Abstract
Objective:
This trial aimed to evaluate the acceptability and efficacy of early time restricted eating plus daily caloric restriction (E-TRE+DCR) as compared to DCR alone within a behavioral weight loss intervention.
Methods:
Participants (n=81, 69 female, mean [SD] age 38.0 [7.8] years, BMI 34.1 [5.7] kg/m2) were randomized to E-TRE (10-hour eating window starting within 3 hours of waking) plus DCR or DCR alone (~35% daily caloric restriction) for 39 weeks. The primary outcome was body weight (measured with digital scale) at week 12. Secondary outcomes measured at week 12 included HbA1c, lipids, energy intake (EI, photographic food records), physical activity (PA, accelerometry) dietary adherence (questionnaires) and body composition (DXA). Weight and body composition were also assessed at week 39.
Results:
Mean [SD] weight loss was not different between groups at week 12 (E-TRE+DCR −6.2 [4.1] kg vs DCR −5.1 [3.2] kg) or at week 39 (E-TRE −4.9 [5.3] kg vs DCR −4.3 [5.3] kg). There were no between-group differences in changes in body composition, dietary adherence, EI, PA, HbA1c or lipids at week 12.
Conclusions:
E-TRE+DCR was found to be an acceptable dietary strategy, resulting in similar levels of adherence and weight loss as compared to DCR alone.
Keywords: Time restricted eating, weight loss, behavioral intervention
Introduction:
Time restricted eating (TRE), the practice of restricting energy intake (EI) to a 6–10 hour window each day, is a dietary approach that has gained attention for weight loss (1–9) and metabolic benefit (3–5, 9–14). Previous studies of TRE have utilized variable timing and duration of eating windows, but there is increasing evidence to suggest that early time-restricted eating windows (E-TRE) may result in greater weight loss and metabolic benefit than restricting energy intake to later in the day (late TRE). Several epidemiological and observational studies have shown associations between breakfast skipping and obesity, type 2 diabetes, and cardiovascular disease (15–18). Food intake at night (as seen with shift work), is linked to obesity independent of EI (19, 20), and a higher percent of total daily EI consumed during the evening has been associated with greater risk of overweight and obesity (21–23). Several human studies evaluating the metabolic effects of short-term, eucaloric E-TRE have found improvements in glucose homeostasis (2, 10), insulin sensitivity (11, 13, 14) and β cell responsiveness (11), increased fat oxidation (12), and reductions in blood pressure (11) and appetite (12). In contrast, similar studies utilizing late TRE are limited and have shown inconsistent metabolic effects (14, 24, 25). Additionally, prospective studies evaluating the effects of TRE on weight loss have either used self-selected eating windows (1–3, 7, 9), or have prescribed eating windows using arbitrary clock times, without considering individual differences in habitual sleep schedules (4, 5, 8). The use of self-selected eating windows in these studies presumes that early eating windows would be difficult to adhere to due to the typically later timing of social eating occasions. These trials have shown modest weight loss (1–4%) over 8–26 weeks (1–6, 8, 9, 26). However, none of these studies included recommendations on caloric restriction or provided the support of a behavioral weight loss intervention (standard of care (27)). Further, only two trials assessed weight change beyond 12 weeks (1, 7).
Therefore, this randomized, parallel-design trial was designed to assess the acceptability and efficacy of a 39-week behavioral weight loss intervention utilizing E-TRE (10-hour window starting within 3 hours of waking) plus DCR as compared to DCR without time restriction in adults with overweight and obesity. We recommended caloric restriction to both groups due to prior findings of only modest weight loss (1–4%) in trials of TRE without caloric restriction (1–6, 8, 9, 26). We chose a 10-hour eating window due to data from Gill and Panda (1) that showed that restricting EI to 10–11 hours/day resulted in weight loss in humans. We hypothesized that E-TRE+DCR would be an acceptable dietary intervention, and that it would result in greater weight loss and more favorable changes in body composition and metabolic outcomes at week 12 as compared to DCR.
Methods:
Participants:
Adults aged 18–50 years with a BMI of 27–45 kg/m2 and weight stable (≤ 5% change by self-report over the previous 6 months) with a self-reported typical eating duration >12 hours per day were recruited for a behavioral weight loss trial from the University of Colorado Anschutz Medical Campus and surrounding community (see Supplementary Materials for additional recruitment information and full inclusion/exclusion criteria). The Colorado Multiple Institutional Review Board approved the study protocol and all participants provided written informed consent prior to participation. This study was conducted in accordance with the principles expressed in the Declaration of Helsinki. Clinicaltrials.gov: NCT03571048.
Study Design:
Screening and Randomization:
Following written informed consent, each participant underwent screening procedures, baseline measures, and were then randomized 1:1 to E-TRE+DCR or DCR (see Supplementary Materials for details).
Behavioral weight loss intervention:
Following completion of the baseline assessments, all participants received a 39-week group-based comprehensive behavioral weight loss program, with randomized groups meeting separately. Groups were taught by registered dieticians and met weekly during the first 12 weeks, then monthly between weeks 13 and 39. The curriculum for the intervention was based on the PreventT2 curriculum and utilized a skills-based approach and cognitive behavioral strategies for lifestyle modification with a dietary focus on daily caloric restriction (28). Participants in both groups were given a personalized calorie goal based on their measured resting energy expenditure (REE, indirect calorimetry) reduced by 10% (~35% caloric restriction). Participants in the DCR group were not given any specific instruction regarding timing of food intake, whereas participants in the E-TRE+DCR group were instructed to eat only during a window of 10 hours, starting within 3 hours of waking. Because participants randomized to E-TRE+DCR in the first cohort of the study reported difficulty focusing on both TRE and DCR within the initial weeks of the intervention, the instructions were modified such that participants in E-TRE+DCR were asked to prioritize eating within their 10-hour windows over meeting calorie goals. Participants in both groups were counseled on the importance of PA for weight loss and received a recommendation to perform 150 min/wk of moderate intensity PA.
COVID-19 Related Intervention Modification:
Participants were recruited and enrolled in 3 cohorts between July 2018 and February 2020. Cohort 1 (n=29) and Cohort 2 (n=26) completed all study measures prior to the start of the 2020 COVID-19 pandemic in the United States. Cohort 3 (n=26) started the intervention in February 2020 and was at week 6 of the intervention when a stay-at-home order was issued in Colorado. The behavioral weight loss intervention was moved to a secure virtual platform and week 12 assessments of body composition, resting energy expenditure and metabolic outcome labs were not performed due to restrictions on in person research. However, participants were able to complete measures that did not require in person visits (measures of EI, meal timing and PA) at week 12 in the same manner as cohorts 1 and 2. Home scale weights were obtained in Cohort 3 from week 6 to week 12. Resumption of in person research allowed for clinic weights and body composition measurements to be obtained at week 39 in cohort 3. Because dual x-ray absorptiometry (DXA) measured weights at week 12 (prespecified primary outcome) were missing in cohort 3, the primary outcome was changed to week 12 scale weights obtained in group classes (cohorts 1 and 2) or at home (cohort 3). Based on guidance regarding protocol modification in the setting of COVID-19 (29), DXA weights are presented on a subset of participants as this was the original primary outcome.
Self-reported adherence:
Self-reported adherence to the E-TRE+DCR and DCR interventions was assessed using questionnaires at baseline and weeks 4, 8 and 12, as described in Supplemental Materials.
Meal timing:
Photographic food records were used to assess the timing of energy intake at baseline and weeks 4, 8 and 12, as described in Supplemental Materials.
Anthropometrics:
Height (without shoes to the nearest cm using a stadiometer) was measured once at baseline. Fasted morning weight (in light clothing) was measured to the nearest 0.1 kg using a digital scale (Cardinal Detecto 6800) and assessment of body composition was performed via dual energy x-ray absorptiometry (DXA; Hologic Discovery W, Bedford, MA) at baseline (all 3 cohorts), week 12 (cohorts 1 and 2) and week 39 (all 3 cohorts). For cohorts 1 and 2, non-fasted body weight (in light clothing) was obtained using a calibrated scale (Tanita HD-351) at weekly classes during the first 12 weeks and monthly from weeks 13–39. For cohort 3, body weight was obtained in class during weeks 1–6 using the same methods used for cohorts 1 and 2. Following the move to virtual classes, cohort 3 obtained home weights weekly for weeks 7–12 and monthly for weeks 13–39 by taking photographs of their home scales while they weighed themselves and then texting the photographs to study personnel.
Energy Intake and Healthy Eating Index:
Photographic food records from a consecutive 3-day period during the 7-day data collection periods were used to estimate EI at baseline and week 12. Data on EI obtained from photographic food records was used to calculate the Healthy Eating Index (HEI) (see Supplemental Materials for additional details).
Appetite and Eating Behaviors:
Appetite (hunger, satiety, prospective food consumption and desire to eat) was measured using visual analog scales (VAS) before and after each meal for the 3 day time period coinciding with photographic food records at baseline and week 12 as previously described (30, 31). The Three Factor Eating Inventory was used to assess predisposition to hunger, dietary restraint and disinhibition (32).
Resting Energy Expenditure (REE):
REE was measured at baseline (all cohorts) and week 12 (cohorts 1 and 2) after an overnight fast using standard indirect calorimetry (33) (Parvo Medics TrueOne 2400, Salt Lake City, UT).
PA and Sedentary Behavior:
Free living PA was measured continuously over 7 days using the ActivPAL3 micro accelerometer (PALTechnologies, Glasgow, Scotland) at baseline and week 12. The ActivPAL was placed on the anterior portion of the participant’s non-dominant thigh and uses accelerometer-derived information to estimate time spent in different body positions. Raw activPAL.datx files were processed using the CREA algorithm in the PALBatch software (34). The output data included estimates of time spent sitting, standing, stepping, and daily energy expenditure expressed in metabolic equivalents per hour (MET-h).
Metabolic Outcomes:
Metabolic outcomes were measured at baseline (all 3 cohorts) and week 12 (cohorts 1 and 2 only). Blood samples were analyzed at the Colorado Clinical and Translational Sciences Institute Core Lab. HbA1c was measured with Siemens DCA Vantage analyzer. Cholesterol, LDL, HDL and triglycerides were measured with Beckman/Coulter AU 480 Chemistry analyzer (Table S6).
Sample Size Determination and Power Analysis:
The prespecified primary outcome was weight change at 12 weeks. We aimed to enroll a total of 80 participants, resulting in a minimum detectable effect size of 0.63 Cohen’s D with 80% power at 5% significance using an intent-to-treat (ITT) analysis of all randomized participants. At the time of study design, the only relevant data regarding weight loss with TRE was from Gill and Panda (1), in which the SD for weight loss with TRE at 16 weeks was 3.4 kg. Thus, the study was powered to detect a between-group difference of 2.2 kg.
Statistical Analysis:
Mean and standard deviation among complete cases are reported for approximately normal variables. Median and interquartile range (25%−75%) are reported for variables violating normality assumptions. Intent-to-treat methodology was used to examine all outcomes. Random intercept mixed models with unstructured covariance matrices were used to test whether there were significant within-group and between-group differences in change from baseline to week 12 or 39. Fixed effects included time, randomized group, and their interaction. Within-group differences and between-group differences were tested using contrasts. Additional details regarding statistical analyses can be found in the Supplementary Materials.
Results:
Participants:
N=95 participants were assessed for eligibility and N=85 were enrolled (Figure 1). Demographic characteristics of participants are shown in Table 1. There were no differences between groups in baseline demographics, body weight or composition, meal timing, EI, HEI, appetite ratings, eating behaviors, PA, clinical labs or REE (all p-values ≥ 0.08). Overall retention was 86% at week 12 and 78% at week 39 and did not differ between groups.
Figure 1.
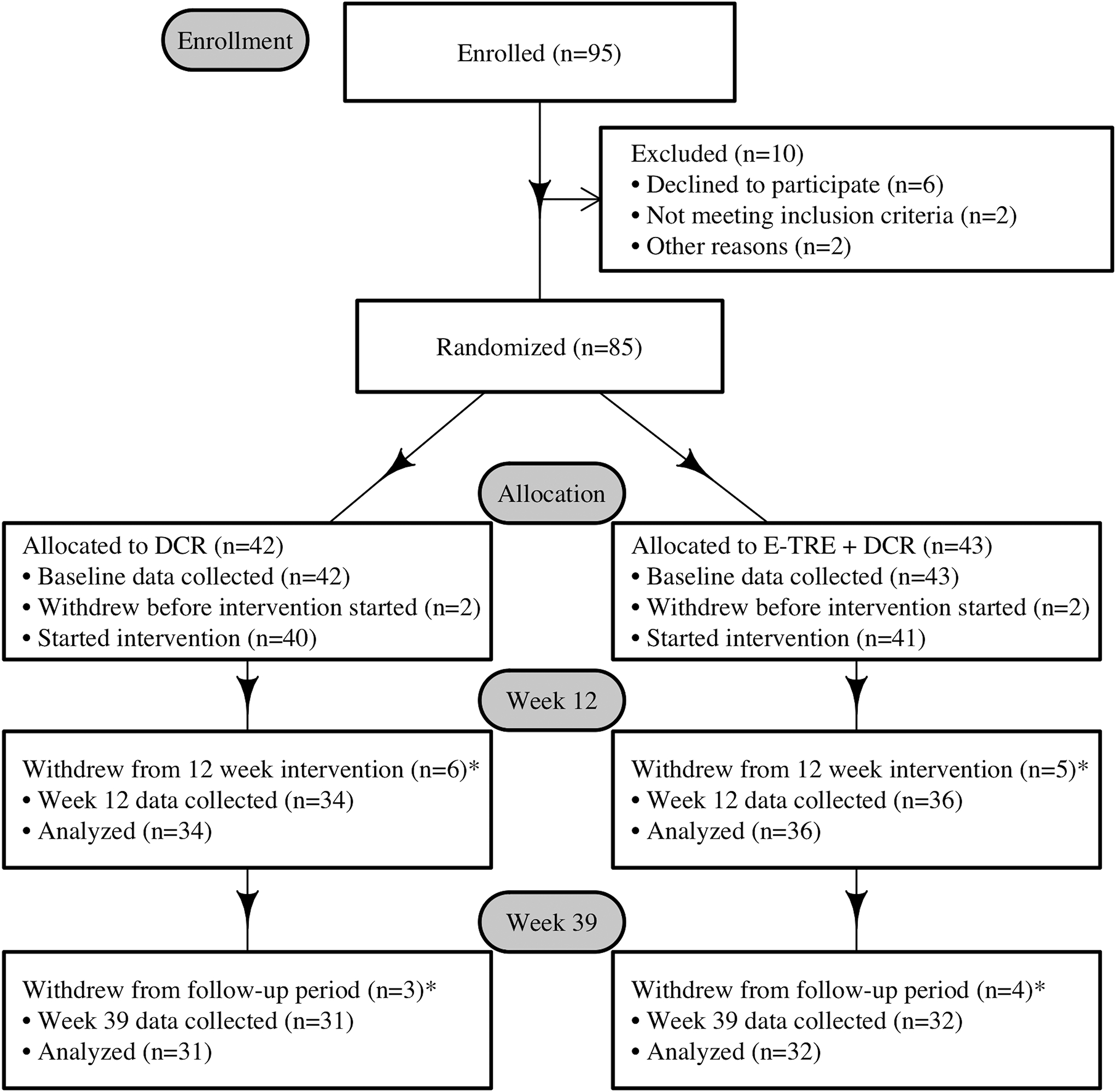
CONSORT Diagram.
* Reasons for withdrawal included scheduling conflicts (n=3), excessive subject burden (n=2), personal reasons (n=3), moved to another state (n=1), pregnancy (n=1), and lost to follow up (n=8).
Table 1.
Participant characteristics at baseline.
| Variable | DCR | E-TRE+DCR | Total | P-value |
|---|---|---|---|---|
| Age, years | 37.8 (7.8) | 38.3 (7.9) | 38.0 (7.8) | 0.79 |
| Sex | 0.56 | |||
| Female | 35 (87.5%) | 34 (82.9%) | 69 (85.2%) | |
| Male | 5 (12.5%) | 7 (17.1%) | 12 (14.8%) | |
| Race | 0.08 | |||
| Asian | 5 (12.5%) | 0 (0.0%) | 5 (6.2%) | |
| Black | 5 (12.5%) | 4 (9.8%) | 9 (11.1%) | |
| White | 30 (75.0%) | 36 (87.8%) | 66 (81.5%) | |
| Not Reported | 0 (0.0%) | 1 (2.4%) | 1 (1.2%) | |
| Ethnicity | 0.55 | |||
| Hispanic or Latino | 7 (17.5%) | 6 (14.6%) | 13 (16.0%) | |
| Not Hispanic or Latino | 32 (80.0%) | 35 (85.4%) | 67 (82.7%) | |
| Not Reported | 1 (2.5%) | 0 (0.0%) | 1 (1.2%) | |
| Height, m | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 0.97 |
| Weight, kg | 93.4 (18.4) | 96.1 (18.1) | 94.8 (18.2) | 0.52 |
| BMI, kg/m 2 | 33.7 (5.6) | 34.6 (5.8) | 34.1 (5.7) | 0.45 |
| Fat mass, kg | 40.1 (11.9) | 41.2 (11.2) | 40.7 (11.5) | 0.66 |
| Fat-free mass, kg | 52.1 (9.1) | 53.7 (9.8) | 52.9 (9.5) | 0.47 |
| Fat mass, % | 43.0 (6.3) | 43.1 (5.9) | 43.1 (6.0) | 0.96 |
Summary statistics are shown as mean (SD) or N (% of total).
Adherence:
There were no significant differences between groups in the average number of days attempted to eat at or below the calorie goal, number of days successful at eating at or below the calorie goal, dietary adherence, adherence difficulty, and number of classes attended during the intervention (Table 2). The E-TRE+DCR group reported greater success eating within their 10-hour windows (mean [SD] 5.1 [1.1] out of 7 days) as compared to eating at or below their calorie goals (4.0 [1.5] out of 7 days; p=0.005).
Table 2.
Mean dietary adherence over initial 12 weeks
| All | DCR | E-TRE+DCR | |||||
|---|---|---|---|---|---|---|---|
| N | Mean + SD or Median (IQR) | N | Mean + SD or Median (IQR) | N | Mean + SD or Median (IQR) | P-value | |
| Days attempted to eat at or below calorie goal (1–7) | 75 | 6.0 (5.0–6.7) | 38 | 5.8 (5.0–6.7) | 37 | 6.0 (5.0–7.0) | 0.46* |
| Days attempted to eat during 10-hour window (1–7) | 37 | 6.2±1.1 | . | 37 | 6.2±1.1 | ||
| Days successful eating at or below calorie goal (1–7) | 75 | 4.1±1.5 | 38 | 3.9±1.5 | 37 | 4.4±1.6 | 0.14 |
| Days successful eating during 10-hour window (1–7) | 37 | 5.5±1.2 | . | 37 | 5.5±1.2 | ||
| Dietary adherence level (1–10) | 75 | 6.2±1.7 | 38 | 5.9±1.8 | 37 | 6.6±1.6 | 0.09 |
| Adherence difficulty (1–10) | 75 | 6.7 (6.0–7.5) | 38 | 6.2 (5.7–8.0) | 37 | 7.0 (6.0–7.3) | 0.69* |
| Likelihood of future adherence to diet (1–10) | 75 | 7.4±1.5 | 38 | 7.5±1.6 | 37 | 7.3±1.5 | 0.71 |
| Number of classes attended during intervention a | 81 | 9.0 (7.0–11.0) | 40 | 8.5 (6.0–11.0) | 41 | 10.0 (8.0–11.0) | 0.47* |
| Number of classes attended during follow up b | 74 | 3.0 (0.0–5.0) | 36 | 3.0 (0.0–5.0) | 38 | 4.0 (1.0–6.0) | 0.27* |
Mean ± SD are presented for variables with approximately normal distributions. Otherwise, Median (P25 - P75) are presented.
A Wilcoxon Two Sample T-Test was used due to non-normal distribution of the data.
Total of 12 classes offered
Total of 6 classes offered
Meal Timing:
Changes in meal timing are shown in Figure 2, S1. Eating duration, first meal timing, and last meal timing changed differently over time between the two groups (p<0.001, p=0.003, and p=0.02). Both groups decreased eating duration from baseline to week 12 (DCR: 11.1 (1.5) hours at baseline to 10.4 (1.5) hours at week 12, p<0.001; E-TRE+DCR: 11.5 (1.7) hours at baseline to 9.1 (0.93) hours at week 12, p<0.001). There was a significant between group difference in week 12 eating duration, with E-TRE+DCR having an eating duration 1.6 (95% CI: −2.6, −1.0, p<0.001) hours shorter than DCR. There was no between group-difference at week 12 in average first mealtime (p=0.11). However, the E-TRE+DCR group ate their last meal 0.93 (95% CI: −1.59, −0.27) hours earlier, on average, than those in DCR (p=0.007) (Figure S2). See Supplemental Materials for additional details regarding meal timing results.
Figure 2.
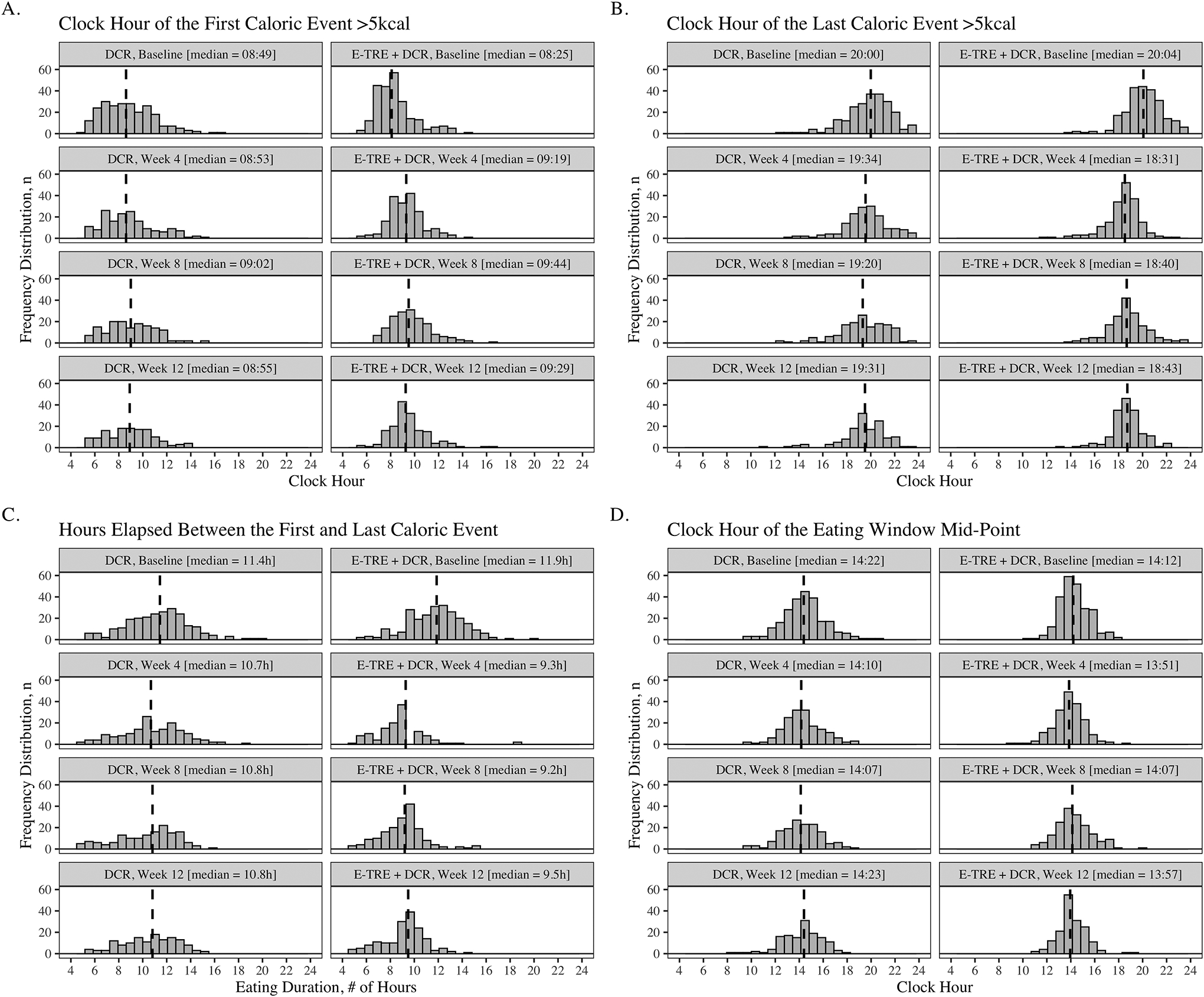
Histogram plots showing the clock hour of the first caloric event (A), clock hour of the last caloric event (B), total eating duration (C), and the mid-point of the eating window (D) at baseline, week 4, week 8, and week 12 of the DCR and E-TRE interventions, respectively. Dashed lines indicate the median response.
Body weight by clinic or home scale:
Among those in DCR, there was a significant decrease in body weight of −5.1 (3.2) kg (−5.5 [3.4] %) at week 12 (p<.001) (Table 3 and Figure 3). In the E-TRE+DCR group, there was a significant decrease in weight of −6.2 (4.1) kg (−6.3 [4.1] %) at week 12 (p<0.001). There was not a significant difference in weight loss over time between the two groups (p=0.28), and there were no significant differences between the groups at week 12 (p=0.69, Table S1). Only 13 participants in DCR and 13 in E-TRE+DCR had a week 39 scale weight collected, so no inferential statistics were calculated for this time point.
Table 3.
Weight change at weeks 12 and 39.
| DCR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12a | Change (Baseline to Week 12) | P for Change (Baseline to Week 12)b | Week 39c | Change (Baseline to Week 39) | P for Change (Baseline to Week 39)b | ||||
| N | Mean (SD) | N | Mean (SD) | Mean (SD) | N | Mean (SD) | Mean (SD) | |||
| Clinic Weight, kg | 40 | 94.2 (18.7) | 27 | 88.9 (19.7) | −5.1 (3.2) | <.0001 | ||||
| DXA Weight, kg | 40 | 93.4 (18.4) | 23 | 87.4 (19.2) | −3.6 (3.3) | <.0001 | 30 | 88.6 (18.6) | −4.3 (5.3) | <.0001 |
| Fat Mass, kg | 40 | 40.1(11.0) | 23 | 37.0 (12.5) | −2.1 (2.6) | <.0001 | 30 | 36.4 (13.2) | −2.6 (4.4) | <.001 |
| Lean Mass, kg | 40 | 52.1 (9.1 | 23 | 49.6 (9.0) | −1.1 (1.8) | 0.001 | 30 | 51.1 (8.3) | −1.5 (1.8) | <.001 |
| E-TRE+DCR | ||||||||||
| Clinic Weight, kg | 39 | 97.0 (18.4) | 33 | 91.2 (18.6) | −6.2 (4.1) | <.0001 | ||||
| DXA Weight, kg | 41 | 96.1 (18.1) | 24 | 90.2 (19.0) | −4.4 (2.6) | <.0001 | 30 | 90.1 (19.8) | −4.9 (5.3) | <.0001 |
| Fat Mass, kg | 41 | 41.3 (11.2) | 24 | 38.5 (11.6) | −2.8 (1.8) | <.0001 | 30 | 36.7 (11.8) | −3.5 (4.0) | <.0001 |
| Lean Mass, kg | 41 | 53.7 (9.8) | 24 | 50.7 (9.7) | −1.5 (1.4) | <.0001 | 30 | 52.5 (10.4) | −1.2 (2.3) | 0.003 |
Due to COVID-related interruptions to the intervention, Week 12 DXA scans were not performed in cohort 3. Cohort 3 is excluded from Week 12 DXA variables.
Random intercept mixed models with unstructured covariance matrices were used to test for significant within-group and between-group changes. Fixed effects included randomized group, time, and a group*time interaction. P-values presented in this table were computed using contrasts.
Week 39 values for clinic weights were not included due to missing data
SD, standard deviation
Figure 3.
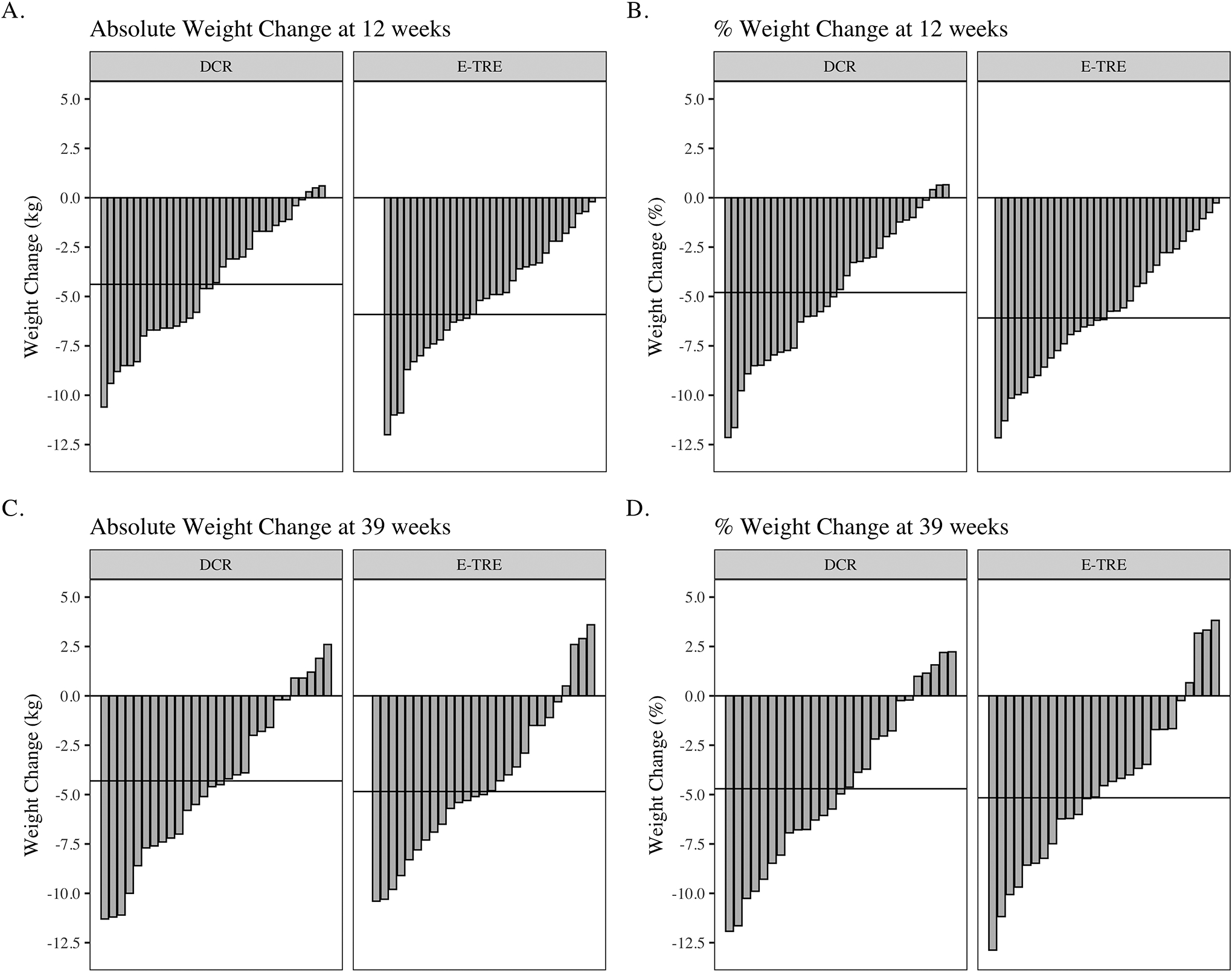
Waterfall plots showing individual (bars) and mean (horizontal lines) weight changes in response to the DCR and E-TRE interventions at study week 12 (A and B) and week 39 (C and D), compared to baseline weight.
Body weight and composition by DXA:
At week 12, the DCR group lost −3.6 (3.3) kg (4.0 [3.7] %) (p<0.001) and the E-TRE+DCR group lost −4.4 (2.6) kg (4.6 [2.7] %) (p<0.001, Table 3). Weight loss, fat mass(FM) loss, and fat free mass (FFM) loss over time did not differ between the groups from baseline to week 12 (p=0.68, p=0.33, and p=0.43). There were no between-group differences in weight, FM or FFM at week 12 (p=0.80, p=0.87 and p=0.79, respectively, Table 3 and Figures S3 and S4). At week 39, the DCR group lost −4.3 (5.3) kg (−4.7 [5.7] %), while the E-TRE group lost −4.9 (5.3) kg (−5.2 [5.9] %). There were no between-group differences in weight, FM or FFM at week 39 (p=0.62, p=0.93 and p=0.39, respectively).
Energy Intake and HEI:
Both groups similarly decreased EI between baseline and week 12 (DCR: 1646 (IQR:1398–2049) kcal/day at baseline to 1166 (IQR:840–1375) kcal/day at week 12, p<0.001; E-TRE+DCR: 1877 (SD: 694) kcal/day at baseline to 1308 (SD: 473) kcal/day at week 12, p<0.001) with no significant difference between groups in week 12 EI (p=0.26). Composite HEI score did not change differently between the groups (p=0.06). In E-TRE+DCR, the composite HEI score increased significantly from baseline (55.4 [12.9]), to week 12 (61.4 [12.2], p=0.005), whereas there was no significant change in DCR from baseline (59.2 [11.8]) to week 12 (60.1 [11.2]) (p=0.87). Week 12 HEI did not differ significantly between groups (p=0.46).
Appetite and Eating Behaviors:
Within each group, average hunger, prospective food consumption, desire to eat and fullness ratings did not significantly change from baseline to week 12 (Figure S5). Week 12 average hunger, prospective food consumption, desire to eat, and fullness did not differ by group (all p-values ≥ 0.17). When assessing appetite outcomes separately by meal, the only significant change in either group was a reduction in hunger at lunch at week 12 in E-TRE+DCR (FDR p=0.04, Table S2). Dietary restraint increased similarly from baseline to week 12 and week 39 in both groups (Table S3). Within each group, disinhibition and predisposition to hunger did not change from baseline at either week 12 or week 39. There were no between-group differences in restraint, disinhibition, or hunger at week 12 or week 39 (p ≥ 0.08).
Activity Behaviors:
There were no significant changes from baseline to week 12 or between group differences at week 12 in measures of PA or sedentary time (Tables 4 and S4). Because lockdown orders imposed significant restrictions on PA for participants in cohort 3 (35), we performed a secondary analysis using data from only cohorts 1 and 2 (see Supplemental Materials and Table S5). These results showed that there was a significant increase in standing time and upright time among both the DCR and E-TRE groups from baseline to week 12 (p<0.01 for both). Among those in the E-TRE group, number of sitting bouts longer than 60 minutes decreased from baseline to week 12 (p=0.02), while in the DCR group, there were no significant differences in number of sitting bouts.
Table 4.
Physical activity at baseline and week 12.
| DCR | |||||
|---|---|---|---|---|---|
| Baseline | Week 12 | P for Comparison (Baseline-Week 12)a | |||
| N | Mean (SD) | N | Mean (SD) | ||
| Number of steps per day | 39 | 7405 (2638) | 34 | 7791 (2703) | 0.36 |
| Upright time, minutes | 39 | 317.9 (109.0) | 34 | 341.7 (117.4) | 0.09 |
| Standing time, minutes | 39 | 225.7 (93.5) | 34 | 243.9 (95.6) | 0.09 |
| Stepping time, minutes | 39 | 92.1 (30.6) | 34 | 97.8 (33.4) | 0.24 |
| Sitting time, minutes | 39 | 628.7 (110.8) | 34 | 622.4 (102.0) | 0.33 |
| Number of sit to stand transitions | 39 | 43.5 (11.6) | 34 | 46.5 (10.5) | 0.17 |
| Activity score, MET.h | 39 | 33.5 (1.1) | 34 | 33.6 (1.2) | 0.31 |
| Number of sitting bouts longer than 30 minutes | 39 | 4.5 (1.6) | 34 | 5.1 (1.9) | 0.11 |
| Number of sitting bouts longer than 60 minutes | 39 | 1.0 (0.8) | 34 | 1.2 (0.8) | 0.48 |
| Time spent in sitting bouts longer than 30 minutes | 39 | 284.9 (107.6) | 34 | 306.9 (101.2) | 0.31 |
| Time spent in sitting bouts longer than 60 minutes | 39 | 138.8 (76.3) | 34 | 140.3 (60.1) | 0.94 |
| E-TRE+DCR | |||||
| Number of steps per day | 41 | 7832 (2285) | 35 | 8455 (3127) | 0.22 |
| Upright time, minutes | 41 | 311.8 (74.6) | 35 | 340.3 (78.1) | 0.08 |
| Standing time, minutes | 41 | 216.3 (60.9) | 35 | 237.6 (58.4) | 0.10 |
| Stepping time, minutes | 41 | 95.5 (25.0 | 35 | 102.7 (31.4) | 0.20 |
| Sitting time, minutes | 41 | 629.0 (69.9) | 35 | 630.2 (94.3) | 1.00 |
| Number of sit to stand transitions | 41 | 44.0 (11.2) | 35 | 45.5 (11.1) | 0.24 |
| Activity score, MET.h | 41 | 33.6 (1.0) | 35 | 33.9 (1.3) | 0.20 |
| Number of sitting bouts longer than 30 minutes | 41 | 4.9 (1.3) | 35 | 4.7 (1.4) | 0.42 |
| Number of sitting bouts longer than 60 minutes | 41 | 1.2 (0.8) | 35 | 1.0 (0.8) | 0.21 |
| Time spent in sitting bouts longer than 30 minutes | 41 | 299.6 (90.1) | 35 | 298.8 (91.9) | 0.82 |
| Time spent in sitting bouts longer than 60 minutes | 41 | 142.2 (72.5) | 35 | 143.3 (74.9) | 0.98 |
Random intercept mixed models with unstructured covariance matrices were used to test for significant within-group and between-group changes. Fixed effects included randomized group, time, and a group*time interaction. P-values presented in this table were computed using contrasts.
Resting Energy Expenditure:
After adjusting for changes in FM and FFM, there was not a significant change from baseline to week 12 in REE in either group (p=0.12 and p=0.87, respectively, Figure 4). There were no significant differences in REE at week 12 between DCR and E-TRE (p=0.63).
Figure 4.
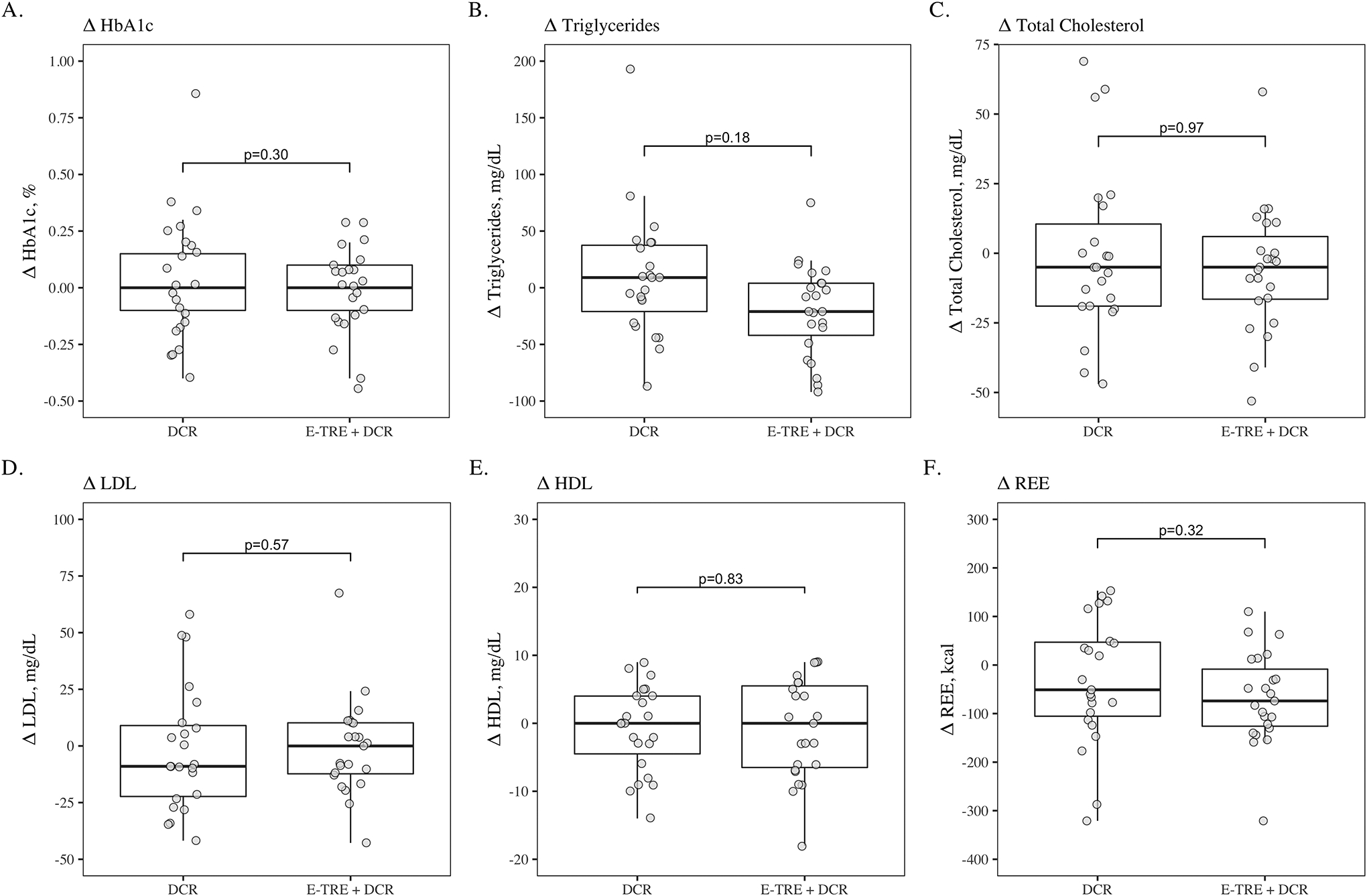
Boxplots comparing baseline to 12-week changes in HbA1c (A), triglycerides (B), total cholesterol (C), LDL cholesterol (D), HDL cholesterol (E), and resting energy expenditure (F) between the DCR and E-TRE interventions, respectively.
Metabolic Outcomes:
HDL, LDL, triglycerides, total cholesterol and HbA1c were not different between groups at week 12 (p ≥ 0.08) (Figure 4). Also, the within group changes from baseline to week 12 were not significant.
Discussion:
We evaluated the acceptability and efficacy of a 39-week weight loss intervention utilizing E-TRE+DCR as compared to DCR in adults with overweight and obesity. While the majority of short-term studies finding metabolic benefit with TRE have utilized early eating windows, most free-living trials of TRE have allowed participants to self-select eating windows to increase adherence. However, we sought to determine the acceptability of a prescribed early eating window within the context of a behavioral weight loss intervention. Overall, we found that E-TRE+DCR was acceptable, with no differences in class attendance, attrition, or self-reported adherence between the E-TRE+DCR and DCR groups.
Our results show similar weight loss in DCR and E-TRE+DCR at 12 weeks (−5.1 kg/5.5% and −6.2 kg/6.3%, respectively) and 39 weeks (−4.3 kg/4.7% and −4.9 kg/5.2%). Previous studies of TRE without caloric restriction or behavioral support have shown variable but overall modest weight loss (1–4%) (1–6, 8, 9, 26). In contrast, our results show that adding behavioral support and recommendations on caloric restriction to a TRE intervention results in clinically significant (>5%) weight loss. In this trial, we provided calorie goals to both groups, but found early in the study that participants in E-TRE+DCR had difficulty focusing on both calories and eating windows. We therefore asked the E-TRE+DCR group to prioritize eating windows over reducing caloric intake. Indeed, participants in the E-TRE+DCR group reported greater adherence to eating within their windows as compared to eating at or below their calorie goals.
Data from photographic food records indicate that both groups reduced caloric intake and increased dietary restraint to a similar extent following the dietary weight loss intervention. In addition, we found an increase in the HEI in the E-TRE+DCR group, indicating improved dietary quality, whereas there was no change in the DCR group. Prior trials have shown inconsistent effects of TRE on dietary quality. Malaeb et al. (36) showed that as compared to control, TRE reduced the frequency of snacks and caffeinated beverages, while Martens et al. (37) showed no difference in dietary quality (assessed with HEI) with TRE as compared to normal eating patterns. Phillips et al. (26) found that TRE did not result in a significant change in dietary quality, while standard dietary advice resulted in a reduction in ultra-processed foods and an increase in unprocessed foods We also found a reduction in hunger in the E-TRE+DCR group at lunch, but no other differences in subjective appetite ratings between groups. Two previous controlled feeding studies have shown reductions in appetite with E-TRE (11, 12), suggesting that future studies should further evaluate effects of TRE on appetite.
Of note, although inclusion criteria for this study required that participants self-report an eating duration of >12 hours at baseline, the mean eating duration derived from photographic food records for the full study sample in this trial was 11.3 hours, which suggests either participants did not log all eating events, or participants over-reported baseline eating duration. Participants in the E-TRE+DCR group were successful in reducing their eating windows as prescribed, from 11.5 to 9.1 hours. However, the DCR group also reduced their mean eating window, from 11.1 to 10.4 hours, despite no instruction to do so. It is possible that some of the individuals in the DCR group attempted to follow a TRE eating pattern even though they were not randomized to that group. The observation that participants in DCR also reduced their eating windows may have resulted in attenuation of the potential effects of E-TRE. However, the E-TRE intervention did result in differences in meal timing between groups, with the E-TRE+DCR group eating over a duration 1.6 hours shorter and ending nearly one hour earlier in the day as compared to DCR.
We did not find significant between-group differences in HbA1c, total cholesterol, triglycerides, LDL cholesterol, or HDL cholesterol at 12 weeks. Two previous trials have shown reductions in triglycerides with both early and late TRE (38, 39), while the majority of other trials evaluating TRE have shown no differences in triglycerides, HDL or LDL cholesterol (2, 4–6, 8, 11). Consistent with our findings, most TRE trials have not demonstrated an effect on HbA1c (2, 3, 6), despite the fact that short-term TRE has been shown to result in improvements in glucose homeostasis (2, 10), insulin sensitivity (11, 13, 14) and β cell responsiveness (11). It is possible that the lack of change in HbA1c in these trials is due to the fact that changes in insulin sensitivity would not be reflected in changes in HbA1c in adults without type 2 diabetes.
Despite providing recommendations to increase PA as part of the behavioral intervention, we did not observe an increase in PA in either group when including data from all 3 cohorts. However, we have previously reported on the negative effects of COVID-19 stay-at-home orders on PA (35). Pooled baseline data from this trial showed that later meal timing was associated with lower PA (23) and in a secondary analysis using data from cohorts 1 and 2, E-TRE reduced sedentary bouts and showed a trend toward increased activity score (p=0.06). Increasing EI early in the day can be hypothesized to increase subjective energy levels, potentially translating to higher levels of PA early in the day. Indeed, in a large, randomized trial of breakfast eating vs skipping, breakfast eating resulted in greater PA thermogenesis in the morning (40). Therefore, the effects of E-TRE on PA and sedentary behavior warrants further evaluation.
Our study has several limitations. First, our third study cohort was interrupted by COVID-19 at week 6 of the intervention, resulting insignificant disruptions to the daily lives of our participants, as previously reported (35). The disruption to research also resulted in missing data (DXA, HbA1c, lipids and REE) and required us to change the primary outcome from DXA weights to clinic and/or home weights. However, to ensure that these weights were as reliable as possible, we asked participants in cohort 3 to take pictures of their home scale while standing on it to verify the reading and to use same scale each time. In addition, weight at week 39 was assessed in person with DXA in cohort 3, and weight loss was similar at week 12 and week 39, suggesting that the home weights were likely reliable. In addition, because participants in DCR also reduced their eating windows during the study, potential effects of E-TRE may have been attenuated. Moreover, generalizability of our results is limited by the study population, which was predominantly non-Hispanic white and female. Despite the fact that obesity is more prevalent in women and women are more likely to attempt weight loss (41), obesity disproportionately affects racial/ethnic minority groups (42, 43) and thus greater efforts will be made in future trials to recruit a more diverse population.
In conclusion, we report that E-TRE+DCR is an acceptable dietary strategy with no differences in class attendance, attrition, or adherence compared to DCR. Moreover, the addition of behavioral support and caloric restriction to an E-TRE dietary intervention results in clinically significant weight loss, a reduction in EI, and improvements in dietary quality.
Supplementary Material
Study Importance Questions:
- What is already known about this subject?
- Short-term studies of early time restricted eating (E-TRE) have shown improvements in glucose homeostasis, increased fat oxidation, and reductions in blood pressure and appetite.
- Results from previous trials of TRE using variable (often self-selected) eating windows without behavioral support or recommendations to reduce caloric intake have shown modest (1–4%) weight loss.
- What are the new findings in your manuscript?
- The present study shows that E-TRE combined with daily caloric restriction (DCR) and behavioral support results in similar, clinically significant weight loss as compared to DCR with behavioral support over a 39-week intervention.
- While prior free-living studies of TRE have allowed participants to self-select eating windows, the present study found that E-TRE combined with DCR was an acceptable intervention, with no between-group differences in retention or adherence to the intervention.
- How might your results change the direction of research or the focus of clinical practice?
- Previous trials of TRE without behavioral support have shown only modest weight loss, so the present findings may change recommendations to individuals pursuing weight loss to include behavioral support for TRE.
- Our study lends further support to the idea that the timing of the eating window may be critical to the success of TRE. However, a confirmatory study directly comparing early to late TRE is needed.
Acknowledgements:
We would like to thank our study participants and the Colorado Clinical and Translational Research Center staff who assisted with data collection.
Funding:
This research was supported NIH/National Center for Research Resources Colorado Clinical and Translational Sciences Institute Grant (UL1 RR025780), NIH/National Institute of Diabetes and Digestive and Kidney Diseases R21 DK117499 (E.A.T., M.A.C., V.A.C., C.A.R), KL2 TR002534 (E.A.T) K01 DK113063 (C.A.R) and Doris Duke Charitable Foundation Grant 2015212 (E.A.T).
Footnotes
ClinicalTrials.gov Identifier: NCT03571048
Disclosure: No conflict of interest to declare.
Data Sharing Plan:
Individual deidentified data used for this article (text, tables, and figures), study protocol, statistical analysis plan, and analytic code are available upon a reasonable request to the corresponding author.
References:
- 1.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell metabolism. 2015;22(5):789–98. Epub 2015/09/29. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity. 2020;28(5):860–9. Epub 2020/04/10. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell metabolism. 2020;31(1):92–104 e5. Epub 2019/12/10. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging. 2018;4(4):345–53. Epub 2018/06/29. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell metabolism. 2020. Epub 2020/07/17. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA internal medicine. 2020. Epub 2020/09/29. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients. 2021;13(3). Epub 2021/04/04. doi: 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. Epub 2016/08/24. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 9.Antoni R, Robertson T, Robertson M, Johnston J A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of Nutritional Science. 2018;7(E22). [Google Scholar]
- 10.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11(6). Epub 2019/06/04. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell metabolism. 2018;27(6):1212–21 e3. Epub 2018/05/15. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity. 2019;27(8):1244–54. Epub 2019/07/25. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19(1):148. Epub 2021/04/15. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison KC, Hopkins CM, Ruggieri M, Spaeth AM, Ahima RS, Zhang Z, et al. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr Biol. 2021;31(3):650–7 e3. Epub 2020/12/02. doi: 10.1016/j.cub.2020.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, et al. Prospective Study of Breakfast Eating and Incident Coronary Heart Disease in a Cohort of Male US Health Professionals. Circulation. 2013;128(4):337–43. Epub 2013/07/24. doi: 10.1161/CIRCULATIONAHA.113.001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. The American journal of clinical nutrition. 2012;95(5):1182–9. Epub 2012/03/30. doi: 10.3945/ajcn.111.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odegaard AO, Jacobs DR Jr., Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes care. 2013;36(10):3100–6. Epub 2013/06/19. doi: 10.2337/dc13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KJ, Gall SL, McNaughton SA, Blizzard L, Dwyer T, Venn AJ. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am J Clin Nutr. 2010;92(6):1316–25. Epub 2010/10/12. doi: 10.3945/ajcn.2010.30101. [DOI] [PubMed] [Google Scholar]
- 19.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. The American journal of clinical nutrition. 2017. Epub 2017/09/08. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun M, Feng W, Wang F, Li P, Li Z, Li M, et al. Meta-analysis on shift work and risks of specific obesity types. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017. Epub 2017/10/05. doi: 10.1111/obr.12621. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Q, Garaulet M, Scheer F. Meal timing and obesity: interactions with macronutrient intake and chronotype. International journal of obesity. 2019;43(9):1701–11. Epub 2019/02/02. doi: 10.1038/s41366-018-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27 Suppl 2:255–62. Epub 2013/07/03. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 23.Thomas EA, Zaman A, Cornier MA, Catenacci VA, Tussey EJ, Grau L, et al. Later Meal and Sleep Timing Predicts Higher Percent Body Fat. Nutrients. 2020;13(1). Epub 2021/01/02. doi: 10.3390/nu13010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–34. Epub 2007/11/14. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. The American journal of clinical nutrition. 2007;85(4):981–8. Epub 2007/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients. 2021;13(3). Epub 2021/04/04. doi: 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2014;63(25 Pt B):2985–3023. Epub 2013/11/19. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.National Diabetes Prevention Program Curricula and Handouts. https://wwwcdcgov/diabetes/prevention/resources/curriculumhtml. 2018.
- 29.Perlis RH, Haneuse S, Rubenfeld GD, Fihn SD, Rivara FP. Reporting Clinical Studies Affected by the COVID-19 Pandemic: Guidelines for Authors. JAMA Netw Open. 2021;4(1):e2036155. Epub 2021/01/15. doi: 10.1001/jamanetworkopen.2020.36155. [DOI] [PubMed] [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. Epub 2000/03/07. [DOI] [PubMed] [Google Scholar]
- 31.Thomas EA, McNair B, Bechtell JL, Ferland A, Cornier MA, Eckel RH. Greater hunger and less restraint predict weight loss success with phentermine treatment. Obesity. 2016;24(1):37–43. Epub 2015/11/21. doi: 10.1002/oby.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research. 1985;29(1):71–83. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 33.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949;109(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ltd. PT. ActivPAL Operating Guide 2010. Available from: www.palt.com.
- 35.Caldwell AE, Thomas EA, Rynders C, Holliman BD, Perreira C, Ostendorf DM, et al. Improving lifestyle obesity treatment during the COVID‐19 pandemic and beyond: New challenges for weight management. Obesity Science & Practice. 2021. Epub June 21 2021. doi: 10.1002/osp4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malaeb S, Harindhanavudhi T, Dietsche K, Esch N, Manoogian ENC, Panda S, et al. Time-Restricted Eating Alters Food Intake Patterns, as Prospectively Documented by a Smartphone Application. Nutrients. 2020;12(11). Epub 2020/11/11. doi: 10.3390/nu12113396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience. 2020. Epub 2020/01/25. doi: 10.1007/s11357-020-00156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity. 2019;27(5):724–32. Epub 2019/04/20. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 39.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. Epub 2016/10/16. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury EA, Richardson JD, Holman GD, Tsintzas K, Thompson D, Betts JA. The causal role of breakfast in energy balance and health: a randomized controlled trial in obese adults. The American journal of clinical nutrition. 2016;103(3):747–56. Epub 2016/02/13. doi: 10.3945/ajcn.115.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos I, Sniehotta FF, Marques MM, Carraca EV, Teixeira PJ. Prevalence of personal weight control attempts in adults: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(1):32–50. Epub 2016/09/23. doi: 10.1111/obr.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA : the journal of the American Medical Association. 2016;315(21):2284–91. Epub 2016/06/09. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS data brief. 2020(360):1–8. Epub 2020/06/04. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual deidentified data used for this article (text, tables, and figures), study protocol, statistical analysis plan, and analytic code are available upon a reasonable request to the corresponding author.


