Abstract
Aims
There is evidence that morbidly obese patients have more intra- and postoperative complications and poorer outcomes when undergoing total hip arthroplasty (THA) with the direct anterior approach (DAA). The aim of this study was to determine the efficacy of DAA for THA, and compare the complications and outcomes of morbidly obese patients with nonobese patients.
Methods
Morbidly obese patients (n = 86), with BMI ≥ 40 kg/m2 who underwent DAA THA at our institution between September 2010 and December 2017, were matched to 172 patients with BMI < 30 kg/m2. Data regarding demographics, set-up and operating time, blood loss, radiological assessment, Harris Hip Score (HHS), International Hip Outcome Tool (12-items), reoperation rate, and complications at two years postoperatively were retrospectively analyzed.
Results
No significant differences in blood loss, intra- and postoperative complications, or implant position were observed between the two groups. Superficial wound infection rate was higher in the obese group (8.1%) compared to the nonobese group (1.2%) (p = 0.007) and relative risk of reoperation was 2.59 (95% confidence interval 0.68 to 9.91). One periprosthetic joint infection was reported in the obese group. Set-up time in the operating table and mean operating time were higher in morbidly obese patients. Functional outcomes and patient-related outcome measurements were superior in the obese group (mean increase of HHS was 52.19 (SD 5.95) vs 45.1 (SD 4.42); p < 0.001), and mean increase of International Hip Outcome Tool (12-items) was 56.8 (SD 8.88) versus 55.2 (SD 5.85); p = 0.041).
Conclusion
Our results suggest that THA in morbidly obese patients can be safely and effectively performed via the DAA by experienced surgeons.
Cite this article: Bone Jt Open 2022;3(1):4–11.
Keywords: Morbid obesity, Total hip arthroplasty, Direct anterior approach, direct anterior approach, Morbidly obese, total hip arthroplasty (THA), BMI, Obesity, Hip, reoperations, periprosthetic joint infection (PJI), superficial wound infections, postoperative complications
Introduction
Obesity is one of the most concerning health issues that pose multiple threats to the life expectancy and quality of life of the affected individuals. After years of controversy regarding its true nature, obesity is now considered a chronic disease by the World Obesity Federation, 1 and associated with numerous other diseases including osteoarthritis (OA). 2 Severe obesity has rapidly increased over the years, 3,4 while morbid obesity has been shown to double healthcare expenditure. 5 According to the World Health Organization (WHO), normal values of BMI are considered to be those between 18.5 kg/m2 and 24.9 kg/m2, and obesity is divided into three classes (class I, moderate with BMI 30 kg/m2 to 34.9 kg/m2; class II, severe with BMI 35 kg/m2 to 39.9 kg/m2; and class III, very severe with BMI ≥ 40 kg/m2). 6 Morbid obesity is technically more vaguely defined and concerns individuals with either BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 who suffer from serious obesity-related comorbidities. 3 However, in current literature, it is a commonly used term, mainly by bariatric surgeons, and usually refers to individuals with a BMI ≥ 40 kg/m2. 7,8
Hip OA is one of the most common and debilitating orthopaedic diseases in the general population, and is also prevalent among obese individuals. 9 To date, total hip arthroplasty (THA) is the treatment of choice in the setting of symptomatic OA and provides satisfactory functional outcomes. 10 Minimally invasive direct anterior approach (DAA) for THA has gained popularity among patients and surgeons, and has been shown to be safe and effective. 11 However, severely obese patients present a significant challenge for arthroplasty surgeons because of their body habitus. 7 Concerns have been raised regarding the complication rate of THA in morbidly obese patients. There are several studies indicating that THA in obese patients leads to more superficial and deep infections, increased blood loss, 7,12,13 suboptimal component positioning, and increased revision rates. How surgical approach affects the aforementioned complications is an issue of extensive research and controversy.
There is currently no clear consensus on the efficacy and safety of the DAA in morbidly obese patients undergoing THA. The purpose of this study was to determine whether DAA is safe and effective in this patient group regarding component positioning, surgical difficulty, complication rate, and clinical and patient-related outcomes compared with nonobese patients.
Methods
This is a single-centre, single-surgeon retrospective study. Ethical approval was obtained from Institute’s (KAT Attica General Hospital) Ethical Committee. A total of 1,547 primary DAA THAs were performed in our centre between September 2010 and December 2017 for the treatment of primary or secondary hip OA. The exclusion criteria were patients with history or signs of active infection, aged under 18 years, and minimum follow-up of less than two years. Among these, 86 THAs were performed in patients with BMI ≥ 40 kg/m2 (group A). These were matched to 172 patients (ratio 1:2) with BMI < 30 kg/m2 (group B) according to their age, sex, and diagnosis. All patients provided signed informed consent.
Preoperative care and set-up
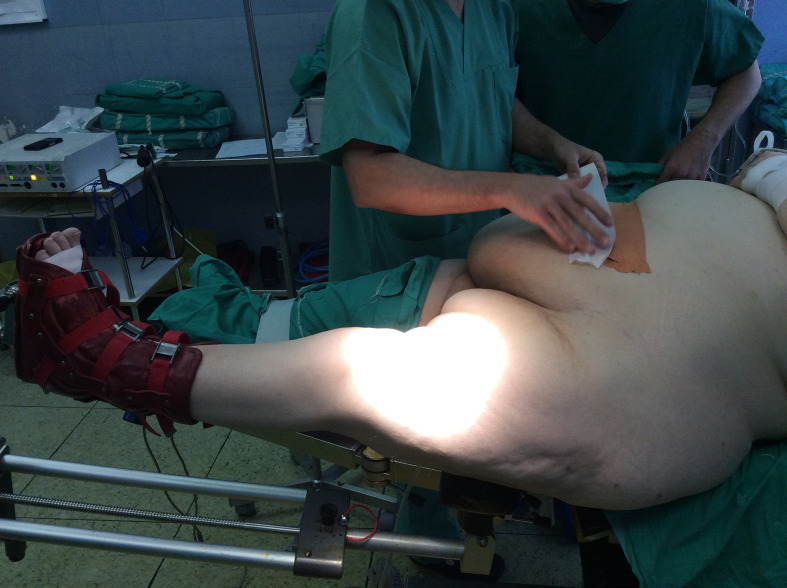
The inguinal area was inspected for skin pathology and appropriate care was taken. Meticulous cleaning with chlorhexidine of the inguinal region and abdominal folds was performed prior entrance of the operating room. This procedure was visually inspected by a member of the team. In case of suspected inguinal skin infection, surgery was postponed. Intravenous (IV) weight-based dosing of vancomycin was administered one hour before surgery unless contraindicated; 1 g of tranexamic acid and 12 mg of dexamethasone were administered 20 minutes before skin incision. Spinal anaesthesia was chosen in the majority of cases. Patients were positioned in supine position on the DAA traction table. In group A, set-up included the retraction of abdominal pannus from the surgical field with adhesive tape. All procedures were performed by the senior author (GAM) and one assistant. Figure 1 shows the positioning of the patient on the traction table.
Fig. 1.
A morbidly obese patient is positioned on the traction table. Abdominal pannus was retracted out of surgical field with taping.
Surgical technique
Skin incision was made 2 cm distal and 2 to 3 cm lateral to the anterior superior iliac spine (ASIS), in oblique direction, parallel to the fibres of the tensor fascia lata muscle. We aimed laterally enough in order to avoid lateral femoral cutaneous nerve (LFCN) injury. Dissection through the interval between tensor fascia lata and sartorius muscles was performed. The ascending branch of the lateral femoral circumflex artery and vein were ligated and cauterized. After capsulotomy, osteotomy was performed in accordance with preoperative templating. The same surgical technique and instrumentation was used in both groups. Uncemented components were used in all cases. The Mathys RM pressfit monoblock acetabular component coupled with the Twinsys Mathys stem and 32 ceramic femoral head (Mathys, Switzerland), or the Trilogy or Continuum modular acetabular component coupled with the Avenir uncemented stem and 32 ceramic femoral head (Zimmer Biomet, Switzerland), were used. The companies were selected according to the hospital’s purchase policy and patient’s hip anatomy. Fluoroscopy was used in every case to check the correct cup positioning and orientation. A drain was not used in either of the groups. Joint capsule and periarticular soft-tissue were infiltrated with 120 ml mixture of ropivacaine (300 mg), plus 8 mg of dexamethasone. Intra-articular injection of 2 gr of tranexamic acid was also administered. The skin was closed with staples. Intraoperative radiological imaging (anteroposterior (AP) hip radiograph) was performed at the end of the procedure.
Postoperative care
Adequate pain control was achieved with Skudexa (75 mg Dexketoprofen and 25 mg tramadol; Menarini, Italy) or morphine. Mobilization and physical therapy were initiated on the first postoperative day. Full weightbearing was allowed with the use of a walker or crutches, which were discontinued three to four weeks postoperatively. Two doses of IV vancomycin were administered at 12 and 24 hours postoperatively. Thromboprophylaxis with subcutaneous fondaparinux injection 2.5 mg/0.5 ml once daily was administered on the first and second postoperative days, followed by oral rivaroxaban 10 mg once daily for one month postoperatively. Radiological imaging (AP and lateral view) was obtained before discharge. All patients were typically discharged home on the second postoperative day. All patients were encouraged to wear compression stockings for one month. Follow-up was performed at one, three, 12, and 24 months.
Wound care protocol
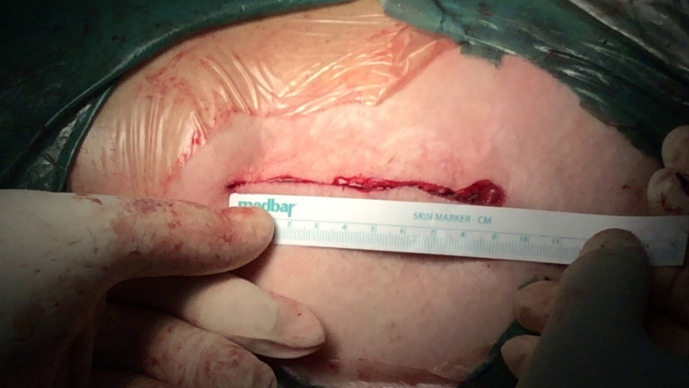
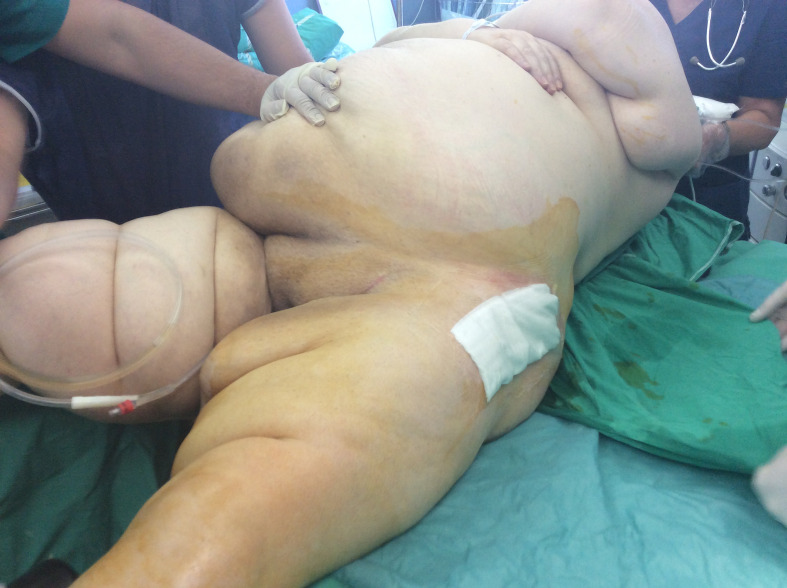
Sterile alginate wound dressings were used to cover the incision intraoperatively and were not changed unless necessary. Suture removal was done at 15 to 17 days postoperatively. Emphasis was placed on the use of the elastic abdominal binder and meticulous regional hygiene in group A. The elastic abdominal binder was introduced as a wound complications prevention method, by retracting the abdominal folds away from the skin incision, and all patients from group A were encouraged to wear it as much as possible. Figures 2 to 4 show the length of the skin incision and the wound dressing under the abdominal pannus.
Fig. 2.
Skin incision. Length of skin incision during wound closure.
Fig. 4.
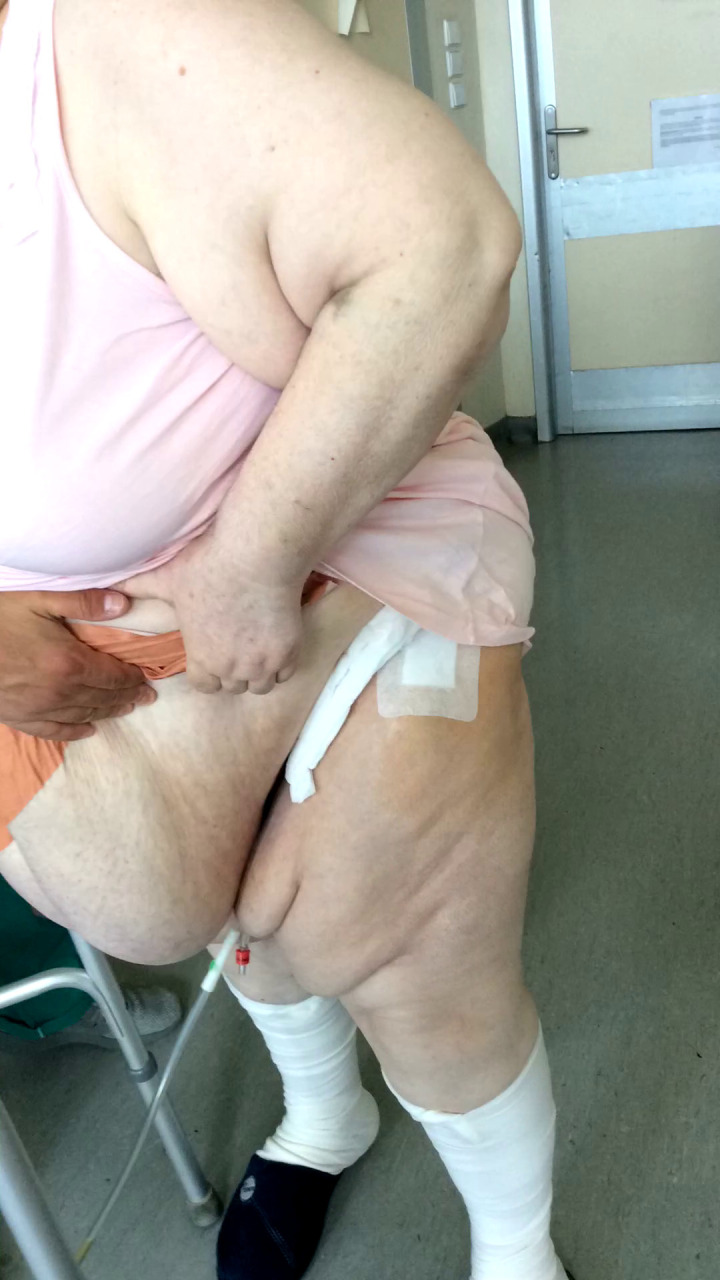
Dressing under abdominal pannus. A protective elastic abdominal binder was used until the removal of the sutures to retract the abdominal folds away from the wound. Meticulous cleaning of the inguinal area, around the dressing, was encouraged in all patients. Wound dressings were not changed unless it was absolutely necessary.
Fig. 3.
Dressing after skin closure.
Variables measured in the study
Data regarding set-up, operating time, incision length, blood loss (evaluated as a percentage of Hb drop preoperatively and at the second postoperative day), and complications (periprosthetic fracture (PPF), LFCN injury (diagnosis was made by patient’s description and sensory clinical examination between the operated and the nonoperated leg), infection, dislocation, thromboembolic event, and other medical complications) were recorded and the reoperation rate was calculated. For the diagnosis of periprosthetic joint infection (PJI) the 2011 Musculoskeletal Infection Society Criteria were used. 14 Clinical and patient-reported outcomes (PROMs), evaluated with Harris Hip Score (HHS) 15 and International Hip Outcome Tool (iHOT-12), 16 were measured preoperatively and at two years postoperatively. Both tools are validated for their use in the Greek population. 17,18 The American Society of Anesthesiologists (ASA) score was reported preoperatively in all patients as a measure of comorbidity index. 19,20
Radiological measurements regarding leg length discrepancy (LLD), acetabular component abduction angle, and cup anteversion were evaluated by two independent radiologists (specializing in musculoskeletal radiology), to assess if obesity had affected the optimal position of the implants. For these measurements, the Sundvall method was used on AP and lateral plain radiographs. 21 Positive LLD indicated longer, and negative indicated shorter, than the nonoperated leg.
Statistical analysis
Data were expressed as mean and standard deviation (SD) for continuous variables and as percentages for categorical data. The Kolmogorov-Smirnov test was used for normality. Comparison of quantitative and qualitative variables between groups was performed using independent-samples t-test or Mann-Whitney U test (in case of violation of normality) and Fisher’s exact test respectively. Relative risk (RR) was calculated to identify whether obese patients have an increased risk of developing postoperative complication or reoperation. Comparison between preoperative and postoperative evaluation of HHS, iHOT-12, and Hb for each group was analyzed using the paired t-test or Wilcoxon test in case of violation of normality. Reliability of the radiological measurements of the two reviewers was calculated with the single-measure intraclass correlation coefficient (ICC) with two-way random effects and absolute agreement. All tests were two-sided, and statistical significance was set at p < 0.05. All analyses were carried out using the statistical package SPSS v. 22.00 (IBM, USA).
Results
Patient characteristics
In group A there were 39 males and 47 females with a mean BMI of 42.33 kg/m2 (SD 2.06) and mean age of 64.9 years (44 to 84), and a mean follow-up of 5.29 years (2 to 9). Group B consisted of 78 males and 94 females, with a mean BMI of 24.97 kg/m2 (SD 2.29), a mean age of 64.67 years (42 to 84), and mean follow-up of 5.24 years (2 to 9). No statistically significant differences were found between the two groups, except for the BMI, ASA grade, diabetes mellitus, and atrial fibrillation (Table I). ASA grades and comorbidities are presented in Table I.
Table I.
Demographics and patients’ characteristics.
| Variable | Group A (n = 86) | Group B (n = 172) | p-value |
|---|---|---|---|
| Mean BMI, kg/m2 (SD; range) | 42.33 (2.06; 40.00 to 49.12) | 24.97 (2.29; 20.00 to 29.92) | < 0.001* |
| Sex, M:F; n (%) | 39 (45.3):47 (54.7) | 78 (45.3):94 (54.7) | N/A |
| Mean age, yrs (SD; range) | 64.90 (10.42; 44 to 84) | 64.67 (9.53; 42 to 84) | 0.858* |
| Mean follow-up, yrs (SD) | 5.29 (1.79) | 5.24 (1.77) | 0.843† |
| Hypertension, n (%) | 48 (55.8) | 83 (48.3) | 0.291‡ |
| Atrial fibrillation, n (%) | 11 (12.7) | 7 (4) | 0.017‡ |
| Diabetes mellitus | 27 (31.4) | 14 (8.1) | < 0.001‡ |
| Coronary artery disease, n (%) | 3 (3.5) | 5 (2.9) | 1.000‡ |
| ESRD, n (%) | 2 (2.3) | 0 (0) | 0.110‡ |
| ASA score, n (%) | |||
| 1 | 0 (0) | 48 (27.9) | < 0.001‡ |
| 2 | 0 (0) | 113 (65.7) | < 0.001‡ |
| 3 | 84 (97.6) | 11 (6.3) | < 0.001‡ |
| 4 | 2 (2.3) | 0 (0) | 0.110‡ |
Group A = patients with BMI ≥ 40 kg/m2
Group B = patients with BMI < 30 kg/m2
Independent-samples t-test
Mann-Whitney U test
Fisher's exact test
ASA, American Society of Anesthesiologists; ESRD, end-stage renal disease; Group A, morbidly obese; Group B, nonobese; N/A, not applicable; SD, standard deviation.
Complications
The results indicated an increased mean set-up time of approximately ten minutes for the morbidly obese patients. Set-up time included positioning of the patient and draping of the surgical site. Mean operating time was 15 minutes longer and incision length was 2 cm longer in group A (Table II). There was no difference in pre- and post-operative haemoglobin levels between the two groups (p = 0.732 and p = 0.225 respectively, independent-samples t-test) and no transfusion was needed in either group (Table III).
Table II.
Comparison of surgical variables between the two groups.
| Variable | Group A (n = 86) | Group B (n = 172) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Mean set-up time on operating table, mins (SD) | 15.03 (1.70) | 5.28 (1.00) | 9.75 (9.42 to 10.09) | < 0.001* |
| Mean operating time, mins (SD) | 70.12 (3.13) | 55.12 (4.45) | 15.00 (14.06 to 15.94) | < 0.001* |
| Mean incision length, cm (SD) | 9.01 (0.73) | 7.12 (0.98) | 1.90 (1.68 to 2.10) | < 0.001† |
| Mean acetabular component inclination, ° (SD, range) | 42.31 (1.31, 40 to 46) | 42.46 (1.60, 40 to 47) | 0.721* | |
| Mean acetabular component anteversion, ° (SD, range) | 20.05 (1.78, 16 to 24) | 20.02 (1.81, 15 to 25) | 0.783* | |
| Mean LLD, mm (SD) | 0.78 (2.04) | 0.81 (2.13) | 0.683* |
Group A = patients with BMI ≥ 40 kg/m2
Group B = patients with BMI < 30 kg/m2
Mann-Whitney U test
ndependent-samples t-test
CI, confidence interval; Group A, morbidly obese; Group B, nonobese; LLD, leg length discrepancy; SD, standard deviation.
Table III.
Comparison of haemoglobin (mg/dl) change from preoperative to second postoperative day between the two groups.
| Mean Hb (SD) | Preoperative | 2nd postoperative day | p-value* |
|---|---|---|---|
| Group A (n = 86) | 13.93 (1.29) | 11.13 (1.00) | < 0.001 |
| Group B (n = 172) | 13.98 (1.13) | 11.30 (1.11) | < 0.001 |
| p-value † | 0.732 | 0.225 |
Group A = patients with BMI ≥ 40 kg/m2
Group B = patients with BMI < 30 kg/m2
Within group, paired-samples t-test
Between groups, independent-samples t-test
Group A, morbidly obese; Group B, nonobese; Hb, haemoglobin; SD, standard deviation.
In group A the overall surgical complication rate was 16.3%: seven superficial wound infections, one PJI, one PPF, two intraoperative trochanteric avulsions treated with internal fixation, and three instances of LFCN temporal paresthesia. No dislocations, clinically relevant thromboembolic events, or other medical complications were reported. The reoperation rate was 5.8%; one PJI was reported two weeks postoperatively, attributed to Candida albicans and treated with two-stage revision THA. Furthermore, three superficial wound infections (presented at 12, 15, and 18 days) were treated with surgical debridement and antibiotics based on antibiogram, whereas the remaining four (presented at ten, 16, 18, and 20 days) with antibiotics only based on antibiogram. One PPF (Vancouver B2) was documented and revised with a longer femoral stem and cerclage wires after 11 months (Table IV).
Table IV.
Surgical complications and reoperations between obese and nonobese groups.
| Variable, n (%) | Group A (n = 86) | Group B (n = 172) | RR (95% CI) | p-value* |
|---|---|---|---|---|
| Any surgical complication | 14 (16.3) | 16 (9.3) | 1.75 (0.90 to 3.41) | 0.104 |
| PJI | 1 (1.2) | 0 (0.0) | 0.333 | |
| LFCN paresthesia | 3 (3.5) | 4 (2.3) | 0.689 | |
| GT avulsion | 2 (2.3) | 6 (3.5) | 0.722 | |
| Superficial wound infection | 7 (8.1) | 2 (1.2) | 0.007 | |
| PPF B1 | 1 (1.2) | 4 (2.3) | 0.667 | |
| Reoperation | 5 (5.8) | 4 (2.3) | 2.59 (0.68 to 9.91) | 0.165 |
Group A = patients with BMI ≥ 40 kg/m2
Group B = patients with BMI < 30 kg/m2
Fisher's exact test
CI, confidence interval; Group A, morbidly obese; Group B, nonobese; GT, greater trochanter; LFCN, lateral cutaneous femoral nerve; PJI, periprosthetic joint infection; PPF, periprosthetic fracture; RR, relative risk.
In group B the overall surgical complication rate was 9.3%: two PPFs (Vancouver B1) treated intraoperatively with cerclage wires, two PPFs (Vancouver B2) needed reoperation, six intraoperative trochanteric avulsions treated with internal fixation, two superficial wound infections, and four instances of LFCN temporal paresthesia. No PJIs, dislocations, clinically relevant thromboembolic events, or other medical complications were noted. The reoperation rate was 2.3%: two superficial wound infections (presented at 12 and 16 days) treated with surgical debridement and antibiotics, and two PPFs (Vancouver B2) (presented at four and 15 months) treated with a longer femoral stem and cerclage wires (Table IV).
Relative risk for the development of overall complications in group A compared to group B was 1.75 (0.90 to 3.41; p = 0.104, Fisher’s exact test) and relative risk of reoperation was 2.59 times higher in group A compared to group B (p = 0.165. Fisher’s exact test) (Table IV).
Radiological measurements
Interobserver reliability between the two independent reviewers was excellent, with single measure ICC = 0.972 for LLD, 0.905 for cup anteversion, and 0.968 for cup inclination. There were no differences in these parameters between the two groups; LLD in both groups was less than 1 mm, cup anteversion was 20°, and cup inclination was 42° (detailed data presented in Table II).
PROMs
Mean modified HHS score increased from 42.52 (SD 4.74) preoperatively to 94.71 (SD 3.21) at two years postoperatively in group A (p < 0.001, paired-samples t-test) and from 51.21 (SD 3.86) to 96.29 (SD 1.95) in group B (p < 0.001, paired-samples t-test). iHOT-12 showed an increase from 31.31 (SD 3.64) preoperatively to 88.10 (SD 4.37) two years postoperatively in group A (p < 0.001, paired-samples t-test) and from 31.21 (SD 4.45) to 86.42 (SD 4.20) in group B (p < 0.001, paired-samples t-test). Mean increase in HHS was 52.19 (SD 5.95) vs 45.1 (SD 4.42) (p < 0.001, Mann-Whitney U test) and mean increase in iHOT-12 was 56.8 (SD 8.88) versus 55.2 (SD 5.85) (p = 0.041, Mann-Whitney U test), indicating greater increase in group A (Table V).
Table V.
Comparison of Modified Harris Hip Score (Greek version) and International Hip Outcome Tool (12-items) (Greek version) change from preoperative to 24 months postoperatively between the two groups.
| Group | Mean preoperative score (SD) | Mean 24 months postoperative score (SD) | p-value* | Mean change (SD) |
|---|---|---|---|---|
| HHS | ||||
| Group A (n = 86) | 42.52 (4.74) | 94.71 (3.21) | < 0.001 | 52.19 (5.95) |
| Group B (n = 172) | 51.21 (3.86) | 96.29 (1.95) | < 0.001 | 45.1 (4.42) |
| p-value† | < 0.001 | |||
| iHOT-12 | ||||
| Group A (n = 86) | 31.31 (3.64) | 88.10 (4.37) | < 0.001 | 56.8 (8.88) |
| Group B (n = 172) | 31.21 (4.45) | 86.42 (4.20) | < 0.001 | 55.20 (5.85) |
| p-value† | 0.041 |
Group A = patients with BMI ≥ 40 kg/m2
Group B = patients with BMI < 30 kg/m2
Paired-samples t-test
Mann-Whitney U test
Group A, morbidly obese; Group B, nonobese; HHS, Harris Hip Score; iHOT-12, International Hip Outcome Tool (12-items); SD, standard deviation.
Discussion
In this study we compared DAA THA in morbidly obese patients with nonobese patients. We chose to set a BMI of 40 as the threshold, as this is the level at which the greatest technical and practical challenges exist. The pre- and postoperative care protocol at our centre has not changed during the study. Morbidly obese patients had an increased ASA score preoperatively, which is expected due to the fact that morbid obesity by itself is a risk factor for developing postoperative complications. Weight loss was advised, but not measured in this study, and was not considered obligatory in order to undergo surgery. The increased set-up and surgical operating time, as well as the increased incision length in the morbidly obese group, were anticipated, and adequate exposure and correct placement of the prosthesis were ensured despite the extensive fatty tissue. As a result, no differences were observed in acetabular component inclination and anteversion between the two groups; further, no dislocations were observed in either group. Regardless of all the precautions taken to avoid risk of wound infection, our results showed that morbidly obese patients are at a higher risk of developing superficial wound infections in nearly one out ten patients, versus one out of 100 in the nonobese patients. As shown by the PJI attributed to C. albicans, inguinal hygiene may be compromised in the morbidly obese undergoing THA with DAA approach, which may predispose them to infections, as opposed to the posterior approach where the skin incision has less proximity to the inguinal crease. Jahng et al 22 and other studies noted an association between DAA and wound infections, due to this proximity and the overhanging abdominal pannus. 23,24 The isolation of C. albicans indicates that the moistness of this area could be another risk factor that should be studied further. Overall risk of reoperation was 2.59 times higher in the morbidly obese, the complication rate was 16.3%, and risk of complications 1.75 times higher as compared to the nonobese. Though these rates are not statistically significant, they should not be overlooked. Functional outcomes were highly satisfactory, especially for the morbidly obese group, whose prior health state was worse. However, morbidly obese patients remain less active than the nonobese. This could explain the increased incidence of postoperative periprosthetic fractures in the nonobese group.
The results of the present study are in line with current literature. There are a limited number of studies with similar design to ours (Table VI). Antoniadis et al 25 compared patients with a BMI > 35 and < 25 who underwent DAA THA and reached comparable results. Purcell et al 26 used 35 as the cut-off point in two DAA THA groups; they concluded that the risk of revision due to deep infection was seven times higher in patients with a BMI > 35. They also suggested the use of an elastic abdominal binder, which was a standard practice in our study. Patients responded well to the use of the binder, with no discomfort reported, and compliance was 100%. Russo et al 27 investigated differences in DAA complications among three groups (BMI < 25, BMI = 25 to 29.9, and BMI ≥ 30). Their results showed an increased risk of wounds and major complications, which were not significant, except for the increased length of hospital stay in the obese group. They also expressed their concern regarding the role of obesity in THA, despite the approach used in each case.
Table VI.
Summary of retrospective studies regarding direct anterior approach total hip arthroplasty in obese versus nonobese individuals.
| Study | Groups by BMI (kg/m2) | Wound complications (obese) | Deep infections (obese) | Dislocation (obese) | Blood loss (obese) | Reoperation (obese) | Mean hospital stay (obese) | Set-up and operating time (obese) | DAA recommendation (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|
| This study | ≥ 40 and < 30 | Increased | NS | NS | NS | NS | N/A | Increased | Yes |
| Antoniadis et al 25 | ≥ 35 (n = 129) and ≤ 25 (n = 125) | Increased | Increased | NS | NS | Increased | Increased | Increased | Yes |
| Purcell et al 26 | < 35 (n = 1,417) and ≥ 25 (n = 204) | NS | Increased | NS | NS | NS | N/A | N/A | Yes |
| Russo et al 27 | < 25 (n = 61), 25 to 29.9 (n = 70), and ≥ 30 (n = 79) | Increased | Increased* | Increased* | Increased* | Increased* | Increased | Increased | Yes |
Deep infections, dislocation, blood loss, and reoperation mentioned together as major complications.
DAA, direct anterior approach; N/A, not available; NS, not significant
Various studies have demonstrated that severe obesity significantly increases the risk of THA complications, especially wound complications and infections. 8 It is well understood that the chronic inflammation associated with obesity negatively impacts the healing process, encouraging infections and wound complications. 28 BMI was also found to be an independent risk factor for wound complications in DAA in a multivariate regression analysis by Jahng et al. 22 Great concern exists regarding the complication rate following DAA. Some studies suggest higher wound complications, infection rate, nerve damage, and PPFs. 29 Nerve damage and fractures have been shown to be significantly higher during the learning curve of DAA, and are less common in experienced hands, while infections and wound complications seem to remain unaffected. 29,30 Based on this evidence, our study included patients operated after the surgeon’s learning curve of DAA has plateaued; cases that were considered to be part of the learning curve were not included in the study. Despite that, some cases of LCFN paresthesia and PPF were still reported. However, recent data are contradictory, showing no difference in wound complication rates, or even lower revision rates, for DAA when compared to posterior approach. 31-33
Current systematic reviews of the literature and meta-analyses report that DAA is associated with earlier recovery and less postoperative pain when compared to other approaches, perhaps due to less muscle trauma. 34-36 We believe that the advantages of the DAA become even more evident when the approach is performed in morbidly obese individuals. First of all, the intramuscular plane between the tensor fascia lata muscle and the sartorius that is exploited in DAA leads to minimal muscle trauma and faster recovery during the early postoperative period, which is extremely important in obese patients where early mobilization is critical in order to avoid postoperative complications. Moreover, in morbidly obese patients the excessive adipose tissue is distributed in the gluteal region, favouring the dissection through DAA plane. In a study by Watts et al, 23 comparing DAA and posterior approaches THA in obese individuals, although the infection rate was similar in both groups, they reported a higher risk of wound complication in obese patients treated with DAA.
There are certain points that we should keep in mind when performing DAA THA in morbidly obese individuals. Appropriate set-up in the operating theatre, with retraction of abdominal pannus away from the incision, is essential in order to achieve better exposure during the procedure. We believe that the use of a traction table enables adequate femoral exposure, which facilitates the optimal position of implants. Fluoroscopy is also important in order to ensure proper positioning of the acetabular component. No special instrumentation is needed for the morbidly obese patients. According to our opinion, the use of a protective elastic abdominal binder in the immediate postoperative period until suture removal, in conjunction with meticulous cleaning of the inguinal crease, is also very critical in avoiding wound complications. Recent reports suggest that the ‘bikini’ incision is of advantage in DAA THA. 37,38
This is the first study, to our knowledge, encompassing a large sample size of patients with a BMI over 40 kg/m2. As a referral centre for DAA THA, we apply a standardized surgical technique and postoperative protocols. However, there are inherent limitations in the present study posed by its retrospective nature. An effort was made to accurately collect all the relevant data. Furthermore, this is a single-surgeon series, eliminating surgeon bias. While we believe this is an advantage, it should be taken into account that the procedures were performed by a surgeon with considerable experience in DAA, so our results may not be safely extrapolated to other surgeons.
In conclusion, we observed that DAA hip arthroplasty in the morbidly obese is associated with increased superficial wound infections and potential increased risk of reoperation. However, functional outcomes are highly satisfactory. Further prospective randomized controlled studies should be designed in the future in order to clarify whether DAA or obesity alone is truly associated with increases in complication rates in the context of THA.
Take home message
- Total hip arthroplasty in morbidly obese patients is a challenge for hip surgeons.
- Despite the increased risk of superficial wound infections, direct anterior approach can be safely performed in this group of patients.
Acknowledgements
Radiological assessment was performed by Dr K. Kokkinis and Dr. K. Kalokairinou. Dr A. Galanos contributed to the statistical analysis.
Footnotes
Author contributions: C. Argyrou: Methodology, Investigation, Formal analysis, Writing – original draft.
D. Tzefronis: Methodology, Investigation, Formal analysis, Writing – original draft.
M. Sarantis: Investigation.
K. Kateros: Writing – review & editing.
L. Poultsides: Writing – review & editing.
G. A. Macheras: Methodology, Supervision.
Funding statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement: The authors have no conflict of interest to declare.
Ethical review statement: The study's protocol was approved by the IRB of KAT Attical General Hospital.
Open access funding: The authors confirm that open access expenses were self-funded.
Contributor Information
Chrysoula Argyrou, Email: chrisa.a@hotmail.com.
Dimitrios Tzefronis, Email: dimtzef@gmail.com.
Michail Sarantis, Email: sarantismichalis@gmail.com.
Konstantinos Kateros, Email: kkateros@hotmail.com.
George A. Macheras, Email: gmacheras@gmail.com.
References
- 1. Bray GA, Kim KK, Wilding JPH, World Obesity Federation . Obesity: A chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes Rev. 2017;18(7):715–723. [DOI] [PubMed] [Google Scholar]
- 2. Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. [DOI] [PubMed] [Google Scholar]
- 3. Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121(7):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hruby A, Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics. 2015;33(7):673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andreyeva T, Sturm R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes Res. 2004;12(12):1936–1943. [DOI] [PubMed] [Google Scholar]
- 6. No authors listed . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 7. Vasarhelyi EM, MacDonald SJ. The influence of obesity on total joint arthroplasty. J Bone Joint Surg Br. 2012;94-B(11 Suppl A):100–102. [DOI] [PubMed] [Google Scholar]
- 8. Workgroup of the American Association of Hip and Knee Surgeons Evidence Based Committee . Obesity and total joint arthroplasty: a literature based review. J Arthroplasty. 2013;28(5):714–721. [DOI] [PubMed] [Google Scholar]
- 9. Dagenais S, Garbedian S, Wai EK. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin Orthop Relat Res. 2009;467(3):623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinedo-Villanueva R, Turner D, Raftery JP. Outcomes after total hip replacement. Osteoarthr Cartil. 2014;22:S214. [Google Scholar]
- 11. Barry JJ, Masonis JL, Mason JB. Recovery and outcomes of direct anterior approach total hip arthroplasty. Annals of Joint. 2018;3(51). [Google Scholar]
- 12. Jeschke E, Citak M, Günster C, et al. Obesity Increases the Risk of Postoperative Complications and Revision Rates Following Primary Total Hip Arthroplasty: An Analysis of 131,576 Total Hip Arthroplasty Cases. J Arthroplasty. 2018;33(7):2287–2292. [DOI] [PubMed] [Google Scholar]
- 13. McCalden RW, Charron KD, MacDonald SJ, Bourne RB, Naudie DD. Does morbid obesity affect the outcome of total hip replacement?: An analysis of 3290 thrs. J Bone Joint Surg Br. 2011;93-B(3):321–325. [DOI] [PubMed] [Google Scholar]
- 14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: From the workgroup of the musculoskeletal infection society. Clin Orthop Relat Res. 2011;469(11):2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;737–755. [PubMed] [Google Scholar]
- 16. Griffin D, Parsons N, Mohtadi N, et al. A short version of the International Hip Outcome Tool (iHOT-12) for use in routine clinical practice. Arthroscopy. 2012;28((5):):611–616. [DOI] [PubMed] [Google Scholar]
- 17. Stasi S, Papathanasiou G, Diochnou A, Polikreti B, Chalimourdas A, Macheras GA. Modified Harris Hip Score as patient-reported outcome measure in osteoarthritic patients: psychometric properties of the Greek version. Hip Int. 2021;31(4):516–525. [DOI] [PubMed] [Google Scholar]
- 18. Stasi S, Stamou M, Papathanasiou G, et al. International Hip Outcome Tool (12-items) as health-related quality-of-life measure in osteoarthritis: validation of Greek version. J Patient Rep Outcomes. 2020;4(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saklad M. Grading of patients for surgical procedures. Anesthesiol. 1941;2(5):281–284. [Google Scholar]
- 20. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55(2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahmood SS, Al-Amiry B, Mukka SS, Baea S, Sayed-Noor AS. Validity, reliability and reproducibility of plain radiographic measurements after total hip arthroplasty. Skeletal Radiol. 2015;44(3):345–351. [DOI] [PubMed] [Google Scholar]
- 22. Jahng KH, Bas MA, Rodriguez JA, Cooper HJ. Risk factors for wound complications after direct anterior approach hip arthroplasty. J Arthroplasty. 2016;31(11):2583–2587. [DOI] [PubMed] [Google Scholar]
- 23. Watts CD, Houdek MT, Wagner ER. High Risk of Wound Complications Following Direct Anterior Total Hip Arthroplasty in Obese Patients. J Arthroplasty. 2015;30(12):2296–2298. [DOI] [PubMed] [Google Scholar]
- 24. Alvi HM, Mednick RE, Krishnan V, Kwasny MJ, Beal MD, Manning DW. The effect of BMI on 30 day outcomes following total joint arthroplasty. J Arthroplasty. 2015;30(7):1113–1117. [DOI] [PubMed] [Google Scholar]
- 25. Antoniadis A, Dimitriou D, Flury A. Is Direct Anterior Approach a Credible Option for Severely Obese Patients Undergoing Total Hip Arthroplasty? A Matched-Control, Retrospective, Clinical Study. J Arthroplasty. 2018;33(8):2535–2540. [DOI] [PubMed] [Google Scholar]
- 26. Purcell RL, Parks NL, Gargiulo JM, Hamilton WG. Severely obese patients have a higher risk of infection after direct anterior approach total hip arthroplasty. J Arthroplasty. 2016;31(9 Suppl):162–165. [DOI] [PubMed] [Google Scholar]
- 27. Russo MW, Macdonell JR, Paulus MC, Keller JM, Zawadsky MW. Increased complications in obese patients undergoing direct anterior total hip arthroplasty. J Arthroplasty. 2015;30(8):1384–1387. [DOI] [PubMed] [Google Scholar]
- 28. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469(2):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christensen CP, Karthikeyan T, Jacobs CA. Greater prevalence of wound complications requiring reoperation with direct anterior approach total hip arthroplasty. J Arthroplasty. 2014;29(9):1839–1841. [DOI] [PubMed] [Google Scholar]
- 31. Miller LE, Gondusky JS, Bhattacharyya S, Kamath AF, Boettner F, Wright J. Does surgical approach affect outcomes in total hip arthroplasty through 90 days of follow-up? A systematic review with meta-analysis. J Arthroplasty. 2018;33(4):1296–1302. [DOI] [PubMed] [Google Scholar]
- 32. Ponzio DY, Poultsides LA, Salvatore A, Lee Y-Y, Memtsoudis SG, Alexiades MM, et al. In-Hospital Morbidity and Postoperative Revisions After Direct Anterior vs Posterior Total Hip Arthroplasty. J Arthroplasty. 2018;33(5):1421–1425. [DOI] [PubMed] [Google Scholar]
- 33. Tissot C, Vautrin M, Luyet A, Borens O. Are there more wound complications or infections with direct anterior approach total hip arthroplasty? Hip Int. 2018;28(6):591–598. [DOI] [PubMed] [Google Scholar]
- 34. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584–596. [DOI] [PubMed] [Google Scholar]
- 35. Yue C, Kang P, Pei F. Comparison of Direct Anterior and Lateral Approaches in Total Hip Arthroplasty: A Systematic Review and Meta-Analysis (PRISMA). Medicine (Baltimore). 2015;94(50):e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao H. Y, Kang P. D, Xia Y. Y, Shi X. J, Nie Y, Pei F. X. Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: A randomized controlled trial. J Arthroplasty. 2017;32(11):3421–3428. [DOI] [PubMed] [Google Scholar]
- 37. Manrique J, Paskey T, Tarabichi M, Restrepo C, Foltz C, Hozack WJ. Total hip arthroplasty through the direct anterior approach using a bikini incision can be safely performed in obese patients. J Arthroplasty. 2019;34(8):1723–1730. [DOI] [PubMed] [Google Scholar]
- 38. Leunig M, Hutmacher JE, Ricciardi BF, Impellizzeri FM, Rüdiger HA, Naal FD. Skin crease “bikini” incision for the direct anterior approach in total hip arthroplasty: a two- to four-year comparative study in 964 patients. Bone Joint J. 2018;100-B(7):853–861. [DOI] [PubMed] [Google Scholar]