Abstract
The development of spinal deformity in children with underlying neurodisability can affect their ability to function and impact on their quality of life, as well as compromise provision of nursing care. Patients with neuromuscular spinal deformity are among the most challenging due to the number and complexity of medical comorbidities that increase the risk for severe intraoperative or postoperative complications. A multidisciplinary approach is mandatory at every stage to ensure that all nonoperative measures have been applied, and that the treatment goals have been clearly defined and agreed with the family. This will involve input from multiple specialities, including allied healthcare professionals, such as physiotherapists and wheelchair services. Surgery should be considered when there is significant impact on the patients’ quality of life, which is usually due to poor sitting balance, back or costo-pelvic pain, respiratory complications, or problems with self-care and feeding. Meticulous preoperative assessment is required, along with careful consideration of the nature of the deformity and the problems that it is causing. Surgery can achieve good curve correction and results in high levels of satisfaction from the patients and their caregivers. Modern modular posterior instrumentation systems allow an effective deformity correction. However, the risks of surgery remain high, and involvement of the family at all stages of decision-making is required in order to balance the risks and anticipated gains of the procedure, and to select those patients who can mostly benefit from spinal correction.
Keywords: Neuromuscular, Myopathic, Scoliosis, Spinal deformity, Surgical treatment, Outcomes, neuromuscular scoliosis, clinical outcomes, spinal deformities, deformities, Deformity correction, medical comorbidities, physiotherapists, postoperative complications, respiratory complications, healthcare professionals
Introduction
Spinal deformity is a common and often most severe musculoskeletal condition affecting children with neurological or myopathic disorders. The treatment of spinal deformity in patients with severe underlying neurodisability is very challenging due to the number of associated comorbidities that cause the potential for major intraoperative or postoperative complications. Meticulous preoperative assessment is required, along with careful consideration of the nature of the deformity and the problems that it is causing. An imbalance in the spinal musculature and poor trunk control can occur due to spasticity or hypotonia, and this leads to the development of scoliosis. The deformity is often more severe when there is poor head control or when the patient is non-ambulatory. 1
Classification of the pattern of deformity and any underlying medical conditions is vital in deciding the strategy for management of the curve. Different types of deformity lead to a variety of clinical problems including back pain, cardiorespiratory compromise, rib on pelvis impingement, sitting imbalance, and difficulty in providing care to the patient. This article will consider the three common patterns of deformity and the specific diagnoses that can cause them. The treatment options for each condition and type of spinal deformity will be discussed, along with a summary of the clinical outcomes that have been reported in the literature.
Patterns of deformity
Scoliosis
A predominantly coronal plane deformity is the most common curve pattern seen in patients with neuromuscular conditions. A long collapsing C-shaped scoliosis develops and this includes the pelvis, leading to marked pelvic obliquity and elevation of the iliac crest on the concave side of the curvature (Figure 1). Pelvic obliquity is affected by contractures either above or below the pelvis (supra- or infra-pelvic). The exact interplay of these forces in creating an abnormal pelvic position and spino-pelvic imbalance is poorly understood, but larger degrees of scoliosis are associated with greater pelvic obliquity.
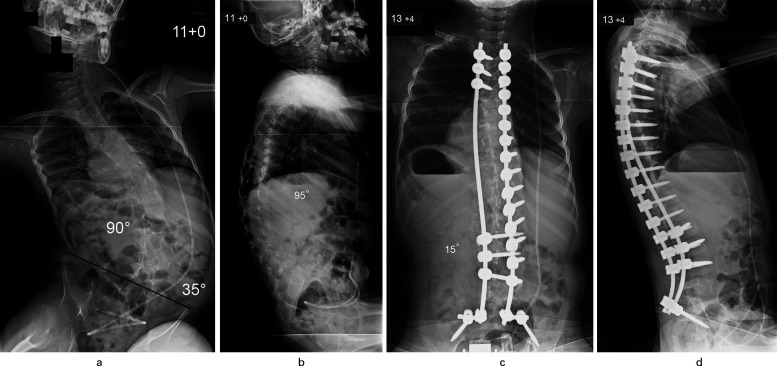
Fig. 1.
Patient aged 11 years with congenital encephalopathy, hydrocephalus, and total body cerebral palsy. a) Typical long C-shaped collapsing thoracolumbar scoliosis (90o) with associated severe pelvic obliquity (35o); b) Thoracolumbar kyphosis producing positive global sagittal balance of the spine. c) Excellent correction of scoliosis to 15o and levelling of the pelvis was achieved through a posterior spinal fusion using segmental pedicle screw/rod instrumentation (aged 13 years and four months). d) Restoration of normal thoracic kyphosis and lumbar lordosis with adequate global sagittal balance noted after spinal surgery.
An imbalance of the muscles below the pelvis has a clear effect on pelvic position in both the coronal and sagittal planes. This can be driven by contractures in the hip flexors, hip adductors, iliotibial band, and hamstrings, as well as by weakness of the hip abductors. Letts et al 2 first described the ‘windblown hip syndrome’, where patients develop the triad of scoliosis, pelvic obliquity, and hip dislocation. The spastic adductors and iliopsoas muscles overpower the weakened hip abductors and extensors. The consequent muscular imbalance can result in a scissor gait (bilateral adduction contracture) or windswept deformity (adduction contracture of one hip and abduction contracture of the other). This creates a challenging clinical picture when deciding on the timing of any surgical intervention. While the deformities affecting the spine, pelvis, and hips are inter-related, it is often not clear which is driving the global spino-pelvic imbalance and subsequent poor sitting posture. Classical teaching has suggested addressing the spino-pelvic relationship first by correcting the scoliosis. However, more recent literature has proposed that the management strategy should be more nuanced, and involves a collaboration multidisciplinary approach, including both hip and spinal surgeons, along with the patients and their families when deciding the priorities and sequence of surgical treatment.
Patients are occasionally referred to the scoliosis clinic with their hips already operated (hip adductor and flexor releases performed in child life; hip reconstruction by proximal femoral and periacetabular osteotomy performed in early puberty), as these have become symptomatic at a younger age before the spinal deformity progresses and requires treatment. The typical example of this clinical scenario is children with quadriplegic cerebral palsy. In contrast, if the patients present with a structural spinal deformity and associated marked pelvic obliquity, as well as hip subluxation/dislocation, it is the authors’ practice to correct the spinal balance and level the pelvis before hip reconstruction is considered. It should be noted that a previously asymptomatic hip subluxation/dislocation, especially in quadriplegic patients, can become persistently symptomatic and require surgical reconstruction after the spinal deformity and associated pelvic imbalance has been corrected through a posterior spinal fusion. The patients and their families should therefore be advised as part of the consent process for scoliosis surgery of the high probability to need a separate hip procedure at a later stage.
Patients with less severe neuromuscular imbalance (hemiplegia, Friedrich’s ataxia, ambulatory Charcot-Marie-Tooth disease) often develop a more balanced curve pattern without pelvic involvement. These curve types behave more like an idiopathic scoliosis, and they should be treated following the same principles. In these groups of patients, in our practice the spinal fusion extends most commonly from the upper thoracic (T2 or T3) to the low lumbar region (L3 or L4), which preserves distal spinal flexibility as this can facilitate ambulation and day-to-day functional abilities.
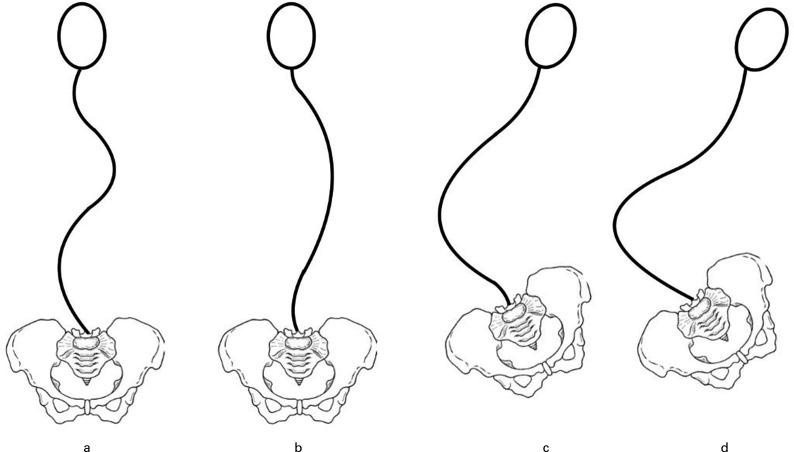
Lonstein and Akbarnia 3 described one of the most commonly used classification systems for patients with scoliosis due to cerebral palsy or intellectual disability (Figure 2). Group 1 includes curve types with a compensated trunk; 1 A involves a balanced double curve pattern; and 1B presents as a major thoracic curve with a small fractional lumbar curve. Group 2 includes patients with a decompensated trunk and associated pelvic obliquity.
Fig. 2.
Classification of scoliosis in patients with cerebral palsy. a) Group 1 curves: trunk compensated and presenting as well-balanced double curve. b) Group 1 curves: trunk compensated and presenting as major thoracic curve with small fractional lumbar curve. c) Group 2 curves: decompensated trunk with pelvic obliquity and a small fractional curve between the caudal end of the main curve and the sacrum. d) Group 2 curves: decompensated trunk with pelvic obliquity and the main curve extending distally to include the sacrum.
Thoracic/thoracolumbar hyperkyphosis
Deformity affecting the sagittal plane is less common but it is also intimately related to pelvic position and trunk control. In children with total body cerebral palsy and other conditions associated with poor muscle tone, hamstring contractures can lead to retroversion of the pelvis and loss of lumbar lordosis. When this occurs, the lack of muscle tone in the trunk leads to loss of the normal compensatory mechanisms for maintaining sagittal balance and a developing increased kyphosis. The clinical consequences of this sagittal deformity include restriction of lung function due to reduced thoracic height, pain due to muscle fatigue attempting to maintain a forward gaze, difficulty with interpersonal interactions when unable to preserve a forward gaze, and pressure on the soft tissues over the apex of the curve.
In the majority of children, increased thoracic kyphosis with the apex in the thoracolumbar junction develops alongside a typical collapsing scoliosis as a paired deformity. Iatrogenic hyperkyphosis can also occur secondary to multi-level laminectomies in patients undergoing selective dorsal rhizotomies.
Lumbar hyperlordosis
Hyperlordosis of the lumbar spine is much less common, and poses a greater surgical challenge. 4 This can be considered the consequence of early pelvic imbalance during growth, with pelvic anteversion secondary to hip flexor contractures. The increased pelvic flexion predisposes to spondylolysis and spondylolisthesis in ambulatory patients. Due to marked anteversion of the pelvis, there is resultant hyperlordosis and in the presence of weak trunk muscles the normal compensatory mechanisms for maintaining sagittal balance are lost. An overall negative global sagittal balance develops with the head positioned posterior to the pelvis resulting in an uncomfortable seating position that is poorly tolerated and often causes persistent pain.
Specific neuromuscular conditions
Cerebral palsy
Cerebral palsy is a static encephalopathy that occurs in the first two years of life, and can lead to neurological deficits including motor dysfunction. Multiple subtypes are recognized, and ambulatory impairment is best classified using the Gross Motor Function Classification System (GMFCS) (Table I). 5 Reported rates of scoliosis in children with cerebral palsy range from 15% to 90% depending on GMFCS level, patient age, and functional status. 1,6,7
Table I.
Gross Motor Function Classification System (GMFCS). 5
| Level I |
|---|
|
| Level II |
|
| Level III |
|
| Level IV |
|
| Level V |
|
There is association between the incidence and severity of scoliosis and the GMFCS level. Persson-Bunke et al 8 found no relationship between subtype of cerebral palsy and the incidence or severity of scoliosis, but noted that curves over 40o were more likely to occur with GMFCS levels IV and V. Once a curve reaches 40o, progression is likely to be around 2o per month regardless of whether a spinal orthosis is used. 9 Female sex, presence of intractable epilepsy, and history of previous hip surgery are also independent risk factors for development of a severe curve. 10 Patients with total body cerebral palsy are more likely to develop a long collapsing C-shaped curve, hypotonic patients are more at risk of developing hyperkyphosis, and patients with dystonia or athetosis are less likely to develop severe curve patterns.
Myelomeningocele
The treatment of spinal deformity in the presence of myelomeningocele is a complex surgical challenge due to a mixture of a severe multiplanar spinal deformity, absent posterior vertebral elements, often congenital vertebral defects, abnormal anatomy of the pedicles, and subcutaneous dura. In addition, there may be a number of co-existing medical conditions, including hydrocephalus, Chiari malformation, tethered cord, thoracic insufficiency, neurogenic bowel and bladder, lower limb contractures, and insensate skin below the level of the myelomeningocele. These factors all make bracing incredibly challenging if there is evidence of curve progression.
The problem of insensate skin is particularly difficult to manage as the development of pelvic obliquity may alter the pressure distribution across the adjacent soft tissues and lead to pressure sores on insensate areas. The apex of the curve is also prone to skin breakdown. Early involvement of plastic surgery colleagues is recommended, especially if surgical correction is planned. Anterior surgery to treat thoracolumbar/lumbar scoliosis without major associated pelvic obliquity is a valid surgical strategy, and the preferred option for the authors in order to avoid accessing the spine through the area of neural tube closure across the back; this will allow better wound healing and reduce significantly the risk of a wound infection. 11
Kyphectomy over two to three levels followed by posterior stabilization is required to treat a sharp, angular lumbar kyphosis, which is typical in this condition, in order to remove the acute gibbus and relieve pressure to the overlying skin or address already existing pressure sores. Use of low-profile implants to stabilize the spine following a kyphectomy, and often mobilization of local flaps for skin closure over the deformity apex, is required. The authors’ preference is to avoid extending distally the fusion to the sacrum/pelvis when possible in order to preserve lumbosacral flexibility which allows easier propelling of the wheelchair. In very young children, where instrumentation cannot be used to stabilize the spine, autologous fibular strut grafts can be harvested during the procedure and placed across the levels of the kyphectomy, followed by immobilization in a hip spica cast with spinal extension to include the thoracic region, in order to maintain deformity correction and achieve a solid fusion.
Duchenne muscular dystrophy
Duchenne muscular dystrophy is an X-linked recessive disorder where there is a mutation in the gene coding for dystrophin. Dystrophin normally acts to stabilize the sarcolemma and when non-functional, mechanical stress leads to necrosis of muscle fibres and local inflammation, lipomatosis and progressive muscle fibrosis. There is deteriorating muscle weakness and ultimately limited life expectancy due to cardiomyopathy and respiratory decline. Diagnosis of the condition is usually made between two to three years of age and the development of scoliosis is much more common once patients lose their ambulatory function and become wheelchair dependent.
In the last 20 years, the use of long-term glucocorticoids has been shown to slow the decline in muscle strength, and this has significantly reduced the need for scoliosis surgery in these patients, with an 80% reduction in the chance of developing scoliosis by age 20 years. 12 Bracing has not been shown to prevent curve progression in this group of children. 13 Traditionally, surgery has been recommended for small curves at the stage of wheelchair use due to the high risk of scoliosis deterioration. In the advent of long-term corticosteroids, it is now more likely to wait until there is documented progression of the deformity and development of symptoms before considering spinal surgery, and this is the authors’ practice. 14
Spinal muscular atrophy
Spinal muscle atrophy (SMA) is an autosomal recessive neurodegenerative disorder that usually manifests with hypotonia and generalized weakness. Presentation can be variable depending on the subtype and can be in early life or as an adult. Four subtypes exist:
SMA type 1 (Werdnig-Hoffman disease): neonatal presentation with poor swallowing and poor head control. Not able to sit independently.
SMA type 2: presentation between seven to 18 months with delayed milestones but able to sit independently.
SMA type 3 (Kugelberg-Welander disease): presentation after 18 months and usually ambulatory with less severe respiratory disease.
SMA type 4: adult onset often in the second or third decade of life. Mild motor impairment and do not typically suffer from respiratory disease.
Almost all children with severe SMA (types 1 and 2) develop scoliosis or kyphoscoliosis. In addition, children with SMA types 1 and 2 develop contractures of the upper and lower limbs. Collapsing C-shaped curves with pelvic obliquity is the most common pattern of deformity while less severe compensated curve types (without pelvic obliquity) develop more frequently in children with good sitting balance. 15 In children who are ambulatory, the incidence of scoliosis is lower, the age of onset is older (around ten years), and curves tend to be less severe. 16
Other neuromuscular conditions
There are numerous other neuromuscular conditions that can lead to the development of scoliosis. In children who suffer a spinal cord injury before the age of ten years, the incidence of scoliosis is almost 100% compared to 67% if the injury occurs before skeletal maturity. 17 There is no clear treatment benefit of bracing for paralytic scoliosis, and almost two thirds of patients who sustain a cord injury before maturity will require a spinal fusion for progressive scoliosis. 18,19
Rett’s syndrome, Friedreich’s ataxia, Charcot-Marie-Tooth, and arthrogryposis multiplex congenita are four neuromuscular conditions that frequently lead to a spinal deformity, and where the literature regarding treatment is sparse. These patients should be subject to a careful work up and consideration of surgical treatment where curves progress and affect patient function. A multidisciplinary approach is recommended, with regular medical care and spinal monitoring during stages of skeletal growth to detect scoliosis progression that may require treatment. Several disease specific treatment strategies have been suggested based on previous case series, but none has shown a clear clinical advantage. 20-22
Treatment options
Children with neuromuscular scoliosis present the unique challenge of a patient with multiple medical comorbidities and complex care requirements who needs major deformity correction that carries high anaesthetic and surgical risks. A multidisciplinary approach to care is therefore required, and treatment should be tailored to the individual needs of the patients and their families. Combined review with the paediatricians and neurologists involved in their care is advantageous and careful planning with anaesthetic and critical care colleagues before surgery is mandatory. Joint meetings with paediatric orthopaedic colleagues will help plan the order of any required surgical interventions.
Observation
Postural changes in the shape of a child’s back are often identified early in life and can be observed by the treating doctors or physiotherapists. This normally forms part of a six-monthly to yearly review of care to avoid repeat hospital appointments. When the curve seems to progress early, assessment with x-rays and a referral to the spinal service is recommended. In some conditions, such as Duchenne muscular dystrophy or Friedreich’s ataxia, patients develop a cardiomyopathy and there will be a point when surgery becomes unsafe. In these patients, the timing of scoliosis correction is critical so that the risks of surgery are minimized. This principle applies also for patients with severe respiratory compromise due to underlying progressive muscle weakness. The advent of non-invasive ventilation and meticulous pulmonary management has allowed children with limited respiratory reserve and progressive spinal deformities to become surgical candidates.
Bracing
There is no clear evidence to support the use of bracing to prevent the need for surgery in neuromuscular scoliosis. Bracing can be applied to improve sitting balance and posture, especially when the patients are not using their wheelchair which has built-in seating adaptations. Anecdotally, use of an underarm brace may delay progression of the curve in less severely affected children, but this is not a consistent and predictable result. Bracing appears to be more successful in ambulatory patients but, even then, the ability to prevent scoliosis progression is limited, and the final treatment outcome is unchanged. 23 Application of a brace can delay the age at which surgery is required when used following a spinal cord injury. 18 When bracing is considered for postural support, we use an underarm bi-valve or a full contact soft plastazote brace, which are more appropriate compared to a rigid moulded thoraco-lumbo-sacral orthosis commonly applied in children with idiopathic scoliosis.
Wheelchair modification
In patients who use a wheelchair to mobilize, there is a continuous need to adapt and optimize their wheelchair as their spine grows. This includes using moulded seat inserts, chest lateral supports, shoulder or waist harnesses and straps, foot and neck supports, and a pad between the thighs to prevent hip adduction/abduction deformity. The seating adaptations will help with comfort and improve sitting tolerance, but there is no evidence that these can affect curve progression or reduce the need for surgery. Many chairs have the ability to tilt backwards so that the patients can recline and stretch when they have been seated for a prolonged period (for example, during school time); this is a very useful wheelchair function in children with hyperkyphosis and poor neck control.
Indications for surgery
The indications to proceed with surgery are multi-faceted, and must involve a shared decision-making approach with the patients and their families. Surgery should be considered when there is significant impact on the patients’ quality of life, and this is usually due to poor sitting balance, back or costo-pelvic pain, respiratory complications, or problems with self-care and feeding. Most deformities will progress once the curve deteriorates beyond 40o, while surgery for curves greater than 90° carries an increased risk of complications, and can result in suboptimal deformity correction. Discussions on the need for spinal correction should occur early during treatment, and once a decision has been made, preoperative optimization and prompt surgery is required.
Surgical techniques
Early onset neuromuscular scoliosis (before the age of ten years) can be treated using growth modulation techniques. However, the outcomes are unpredictable and growth preservation surgery is associated with a high rate of complications, increased morbidity, and reoperation rates in patients where repeat surgeries are generally contraindicated due to associated medical comorbidities. In our opinion, these techniques are more suited to early onset scoliosis (EOS) in non-ambulatory children with hypotonia due to myopathic conditions in order to control the deformity, and delay the need for definitive fusion while preserving growth of the spine and chest development.
Growth preserving techniques
The available techniques include traditional or magnetically controlled growing rods, the modern self-growing Luque trolley, 24 and the Shilla technique. 25 The Shilla technique is based on the original Luque trolley system, 26 where segmental fixation using sublaminar wires allowed correction onto two relatively soft rods and these fixation points could move along the rods as the spine grew. The Shilla technique involves an instrumented apical fusion and non-locking polyaxial screws proximally and distally, with the rods left long to accommodate longitudinal growth of the spine. When compared to growing rods, the Shilla technique requires fewer reoperations but has achieved reduced spinal growth (T1 to S1 length) and lesser scoliosis correction with higher complication rates. 27 Therefore, its use has become limited to selective cases which need apical control. The modern Luque trolley technique with proximal and distal pedicle screw fixation seems to be more effective in non-ambulatory myopathic conditions as opposed to idiopathic or cerebral palsy EOS where a high rate of rod failure was observed occurring at the thoracolumbar junction. 24
Miladi et al 28 introduced a fusionless bipolar system consisting of proximal upper thoracic spinal and distal pelvic anchors attached to rods and parallel connectors. This technique uses specific ilio-sacral screws distally, as well as supralaminar and pedicle hooks proximally. Repeat lengthenings can be performed, but the instrumentation can also be left as a definitive construct in low physical demand, wheelchair-bound patients. The authors reported 100 patients (aged 5 to 21 years) with neuromuscular scoliosis, and recorded mean scoliosis correction from 89° to 33° and correction of pelvic obliquity of 17°. In all, 72 patients underwent one to two lengthening procedures during growth, while the remaining 28 required no lengthenings. There was a high rate of complications that occurred in 26 out of the 100 patients that participated in this study.
Definitive spinal fusion
In our opinion, it is preferable, if possible, to avoid the need for repetitive procedures at a young age while the patient remains under close monitoring, delay scoliosis surgery, and plan a definitive spinal fusion close to puberty and the adolescent growth spurt which, in children with severe neurodisability, often comes at an older age.
Definitive correction is usually performed through a posterior only approach, and this can be achieved with a number of different techniques. The Galveston technique used paired Luque rods anchored to the ilium to provide spino-pelvic fixation. 29 The rods were soft and small diameter that could move independently in the coronal plane, which resulted in high rates of implant failure and nonunion with subsequent loss of deformity correction.
Greater stability was achieved by joining the rods at the top to form an inverted U in a sagittally pre-contoured single rod that became known as the Unit rod technique. 30 This allowed segmental correction using sublaminar wires with stable fixation to the pelvis and the ability to restore coronal and sagittal balance, as well as pelvic obliquity. Tsirikos et al 31 in a review of 287 children and adolescents treated using the Unit rod technique reported an excellent scoliosis correction of 68% and correction of pelvic obliquity of 71%. The Unit rod technique produced levelling of the pelvis, but had a number of limitations. It relied on a cantilever technique using the pelvis as a base with no ability to compress across the posterior elements that could additionally control existing hyperkyphosis. There was also risk of increased blood loss and neurological injury during the laminotomies and placement of segmental sublaminar wires. We find insertion of the pelvic legs of the Unit rod technically very challenging in the presence of severe lumbar hyperlordosis with associated marked anterior pelvic tilt, in which case the Unit rod often has to be cut and its proximal and distal ends cross-connected in the upper lumbar spine to finalize the construct.
The rise in popularity of pedicle screw constructs meant that these were soon considered a viable alternative to the Unit rod technique. These could combine the standard cantilever technique with the added ability to effect compression, translation, and derotation during deformity correction (Figure 1). Modular systems allowed the use of pedicle screws, hooks, and wires to achieve a safe correction and are now the standard instrumentation techniques used for neuromuscular deformity. Tsirikos and Mains 32 reviewed 45 consecutive patients with severe cerebral palsy undergoing deformity surgery using a segmental pedicle screw technique, and reported mean scoliosis correction of 74% and correction of pelvic obliquity of 83% with low rates of complications.
Balancing the spine over a level pelvis is essential to achieve global trunk balance and spino-pelvic alignment which will facilitate postural wheelchair support and increase seating tolerance. In our opinion, the presence of severe pelvic obliquity producing a rigid spinopelvic imbalance requires extension of the instrumented fusion to include the sacrum and pelvis, especially in skeletally immature patients. This has been shown to not compromise ambulatory function in patients with cerebral palsy as long as the sagittal spinal balance is restored. 33 Pelvic fixation can be achieved with bilateral iliac screws that are connected to the distal end of the spinal rods or by using the S2-alar-iliac screw fixation technique that allows a lower profile of the screw and rod interface, and is preferable when operating on markedly underweight children. However, if the pelvic obliquity is not severe and mostly corrects in preoperative supine traction radiographs in adolescent patients, when performing the instrumented fusion we elect to spare the lumbosacral junction. This preserves distal spinal flexibility, reduces surgical time, and blood loss, and avoids the risk of implant prominence, which occasionally occurs when iliac screws have been used (Figure 3).
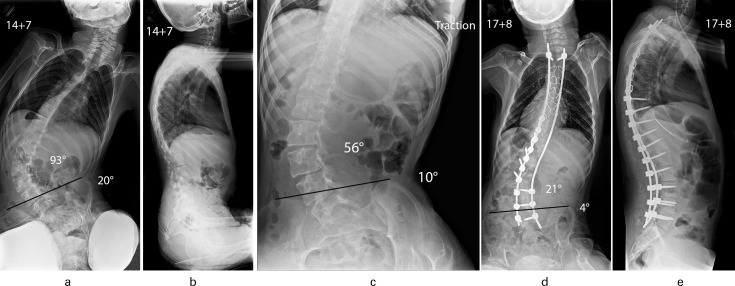
Fig. 3.
Patient aged 14 years and seven months with quadriplegic cerebral palsy. a) Collapsing lumbar scoliosis (93o) with associated pelvic obliquity (20o). b) Increased thoracic kyphosis with elimination of normal lumbar lordosis and positive global sagittal balance of the spine. c) Supine traction radiograph shows the scoliosis to improve to 56o and the pelvic obliquity to retain flexibility and correct to 10o. d) Excellent correction of scoliosis to 21o and marked improvement of pelvic obliquity to 4o was maintained at follow-up (aged 17 years and eight months) after a posterior spinal fusion using a hybrid segmental pedicle screw/sublaminar wire/rod construct extending distally to L5; E: Spinal surgery restored thoracic kyphosis/lumbar lordosis and balanced the spine.
Correction of severe deformities is possible using modern segmental posterior instrumentation. Where there is a severe and stiff deformity, posterior based osteotomies can increase spinal flexibility and facilitate curve correction. In the rare case of an extreme and rigid deformity, three column osteotomies, or anterior surgery if the patient can medically tolerate it, may be required. Both approaches carry significant risks for major morbidity and potential mortality, and so all efforts should be taken to make the surgery as safe as possible. This includes staging anterior and posterior procedures in two anaesthetics three to five days apart, which is our preference, 34 using two spinal surgeons operating together to reduce surgical time (especially if a posterior vertebral column resection is performed), or presence of an approach surgeon if the spinal surgeon is not familiar with the anterior approach. Anterior-only procedures are most useful in patients with poor posterior soft-tissue coverage, such as when treating spinal deformity secondary to myelomeningocele. 11
We use spinal cord monitoring (IOM) in ambulatory patients recording multimodal somatosensory and motor evoked potentials. We also monitor continent patients with myopathic conditions recording cervical SSEPs. Muscle relaxant is used to facilitate spinal exposure. Hypotensive anaesthesia, tranexamic acid, and cell salvage along with a meticulous surgical technique are essential to reduce intraoperative blood loss.
Outcomes
Surgical correction of neuromuscular spinal deformity is associated with much higher rates of complications than idiopathic scoliosis. 35 Careful preoperative workup and planning is required to minimize this risk. Even with meticulous preparation, there is still approximately 1% in-hospital mortality, 30 and major complication rates as high as 63%. 36 The most commonly encountered postoperative complications include surgical site infection, pseudarthrosis, prominent instrumentation causing skin irritation or breakdown, and medical complications related to the underlying neurological condition. The rate of neurological injury is low and has been reported to be between 0.003% to 1.03%. 30,36 Ongoing research and a multidisciplinary approach to the perioperative management of these patients have improved the safety of the procedure, and this has widened the indications for scoliosis surgery in children with severe underlying neurodisability.
The long-term outcomes of surgery for neuromuscular spinal deformity are difficult to quantify. Survival following spinal deformity correction has been reported as being greater than 11 years in 70% of patients with total body cerebral palsy. 37 It is, therefore, vital to make sure a good clinical outcome is achieved. Health-related quality of life (QoL) measures are difficult to collect and very challenging to interpret as many patients are cognitively unable to respond. Despite high complication rates, parents and caregivers report high satisfaction rates following surgery, with improvement in sitting balance and overall QoL. 11,38 Consistent collection of patient and caregiver reported outcomes through the use of national or international spinal registries is likely to help validate some of the available health-related QoL outcome measures, and guide decision-making to optimize provision of care and maximize QoL for these groups of patients.
In conclusion, spinal deformity due to neuromuscular disease is difficult to treat because it encompasses a wide range of conditions with numerous associated comorbidities that must be assessed before and during treatment. A multidisciplinary approach is required throughout patient management to make sure that all nonoperative measures have been considered, and that the goals of treatment have been clearly defined. This will necessitate input from multiple medical specialities, including allied healthcare professionals, such as physiotherapists and wheelchair services. Surgery is indicated when the deformity has a significant impact on the patients’ ability to perform on a daily basis and their Qol, as well as affects nursing care. A meticulous preoperative assessment is recommended to balance the risks and benefits of surgery over pre-existing medical comorbidities that can severely compromise surgical outcomes. Careful preoperative planning is greatly advised, but surgery can lead to excellent deformity correction and high levels of satisfaction reported from the patients and their carers. Modern posterior instrumentation techniques allow an effective curve correction; however, the risks of surgery remain high, and so involvement of the family at all stages of decision-making is mandatory to achieve optimum results and meet realistic expectations.
Take home message
- Spinal deformity is a common and severe musculoskeletal condition affecting children with neurological or myopathic disorders.
- Surgical correction is indicated for progressive deformities, which produce functional
- limitations and severe symptoms in growing children.
- Deformity correction and spinal fusion should only be performed in major spinal centres with adequate medical support by well-trained surgeons who are familiar with the surgical techniques in order to minimize complications and produce optimum outcomes.
- The selection of operative technique and instrumentation type depends on the experience of individual surgeons, and aims to achieve adequate deformity correction
- and a balanced spine while reducing complication rates.
Footnotes
Author contributions: P. R. Loughenbury: Data curation, Investigation, Writing – original draft.
A. I. Tsirikos: Data curation, Investigation, Writing – review & editing.
Funding statement: The authors received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement: The authors confirm that they have no conflicts of interest.
Open access funding: The authors confirm that the open access funding for this manuscript was self-funded.
Contributor Information
Peter R. Loughenbury, Email: prloughenbury@hotmail.com, prloughenbury@gmail.com.
Athanasios I. Tsirikos, Email: atsirikos@hotmail.com.
References
- 1. Madigan RR, Wallace SL. Scoliosis in the institutionalized cerebral palsy population. Spine. 1981;6(6):583–590. [DOI] [PubMed] [Google Scholar]
- 2. Letts M, Shapiro L, Mulder K, Klassen O. The windblown hip syndrome in total body cerebral palsy. J Pediatr Orthop. 1984;4(1):55–62. [DOI] [PubMed] [Google Scholar]
- 3. Lonstein JE, Akbarnia A. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation. An analysis of one hundred and seven cases. J Bone Joint Surg Am. 1983;65-A(1):43–55. [PubMed] [Google Scholar]
- 4. Karampalis C, Tsirikos AI. The surgical treatment of lordoscoliosis and hyperlordosis in patients with quadriplegic cerebral palsy. Bone Joint J. 2014;96-B(6):800–806. [DOI] [PubMed] [Google Scholar]
- 5. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 2008;39(4):214–223. [DOI] [PubMed] [Google Scholar]
- 6. Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351(9117):1687–1692. [DOI] [PubMed] [Google Scholar]
- 7. McCarthy JJ, D’Andrea LP, Betz RR, Clements DH. Scoliosis in the child with cerebral palsy. J Am Acad Orthop Surg. 2006;14(6):367–375. [DOI] [PubMed] [Google Scholar]
- 8. Persson-Bunke M, Hägglund G, Lauge-Pedersen H, Ma PW, Westbom L. Scoliosis in a total population of children with cerebral palsy. Spine. 2012;37(12):E708–E713. [DOI] [PubMed] [Google Scholar]
- 9. Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16(3):332–335. [DOI] [PubMed] [Google Scholar]
- 10. Bertoncelli CM, Solla F, Loughenbury PR, Tsirikos AI, Bertoncelli D, Rampal V. Risk factors for developing scoliosis in cerebral palsy: a cross-sectional descriptive study. J Child Neurol. 2017;32(7):657–662. [DOI] [PubMed] [Google Scholar]
- 11. Tsirikos AI, Wordie SJ. The surgical treatment of spinal deformity in children with non-ambulatory myelomeningocele. Bone Joint J. 2021;103-B(6):1133–1141. [DOI] [PubMed] [Google Scholar]
- 12. Alman BA, Raza SN, Biggar WD. Steroid treatment and the development of scoliosis in males with duchenne muscular dystrophy. J Bone Joint Surg Am. 2004;86-A(3):519–524. [DOI] [PubMed] [Google Scholar]
- 13. Colbert AP, Craig C. Scoliosis management in Duchenne muscular dystrophy: prospective study of modified Jewett hyperextension brace. Arch Phys Med Rehabil. 1987;68(5 Pt 1):302–304. [PubMed] [Google Scholar]
- 14. Cervellati S, Bettini N, Moscato M, Gusella A, Dema E, Maresi R. Surgical treatment of spinal deformities in Duchenne muscular dystrophy: a long term follow-up study. Eur Spine J. 2004;13(5):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujak A, Raab W, Schuh A, Richter S, Forst R, Forst J. Natural course of scoliosis in proximal spinal muscular atrophy type II and IIIa: descriptive clinical study with retrospective data collection of 126 patients. BMC Musculoskelet Disord. 2013;14(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granata C, Merlini L, Magni E, Marini ML, Stagni SB. Spinal muscular atrophy: natural history and orthopaedic treatment of scoliosis. Spine. 1989;14(7):760–762. [DOI] [PubMed] [Google Scholar]
- 17. Dearolf WW, Betz RR, Vogel LC, Levin J, Clancy M, Steel HH. Scoliosis in pediatric spinal cord-injured patients. J Pediatr Orthop. 1990;10(2):214–218. [PubMed] [Google Scholar]
- 18. Mehta S, Betz RR, Mulcahey MJ, McDonald C, Vogel LC, Anderson C. Effect of Bracing on Paralytic Scoliosis Secondary to Spinal Cord Injury. J Spinal Cord Med. 2016;27(sup1):S88–S92. [DOI] [PubMed] [Google Scholar]
- 19. Tsirikos AI, Markham P, McMaster MJ. Surgical correction of spinal deformities following spinal cord injury occurring in childhood. J Surg Orthop Adv. 2007;16(4):174–186. [PubMed] [Google Scholar]
- 20. Larsson E-L, Aaro S, Ahlinder P, Normelli H, Tropp H, Öberg B. Long-term follow-up of functioning after spinal surgery in patients with Rett syndrome. Eur Spine J. 2009;18(4):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker JL, Nelson KR, Stevens DB, Lubicky JP, Ogden JA, VandenBrink KD. Spinal deformity in Charcot-Marie-Tooth disease. Spine. 1994;19(9):1044–1047. [DOI] [PubMed] [Google Scholar]
- 22. Tsirikos AI, Smith G. Scoliosis in patients with Friedreich’s ataxia. J Bone Joint Surg Br. 2012;94-B(5):684–689. [DOI] [PubMed] [Google Scholar]
- 23. Olafsson Y, Saraste H, Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop. 1999;19(3):376–379. [PubMed] [Google Scholar]
- 24. Mehdian H, Haddad S, Pasku D, Nasto LA. Mid-term results of a modified self-growing rod technique for the treatment of early-onset scoliosis. Bone Joint J. 2020;102-B(11):1560–1566. [DOI] [PubMed] [Google Scholar]
- 25. McCarthy RE, Sucato D, Turner JL, Zhang H, Henson MAW, McCarthy K. Shilla growing rods in a caprine animal model: A pilot study. Clin Orthop Relat Res. 2010;468(3):705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luque ER. Paralytic scoliosis in growing children. Clin Orthop Relat Res. 1982;163(NA):202. [PubMed] [Google Scholar]
- 27. Andras LM, Joiner ERA, McCarthy RE, et al. Growing rods versus shilla growth guidance: Better Cobb angle orrection and T1-S1 length increase but more surgeries. Spine Deform. 2015;3(3):246–252. [DOI] [PubMed] [Google Scholar]
- 28. Miladi L, Gaume M, Khouri N, Johnson M, Topouchian V, Glorion C. Minimally invasive surgery for neuromuscular scoliosis. Results and complications in a series of one hundred patients. Spine. 2018;43(16):E968–E975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen BL Jr, Ferguson RL. The Galveston technique of pelvic fixation with L-rod instrumentation of the spine. Spine. 1984;9(4):388–394. [DOI] [PubMed] [Google Scholar]
- 30. Bell DF, Moseley CF, Koreska J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine. 1989;14(12):1301–1307. [DOI] [PubMed] [Google Scholar]
- 31. Tsirikos AI, Lipton G, Chang WN, Dabney KW, Miller F. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the Unit rod instrumentation. Spine. 2008;33(10):1133–1140. [DOI] [PubMed] [Google Scholar]
- 32. Tsirikos AI, Mains E. Surgical correction of spinal deformity in patients with cerebral palsy using pedicle screw instrumentation. J Spinal Disord Tech. 2012;25(7):401–408. [DOI] [PubMed] [Google Scholar]
- 33. Tsirikos AI, Chang WN, Shah SA, Dabney KW, Miller F. Preserving ambulatory potential in pediatric patients with cerebral palsy who undergo spinal fusion using unit rod instrumentation. Spine. 2003;28(5):480–483. [DOI] [PubMed] [Google Scholar]
- 34. Tsirikos AI, Chang WN, Dabney KW, Miller F. Comparison of one-stage versus two-stage anteroposterior spinal fusion in pediatric patients with cerebral palsy and neuromuscular scoliosis. Spine. 2003;28(12):1300–1305. [DOI] [PubMed] [Google Scholar]
- 35. Duckworth AD, Mitchell MJ, Tsirikos AI. Rate and risk factors for postoperative complications in patients with Duchenne Muscular Dystrophy undergoing scoliosis surgery: a comparison to other neuromuscular conditions. Bone Joint J. 2014;96-B(7):943. [DOI] [PubMed] [Google Scholar]
- 36. Reames DL, Smith JS, Fu K-M, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the scoliosis research society morbidity and mortality database. Spine. 2011;36(18):1484–1491. [DOI] [PubMed] [Google Scholar]
- 37. Tsirikos AI, Chang WN, Dabney KW, Miller F, Glutting J. Life expectancy in pediatric patients with cerebral palsy and neuromuscular scoliosis who underwent spinal fusion. Dev Med Child Neurol. 2003;45(10):677–682. [DOI] [PubMed] [Google Scholar]
- 38. Tsirikos AI, Chang WN, Dabney KW, Miller F. Comparison of parents’ and caregivers’ satisfaction after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 2004;24(1):54–58. [DOI] [PubMed] [Google Scholar]