Abstract
The mouse genetic revolution has shown repeatedly that most organs have more functions than expected. This has led to the realization that, in addition to a molecular and cellular approach, there is a need for a whole-organism study of physiology. The skeleton is an example of how a whole-organism approach to physiology can broaden the functions of a given organ, reveal connections of this organ with others such as the brain, pancreas and gut, and shed new light on the pathogenesis of degenerative diseases affecting multiple organs.
There are two definitions of physiology. In its most modern incarnation, physiology focuses on signalling and gene expression events occurring within cells as they relate to a given function and is often referred to as molecular and cellular physiology. An older physiology deals with events occurring and molecules acting outside the cell. It can be called whole-organism physiology and aims at identifying interactions taking place between organs through what Claude Bernard called the “milieu intérieur”1. Its study relies on three basic principles. First, the premise of whole-organism physiology is that no function is determined by one organ alone; this hypothesis has been verified multiple times through the use of model organisms2-4. Second, homeostasis, a principle positing that different organs exert opposite influences on the same function to regulate it tightly, applies to most physiological functions5. Closely related to homeostasis is the cardinal rule of endocrinology, that is feedback regulation: a regulated organ talks back to a regulating one to limit its influence6. Third is the principle in which whole-organism physiology resembles any other aspect of biology: regulatory molecules appear during evolution with the functions they regulate, not millions of years afterwards.
In vertebrates the modern study of whole-organism physiology uses two main tools. The experimental one, which favoured the renaissance of this physiology, is mouse genetics. This has allowed us to decipher, one gene at a time, how organs influence each other. For the focus of this Review, namely skeleton physiology, mouse genetics has proved to be an extremely reliable tool, possibly because bone is one of the last tissues to appear during evolution, and consequently most genes involved in skeletal biology have conserved functions from mice to humans. The second tool is the observation of internal medicine: mirror images of many physiological processes may be found in disease symptoms or drug side effects. Both tools are equally useful in extending the range of functions of the skeleton and our understanding of their molecular bases.
In this Review, we show how a whole-organism approach to physiology has modified our view of the skeleton. To that end, we describe work pointing towards a coordinated regulation of bone mass, energy metabolism and fertility; a central control of bone mass; and the roles of leptin, serotonin, insulin and osteocalcin in these pathways.
The skeleton and whole-organism physiology
A strategy that could be used to determine whether the skeleton is influenced by organs that are not classically associated with it and whether it influences other organs is to confront features of bone that are unique to clinical and experimental observations. This approach may indicate physiological functions that could affect or be influenced by bone. The reality of such interactions could then be tested genetically.
A striking feature of bone is that it is the only tissue that contains a cell type, the osteoclast, whose unique function is to resorb (destroy) the host tissue7. This does not occur at random but rather in the context of a true homeostatic function, which is called bone modelling during childhood and remodelling during adulthood. This function, hereafter referred to as bone (re)modelling, is characterized by alternating phases of destruction by osteoclasts and bone formation by osteoblasts8. Bone modelling allows longitudinal growth, without which most vertebrates could not ambulate and, therefore, could not live. It is, by definition, a survival function. Bone (re)modelling occurs daily in multiple locations in an organ covering a very large surface area. Both the cellular events it entails and the surface area of the organ in which they occur suggest there is a high energetic cost. Clinical observations add credibility to this view of bone (re) modelling as an energy-demanding process. Specifically, the absence of food — that is, energy — intake, as in patients with anorexia nervosa, causes a near-total arrest of growth in children and low bone mass in adults9,10. Moreover, in a manner unrelated to food intake, gonadal failure leads to low bone mass in both sexes, thereby suggesting a link between bone mass accrual and fertility11,12. Although compelling, such clinical evidence remained correlative. It became an incentive for laboratory investigation because of an experimental observation that was striking because it was unexpected. Osteocalcin was, at the time the encoding gene was inactivated in the mouse, the only osteoblast-specific secreted protein13. This by itself justified the study of its function in vivo with the hope of learning more about bone biology. Surprisingly, osteocalcin deletion resulted in mice that were abnormally obese and that bred poorly14,15. These phenotypes suggested that, in ways that still need to be explained, bone was affecting fat accumulation and possibly other aspects of energy metabolism and reproduction.
Taken together, this view of bone (re)modelling, clinical observations and the phenotypes of mice deficient in the osteocalcin gene suggested that there might have been a coordinated regulation of bone mass or growth, energy metabolism and reproduction. Because this hypothesis was triggered by the energy cost of bone (re)modelling, one would expect that the hormones orchestrating it would appear with the skeleton during evolution. Several laboratories have tested this hypothesis in the past 10 years.
A whole-organism physiology view of leptin
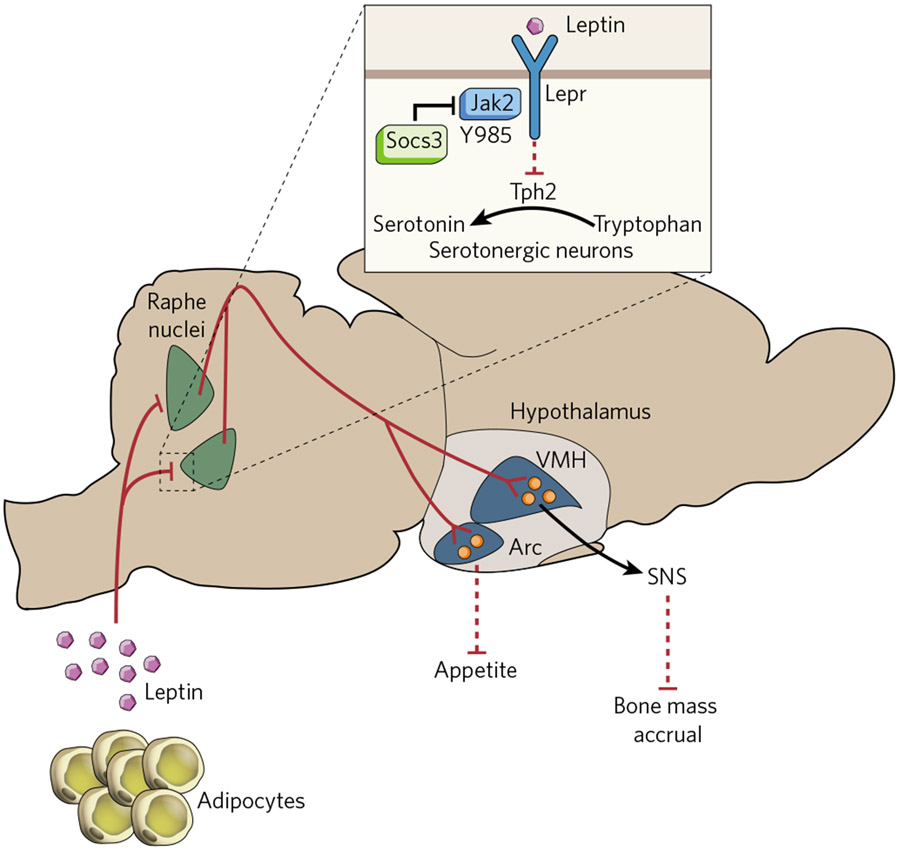
For a bone biologist in the late 1990s, it seemed easier to explore the aforementioned assumption by studying a hormone for which the biology has been characterized and the receptor identified. This hormone was leptin, an adipocyte-specific molecule identified for its ability to limit appetite and to favour energy expenditure and reproduction, two tenets of the overarching hypothesis16-18. Remarkably, and this is important in view of its function described here, leptin does not appear during evolution with any aspect of energy metabolism or reproduction, but is instead associated with bone (re)modelling. This would be mere coincidence if ob/ob (obese) or db/db (diabetic) mice, which lack leptin or its receptor, respectively, did not demonstrate a high bone mass because of a massive increase in bone formation19. This is a biological tour de force because these mice have no functional gonads, a situation that would otherwise increase bone resorption and decrease bone mass. Subsequently, leptin regulation of bone mass was verified in sheep and humans20-22. The high bone mass (despite hypogonadism) observed in the absence of leptin signalling and the fact that leptin is a vertebrate invention suggested that bone was a major target of this hormone; a model of partial gain-of-function of leptin signalling allowed this hypothesis to be tested. To mediate its functions, leptin binds to a receptor (Lepr) linked to the tyrosine kinase Jak2. Leptin binding activates Jak2, resulting in the phosphorylation of several residues on Lepr. One of these, Tyr 985, binds Socs3, an event that attenuates signalling through Lepr23 (Fig. 1). Accordingly, mutation of this residue in Lepr (Y985L) in l/l mice, which are homozygous for this substitution, results in a partial gain of function of leptin signalling24. Because the increase in signalling is only partial, phenotypes displayed by l/l mice reveal one or more functions of leptin that require its lowest threshold of signalling24. Hence, l/l mice breed normally and have normal appetite when fed on normal chow, but are osteoporotic; these observations suggest that the threshold of leptin signalling necessary to affect bone mass is lower than that needed to affect appetite and reproduction25.
Figure 1 ∣. Leptin co-regulates appetite and bone mass.
Leptin signals through its receptor (Lepr), expressed in serotonin-producing neurons of the raphe nuclei in the brainstem, decreasing the expression of tryptophan hydrxylase 2 (Tph2), the gene encoding the initial enzyme for serotonin biosynthesis. Signalling by Lepr requires Jak2 and is negatively regulated by Socs3. Serotonin-producing neurons of the raphe nuclei project to the ventromedial (VMH) and the arcuate (Arc) hypothalamic nuclei. Serotonin signalling in VMH neurons decreases the activity of the sympathetic nervous system (SNS), an inhibitor of bone mass accrual. In Arc neurons, serotonin signalling has anti-anorexigenic effects.
Several genetic pieces of evidence indicate that leptin acts centrally to inhibit the accrual of bone mass. The most convincing argument, although not the only one, is that a neuron-specific deletion of Lepr recapitulates the bone phenotype of ob/ob mice, whereas an osteoblast-specific one does not25. This is consistent with what has been shown for other functions of leptin that also occur through a central relay. In broader terms this revealed for the first time a central control of bone mass; its existence has now been verified by other laboratories studying how neuropeptide Y or neuromedin U regulate bone mass26,27. Over the years it has been proposed that, in contrast to most of its functions, leptin could also regulate bone mass through local means. This hypothesis is based on studies that injected large amounts (micrograms per day) of leptin into wild-type mice28 or infused leptin into the third ventricle of ob/ob mice29. That leptin did not decrease the percentage of fat in the latter study, in contrast to expectations, raises questions about the efficacy of these infusions.
Let us concentrate here on the central mode of action of leptin. We needed to know where it signals in the brain to fulfil this function and the regulation of energy metabolism (we viewed these two functions as co-regulated). Initially, it seemed that the hypothalamus was where all the action was taking place. The leptin receptor is highly expressed in ventromedial (VMH) and arcuate neurons of the hypothalamus, and chemical lesioning of these neurons had demonstrated their involvement in the regulation of appetite and bone mass; moreover, leptin infusions in the third ventricle of ob/ob mice decreased bone mass and appetite only if these hypothalamic neurons were intact30. This simple view was shattered by landmark studies showing that the selective inactivation of Lepr in VMH or arcuate neurons did not affect appetite or the accrual of bone mass in mice fed on a normal diet, the diet on which ob/ob mice display hyperphagia31-33. One interpretation of these seemingly contradictory results is that they are instead complementary in suggesting that leptin requires the integrity of hypothalamic neurons to regulate appetite and bone mass, but it need not bind to them. In other words, leptin might signal elsewhere in the brain to regulate the synthesis of neurotransmitters that will then act in the hypothalamus. Admittedly, this is a shift in the view of leptin signalling in the brain, but a novel approach was needed because cell-specific deletion of Lepr in hypothalamic neurons did not reproduce phenotypes seen in ob/ob mice fed on normal chow. The fact that patients chronically treated with serotonin reuptake inhibitors can develop low bone mass suggested that serotonin and leptin signalling intersect in the brain34-36.
In some instances cell-specific gene inactivation can be achieved without using sophisticated techniques. These are favourable circumstances because cell-specific gene deletion techniques are not without risks. This situation presented itself for leptin and for brain-derived serotonin, which is made only in neurons of the raphe nuclei and does not cross the blood–brain barrier37. It could therefore be eliminated from the brain by conventional inactivation of tryptophan hydroxylase 2 (Tph2), the rate-limiting enzyme of serotonin synthesis38. The fact that Tph2−/− mice were distinctly osteoporotic and anorectic established that brain-derived serotonin favours bone mass accrual and appetite and, because it does not cross the blood–brain barrier, identified serotonin as the first neurotransmitter regulating bone mass accrual31. Axonal connections between serotonin-producing neurons and hypothalamic neurons can exist and are probably functionally important, because ablations of serotonin receptors in specific neurons of the hypothalamus result in osteoporosis or anorexia in the same way as Tph2 deletion does31,39,40. This work, suggesting a direct brainstem–hypothalamus axis, does not contradict or exclude a more recent study proposing that serotonin signalling in the hypothalamus occurs through an interneuron41. This interneuron will need to be identified for the proper evaluation of the respective importance of these two modes of action.
Leptin inhibits Tph2 expression in, and serotonin release from, brainstem neurons; consequently, serotonin content in ob/ob hypothalami is abnormally high. Removing one allele of Tph2 from ob/ob mice sufficed to normalize their brain serotonin content, with remarkable consequences31. Indeed, ob/ob;Tph2+/− mice, despite having no leptin, had normal appetite, energy expenditure, body weight and bone mass. Subsequently, the molecular bases of this leptin–serotonin–hypothalamus axis were deciphered. They include calmodulin signalling in VMH neurons activating the transcription factor cycli-cAMP-response-element-binding protein (CREB), which induces the expression of genes involved in catecholamine synthesis39. This is important because the mediator acting as a bridge between leptin signalling in the brain and in bone cells is the sympathetic nervous system acting on osteoblasts through the β2 adrenergic receptor (Adrb2)30,42 (Fig. 1) and because β-blockers can limit the risk of osteoporotic fractures43.
The fact that leptin inhibits both bone mass accrual and appetite is consistent with the hypothesis that bone acquisition, an energy-expensive process, must be linked to energy (food) intake, otherwise the risk of organ failure anywhere else in the body, at the time of a growth spurt for instance, could be high. This broader view of leptin proposes a simple explanation of why this hormone appeared during evolution with bone and when food was scarce.
Osteocalcin and the endocrine nature of bone
Implicit in the hypothesis that there is coordinated regulation of bone mass, energy metabolism and fertility is the notion that bone is not only a recipient of hormonal inputs but also an endocrine organ affecting the other two functions. It was already known that, through fibroblast growth factor 23 (FGF 23), bone acts as an endocrine organ, but the function regulated by FGF23, namely mineral metabolism, is intimately linked to bone health44,45. The question asked at this point was different: does bone regulate physiological functions that, a priori, have nothing to do with bone health?
Although this hypothesis was initially formulated on inspection of mice deficient in the osteocalcin gene, it was the study of another gene and of another mouse model that shaped the concept into the form of a genetic pathway. Embryonic stem-cell phosphatase (ESP) is an obscure protein tyrosine phosphatase without any known substrate that is expressed in only three cell types: the embryonic stem cell, the Sertoli cell of the testes and the osteoblast15. This restricted pattern of expression justified the study of its function in vivo, and for that purpose Esp was deleted either in all cells or only in osteoblasts. The observation that both mouse models developed the same phenotypes implied that the biological functions they reveal take place in osteoblasts15.
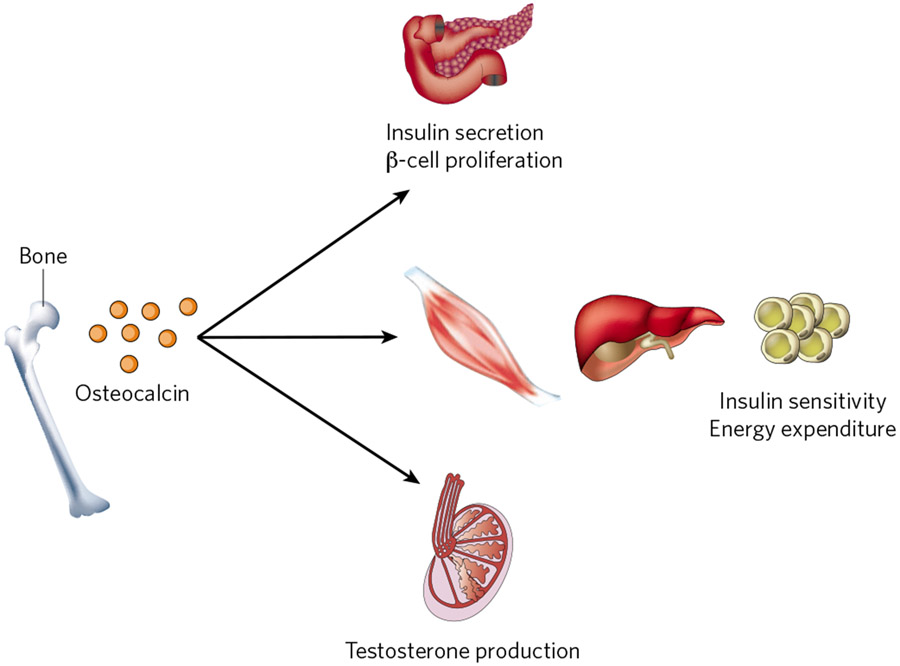
The absence of Esp in osteoblasts prompted perinatal death of mice, resulting from severe hypoglycaemia. This happened because the functions of this intracellular tyrosine phosphatase are, through its expression in osteoblasts, to inhibit the expression and secretion of insulin by pancreatic β-cells, to limit insulin sensitivity in liver, muscle and white adipose tissue, and to decrease energy expenditure15. All of the phenotypes of Esp-deficient mice mirrored those observed in mice deficient in the osteocalcin gene, and genetically Esp inhibits osteocalcin’s functions15. The fact that wild-type, but not osteocalcin-gene-deficient, osteoblasts induce insulin secretion from isolated islets established that osteocalcin is a hormone promoting β-cell proliferation and insulin expression and secretion. It also increases insulin sensitivity and energy expenditure15 (Fig. 2).
Figure 2 ∣. Osteocalcin, a bone-derived multifunctional hormone.
Undercarboxylated osteocalcin stimulates insulin secretion and β-cell proliferation in the pancreas, energy expenditure by muscle, and insulin sensitivity in adipose tissue, muscle and liver. In addition, it promotes male fertility by stimulating testosterone synthesis in Leydig cells of the testis through the activation of its receptor, GPRC6A, in these cells.
Before considering other aspects of osteocalcin biology one needs to address the relevance of these findings to human physiology. To the best of our knowledge no molecule identified as a hormone in the mouse has lost this function in humans. Although no mutation in osteocalcin or its receptor has yet been reported, this assumption seems to extend to osteocalcin, which has become an increasingly accepted biomarker of insulin resistance in human studies46,47.
Because most hormones have several functions, the next question was whether this was the case for osteocalcin. This was even more relevant because osteocalcin-gene-deficient mice breed poorly. A study of this phenotype revealed that osteocalcin promotes testosterone synthesis by Leydig cells of the testis and fertility in male mice14. For that purpose osteocalcin upregulates, in testes but not in ovaries, the synthesis of enzymes necessary for testosterone biosynthesis, without affecting the expression of the gene encoding Cyp19 (the enzyme converting testosterone to oestradiol). This male-specific function of osteocalcin implied that its putative receptor (OSTR) may be expressed only in testes. This sexual dichotomy allowed the identification of GPRC6A, a G-protein-coupled receptor, as an osteocalcin receptor, that is expressed in mice and humans in Leydig cells of the testis, but not in follicular cells of the ovary14 (Fig. 2). A pioneering study had previously proposed that GPR6CA could be an osteocalcin receptor, although no binding experiments were done48.
Osteocalcin puts bone in the thick of things
If the notion that bone affects energy metabolism and reproduction was implicit in the overall hypothesis, what emerged next — that bone is an endocrine hub on which several hormones converge to recruit osteocalcin as their ultimate relay — was not expected. To appreciate this notion, which was an outcome of the study of the relationship between ESP and osteocalcin, one needs to understand how osteocalcin is modified post-translationally.
Osteocalcin is carboxylated on three glutamic acid residues, a modification that confers on proteins a high affinity for minerals such as the hydroxyapatite crystal present in the mineralized bone matrix13. Nevertheless, a small but measurable proportion of undercarboxylated osteocalcin is found in the serum49, indicating that either some osteocalcin is secreted in an incompletely carboxylated form by osteoblasts or that osteocalcin becomes decarboxylated outside the cell. This was an important question to address because cell-based and in vivo investigations had shown that it is the undercarboxylated form of osteocalcin (the one in which Glu 13 is not carboxylated) that is active on β-cells and Leydig cells14,50.
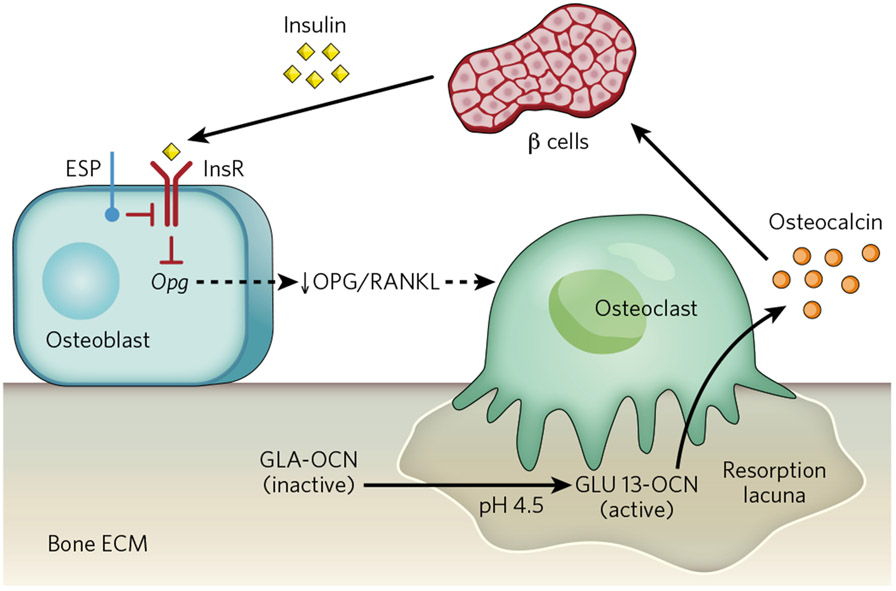
The search for substrates of ESP identified the insulin receptor as such a molecule51. The Esp-null mouse, therefore, is a gain-of-function model of insulin signalling in osteoblasts. Subsequently, two groups working independently showed that mice lacking the insulin receptor in osteoblasts (InsRosb−/− mice) were glucose intolerant and insulin insensitive when fed on normal chow; that is, they were a phenocopy of the osteocalcin-gene-deficient mice51,52. Because mice lacking the insulin receptor in skeletal muscle or white adipose tissue do not display glucose intolerance when fed on a normal diet53,54, insulin must act in additional tissues to achieve glucose homeostasis. The fact that bone is such a tissue legitimizes the notion that this tissue is necessary for glucose homeostasis. In addition, InsRosb−/− mice had significantly less biologically active (undercarboxylated) osteocalcin in their sera, revealing that insulin signalling in osteoblasts is a determinant of osteocalcin bioactivity53,54. In a manner that is both elegant and economical, insulin uses the interplay between osteoblasts and osteoclasts for that purpose. Specifically, insulin inhibits the expression in osteoblasts of the gene encoding osteoprotegerin (Opg)51, which hampers osteoclast differentiation. In other words, insulin signalling in osteoblasts favours bone resorption, a process that occurs at pH 4.5 (ref. 55). Acidic pH is the only mechanism known to achieve decarboxylation of proteins56, therefore, bone resorption decarboxylates and activates osteocalcin51. Thus, in a feedforward loop, insulin signalling in osteoblasts promotes its own secretion by activating osteocalcin (Fig. 3), and mice and humans in which bone resorption is genetically impaired show a decrease in the undercarboxylated form of osteocalcin, resulting in glucose intolerance51. The functional equivalent of ESP in human osteoblasts is protein tyrosine phosphatase 1B (PTP-1B), a tyrosine phosphatase previously known for its ability to inactivate the insulin receptor in other cell types51,57-59.
Figure 3 ∣. A feedforward loop links insulin, bone resorption and osteocalcin activity.
Insulin signalling in osteoblasts decreases the expression of Opg. The decrease in the ratio of osteoprotegerin (OPG) to receptor activator of nuclear factor–κB ligand (RANKL) increases bone resorption by osteoclasts. The acidic pH (4.5) in resorption lacunae decarboxylates (that is, activates) osteocalcin (GLA-OCN) stored in the bone extracellular matrix. Undercarboxylated active osteocalcin (GLU13-OCN) then stimulates insulin secretion by the β-cells of the pancreatic islets and promotes insulin sensitivity in peripheral organs. ECM, extracellular matrix; InsR, insulin receptor.
If insulin enhances osteocalcin activity, which in turn favours insulin secretion, how do vertebrates prevent hypoglycaemia? The answer is leptin60. Young ob/ob mice, before they become obese, have no molecular or metabolic evidence of insulin resistance, yet they are markedly hyperinsulinaemic and hypoglycaemic, indicating that a function of leptin is to inhibit insulin secretion3. As is true of its other functions, leptin by and large does not act locally on islets to affect insulin secretion, but through a neuronal relay60. Because the sympathetic nervous system acting through Adrb2 expressed in osteoblasts mediates the inhibition of bone mass accrual by leptin, it was tempting to ask whether the same pathway might inhibit insulin secretion by modulating osteocalcin expression or activity. Molecular evidence showed that the sympathetic tone mediates the upregulation of Esp expression by leptin and that ob/ob mice have more active osteocalcin in their circulation60. Three pieces of genetic evidence demonstrate that leptin regulation of osteocalcin activity occurs through sympathetic signalling in osteoblasts. First, mice lacking Adrb2 in osteoblasts are hypoglycaemic and hyperinsulinaemic. Second, mice lacking one copy of the leptin gene and one copy of Adrb2 in osteoblasts are also hyperinsulinaemic and hypoglycaemic. Third, ob/ob mice that lack the osteocalcin gene have normal expression of the insulin genes and are normo-insulinaemic for significantly longer than ob/ob mice60.
Altogether, the metabolic functions of osteocalcin and their regulation by leptin and insulin reveal how intertwined energy metabolism and bone (re)modelling are. They also underscore the importance of the interplay between osteoblasts and osteoclasts for glucose metabolism and indicate that the regulation of osteocalcin activity occurs at both the transcriptional and post-translational levels.
Energy metabolism, bone mass and bone diseases
Energy metabolism is a multistep process initiated by food absorption along the gastrointestinal tract. Thus, in considering whether there is coordinated regulation of bone mass and energy metabolism one needs to determine whether any part or function of the gastrointestinal tract influences the accrual of bone mass. This is important in view of the fact that the gastrointestinal tract is an endocrine organ secreting several hormones whose functions are not all known. Clinically there is reason to ask whether the gastrointestinal tract affects bone mass: osteoporosis is often seen in patients with inflammatory bowel diseases61,62.
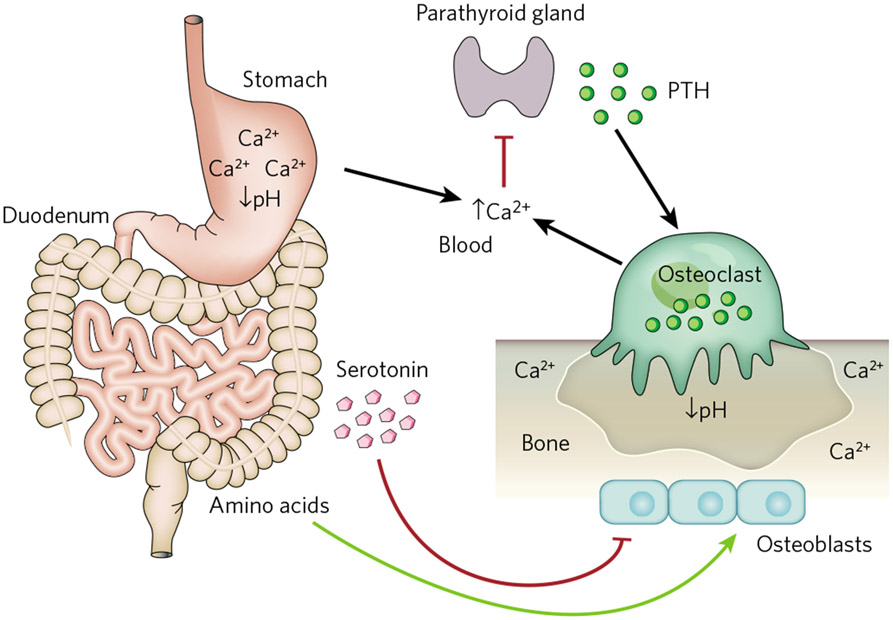
The study of mouse models of two human genetic diseases, the Coffin–Lowry syndrome (a learning disability syndrome with low bone mass) and the skeletal manifestations of neurofibromatosis 1 (NF1), established the existence of a connection between food absorption and the accrual of bone mass. In these disorders, bone mass abnormalities are due to a decrease or an increase, respectively, in the activity of activating transcription factor 4 (ATF4), a transcription factor that favours bone formation, in part by promoting amino acid import into osteoblasts63,64. One could, therefore, put this function of ATF4 to work to correct the skeletal manifestations of Coffin–Lowry syndrome or NF1 in the mouse by modulating protein content in their diets63 (Fig. 4). Another study looked at the influence of the gastrointestinal tract on mineral metabolism. Given that bone is a mineralized tissue and that the mineral crystal in the bone is made of calcium and phosphate, it is not surprising that significant variations in calcium or phosphate metabolism affect bone mass. One reason for that is that a high extracellular concentration of calcium inhibits secretion by parathyroid glands of parathyroid hormone (PTH), whose physiological role is to increase bone resorption65. A study relying on mouse genetics and human pathology showed that the high acidity (low pH) present in the stomach, the entry point of the gastrointestinal tract, is required for proper calcium absorption and, therefore, for normal PTH secretion and bone resorption66 (Fig. 4). This established a simple connection between the gastrointestinal tract and bone mass that could be exploited for therapeutic purposes. These examples of an influence of the gastrointestinal tract on bone mass raised the possibility that hormones made by the gastrointestinal tract regulate bone mass. A peculiar combination of scientific frustration and luck showed that this was not only the case, but also that this regulation is potentially important for the treatment of the most frequent bone degenerative disease, osteoporosis.
Figure 4 ∣. Interactions between the gastrointestinal tract and bone mass.
Stomach acidity (low pH) is required for the proper absorption of calcium (Ca2+) and is, therefore, essential to maintain normal levels of serum calcium. Serum calcium, in turn, negatively regulates secretion from the parathyroid gland of PTH, a hormone that stimulates osteoclast differentiation and bone resorption. Bone resorption by osteoclasts also occurs at low pH and contributes to the maintenance of serum calcium. Peripheral serotonin is produced by the duodenum and inhibits bone formation by osteoblasts, whereas dietary intake of amino acids (proteins) favours collagen synthesis by osteoblasts.
The cell-surface molecule Lrp5 (low-density-lipoprotein receptor related protein 5), despite having no identifiable ligand and no signalling pathway, is remarkably important in bone biology for medical reasons. First, LRP5 is mutated in two human diseases. In osteoporosis pseudoglioma (OPPG), a disease characterized by the appearance of osteoporosis several years after birth, it is inactivated67; in high bone mass syndrome (HBM) there is a missense mutation in LRP5 that is thought to be a gain-of-function mutation68,69. Patients harbouring the latter mutation have no detectable symptoms before adulthood, again arguing against a developmental defect. In fact, the main symptom is that post-menopausal female patients with HBM do not develop osteoporosis68,69. Another reason to draw much attention to Lrp5 is that it affects bone mass only by acting on bone formation70. In other words, the Lrp5-dependent signalling pathway holds the key to an anabolic treatment for osteoporosis, the ultimate objective for the most frequent bone degenerative disease.
Lrps are known to be able to bind multiple ligands; nevertheless, because of its homology to Arrow71 (a co-receptor in Drosophila for Wingless, a homologue of Wnt) it is as a potential co-receptor for Wnt proteins that Lrp5 has been studied for the longest time. This was a reasonable assumption to test, and cell culture experiments showed repeatedly that Lrp5, like other Lrps, can function as a Wnt co-receptor in vitro and in controlling eye vascularization71-75. However, when it came to its role in bone, there were discrepancies between expectations and results obtained through the study of animal models. A few examples follow. Lrp5-null osteoblasts proliferate poorly in vivo, but normally in cell culture76. Because Lrp5 is a receptor, this observation is inconsistent with the notion that its signalling is affected in osteoblasts. Furthermore, microarray analyses showed that genes regulated by Lrp5 (cell-cycle regulators) and by the canonical Wnt signalling (Opg) in bone specimens were different77,78. Accordingly, in mice, inactivation of the canonical Wnt signalling pathway in osteoblasts or osteocytes does not affect bone formation but bone resorption instead78,79. In humans, patients with HBM who have been followed up for more than 20 years have not yet developed bone tumours (M. Kassem, personal communication), even though many Wnt family members are aggressive oncoproteins80.
In an unforeseen turn of events, attempts to understand these discrepancies have brought serotonin back into the picture. While studying the role of brain-derived serotonin through the inactivation of Tph2, the function of Tph1, the enzyme responsible for peripheral serotonin synthesis, was investigated as a negative control. Tph1−/− mice had already been generated when it was realized that the most overexpressed gene in the absence of Lrp5 was in fact Tph1 (ref. 76). From being an internal control in another project Tph1 became, overnight, the topic of an independent one. Two groups have generated mouse models of Lrp5 deletion in a cell-specific manner and have obtained different results76,81. The reason for this discrepancy is unknown. Because we ourselves generated one of these models, we believe it would be inappropriate to use the tribune provided by this review to make our case. It is also unnecessary, because, as important as mouse genetics is, it remains a surrogate for human genetics, and so far all patients with OPPG or HBM for whom measurements of circulating serotonin have been published have shown high and low circulating levels of serotonin, respectively76,82-84. Similarly, serotonin-producing tumours cause osteoporosis85, and patients with osteoporosis have high circulating serotonin levels86 (Fig. 4). As a result, the regulation of bone mass by serotonin has now acquired a (medical) life of its own and deserves to be studied because it holds great promise for the anabolic treatment of osteoporosis. This is even more true given that two proof-of-principle studies have shown that an inhibitor of serotonin synthesis in the gut, shown to be safe in humans in a phase I clinical trial87,88, cured bone disease in Lrp5-null mice and gonadectomy-induced osteoporosis in rodents. In both cases this was achieved through a purely anabolic mode of action, just as Lrp5 affects bone mass89,90.
Perspective
The most appropriate way to look forward is to formulate the questions that will identify the next frontiers in this aspect of physiology. For instance, are all of the functions of osteocalcin known? What are the genes downstream of osteocalcin in all of its target cells? Is osteocalcin the only bone-derived hormone affecting energy metabolism? Is there a counterpart of osteocalcin in females that affects their reproduction? We will also venture a general and humbling statement: if we have learned so much in so brief a time about the physiology of one organ, it is likely that there are many aspects of whole-organism physiology to be discovered through the use of model organisms. ■
Acknowledgements
We thank T. Clemens, P. Ducy, M. Gershon, M. Kassem, S. Kousteni and members of the Karsenty laboratory for comments on the manuscript. We apologize to our colleagues whose work is not directly discussed and/or cited in this article because of space constraints. G.K. is supported by the National Institutes of Health, and M.F. by the Canadian Diabetes Association.
Footnotes
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Bernard C Introduction à l’Etude de la Médecine Expérimental (Flammarion, 1865). [Google Scholar]
- 2.Gurney CW Erythropoietin, erythropoiesis, and the kidney. J. Am. Med. Assoc 173, 1828–1829 (1960). [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM & Halaas JL Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Chen SK et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon WB The Wisdom of the Body (Norton, 1932). [Google Scholar]
- 6.Monod J & Jacob F Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb. Symp. Quant. Biol 26, 389–401 (1961). [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum SL Bone resorption by osteoclasts. Science 289, 1504–1508 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Ducy P, Schinke T & Karsenty G The osteoblast: a sophisticated fibroblast under central surveillance. Science 289, 1501–1504 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Legroux-Gerot I, Vignau J, Collier F & Cortet B Bone loss associated with anorexia nervosa. Joint Bone Spine 72, 489–495 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Misra M & Klibanski A The neuroendocrine basis of anorexia nervosa and its impact on bone metabolism. Neuroendocrinology 93, 65–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggs BL, Khosla S & Melton LJ A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res 13, 763–773 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL & Melton LJ Involutional osteoporosis. N. Engl. J. Med 314, 1676–1686 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Hauschka PV, Lian JB, Cole DE & Gundberg CM Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev 69, 990–1047 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Oury F et al. Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee NK et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007). This paper describes for the first time the endocrine function of bone.
- 16. Zhang Y et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This report describes the identification of the leptin gene.
- 17.Halaas JL et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Chehab FF, Lim ME & Lu R Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature Genet. 12, 318–320 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Ducy P et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Pogoda P et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J. Bone Miner. Res. 21, 1591–1599 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Elefteriou F et al. Serum leptin level is a regulator of bone mass. Proc. Natl Acad. Sci. USA 101, 3258–3263 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson WT et al. Congenital leptin deficiency due to homozygosity for the Δ133G mutation: report of another case and evaluation of response to four years of leptin therapy. J. Clin. Endocrinol. Metab 89, 4821–4826 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE & Flier JS Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Bjornholm M et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J. Clin. Invest 117, 1354–1360 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc. Natl Acad. Sci. USA 105, 20529–20533 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldock PA et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS ONE 4, e8415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S et al. Central control of bone remodeling by neuromedin U. Nature Med. 13, 1234–1240 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Cornish J et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol 175, 405–415 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Bartell SM et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J. Bone Miner. Res 26, 1710–1720 (2011). [DOI] [PubMed] [Google Scholar]
- 30. Takeda S et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002). This paper reports that leptin regulation of bone mass requires the sympathetic nervous system.
- 31. Yadav VK et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 (2009). This study identifies brain serotonin as a critical mediator of the central action of leptin.
- 32.Balthasar N et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Dhillon H et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Warden SJ et al. Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone 46, 985–992 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bliziotes M Update in serotonin and bone. J. Clin. Endocrinol. Metab 95, 4124–4132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaye W, Gendall K & Strober M Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol. Psychiatry 44, 825–838 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Mann JJ et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch. Gen. Psychiatry 49, 442–446 (1992). [DOI] [PubMed] [Google Scholar]
- 38. Walther DJ et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76 (2003). This report describes the cloning of the Tph2 gene, which is responsible for brain serotonin synthesis.
- 39.Oury F et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 24, 2330–2342 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav VK et al. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J. Exp. Med 208, 41–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam DD et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 13, 584–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elefteriou F et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Bonnet N et al. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40, 1209–1216 (2007). [DOI] [PubMed] [Google Scholar]
- 44.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genet. 26, 345–348 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Hori M, Shimizu Y & Fukumoto S Fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 152, 4–10 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Motyl KJ, McCabe LR & Schwartz AV Bone and glucose metabolism: a two-way street. Arch. Biochem. Biophys 503, 2–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollock NK et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J. Clin. Endocrinol. Metab 96, E1092–E1099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pi M et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J. Biol. Chem 280, 40201–40209 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merle B & Delmas PD Normal carboxylation of circulating osteocalcin (bone Gla-protein) in Paget’s disease of bone. Bone Miner. 11, 237–245 (1990). [DOI] [PubMed] [Google Scholar]
- 50.Ferron M, Hinoi E, Karsenty G & Ducy P Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl Acad. Sci. USA 105, 5266–5270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferron M et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010). This study identifies a feed-forward loop linking insulin and osteocalcin.
- 52. Fulzele K et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010). In this report, insulin signalling in osteoblasts is identified as a key regulator of bone mass and of energy metabolism.
- 53.Bluher M et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3, 25–38 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Bruning JC et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2, 559–569 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Silver IA, Murrills RJ & Etherington DJ Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res 175, 266–276 (1988). [DOI] [PubMed] [Google Scholar]
- 56.Poser JW & Price PA A method for decarboxylation of γ-carboxyglutamic acid in proteins. Properties of the decarboxylated γ-carboxyglutamic acid protein from calf bone. J. Biol. Chem 254, 431–436 (1979). [PubMed] [Google Scholar]
- 57.Elchebly M et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Delibegovic M et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58, 590–599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delibegovic M et al. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol. Cell. Biol 27, 7727–7734 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinoi E et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol 183, 1235–1242 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miheller P, Lorinczy K & Lakatos PL Clinical relevance of changes in bone metabolism in inflammatory bowel disease. World J. Gastroenterol 16, 5536–5542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Bores L, Barahona-Garrido J & Yamamoto-Furusho JK Basic and clinical aspects of osteoporosis in inflammatory bowel disease. World J. Gastroenterol 13, 6156–6165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elefteriou F et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 4, 441–451 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell 117, 387–398 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Raisz LG Stimulation of bone resorption by parathyroid hormone in tissue culture. Nature 197, 1015–1016 (1963). [DOI] [PubMed] [Google Scholar]
- 66.Schinke T et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nature Med.15, 674–681 (2009). [DOI] [PubMed] [Google Scholar]
- 67. Gong Y et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001). This paper identifies LRP5 as the gene mutated in OPPG.
- 68.Little RD et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet 70, 11–19 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boyden LM et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med 346, 1513–1521 (2002). This study identifies a mutation in LRP5 as causing HBM syndrome in humans.
- 70.Kato M et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol 157, 303–314 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamai K et al. LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Hay E et al. Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J. Biol. Chem 280, 13616–13623 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Tolwinski NS et al. Wg/Wnt signal can be transmitted through Arrow/LRP5,6 and Axin independently of Zw3/Gsk3β activity. Dev. Cell 4, 407–418 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Li X et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem 280, 19883–19887 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Ye X et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139, 285–298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yadav VK et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson A et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone 36, 585–598 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Glass DA et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Kramer I et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol 30, 3071–3085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reya T & Clevers H Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Cui Y et al. Lrp5 functions in bone to regulate bone mass. Nature Med. 17, 684–691 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saarinen A et al. Low density lipoprotein receptor-related protein 5 (LRP5) mutations and osteoporosis, impaired glucose metabolism and hypercholesterolaemia. Clin. Endocrinol 72, 481–488 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Frost M et al. Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin. J. Bone Miner. Res 25, 673–675 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Frost M et al. Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high bone mass phenotype due to a mutation in Lrp5. J. Bone Miner. Res 26, 1721–1728 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Vilaca T, Yamamoto RM, Carvalho AB & Lazaretti-Castro M Neuroendocrine tumor associated with severe osteoporosis in a male patient. Endocr Rev, 32, abstr. P3–123 (2011) [Google Scholar]
- 86.Modder UI et al. Relation of serum serotonin levels to bone density and structural parameters in women. J. Bone Miner. Res 25, 415–422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi ZC et al. Modulation of peripheral serotonin levels by novel tryptophan hydroxylase inhibitors for the potential treatment of functional gastrointestinal disorders. J. Med. Chem 51, 3684–3687 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Liu Q et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J. Pharmacol. Exp. Ther 325, 47–55 (2008). [DOI] [PubMed] [Google Scholar]
- 89.Inose H et al. Efficacy of serotonin inhibition in mouse models of bone loss. J. Bone Miner. Res 26, 2002–2011 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Yadav VK et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nature Med. 16, 308–312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]