Abstract
Recent research has identified endogenous cationic antimicrobial peptides as important factors in the innate immunity of many organisms, including fish. It is known that antimicrobial activity, as well as lysozyme activity, can be induced in coho salmon (Oncorhynchus kisutch) mucus after exposure of the fish to infectious agents. Since lysozyme alone does not have antimicrobial activity against Vibrio anguillarum and Aeromonas salmonicida, a four-step protein purification protocol was used to isolate and identify antibacterial fractions from bacterially challenged coho salmon mucus and blood. The purification consisted of extraction with hot acetic acid, extraction and concentration on a C18 cartridge, gel filtration, and reverse-phase chromatography on a C18 column. N-terminal amino acid sequence analyses revealed that both the blood and the mucus antimicrobial fractions demonstrated identity with the N terminus of trout H1 histone. Mass spectroscopic analysis indicated the presence of the entire histone, as well as fragments thereof, including a 26-amino-acid N-terminal segment. These fractions inhibited the growth of antibiotic-supersuscptible Salmonella enterica serovar Typhimurium, as well as A. salmonicida and V. anguillarum. Synthetic peptides identical to the N-terminally acetylated or C-terminally amidated 26-amino-acid fragment were inactive in antimicrobial assays, but they potentiated the antimicrobial activities of the flounder peptide pleurocidin, lysozyme, and crude lysozyme-containing extracts from coho salmon. The peptides bound specifically to anionic lipid monolayers. However, synergy with pleurocidin did not appear to occur at the cell membrane level. The synergistic activities of inducible histone peptides indicate that they play an important role in the first line of salmon defenses against infectious pathogens and that while some histone fragments may have direct antimicrobial effects, others improve existing defenses.
Mammalian mechanisms of nonspecific host resistance include physiological barriers at the portal of entry (skin or mucosa) and relatively unspecific innate immunity (e.g., phagocytosis by polymorphonuclear leukocytes and macrophages, the reticuloendothelial system, biochemical tissue constituents, and the inflammatory response, including fever). While macrophages and granulocytes remain central cellular components of innate immunity, fish lack bone marrow and lymph nodes. The thymus, kidney, and spleen are the most important lymphomyeloid tissues (5). It has long been known that while mammalian species are relatively sensitive to endotoxic lipopolysaccharide (LPS), some fish, including coho salmon and rainbow trout, fail to produce any clinical signs even when given 200 mg of Escherichia coli LPS kg of body weight−1 (2, 32). This, taken together with reports of salmonids developing resistance to bacterial infections prior to producing detectable levels of antibodies, which cannot be explained solely by macrophage activation or C-reactive protein-mediated opsonization (17, 18), draws attention to the existence of other important elements of fish defenses.
Endogenous cationic antimicrobial peptides have been isolated from a multitude of animal and plant species (reviewed by Hancock and Lehrer [11]), including fish (4, 7, 15, 19, 24, 29), and are recognized as components of the nonspecific defense system (3, 12). Each animal can contain several antimicrobial cationic peptides, which are either redundant or possibly complementary in their activities and which occur in single locations, such as the intestinal mucosa or the neutrophils (10). In some instances, antimicrobial proteins and peptides have been shown to be processed products of larger proteins, including lactoferrin (6) and cathepsin G (26). Another example is an antimicrobial peptide derived from H2A histones, which has been isolated from the stomach tissue of the Asian toad by Park et al. (23).
In this research we identified a peptide whose presence in salmon mucus and blood correlates with the increase in lysozyme activity following a disease challenge. We further constructed synthetic homologues of the peptide and investigated their ability to synergize with the flounder peptide pleurocidin, lysozyme, and crude mucus and serum extracts. An amidated version of pleurocidin had previously been shown to protect coho salmon from Vibrio anguillarum infections in vivo (16).
MATERIALS AND METHODS
Bacterial strains and growth media.
Field isolates of the salmonid pathogens Aeromonas salmonicida and V. anguillarum were identified by typing and kindly provided by Julian Thornton, Microtek International Inc., Victoria, British Columbia, Canada. The defensin-supersusceptible strain (MS7953s) of Salmonella enterica serovar Typhimurium, with a mutation in its PhoP-PhoQ two-component regulatory system, was described by Fields et al. (8). Serovar Typhimurium was maintained at 37°C in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.), while the fish bacteria were maintained at 16°C in tryptic soy broth (Difco) (5g of NaCl per liter). All strains were stored at −70°C until they were thawed for use and subcultured daily.
Synthesis of peptides.
The two peptides based in trout H1 histone were produced, using N-(9-fluorenyl)methoxycarbonyl chemistry, by the Nucleic Acid and Protein Service, University of British Columbia, Vancouver, Canada. Peptide sequences are shown in Table 1.
TABLE 1.
Identity of the active component
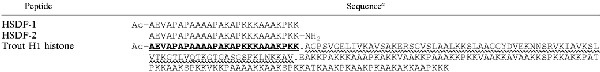 |
The 26-amino-acid sequence of the FPLC peak derived from the LPS-MUC sample is identical to the N-terminal sequence from trout H1 histone (underlined). The 8-amino-acid sequence obtained from the VIB-SER-derived peak was also identical to the N terminus of the histone. Mass spectroscopic data for both samples revealed a number of histone fragments. The most probable fragmentation pattern is shown by separating the fragments with periods. The section indicated by the wavy underlined likely resulted in fragmentation into many short (∼15-amino-acid) sections. The sequences of the synthetic peptides (HSDF-1 and HSDF-2) used in further experiments are also shown. Ac, acetylation of the N terminus, NH2, C-terminal amidation.
Lysozyme activity and initial antimicrobial activity assessment.
One of three groups of six coho salmon weighing 120 to 150 g was challenged with LPS isolated from Pseudomonas aeruginosa, S. enterica serovar Minnesota, and E. coli. Mixed LPS in saline at the concentration of 7.5 mg/kg of fish was injected intraperitoneally. Of the remaining two (control) groups, one was injected with saline alone, while the other was left untreated. At 2 days postinjection mucus and blood were obtained from each fish, and the blood was separated into cells and serum. Mucus and serum were diluted serially, and the dilutions were tested for inhibitory activity against defensin-susceptible S. enterica serovar Typhimurium. The level of lysozyme activity in the serum was evaluated by the modified lysoplate assay of Osserman and Lawlor (22), which utilizes Micrococcus lysodeikticus as the test agent.
Peptide purification.
Three groups of 20 coho salmon weighing 120 to 150 g were challenged. The challenges consisted of LPS isolated from P. aeruginosa, S. enterica serovar Minnesota, and E. coli; clinical isolates of V. anguillarum; and handling stress. Mixed LPS in saline at the concentration of 7.5 mg/kg of fish was injected intraperitoneally, while V. anguillarum was introduced by immersing the fish for 15 min in a bath of 109 bacteria per liter of Cortland saline. Handling stress consisted of holding the fish out of water for less than 5 min in a dip net. Following the challenge, fish were released into tanks and allowed to mount an immune response. When an adequate response was developed, as determined by measuring the levels of lysozyme in optimization studies, fish were sacrificed. The blood was obtained from each fish and separated into cells and serum. The head kidney, spleen, mucus, and gills were also harvested. All samples were pooled based on the sample type and challenge type. For example, all spleens from the 20 LPS-challenged fish were pooled as one sample, while all spleens from the 20 V. anguillarum-challenged fish were pooled as a separate sample. The resulting 18 cross-pooled samples were then extracted by boiling in 10% acetic acid. Solid-phase extraction with a Sep-Pak C18 cartridge (Waters) was performed using 60% acetonitrile in 0.1% trifluoroacetic acid (TFA) to elute the cartridge. The samples were lyophilized, resuspended in water, and tested for antimicrobial activity against a defensin-supersusceptible S. enterica serovar Typhimurium strain as described below. Samples exhibiting antimicrobial activities were applied to a Bio-Gel P-30 size exclusion chromatography column (Bio-Rad), eluted with 50 mM ammonium formate, lyophilized, resuspended in water, and retested for antimicrobial activity. Active samples were applied to a reverse-phase fast-performance liquid chromatography (RP-FPLC) column (PepRPC; Pharmacia Biotech) in 0.1% TFA and eluted with a 0 to 60% gradient of acetonitrile in 0.1% TFA, and fractions were collected, monitored at 220 nm, and tested for antimicrobial activity. The total protein content of the samples was assessed using the modified Lowry method (20). The purity of active samples was assessed using acid-urea polyacrylamide gel electrophoresis (AU-PAGE) (31). Following the purity assessment, active samples were subjected to amino acid analysis and Edman microsequencing at the Protein Microsequencing Laboratory at the University of Victoria, Victoria, Canada. In addition, matrix-assisted laser desorption ionization mass spectroscopy was performed by the Mass Spectroscopy Facility, Department of Chemistry, University of British Columbia, Vancouver, Canada.
Histone protein expression in fish extracts.
Seven coho salmon were challenged with V. anguillarum as described above, along with six control fish. Blood samples obtained at 72 h postincubation were separated into cells and serum, and the latter was tested for antimicrobial activity. Acid extracts of the serum from each fish were also subjected to AU-PAGE (31) along with the previously purified histone peptide.
Synergy of histone peptides with other natural fish antibiotics.
The checkerboard microtiter assay was used to determine peptide-peptide synergy (1). Serial dilutions of each peptide were made in tryptic soy broth medium in 96-well polypropylene microtiter plates (Costar, Cambridge, Mass.). Each well was inoculated with 10 μl of a solution of the test organism at approximately 5 × 106 CFU/ml. Samples of the bacterial inoculum were plated to ensure they were within the proper range. The fractional inhibitory concentration (FIC) was determined after 48 h of incubation of the plates at 16°C. Synergy was defined as an FIC index of less than 0.5, calculated according to the following formula: FIC index = [A]/MICA + [B]/MICB, where [A] was the concentration of drug A in a well that represented the lowest inhibitory concentration in its row, MICA was the MIC of drug A alone, [B] was the concentration of drug B in a well that represented the lowest inhibitory concentration in its row, and MICB was the MIC of drug B alone. Since many MICs in the assay were higher than the maximal level of compound tested, they were assumed to equal the highest concentration tested. When MICs alone were required, the method of Wu and Hancock (33) was used. It was essentially identical to the FIC protocol described above, except that serial dilutions of only one peptide per well were made.
Cytoplasmic membrane permeabilization assay.
The cytoplasmic membrane depolarization activities of the peptides were determined using the membrane potential-sensitive dye 3,3-dipropylthiacarbocyanine (diSC35) (30, 33) and V. anguillarum. Bacterial cells in the mid-logarithmic phase of growth (optical density at 600 nm of 0.5) were centrifuged, washed in 5 mM HEPES (pH 7.8) and resuspended in the same buffer to an optical density at 600 nm of 0.05. A stock solution of diSC35 was added to a final concentration of 0.4 μM, and quenching was allowed to occur at room temperature for 45 min. Then KCl was added to the cell suspension to a final concentration of 100 mM and allowed to equilibrate for 15 min. A 2-ml cell suspension was placed in a 1-cm cuvette, and the desired concentration of tested peptide was added. Changes in fluorescence due to the disruption of the membrane potential in the cytoplasmic membrane were continuously recorded using a Perkin-Elmer model 650-10S spectrofluorimeter at an excitation wavelength of 622 nm and an emission wavelength of 670 nm.
Langmuir monolayer assay.
Lipid monolayers were formed by applying the appropriate lipids dissolved in hexane or chloroform onto water contained in a circular Teflon trough (diameter, 4.5 cm; total volume, 11.5 ml). Monolayers were allowed to equilibrate until a stable surface pressure was obtained (<0.2 mN/m drift in Δπ). A small port in the side of the trough enabled injection of reagents into the subphase without disruption of the monolayer. The subphase was gently mixed with a magnetic stir bar at 45 rpm. Surface pressure measurements were obtained by using the Whilhelmy plate method (21). The plate was cleaned with methanol three times and thoroughly rinsed with double-distilled water prior to each surface pressure measurement. The experiments were run at 23°C.
RESULTS
Lysozyme activity and antimicrobial activity in crude samples.
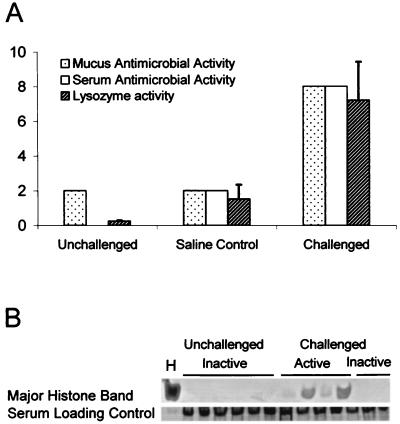
Antimicrobial activities of the serum and mucus of LPS-challenged coho salmon were at least fourfold higher than those of the controls at 48 h postinjection (Fig. 1A). A concurrent increase in lysozyme activity was observed in the serum of challenged fish. While the increase in lysozyme activity may have contributed to the increase in antimicrobial activity, lysozyme alone is not normally active against gram-negative bacteria. AU-PAGE analysis of acid extracts from the sera of unchallenged and challenged salmon revealed a band which was detectable in the microbicidal serum of challenged salmon but not in samples from unchallenged salmon or in challenged but nonmicrobicidal samples (Fig. 1B). The position of the band on the gel corresponded to that of an antimicrobial factor, HSDF-1 (see below), independently purified from the mucus and serum of coho salmon. The identities of two of the strongest bands were further confirmed through N-terminal Edman microsequencing.
FIG. 1.
Induction of lysozyme activity, antimicrobial activity, and H1 histone-derived peptide after a disease challenge of coho salmon. (A) Lysozyme activity in coho salmon serum, as well as antimicrobial activity of coho serum and mucus, are shown. The numbers on the y axis represent two separate units. For the lysozyme activity, the numbers represent activity units (106) in 1 ml of serum. For the mucus and serum antimicrobial activities the y-axis units are the inverse of the highest dilutions of the sample capable of inhibiting the growth of the supersusceptible S. enterica serovar Typhimurium C610. The average and standard deviation from three separate experiments are shown for each lysozyme result. (B) AU–15% PAGE and Coomassie blue staining were used to analyze acid extracts of serum samples from individual disease-challenged and unchallenged fish. Four of the challenged samples had antimicrobial activity against serovar Typhimurium C610 and have been designated active. A band which appeared only in active samples from challenged fish and which corresponded to an independently purified and identified histone fragment peptide, HSDF-1 (H), is shown.
Purification of active protein fractions.
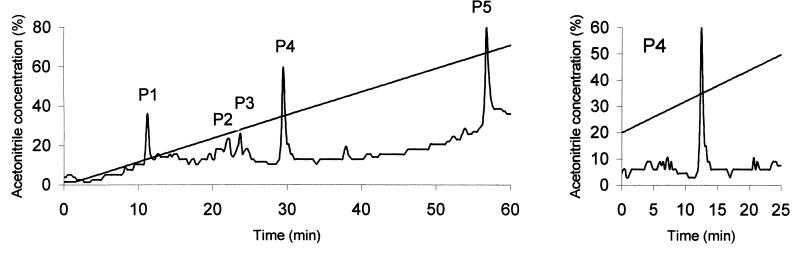
Following an initial screen of 18 separate acetic acid extracts, two extracts were fractionated on two separate columns and finally obtained as active single-peak samples eluted from an RP-FPLC column. In each case, every protein-containing peak was collected and tested (P1 to P5 in Fig. 2), as well as the samples immediately before and after each peak. When a single peak extended over two or more eluate samples, these eluates were pooled. After every use, the column was washed in 100% acetonitrile in 0.1% TFA to ensure that no peptides were left on the column.
FIG. 2.
Purification of the active component from the mucus of LPS-challenged coho salmon. Following the acid extraction step, C18 extraction and concentration step, and gel filtration, the active sample was applied to an RP-FPLC column in 0.1% TFA. The column was eluted with acetonitrile in 0.1% TFA. The protein content of the eluates, monitored at 220 nm, is shown. The acetonitrile concentration gradient is represented by the straight line. Each peak (P1 to P5) was collected and tested for antimicrobial activity. Peak P4 was the only peak with activity, and it was reapplied to the column to ensure its homogeneity. Peak P4 was active against S. enterica serovar Typhimurium, V. anguillarum, and A. salmonicida. An identical peak, which eluted at the same acetonitrile concentration, was isolated from the serum of V. anguillarum-challenged salmon.
Of all peaks present in the sample derived from the mucus of LPS-stimulated fish (LPS-MUC), only peak P4 showed antimicrobial activity after being lyophilized and resuspended in water (Fig. 2). Samples immediately before and after the peak had no activity. Peak P4 was subsequently reapplied to the C18 column to ensure its purity. An identical single peak was purified from the sample obtained from the serum of Vibrio-infected fish (VIB-SER).
The respective MICs of the LPS-MUC and VIB-SER samples, expressed in micrograms of total protein per milliliter, were 100 and 50 against S. enterica serovar Typhimurium C610, 50 and 25 against A. salmonicida, and 50 and 50 against V. anguillarum. The amino acid composition of the LPS-MUC sample revealed a high proportion of lysine, alanine, and proline. Screening of the VIB-SER sample against the SWISSPROT database using the web-based Expert Protein Analysis System AACompIdent tool resulted in several matches, all of which were histone proteins from various species, including trout. The amino acid sequencing data were similar for both samples and revealed sequences identical to the N terminus of trout H1 histone, as depicted in Table 1. The lengths of the sequences obtained for the LPS-MUC and VIB-SER samples were, respectively, 26 and 8 amino acids. Matrix-assisted laser desorption ionization mass spectroscopic analysis of each sample revealed a number of histone fragments, as indicated in Table 1, suggesting that the samples were not homogenous despite appearing as a single RP-FPLC peak. Species in the 20-kDa range were identified, indicating that the entire histone might be present. Also, species in the 10-kDa range, species corresponding to the N-terminally acetylated 26-amino-acid peptide identical to the N terminus of the trout H1 histone, and several smaller species were identified. We named the 26-amino-acid species HSDF-1 (for histone-derived fragment 1). Amino acid analysis of the FPLC-purified samples from mucus and serum showed HSDF-1 concentrations to be 12 and 207 μg/ml, respectively. Assuming 100% yield from the purification protocol, HSDF-1 concentrations in the original samples were estimated as 13 μg/ml in salmon mucus and 23 μg/ml in salmon blood. Given the unlikelihood of the assumed 100% yield, these concentrations almost certainly underestimate the actual values.
Synergy of histone peptides with other natural fish antibiotics.
Synthetically produced 26-amino-acid peptides equivalent to the N-terminally acetylated (native histones are acetylated) and C-terminally amidated variants of the N terminus of histone H1 (Table 1) had no detectable inhibitory activities against A. salmonicida and V. anguillarum, even when used at concentrations in excess of 1 mg/ml. However, at concentrations as low as 16 to 32 μg/ml, they potentiated the activities of the flounder peptide pleurocidin, hen lysozyme, and extracts from the mucus and serum of coho salmon against V. anguillarum. Both histone peptides potentiated the activity of pleurocidin against A. salmonicida, but neither potentitated the activity of hen lysozyme or extracts from the mucus and serum of coho salmon against this bacterium (results not shown). As the sources of serum and mucus were prechallenged with V. anguillarum, these samples contained lysozyme and other pathogen-specific defenses, which could apparently be potentiated by histone peptides.
The synergistic activities of histone peptides and natural fish antibiotics are shown in terms of actual reductions in inhibitory concentrations, as well as the FIC indices, in Table 2. While the latter is the common way of expressing synergy, the former provides a more intuitive visualization of the experimental data, given that the histone peptides had no detectable MICs by themselves.
TABLE 2.
FICs of natural fish antibioticsa
| Antibiotic | MIC (μg/ml) | FIC (μg/ml) with HSDF-1 concn (μg/ml) of:
|
FIC index with HSDF-1 | FIC (μg/ml) with HSDF-2 concn (μg/ml) of:
|
FIC index with HSDF-2 | ||
|---|---|---|---|---|---|---|---|
| 32 | 128 | 32 | 128 | ||||
| None | |||||||
| A. salmonicida | >1,024 | >1,024 | >1,024 | >1,024 | |||
| V. anguillarum | >1,024 | >1,024 | >1,024 | >1,024 | |||
| Pleurocidin | |||||||
| A. salmonicida | 2 | 0.125 | <0.12 | <0.08 | 0.125 | <0.12 | <0.09 |
| V. anguillarum | 16 | 8 | 4 | <0.31 | 4 | 1 | <0.19 |
| Hen egg white lysozyme (V. anguillarum) | >256 | 128 | 32 | <0.25 | 128 | 64 | <0.38 |
| Mucus (V. anguillarum)b | >256 | 256 | 32 | <0.25 | 256 | 64 | <0.38 |
| Serum (V. anguillarum)b | >256 | 128 | 32 | <0.19 | 128 | 32 | <0.25 |
Inhibitory concentrations of natural fish antibiotics, as well as hen egg white lysozyme, in the presence of HSDF-1 or HSDF-2 are shown. Two concentrations of each histone peptide are presented for two separate bacteria: V. anguillarum and A. salmonicida. FIC indices for natural fish antibiotics and histone peptides have been calculated according to the previously described formula and are shown. See text for details of calculations.
Values are lysozyme concentrations in mucus or serum. Estimates of lysozyme concentration in the extracts were performed using a standard M. lysodeikticus assay (22).
Membrane activities of histone-derived peptides.
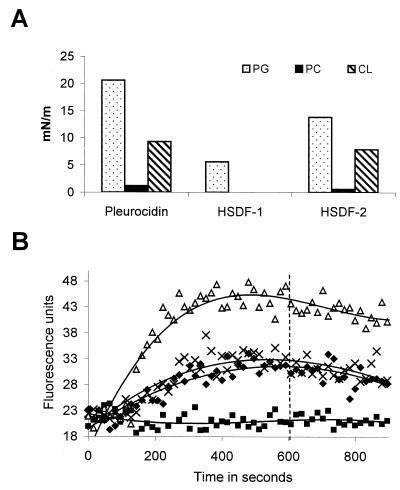
The ability of pleurocidin and histone peptides to insert into lipid monolayers and V. anguillarum membranes was investigated. All peptides appeared to insert into the anionic lipid egg phosphatidyl-dl-glycerol monolayer, and pleurocidin and HSDF-2 inserted into the anionic cardiolipin monolayer (Fig. 3A). None of the three peptides inserted into the neutral phosphatidylcholine monolayer. This indicates that the peptides are specific for anionic lipids. In order to test whether HSDF-1 had the ability to permeabilize V. anguillarum cell membranes, membrane potential-dependent quenching of a fluorescent dye was monitored. The HSDF-1 peptide did not permeabilize the cytoplasmic membrane or potentiate permeabilization caused by the flounder pleurocidin in this assay (Fig. 3B).
FIG. 3.
Membrane activities of histone-derived peptides. (A) Ability of pleurocidin and histone-derived peptides to insert into lipid monolayers. Peptides were added to the monolayers to a final concentration of 6.4 μg/ml. Single-lipid monolayers composed of egg phosphatidyl-dl-glycerol (PG), phosphatidylcholine (PC), and cardiolipin (CL) were tested. The maximal surface pressure increase caused by each peptide addition is shown. Results are averages from two experiments. (B) Ability of HSDF-1 at 50 μg/ml (squares), pleurocidin at 6.4 μg/ml (diamonds), pleurocidin at 12.8 μg/ml (triangles), and pleurocidin at 6.4 μg/ml with HSDF-1 at 50 μg/ml (crosses) to permeabilize V. anguillarum cell membranes. An increase in fluorescence is indicative of membrane permeabilization. An additional 50 μg of HSDF-1 per ml was added at 600 s.
DISCUSSION
We have identified the H1 histone and its fragments in the antimicrobial fractions at two separate sites in coho salmon challenged with distinct agents, indicating that histone proteins may be a relatively ubiquitous component of the host defenses. This finding is consistent with other histone research published to date. Human wound fluid contains histone H2B fragments (9), and histone-like cationic proteins have been isolated from the cytoplasm of murine macrophages (13). Recently, the presence of histone H1 in the cytoplasm and supernatants of villus epithelial cells has been reported (28), and antimicrobial activity in the skin of channel catfish has been attributed to histone-like cationic proteins (27). The antimicrobial activity of calf thymus histone has been known since 1958 (14), but the recent findings described above identify several extranuclear sites where histone proteins may play an important protective role in vivo.
The presence of defined histone fragments in antimicrobial extracts has been shown previously in toads and catfish (Ictalurus punctatus) (23, 25), consistent with our finding of the 26-amino-acid N-terminal fragment of H1 histone in coho salmon. Although mass spectroscopy identified multiple species of various sizes, the total amino acid content of the serum sample matched unambiguously that of other H1 histones, suggesting that all mass spectroscopy species may be of histone origin. In fact, specific histone processing, rather than increased histone production, could account for the increased presence of the 26-amino-acid histone fragment in active samples. It remains unclear whether the histone proteins were secreted or if they represent active debris from host cells damaged in the disease process, but precedents for the latter exist (13, 28).
The lack of antimicrobial activity of the artificially synthesized peptides HSDF-1 and HSDF-2 against V. anguillarum and A. salmonicida, as well as the increase in lysozyme activity in the original samples, led to the hypothesis that the antimicrobial activity associated with the presence of the 26-amino-acid peptide may have arisen from its ability to synergize with other histone fragments and, in early extracts, other natural fish antibiotics. The ability of the two synthetic peptides to potentiate the flounder peptide pleurocidin, as well as lysozyme and lysozyme-containing serum and mucus extracts, lent strong support to the synergy hypothesis. Synergy with pleurocidin is of particular significance, as this peptide has been shown to protect coho salmon from V. anguillarum infections in vivo (16). The diSC35 system did not indicate that synergy took place at the cell membrane level. Indeed, HSDF-1 was a poor membrane pearmeabilizer. Hence, if the target of HSDF-1 is intracellular, it may have to rely on other agents to facilitate its passage through the membranes. However, the preference of the cationic histone peptides for the monolayers of anionic lipids suggests that these peptides may also be capable of membrane interactions of their own, and membrane composition may be a factor in determining bacterial susceptibility to histone peptides.
Trout H1 histone-like protein and its fragments were isolated from the acid extracts of the mucus and serum of challenged coho salmon. Synthetic peptides identical to the N terminus of H1 histone potentiated natural fish antibiotics, including flounder pleurocidin, against fish pathogens. The findings of this research expand our understanding of the extent to which histone proteins are involved in salmon immunity. In the light of recent concerns about residual antibiotics in aquaculture, enhancing natural immune responses presents a unique opportunity for infection control among farmed fish.
ACKNOWLEDGMENTS
This work was funded by a grant from the Natural Sciences and Engineering Council to R.E.W.H. and by separate grants from the Canadian Bacterial Diseases Network to R.E.W.H. and G.K.I. R.E.W.H. was a recipient of the Medical Research Council of Canada Distinguished Scientist Award.
The technical assistance of Sandy Kielland at the Protein Microsequencing Laboratory at the University of Victoria, Victoria, British Columbia, Canada, is gratefully acknowledged.
REFERENCES
- 1.Amsterdam D. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. Susceptibility testing of antimicrobials in liquid media; pp. 55–112. [Google Scholar]
- 2.Berczi I, Bertok L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966;12:1070–1071. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 3.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immun. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 4.Cole A M, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 5.Dalmo R, Ingebrigsten K, Boegwald J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES) J Fish Dis. 1997;20:241–273. [Google Scholar]
- 6.Dionysius D A, Milne J M. Antibacterial peptides of bovine lactoferrin: purification and characterization. J Dairy Sci. 1997;80:667–74. doi: 10.3168/jds.S0022-0302(97)75985-X. [DOI] [PubMed] [Google Scholar]
- 7.Ebran N, Julien S, Orange N, Saglio P, Lemaitre C, Molle G. Pore-forming properties and antibacterial activity of proteins extracted from epidermal mucus of fish. Comp Biochem Physiol A. 1999;122:181–189. doi: 10.1016/s1095-6433(98)10165-4. [DOI] [PubMed] [Google Scholar]
- 8.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–62. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 9.Frohm M, Gunne H, Bergman A C, Agerberth B, Bergman T, Boman A, Liden S, Jornvall H, Boman H G. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem. 1996;237:86–92. doi: 10.1111/j.1432-1033.1996.0086n.x. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsson G H, Agerberth B. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J Immunol Methods. 1999;232:45–54. doi: 10.1016/s0022-1759(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E W, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 12.Hancock R E W, Diamond G. Cationic antimicrobial peptides: a major element in mammalian innate immune defenses against infection. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 13.Hiemstra P S, Eisenhauer P B, Harwig S S, van den Barselaar M T, van Furth R, Lehrer R I. Antimicrobial proteins of murine macrophages. Infect Immun. 1993;61:3038–3046. doi: 10.1128/iai.61.7.3038-3046.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsh J. Bactericidal action of histone. J Exp Med. 1958;108:925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwanaga S, Muta T, Shigenaga T, Miura Y, Seki N, Saito T, Kawabata S. Role of hemocyte-derived granular components in invertebrate defense. Ann NY Acad Sci. 1994;712:102–116. doi: 10.1111/j.1749-6632.1994.tb33566.x. [DOI] [PubMed] [Google Scholar]
- 16.Jia X, Patrzykat A, Devlin R H, Ackerman P A, Iwama G K, Hancock R E W. Antimicrobial peptides protect coho salmon from Vibrio anguillarum infections. Appl Environ Microbiol. 2000;66:1928–1932. doi: 10.1128/aem.66.5.1928-1932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodama H, Honda A, Mikami T, Izawa H. Detection of fish antibody against protein antigen of Aeromonas salmonicida by enzyme-linked immunoabsorbant assay using biotin-avidin system. Res Vet Sci. 1987;43:78–84. [PubMed] [Google Scholar]
- 18.Kodama H, Yamada F, Murai T, Nakanishi Y, Mikami T, Izawa H. Activation of trout macrophages and production of CRP after immunization with Vibrio anguillarum. Dev Comp Immunol. 1989;13:123–132. doi: 10.1016/0145-305x(89)90027-x. [DOI] [PubMed] [Google Scholar]
- 19.Lemaitre C, Orange N, Saglio P, Saint N, Gagnon J, Molle G. Characterization and ion channel activities of novel antibacterial proteins from the skin mucosa of carp (Cyprinus carpio) Eur J Biochem. 1996;240:143–149. doi: 10.1111/j.1432-1033.1996.0143h.x. [DOI] [PubMed] [Google Scholar]
- 20.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 21.Mayer L D, Nelsestuen G L, Brockman H L. Prothrombin association with phospholipid monolayers. Biochemistry. 1983;22:316–321. doi: 10.1021/bi00271a013. [DOI] [PubMed] [Google Scholar]
- 22.Osserman E F, Lawlor D P. Serum and urinary lysozyme (muramidase) in monocytic and monomecelocytic leukemia. J of Exp Medi. 1966;124:921–951. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C B, Kim M S, Kim S C. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochem Biophys Res Commun. 1996;218:408–413. doi: 10.1006/bbrc.1996.0071. [DOI] [PubMed] [Google Scholar]
- 24.Park C B, Lee J H, Park I Y, Kim M S, Kim S C. A novel antimicrobial peptide from the loach, Misgurnus anguillicaudatus. FEBS Lett. 1997;411:173–178. doi: 10.1016/s0014-5793(97)00684-4. [DOI] [PubMed] [Google Scholar]
- 25.Park I Y, Park C B, Kim M S, Kim S C. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998;437:258–262. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- 26.Pereira H A, Erdem I, Pohl J, Spitznagel J K. Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci USA. 1993;90:4733–4737. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinette D, Wada S, Arroll T, Levy M G, Miller W L, Noga E J. Antimicrobial activity in the skin of the channel catfish Ictalurus punctatus: characterization of broad-spectrum histone-like antimicrobial proteins. Cell Mol Life Sci. 1998;54:467–75. doi: 10.1007/s000180050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose F R, Bailey K, Keyte J W, Chan W C, Greenwood D, Mahida Y R. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun. 1998;66:3255–3263. doi: 10.1128/iai.66.7.3255-3263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shai Y, Fox J, Caratsch C, Shih Y L, Edwards C, Lazarovici P. Sequencing and synthesis of pardaxin, a polypeptide from the Red Sea Moses sole with ionophore activity. FEBS Lett. 1988;242:161–166. doi: 10.1016/0014-5793(88)81007-x. [DOI] [PubMed] [Google Scholar]
- 30.Sims P J, Waggoner A S, Wang C H, Hoffman J F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 31.Smith B J. Acetic acid-urea polyacrylamide gel electrophoresis of proteins. Methods Mol Biol. 1994;32:39–47. doi: 10.1385/0-89603-268-X:39. [DOI] [PubMed] [Google Scholar]
- 32.Wedemeyer G, Ross A. Some metabolic effects of bacterial endotoxins in salmonid fishes. J Fish Board Can. 1960;26:115–122. [Google Scholar]
- 33.Wu M, Hancock R E W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]