Abstract
Objective
Oral tyrosine kinase inhibitors (TKIs) are first line therapy for chronic myeloid leukemia (CML). A complete cytogenetic response (CCyR) correlates with increased overall survival, however only 66%–88% of patients achieve CCyR after one year of TKI treatment. Because TKI therapy alone cannot eliminate CML stem cells, strategies aimed at achieving faster and deeper responses are needed to improve long-term survival. Metformin is a widely prescribed glucose-lowering agent for patients with diabetes and in preclinical studies, has been shown to suppress cell viability, induce apoptosis, and downregulate the mTORC1 signaling pathway in imatinib resistant CML cell lines (K562R). This study aims to investigate the utility of metformin added to TKI therapy in patients with CML.
Data Sources
An observational study at an academic medical center (Salt Lake City, UT) was performed for adults with newly diagnosed, chronic-phase CML to evaluate attainment of CCyR from TKI therapy with or without concomitant metformin use. Descriptive analyses were used to describe baseline characteristics and attainment of response to TKI therapy.
Data Summary
Fifty-nine patients were evaluated. One hundred percent (5 of 5) in the metformin group and 73.6% (39 of 54) in the non-metformin group achieved CCyR. Approximately 20% of patients in both groups relapsed (defined by a loss of CCyR during study) after a median 34.5 months of follow-up.
Conclusions
Future research is warranted to validate these findings and determine the utility of metformin added to TKI therapy.
Keywords: Metformin, chronic myeloid leukemia, tyrosine kinase inhibitor, cytogenetic response, treatment free remission
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm accounting for approximately 15% of adult leukemias with an expected 8450 new diagnoses in the United States in 2020. 1 Central pathogenesis of CML is defined by the presence of the Philadelphia chromosome (Ph+), which results from a translocation between the Abelson murine leukemia (ABL1) gene and breakpoint cluster region (BCR) on chromosomes 9 and 22, respectively.2,3 The expression of BCR-ABL1 fusion protein results in deregulation of tyrosine kinase activity and constitutively active downstream intracellular signaling, leading to aberrant leukemogenesis. 4
Current treatment paradigm
For the majority of patients, tyrosine kinase inhibitor (TKI) therapy has changed CML into a manageable, chronic condition requiring frequent initial monitoring (every 3 months for the first 12 months) to assess response. Response milestones, in the order in which they are achieved, include normalization of peripheral blood counts (hematologic response; 1 log reduction in BCR-ABL1 copies), reduction in Ph+ chromosome metaphases (cytogenetic response; 2 log reduction in BCR-ABL1 copies), and decline in the number of BCR-ABL1 mRNA transcripts (molecular response; 3 log reduction in BCR-ABL1 copies). 2 Normalization of peripheral counts typically occurs within the first two to four weeks of TKI initiation when the bone marrow regains normal hematopoietic function. The next milestone, a complete cytogenetic response (CCyR; BCR-ABL1 < 1% per International Scale 5 ) to be achieved within 12 months of starting first-line therapy, has been correlated with improved overall survival.2,6,7 Once CCyR is achieved, ongoing molecular response assessment is required to detect BCR-ABL1 transcripts despite elimination of Ph+ chromosome metaphases. A deeper, molecular response is then assessed every 3 months with the goal of achieving specific milestones to guide both dose reductions and treatment-free remission (TFR) trials. 2
Standard of care for chronic-phase CML prior to the early 2000s produced dismal CCyR rates of only 10%. 8 Since the introduction of BCR-ABL1-directed TKIs, CCyR rates have increased to 66%–88% after just one year of therapy.9–13 Despite these advances, clearly not all patients attain CCyR. Numerous variables account for suboptimal response rates, and while the most important remains adherence to TKI therapy, 14 perfect adherence does not guarantee response. Therefore, the ability to increase the percentage of patients who attain a CCyR within 12 months of TKI therapy initiation is crucial for improved overall outcomes.
Opportunity for improvement
Although TKIs have forever changed CML management, the development of resistance, manifesting most commonly as amino acid substitutions (single, or compound) in the ATP-binding pocket, is still a relatively common occurrence.15,16 Because TKIs alone have not been shown to fully eradicate CML stem cells they cannot be considered curative.17–21 Additionally, TFR trials are not always successful; TFR failures have been observed as far out as 6 years post-TKI discontinuation, a timepoint where certain other hematologic malignancies such as acute myeloid leukemia and many lymphomas may be considered cured. Thus, novel strategies aimed at interfering with molecular-level resistance mechanisms employed by CML stem cells are still needed. In recent years, a growing body of in vitro and murine data has investigated the potential antileukemic activity of antidiabetic agents. Metformin is a widely prescribed glucose-lowering agent for patients with diabetes and in preclinical studies, has been shown to suppress cell viability, induce apoptosis, and downregulate the mTORC1 signaling pathway in imatinib resistant CML cell lines (K562R). 4 Previously proposed molecular pathways for BCR-ABL1 stem cell survival and possible metformin-induced impact are shown in Figure 1.4,22–42 Synergism between TKIs and metformin, should it exist, could have dramatic clinical implications on CML research. Therefore, we conducted a single-center, observational study to evaluate CCyR in patients with CML on TKI therapy also taking metformin.
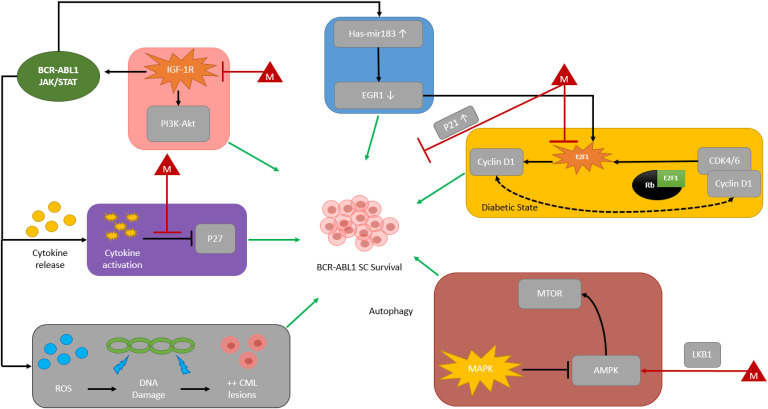
Figure 1.
Proposed mechanisms for BCR-ABL1 SC survival & relationship with metformin.4,22–52
Colored boxes denote proposed molecular pathways involved in BCR-ABL1-independent and BCR-ABL1-dependent SC survival. Each red triangle denoted with the letter “M” are proposed metformin effects on BCR-ABL1 stem cell survival.
(Purple Box) Cytokine release leads to inhibition of P27 (cell cycle inhibitor) and BCR-ABL1 SC survival. Metformin has been proposed to inhibit this mechanism leading to cell cycle inhibition of BCR-ABL1 SCs. (Pink Box) IGF-1R is activated through autocrine and non-autocrine signaling. This leads to PI3K-Akt pathway activation and direct BCR-ABL1 activation. Metformin inhibits IGF-1R activation, which inhibits BCR-ABL1 activation. (Blue Box) BCR-ABL1 protein kinase-dependent pathway is mediated by upregulation of hsa-mir183, subsequent downregulation of EGR1, and therefore activation of transcription factor E2F1 leading to downstream signal transduction and BCR-ABL1 SC survival. (Yellow Box) In the diabetic state, hyperactivation of CDK4-RB1-E2F1 pathway is associated with downstream signal transduction targets of E2F1 (i.e., Cyclin D1) contributing to SC survival, further hyperactivation of the CKD4-RB1-E2F1 pathway via Cyclin D1, and hyperglycemia during insulin resistance. Metformin has been proposed to inhibit E2F1 and therefore may further inhibit BCR-ABL1 SC proliferation as well as upregulate p21 (cell cycle inhibitor) through a separate mechanism. (Red Box) MAPK pathway activation suppresses AMPK which leads to subsequent activation of mTOR pathway and BCR-ABL1 SC survival. Metformin has been proposed to cause direct-AMPK activation. Metformin utilization of LKB1 phosphorylates and activates AMPK, leading to inhibition of mTOR and downstream signaling cascades. (Grey Box) Downstream effects of ROS lead to DNA damage and aberrant proliferation of CML lesions.
Figure Legend: AMPK = 5’ adenosine monophosphate-activated protein kinase, Akt = protein kinase B, BCR-ABL1 = breakpoint cluster region-Abelson murine leukemia oncogene, CDK4/6 = cyclin-dependent kinase 4/6, CML = chronic myeloid leukemia, DNA = deoxyribonucleic acid, E2F1 = E2F transcription factor 1, EGR1 = early growth response 1, hsa-mir183 = homo sapiens microRNA 183 cluster, IGF-1R = type 1 insulin-like growth factor receptor, JAK2 = Janus kinase 2, LKB1 = liver kinase B1, mTOR = mammalian target of rapamycin, P21 = cyclin-dependent kinase inhibitor 1, p27 = cyclin dependent kinase inhibitor 1B, PI3K = phosphatidylinositol 3-kinase, Rb = retinoblastoma protein, ROS = reactive oxygen species, SC = stem cell, STAT3/5 = signal transducer and activator of transcription 3/5; autophagy = degradation of cytosolic components and recycled through lysosomes, autocrine signaling = cell secretes signaling molecules and binds to the signaling-secreting cell.
Methods
Study design
This observational study was conducted at Huntsman Cancer Institute in Salt Lake City, Utah. Patients ≥ 18 years old diagnosed with CML who initiated TKI therapy on or after May 2014 (date of electronic medical record implementation) to January 2019 were queried for data analysis using the local institution's cancer clinical research database. The study included patients with newly diagnosed, chronic-phase CML who were treated with TKI therapy. Groups were divided based on concomitant use of metformin (metformin group) or not (non-metformin group). Patients were excluded if assessment of CCyR was unattainable (e.g., patient was deceased prior to assessment of CCyR or had not been on treatment for at least one year from TKI initiation). This study was approved by the local Institutional Review Board. Research reported in this publication utilized the Research Informatics Shared Resource at Huntsman Cancer Institute at the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The primary endpoint sought to determine the proportion of patients who achieved CCyR on TKI therapy with or without metformin at one year. At our institution, BCR-ABL1 fusion transcripts were quantified according to standard of care and reported using the international scale (IS). CCyR was defined as the absence of Ph+ chromosome-positive metaphases from the bone marrow or a BCR-ABL1 transcript level of ≤ 1%. Secondary endpoints included proportion of patients who achieved major molecular response (MMR) or complete molecular response (CMR), time to molecular response, rate of relapse, characterization of BCR-ABL1 mutation profiles (Y253H, E255K/V, F359V/C/I, F317L/V/I/C, T315A, V299L, or T315I), rates of stem cell transplant, and initial prescribed daily dose of metformin. MMR and CMR were defined as BCR-ABL1 transcript levels of ≤ 0.1% and ≤ 0.0032%, respectively. Relapse was defined as loss of complete cytogenetic response.
Data collection
Data were collected from the medical record and the institution's internal cancer clinical research database utilizing a standardized case report form in REDCap®. Data included baseline demographics (age, gender, and race) and CML history (date of CML diagnosis if known, Sokal risk score, mutation status, and name of first-line TKI therapy). For patients with a history of diabetes, data collected included date of diabetes diagnosis (if known for those patients with diabetes), hemoglobin A1c, and class of glucose-lowering agents used. Initial dose of metformin was recorded for patients who were on metformin. Response milestones and characterization of relapse were also captured. Descriptive analyzes were used to describe all endpoints. Time to molecular response rates were calculated using Kaplan-Meier method. Analyses were performed by using JMP Pro® version 14 (SAS Institute, Cary, NC).
Results
Baseline characteristics
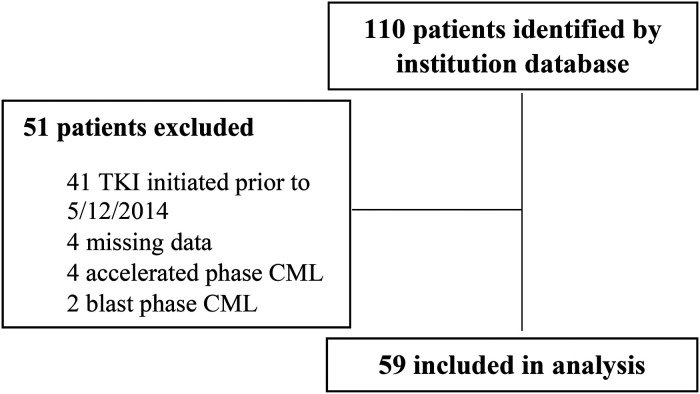
One-hundred and ten patients were identified for inclusion based on the institution's internal clinical cancer research database (Figure 2). Fifty-one patients were subsequently excluded due to initiating a TKI prior to May 2014 (n = 41), missing data (n = 4), or an initial diagnosis of non-chronic phase CML (n = 6). A total of 59 patients were included in this study, 5 on TKI therapy and metformin and 54 on TKI therapy without metformin. Baseline characteristics were well balanced between both groups (Table 1). Median age at diagnosis was 54, over half of the total population (57%) was male, and approximately 90% were Caucasian. There was a larger distribution in CML risk stratification in the non-metformin group, with 38.9% (n = 21) having low risk disease compared to 60% (n = 3) in the metformin group. In both groups, the most common TKI therapy initiated was imatinib (55.9%) followed by dasatinib (23.7%). All patients in the metformin group had a diagnosis of diabetes compared to 14.8% in the non-metformin group.
Figure 2.
CONSORT diagram.
Table 1.
Baseline characteristics.
| Characteristics | TKI with metformin n = 5 | TKI without metformin n = 54 | Total n = 59 |
|---|---|---|---|
| Age, years (range) | 56 (48–63) | 53 (20–86) | 54 (20–86) |
| Male (%) | 2 (40) | 32 (59.3) | 34 (57.6) |
| Race (%) | |||
| Caucasian | 4 (80) | 49 (90.7) | 53 (89.8) |
| Other | 1 (20) | 5 (9.3) | 6 (10.2) |
| Sokal risk | |||
| High | 1 (20) | 7 (12.9) | 8 (13.6) |
| Intermediate | 0 | 9 (16.7) | 9 (15.2) |
| Low | 3 (60) | 21 (38.9) | 24 (40.7) |
| Unknown | 1 (20) | 17 (31.5) | 18 (30.5) |
| First line TKI therapy | |||
| Bosutinib | 0 | 3 (5.6) | 3 (5.1) |
| Dasatinib | 1 (20) | 13 (24) | 14 (23.7) |
| Imatinib | 4 (80) | 29 (53.7) | 33 (55.9) |
| Nilotinib | 0 | 9 (16.7) | 9 (15.3) |
| Diagnosis of diabetes | 5 (100) | 8 (14.8) | 13 (22) |
| Hemoglobin A1c (%) (range) | 5.6 (5.5–5.6)a | 5.7 (4.9–6.2)b | 5.6 (4.9–6.2) |
a2 patients, b8 patients.
Outcomes
Cytogenetic and molecular response results are shown in Table 2. The primary endpoint was the proportion of patients who achieved CCyR at one-year post-TKI initiation. One-hundred percent of patients in the metformin group achieved CCyR at one year compared to 74% in the non-metformin group (Table 2).
Table 2.
Primary and secondary endpoint results.
| TKI with metformin (n = 5) | TKI without metformin (n = 54) | |
|---|---|---|
| Primary endpointa | ||
| CCyR | ||
| Response rate (%) | 5 (100) | 39 (73.6) |
| Secondary Endpoints | ||
| MMR | ||
| Response rate (%) | 3 (60) | 34 (62.9) |
| Median time to response, months | 11.1 | 19.5 |
| CMR | ||
| Response rate (%) | 2 (40) | 22 (40.7) |
| Median time to response, months | 37.4 | NR |
| Other Secondary Endpoints | ||
| Stem cell transplant (%) | 0 | 2 (3.7) |
| BCR-ABL1 mutation (%) | ||
| Y253H | 0 | 2 (3.7) |
| T315I | 0 | 1 (1.9) |
| Relapse (%) | 1 (20) | 11 (20.4) |
| Initial dose of metformin, mgb [median (range)] | 1000 (500–2000) | -- |
| Patient 5 | 1000 | |
| Patient 6 | 500 | |
| Patient 12 | 2000 | |
| Patient 45 | 1000 | |
| Patient 61 | 2000 |
CCyR: complete cytogenetic response; MMR: major molecular response; CMR: complete molecular response; a53 patients available for CCyR analysis in non-metformin group; NR: not reached; bDefined as the initial starting dose (mg) per day.
Secondary endpoints are shown in Table 2. Approximately 60% of patients in both groups achieved MMR, with median time to MMR numerically shorter in the metformin group compared to the non-metformin group (11.1 vs. 19.5 mos). The proportion of patients achieving CMR was similar between both groups (approximately 40%), with median time to CMR 37.4 mos in the metformin group and not reached in the non-metformin group. Two patients taking a TKI without metformin received stem cell transplantation, whereas none of the 5 patients taking metformin received transplantation. Further, no patient taking a TKI plus metformin developed BCR-ABL1 mutation compared to three in the non-metformin group. Approximately 20% of patients in both groups had relapsed (loss of CCyR).
Discussion
Current initial standard of care for CML is TKI therapy. Patients achieving a deep molecular response and maintaining this for a minimum of 2 years on TKI therapy, may qualify for a TFR trial. However, not all who attempt to discontinue therapy are able to maintain their response. Because TKIs do not fully eliminate CML stem cells, TFR rates are imperfect (usually ∼40%–50% of patients maintain TFR).53–55 When TFR is unsuccessful, treatment re-initiation not only increases costs, but potentially decreases quality of life resulting from ongoing therapy-related adverse events.56–58 Adherence, at any point in time, may be hampered by adverse events leading to diminished dose intensity and lack, or loss of response. Ultimately, this unfortunately translates to diminished overall survival for some. Moreover, despite perfect adherence, some patients still fail to achieve CMR, which diminishes the chance that they may someday qualify for a TFR trial. Because suboptimal TFR rates could be substantially improved, the ability to eliminate CML stem cells remains a priority. Due to resistance of CML stem cells despite BCR-ABL1 inhibition, novel strategies are needed if we hope to eradicate CML stem cells, improve upon current TFR rates, and improve survival.
Several molecular pathways have been proposed as mediators of CML stem cell resistance and persistence in the face of continued TKI therapy. In recent years, a growing body of in vitro and murine literature has elucidated the potential antileukemic activity of antidiabetic agents. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists are a common class of medications prescribed for patients with diabetes. PPAR-γ agonists in combination with TKI therapy were initially thought to downregulate transcription of STAT5 and transition CML stem cells out of quiescence, thereby sensitizing them to TKI therapy.41,43–45 While Rousselot and colleagues reported that in CML patients on imatinib for a minimum of 2 years who already achieved MMR, the addition of pioglitazone increased deep molecular response rates. 45 More recent data revealed that pioglitazone did not appear to improve TFR trial results. 59 Although these data require confirmation within the constructs of a randomized clinical trial, they support PPAR-γ agonism and interference with cellular energy availability as a potential synergistic mediator of CML stem cell sensitization to TKI therapy.
Metformin, a more commonly used antidiabetic agent, has been extensively studied in solid tumor malignancies for its antineoplastic properties and has been shown in vitro to inhibit mTOR activity through 5’ adenosine monophosphate-activated protein kinase (AMPK)-dependent inhibitory effects. Of great interest owing to metformin's mechanism of action, accelerated phase and blast phase CML cells may express additional type 1 insulin-like growth factor receptors (IGF-1R) or increase IGF-1 ligand expression to upregulate BCR/ABL1 amplification through a phosphatidylinositol 3-kinase (PI3K)-, protein kinase B (AKT)-, mammalian target of rapamycin (mTOR)-dependent autocrine signaling mechanism.38,49–52,60 The proposed IGF-1R signaling cascade leading to stimulation of BCR-ABL1-independent growth signaling is shown in Figure 3. In this pathway, expression of IGF-1 receptors or upregulation of autocrine signaling by increased IGF-1 ligand expression may be inhibited by upregulation of AMPK or direct inhibition of IGF-1R by metformin. Additionally, increased IGF-1R signaling has also been shown as a potential TKI-resistance mechanism in non-BCR-ABL-directed TKI therapy. For example, crizotinib is an oral multitargeted TKI recommended for patients with non-small cell lung cancer (NSCLC) harboring specific tyrosine kinase rearrangements. In an in vitro model with crizotinib-resistant human lung cancer cells with increased IGF-1 signaling, metformin restored sensitivity by inhibiting this signaling pathway. 49 Other proposed molecular mechanisms for metformin's antileukemic activity and synergy with TKI therapy are illustrated in Figure 1 and have also previously been reported elsewhere.22–32,37,39 Based on myriad in vitro data across multiple tumor types, it is plausible that metformin possesses undiscovered antileukemic properties, such as those previously discussed.
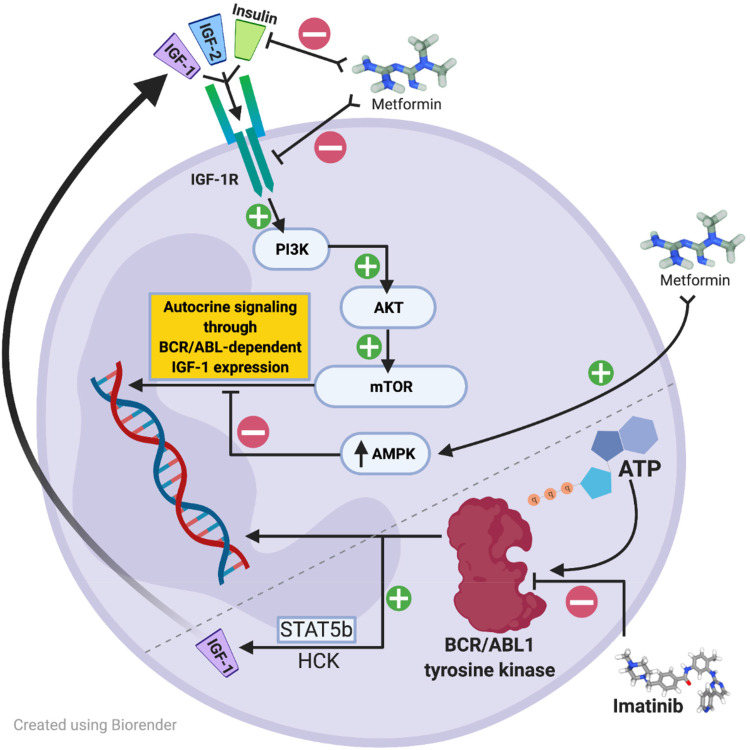
Figure 3.
Proposed signaling cascade in CML cell.38,49–52,60
In AP and BP, CML cells may express additional IGF-1 receptors or, in response to BCR/ABL1 inhibition by TKI therapy such as imatinib, may upregulate autocrine signaling by increasing IGF-1 ligand expression to stimulate BCR/ABL1-independent growth signals mediated by the PI3K, AKT, mTOR pathway. Metformin has been shown in vitro to inhibit mTOR through upregulation of AMPK inhibitory effects. Additionally metformin alone or in combination with crizotinib has been shown in a lung cancer cell model to decrease IGF-1R phosphorylation, when the use of crizotinib alone, a TKI aimed at ALK inhibition, enhanced IGF-1R phosphorylation. Metformin may also indirectly act to down-regulate insulin.
Figure Legend: AKT = protein kinase B, AMPK = 5’ adenosine monophosphate-activated protein kinase, ATP = adenosine triphosphate, HCK = Hematopoietic cell kinase, IGF = insulin-like growth factor, IGF-1R = type 1 insulin-like growth factor receptor, BCR/ABL = breakpoint cluster region-Abelson murine leukemia oncogene, PI3K = phosphatidylinositol 3-kinase, STAT = signal transducer and activator of transcription.
This observational study is the first to report on the use of metformin in combination with CML-directed TKI therapy. The percentage of patients achieving CCyR in the metformin group and the non-metformin group was 100% and 73.6%, respectively, with attainment of MMR and CMR numerically similar between both groups (Table 2). Median time to MMR and CMR were numerically shorter in the metformin group compared to the non-metformin group (11.1 mo vs. 19.5 mo; 37.4 mo vs. NR).
The main limitation of the current study was small sample size. A greater percentage of patients in the metformin group had low-risk disease, with 4 out of 5 (80%) initiated on a first generation TKI. Additionally, medication adherence and TKI dose interruptions/reductions were not fully assessed due to limitations inherent to an observational study design.
In conclusion, the results of this study suggest that metformin together with TKI therapy may increase the proportion of CML patients who achieve CCyR and decrease time to both MMR and CMR. The impact of metformin on TKI response rates warrants further investigation into whether metformin adds antileukemic synergistic properties. Confirmation of clinical benefit requires a large, prospective randomized controlled trial.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Rebecca Pokorny https://orcid.org/0000-0002-4431-1076
References
- 1.American Cancer Society. Key statistics for chronic myeloid leukemia. https://www.cancer.org/cancer/chronic-myeloid-leukemia/ about/statistics.html (2019, accessed 6 March 2020).
- 2.National Comprehensive Cancer Network. Chronic Myeloid Leukemia (Version 3.2021). https://www.nccn.org/professionals/ physician_gls/pdf/cml.pdf (accessed 2 July 2021).
- 3.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol 2018; 93: 442–459. [DOI] [PubMed] [Google Scholar]
- 4.Shi R, Lin J, Gong Y, et al. The antileukemia effect of metformin in the Philadelphia chromosome-positive leukemia cell line and patient primary leukemia cell. Anti-Cancer Drugs 2015; 26: 913–922. [DOI] [PubMed] [Google Scholar]
- 5.Müller MC, Cross NC, Erben P, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia 2009; 23: 1957–1963. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood 2011; 118: 4541–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chronic Myeloid Leukemia Trialists’ Collaborative Group. Interferon alfa versus chemotherapy for chronic myeloid leukaemia: a meta-analysis of seven randomized trials. J Natl Cancer Inst 1997; 89: 1616–1620. [PubMed] [Google Scholar]
- 9.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase myeloid leukemia. N Engl J Med 2010; 362: 2260–2270. [DOI] [PubMed] [Google Scholar]
- 10.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol 2016; 34: 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016; 30: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol 2018; 36: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brümmendorf TH, Cortes JE, de Souza CA, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol 2015; 168: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28: 2381–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekhar C, Kumar PS, Sarma PVGK. Novel mutations in the kinase domain of BCR-ABL gene causing imatinib resistance in chronic myeloid leukemia patients. Sci Rep 2019; 9: 2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deininger MW, Hodgson JG, Shah NP, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood 2016; 127: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichim CV. Kinase-independent mechanisms of resistance of leukemia stem cells to tyrosine kinase inhibitors. Stem Cells Transl Med 2014; 3: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 2011; 121: 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 2003; 101: 4701–4707. [DOI] [PubMed] [Google Scholar]
- 20.Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319–325. [DOI] [PubMed] [Google Scholar]
- 21.Konig H, Holtz M, Modi H, et al. Enhanced BCR-ABL kinase inhibition dose not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia 2008; 22: 748–755. [DOI] [PubMed] [Google Scholar]
- 22.Pellicano F, Park L, Hopcroft LEM, et al. has-mir183/EGR-1-mediated regulation of E2F1 is required for CML/stem/progenitor cell survival. Blood 2018; 131: 1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009; 8: 909–915. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Guo P, Zhang Y, et al. The antidiabetic drug metformin inhibits the proliferation of bladder cancer cells in vitro and in vivo. Int J Mol Sci 2013; 14: 24603–24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valent P. Targeting the JAK2-STAT5 pathway in CML. Blood 2014; 124: 1386–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang G, Guo J, Zhu Y, et al. Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation. Int J Oncol 2018; 52: 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado-Neto JA, Fenerich BA, Scopim-Ribeiro R, et al. Metformin exerts multitarget antileukemia activity in JAK2V617F-positive myeloproliferative neoplasms. Cell Death Dis 2018; 9: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause DS. Et tu, E2F1? The assassins of CML stem cells. Blood 2018; 131: 1499–1500. [DOI] [PubMed] [Google Scholar]
- 29.Shi R, Lin J, Gong Y, et al. The antileukemic effect of metformin in the Philadelphia chromosome-positive leukemia cell line and patient primary leukemia cell. Anticancer Drugs 2015; 26: 913–922. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Hu D, Chen H. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018; 559: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cilloni D, Saglio G. Molecular pathways: BCR-ABL. Clin Cancer Res 2012; 18: 930–937. [DOI] [PubMed] [Google Scholar]
- 32.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310: 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins CR, Shevchuk OO, Giambra V, et al. IGF Signaling contributes to malignant transformation of hematopoietic progenitors by the MLL-AF9 oncoprotein. Exp Hematol 2012; 40: 715–723. [DOI] [PubMed] [Google Scholar]
- 34.Mitslades CS, Mitslades NS, McMullan CJ, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004; 5: 221–230. [DOI] [PubMed] [Google Scholar]
- 35.Pastural E, Takahashi N, Dong WF, et al. RIZ1 Repression is associated with insulin-like growth factor-1 signaling activation in chronic myeloid leukemia cell lines. Oncogene 2007; 26: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 36.Lakshmikuttyamma A, Pastural E, Takahashi N, et al. Bcr-Abl induces autocrine IGF-1 signaling. Oncogene 2008; 27: 3831–3844. [DOI] [PubMed] [Google Scholar]
- 37.Lei Y, Yi Y, Liu Y, et al. Metformin targets multiple signaling pathways in cancer. Chin J Cancer 2017; 36: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi P, Chandra J, Sun X, et al. Inhibition of IGF-IR tyrosine kinase induces apoptosis and cell cycle arrest in imatinib-resistant chronic myeloid leukaemia cells. J Cell Mol Med 2010; 14: 1777–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giralt A, Denechaud PD, Lopez-Mejia IC, et al. E2f1 promotes hepatic gluconeogenesis and contributes to hyperglycemia during diabetes. Mol Metab 2018; 11: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland W, Morrison T, Chang Y, et al. Metformin (glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol 2004; 67: 2081–2091. [DOI] [PubMed] [Google Scholar]
- 41.Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood 2017; 129: 1595–1606. [DOI] [PubMed] [Google Scholar]
- 42.Lin YC, Wu MH, Wei TT, et al. Metformin sensitizes anticancer effect of dasatinib in head and neck squamous cell carcinoma cells through AMPK-dependent ER stress. Oncotarget 2014; 5: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prost S, Relouzat F, Spentchian M, et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature 2015; 525: 380–383. [DOI] [PubMed] [Google Scholar]
- 44.Goldkowska-Mrowka E, Manda-Handzlik A, Stelmaszczyk-Emmel A, et al. PPARγ ligands increase antileukemic activity of second- and third-generation tyrosine kinase inhibitors in chronic myeloid leukemia cells. Blood Cancer J 2016; 6: e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousselot P, Prost S, Guilhot J, et al. Pioglitazone together with imatinib in chronic myeloid leukemia: a proof of concept study. Cancer 2017; 123: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullrich A, Bell JR, Herrera R, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 1985; 313: 756–761. [DOI] [PubMed] [Google Scholar]
- 47.Stadtmauer LA, Rosen OM. Phosphorylation of exogenous substrates by the insulin receptor-associated protein kinase. J Biol Chem 1983; 258: 6682–6685. [PubMed] [Google Scholar]
- 48.Pike LJ, Kuenzel EA, Casnellie JE, et al. A comparison of the insulin- and epidermal growth factor-stimulated protein kiases from human placenta. J Biol Chem 1984; 259: 9913–9921. [PubMed] [Google Scholar]
- 49.Li L, Wang Y, Peng T, et al. Metformin restores crizotinib sensitivity in crizotinib-resistant human lung cancer cells through inhibition of IGF1-R signaling pathway. Oncotarget 2016; 7: 34442–34452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klement RJ, Fink MK. Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis 2016; 5: e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie J, Chen X, Zheng J, et al. IGF-IR determines the fates of BCR/ABL leukemia. J Hematol Oncol 2015; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell R, Hopcroft LEM, Baquero P, et al. Targeting BCR-ABL-independent TKI resistance in chronic myeloid leukemia by mTOR and autophagy inhibition. J Natl Cancer Inst 2018; 110: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016; 128: 17–23. [DOI] [PubMed] [Google Scholar]
- 54.Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre stop imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 55.Etienne G, Guilhot J, Réa D, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol 2017; 35: 298–305. [DOI] [PubMed] [Google Scholar]
- 56.Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013; 122: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015; 33: 4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011; 118: 4554–4560. [DOI] [PubMed] [Google Scholar]
- 59.Pagnano KBB, Lopez ABP, Miranda EC, et al. Efficacy and safety of pioglitazone in a phase 1/2 imatinib discontinuation trial (EDI-PIO) in chronic myeloid leukemia with deep molecular response. Am J Hematol 2020; 95: E321–E323. [DOI] [PubMed] [Google Scholar]
- 60.Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer 2008; 8: 915–928. [DOI] [PubMed] [Google Scholar]