Abstract
Biological drugs, termed biologics, are medications that contain or are derived from a living organism (human, animal, or microorganism). With new biological agents being approved by the Food and Drug Administration (FDA) every year, clinicians need to know potential ocular adverse effects that are associated with these drugs. This review provides an overview of ocular adverse effects of biological medications used to treat both ophthalmic and non-ophthalmic diseases. We searched PubMed for relevant case reports, case series, reviews, and clinical trials reporting ocular adverse effects caused by biologics. This review was conducted in June 2021 and investigated the drugs listed in the most updated (April 2021) FDA Purple Book Database of Licensed Biological Products. This review focuses on monoclonal antibodies, interleukins, and receptor fusion proteins. We explore ocular side effects of 33 biological drugs, stating whether they are frequent, common, or rare.
Keywords: biological medications, biologics, ocular adverse effects, ocular side effects, ocular toxicity, ophthalmic side effects
Introduction
Biologics include drugs derived from cells, allergens, blood components, and tissues, as well as vaccines and recombinant proteins. This review focuses on interferons, interleukins, monoclonal antibodies, and receptor fusion proteins. Targeted agents include biologics and small molecule inhibitors. Biologics (primarily monoclonal antibodies and immune modulators) are large molecules with a long half-life that target specific cellular components to alter molecular pathways. Conversely, small-molecule inhibitors (mostly kinases) are molecules with short half-lives that target rapidly dividing cells. This review will not include traditional chemotherapeutic agents (e.g. cisplatin, methotrexate) and small molecular inhibitors (e.g. imatinib, vemurafenib); instead, we focus on therapeutic biologics used for ophthalmic or systemic diseases that are associated with ocular adverse effects.
Discoveries in immunology and genetics have generated novel targets for biological drugs. Biologics are involved in ubiquitous processes throughout the body and can have seemingly unrelated adverse effects. Previous reviews have discussed ocular adverse effects of biologics that were specific to oncology,1,2 neuro-ophthalmology, 3 or the retina. 4 To our knowledge, this is the first study to review ocular adverse effects of currently available biological medication listed in the FDA’s Purple Book 5 according to our criteria of inclusion.
The biologics with ocular adverse effects are organized by their targets of therapy. For each group of drugs, we also present a summary table. In these tables, frequent side effects were defined as occurring in more than 10% of patients, common side effects were defined as occurring in more than 1% of patients, rare side effects were those acknowledged by the drug manufacturer on their FDA label to rarely occur, and extremely rare side effects were defined as the ocular side effects that were only accounted for by anecdotal evidence found in case reports, case series, or review articles. In addition, when available, we added information to the tables about how to proceed when these side effects occur. Information from FDA labels for each drug was reviewed to identify frequent and common adverse effects. Although many of these drugs are used off-label to treat ophthalmological diseases, such as uveitis, we only present FDA-approved indications in the tables.
Methods
The review was performed via a three-step approach. First, we downloaded the most current FDA Purple Book at the time of review and excluded allergens, immunoglobulins, blood products, vaccines, antitoxins, hormone analogs, proteases, and gene therapy products. Of note, small molecule inhibitors, as a class, are a different group of targeted agents; thus, they were not included in this review. For each remaining biologic medication, we exclusively searched PubMed using the term: ( ‘generic name of biologic’) AND (side effect OR adverse effect OR adverse OR immune-related adverse effect OR IRAE OR complications) AND (ocular OR ophthalmic OR eye OR vision OR retina OR cornea OR conjunctiva OR sclera OR macula). Third, when the search revealed relevant results, we downloaded the FDA label for each of these drugs to review the frequency, manifestations, and acknowledgment of ocular side effects. The review was conducted in June 2021 and included all articles indexed in PubMed up to May 2021. We reviewed the most updated (April 2021) FDA Purple Book Database of Licensed Biological Products, and only included biologicals with reported cases of ocular side effects. Our search included only the names of generic drugs and did not differentiate between the different formulations of the same drug.
Ocular adverse effects of biologics by targets of therapy
We group biologics into seven different groups according to the targets of each drug: clusters of differentiation (CD), epidermal growth factor receptor (EGFR), immune checkpoints, interleukins, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), and others.
Cluster of differentiation (CD)
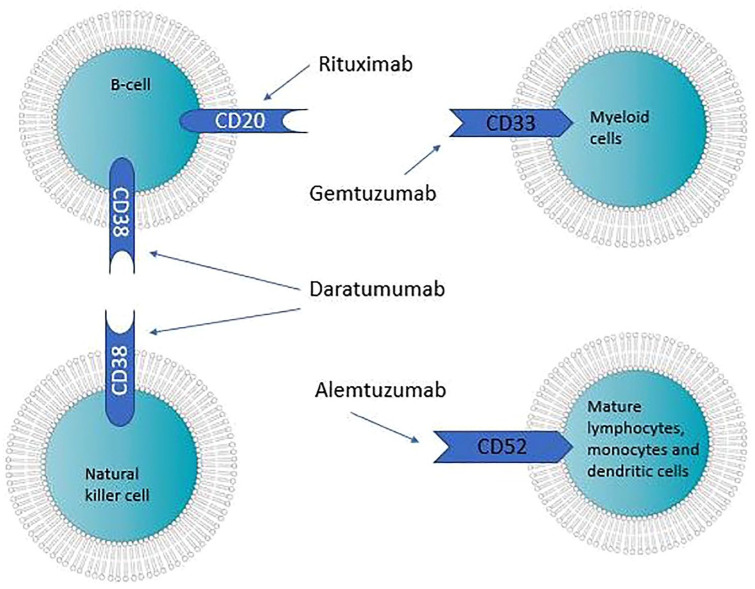
Cluster of differentiation (CD) is a nomenclature protocol used in the identification and investigation of cell surface molecules that usually function as receptors or ligands during cellular processes. This protocol is used to identify cell markers in immunophenotyping, as different cells in the immune system have different CD markers. Consequently, it is possible to target immune or tumor cells with CDs specific to them (Figure 1). For example, giving patients monoclonal antibodies against a specific CD activates immune cells to kill the cells that now have those antibodies attached to them. Alternatively, biologics can function as competitive antagonists against growth factor receptors (e.g. EGFR), and, thus, inhibit the action of growth factors (Table 1).
Figure 1.
Cluster of differentiation and cell types targeted by different monoclonal antibodies.
Table 1.
Biologics targeting different clusters of differentiation (CD)–their indications and ocular adverse effects.
| Cluster of differentiation (CD) | |||
|---|---|---|---|
| Targets (Biologic) | Indications | Frequency: side effects | References |
| CD20 (Rituximab / RITUXAN) | RA, CLL, NHL | Rare: JC virus reactivation (PML) | 3,7 |
| Extremely rare: acute retinal necrosis | |||
| CD33 (Gemtuzumab ozogamicin / MYLOTARG) | AML | Rare: Ocular bleeding | 8 |
| CD38 (Daratumumab/ DARZALEX) | multiple myeloma | Extremely rare: Acute angle closure glaucoma | 9 |
| CD52 (Alemtuzumab/ LEMTRADA) | multiple sclerosis | Common: Graves orbitopathy | 10–13 |
| Extremely rare: CMV reactivation, ocular Aspergillus spp infection, acute posterior multifocal placoid pigment epitheliopathy (APMPPE) | |||
| CD80/CD86 (Belatacept/ NULOJIX) | prophylaxis against kidney transplant rejection |
Extremely rare: CMV retinitis reactivation | 14 |
AML, acute myeloid leukemia; APMPPE, acute posterior multifocal placoid pigment epitheliopathy; CD, Cluster of differentiation; CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; JC, John Cunningham; NHL, Non-Hodgkin’s lymphomas; PML, progressive multifocal leukoencephalopathy; RA, rheumatoid arthritis.
CD20
CD-20 is found on most B-cells (Figure 1); it regulates B-cell activation and proliferation by modulating transmembrane Ca2 + conductance and cell cycle. 6 Thus, its inhibition can be used to treat malignancies that heavily express B-cells, such as Non-Hodgkin’s lymphomas (NHL), chronic lymphocytic leukemia (CLL), and autoimmune diseases, such as rheumatoid arthritis (RA), by decreasing the production of autoantibodies by B-cells. Rituximab (RITUXAN, Genentech, Inc., South San Francisco, CA) is a chimeric human-mouse IgG1 antibody against CD-20. Rituximab has been used off-label to treat many ophthalmic diseases such as Graves ophthalmopathy, myasthenia gravis, and Sjogren disease.
Because B cells play a role in activating T cells, it is theorized that by decreasing the number of B-cells, CD4+ memory T cells are lost, leading to an increase in reactivation of many viral infections. The reactivation of the John Cunningham (JC) virus can cause progressive multifocal leukoencephalopathy (PML), which often presents with ocular complaints and visual field defects. The risk of reactivation of JC virus is estimated to be 1:30.000. 3 Rituximab should be discontinued if PML is suspected. One case of acute retinal necrosis associated with the use of rituximab has also been reported. 7 No ocular adverse events have been reported to be related to ibritumomab, obinutuzumab, ocrelizumab, and ofatumumab, four other monoclonal antibodies targeting CD20.
CD33
CD33 is a transmembrane receptor found on myeloid cells. 6 Thus, Gemtuzumab ozogamicin (MYLOTARG, Wyeth Pharmaceuticals Inc., Philadelphia, PA), a monoclonal antibody that binds cells expressing the CD33 antigen, is used to treat CD33-positive acute myeloid leukemia (AML) in adults. Ocular hemorrhage, likely due to myelosuppression, has been reported as an adverse ocular effect in a patient with acute myeloid leukemia receiving treatment with gemtuzumab ozogamicin plus cytarabine. 8
CD38
CD38 is a glycoprotein found on the cellular membrane of many immune cells, particularly plasma cells and natural killer cells (Figure 1); it modulates cell activation, proliferation, and migration. 6 Daratumumab (DARZALEX, Janssen Biotech, Inc., Horsham, PA) is an anti-CD38 monoclonal antibody used for multiple myeloma. One case report described a patient being treated with daratumumab who developed choroidal effusions leading to acute angle-closure. 9 No ocular adverse effects have been reported to be associated with Isatuximab, another
monoclonal antibody that targets CD38.
CD52
CD 52 is found in multiple cells of the immune system and is known for being a powerful promoter of target cells lysis. 6 Alemtuzumab (LEMTRADA, Genzyme Corporation, Cambridge, MA) is a humanized IgG1 antibody against CD52. It is indicated in the treatment of patients with relapsing forms of multiple sclerosis (MS). Because alemtuzumab attacks all mature lymphocytes, patients are at risk of developing secondary autoimmune disorders from immune reconstitution. Graves orbitopathy is the most common (more than 2% of patients) ocular adverse effect involved with its use, thus thyroid function should be regularly monitored. 10 In addition, a case of acute posterior multifocal placoid pigment epitheliopathy (APMPPE) has also been reported. 11 Infection reactivation is a systemic adverse effect of alemtuzumab that can lead to ocular complications such as cytomegalovirus (CMV) retinitis and ocular infection with Aspergillus fumigatus.12,13
CD80 and CD86
CD80 and CD86 are transmembrane proteins that are closely related and often work in tandem; they interact with costimulatory receptors CD28 and CTLA-4 to modulate the immune response, particularly of T-cells (Figure 3). 6 Belatacept (NULOJIX, Bristol-Myers Squibb Company Princeton, NJ) binds to CD80 and CD86 on antigen-presenting cells, preventing CD28 mediated stimulation of T lymphocytes. It is used prophylactically against organ rejection in patients receiving kidney transplants. There is a case report of belatacept-associated CMV retinitis reactivation in a kidney transplant recipient. 14
Figure 3.
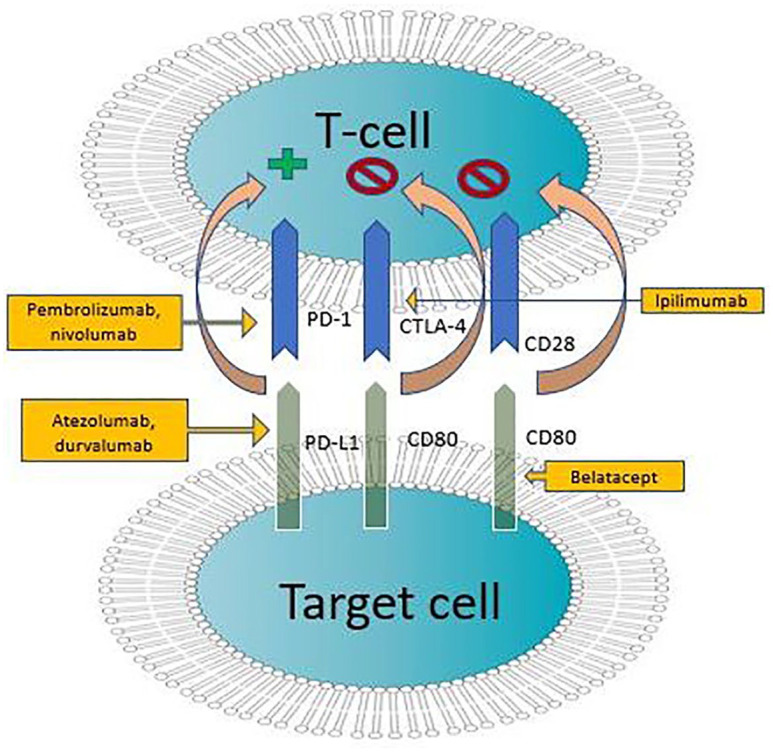
Immune checkpoint inhibitors influencing interactions between T-cells and target cells via different ligands.
Growth factor modulators
EGFR inhibitors
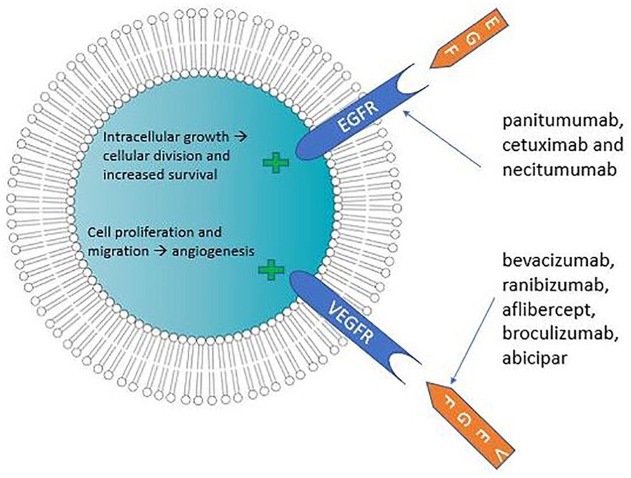
Epidermal growth factor receptors (EGFR) are transmembrane polypeptide proteins that activate signal transduction molecules including protein kinase B, the tyrosine kinase Src, c-Jun kinase, and signal transducers and activators of transcription (STATs). 15 EGFR is overexpressed in most solid tumors and is often associated with a poor prognosis, as this overexpression hyperactivates downstream signaling pathways, leading to very aggressive cancers. Abnormalities in the EGFR can cause (cancer) cells to divide more quickly (Figure 2). Consequently, EGFR inhibitors are often used as antineoplastics. The drugs in the class can be used to treat squamous cell carcinoma of the head and neck (SCCHN), colorectal carcinoma (CRC), and non-small cell lung cancer (NSCLC) (Table 2).
Figure 2.
Cellular responses to interactions between growth factors and receptors.
Table 2.
Growth factor modulators–their indications and adverse effects.
| Growth factor modulators | |||
|---|---|---|---|
| Targets (Biologic) | Indications | Frequency: ocular adverse effects | References |
| EGFR (cetuximab/ ERBITUX) | colorectal cancer, squamous cell carcinoma of the head and neck (SCCHN) | Common: conjunctivitis, ocular hyperemia, increased lacrimation, blepharitis, trichomegaly, dry eye, and eye/eyelid irritation | 15–17 |
| EGFR (necitumumab/ PORTRAZZA) | squamous non-small cell lung cancer (NSCLC) | ||
| EGFR (panitumumab/ VECTIBIX) | colorectal cancer | ||
| HER-2 (trastuzumab / HERCEPTIN) | HER-2 positive breast cancer | Common: dry eye, increased lacrimation, conjunctivitis, vision blurred, keratitis | 18–21 |
| Extremely Rare: retinal ischemia | |||
| Anti-VEGF intravitreal injections: aflibercept (EYLEA), bevacizumab (AVASTIN), broculizumab (BEOVU), ranibizumab (LUCENTIS) | neovascular age-related macular degeneration (AMD), diabetic retinopathy, retinal vein occlusions (RVOs), neovascular glaucoma, retinopathy of prematurity (ROP) | Frequent: eye pain, conjunctival hemorrhage, increased intraocular pressure, blurred vision | 22–38 |
| Common: Intraocular inflammation, vitreous floater, vitreous detachment, foreign body sensation, dry eye pruritus, ocular discomfort, conjunctival hyperemia, injection site hemorrhage, cataract, increased lacrimation, conjunctivitis | |||
| Rare: Endophthalmitis, retinal detachment, retinal hemorrhage, retinal tear, retinal artery occlusion, sixth nerve palsy, anterior ischemic optic neuropathy, maculopathy, blindness | |||
AMD, age-related macular degeneration; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor-2; NSCLC, non-small cell lung cancer; ROP, retinopathy of prematurity; RVO, retinal vein occlusion; SCCHN, squamous cell carcinoma of the head and neck.
Epidermal growth factor receptors (EGFR) are found throughout the anterior surface of the eye (eyelid, lacrimal glands, conjunctiva, cornea) and periocular tissue. Thus, ocular toxicities associated with the use of EGFR most commonly affect the eyelids (blepharitis, trichomegaly), the cornea, and the tear film. These complications occur due to meibomian gland dysfunction and damage to corneal epithelial cells, provoking dry-eye and foreign-body sensation and scarring.16,17 It is estimated that one-third of patients taking EGFR inhibitors will have ocular side effects. 16 Current FDA-approved EGFR inhibitors are panitumumab (VECTIBIX, Amgen Inc., Thousand Oaks, CA), cetuximab (ERBITUX, ImClone LLC, Branchburg, NJ), and necitumumab (PORTRAZZA, Eli Lilly and Company, Indianapolis, IN).
HER-2
HER-2 (human epidermal growth factor receptor-2) belongs to the same family as EGFR (erbB receptor family), and thus shares similar characteristics; HER-2 is a common target for solid tumors as it is an epidermal growth factor receptor involved in cell growth and division (Figure 2). 15 Mutations and abnormalities in its function are strongly associated with some types of breast cancer. Corneal epithelial cells express HER-2, and may, consequently, may suffer the adverse effects of therapies targeting HER-2. 18
Trastuzumab (HERCEPTIN, Genentech Inc., South San Francisco, CA) is a humanized monoclonal antibody that targets HER-2 expression used in breast cancer treatment. Only one case report describing retinal ischemia in a patient taking trastuzumab was found. 19 However, in a study investigating the side effects of a related compound, trastuzumab-emtansine (KADCYLA, Genentech Inc., South San Francisco, CA), ophthalmic adverse effects included dry eye, increased lacrimation, and conjunctivitis. 20 In a clinical trial evaluating the safety of trastuzumab duocarmazine, patients often presented with conjunctivitis, dry eyes, increased lacrimation, or keratitis. 21 No ocular adverse events have been reported in patients treated with pertuzumab, a HER dimerization inhibitor, or margetuximab-cmkb, another monoclonal antibody that targets HER-2.
Vascular endothelial growth factor (VEGF)
Vascular endothelial growth factor (VEGF) activates a signaling cascade that induces angiogenesis and the abnormal phenotype of blood vessels seen in tumors (Figure 2). As normal adult vasculature is believed not to be affected by VEGF, anti-VEGF treatment can be used to combat tumors that use VEGF as a survival factor without harming healthy vessels. 22 Anti-VEGF is the first-line treatment for neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), and diabetic macular edema (DME), and it has also been used to treat conditions such as neovascular glaucoma and retinopathy of prematurity (Table 2). When used in the eye through intravitreal injections, it works by inhibiting angiogenic processes that often lead to visual loss and retinal complications. 23
The most frequently utilized biologics in the class are bevacizumab (AVASTIN, Genentech Inc., South San Francisco, CA), ranibizumab (LUCENTIS, Genentech Inc., South San Francisco, CA), aflibercept (EYLEA, Regeneron Pharmaceuticals Inc., Tarrytown, NY), and broculizumab (BEOVU, Novartis Pharmaceuticals Corporation, East Hanover, NJ).
Intravitreal injections can lead to subconjunctival hemorrhage, infectious endophthalmitis, and rhegmatogenous retinal detachment. In addition, as a group, the ophthalmic side effect profile of anti-VEGF drugs includes increased risk of intraocular pressure elevation, tractional retinal detachment, IOI, and microvascular ocular events. 23 To reduce the incidence of complication when administering intravitreal injections, the American Academy of Ophthalmology recommends that practitioners abide by the following steps:
1) Apply a topical anesthetic; 2) apply 5 percent or 10 percent povidone-iodine drops and/or periocular povidone-iodine eyelid preparation; 3) insert a sterile speculum to separate the lids, and 4) reapply povidone-iodine immediately over the injection site prior to injection. 24
In addition, it is recommended that that talking, coughing, or sneezing be minimized during the injection procedure, as common respiratory flora from vitreous culture aspirates of patients with endophthalmitis after intravitreal injection.
Although a transient increase in intraocular pressure is expected after the injection of intravitreal anti-VEGF, a sustained increase in intraocular pressure was reported to be more likely in ranibizumab and aflibercept. 25
Intraocular inflammation is a common side effect of anti-VEGF agents and can present in mild or severe forms. Previous studies have not reported a significant difference in the incidence of IOI among bevacizumab and ranibizumab, 26 although the frequency of IOI has been reported to be higher with aflibercept injections. 27 Brolucizumab and abicipar are newer anti-VEGF agents. In clinical trials, despite achieving effective treatment with less frequent dosing, rates of IOI in these new drugs were higher than in older anti-VEGF.28,29
A multicenter study has shown that retinal vascular events may be associated with the use of intravitreal bevacizumab. Through a multi-center retrospective case series, the authors identified four cases of central retinal artery occlusion, and one case each of branch retinal artery occlusion, capillary occlusion, central retinal vein occlusion, and branch retinal vein occlusion shortly (0–55 days) after administration of intravitreal bevacizumab. 30 In addition, there are case reports of acute ocular ischemic change leading to acute vision loss, 31 isolated sixth nerve palsy, 32 and two case reports of anterior ischemic optic neuropathy after intravitreal injection of bevacizumab.23,33,34 Similarly, other case reports have described the development of retinal artery occlusion 35 and hemorrhagic macular infarction after using intravitreal bevacizumab. 36 A case report described a patient developing central retinal artery occlusion within one month of an intravitreal injection of ranibizumab. 35 Brolucizumab has also been associated with various retinal vasculitis by numerous case reports and a case series by the American Society of Retinal Specialists. 37 Presentations of IOI and retinal vasculitis as a complication of broculizumab use included arterial sheathing, plaque deposition, retinal whitening, perivascular, and others. 26
In patients with proliferative diabetic retinopathy, the incidence of tractional retinal detachment has been reported to be between 1.45% and 5.2% in patients receiving intravitreal bevacizumab injections. 26
The ocular adverse effects listed in the table for bevacizumab are due to its intravitreal use; the only ocular side effect of intravenous bevacizumab is lacrimation disorder. Despite not being approved by the FDA for intravitreal use, bevacizumab has been widely used off-label for intravitreal injections for the indications listed in Table 2. 38 No ocular adverse events related to ramucirumab, a direct VEGFR2 antagonist used to treat solid tumors, have been reported.
Immune checkpoint inhibitors (ICIs): CTLA-4, PD-1, PD-L1
Immune checkpoints modulate the immune response so that immune cells do not destroy healthy cells. However, sometimes immune checkpoints erroneously inhibit the destruction of tumor cells. Immune checkpoint inhibitors can block proteins involved in these immune checkpoints, thus helping immune cells perform their function better. CTLA-4, PD-1, and PD-L1 are the main target molecules in this process (Figure 3).
A review analyzing the FDA’s Adverse Events Reporting System (FAERS) database between 2003 and 2018 found uveitis, ocular myasthenia, eye inflammation, and dry eye to be associated with immune checkpoint inhibitors (ICIs). 39 Although no ocular side effects have been noted in the FDA labels of avelumab (BAVENCIO, Merck KGaA, Darmstadt, Germany) and abatacept (ORENCIA, Bristol-Myers Squibb Company, Princeton, NJ), and although our literature review did not yield results for ocular adverse effects of dostarlimab (JEMPERLI, GlaxoSmithKline LLC, Philadelphia, PA), intraocular inflammation and dry eye are common side effects of patients taking ICIs.39,40 For instance, the FDA labels of both dostarlimab and cemiplimab (LIBTAYO, Regeneron Pharmaceuticals, Tarrytown, NY) list uveitis, iritis, other ocular inflammatory toxicities as potential side effects. In Table 3, intraocular inflammation is an umbrella term that includes reported cases of anterior chamber cell, anterior chamber flare, anterior chamber inflammation, chorioretinitis, eye inflammation, iridocyclitis, iritis, uveitis, vitreous haze, and vitritis.
Table 3.
Biologics targeting different ICIs–their indications and ocular adverse effects.
| Immune checkpoint inhibitors (ICIs) | |||
|---|---|---|---|
| Targets (Biologic) | Indications | Frequency: ocular adverse effects | References |
| CTLA-1 (ipilimumab / YERVOY) | melanoma, renal cell carcinoma (RCC) | Rare: intraocular inflammation, ocular myositis, neuroretinitis, optic neuropathy, dry eye, and Vogt-Koyanagi-Harada-like syndrome | 39,40,42 |
| PD-1 (nivolumab/ OPDIVO) | melanoma, NSCLC, RCC, mesothelioma, SCCHN, urothelial carcinoma, hepatocellular carcinoma (HCC), and esophageal squamous cell carcinoma (ESCC) | Rare: intraocular inflammation, dry eye, Vogt-Koyanagi-Harada-like syndrome | |
| PD-1 (pembrolizumab / KEYTRUDA) | melanoma, SCCHN, NSCLC, small cell lung cancer (SCLC), urothelial carcinoma, among other malignancies | Rare: intraocular inflammation, retinal detachment, visual impairment, ocular myasthenia, dry eye, bilateral choroidal effusion | 39–47 |
| Extremely rare: retinal vasculitis, Behcet’s-like syndrome, ocular hypotony, and corneal epithelial toxicity | |||
| PD-L1 (atezolizumab /TECENTRIQ) | urothelial carcinoma, NSCLC, SCLC, HCC, triple negative breast cancer | Rare: intraocular inflammation | 39,40,42,48 |
| PD-L1 (durvalumab / IMFINZI) | NSCLC, SCLC | Rare: intraocular inflammation | 39,40,42,49 |
| Extremely rare: extraocular myopathy | |||
CTLA, Cytotoxic T-lymphocyte-associated protein; ESCC, esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; PD-1, Programmed death-1; PD-L1, Program death ligand-1; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck.
When the ocular side effects are notices, providers should administer corticosteroid eye drops to patients who develop intraocular inflammation. In addition, ICIs should be permanently discontinued in patients with immune-mediated ocular inflammation that is unresponsive to corticosteroid eye drops. Finally, consider Vogt-Koyanagi-Harada-like syndrome if intraocular inflammation occurs in tandem with other autoimmune adverse reactions, as this may require the use of systemic steroids to minimize the risk of vision loss. 2
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)
The use of CTLA-4 monoclonal antibodies can lead to adverse events, including ocular inflammation of the iris and conjunctiva, uveitis, dry eyes, and blurry vision, all of which resolve with topical therapy. 41
The use of Ipilimumab (YERVOY, Bristol-Myers Squibb Company, Princeton, NJ), a CTLA-4 inhibitor, is indicated to treat melanoma and renal cell carcinoma. It is associated with an increased risk for dry eye, and intraocular inflammation (IOI). 39 Episcleritis, neuroretinitis, optic neuropathy, and Vogt-Koyanagi-Harada-like syndrome have also been reported. 42
Programmed death-1 (PD-1)
Nivolumab (OPDIVO, Bristol-Myers Squibb Company, Princeton, NJ), a PD-1 inhibitor used to treat multiple types of cancer (Table 3), has the strongest association with ocular myasthenia compared to other ICIs and was also associated with IOI and dry eye. 39 There is one case report of nivolumab inducing Vogt-Koyanagi-Harada-like syndrome. 42
Pembrolizumab (KEYTRUDA, Merck Sharp & Dohme Corp, Whitehouse Station, NJ), another PD-1 inhibitor that can treat multiple malignancies (Table 3), is associated with ocular myasthenia, uveitis, dry eye, bilateral choroidal effusion, and focal exudative retinal detachment.39,43 There are case reports of retinal vasculitis, 42 Behcet’s-like syndrome, 44 ocular hypotony,45,46 and corneal epithelial toxicity. 47
Program death ligand-1 (PD-L1)
Atezolizumab (TECENTRIQ, Genentech Inc., South San Francisco, CA), a PD-L1 inhibitor used to treat multiple cancers (Table 3), has the strongest association with IOI compared to other immune checkpoint inhibitors. 39 Acute macular neuroretinopathy has also been associated with atezolizumab. 48 There is a case report of extra-ocular myopathy in a patient treated with a combination of durvalumab (IMFINZI, Janssen Biotech Inc., Horsham, PA) and tremelimumab. 49
Interleukins (antagonists and agonists)
Interleukins (IL) are cytokines that directly influence the behavior of immune cells, by binding cell surface receptors, and consequently modulating their activation, differentiation, proliferation, and migration. Therefore, they play a crucial role in the immune and inflammatory responses.
IL-2 receptor α chain antagonist
IL-2 receptor α chain (CD25) is expressed by T-cells, activated B-cell and monocytes, and plays an important role in immunomodulation by regulatory T-cells. 6 Denileukin diftitiox (ONTAK, Eisai Medical Research Inc., Ridgefield Park, NJ) is a CD25-directed cytotoxin designed for the treatment of persistent or recurrent CD25 positive cutaneous T-cell lymphoma. Denileukin diftitiox has been associated with loss of visual acuity and color vision, often associated with pigmentary retinopathy. The incidence of retinopathy is estimated to be less than 1 in 4,000, thus visual acuity and color vision should be monitored regularly. 4 The mechanism of this adverse effect is not completely understood.
IL-4 receptor α antagonists
Interleukin 4 is produced by CD4 + T cells, type 2 innate lymphoid cells (ILCs), basophils, mast cells, and eosinophils. It helps with B-cell proliferation and differentiation, Th2 cell differentiation, and IgE class switching. 50 Dupilumab (DUPIXEN, Regeneron Pharmaceuticals Inc., Tarrytown, NY) is a monoclonal antibody that binds the IL-4 receptor α subunit, inhibiting IL-4 and IL-13 signaling. It is used to treat atopic dermatitis and asthma, reducing the effects of Th2 cells and, consequently, the production of IgE by B-cells.
A systematic review and meta-analysis showed that more than half of patients treated for atopic dermatitis with dupilumab, an IL-4 receptor antagonist, developed conjunctivitis as a side effect; eye pruritus, dry eye, blepharitis, and keratitis were less commonly reported. 51
IL-6 receptor antagonists
IL-6 is a cytokine that plays an important role in hematopoiesis, acute-phase responses, and the development of systemic autoimmune diseases. It is produced by endothelial cells, fibroblasts, monocytes, and macrophages in response to distinct stimuli. 50 When in excess, this cytokine inhibits the action of regulatory T-cells, making autoimmune processes more likely. In patients with uveitis, IL-6 is consistently elevated in the vitreous and aqueous humor. 52 Thus, IL-6 antagonists can help treat non-infectious uveitis, thyroid eye disease, and giant cell arteritis (Table 4).
Table 4.
Interleukin analogs and antagonist–their indications and ocular adverse effects.
| Targets (Biologic) | Indications | Frequency: ocular adverse effects | References |
|---|---|---|---|
| Interleukin antagonists | |||
| IL-2α (Denileukin difitox/ ONTAK) 4 | CD25 + cutaneous T-cell lymphoma | Rare: Loss of visual acuity and color vision, Pigmentary retinopathy |
4 |
| IL-4 (Dupilumab/ DUPIXENT) 51 | atopic dermatitis, asthma | Frequent: conjunctivitis | 51 |
| Common: dry eye, blepharitis, eye pruritus | |||
| Rare: keratitis | |||
| IL-6 (Sarilumab/ KEVZARA) | rheumatoid arthritis (RA) | Extremely rare: uveitis, retinal infiltrates | 52 |
| IL-6 (Tocilizumab/ ACTEMRA) 53 | RA, giant cell arteritis (GCA), polyarticular juvenile idiopathic arthritis (PJIA), systemic juvenile idiopathic arthritis (SJIA), cytokine release syndrome (CRS) | Common: increased intraocular pressure | 53 |
| Interferon analog | |||
| Interferon-alfa 2b (INTRON A) | ocular surface squamous neoplasia, hairy cell leukemia, malignant melanoma, follicular lymphoma, condyloma acuminata, AIDS-related Kaposi’s sarcoma, chronic hepatitis C, chronic hepatitis B | Frequent: retinopathy (macular edema, retinal artery or vein thrombosis, retinal hemorrhages, and cotton wool spots) | 1,54 |
| Common: anterior ischemic optic neuropathy (AION) | |||
| Extremely rare: myasthenia gravis, sixth nerve palsy | |||
| TNF-α inhibitors | |||
| TNF-α (adalimumab/ HUMIRA) | RA, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis | Rare: optic neuritis | 58–68 |
| Extremely rare: uveitis, optic neuritis/neuropathy, endophthalmitis, corneal infiltrates, retinal toxicity, ophthalmoplegia | |||
| TNF-α (certolizumab pegol / CIMZIA) | Crohn’s disease, RA, psoriatic arthritis, ankylosing spondylitis | Rare: optic neuritis | 77 |
| TNF-α (golimumab/ SIMPONI) | RA, psoriatic arthritis, ankylosing spondylitis | Rare: optic neuritis | 68 |
| TNF-α (etanercept / ENBREL) | RA, PJIA, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis | Rare: uveitis, optic neuritis, scleritis | 69–75 |
| Extremely rare: ocular myositis | |||
| TNF-α (infliximab/ REMICADE) | Crohn’s disease, ulcerative colitis, RA, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis | Rare: optic neuritis | 68,78–88 |
| Extremely rare: Uveitis, endophthalmitis | |||
AION, anterior ischemic optic neuropathy; CD, clusters of differentiation; CRS, cytokine release syndrome; GCA, giant cell arteritis; IL, Interleukins; PJIA, polyarticular juvenile idiopathic arthritis; SJIA, systemic juvenile idiopathic arthritis.
In the STOP-Uveitis study, increased intraocular pressure was seen in three eyes out of 74 eyes treated with tocilizumab (ACTEMRA, Genentech Inc., South San Francisco, CA), with only one eye requiring intervention. 53 A clinical trial showed that retinal infiltrates and worsening of uveitis were the most common ocular adverse events in patients who were treated with sarilumab (KEVZARA, Sanofi-Aventis U.S. LLC, Bridgewater, NJ). 52 No adverse ocular events associated with satralizumab or siltuximab have been reported.
Interferon alfa 2b
Interferons are cytokines produced by the immune system that fight viral infections and multiple malignancies by creating adaptive immune responses. They boost immune response through a variety of mechanisms, including stimulation of apoptosis of tumor cells, macrophage antibody-dependent cytotoxicity, and proliferation of B1 cells (Table 4). 50 Interferon alfa-2b (INTRON A, Schering Corporation Inc., Whitehouse Station, NJ), an interferon analog, is commonly used to treat chronic viral infections, such as hepatitis C, and cancers such as follicular lymphoma and malignant melanoma. In ophthalmology, according to the American Joint Committee on Cancer classification, topical interferon alfa-2b can be used to effectively treat ocular surface squamous neoplasia. 54
More than one-third of patients treated with interferon developed interferon-associated retinopathy associated with retinal vascular changes. These microvascular changes can commonly cause anterior ischemic optic neuropathy (AION). There are also case reports associating interferon use with myasthenia gravis and sixth nerve palsy. 1 The drug manufacturer recommends the discontinuation of the medication and a complete eye examination if ocular side effects are suspected.
Tumor necrosis factor-α (TNF-α) antagonist
TNF-α is a pro-inflammatory cytokine secreted mostly by macrophages, monocytes, and other immune cells during the inflammatory response that targets nucleated cells; it activates cell necrosis or apoptosis and can function as an immunomodulator by limiting the intensity of inflammatory processes. 50 TNF-α inhibitors are widely used to treat autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, and ulcerative colitis (Table 4). It has been used to treat uveitis, but, paradoxically, it has also been linked to increases in uveitis due to cytokine imbalance or dysregulation. 55 Both soluble and transmembrane TNF-α can bind receptors in the iris, ciliary body, retinal pigment epithelium, and retina. 56
Adalimumab (HUMIRA, AbbVie Inc., North Chicago, IL) is a monoclonal antibody that binds TNF-α and can be used to treat multiple autoimmune diseases (Table 4), including uveitis. Reported ophthalmic side effects include uveitis, optic neuritis/neuropathy, endophthalmitis, corneal infiltrates, retinal toxicity, and ophthalmoplegia.57–67
Certolizumab pegol (CIMZIA, UCB Inc., Smyrna, GA) and Golimumab (SIMPONI, Janssen Biotech Inc., Horsham, PA) are newer TNF-α monoclonal antibodies used in ophthalmology to treat uveitis. Optic neuritis has been previously reported to be a side effect of both drugs. 68
Etanercept (ENBREL, Immunex Corporation, Thousand Oaks, CA) is a soluble TNF-α inhibitor used to treat uveitis and scleritis. Ophthalmic side effects previously described in case reports and case series include uveitis, optic neuritis, scleritis, and ocular myositis.57,69–77 Patients being treated with etanercept were found to have significantly higher numbers of drug-induced uveitis when compared to infliximab and adalimumab. 55
Infliximab (REMICADE, Janssen Biotech Inc., Horsham, PA) is a chimeric TNF-α monoclonal antibody used to treat multiple autoimmune diseases (Table 4) and shown to have therapeutic benefits for refractory non-inflammatory uveitis, uveitis secondary to Behcet’s disease, and retinal vasculitis. 56 Ophthalmic side effects related to infliximab include uveitis, optic neuritis, and endophthalmitis.57,58,70,78–88 The association with endophthalmitis is likely related to the immunosuppressive effect of infliximab; it is also theorized that scleral thinning due to scleritis may also increase the risk of endophthalmitis during anti-TNF-α therapy. 84
Others
Abciximab
Abciximab (REOPRO, Centocor B.V., Leiden, The Netherlands) is a monoclonal antibody that inhibits platelet aggregation by binding to the glycoprotein IIb/IIIa receptor of platelets (Table 5). One case of subconjunctival hemorrhage was reported after abciximab treatment. 89
Table 5.
Other biologics–their targets, indications, and ocular adverse effects.
| Others | |||
|---|---|---|---|
| Targets (Biologic) | Indications | Frequency: Side effects | References |
| glycoprotein IIb/IIIa (abciximab /REOPRO) | Adjunct to percutaneous coronary intervention | Extremely rare: subconjunctival hemorrhage | 89 |
| BCMA (belantamab mafodotin/ BLENREP) | multiple myeloma | Frequent: keratopathy, changes in visual acuity, blurred vision, and dry eyes | 90 |
| Common: photophobia, eye pruritus | |||
| human nerve growth factor (cenergemin/ OXERVATE) | neurotrophic keratitis | Frequent: eye pain | 91,92 |
| Common: corneal deposits, foreign body sensation, ocular hyperemia, intraocular inflammation, and tearing | |||
BCMA, B-cell maturation antigen.
Belantamab mafodotin
Belantamab mafodotin (BLENREP, GlaxoSmithKline Intellectual Property Development Ltd. England, Brentford, Middlesex, UK) is a drug that acts as an anti-B-cell maturation antigen (BCMA) agent and is used to treat relapsed and refractory multiple myeloma (RRMM) (Table 5).
Ocular toxicity is dose-dependent and attributed to the drug’s microtubule-disrupting monomethyl auristatin-F (MMAF), which produces an off-target injury to corneal epithelial cells. 90 More than half of the patients in the clinical trials of this drug had at least one of the following side effects: keratopathy, changes in visual acuity, blurry vision, and dry eyes. 90 Photophobia, eye irritation, infective keratitis, ulcerative keratitis were also noted in trials but were less frequent.
Cenergemin
Human nerve growth factor directly promotes tear production, corneal re-innervation, limbal epithelial stem cell potential, and the growth and survival of corneal epithelial cells. 91 Cenergemin (OXERVATE, Dompé farmaceutici S.p.A, L’Aquila, Italy) is a topical recombinant human nerve growth factor used to treat neurotrophic keratopathy (Table 5). The most common side effects associated with it are eye pain, ocular hyperemia, eye inflammation, and increased lacrimation. 92 The drug manufacturer recommends dosage modifications based on the severity of corneal adverse events.
Conclusion
The use of biologics has increased rapidly to treat a variety of systemic and ocular diseases. Not only are new drugs regularly designed and commercialized, but their use has also become much more frequent, as these drugs offer a more targeted treatment alternative. However, as with other medications, biologics have side effect profiles that physicians should be aware of. Ophthalmologists and eye care providers should be aware of both common and rare ocular adverse effects in patients treated with biologics. Primary care providers, internists, rheumatologists, oncologists, and other health providers should continue to coordinate with ophthalmologists to care for patients with ocular adverse events to prevent the occurrence of sight-threatening complications.
Footnotes
Author contributions: Helio V. Neves da Silva: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
John Placide: Investigation; Project administration; Validation; Writing – review & editing.
Anne Duong: Methodology; Project administration; Writing – original draft.
Yasmyne Ronquillo: Conceptualization; Project administration; Resources; Supervision; Writing – review & editing.
Shannon McCabe: Conceptualization; Project administration; Supervision; Writing – review & editing.
Majid Moshirfar: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Resources; Supervision; Visualization; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an unrestricted grant from Research to Prevent Blindness (RPB), 360 Lexington Avenue, 22nd Floor New York, NY 10017. No support was received for the publication of this article.
Ethical board, IRB and consent: This is a literature review that did not involve patients or protected health information, thus approval by the ethical board and informed consent from patients are not applicable.
Publication originality statement: We confirm that this publication is original.
ORCID iDs: Helio V. Neves da Silva  https://orcid.org/0000-0002-8572-9946
https://orcid.org/0000-0002-8572-9946
Yasmyne Ronquillo  https://orcid.org/0000-0002-8852-4380
https://orcid.org/0000-0002-8852-4380
Majid Moshirfar  https://orcid.org/0000-0003-1024-6250
https://orcid.org/0000-0003-1024-6250
Contributor Information
Helio V. Neves da Silva, University of Colorado School of Medicine, Aurora, CO, USA
John Placide, McGovern Medical School, The University of Texas Health Science Center, Houston, TX, USA.
Anne Duong, McGovern Medical School, The University of Texas Health Science Center, Houston, TX, USA.
Yasmyne Ronquillo, Hoopes Vision Research Center, Draper, UT, USA.
Shannon McCabe, Hoopes Vision Research Center, Draper, UT, USA; Mission Hills Eye Center, Pleasant Hill, CA, USA.
Majid Moshirfar, Hoopes Research Center, 11820 South State Street, Suite 200, Draper, UT 84020, USA; John A. Moran Eye Center, The University of Utah, Salt Lake City, UT, USA; Utah Lions Eye Bank, Murray, UT, USA.
References
- 1. Hager T, Seitz B. Ocular side effects of biological agents in oncology: what should the clinician be aware of? Onco Targets Ther 2013; 7: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fortes BH, Tailor PD, Dalvin LA. Ocular toxicity of targeted anticancer agents. Drugs 2021; 81: 771–823. [DOI] [PubMed] [Google Scholar]
- 3. Bhatti MT, Salama AKS. Neuro-ophthalmic side effects of molecularly targeted cancer drugs. Eye 2018; 32: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu CY, Francis JH, Brodie SE, et al. Retinal toxicities of cancer therapy drugs: biologics, small molecule inhibitors, and chemotherapies. Retina 2014; 34: 1261–1280. [DOI] [PubMed] [Google Scholar]
- 5. Purple book: lists of licensed biological products with reference product exclusivity and biosimilarity or interchangeability evaluations. FDA, https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-products-reference-product-exclusivity-and-biosimilarity-or (accessed 14 June 2021). [Google Scholar]
- 6. Cluster of differentiation (CD) antigens. In: Cruse JM, Lewis RE, Wang H. (eds) Immunology guidebook. Amsterdam: Elsevier, 2004, pp. 47–124. [Google Scholar]
- 7. Schuler S, Brunner M, Bernauer W. Rituximab and acute retinal necrosis in a patient with scleromalacia and rheumatoid arthritis. Ocul Immunol Inflamm 2016; 24: 96–98. [DOI] [PubMed] [Google Scholar]
- 8. Piccaluga PP, Martinelli G, Rondoni M, et al. First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leuk Res 2004; 28: 987–990. [DOI] [PubMed] [Google Scholar]
- 9. Edwards RG, Vanderhoof S, Palestine A, et al. Bilateral secondary angle closure during daratumumab infusion: a case report and review of the literature. J Glaucoma 2020; 29: e83–e86. [DOI] [PubMed] [Google Scholar]
- 10. Roos JCP, Moran C, Chatterjee VK, et al. Immune reconstitution after alemtuzumab therapy for multiple sclerosis triggering Graves’ orbitopathy: a case series. Eye 2019; 33: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao J, Jones J, Damato EM, et al. Acute posterior multifocal placoid pigment epitheliopathy after alemtuzumab treatment for relapsing–remitting multiple sclerosis. J Neurol 2019; 266: 1539–1540. [DOI] [PubMed] [Google Scholar]
- 12. Song WK, Min YH, Kim YR, et al. Cytomegalovirus retinitis after hematopoietic stem cell transplantation with alemtuzumab. Ophthalmology 2008; 115: 1766–1770. [DOI] [PubMed] [Google Scholar]
- 13. Anoop P, Stanford M, Saso R, et al. Ocular and cerebral aspergillosis in a non-neutropenic patient following alemtuzumab and methyl prednisolone treatment for chronic lymphocytic leukaemia. J Infect Chemother 2010; 16: 150–151. [DOI] [PubMed] [Google Scholar]
- 14. Deliège PG, Bastien J, Mokri L, et al. Belatacept associated – cytomegalovirus retinitis in a kidney transplant recipient: a case report and review of the literature. BMC Ophthalmol 2020; 20: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamath S, Buolamwini JK. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med Res Rev 2006; 26: 569–594. [DOI] [PubMed] [Google Scholar]
- 16. Basti S. Ocular toxicities of epidermal growth factor receptor inhibitors and their management. Cancer Nurs 2007; 30(4, Suppl. 1): S10–S16. [DOI] [PubMed] [Google Scholar]
- 17. Shin E, Lim DH, Han J, et al. Markedly increased ocular side effect causing severe vision deterioration after chemotherapy using new or investigational epidermal or fibroblast growth factor receptor inhibitors. BMC Ophthalmol 2020; 20: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuda M, Takano Y, Shigeyasu C, et al. Abnormal corneal lesions induced by trastuzumab emtansine: an antibody-drug conjugate for breast cancer. Cornea 2016; 35: 1378–1380, www.corneajrnl.com (accessed 16 October 2021). [DOI] [PubMed] [Google Scholar]
- 19. Saleh M, Bourcier T, Noel G, et al. Bilateral macular ischemia and severe visual loss following trastuzumab therapy. Acta Oncol 2011; 50: 477–478. [DOI] [PubMed] [Google Scholar]
- 20. Burris HA, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2) -positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398–405. [DOI] [PubMed] [Google Scholar]
- 21. Banerji U, van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019; 20: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 22. Kamba T, Mcdonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007; 96: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 2013; 27: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson ME, Scott AW. How to give intravitreal injections. EyeNet Magazine, April 2013, https://www.aao.org/eyenet/article/how-to-give-intravitreal-injections (accessed 19 October 2021).
- 25. Atchison EA, Wood KM, Mattox CG, et al. The real-world effect of intravitreous anti–vascular endothelial growth factor drugs on intraocular pressure: an analysis using the IRIS Registry. Ophthalmology 2018; 125: 676–682. [DOI] [PubMed] [Google Scholar]
- 26. Iyer PG, Albini TA. Drug-related adverse effects of antivascular endothelial growth factor agents. Curr Opin Ophthalmol 2021; 32: 191–197. [DOI] [PubMed] [Google Scholar]
- 27. Souied EH, Dugel PU, Ferreira A, et al. Severe ocular inflammation following ranibizumab or aflibercept injections for age-related macular degeneration: a retrospective claims database analysis. Ophthalmic Epidemiol 2016; 23: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khurana RN, Kunimoto D, Yoon YH, et al. Two-year results of the phase 3 randomized controlled study of abicipar in neovascular age-related macular degeneration. Ophthalmology 2021; 128: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 29. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020; 127: 72–84. [DOI] [PubMed] [Google Scholar]
- 30. Mansour AM, Bynoe LA, Welch JC, et al. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol 2010; 88: 730–735. [DOI] [PubMed] [Google Scholar]
- 31. Huang ZL, Lin KH, Lee YC, et al. Acute vision loss after intravitreal injection of bevacizumab (Avastin) associated with ocular ischemic syndrome. Ophthalmologica 2010; 224: 86–89. [DOI] [PubMed] [Google Scholar]
- 32. Çakmak HB, Toklu Y, Yorgun MA, et al. Isolated sixth nerve palsy after intravitreal bevacizumab injection. Strabismus 2010; 18: 18–20. [DOI] [PubMed] [Google Scholar]
- 33. Ganssauge M, Wilhelm H, Bartz-Schmidt KU, et al. Non-arteritic anterior ischemic optic neuropathy (NA-AION) after intravitreal injection of bevacizumab (Avastin®) for treatment of angoid streaks in pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1707–1710. [DOI] [PubMed] [Google Scholar]
- 34. Hosseini H, Razeghinejad MR. Anterior ischemic optic neuropathy after intravitreal injection of bevacizumab. J Neuroophthalmol 2009; 29: 160–161. [DOI] [PubMed] [Google Scholar]
- 35. von Hanno T, Kinge B, Fossen K. Retinal artery occlusion following intravitreal anti-VEGF therapy. Acta Ophthalmol 2010; 88: 263–266. [DOI] [PubMed] [Google Scholar]
- 36. Furino C, Boscia F, Cardascia N, et al. Hemorrhagic macular infarction after intravitreal bevacizumab for central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. Epub ahead of print 9 March 2010. DOI: 10.3928/15428877-20100215-57. [DOI] [PubMed] [Google Scholar]
- 37. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis 2020; 4: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bro T, Derebecka M, Jørstad ØK, et al. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol 2020; 258: 503–511. [DOI] [PubMed] [Google Scholar]
- 39. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol 2019; 31: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warner BM, Baer AN, Lipson EJ, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019; 24: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016; 60: 210–225. [DOI] [PubMed] [Google Scholar]
- 42. Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina 2018; 38: 1063–1078. [DOI] [PubMed] [Google Scholar]
- 43. Telfah M, Whittaker TJ, C Doolittle G. Vision loss with pembrolizumab treatment: a report of two cases. J Oncol Pharm Pract 2019; 25: 1540–1546. [DOI] [PubMed] [Google Scholar]
- 44. Thomas S, Bae C, Joy-Ann T, et al. Behcet’s-like syndrome following pembrolizumab: an immune-related adverse event associated with programmed death receptor-1 inhibitor therapy. J Oncol Pharm Pract 2020; 26: 995–999. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen M, Islam MR, Lim SW, et al. Pembrolizumab induced ocular hypotony with near complete vision loss, interstitial pulmonary fibrosis and arthritis. Front Oncol 2019; 9: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Basilious A, Lloyd JC. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol 2016; 51: e4–e6. [DOI] [PubMed] [Google Scholar]
- 47. Hsiao CC, Yao M, Liu JH, et al. Pembrolizumab induced acute corneal toxicity after allogeneic stem cell transplantation. Clin Exp Ophthalmol 2018; 46: 698–700. [DOI] [PubMed] [Google Scholar]
- 48. Ramtohul P, Freund KB. Clinical and morphological characteristics of anti–programmed death ligand 1–associated retinopathy: expanding the spectrum of acute macular neuroretinopathy. Ophthalmol Retina 2020; 4: 446–450. [DOI] [PubMed] [Google Scholar]
- 49. Carrera W, Baartman BJ, Kosmorsky G. A case report of drug-induced myopathy involving extraocular muscles after combination therapy with tremelimumab and durvalumab for non-small cell lung cancer. Neuroophthalmology 2017; 41: 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2016; 138: 984–1010. [DOI] [PubMed] [Google Scholar]
- 51. Halling AS, Loft N, Silverberg JI, et al. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol 2021; 84: 139–147. [DOI] [PubMed] [Google Scholar]
- 52. Heissigerová J, Callanan D, de Smet MD, et al. Efficacy and safety of sarilumab for the treatment of posterior segment noninfectious uveitis (SARIL-NIU):: the Phase 2 SATURN Study. Ophthalmology 2019; 126: 428–437. [DOI] [PubMed] [Google Scholar]
- 53. Sepah YJ, Sadiq MA, Chu DS, et al. Primary (month-6) outcomes of the STOP-uveitis study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol 2017; 183: 71–80. [DOI] [PubMed] [Google Scholar]
- 54. Shah SU, Kaliki S, Kim HJ, et al. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on american joint committee on cancer classification. Arch Ophthalmol 2012; 130: 159–164. [DOI] [PubMed] [Google Scholar]
- 55. Arepalli S, Rosenbaum JT. The use of biologics for uveitis associated with spondyloarthritis. Curr Opin Rheumatol 2019; 31: 349–354. [DOI] [PubMed] [Google Scholar]
- 56. Touhami S, Diwo E, Sève P, et al. Expert opinion on the use of biological therapy in non-infectious uveitis. Expert Opin Biol Ther 2019; 19: 477–490. [DOI] [PubMed] [Google Scholar]
- 57. Nicolela Susanna F, Pavesio C. A review of ocular adverse events of biological anti-TNF drugs. J Ophthalmic Inflamm Infect 2020; 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? Arthritis Rheum 2007; 56: 3248–3252. [DOI] [PubMed] [Google Scholar]
- 59. Seve P, Varron L, Broussolle C, et al. Sarcoid-related uveitis occurring during adalimumab therapy. Ocul Immunol Inflamm 2012; 20: 59–60. [DOI] [PubMed] [Google Scholar]
- 60. Kim A, Saffra N. A case report of adalimumab-associated optic neuritis. J Ophthalmic Inflamm Infect 2012; 2: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saffra N, Astafurov K. Visual loss induced by adalimumab used for plaque psoriasis. Case Rep Dermatol 2017; 9: 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chung JH, van Stavern GP, Frohman LP, et al. Adalimumab-associated optic neuritis. J Neurol Sci 2006; 244: 133–136. [DOI] [PubMed] [Google Scholar]
- 63. von Jagow B, Kohnen T. Anterior optic neuropathy associated with adalimumab. Ophthalmologica 2008; 222: 292–294. [DOI] [PubMed] [Google Scholar]
- 64. Matet A, Daruich A, Beydoun T, et al. Systemic adalimumab induces peripheral corneal infiltrates: a case report. BMC Ophthalmology 2015; 15: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Montero JA, Ruiz-Moreno JM, Rodríguez AE, et al. Endogenous endophthalmitis by Propionibacterium acnes associated with leflunomide and adalimumab therapy. Eur J Ophthalmol 2006; 16: 343–345. [DOI] [PubMed] [Google Scholar]
- 66. Drury J, Hickman SJ. Internuclear ophthalmoplegia associated with anti-TNFα medication. Strabismus 2015; 23: 30–32. [DOI] [PubMed] [Google Scholar]
- 67. Schechet SA, Garff K, Klima K, et al. Acute retinal necrosis after administration of adalimumab, a systemic antitumor necrosis factor antibody. Retin Cases Brief Rep 2018; 12: 307–309. [DOI] [PubMed] [Google Scholar]
- 68. Winthrop KL, Chen L, Fraunfelder FW, et al. Initiation of anti-TNF therapy and the risk of optic neuritis: from the safety assessment of biologic ThERapy (SABER) study. Am J Ophthalmol 2013; 155: 183–189.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tauber T, Turetz J, Barash J, et al. Optic neuritis associated with etanercept therapy for juvenile arthritis. J AAPOS 2006; 10: 26–29. [DOI] [PubMed] [Google Scholar]
- 70. Simsek I, Erdem H, Pay S, et al. Optic neuritis occurring with anti-tumour necrosis factor α therapy. Ann Rheum Dis 2007; 66: 1255–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Noguera-Pons R, Borrás-Blasco J, Romero-Crespo I, et al. Optic neuritis with concurrent etanercept and isoniazid therapy. Ann Pharmacother 2005; 39: 2131–2135. [DOI] [PubMed] [Google Scholar]
- 72. Le Garrec J, Marcelli C, Mouriaux F. Can tumor necrosis factor inhibitors induce sclero-uveitis? J Fr Ophtalmol 2009; 32: 511.e1–e6. [DOI] [PubMed] [Google Scholar]
- 73. Gaujoux-Viala C, Giampietro C, Gaujoux T, et al. Scleritis: a paradoxical effect of etanercept? Etanercept-associated inflammatory eye disease [J Rheumatol 2012; 39: 233–239]. J Rheumatol 2012; 39: 881. [DOI] [PubMed] [Google Scholar]
- 74. Caramaschi P, Biasi D, Carletto A, et al. Orbital myositis in a rheumatoid arthritis patient during etanercept treatment. Clin Exp Rheumatol 2003; 21: 136–137. [PubMed] [Google Scholar]
- 75. Couderc M, Mathieu S, Tournadre A, et al. Acute ocular myositis occurring under etanercept for rheumatoid arthritis. Joint Bone Spine 2014; 81: 445–446. [DOI] [PubMed] [Google Scholar]
- 76. Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum 2007; 56: 3248–3252. [DOI] [PubMed] [Google Scholar]
- 77. Hashkes PJ, Shajrawi I. Sarcoid-related uveitis occuring during etanercept therapy. Clin Exp Rheumatol 2003; 21: 645–646, https://europepmc.org/article/med/14611117 (accessed 31 May 2021). [PubMed] [Google Scholar]
- 78. Wendling D, Paccou J, Berthelot JM, et al. New onset of uveitis during anti-tumor necrosis factor treatment for rheumatic diseases. Semin Arthritis Rheum 2011; 41: 503–510. [DOI] [PubMed] [Google Scholar]
- 79. Thomas DA. Retrobulbar optic neuritis associated with infliximab. J Neuroophthalmol 2003; 23: 94–95, https://journals.lww.com/jneuro-ophthalmology/Fulltext/2003/03000/Retrobulbar_optic_neuritis_associated_with.28.aspx [Google Scholar]
- 80. Landais A, Fanhan R. Optic neuritis associated to treatment with infliximab. Presse Med 2017; 46: 337–341. [DOI] [PubMed] [Google Scholar]
- 81. Clemmensen K, Akrawi N, Stawowy M. Irreversible optic neuritis after infliximab treatment in a patient with ulcerative colitis. Scand J Gastroenterol 2015; 50: 1508–1511. [DOI] [PubMed] [Google Scholar]
- 82. ten Tusscher MPM, Jacobs PJC, Busch MJWM, et al. Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003; 326: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chan JW, Castellanos A. Infliximab and anterior optic neuropathy: case report and review of the literature. Graefes Arch Clin Exp Ophthalmol 2010; 248: 283–287. [DOI] [PubMed] [Google Scholar]
- 84. Yoshida M, Kiyota N, Maruyama K, et al. Endogenous Fusarium endophthalmitis during treatment for acute myeloid leukemia, successfully treated with 25-gauge vitrectomy and antifungal medications. Mycopathologia 2018; 183: 451–457. [DOI] [PubMed] [Google Scholar]
- 85. Jin XH, Namba K, Saito W, et al. Bacterial endophthalmitis caused by an intraocular cilium in a patient under treatment with infliximab. J Ophthalmic Inflamm Infect 2013; 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Agarwal PK, Gallaghar M, Murphy E, et al. Endogenous endophthalmitis in a rheumatoid patient on tumor necrosis factor alpha blocker. Indian J Ophthalmol 2007; 55: 230–232. [DOI] [PubMed] [Google Scholar]
- 87. Kochhar R, Gupta V, Dutta U, et al. Infliximab induced endophthalmitis in a patient of fistulizing Crohn’s disease. Indian J Gastroenterol 2011; 30: 241–242. [DOI] [PubMed] [Google Scholar]
- 88. Ouakaa-Kchaou A, Gargouri D, Trojet S, et al. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn’s disease. J Crohns Colitis 2009; 3: 131–133. [DOI] [PubMed] [Google Scholar]
- 89. Kul S, Sayın MR. Bilateral subconjunctival hemorrhage secondary to abciximab use: case report. Sao Paulo Med J 2019; 137: 209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wahab A, Rafae A, Mushtaq K, et al. Ocular toxicity of belantamab mafodotin, an oncological perspective of management in relapsed and refractory multiple myeloma. Front Oncol 2021; 11: 678634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sheha H, Tighe S, Hashem O, et al. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol 2019; 13: 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology 2020; 127: 14–26. [DOI] [PubMed] [Google Scholar]