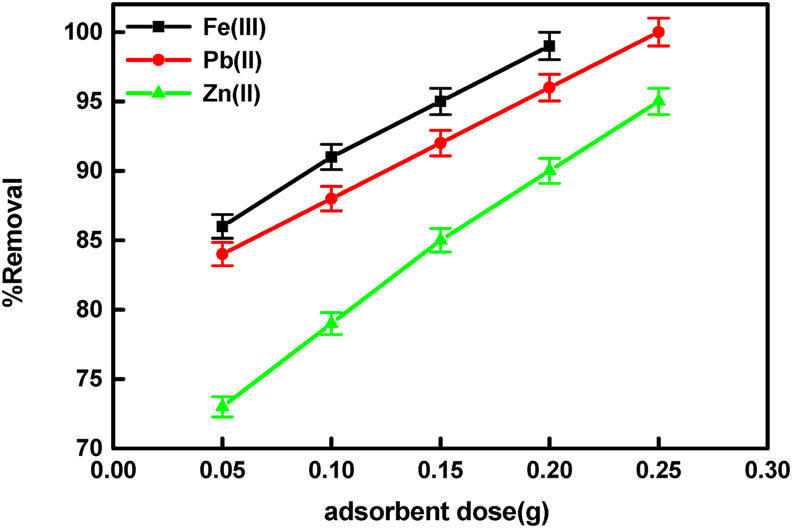
3.2.2. Adsorbent dose effect
The adsorption efficiency of the batch adsorption process is substantially governed by the quantity of adsorbent dose and adsorbent/solution ratio [37]. The removal percentages of Zn (II), Pb (II), and Fe(III) ions were obtained at different doses of BLP/AgNPs and fixed metal ions concentrations. As shown in Figure 5 the removal percentage increased from (73–95%), (84–100%), and (86–100%) for Zn (II), Pb (II), and Fe (III) ions, respectively, by increasing the amount of BLP/AgNPs dose from 0.05 to 0.25 g. This behavior may be due to the fact that, the higher quantity of adsorbent dose in adsorption process, the greater surface area, hence available exchangeable sites for the ions [38].
Figure 5.
Effect of adsorbent dose on the removal percentage of Zn (II), Pb (II), and Fe (III) ions.
3.2.3. The effect of initial Zn (II), Pb (II), and Fe (III) ion concentrations
In order to examine the effect of the initial concentration of Zn (II), Pb (II), and Fe (III) ions on adsorption reactions, the metal ion concentration was varied in the range of 50–250 ppm with a fixed dose (0.1) g of BLP/AgNPs. Figure 6 shows that, the removal percentage of Zn (II), Pb (II), and Fe (III) ions decreases with increasing the initial concentration from (84–60%), (90–73.6%) and (98–76%), respectively, as the metal ions concentration increases from 50 to 250 ppm. The percentage of removal depended upon the initial metal ions concentration may be due to the presence of abundant active sorption sites on the BLP/AgNPs surface at low concentrations of metal ions, and free BLP/AgNPs surface of metal ions, but at high concentrations, a few active sites on the BLP/AgNPs surface hinder the adsorption of more metal ions present in the solution [39].
Figure 6.
Effect of metal ions' concentration on the removal percentage of Zn (II), Pb (II), and Fe (III) ions.
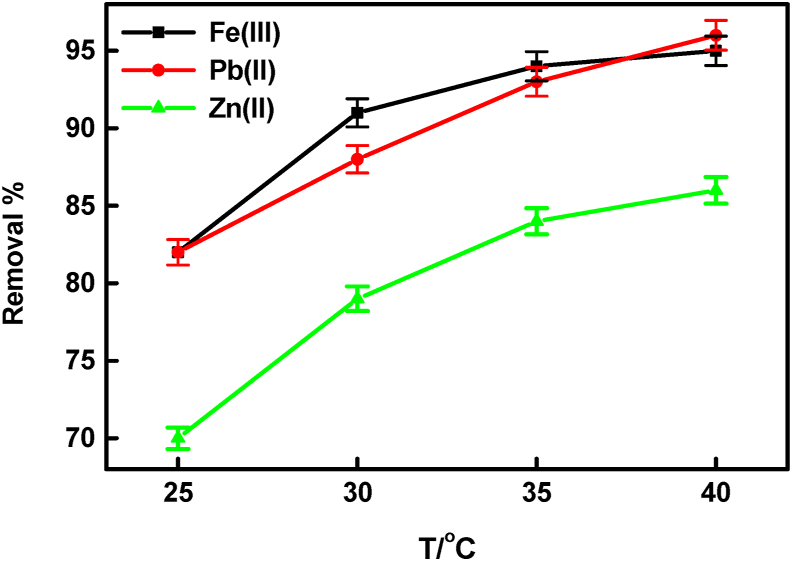
3.2.4. The influence of temperature
The experimental results confirmed the great effect of temperature on the adsorption capacity of BLP/AgNPs, and Zn (II), Pb (II), and Fe (III) ions removed from aqueous solutions. The adsorption experiments were conducted at defined temperatures in the range of 25 °C–40 °C Figure 7 depicts an increase in the percentage of Zn(II), Pb(II), and Fe(III) ions removed by (BLP/AgNPs) from (70–86%), (82–96%), and (82–95%), respectively, as temperature increases from 25 to 40 °C). This improvement in Zn(II), Pb(II), and Fe(III) ion removal with high temperature could be attributed to an increase in the sorbate diffusion rate within the pores of the sorbent, as well as a reduction in liquid viscosity [40]. This reaction behavior confirms the endothermic nature of the adsorption process.
Figure 7.
Influence of temperature on the removal percentage of Zn (II), Pb (II), and Fe (III) ions.
3.2.4.1. Thermodynamic parameters
Thermodynamic behavior of Zn (II), Pb (II), and Fe (III) ions adsorption onto (BLP/AgNPs) surface and the changes in the energies can be described via parameters such as the change in Gibbs free energy (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) which were determined by Eq. (3),
| (3) |
where Kc represents the distribution coefficient of the solute, which is equal to qe/Ce, R is the gas constant (8.314 J/mol K). The Kc values are used in Eq. (3) determine (ΔG°), (ΔH°) and (ΔS°) by Eq. (4).
| ΔG° = ΔH° - T ΔS° | (4) |
The values of other thermodynamic parameters may be obtained from the Van't Hoff equation, which is given by Eq. (5),
| (5) |
Thermodynamic parameters were extracted from van't Hoff linear plots (lnKc vs 1/T) by determining the slope and intercept (Figure 8). According to results tabulated in Table 1, the positive values of ΔH° refer to endothermic adsorption of Zn(II), Pb(II), and Fe(III) ions onto (BLP/AgNPs), The positive values of ΔS° propose increased randomness at the solid/solution interface. The spontaneity and feasibility of Zn (II), Pb (II), and Fe (III) ions adsorption were confirmed by the negative values of ΔG° [41].
Figure 8.
Plot of ln Kc and 1/T at the temperature range under consideration.
Table 1.
Thermodynamic parameters of Zn (II), Pb (II), and Fe (III) ions adsorption onto the (BLP/AgNPs) surface.
| Metal ion | ΔH° (Jmol−1) | ΔS° (Jmol−1K−1) | ΔG° (J.mol−1) |
|||
|---|---|---|---|---|---|---|
| Temperature (K) | ||||||
| 298 | 303 | 308 | 313 | |||
| Zn(II) | 694 | 2.44 | - 2099.2 | - 3337.6 | - 4246.2 | - 4723.9 |
| Pb(II) | 1195.9 | 4.1 | - 3756.8 | - 5019.2 | - 6623.7 | - 8270.2 |
| Fe(III) | 819 | 2.97 | - 4497.5 | - 5828.3 | - 7045.9 | - 7662.2 |
3.2.5. The influence of agitation speed
Agitation speed of the reaction mixture is an intrinsic factor that influences the adsorption process by affecting the dispersion of the reactants, adsorbent/adsorbate rapprochement, and mass transfer. Figure 9 exhibits experimental results for the effect of agitation speeds of (100, 200, 300, and 400) rpm, on the adsorption of Zn(II), Pb(II), and Fe(III) ions onto the BLP/AgNPs surface, at constant other parameters. The removal efficiency increased from (63–82 %), (76–92 %) and from (80–94 %) for Zn(II), Pb(II), and Fe(III), respectively, as the agitation speed increased. The increase in removal percentage may be due to an increase in the diffusion rate of Zn(II), Pb(II), and Fe(III) ions in the bulk solution, decrease of the boundary layer, and increases the transfer rate of the ions in solution [42], whereas the slow agitation speed may cause adsorbent particle aggregation in the solution.
Figure 9.
Agitation speed effect on the removal percentage of Zn(II), Pb(II), and Fe(III) ions.
3.2.6. The influence of solution pH
The solution pH has a great influence on the adsorption of heavy metal ions by affecting the solution chemistry of the heavy metals and the surface charge density of the adsorbent [43]. The effect of pH on the removal of Zn (II), Pb (II), and Fe (III) ions by the BLP/AgNPs composite was investigated, and the results are shown in Figure 10. The adsorption process was investigated at pH < 7 because, at higher pH values, heavy metal ions started to form insoluble metal hydroxides, which restricts true adsorption studies [44]. Figure 10 shows that for Zn (II), Pb (II), and Fe (III) ions, the removal percentage increased as a function of pH from (27–77%), (23–88%), and (30–91%), respectively. With Zn (II), Pb (II), and Fe (III) ions, removal percentages reached maximum values of 79% at pH (5), 88%, and 91% at pH (6), respectively. At low pH, the low adsorption and removal efficiency of Zn(II), Pb(II), and Fe(III) ions could be attributed to the competition of the binding sites on BLP/AgNPs surface for hydrogen ions, so metal ions cannot easily bond to the adsorbent surface [45]. In contrast, the removal percentage increases as pH increases due to the decrease in competition between protons (H+) and positively charged metal ions at the surface sites. The BLP/AgNPs adsorbent is expected to have high adsorption efficiency at neutral or higher pH values, but precipitation of heavy metal ions as insoluble metal hydroxides above pH 6 limits true evaluation of the adsorption process [46]. The metal ions may be bind strongly to the functional groups on the BLP/AgNPs surface with high stability via complexation formation. According to the experimental findings, the present adsorbents exhibited high Zn (II), Pb (II), and Fe (III) ions removal efficiency, and high adsorption capacities in regard to agricultural waste-based adsorbents.
Figure 10.
Effect of pH on the removal percentage of Zn(II), Pb(II), and Fe(III) ions.
3.3. Adsorption isotherms
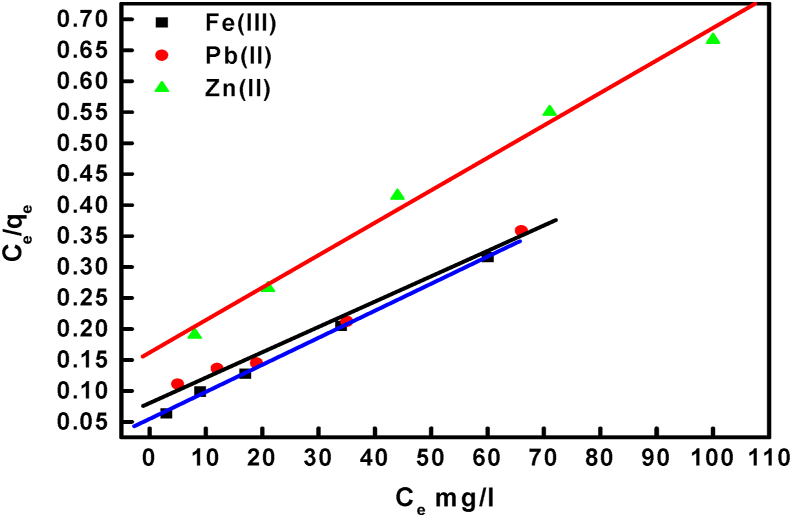
The adsorption isotherms models are used to determine the mechanism of the adsorption process and indicate the distribution of adsorbate between solution and adsorbent at equilibrium [47]. The data obtained from adsorption of Zn (II), Pb (II), and Fe (III) ions onto BLP/AgNPs were analyzed using three isotherm models: Langmuir, Freundlich, and Temkin. The Langmuir adsorption isotherm assumes adsorption of homogeneous and monolayer surfaces. Eq. (6) defines the Langmuir adsorption isotherm,
| (6) |
Where Ce is the concentration of the metal in solution at equilibrium (mg/L), qe is a capacity of adsorption process at equilibrium (mg/g), qm is a maximum capacity of active site (mg/g), and KL (L mg−1) is the Langmuir constant to determine the energy of adsorption. By drawing relation of Ce/qe against Ce as shown in Figure 11, the values of qm and KL can be calculated from the slope and intercept of the plots, respectively, and summarized in Table 2. In Langmuir isotherm model the affinity between the adsorbent and adsorbate were indicated via dimensionless separation factor (RL) which is given by Eq. (7);
| (7) |
Figure 11.
The Langmuir isotherm for Zn (II), Pb (II), and Fe (III) ions adsorption onto BLP/AgNPs surface.
Table 2.
Isotherms data for adsorption of Zn(II), Pb(II), and Fe(III) ions onto BLP/AgNPs surface.
| Adsorption isotherm | Parameters | Values for metal ions |
||
|---|---|---|---|---|
| Zn(II) | Pb(II) | Fe(III) | ||
| Langmuir | KL | 0.032 l g−1 | 0.05 l g−1 | 0.08 l g−1 |
| qm | 190 mg g−1 | 244 mg g−1 | 228 mg g−1 | |
| R2 | 0.995 | 0.993 | 0.999 | |
| Freundlich | KF | 16.2 mol g−1 | 21 mol g−1 | 30.5 mol g−1 |
| 1/n | 0.49 | 0.55 | 0.47 | |
| R2 | 0.97 | 0.96 | 0.98 | |
| Temkin | Bt | 41.7 J mol−1 | 58 J mol−1 | 49 J mol−1 |
| A | 0.324 l g−1 | 0.81 l g−1 | 0.81 l g−1 | |
| R2 | 0.99 | 0.99 | 0.99 | |
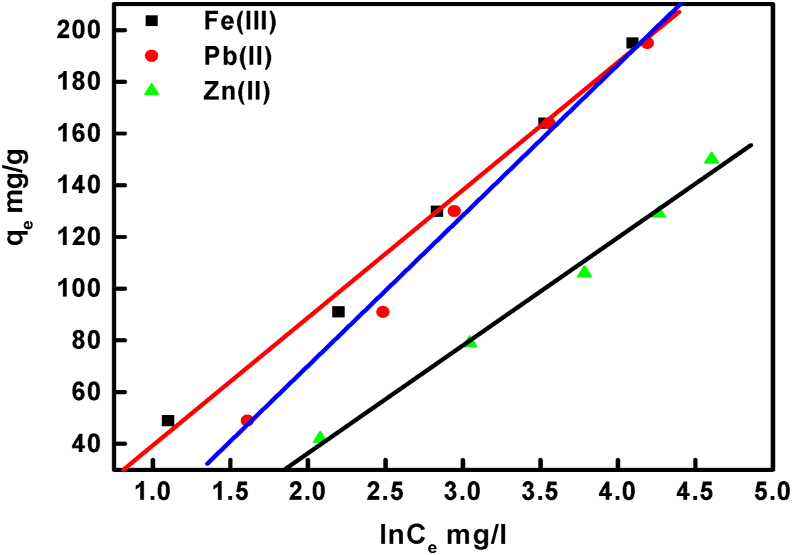
Co represents initial metal ion concentration (mg/L). The characteristics of the RL value indicate the nature of adsorption as favorable when its values lie between zero and one. The values of RL calculated for Zn(II), Pb(II), and Fe(III) ions adsorption onto BLP/AgNPs are respectively 0.38, 0.28 and 0.2 at 50 mg L−1, which indicate a favorable adsorption reactions. The empirical Freundlich equation is based on multilayer adsorption and a heterogeneous surface. The linear form of the Freundlich isotherm can be defined by Eq. (8).
| (8) |
where KF and n are respectively determine the adsorption capacity and the adsorption intensity, and were determined from the intercept and slope of the plot of lnqe versus lnCe, as shown in Figure 12. The resulted 1/n values which given in Table 2, confirm a great adsorption capacity of BLP/AgNPs. Temkin isotherm assumes a uniform distribution of binding energies and contains a factor that considers the heat of adsorbent interactions [48]. The model is given in linear form by Eq. (9),
| (9) |
where Bt = RT/b, b is corresponding to the heat of sorption (J/mol), and A is the equilibrium constant related to the maximum binding energy (l/g), T and R, are respectively, the absolute temperature (K) and gas constant (8.314 J/mol K), A graph of qe against lnCe are shown in (Figure 13) and the values of isotherm constants were determined and listed in Table 2. According to the adsorption constants obtained in Table 2, The Langmuir isotherm was found to be the most suitable model to describe the Zn(II), Pb(II), and Fe(III) ions adsorption onto BLP/AgNPs surface with correlation coefficient values is almost unity. The Langmuir isotherm agreement with the experimental findings, suggest a homogeneous surface and monolayer coverage. For evaluation of BLP/AgNPs as an adsorbent, the adsorption capacity of the BLP/AgNPs was compared with that of other previously studied adsorbents and listed in Table 3. Some previous studies discussed the employment of banana leaves, as adsorbent and exhibited moderate adsorption capacity [49], so there was an urgent need to improve the adsorption capacity of cheap and abundantly available adsorbents (banana leaves). This paper studied the modification of banana leaves surfaces by AgNPs to improve adsorption capacity. The new adsorbent BLP/AgNPs composite is employed to remove all coexisting metal ions and impurities, and will be an accurate relevant adsorbent for various effluents and wastewater.
Figure 12.
The Freundlich isotherm for Zn (II), Pb (II), and Fe (III) ions adsorption onto BLP/AgNPs surface.
Figure 13.
The Temkin isotherm for Zn (II), Pb (II), and Fe (III) ions adsorption onto BLP/AgNPs surface.
Table 3.
Adsorption capacities of BLP/AgNPs composite for Zn (II), Pb (II), and Fe (III) ions compared to other previously studied adsorbents.
| Adsorbents | qm (mg/g) for metal ions as adsorbate |
Reference | ||
|---|---|---|---|---|
| Zn(II) | Pb(II) | Fe(III) | ||
| BLP/AgNPs composite | 190 | 244 | 228 | This study |
| flax fibres | 8.453 | 10.741 | [50] | |
| Albizia lebbeck pods | 8.26 | 7.17 | [51] | |
| manganese dioxide-modified green biochar | 22.38 | 70.67 | [52] | |
| bael leaves | 125 | [53] | ||
| tannic acid immobilised activated carbon | 1.8 | 2.8 | [54] | |
3.4. Adsorption kinetics
To further understand the adsorption behaviors; whether chemical reaction or mass transfer, and describe the metal ions' uptake, several kinetic models were used to model the rate of adsorption of Zn (II), Pb (II), and Fe (III) ions adsorption onto the BLP/AgNPs surface.
3.4.1. Pseudo-first-order kinetic model
This model describes the adsorption rate based on adsorption capacity, where it assumes that the heavy metal ions removal rates are associate with the quantity of the vacant adsorptive sites [55]. Eq. (10) is a general expression for the first-order Lagergren model.
| (10) |
Where qe and qt are, respectively, the amounts of heavy metal ions adsorbed onto the BLP/AgNPs surface (mg/g) at equilibrium and at time t (min). The slope and intercept of plotting vs t, (not shown) are used to determine the Pseudo-first-order kinetic constant k1 (1/min) and the equilibrium capacity qe, respectively. The resultant values of qe, k1, and R2 are tabulated in Table 4. The calculated values of qe were greatly different from the experimental values, which suggest that, the adsorption of Zn (II), Pb (II), and Fe (III) ions onto BLP/AgNPs is not compatible with the Pseudo-first-order kinetic model.
Table 4.
Adsorption kinetics models for the adsorption of Zn (II), Pb (II), and Fe (III) ions onto BLP/AgNPs.
| Metal ion (adsorbate) | C0 mg/l | qe,exp mg/g | Pseudo-first-order model |
Pseudo -second-order model |
||||
|---|---|---|---|---|---|---|---|---|
| qe,cal mg/g | K1X10−3 min−1 | R2 | qe, cal mg/g | K2X10−3 min−1 | R2 | |||
| Zn(II) | 50 | 42 | 30.3 | 6.9 | 0.98 | 45.2 | 2.2 | 0.999 |
| 100 | 79 | 61.2 | 6.21 | 0.99 | 86.4 | 0.91 | 0.999 | |
| 150 | 106 | 66.9 | 4.4 | 0.95 | 112 | 1 | 0.999 | |
| 200 | 129 | 84.6 | 4 | 0.95 | 138 | 0.81 | 0.999 | |
| 250 | 150 | 104 | 3 | 0.93 | 158 | 0.73 | 0.998 | |
| Pb(II) | 50 | 45 | 35.3 | 3.8 | 0.97 | 50.2 | 1.4 | 0.999 |
| 100 | 88 | 54.9 | 5.2 | 0.96 | 94.4 | 1.2 | 0.999 | |
| 150 | 131 | 82.3 | 5.1 | 0.95 | 136.7 | 0.79 | 0.997 | |
| 200 | 165 | 107 | 6.8 | 0.98 | 173.3 | 0.75 | 0.998 | |
| 250 | 184 | 114 | 3.9 | 0.95 | 191.6 | 0.82 | 0.999 | |
| Fe(III) | 50 | 47 | 31.7 | 6.7 | 0.95 | 49.6 | 2.5 | 0.999 |
| 100 | 91 | 63.5 | 6.7 | 0.98 | 96.7 | 1.1 | 0.999 | |
| 150 | 133 | 80.7 | 6.5 | 0.977 | 135.8 | 1.06 | 0.999 | |
| 200 | 166 | 98.8 | 5.2 | 0.95 | 171.5 | 0.94 | 0.999 | |
| 250 | 190 | 113 | 6.4 | 0.98 | 197.6 | 0.9 | 0.999 | |
3.4.2. Pseudo-second-order kinetic model
Pseudo-second-order reaction measures the metal ions adsorbed at time t and that adsorbed at equilibrium on the adsorbent surface [56, 57]. It is represented by Eq. (11);
| (11) |
The t/qt versus t plots (not shown) are used to determine parameters such as k2, qe, and R2. Calculated values of the second-order rate constant k2 (g mol−1 min−1), and qe (mg/g) were respectively extracted from the intercept and slope of the plot and presented in Table 4. The initial adsorption rate (h) was determined by calculated values of k2 and qe and defined by Eq. (12),
| h = k2qe2 | (12) |
The high correlation coefficient close to unity, R2 = 0.999 and the satisfactory fitting of kinetic data in the second-order-model with obtained experimental results shows the compatibility of this model with the Zn(II), Pb(II), and Fe(III) ions adsorption onto the BLP/AgNPs surface, as shown in Table 4.
3.4.3. Intraparticle diffusion model
The previous pseudo-second-order models were unfitted to determine the diffusion mechanism, Weber and Morris developed Intraparticle diffusion model to study the steps of the diffusion mechanism, and investigate the nature of the rate-determining step [58]. The intra-particle diffusion is given by Eq. (13),
| (13) |
where qt (mg g−1) is the amount of metal ions (Zn(II), Pb(II), and Fe(III)) adsorbed onto the adsorbents (BLP/AgNPs) at time t, Kp (mg/g min0.5) is the intraparticle rate constants and C indicates to the boundary layer thickness caused by Zn(II), Pb(II), and Fe(III) adsorption onto BLP/AgNPs. The intraparticle diffusion plot (not shown), confirm that the adsorption process passes through two different stages. The first linear segment describes the external surface adsorption. The second linear segment describes the second stage in which the Zn (II), Pb (II), and Fe (III) ions are gradually adsorbed within the pores of BLP/AgNPs particles The rate constants of the two stages kp1 and kp2 are presented in Table 5, Additionally, the plot exhibited deviation from the origin which refers to that the intraparticle diffusion is not the only rate-limiting step and Zn(II), Pb(II), and Fe(III) ions adsorption process may be controlled by other kinetic models [59, 60].
Table 5.
Intraparticle diffusion model for Zn(II), Pb(II), and Fe(III) ions adsorption onto BLP/AgNPs.
| Metal ion | Co mg/l | Intraparticle diffusion model |
|||
|---|---|---|---|---|---|
| Kp1 mg/g min0.5 | R2 | Kp2 mg/g min0.5 | R2 | ||
| Zn(II) | 50 | 3 | 0.98 | 0.62 | 0.97 |
| 100 | 4.8 | 0.99 | 1.6 | 0.98 | |
| 150 | 5.5 | 0.98 | 2.1 | 0.94 | |
| 200 | 6.1 | 0.99 | 2.3 | 0.94 | |
| 250 | 6.8 | 0.98 | 2.9 | 0.97 | |
| Pb(II) | 50 | 6.6 | 0.99 | 1.5 | 0.98 |
| 100 | 9.7 | 0.99 | 3.3 | 0.95 | |
| 150 | 11.9 | 0.99 | 3.7 | 0.98 | |
| 200 | 12.3 | 0.99 | 4.3 | 0.94 | |
| 250 | 15.3 | 0.98 | 6.3 | 0.97 | |
| Fe(III) | 50 | 6.6 | 0.99 | 1.4 | 0.98 |
| 100 | 9.7 | 0.99 | 3.3 | 0.95 | |
| 150 | 11.9 | 0.99 | 3.7 | 0.98 | |
| 200 | 12.3 | 0.99 | 4.3 | 0.94 | |
| 250 | 15.3 | 0.98 | 6 | 0.97 | |
3.4.4. Reaction mechanism
The adsorption mechanisms of Zn(II), Pb(II), and Fe(III) ions on the BLP/AgNPs composite could be due to physical adsorption and/or precipitation of metal ions. It is also suggested that the metal ions adsorption process may be due to chemisorption, electrostatic attraction between positively charged metal ions and nanosilver (Ago), and complex formation of reduced metal ions. Reduction and complexation may occur according to Eqs. (14) and (15),
| Agom + (m/2) Zn2+ ⟶ mAg++ (m/2) Zno | (14) |
| Agon + Zn2+ ⟶ Ago(n-2) Zno+2Ag+ | (15) |
The functional groups on (BLP) surfaces may contribute to the adsorption of Zn(II), Pb(II), and Fe(III) ions as metal–H ion exchange or metal ion surface complexation adsorption or both. The adsorption of heavy metal ions may occur according to Eq. (16),
| BLP/AgNPs + Fe3+(aq)→Fe3+-BLP/AgNPs | (16) |
The experimental study confirmed that the adsorption of heavy metal ions is reduced in an acidic medium and enhanced by increasing pH.
4. Conclusion
The present study focused on the fabrication of a cost-effective, new adsorbent. On banana leaves powder (BLP), silver nanoparticles (AgNPs) were synthesized and supported. The formation of (AgNPs) was confirmed spectrophotometrically at λmax = 420 nm, impregnation of (AgNPs) with (BLP) was confirmed by the scanning electron microscopy, X-ray diffraction (XRD) patterns, and Fourier transform infrared spectroscopy analysis. The produced adsorbents (BLP/AgNPs) tested for Zn (II), Pb (II), and Fe (III) ions adsorption from aqueous solutions, The experimental findings revealed that the adsorption capacities of the adsorbent and sensitivity to Zn (II), Pb (II), and Fe (III) ions were greatly affected by the conditions of the adsorption process, the adsorption increased with increasing contact time, the dose of adsorbents, agitation speed, and temperature. It was also enhanced at low initial adsorbate concentrations but only slightly at high concentrations. The metal ions uptake was pH-controlled and the maximum sorption uptake was achieved at an optimum pH = 5 for Zn (II) ion, pH = 6 for Pb (II) and Fe (III) ions. The Zn (II), Pb (II), and Fe (III) ions uptake followed pseudosecond-order kinetics together with the intraparticle diffusion model. The adsorption processes were best fitted with Langmuir model, the removal of Zn (II), Pb (II), and Fe (III) ions by BLP/AgNPs were spontaneous and endothermic. The great adsorption capacity of BLP/AgNPs qualifies their use with effluents from a wide variety of industries.
Declarations
Author contribution statement
Mona A. Darweesh, Mohamed I. Ayad, Abdel Monem M. Ahmed: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mahmoud Y. Elgendy, W. A. Hammad: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
N. M. Kamel Elsayed: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Pradhan S.K., Panwar J., Gupta S. Enhanced heavy metal removal using silver-yttrium oxide nanocomposites as novel adsorbent system. J. Environ. Chem. Eng. 2017;5(6):5801–5814. . [Google Scholar]
- 2.Shittu K.O., Ihebunna O. Purification of simulated waste water using green synthesized silver nanoparticles of Piliostigma thonningii aqueous leave extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8(4) [Google Scholar]
- 3.Dubey R.S., Xavier R. Study on removal of toxic metals using various adsorbents from aqueous environment: a review. Sci. J. Eng. 2015;1(1):30–36. [Google Scholar]
- 4.Ge F., Li M.M., Ye H., Zhao B.X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard Mater. 2012;211:366–372. doi: 10.1016/j.jhazmat.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Guo Y., Yang L., Han M., Zhao J., Cheng X. Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J. Environ. Anal. Toxicol. 2012;2(7):154. [Google Scholar]
- 6.Afroze S., Sen T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water, Air. Soil Pollut. 2018;229(7):1–50. [Google Scholar]
- 7.Ali A., Mannan A., Hussain I., Hussain I., Zia M. Effective removal of metal ions from aquous solution by silver and zinc nanoparticles functionalized cellulose: isotherm, kinetics and statistical supposition of process. Environ. Nanotechnol. Monit. Manag. 2018;9:1–11. [Google Scholar]
- 8.Pereira A.G.B., Martins A.F., Paulino A.T., Fajardo A.R., Guilherme M.R., Faria M.G.I., Muniz E.C. Recent advances in designing hydrogels from chitin and chitin-Derivatives and their impact on environment and agriculture: a. Rev. Virtual Quim. 2017;9 [Google Scholar]
- 9.Ali I., Gupta V.K. Advances in water treatment by adsorption technology. Nat. Prot. 2006;1(6):2661–2667. doi: 10.1038/nprot.2006.370. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan G., Chauhan P.U.K. Risk assessment of heavy metal toxicity through contaminated vegetables from waste. Int. J. Adv. Technol. Eng. Sci. 2014;51:444–460. [Google Scholar]
- 11.Hakami O., Zhang Y., Banks C.J. Thiol-functionalised mesoporous silica-coated magnetite nanoparticles for high efficiency removal and recovery of Hg from water. Water Res. 2012;46(12):3913–3922. doi: 10.1016/j.watres.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Uddin M.K. A review on the adsorption of heavy metals by clay minerals with special focus on the past decade. Chem. Eng. J. 2017;308:438–462. [Google Scholar]
- 13.Gupta A., Vidyarthi S.R., Sankararamakrishnan N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard Mater. 2014;274:132–144. doi: 10.1016/j.jhazmat.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V.K., Nayak A., Agarwal S. Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ. Eng. Res. 2015;20(1):1–18. [Google Scholar]
- 15.fao . 2014. Banana Market Review and Banana Statistics 2012–2013. Market and Policy Analyses of Raw Materials, Horticulture and Tropical (RAMHOT) Products Team. Rome. [Google Scholar]
- 16.Al-Qahtani K.M. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt. J. Aqua. Res. 2017;43:269–274. [Google Scholar]
- 17.Zhang Y., Wu B., Xu H., Liu H., Wang M., He Y., Pan B. Nanomaterials-enabled water and wastewater treatment. NanoImpact. 2016;3:22–39. . [Google Scholar]
- 18.El-Wakeel S.T., El-Tawil R.S., Abuzeid H.A., Abdel-Ghany A.E., Hashem A.M. Synthesis and structural properties of MnO2 as adsorbent for the removal of lead (Pb2+) from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017;72:95–103. [Google Scholar]
- 19.Kim S.G., Hagura N., Iskandar F., Okuyama K. Characterization of silica-coated Ag nanoparticles synthesized using a water-soluble nanoparticle micelle. Adv. Powder Technol. 2009;20:94–100. [Google Scholar]
- 20.Vélez E., Campillo G.E., Morales G., Hincapié C., Osorio J., Arnache O., Jaramillo F. Mercury removal in wastewater by iron oxide nanoparticles. J. Phys. C. 2016;687(1) [Google Scholar]
- 21.El-Tawil R.S., El-Wakeel S.T., Abdel-Ghany A.E., Abuzeid H.A., Selim K.A., Hashem A.M. Silver/quartz nanocomposite as an adsorbent for removal of mercury (II) ions from aqueous solutions. Heliyon. 2019;5(9) doi: 10.1016/j.heliyon.2019.e02415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem M.A., Elsharkawy R.G., Ayad M.I., Elgendy M.Y. Silver nanoparticles deposition on silica, magnetite, and alumina surfaces for effective removal of Allura red from aqueous solutions. J. Sol. Gel Sci. Technol. 2019;91(3):523–538. [Google Scholar]
- 23.Liou T.H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010;158(2):129–142. [Google Scholar]
- 24.Barpanda P., Fanchini G., Amatucci G.G. Structure, surface morphology and electrochemical properties of brominated activated carbons. Carbon. 2011;49(7):2538–2548. . [Google Scholar]
- 25.Ashish C., Balbir K. XRD and physicochemical evaluation of Hibiscus sabdariffa cellulose-butyl acrylate-co-vinyl monomer graft. Am. J. Biochem. Mol. Bio. 2013;3(1):61–70. . [Google Scholar]
- 26.Kaith B.S., Singha A.S., Sharma S.K. Synthesis of graft copolymers of binary vinyl monomer mixtures and flax fiber using FAS-KPS redox system. Int. J. Chem. Sci. 2004;2:37–43. . [Google Scholar]
- 27.Du X., He J., Zhu J., Sun L., An S. Ag-deposited silica-coated Fe3O4 magnetic nanoparticles catalyzed reduction of p-nitrophenol. Appl. Surf. Sci. 2012;258:2717–2723. [Google Scholar]
- 28.Chen Q., Zheng J., Wen L., Yang C., Zhang L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: performance and quantum chemical mechanism. Chemosphere. 2019;224:509–518. doi: 10.1016/j.chemosphere.2019.02.138. [DOI] [PubMed] [Google Scholar]
- 29.Popescu C.M., Singurel G., Popescu M.C., Vasile C., Argyropoulos D.S., Willför S. Vibrational spectroscopy and X-ray diffraction methods to establish the differences between hardwood and softwood. Carbohydr. Polym. 2009;77(4):851–857. . [Google Scholar]
- 30.Over L.C., Meier M.A. Sustainable allylation of organosolv lignin with diallyl carbonate and detailed structural characterization of modified lignin. Green Chem. 2016;18(1):197–207. [Google Scholar]
- Dos Santos Dias D., Faria F.A., Mattiolli L., Capela M.V., Capela J.M.V., Crespi M.S., Ribeiro C.A. Moisture sorption of biochar from banana pseudostem fibers according to the pyrolysis temperature. J. Therm. Anal. Calorim. 2019;138(5):3825–3832. [Google Scholar]
- 32.Li W., Zhu Y. Structural characteristics of coal vitrinite during pyrolysis. Energy Fuels. 2014;28(6):3645–3654. [Google Scholar]
- 33.Adapa P.K., Karunakaran C., Tabil L.G., Schoenau G.J. Potential applications of infrared and Raman spectromicroscopy for agricultural biomass. Agric. Eng. Int.: CIGR J. 2009 [Google Scholar]
- 34.Xiao R., Wang S., Li R., Wang J.J., Zhang Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017;141:17–24. doi: 10.1016/j.ecoenv.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Chia C.H., Gong B., Joseph S.D., Marjo C.E., Munroe P., Rich A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012;62:248–257. [Google Scholar]
- 36.Awual M.R., Ismael M., Khaleque M.A., Yaita T. Ultra-trace copper (II) detection and removal from wastewater using novel meso-adsorbent. J. Ind. Eng. Chem. 2014;20(4):2332–2340. [Google Scholar]
- 37.Murugan T., Ganapathi A., Valliappan R. Removal of dyes from aqueous solution by adsorption on biomass of mango (Mangifera indica) leaves. E-j. Chem. 2010;7(3):669–676. [Google Scholar]
- Kaliannan D., Palaninaicker S., Velayutham P., Palanivel V., Kumar A.J. Novel synthesis of Chrysanthemum indicum flower as an adsorbent for the removal of direct Congo red from aqueous solution. Desalination Water Treat. 2018;113:270–280. . [Google Scholar]
- 39.Abdullah N.H., Shameli K., Abdullah E.C., Abdullah L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. B Eng. 2019;162:538–568. [Google Scholar]
- 40.Ding H., Luo X., Zhang X., Yang H. Alginate-immobilized Aspergillus Niger: characterization and biosorption removal of thorium ions from radioactive wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2019;562:186–195. [Google Scholar]
- 41.Waseem M., Muntha S.T., Nawaz M., Rehman W., Rehman M.A., Shah K.H. Effect of heat treatment on the efficient adsorption of Cd2+ ions by nanosized SiO2, TiO2 and their composite. Mater. Res. Express. 2017;4(1) . [Google Scholar]
- 42.Bhaumik R., Mondal N.K. Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl. Water Sci. 2016;6(2):115–135. . [Google Scholar]
- 43.Gong R., Ding Y., Liu H., Chen Q., Liu Z. Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere. 2005;58(1):125–130. doi: 10.1016/j.chemosphere.2004.08.055. . [DOI] [PubMed] [Google Scholar]
- 44.Elouear Z., Amor R.B., Bouzid J., Boujelben N. Use of phosphate rock for the removal of Ni2+ from aqueous solutions: kinetic and thermodynamics studies. J. Environ. Eng. 2009;135(4):259–265. [Google Scholar]
- 45.Saeed A., Iqbal M., Akhtar M.W. Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk) J. Hazard Mater. 2005;117(1):65–73. doi: 10.1016/j.jhazmat.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Liang X., Wang Y., Wang X., Liu M., Yin D., Zhang Y. Adsorption of Copper (II) onto activated carbons from sewage sludge by microwave-induced phosphoric acid and zinc chloride activation. Desalination. 2011;278(1-3):231–237. . [Google Scholar]
- 47.Soliman E.M., Ahmed S.A., Fadl A.A. Reactivity of sugar cane bagasse as a natural solid phase extractor for selective removal of Fe (III) and heavy-metal ions from natural water samples. Arab. J. Chem. 2011;4(1):63–70. . [Google Scholar]
- 48.Kaliannan D., Palaninaicker S., Palanivel V., Mahadeo M.A., Ravindra B.N., Jae-Jin S. A novel approach to preparation of nano-adsorbent from agricultural wastes (Saccharum officinarum leaves) and its environmental application. Environ. Sci. Pollut. Res. 2019;26(6):5305–5314. doi: 10.1007/s11356-018-3734-z. [DOI] [PubMed] [Google Scholar]
- 49.Darweesh M.A., Elgendy M.Y., Ayad M.I., Ahmed A.M., Elsayed N.K., Hammad W.A. Adsorption isotherm, kinetic, and optimization studies for copper (II) removal from aqueous solutions by banana leaves and derived activated carbon. S. Afr. J. Chem. Eng. 2022;40:10–20. [Google Scholar]
- 50.Abbar B., Alem A., Marcotte S., Pantet A., Ahfir N.D., Bizet L., Duriatti D. Experimental investigation on removal of heavy metals (Cu2+, Pb2+, and Zn2+) from aqueous solution by flax fibres. Process Saf. Environ. Protect. 2017;109:639–647. [Google Scholar]
- 51.Mustapha S., Shuaib D.T., Ndamitso M.M., Etsuyankpa M.B., Sumaila A., Mohammed U.M., Nasirudeen M.B. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb (II), Cd (II), Zn (II) and Cu (II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019;9(6):1–11. . [Google Scholar]
- 52.Maneechakr P., Karnjanakom S. Environmental surface chemistries and adsorption behaviors of metal cations (Fe3+, Fe2+, Ca2+ and Zn2+) on manganese dioxide-modified green biochar. RSC Adv. 2019;9(42):24074–24086. doi: 10.1039/c9ra03112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravarty S., Mohanty A., Sudha T.N., Upadhyay A.K., Konar J., Sircar J.K., Gupta K.K. Removal of Pb (II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos) J. Hazard Mater. 2010;173(1-3):502–509. doi: 10.1016/j.jhazmat.2009.08.113. [DOI] [PubMed] [Google Scholar]
- 54.Üçer A., Uyanik A., Aygün Ş.F. Adsorption of Cu (II), Cd (II), Zn (II), Mn (II) and Fe (III) ions by tannic acid immobilised activated carbon. Sep. Purif. Technol. 2006;47(3):113–118. . [Google Scholar]
- 55.Karim M.R., Aijaz M.O., Alharth N.H., Alharbi H.F., Al-Mubaddel F.S., Awual M.R. Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotox. Environ. Safety. 2019;169:479–486. doi: 10.1016/j.ecoenv.2018.11.049. . [DOI] [PubMed] [Google Scholar]
- 56.Kismir Y., Aroguz A.Z. Adsorption characteristics of the hazardous dye Brilliant Green on Saklıkent mud. Chem. Eng. J. 2011;172(1):199–206. [Google Scholar]
- 57.Ahmed A.M., Ayad M.I., Eledkawy M.A., Darweesh M.A., Elmelegy E.M. Removal of iron, zinc, and nickel-ions using nano bentonite and its applications on power station wastewater. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya K., Pehlivan E., Schmidt C., Bahadir M. Use of modified wheat bran for the removal of chromium (VI) from aqueous solutions. Food Chem. 2014;158:112–117. doi: 10.1016/j.foodchem.2014.02.107. . [DOI] [PubMed] [Google Scholar]
- 59.Belkhouche N.E., Benyahia N. Modeling of adsorption of Bi (III) from nitrate medium by impregnated resin D2EHPA/XAD-1180. J. Surf. Eng. Mater. Adv. Technol. 2011;1(2):30–34. [Google Scholar]
- 60.El-Sawy A.M., Moa’mena H.A., Darweesh M.A., Salahuddin N.A. Synthesis of modified PANI/CQDs nanocomposite by dimethylgyloxime for removal of Ni (II) from aqueous solution. Surface. Interface. 2021;26:101392. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.