Abstract
In 1996, Serratia marcescens KU3838 was isolated from the urine of a patient with a urinary tract infection at a hospital in northern Japan and was found to contain the plasmid pKU501. Previously, we determined that pKU501 carries blaIMP and the genes for TEM-1-type β-lactamases as well as producing both types of β-lactamases (H. Yano, A. Kuga, K. Irinoda, R. Okamoto, T. Kobayashi, and M. Inoue, J. Antibiot. 52:1135–1139, 1999). pKU502 is a recombinant plasmid that contains a 1.5-kb DNA fragment, including the metallo-β-lactamase gene, and is obtained by PCR amplification of pKU501. The sequence of the metallo-β-lactamase gene in pKU502 was determined and revealed that this metallo-β-lactamase gene differed from the gene encoding IMP-1 by one point mutation, leading to one amino acid substitution: 640-A in the base sequence of the IMP-1 gene was replaced by G, and Ser-196 was replaced by Gly in the mature enzyme. This enzyme was designated IMP-6. The strains that produced IMP-6 were resistant to carbapenems. The MICs of panipenem and especially meropenem were higher than the MIC of imipenem for these strains. The kcat/Km value of IMP-6 was about sevenfold higher against meropenem than against imipenem, although the MIC of meropenem for KU1917, which produced IMP-1, was lower than that of imipenem, and the MIC of panipenem was equal to that of imipenem. These results support the hypothesis that IMP-6 has extended substrate profiles against carbapenems. However, the activity of IMP-6 was very low against penicillin G and piperacillin. These results suggest that IMP-6 acquired high activity against carbapenems, especially meropenem, via the point mutation but in the process lost activity against penicillins. Although IMP-6 has reduced activity against penicillins due to this point mutation, pKU501 confers resistance to a variety of antimicrobial agents because it also produces TEM-1-type enzyme.
Carbapenems such as imipenem, panipenem, and meropenem are the most potent agents for treatment of gram-negative infections due to the stability of these agents against the majority of β-lactamases and their high rate of permeation through bacterial outer membranes. In addition, imipenem, panipenem, and meropenem have a high affinity for penicillin-binding protein (PBP) 2 of gram-negative bacteria except for Pseudomonas aeruginosa. In P. aeruginosa, imipenem and panipenem have a high affinity for PBP 2 and meropenem has an affinity for PBP 3 (6, 19, 40).
However, some clinical isolates have been reported to be resistant to carbapenems due to production of metallo-β-lactamases. There have been increasing reports, especially from Japan (7, 14, 21, 27, 35), of gram-negative bacteria that carry the transferable carbapenem resistance gene blaIMP, including isolates of P. aeruginosa and Serratia marcescens. The metallo-β-lactamases are class B enzymes in Ambler's molecular classification (1) and belong to Bush group 3 (3, 31). Most of these metallo-β-lactamases confer resistance not only to carbapenems but also to other β-lactams and are poorly inhibited by the presence of β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam (31). Therefore, there is a possibility that therapeutic failures occur in patients infected with the strains that produce metallo-β-lactamase, and the spread of these strains has resulted in serious medical problems in hospitals.
Previously, we described pKU501 as a novel plasmid carrying not only blaIMP but also the genes for TEM-1-type β-lactamases as well as producing both types of β-lactamases (41). A strain carrying pKU501 was more resistant to meropenem and panipenem, especially meropenem, than to imipenem. In this report, we describe the metallo-β-lactamase encoded by pKU501 that hydrolyzes carbapenems, especially meropenem.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. In 1996, Serratia marcescens KU3838 was isolated from the urine of a patient with a urinary tract infection at a hospital in northern Japan (41) and was found to contain plasmid pKU501. Escherichia coli K12 ML4901 (18) was used as the recipient for plasmids. E. coli KU3999 is a transconjugant that contains the conjugative plasmid pKU501 from S. marcescens KU3838 to E. coli K12 ML4901. Plasmids pHSG398 (37) and pBluescript (36) are vector plasmids that confer resistance to chloramphenicol and tetracycline-ampicillin, respectively. E. coli KU1917 is a transformant that contains pMS361 (16), which carries the 4.1-kb fragment encoding IMP-1 on the multicloning region of pHSG398.
TABLE 1.
Bacterial strains and plasmids used in this study
| Designation | Relevant markersa | Source, derivation, and/or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli K12 | ||
| ML4901 | F−, galK2 galT22 hsdR lacY1 metB1 relA supE44 NA25 | NA mutant from χ1037 (18) |
| KU3999 | ML4901 carrying pKU501, which produces TEM-1 and IMP-6 β-lactamases | Conjugation from KU3838 |
| KU4866 | ML4901 carrying pKU502, which produces TEM-1 and IMP-6 β-lactamases | Transferred pKU502 to ML4901 |
| KU4867 | ML4901 carrying pKU503, which produces IMP-6 β-lactamase | Transferred pKU503 to ML4901 |
| KU1917 | ML4901 carrying pMS361, which produces IMP-1 β-lactamase | Transferred pMS361 to ML4901 (16) |
| S. marcesens | ||
| KU3838 | Clinical isolates carrying pKU501 | This paper |
| Plasmids | ||
| pKU501 | Isolated from KU3838 | This paper |
| pKU502 | 1.5-kb fragment containing IMP-6 gene from pKU501 cloned into pBluescript | This paper |
| pKU503 | 1.5-kb fragment containing IMP-6 gene from pKU502 cloned into pHSG398 | This paper |
| pBluescript | Cloning vector, TETr, AMPr | 36 |
| pHSG398 | Cloning vector, CHLr | 37 |
| pMS361 | pHSG398 with insertion of 4.1-kb fragment of pMS354, a segregant plasmid derived from pMS350 | 16 |
Abbreviations: CHL, chloramphenicol; TET, tetracycline; AMP, ampicillin.
Antibacterial agents.
The antibacterial agents listed below were used in this study. Reference powders of known potency for the drugs were provided by the respective manufacturers. Penicillin G (Meiji Seika Kaisha., Ltd., Tokyo, Japan) and piperacillin (Toyama Chemical Co., Ltd., Toyama, Japan) were used as representative penicillins, while cephalothin (Shionogi and Co., Ltd., Osaka, Japan), cefotiam (Takeda Chemical Industries, Ltd., Osaka, Japan), cefmetazole (Sankyo Co., Ltd., Tokyo, Japan), cefotaxime (Nippon Hoechst Marion Roussel, Tokyo, Japan), and cefepime (Bristol-Myers Squibb K. K., Tokyo, Japan) were used as representative cephems. Other β-lactams, including imipenem (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan), panipenem (Sankyo Co.), meropenem (Sumitomo Pharmaceutical Ltd., Osaka, Japan), and aztreonam (Eizai Co., Ltd., Tokyo, Japan), as well as chloramphenicol (Sankyo Co.), tetracycline (Lederle Japan, Tokyo, Japan), streptomycin (Meiji Seika Kaisha), and nalidixic acid (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan) were also used. Clavulanic acid (SmithKline Beecham Pharmaceuticals, Tokyo, Japan) was used as a representative β-lactamase inhibitor.
Transconjugation.
Conjugation was done by broth method, as previously described (10). Exponential-phase L-broth (24) cultures of donor strain ML4901 and recipient strain KU3838 were mixed at a volume ratio of 1:10. This mating mixture was incubated for 2 h at 35°C. The transconjugants were selected on sensitivity disk agar (Nissui Pharmaceutical Co., Tokyo, Japan) containing 32 μg of nalidixic acid and cefotaxime per ml.
PCR amplification, cloning, and sequencing of the metallo-β-lactamase gene.
Isolation of plasmid DNA using the small-scale alkaline method (33) and gene amplification by PCR (29, 41) were performed as previously described. Primer pairs P1 (5′-CGG ATG AAG GCA CGA ACC CA-3′) and P2 (5′-AAG CAG ACT TGA CCT GAT AGT-3′) (TaKaRa Shuzo Co., Ltd. Kyoto, Japan), which were chosen on the basis of the published pMS350 integron and 3′-conserved region sequence (17), were used in the PCR experiments. pKU501 was used as a template DNA. The PCR experiments involved using a commercially available PCR kit (Gene Amp PCR reaction kit with Ampli Taq DNA polymerase; TaKaRa Shuzo Co.) and a DNA thermal cycler PH2000 (Perkin-Elmer Cetus Instruments, Emeryville, Calif.) and involved 25 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and elongation at 72°C for 1 min, followed by heating at 72°C for 30 min. The 1.5-kb DNA fragment obtained by PCR amplification of pKU501 was purified and cloned into the EcoRV site of pBluescript using the TA-cloning technique (9) with T4 DNA ligase (Nippon Gene, Tokyo, Japan). The recombinant DNAs were introduced into E. coli K12 ML4901 by electroporation with a Bio-Rad gene pulser and a pulse controller unit (Bio-Rad Laboratories, Hercules, Calif.).
The nucleotide sequence of both strands of four cloned amplicons, obtained from independent PCRs, was determined using the dideoxy chain termination method of Sanger et al. (34) with the ALFred DNA sequencer (Amersham Pharmacia Biotech, Tokyo, Japan) and the Thermo Sequenase fluorescence-labeled primer sequencing kit (Amersham Pharmacia Biotech). After we confirmed that these recombinant DNAs had identical sequences, this plasmid was designated pKU502. The transformant that contained the pKU502 for E. coli K12 ML4901 was designated KU4866.
In order to eliminate the ampicillin resistance gene in pBluescript, DNA from pKU502 was digested with SalI and EcoRI (TaKaRa Shuzo Co.). The fragments were then ligated into the SalI and EcoRI sites of pHSG398. This recombinant plasmid was designated pKU503. pKU503 was introduced into E. coli K12 ML4901 by electroporation, and the transformant was designated KU4867.
Drug susceptibility assay.
MICs were determined by the agar dilution method with sensitivity disk agar (Nissui Pharmaceutical Co.) in accordance with the specified drug sensitivity assay methods of the Japan Society of Chemotherapy, except for the antibiotic concentrations used (12).
β-Lactamase preparation and purification.
Purification of the metallo-β-lactamase was performed according to a previously reported method (26). E. coli KU4867 harboring plasmid pKU503 was cultured in Mueller-Hinton broth (Nissui Pharmaceutical Co.), diluted 100-fold into 5 liters of the same broth, and incubated overnight with shaking at 35°C. The cells were harvested by centrifugation at 6,000 × g for 10 min and suspended in 50 mM phosphate buffer (pH 7.0) with 10 μM ZnCl2. The cells were disrupted by sonication (model UD-201; Tomy Seiko, Tokyo, Japan), and the cellular debris was removed by centrifugation at 18,000 × g for 30 min at 4°C. Streptomycin (2% [wt/vol]) was added to the supernatant, and the mixture was agitated for 2 h at 4°C. After centrifugation at 18,000 × g for 30 min at 4°C, the supernatant was dialyzed against 5 mM phosphate buffer (pH 7.0) with 10 μM ZnCl2. The dialysate was centrifuged at 18,000 × g for 30 min at 4°C, and the supernatant then obtained was applied to a carboxymethyl cellulose CM52 column (Whatman Ltd., Kent, United Kingdom). The metalloenzyme was eluted in 50 mM phosphate buffer (pH 7.0) with 10 μM ZnCl2, and the active enzyme fractions were pooled and concentrated. The concentrated enzyme was applied to a Sephadex G-75 column (Amersham Pharmacia Biotech) and eluted with 5 mM phosphate buffer (pH 7.0)–10 μM ZnCl2. The active enzyme fractions were pooled and concentrated. The concentrated enzyme was applied to a Mono S column equipped with fast-protein-liquid-chromatography equipment (Amersham Pharmacia Biotech). The enzyme was eluted with a 0 to 0.5 M linear gradient of sodium chloride and detected by absorbance at 280 nm and by enzyme activity. The purity of the preparation was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (39), and final preparation showed a single band with more than 95% purity.
Assay of β-lactamase.
Enzyme activity was determined by spectrophotometry (UV2000; Shimazu Corp., Tokyo, Japan) at 30°C in 50 mM phosphate buffer (pH 7.0) with 10 μM ZnCl2 (41). One unit of enzyme activity was defined as the amount of enzyme hydrolyzing 1 μmol of substrate in 1 min at 30°C. Protein concentrations were determined by using the Bio-Rad protein assay (Bio-Rad Laboratories) as the standard.
Values for the Michaelis constant (Km) and the maximum rate of hydrolysis (Vmax) were determined from the direct fitting of the data to the Henri- Michaelis-Menten equation (4, 8) with eight different substrates at concentrations from 12.5 to 100 μM for cephalothin, cefotaxime, aztreonam, and meropenem and from 50 to 1,000 μM for penicillin G, piperacillin, imipenem, and panipenem. The turnover number (kcat) was derived by dividing the Vmax by the number of moles of the enzyme present. The specific constant (kcat/Km) was also calculated from these values.
When the Km value was less than 10 μM, it was measured as a Ki in a competition experiment with imipenem as the substrate at concentrations from 25 to 50 μM, after preincubation with a β-lactamase inhibitor at concentrations from 50 to 100 μM for 5 min, and data were analyzed using a Dixon plot (13).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases with the accession number AB040994.
RESULTS
Sequence of the metallo-β-lactamase gene.
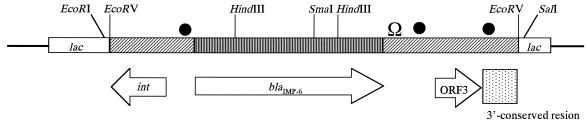
A schematic representation of the structure of the metallo-β-lactamase gene and its flanking regions in pKU502 is shown in Fig. 1. The putative integrase gene and a gene of unknown function were identified in the upstream and downstream regions of the metallo-β-lactamase gene, respectively.
FIG. 1.
Schematic representation of the structure of IMP-6 and its flanking regions in pKU502. The 1,523-bp fragment encoding IMP-6 was ligated into the EcoRV site of pBluescript. Open reading frames (ORFs) are indicated by arrows. ●, position of the sequences similar to the GTTRRRY sequence; Ω, position of the inverted repeat.
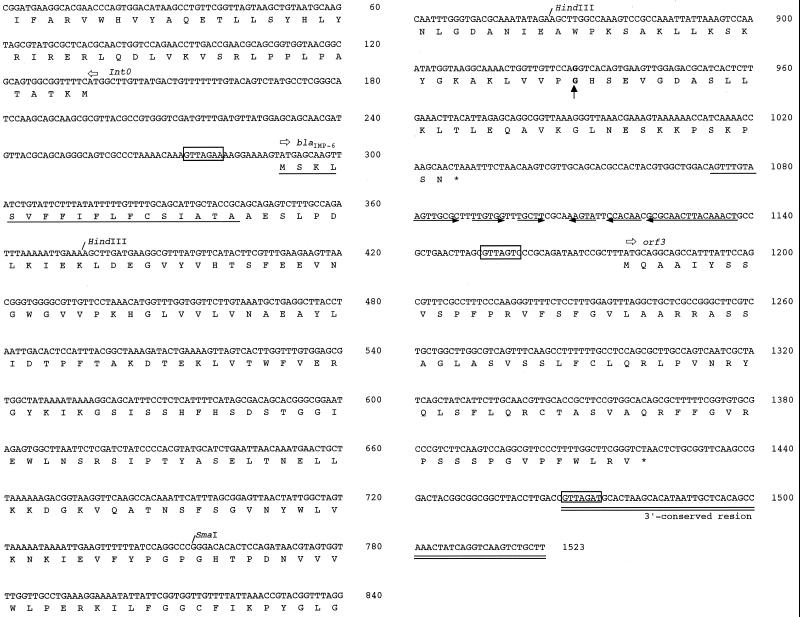
The 1,523-bp sequence of the metallo-β-lactamase gene and its flanking regions in pKU502 is shown in Fig. 2. Sequence analysis revealed that this metallo-β-lactamase gene differed from that carried by pMS350 (IMP-1) by one point mutation, leading to one amino acid substitution: 640-A in the base sequence of IMP-1 was converted to G, and Ser-196 was replaced by Gly in the mature enzyme (Fig. 2). Since this one amino acid substitution has not been previously described in IMP-type enzymes produced by clinical strains, the enzyme in the current study was designated IMP-6.
FIG. 2.
Nucleotide sequence of IMP-6 and its flanking regions. The 1,523-bp sequence determined is shown, and nucleotide 1 corresponds to the first nucleotide of the EcoRV site upstream of the putative integron (In0). The start codon is indicated by horizontal open arrows, and the corresponding translated protein sequence is shown below the nucleotide sequence. The signal peptide for secretion of IMP-6 protein is underlined (23, 30). Sequences similar to the GTTRRRY sequences are indicated by boxes. The region containing a large inverted repeat sequence is indicated by horizontal arrows. The 3′-conserved region is double-underlined. A vertical arrow indicates a nucleotide mutation leading to an amino acid substitution: 640-A in the base sequence encoding IMP-1 is converted to G, and Ser-196 is replaced by Gly in the mature enzyme.
Susceptibility to antibiotics.
The MICs of β-lactams for S. marcescens KU3838 and the transconjugant strain of E. coli KU3999, which acquired pKU501 by conjugation at a frequency of 10−5, as well as those of the transformant strains of E. coli KU4866, KU4867, and KU1917 are shown in Table 2.
TABLE 2.
MICs of different antibiotics for bacterial strains
| Strain | Plasmid | β-Lactamase(s) | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIP | PIP/CLAb | CEF | CMZ | CTM | CTX | FEP | IPM | PAM | MEM | ATM | |||
| S. marcescens KU3838 | pKU501 | TEM-1, IMP-6 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 32 | >128 | >128 | 128 |
| E. coli KU3999 | pKU501 | TEM-1, IMP-6 | >128 | 32 | >128 | >128 | 128 | >128 | 16 | 8 | 32 | 64 | 0.25 |
| E. coli KU4866 | pKU502 | TEM-1, IMP-6 | >128 | 1 | >128 | >128 | >128 | >128 | 16 | 2 | 8 | 64 | 0.25 |
| E. coli KU4867 | pKU503 | IMP-6 | 1 | 0.5 | >128 | >128 | >128 | >128 | 32 | 2 | 4 | 64 | 0.06 |
| E. coli KU1917 | pMS361 | IMP-1 | 4 | 4 | >128 | 128 | 128 | 64 | 0.5 | 2 | 2 | 0.5 | 0.06 |
| E. coli ML4901 | 0c | 0d | 2 | 0.5 | 4 | 0.25 | 0.03 | 0.13 | 0.13 | 0.25 | 0.5 | 0.25 | 0.06 |
Abbreviations: PIP, piperacillin; CLA, clavulanic acid; CEF, cephalothin; CMZ, cefmetazole; CTM, cefotiam; CTX, cefotaxime; FEP, cefepime; IPM, imipenem; PAM, panipenem; MEM, meropenem; ATM, aztreonam.
MICs were determined in the presence of clavulamic acid (5 μg/ml).
Contains no plasmid.
Does not produce TEM-1, IMP-1, and IMP-6.
S. marcescens KU3838 was highly resistant to almost all β-lactam antibiotics, including piperacillin, piperacillin/clavulanic acid, aztreonam, and carbapenems. The MICs of all selected carbapenems were 0.5 μg/ml or less for the parent E. coli K12 ML4901 strain and increased for both the transconjugant (KU3999) and the transformants (KU4866 and KU4867) all of which bear carbapenem resistance genes. The MICs of cephalosporins were increased significantly compared with those for the parent E. coli K12 ML4901 strain.
Moreover, KU4866, the transformant that contained pKU502, was resistant to piperacillin, cephalosporins, and carbapenems. In the presence of clavulanic acid (5 μg/ml), the MIC of piperacillin was decreased. KU4867, the transformant that contained pKU503, was resistant to cephalosporins and carbapenems but susceptible to piperacillin. As with KU3999, the MICs of meropenem and panipenem, especially of meropenem, for both KU4866 and KU4867 were also higher than that of imipenem, and both strains were sensitive to aztreonam. The MIC of meropenem for KU1917, the transformant carrying an IMP-1 type β-lactamase gene, was lower than that of imipenem, and the MIC of panipenem was equal to that of imipenem.
Assay of β-lactamase.
IMP-6 was purified from the culture supernatant of KU4867 as described in Materials and Methods. IMP-6 was completely purified by a carboxymethyl cellulose CM52, a Sephadex G-75, and Mono S column chromatography, and the specific activity of purified enzyme (38.6 U/mg of protein) was 701-fold higher than that of the crude extract (0.055 U/mg of protein), using 100 μM of imipenem as the substrate. The values of kcat, Km, and kcat/Km for eight antibiotics are shown in Table 3. As for carbapenems, the kcat/Km value of the IMP-1 for meropenem was almost equal than that for imipenem (22). However, the kcat/Km value of the IMP-6 for meropenem was about sevenfold higher than that for imipenem. In addition, the values of kcat/Km of the IMP-6 for cephalothin and cefotaxime were higher than that of IMP-1. On the other hand, the values of kcat/Km of the IMP-6 for penicillins were low compared with those of the IMP-1. No hydrolysis of aztreonam was detected.
TABLE 3.
Hydrolysis of various β-lactam antibiotics by IMP-6 and IMP-1 type metallo-β-lactamases
| Substrate | IMP-6a
|
IMP-1b
|
||||
|---|---|---|---|---|---|---|
| kcat (s−1)c | Km (μM)c | kcat/Km (μM−1 s−1) | kcat (s−1)c | Km (μM)c | kcat/Km (μM−1 s−1) | |
| Penicillin G | 51 ± 4.9 | 220 ± 30 | 0.23 | 320 ± 30 | 520 ± 30 | 0.62 |
| Piperacillin | 22 ± 0.31 | 230 ± 31 | 0.09 | NDd | ND | 0.72 |
| Cephalothin | 374 ± 6.6 | 4.7 ± 0.31e | 79.6 | 48 ± 4 | 21 ± 2 | 2.4 |
| Cefotaxime | 55 ± 3.1 | 3.8 ± 0.15e | 14.5 | 1.3 ± 0.5 | 4 ± 0.5 | 0.35 |
| Aztreonam | NHf | ND | ND | >0.01 | >1,000 | <0.0001 |
| Imipenem | 68 ± 7.0 | 110 ± 7.5 | 0.61 | 46 ± 3 | 39 ± 4 | 1.2 |
| Panipenem | 180 ± 19.5 | 250 ± 21 | 0.71 | 50 ± 5 | 10 ± 2 | 0.12 |
| Meropenem | 32 ± 0.99 | 7.6 ± 0.21e | 4.2 | 44 ± 6 | 30 ± 3 | 1.5 |
IMP-6 was purified from KU4867.
IMP-1 values were reported by Laraki et al. (22).
Data represent the mean±standard deviation of more than three measurements.
ND, not determined.
Km was obtained as the Ki value.
NH, no hydrolysis detected.
DISCUSSION
Antibiotic resistance has evolved over the past 50 years from the rapid microbiological development of resistant strains to a serious medical problem in hospitals all over the world (11). The use of broad-spectrum antimicrobial agents has had a profound effect on the emergence of antibiotic resistance (28). Resistance to various classes of antibiotics, including recently developed ones, has been reported in almost all species, for example, gram-negative bacteria possessing carbapenem-hydrolyzing metallo-β-lactamases (2).
The majority of the carbapenem-hydrolyzing metallo-β-lactamase genes are chromosomally encoded (31). After a plasmid-mediated metallo-β-lactamase carried by P. aeruginosa was reported by Watanabe et al. (39) in 1991, the metallo-β-lactamases have been found frequently among gram-negative isolates, such as members of the family Enterobacteriaceae and P. aeruginosa (7, 14, 21, 27, 35). The majority of isolates of IMP-1 type metallo-β-lactamase-producing gram-negative bacteria have been reported from Japan. In addition, variants of blaIMP, such as IMP-2 and IMP-3, have been reported recently by Cornaglia et al. (5), Riccio et al. (32), and Iyobe et al. (15). The transfer of a plasmid carrying a metallo-β-lactamase gene suggests the possibility of clinical spread of plasmid-encoded metallo-β-lactamases by cell-to-cell contact. Since metallo-β-lactamases confer resistance not only to carbapenems but also to other β-lactams except for monobactams, antibiotics are frequently ineffective against gram-negative rods carrying this enzyme. Therefore, the appearance of metallo-β-lactamase-producing bacterial pathogens is a matter of major concern for antimicrobial chemotherapy. In this study, we characterized a new carbapenem-hydrolyzing metallo-β-lactamase, IMP-6.
KU3999, KU4866, and KU4867, which produce IMP-6, were resistant to carbapenems. The MICs especially of meropenem but also of panipenem were higher than the MIC of imipenem for these strains. However, the MIC of meropenem for KU1917, which produced IMP-1, was lower than that of imipenem, and the MIC of panipenem was equal to that of imipenem. The kcat/Km values of the IMP-6 against meropenem and panipenem, especially against meropenem, were higher than that against imipenem. In previous studies on IMP-1 (22, 26), the kcat/Km values against panipenem and meropenem were almost equal to or less than the kcat/Km against imipenem. These results support the hypothesis that IMP-6 is extended substrate profiles against carbapenems. The DNA sequence of the IMP-6 gene shows that its high activity against carbapenems is due to a point mutation that changes Ser-196 of IMP-1 to Gly. This suggests that the hydroxyl group of Ser-196 plays an important role in meropenem hydrolysis.
Metallo-β-lactamases confer resistance not only to carbapenems but also to penicillins and cephems (31). However, the kcat/Km values of KU4867, carrying a plasmid pKU503 with deletion of the ampicillin resistance gene in pBluescript, were very low against penicillin G and piperacillin. The MICs of piperacillin and piperacillin/clavulanic acid for this strain were 1 and 0.5 μg/ml, respectively, as with the recipient E. coli strain ML4901. These results showed that IMP-6 hydrolyzes penicillins very poorly and also suggest that IMP-6 acquired high activity against carbapenems, especially meropenem, via the point mutation but lost activity against penicillins. pKU501 carries blaIMP and the genes for TEM-1-type β-lactamases and produces both enzymes, as we have reported previously (41). Therefore, although IMP-6 is inactive against penicillins due to this point mutation, pKU501 confers resistance to a variety of antimicrobial agents because it also produces TEM-1 type enzyme.
Iyobe et al. (15) have reported an IMP-3 metallo-β-lactamase in which the gene differed from IMP-1 by a seven-point mutation, leading to two amino acid substitutions: both 314- and 640-A in the base sequence of IMP-1 were converted to G, and both Glu-87 and Ser-196 were replaced by Gly in the mature enzyme. Iyobe et al. obtained pMS402, which has a hybrid bla gene from blaIMP-1 and blaIMP-3, by DNA recombination techniques and found that pMS402 has identical amino acid sequences to IMP-6, in which Ser-196 was replaced by Gly. They showed that the kinetic parameters between the hybrid and IMP-3 had similar patterns against various β-lactams and that the values of kcat/Km of both the hybrid and IMP-3 for penicillins were much lower than those of IMP-1. Iyobe et al. mentioned that replacement of Ser-196 by Gly is important to hydrolyze β-lactams (15). However, they did not evaluate the kinetic parameters for hydrolysis of carbapenems such as panipenem and meropenem. This study is a first report of IMP-6 in a clinical sample and shows that IMP-6 acquired high activity against carbapenems, especially meropenem.
Recently, clinical isolates producing class A extended-spectrum β-lactamases (ESBLs) that differ by a few point mutations have been described (13, 20, 25, 38). These enzymes hydrolyze cephalosporins and monobactams, in addition to ampicillin and piperacillin. However, ESBLs do not hydrolyze cefoxitin and cefmetazole, which belong to the cephamycin group, or carbapenems. The IMP-6 gene differed from IMP-1 by a one-point mutation, like ESBLs. More rational and appropriate use of antibiotics may reduce the selective pressure for resistance mutations such as IMP-6.
ACKNOWLEDGMENTS
We thank Shizuko Iyobe, Laboratory of Drug Resistance in Bacteria, Gunma University School of Medicine, for kindly providing the PCR primers. We also thank Takeshi Nakamura, Department of Parasitology, Kitasato University School of Medicine, for technical assistance.
This work was supported in part by grant-in-aid 12670264 from the Ministry of Science, Education and Culture of Japan.
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc London Biol. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Bush K. Metallo-β-lactamases: a class apart. Clin Infect Dis. 1998;27(Suppl. 1):S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Sykes R B. Methodology for the study of β-lactamases. Antimicrob Agents Chemother. 1986;30:6–10. doi: 10.1128/aac.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornaglia G, Riccio M L, Mazzariol A, Lauretti L, Fontana R, Rossolini G M. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet. 1999;353:899–900. doi: 10.1016/s0140-6736(98)05954-6. [DOI] [PubMed] [Google Scholar]
- 6.Cornaglia G, Russell K, Satta G, Fontana R. Relative importances of outer membrane permeability and group 1 β-lactamase as determinants of meropenem and imipenem activities against Enterobacter cloacae. Antimicrob Agents Chemother. 1995;39:350–355. doi: 10.1128/aac.39.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirakata Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, Matsuda J, Nakano M, Tomono K, Maesaki S, Kaku M, Yamada Y, Kamihira S, Kohno S. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–2011. doi: 10.1128/aac.42.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraoka M, Masuyoshi S, Mitsuhashi S, Tomatsu K, Inoue M. Cephalosporinase interactions and antimicrobial activity of BMY-28142, ceftazidime and cefotaxime. J Antibiot. 1988;41:86–93. doi: 10.7164/antibiotics.41.86. [DOI] [PubMed] [Google Scholar]
- 9.Holton T A, Graham M W. A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res. 1991;19:1156. doi: 10.1093/nar/19.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M, Itoh J, Mitsuhashi S. pMS76, a plasmid capable of amplification by treatment with chloramphenicol. Plasmid. 1983;9:86–97. doi: 10.1016/0147-619x(83)90033-1. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Kuga A, Shimauchi C, Yano H, Okamoto R. Why do antimicrobial agents become ineffectual? Yonsei Med J. 1998;39:502–513. doi: 10.3349/ymj.1998.39.6.502. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Okamoto R, Okubo T, Inoue K, Mitsuhashi S. Comparative in vitro activity of RP59500 against clinical bacterial isolates. J Antimicrob Chemother. 1992;30(Suppl. A):45–51. doi: 10.1093/jac/30.suppl_a.45. [DOI] [PubMed] [Google Scholar]
- 13.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyobe S, Kusadokoro H, Ozaki J, Matsumura N, Minami S, Haruta S, Sawai T, O'Hara K. Amino acid substitutions in a variant of IMP-1 metallo-β-lactamase. Antimicrob Agents Chemother. 2000;44:2023–2027. doi: 10.1128/aac.44.8.2023-2027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyobe S, Tsunoda M, Mitsuhashi S. Cloning and expression in Enterobacteriaceae of the extended-spectrum β-lactamase gene from a Pseudomonas aeruginosa plasmid. FEMS Microbiol Lett. 1994;121:175–180. doi: 10.1111/j.1574-6968.1994.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 17.Iyobe S, Yamada H, Minami S. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 18.Katsu K, Inoue M, Mitsuhashi S. Transposition of the carbenicillin-hydrolyzing β-lactamase gene. J Bacteriol. 1982;150:483–489. doi: 10.1128/jb.150.2.483-489.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzis M D, Acar J F, Gutmann L. Antibacterial activity of meropenem against gram-negative bacteria with a permeability defect and against staphylococci. J Antimicrob Chemother. 1989;24:125–132. doi: 10.1093/jac/24.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- 20.Kunugita C, Higashitani F, Hyodo A, Unemi N, Inoue M. Characterization of a new plasmid-mediated extended-spectrum β-lactamase from Serratia marcescens. J Antibiot. 1995;48:1453–1459. doi: 10.7164/antibiotics.48.1453. [DOI] [PubMed] [Google Scholar]
- 21.Kurokawa H, Yagi T, Shibata N, Shibayama K, Arakawa Y. Worldwide proliferation of carbapenem-resistant gram-negative bacteria. Lancet. 1999;354:955. doi: 10.1016/S0140-6736(05)75707-X. [DOI] [PubMed] [Google Scholar]
- 22.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, de Pauw E, Amicosante G, Frere J M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frere J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1995;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 25.Livermore D M, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 26.Marumo K, Takeda A, Nakamura Y, Nakaya K. Purification and characterization of metallo-β-lactamase from Serratia marcescens. Microbiol Immunol. 1995;39:27–33. doi: 10.1111/j.1348-0421.1995.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 27.Minami S, Akama M, Araki H, Watanabe Y, Narita H, Iyobe S, Mitsuhashi S. Imipenem and cephem resistant Pseudomonas aeruginosa carrying plasmids coding for class B β-lactamase. J Antimicrob Chemother. 1996;37:433–444. doi: 10.1093/jac/37.3.433. [DOI] [PubMed] [Google Scholar]
- 28.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto R, Okubo T, Inoue M. Detection of genes regulating β-lactamase production in Enterococcus faecalis and Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2550–2554. doi: 10.1128/aac.40.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riccio M L, Franceschini N, Boschi L, Caravelli B, Cornaglia G, Fontana R, Amicosante G, Rossolini G M. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob Agents Chemother. 2000;44:1229–1235. doi: 10.1128/aac.44.5.1229-1235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short J M, Fernandez J M, Sorge J A, Huse W D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 38.Tenover F C, Mohammed M J, Gorton T S, Dembek Z F. Detection and reporting of organisms producing extended-spectrum β-lactamases: survey of laboratories in Connecticut. J Clin Microbiol. 1999;37:4065–4070. doi: 10.1128/jcm.37.12.4065-4070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagata T, Momoi M Y, Murai K, Ikematsu K, Suwa K, Sakamoto K, Fujimura A. Panipenem-betamipron and decreases in serum valproic acid concentration. Ther Drug Monit. 1998;20:396–400. doi: 10.1097/00007691-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Yano H, Kuga A, Irinoda K, Okamoto R, Kobayashi T, Inoue M. Presence of genes for β-lactamases of two different classes on a single plasmid from a clinical isolate of Serratia marcescens. J Antibiot. 1999;52:1135–1139. doi: 10.7164/antibiotics.52.1135. [DOI] [PubMed] [Google Scholar]