Abstract
Antibiotics are among the essential veterinary medicine compounds associated with animal feed and food animal production. The use of antibiotics for the treatment of bacterial infections is almost unavoidable, with less need to demonstrate their importance. Although banned as a growth factor for a few years, their use in animals can add residues in foodstuffs, presenting several environmental, technological, animal health, and consumer health risks. With regard to human health risks, antibiotic residues induce and accelerate antibiotic resistance development, promote the transfer of antibiotic-resistant bacteria to humans, cause allergies (penicillin), and induce other severe pathologies, such as cancers (sulfamethazine, oxytetracycline, and furazolidone), anaphylactic shock, nephropathy (gentamicin), bone marrow toxicity, mutagenic effects, and reproductive disorders (chloramphenicol). Antibiotic resistance, which has excessively increased over the years, is one of the adverse consequences of this phenomenon, constituting a severe public health issue, thus requiring the regulation of antibiotics in all areas, including animal breeding. This review discusses the common use of antibiotics in agriculture and antibiotic residues in food/feed. In-depth, we discussed the detection techniques of antibiotic residues, potential consequences on the environment and animal health, the technological transformation processes and impacts on consumer health, and recommendations to mitigate this situation.
Keywords: animal breeding, antibiotic resistance, antibiotics residues, food and feed, public health
Introduction
Antibiotics are among the essential veterinary medicine compounds related to food animal production, defined as substances that can kill or inhibit the growth of bacteria [1]. Its application is almost unavoidable in the treatment of bacterial infections in animals and humans [2]. The global consumption of antibiotics in animals is almost twice that of humans [3]. Globally, 63.1±1.5 tons of antibiotics are annually used in livestock [4], estimating that over 80% of the animals for food production are currently being treated with these compounds [5].
Antibiotics help treat and prevent various infections in animals, such as mastitis, arthritis, respiratory diseases, and gastrointestinal and other bacterial infections [1], and are used as a growth factor with imminent consequences [6]. However, if they are misused, they can end up as residues in foodstuffs, such as milk, eggs, and meat, thus exerting harmful effects on the health of consumers [7]. Furthermore, among the different forms of administration (oral, parenteral, or topical), it was reported that antibiotic residues exceeding the standards are usually encountered when administered through injection [8]. Therefore, various authors have reported that antibiotic residues in food are likely to induce and accelerate the development of antibiotic resistance in bacteria, promote the transfer of antibiotic-resistant bacteria to humans, cause allergies (penicillin), and induce other more severe pathologies, such as cancers (sulfamethazine, oxytetracycline, and furazolidone), anaphylactic shock, nephropathy (gentamicin), bone marrow toxicity, mutagenic effects, and reproductive disorders (chloramphenicol) in humans [1].
Therefore, the World Health Organization (WHO), the American Medical Association, and the American Public Health Association have urged a ban on growth-promoting antibiotics [9] and established standards to limit this phenomenon [10]. Consequently, drugs or antibiotic residues in food above the maximum level globally recognized by various public authorities are illegal [10,11]. Furthermore, observation of the waiting or withdrawal time and physicochemical analyses is mandatory to ensure that the antibiotics used or their analogs do not exceed the maximum residue limit (MRL) before the food is marketed.
With regard to the issue of residues as a topical public health concern and the excessive growth of antibiotic resistance over the years [12]. This review, discussed the regular application of antibiotics in agriculture, antibiotic residues in food and feed, detection techniques, potential consequences on the environment and animal health, technological transformation processes and impacts on consumer health, and recommendations to mitigate these situations.
Review Methodology
This review article was conducted by exploiting numerous review articles, original articles, and related books from reputable databases, such as Web of Science, PubMed, and Scopus. The papers published with toll access have been made available using the facilities provided by the Peoples’ Friendship University of Russia, Moscow, Russia. The literature investigation process was conducted in English and French between October 2020 and April 2021. The keywords explored during the literature search consisted of combinations of the following words: “antibiotic,” “animals,’’ “residue,” “food,” “antimicrobial drugs,” “detection,” “meat,” “milk,” “eggs,” “antibiotique,” “animaux,” “résidus,” “aliments,” and “médicaments antimicrobiens.”
The Common Application of Antibiotics in Agriculture
Antibiotics are used for food production for several reasons, including their primary use as preventive or curative measures against animal infection. Others are used as growth promoters for improved feed conversion efficiency, carcass quality, and economic production [4]. However, in the use of antibiotics in agriculture on a larger scale, the first property is its safety and effectiveness [1]. This property is essential because it aims to protect consumers from severe infections transferrable to humans through contact with the infected animal, consumption of contaminated food, or proliferation in the environment [13]. The frequently used antibiotics in veterinary medicine are b-lactams (penicillin and cephalosporin), macrocyclines (ansamycins, glycopeptides, and aminoglycosides), tetracyclines, chloramphenicols, macrolides, spectinomycin, lincosamide, sulfonamides, nitrofurans, nitroimidazoles, trimethoprim, polymyxins, and quinolones [14]. However, Bacanlı and Başaran [1] reported that the combination of streptomycin and oxytetracycline or streptomycin alone could sometimes be used during the treatment and prophylaxis of plant diseases. Although its main purpose is to fight against pathogens, antibiotics are used explicitly with the disease of concern to be treated. According to the breed, the bacteria responsible for infections vary from one animal to another. The choice of antibiotics and the antimicrobial consumption pattern demonstrate geographical variation across the continents influenced by the food animal species, regional production patterns and types of the production system, intensive or extensive farming, the purpose of farming (commercial or industrial or domestic), unclear legislative framework or policies on the use of antibiotics, and size and socioeconomic status of the population and farmers in particular [15].
Antibiotic Residues
Some published work showing the presence of antibiotic residues in common foods
Table-1 recorded some studies that reported antibiotic residues in food [16-47]. These foods are mainly obtained from poultry (chicken meat and eggs), bovines (bovine carcasses, milk, and meat), and pigs (pork meat). We also reported at least one positive case per continent. However, interestingly, during the literature investigation, we found it easier to obtain work reporting the presence of antibiotic residues in developing countries (mainly in African countries) than in developed countries. This trend is attributed to the lack of legislation and its non-strict application [48], non-systematic detection of residues [49], and improper use or use without a prescription of veterinary antibiotics [15-17].
Table-1.
Antibiotic contamination in various foodstuffs consumed in different countries.
| Country | Antibiotic | Foodstuff | Reference |
|---|---|---|---|
| America | Tetracyclines | Imported Chicken Meat | [18] |
| Brazilia | Tetracyclines, fluoroquinolone | ||
| Bangladesh | Amoxicillin | Milk, eggs | [19] |
| Cameroon | Tetracyclines | Chicken | [16] |
| China | Quinolones, tetracyclines, sulfonamides | Chicken, chicken giblets, and eggs | [20] |
| Beta-lactams, tetracyclines, sulfonamides, and quinolones | Milk | [21] | |
| Egypt | Tetracyclines | Bovine carcasses | [22] |
| Tetracyclines | Chicken meat | [23] | |
| β-lactams Cephalosporines | Eggs | [17] | |
| β-lactams Cephalosporines | Rabbit meat Rabbit liver Rabbit kidney |
[24] | |
| Ethiopia | Tetracyclines | Meat and edible tissue | [25] |
| Ghana | Tetracyclines | Milk | [26] |
| Greece | Nitrofuran | Pork | [27] |
| India | Oxytetracycline and erythromycin | Honeys | [28] |
| Enrofloxacin, oxytetracycline penicillin G and sulfamethoxazole | Milk | [29] | |
| India | Tetracycline, Oxytetracycline, Sulfadimidine, Sulfamethoxazole | Raw milk | [30] |
| Iran | Penicillin, chloramphenicol, gentamicin, tylosin, tetracycline, and sulfonamide | Honey | [31] |
| Italy | Nitrofuran | pork | [27] |
| Kenya | Tetracyclines | Beef, liver and kidney | [32] |
| Tetracyclines, sulfamethazine, beta-lactams, and gentamicin | Milk | [33] | |
| b-lactams | Milk | [34] | |
| Malaysia | Sulfonamides | Chicken | [35] |
| Mexico | Penicillin | Milk | [36] |
| Nigeria | Tetracyclines | Meat | [37] |
| Tetracyclines | Eggs | [38] | |
| Clhoramphenicol | Eggs | [39] | |
| b-lactams | Cattle meats | [40] | |
| Portugal | Nitrofuran | Pork | [27] |
| South Africa | Ciprofloxacin, streptomycin, tetracycline, and sulfanilamide | Beef; chicken; pork | [41] |
| Tetracycline | Chicken livers | [42] | |
| Sudan | Macrolides | Milk | [43] |
| Tanzania | Tetracyclines | Milk | [44] |
| Tanzania | Tetracyclines | Eggs | [45] |
| Turkey | Quinolones | Chicken, beef | [46] |
| Zambia | Oxytetracycline and Sulfamethazine | Beef | [47] |
Movements of Antibiotic Residues through the Animal–human Interface
Several factors occur before antibiotic residues are found in food. We adapted the conceptual representation proposed by Sharma et al. [50] (movement of antibiotic-resistant bacterial strains/genes between different ecosystems) because we judged that the movement of residues obeyed a similar dynamic. Figure-1 [50] shows that any direct or indirect interaction between humans and animals may cause antibiotic residue transmission. Direct interaction occurs when the antibiotics given to the animals or applied to plant cultures become directly residual in the foodstuffs after slaughter or harvest. On the contrary, direct interaction occurs when the residues accumulated in water and soil through manure (containing antibiotic residues from animals) or human excretions (containing residues of antibiotics) are found in food (especially in vegetables by watering or contamination with animal feces) [51]. Although the latter is unlikely to occur compared with the former, several studies have reported significant amounts of antibiotic residues in manure in soils, concluding that these residues can end up in plant foods [52-56]. For the direct mechanism, it is important to stress that the types and amounts of antibiotics administered to the animals also play a significant role, including the mode of administration. Apparently, among the different forms of administration (oral, parenteral, or topical), it has been reported that antibiotic residues exceeding the standards are mainly encountered when administered through injection [8]. This mode of administration might cause the accumulation of drugs in the adipose tissue, limiting the metabolism and the elimination of this drug, which could therefore make them persist in the tissues of animals even after slaughter.
Figure-1.

Conceptual representation of possible movement of antibiotic residues between different ecosystems [50].
The Main Techniques for Detecting Antibiotic Residues
The methods for detecting antibiotic residues can be divided into two categories: screening method (1) and confirmatory method (2). These two methods differ because the first one is globally qualitative or semiquantitative. In contrast, the second one ensures its determination with high precision of the type and concentration of the investigated residue.
Screening Method
All screening methods are essentially microbiological or immunological. The most well-known and earliest microbiological technique is the so-called “four plates” technique. This method inhibits the growth of Micrococcus luteus and Bacillus subtilis. Inhibitory zones lacking bacterial colonies around the sample deposit sites indicate the potential presence of antibiotics [57]. This method is widely used to investigate the presence of antibiotic residues in different meats, fish, and eggs [57-60]. Similarly, acidification is also a well-known microbiological technique used to detect the presence of antibiotic residues in milk. This test uses a culture of a bacterium capable of degrading lactose into lactic acid and a colored indicator, bromocresol purple, which detects if the acidification of the medium has been done. The most suitable strain for this method is Bacillus stearothermophilus var. calidolactis C953 (strain C953, CIP 5281) [57].
Furthermore, if the milk analyzed contains antibiotics, the bacteria will not degrade the lactose, and the color of the medium will remain unchanged. On the other hand, the absence of antibiotics results in a color change from blue to yellow, indicating acidification. However, despite these techniques being accessible, inexpensive, and performed by a nonprofessional, there is a lack of specificity and long incubation time. Thus, to overcome these disadvantages, several companies have manufactured different commercial kits under different trade names (e.g., BR test, Eclipse test, Copan test, Delvotest, Lumac, and Arla). Such tests detect numerous antibiotics at thresholds generally close to the MRL [14].
Compared with microbiological methods, immunological methods such as enzyme-linked immunosorbent assay, fluoroimmunoassay, and time-resolved fluoroimmunoassay are highly specific, highly sensitive, simple, and cost effective. In addition, rapid-detection immunochromatographic kits (on strips) have been developed (TwinSensor), allowing within one assay the simultaneous screening of penicillins and cephalosporins (TwinSensor KIT034); beta-lactam and tetracycline (TwinSensor KIT020); beta-lactam antibiotics tetracyclines, streptomycin, and chloramphenicol (4Sensor BSCT-KIT060); beta-lactam antibiotics, sulfonamides, tetracyclines, and (Fluoro) quinolones (4Sensor BSTQ-KIT072) [61-64].
Confirmatory Methods
The main advantage of confirmatory methods is their high specificity, but they are expensive, time-consuming, and require personnel and an adequate laboratory [1]. Confirmatory methods are essentially chromatographic methods (mainly liquid chromatography) coupled to mass spectrometry or ultraviolet (UV) [1]. However, capillary electrophoresis (CE) [65], CE–laser-induced fluorescence [66], surface-enhanced Raman spectroscopy [67], and high-performance liquid chromatography (HPLC) with spectroscopic fluorometric detection (HPLC– RF) or with a spectroscopic HPLC–photodiode array detector [42] are also shown to be effective detectors of antibiotic residues.
New Fully Automatic Approach
Fully automatic biosensors are becoming increasingly important in detecting antibiotics in food [1]. The biosensors can be classified according to the biological element (enzymatic, immunosensory, and microbiological), transducer (piezoelectric, electrochemical, optical, thermal, impedimetric, and calorimetric), and biological element immobilization procedure on solid support (adsorption, covalent bonding, cross-linking, entrapment, and encapsulation) [14]. Cháfer-Pericás et al. [14], in their work, reported most of the existing techniques for the rapid detection of antibiotic residues in foods. They concluded that fully automatic biosensors consist of a combination of biological element/transducer(such as microbiological cell/electrochemical, antibody/impedimetric, and oligonucleotide/electrochemical with microbiological cell) represent an interesting screening approach due to its quick and fully automated operability [14]. They also reported the advantages of biosensors, specifically the biorecognition element used, which allows rapid, continuous control, and onsite applications. However, the main limitations of these instruments are (1) the potential loss of stability of the biological detection component due to exposure to environmental stresses, such as pH, temperature, or ionic strength, and (2) the limited size of the physicochemical transducers used.
National and International Legislation on Antibiotic Residues in Food
It is challenging to control the use of antibiotics in agriculture in a uniform way because their use varies significantly from one country to another . For example, it was reported that China is the first country to use antibiotics in food animals (23%), followed by the United States (13%), Brazil (9%), India (3%), and Germany (3%) [4]. This statistic seems disproportionate because it assumes that other countries use fewer antibiotics than the five countries. However, international and national regulatory agencies, such as Food and Agriculture Organization/WHO, Food and Drug Administration, Canadian Food Inspection Agency, the Australian Pesticides and Veterinary Medicines Authority, European Commission, European Food Safety Authority, and the Ministry of Health of each country, continuously attempt to regulate antibiotic use with international standards, considering the specific realities of each country. This harmonization mainly involves the control of parameters, such as (1) acceptable daily intake (ADI), which is a critical standard set from toxicological studies based on the no-observable-effect level and safety factor [68]; (2) withdrawal period or waiting time (WT), which refers to the minimum period from the administration of the last dose of medication and the production of meat or other animal-derived products for food (Figure-2) [68,69]; and (3) MRL, which is the highest level of an antibiotic residue or its metabolites that is legally tolerated in food when antibiotics are correctly applied following Good Agricultural Practice [20]. Although the ADI, WT, and MRL for most antibiotics have been established (for each food) and efforts have been made to regulate the MRL worldwide under the aegis of the World Trade Organization and the Codex Alimentarius, MRLs still vary from one geographical location to another. Meanwhile, although this situation seems to be under control in the European Union countries and other developed countries [68], the problem of antibiotic residues remains topical with unprecedented danger in developing countries due to the lack of control mechanisms despite the existing legislation.
Figure-2.

Consequences of the Presence of Antibiotic Residues in Food and Feed
Adverse consequences of antibiotic residues can be seen on four levels (Figures-2 and 3) [70]: (1) On the health of the animals themselves, (2) on the environment, (3) on the transformation processes (technological risks), and (4) on consumer health.
Figure-3.
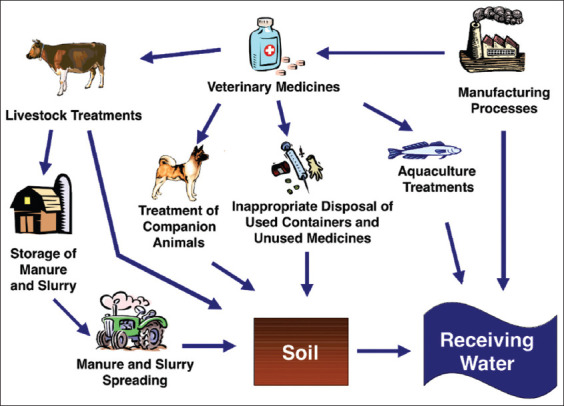
Potential pathways for veterinary medicines in soil and water [70].
Consequences and Risks on Animal Health
We will not discuss the consequences of antibiotics on animals, only those of their residues. It is well established that antibiotics administered to animals can be found in their feces [52-56]. The bacteria present in these feces or those responsible for infections are likely to be exposed to low doses of antibiotics, making them more virulent and more resistant [2,12,71,72] and leading to significant losses [73]. The animal digestive flora disturbance may also lead to the same result.
Environmental Risks
The indiscriminate and improper use of antibiotics can result in higher concentrations of antibiotics in the environment, which is referred to as antibiotic pollution [15]. Most definitely, it is estimated that approximately 75% of antibiotics are not absorbed by animals but are excreted as waste [74]. In addition to this source, as presented in Figure-3, other factors in the production chain, including manure and slurry spreading, aquaculture treatment, and inappropriate disposal of used containers and unused medicines, can lead to the dumping of antibiotic residues in water and soils [70]. Recently, residues of antibiotics like fluoroquinolones (e.g., ciprofloxacin) and sulfonamides (e.g., sulfamethoxazole), which are chemically stable, are often detected in the environment, and the resistance to these antibiotics is frequently reported [75]. These residues are significantly considered pollutants since they disturb the normal flora of soils and water, consequently leading to the production of resistant bacteria through selective pressure. The mechanism of resistance acquisition is mainly attributed to the selection of resistance genes capable of causing enzymatic degradation of antibiotics by bacteria [76], modification of the target of the antibiotic [77], change in membrane permeability [78], and establishment of alternative metabolic pathways [79]. These resistance genes in bacteria can subsequently be transmitted to the next generation of bacteria through a vertical (from one generation to another) or horizontal (from one bacterium to another through a plasmid) gene transfer [75].
Technological Risks
The main technological risks presented by antibiotic residues in foodstuffs are applied to products processed by microbial fermentation, such as milk (fermented milk products and cheese), meat (chorizo, corned beef, pepperoni, soudjouk, salami, and sausage), and fish (bagoong, fesikh, garum, gravlax, shrimp paste, fish sauce, and surströmming). Indeed, antibiotic residues in these raw materials can interfere with the fermentation process by inhibiting the starter cultures, leading to manufacturing accidents and poor-quality foods.
Consequences on Consumer Health
Antibiotic residues are a severe public health issue [80], with their presence in foods capable of causing mild to adverse complications that are difficult to manage. Therefore, we have divided the toxic consequences into two subgroups: (1) Direct toxicity and allergic reactions and (2) resistance to antibiotics as indirect consequences.
Direct Toxicity and Allergic Reactions
Allergic reactions
Recently, several studies have reported that antibiotic residues can elicit allergic reactions. Most of the reported allergies are related to beta-lactam antibiotic residues, especially penicillin and cephalosporins. They include skin rashes, serum sickness, thrombocytopenia, erythema multiforme, hemolytic anemia, vasculitis, acute interstitial nephritis, Stevens–Johnson syndrome, and toxic epidermal necrolysis [81]. For example, allergic reactions have been reported in people who consumed milk [48,81], meat [82], and pork [83], all containing penicillin residues. Furthermore, some studies have mentioned that aminoglycoside, sulfonamide, and tetracycline residues can also cause allergic reactions [1,84].
Hepatotoxicity
Limited data exist on the consequences of antibiotic residues on the liver, possibly due to a lack of investigations pointing toward this direction. However, the hepatotoxic effects of some antibiotics are well known. For example, Hautekeete [85] reported that penicillin, oxacillin, cloxacillin, flucloxacillin, and amoxicillin-clavulanate could cause hepatitis (mainly cholestatic). He also reported that tetracyclines could cause a syndrome mimicking acute fatty liver of pregnancy, indicating the potential of erythromycin and several other macrolides to cause hepatitis (usually cholestatic) [85]. Furthermore, Van Gerven et al. [86] and Hautekeete [85] reported that nitrofurantoin could cause chronic hepatitis mimicking chronic autoimmune hepatitis acute cholestatic and hepatocellular reactions. Finally, it has been reported that ceftriaxone can cause drug-induced gallstones and quinolone cholestasis, and sulfamethoxazole/trimethoprim can cause severe hepatotoxicity, especially in patients with acquired immunodeficiency syndrome [85]. Consequently, we can hypothesize that the residues of the aforementioned antibiotics, whose hepatotoxic effects are well known, can also have harmful consequences on the liver if present in high concentrations.
Destruction of normal and/or useful intestinal flora and indigestion
The intestinal flora contains several microorganisms (with nearly 1000 species) that play an important role in human physiology and health [81]. Despite the inadequate information on the direct impact of residues of antibiotics in food, Kyuchukova [81] and Beyene [80] pointed out that a broad spectrum of antibiotics used in feed can end up in food, adversely affecting the intestinal flora and subsequently causing the gastrointestinal disturbance.
Mutagenicity, reproductive disorders, and teratogenicity
In a recent study summarizing data from 73 scientific studies reporting antimicrobial residues in animal products readily available for sale, Treiber and Beranek-Knauer [87] highlighted that the frequency of mutations is increased with antibiotic residues. In addition, Botsoglou and Fletouris [88] found that several drugs, including doxorubicin, elicit mutagenic activities. Similarly, Beyene [80] reported antibiotic residues as a probable threat to the human population as they adversely affect human fertility. Finally, in their reviews of the consequences of antibiotic residues on humans, Kyuchukova [81] and Darwish et al. [48] mentioned that antibiotic residues could produce reproductive disorders and teratogenic effects.
Carcinogenicity and other effects
Some antibiotic residues, such as sulfamethazine, oxytetracycline, and furazolidone, have carcinogenic effects [1,48,87]. Aside from their carcinogenicity, other effects, including bone marrow toxicity (mainly due to chloramphenicol) and nephropathy (mainly due to gentamicin), were also reported [1]. Beyond all these adverse effects, antibiotic resistance, an indirect consequence of antibiotic residues in food, is the most catastrophic issue, with the WHO estimating that if nothing is done to address this problem, drug-resistant diseases may cause 10 million deaths each year by 2050, consequently damaging the economy as catastrophic as the 2008-2009 global financial crisis [89].
Resistance to Antibiotics
The current transmission mechanisms of antibiotic resistance are better understood than those in the past. It has been established that the exposure of bacteria to low doses of antibiotics is likely to lead to bacterial adaptation, making them more resistant and more virulent [90-94]. Since antibiotic residues can be considered low or subtherapeutic doses, we assume that bacterial exposure can lead to adaptation. This mode of resistance acquisition is mainly based on spontaneous mutations and positive selection [95,96], alteration of the target with decreased affinity for the antibiotic [77,96], alternative metabolic pathways [79], change in membrane permeability, and efflux pumps [78,91]. The transmission of resistance between bacteria through a vertical (from one generation to another) or horizontal (transfer of resistance genes from one bacterium to another through conjugative plasmids, which play an essential role in the dissemination of resistance genes) gene transfer has worsened the situation [75], given that any direct or indirect interaction between humans and animals may lead to zoonotic transmission of antibiotic-resistant strains and genes from food animals to humans (Figure-1) [50]. Therefore, antibiotics should be carefully used to reduce the risks of resistance development and their complications in the management of diseases induced in humans. The most resistant germs found in breeding and food, which are implicated the most in these phenomena, are essentially resistant salmonellae, glycopeptide, or streptogramin-resistant enterococci, multiresistant Escherichia coli, and macrolide or fluoroquinolone-resistant campylobacters [1]. Since carbapenems are strictly prohibited in food-producing animals, the carbapenemase gene plasmids are not expected to end up in the food chain. However, studies reported the presence of carbapenem resistance on plasmids in animal breeding, such as blaOXA-181 in pigs from Italy, blaNDM-1 in pigs, and blmNDM-17 in chicken from China [1]. This result validates the disproportion in the use of antibiotics in some countries. The studies conducted worldwide report an increasing and alarming number of resistance genes to antibiotics in livestock each year. Therefore, measures must be taken to curb this phenomenon because the consequences of infections in humans by multidrug-resistant bacteria are highly expensive and often result in death.
Potential Solutions and Recommendations
Solutions to the problems of antibiotic residues in foods require the implication of both producers and legal organizations. With regard to the responsibilities of legal organizations, we suggest (1) that more rigorous control over the types and concentrations of antibiotics used should be established, (2) antibiotics should be marketed only by professionals who should sell them only with a prescription from a veterinarian, (3) prohibition of antibiotics whose toxicity is established (i.e., chloramphenicol, furazolidone, nitrofurazone, sulfonamides, and fluoroquinolones) and those more likely to induce direct or cross-resistance to antibiotics, (4) mandatory quality controls of food before marketing and the fight against black market products, and (5) permanent awareness of the dangers posed by antibiotic residues in food and resistance to antibiotics. Similarly, producers must (1) use antibiotics only when necessary and under the prescription of a veterinarian, (2) respect withdrawal times and other good practices related to antibiotics, (3) systematically test the presence of residues of the antibiotics used in their production, and (4) educate themselves on the regulations in force and respect them. In a similar manner, economic and straightforward field tests should be developed to identify antibiotic residues in animal products quickly. Meanwhile, methods such as heat treatment, activated charcoal, resin, and UV irradiation, may help inactivate antibiotics. Finally, probiotics and active phytochemical compounds should be further investigated and used as alternatives to antibiotics.
Conclusion
The residues of antibiotics in food are of severe public health concern. Although necessary, even essential in agriculture, they should nevertheless be used more sparingly to avoid the consequences (direct toxicity and resistance to antibiotics) they can cause. Comprehensive antimicrobial use databases should be set up to identify global hotspots where antimicrobials are disproportionately used. Furthermore, risk assessment approaches for preventing diseases, including developing and spreading of antimicrobial resistant bacteria, need to be established. In a similar manner, inexpensive and easy-to-use tests should be developed to detect antibiotic residues in foods rapidly. Finally, healthier approaches such as probiotics and herbal remedies should be used.
Authors’ Contributions
MMJA, AKLD, and PIV: Conceptualized and designed review, literature search, and wrote the first manuscript draft. MMJA, SLA, SS, IK, DMS: Edited and revised the draft of the manuscript. All authors critically reviewed the manuscript and approved the final manuscript.
Acknowledgment
This publication has been supported by the RUDN University Strategic Academic Leadership Program.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Bacanlı M, Başaran N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019;125:462–466. doi: 10.1016/j.fct.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Arsene M.M.J, Viktorovna P.I, Davares A.K.L, Esther N, Nikolaevich S.A. Urinary tract infections:Virulence factors, resistance to antibiotics, and management of uropathogenic bacteria with medicinal plants a review. J. Appl. Pharm. Sci. 2021;11(7):1–12. [Google Scholar]
- 3.Aarestrup F. Sustainable farming:Get pigs off antibiotics. Nature. 2012;486(7404):465–466. doi: 10.1038/486465a. [DOI] [PubMed] [Google Scholar]
- 4.Boeckel T.P, Brower C, Gilbert M, Grenfell B.T, Levin S.A, Robinson T.P, Teillant A, Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlov A, Lashev L, Vachin I, Rusev V. Residues of antimicrobial drugs in chicken meat and offals. Trakia J. Sci. 2008;6(1):23–25. [Google Scholar]
- 6.Liu Y.L, Yan T, Li X.Y, Duan Y.L, Yang X, Yang X.J. Effects of Bacillus subtilis and antibiotic growth promoters on the growth performance, intestinal function and gut microbiota of pullets from 0 to 6 weeks. Animal. 2020;14(8):1619–1628. doi: 10.1017/S1751731120000191. [DOI] [PubMed] [Google Scholar]
- 7.Kantati Y.T. Détection des Résidents D'antibiotiques Dans les Viandes des Bovins Prélevées aux Abattoirs de Dakar Ecole Inter-Etat des Sciences et Médecin Vétérinaires de Dakar (EISMU) Mémoire de Master, Dakar. 2011 [Google Scholar]
- 8.Katz S.E, Brady M.S. Antibiotic residues in food and their significance. Food Biotechnol. 2000;14(3):147–171. [Google Scholar]
- 9.Graham J.P, Boland J.J, Silbergeld E. Growth promoting antibiotics in food animal production:An economic analysis. Public Health Rep. 2007;122(1):79–87. doi: 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempe M, Verachtert B. Cartridges with Molecularly Imprinted Recognition Elements for Antibiotic Residues Monitoring in Milk Cream. Pure and Applied Biochemistry, Lunds Universitét Centre for Chemistry and Chemical Engineering Getingevagen, Lund Sweden. 2000:1–10. [Google Scholar]
- 11.Milhaud G, Pinault L. Evaluation des Médicaments Vétérinaires:Autorisation de Mise sur le Marché(AMM), limites Maximales de Résidus (LMR) Editions Le point Vétérinaire. 2001:25–40. [Google Scholar]
- 12.Manga M.J.A, Davares A.K, Andreevna S.L, Vladimirovich E.A, Carime B.Z, Marouf R, Khelifi I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World. 2021;14(2):319. doi: 10.14202/vetworld.2021.319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busch G, Kassas B, Palma M.A, Risius A. Perceptions of antibiotic use in livestock farming in Germany, Italy and the United States. Livest. Sci. 2020;241:104251. [Google Scholar]
- 14.Cháfer-Pericás C, Maquieira A, Puchades R. Fast screening methods to detect antibiotic residues in food samples. TrAC. 2010;29(9):1038–1049. [Google Scholar]
- 15.Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources:Potential public health implications. Molecules. 2018;23(4):795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guetiya-Wadoum R.E, Zambou N.F, Anyangwe F.F, Njimou J.R, Coman M.M, Verdenelli M.C, Cecchini C, Silvi S, Orpianesi C, Cresci A, Colizzi V. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016;57(4):483–493. doi: 10.1080/00071668.2016.1180668. [DOI] [PubMed] [Google Scholar]
- 17.Khattab W.O, Elderea H.B, Salem E.G, Gomaa N.F. Transmission of administered amoxicillin drug residues from laying chicken to their commercial eggs. J. Egypt. Public Health Assoc. 2010;85(5-6):297–316. [PubMed] [Google Scholar]
- 18.Mahatmi H, da Costa M, Puja I.K. Residues of antibiotic in chicken meat imported from Brasil and United States of America through quarantine stations in Dili, Timor Laste. IOSR J. Agric. Vet. Sci. 2019;12(6):39–43. [Google Scholar]
- 19.Chowdhury S, Hassan M.M, Alam M, Sattar S, Bari M.S, Saifuddin A.K.M, Hoque M.A. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Vet. World. 2015;8(4):467–471. doi: 10.14202/vetworld.2015.467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Qiu W, Li Y, Liu L. Antibiotic residues in poultry food in Fujian Province of China. Food Addit. Contam. Part B Surveill. 2020;13(3):177–184. doi: 10.1080/19393210.2020.1751309. [DOI] [PubMed] [Google Scholar]
- 21.Zheng N, Wang J, Han R, Xu X, Zhen Y, Qu X, Yu Z. Occurrence of several main antibiotic residues in raw milk in 10 provinces of China. Food Addit. Contam. Part B. 2013;6(2):84–89. doi: 10.1080/19393210.2012.727189. [DOI] [PubMed] [Google Scholar]
- 22.Salama N.A, Abou-Raya S.H, Shalaby A.R, Emam W.H, Mehaya F.M. Incidence of tetracycline residues in chicken meat and liver retailed to consumers. Food Addit. Contam. B. 2011;4(2):88–93. doi: 10.1080/19393210.2011.585245. [DOI] [PubMed] [Google Scholar]
- 23.Morshdy A.E, El-Atabany A.I, Hussein M.A, Darwish W.S. Oxytetracycline residues in bovine carcasses slaughtered at Mansoura Abattoir, Egypt. Jpn. J. Vet. Res. 2013;61(Suppl):S44–S47. [PubMed] [Google Scholar]
- 24.Abd El-Aty A.M, Goudah A, Abo El Sooud K. Pharmacokinetics, intramuscular bioavailability and tissue residue profiles of ceftazidime in a rabbit model. Dtsch. Tierarztl. Wochenschr. 2001;108(4):168–71. [PubMed] [Google Scholar]
- 25.Myllyniemi A.L, Rannikko R, Lindfors E, Niemi A, Bäckman C. Microbiological and chemical detection of incurred penicillin G, oxytetracycline, enrofloxacin and ciprofloxacin residues in bovine and porcine tissues. Food Addit. Contam. 2000;17(12):991–1000. doi: 10.1080/02652030050207774. [DOI] [PubMed] [Google Scholar]
- 26.Addo K.K, Mensah G.I, Aning K.G, Nartey N, Nipah G.K, Bonsu C, Akyeh M.L, Smits H.L. Microbiological quality and antibiotic residues in informally marketed raw cow milk within the coastal savannah zone of Ghana. Trop. Med. Int. Health. 2011;16(2):227–232. doi: 10.1111/j.1365-3156.2010.02666.x. [DOI] [PubMed] [Google Scholar]
- 27.O'Keeffe M, Conneely A, Cooper K.M, Kennedy D.G, Kovacsics L, Fodor A, Trigueros G. Nitrofuran antibiotic residues in pork:The FoodBRAND retail survey. Anal. Chim. Acta. 2004;520(1-2):125–131. [Google Scholar]
- 28.Kumar A, Gill J.P.S, Bedi J.S, Chhuneja P.K, Kumar A. Determination of antibiotic residues in Indian honeys and assessment of potential risks to consumers. J. Apic. Res. 2020;59(1):25–34. [Google Scholar]
- 29.Moudgil P, Bedi J.S, Aulakh R.S, Gill J.P.S. Antibiotic residues and mycotoxins in raw milk in Punjab (India):A rising concern for food safety. J. Food Sci. Technol. 2019;56(11):5146–5151. doi: 10.1007/s13197-019-03963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nirala R.K, Anjana K, Mandal K.G, Jayachandran C. Persistence of antibiotic residue in milk under the region of Bihar, India. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(3):2296–2299. [Google Scholar]
- 31.Mahmoudi R, Norian R, Pajohi-Alamoti M. Antibiotic residues in Iranian honey by ELISA. Int. J. Food Prop. 2014;17(10):2367–2373. [Google Scholar]
- 32.Muriuki F.K, Ogara W.O, Njeruh F.M, Mitema E.S. Tetracycline residue levels in cattle meat from Nairobi slaughterhouse in Kenya. J. Vet. Sci. 2001;2(2):97–101. [PubMed] [Google Scholar]
- 33.Kosgey A, Shitandi A, Marion J.W. Antibiotic residues in milk from three popular Kenyan milk vending machines. Am. J. Trop. Med. Hyg. 2018;98(5):1520. doi: 10.4269/ajtmh.17-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shitandi A, Sternesjö Å.S.E. Detection of antimicrobial drug residues in Kenyan milk. J. Food Saf. 2001;21(4):205–214. doi: 10.4315/0362-028x-67.2.399. [DOI] [PubMed] [Google Scholar]
- 35.Cheong C.K, Hajeb P, Jinap S, Ismail-Fitry M.R. Sulfonamides determination in chicken meat products from Malaysia. Int. Food Res. J. 2010;17(4?):885–892. [Google Scholar]
- 36.Nascimento G.G.F, Maestro V, Campos M.S.P. The occurrence of antibiotic residues in milk in commercial establishments in the city of Piracicaba, Sao Paulo, Brazil. Rev. Nutr. 2001;14(2):119–124. [Google Scholar]
- 37.Olufemi O.I, Ehinmowo A.A.E. Oxytetracycline residues in edible tissues of cattle slaughtered in Akure, Nigeria. Int. J. Food Saf. 2009;11(2?):62–66. [Google Scholar]
- 38.Ezenduka E.V, Oboegbulem S.I, Nwanta J.A, Onunkwo J.I. Prevalence of antimicrobial residues in raw table eggs from farms and retail outlets in Enugu State, Nigeria. Trop. Anim. Health Prod. 2011;43(3):557–9. doi: 10.1007/s11250-010-9730-z. [DOI] [PubMed] [Google Scholar]
- 39.Omeiza G.K, Kabir J, Mamman M, Ibrahim H, Fagbamila I.O. Response of Nigerian farmers to a questionnaire on chloramphenicol application in commercial layers. Vet. Ital. 2012;48(1):87–93. [PubMed] [Google Scholar]
- 40.Ibrahim A.I, Junaidu A.U, Garba M.K. Multiple antibiotic residues in meat from slaughtered cattle in Nigeria. Int. J. Vet. Med. 2010;8(1?):1. [Google Scholar]
- 41.Ramatla T, Ngoma L, Adetunji M, Mwanza M. Evaluation of antibiotic residues in raw meat using different analytical methods. Antibiotics. 2017;6(4):34. doi: 10.3390/antibiotics6040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adesiyun A.A, Fasina F.O, Abafe O.A, Mokgoatlheng-Mamogobo M, Adigun O, Mokgophi T, Majokweni Z. Occurrence and concentrations of residues of tetracyclines, polyether ionophores, and anthelmintics in livers of chickens sold in the informal market in Gauteng Province, South Africa. J. Food Sci. 2021;84(4):655–663. doi: 10.4315/JFP-20-312. [DOI] [PubMed] [Google Scholar]
- 43.Goudah A, Shah S.S, Shin H.C, Shim J.H, Abd El-Aty A.M. Pharmacokinetics and mammary residual depletion of erythromycin in healthy lactating ewes. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007;54(10):607–11. doi: 10.1111/j.1439-0442.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 44.Kurwijila L.R, Omore A, Staal S, Mdoe N.S. Investigation of the risk of exposure to antimicrobial residues present in marketed milk in Tanzania. J. Food Prot. 2006;69(10):2487–92. doi: 10.4315/0362-028x-69.10.2487. [DOI] [PubMed] [Google Scholar]
- 45.Nonga H.E, Simon C, Karimuribo E.D, Mdegela R.H. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro municipality, Tanzania. Zoonoses Public Health. 2010;57(5):339–344. doi: 10.1111/j.1863-2378.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 46.Er B, Onurdağ F.K, Demirhan B, Özgacar S.Ö, Öktem A.B, Abbasoğlu U. Screening of quinolone antibiotic residues in chicken meat and beef sold in the markets of Ankara, Turkey. Poult. Sci. 2013;92(8):2212–2215. doi: 10.3382/ps.2013-03072. [DOI] [PubMed] [Google Scholar]
- 47.Nchima G, Choongo K, Muzandu K, Nalubamba K, Muma J, Bumbangi F, Monga G, Kangwa H. Determination of oxytetracycline and sulphamethazine residues in marketed beef from selected parts of Zambia to assess compliance with maximum residual limits. Am. J. Res. Commun. 2017;5:42–64. [Google Scholar]
- 48.Darwish W.S, Eldaly E.A, El-Abbasy M.T, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic residues in food:The African scenario. Jpn. J. Vet. Res. 2013;61(Suppl):S13–S22. [PubMed] [Google Scholar]
- 49.Brown K, Mugoh M, Call D.R, Omulo S. Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera, Nairobi. PLoS One. 2020;15(5):e0233413. doi: 10.1371/journal.pone.0233413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma C, Rokana N, Chandra M, Singh B.P, Gulhane R.D, Gill J.P.S, Ray P, Puniya A.K, Panwar H. Antimicrobial resistance:Its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 2018;4:237. doi: 10.3389/fvets.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, Waddell J. Does the use of antibiotics in food animals pose a risk to human health?A critical review of published data. J. Antimicrob. Chemother. 2004;53(1):28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 52.Christian T, Schneider R.J, Färber H.A, Skutlarek D, Meyer M.T, Goldbach H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003;31(1):36–44. [Google Scholar]
- 53.Xian-Gang H.U, Yi L.U.O, Qi-Xing Z.H.O, Lin X.U. Determination of thirteen antibiotics residues in manure by solid-phase extraction and high-performance liquid chromatography. Chin. J. Anal. Chem. 2008;36(9):1162–1166. [Google Scholar]
- 54.Zhao L, Dong Y.H, Wang H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010;408(5):1069–1075. doi: 10.1016/j.scitotenv.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Chen C.Q, Zheng L, Zhou J.L, Zhao H. Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China. Sci. Total Environ. 2017;580:1175–1184. doi: 10.1016/j.scitotenv.2016.12.075. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X, Wang J, Lu C, Liao Q, Gudda F.O, Ling W. Antibiotics in animal manure and manure-based fertilizers:Occurrence and ecological risk assessment. Chemosphere. 2020;255:127006. doi: 10.1016/j.chemosphere.2020.127006. [DOI] [PubMed] [Google Scholar]
- 57.Zeghilet N, El Hadef E.O.S. Optimisation des Paramètres de Détection et de Quantification des Résidus D'antibiotiques dans la Viande Blanche par Chromatographie Liquide Haute Performence (HPLC) UMC, Theses. 2009 [Google Scholar]
- 58.Cantwell H, O'keeffe M. Evaluation of the Premi®Test and comparison with the One-Plate Test for the detection of antimicrobials in kidney. Food Addit. Contam. 2006;23(2):120–125. doi: 10.1080/02652030500357433. [DOI] [PubMed] [Google Scholar]
- 59.Kilinc B, Cakli S. Screening for antibiotic residues in the trout by the Four Plate test, Premi test and ELISA test. Eur. Food Res. Technol. 2008;226(4):795–799. [Google Scholar]
- 60.Rahimi Z, Shahbazi Y, Ahmadi F. Comparative screening of chloramphenicol residue in chicken tissues using four plate test and Premi®test methods. Pharm. Sci. 2018;24(2):157–162. [Google Scholar]
- 61.Kyselková M, Jirout J, Vrchotová N, Schmitt H, Elhottová D. Spread of tetracycline resistance genes at a conventional dairy farm. Front. Microbiol. 2015;6:536. doi: 10.3389/fmicb.2015.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.?Mensah S.E.P, Koudande O.D, Aboh B.A, Adjahoutonon K.Y.K, Salifou S, Mensah G.A, Abiola F.A. Evaluation des résidus de tétracyclines et de bêta-lactamines dans le lait de vache produit au Centre Bénin. Rev Élev. Méd. Vét. Pays Tro. 2019;72(4):181–185. [Google Scholar]
- 63.Siljanoski A, Ciglarič R, Pezdir T, Lainšček P. R, Dolenc J, Starič J, Šinigoj-Gačnik K. Detection of tetracycline and other antimicrobial residues in milk from cows with clinical mastitis treated by combination therapy. J Dairy Res. 2018;85(3):321–326. doi: 10.1017/S0022029918000389. [DOI] [PubMed] [Google Scholar]
- 64.Broekaert K, Ooghe S, Reybroeck W. Validation Report of 4SENSOR BSTQ (KIT072) a Rapid Test for ß-lactams, Sulfonamides. Tetracyclines and (Fluoro) Quinolones Detection in Milk (UNISENSOR sa, Seraing, Belgium) 2020 [Google Scholar]
- 65.Huang Y, Han X, Yu X, Wang S, Zhai H. Capillary electrophoresis-indirect laser-induced fluorescence detection of neomycin in fish. Chromatographia. 2021;84(9):1–8. [Google Scholar]
- 66.Khatibi S.A, Hamidi S, Siahi-Shadbad M.R. Current trends in sample preparation by solid-phase extraction techniques for the determination of antibiotic residues in foodstuffs:A review. Crit. Rev. Food. 2020;61(20):3361–3382. doi: 10.1080/10408398.2020.1798349. [DOI] [PubMed] [Google Scholar]
- 67.Xie Y, Zhu X, Sun Y, Wang H, Qian H, Yao W. Rapid detection method for nitrofuran antibiotic residues by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2012;235(3):555–561. [Google Scholar]
- 68.Okocha R.C, Olatoye I.O, Adedeji O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018;39(1):21. doi: 10.1186/s40985-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aroeira C.N, Feddern V, Gressler V, Contreras-Castillo C.J, Hopkins D.L. Growth promoters in cattle and pigs:A review of legislation and implications for human health. Food Rev. Int. 2021. [Retrieved on 10-03-2022]. Avaialbe from: https://doi.org/10.1080/∳9129.2021.1961268 .
- 70.US Geological Survey. Potential Pathways for Veterinary Medicines in Soil and Water. 2020. [Retrieved on 13-07-2020]. Available from: https://www.usgs.gov/media/images/potential-pathways-veterinary-medicines-soil-and-water .
- 71.Mbarga M.M.J, Andreevna S.L, Viktorovna P.I. Evaluation of apparent microflora and study of antibiotic resistance of coliforms isolated from the shells of poultry eggs in Moscow-Russia. J. Adv. Microbiol. 2020;20(4):70–77. [Google Scholar]
- 72.Joseph M.M.A, Podoprigora I.V, Volina E.G, Ermolaev A.V, Smolyakova L.A. Evaluation of changes induced in the probiotic Escherichia coli M17 following recurrent exposure to antimicrobials. J. Pharm. Res. Int. 2021;33(29B):158–167. [Google Scholar]
- 73.Muhammad J, Khan S, Su J.Q, Hesham A.E.L, Ditta A, Nawab J, Ali A. Antibiotics in poultry manure and their associated health issues:A systematic review. J. Soils Sediments. 2020;20(1):486–497. [Google Scholar]
- 74.Chee-Sanford J.C, Mackie R.I, Koike S, Krapac I.G, Lin Y.F, Yannarell A.C, Aminov R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009;38(3):1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- 75.Lundborg C.S, Tamhankar A.J. Antibiotic residues in the environment of South East Asia. BMJ. 2017;358:j2440. doi: 10.1136/bmj.j2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reis A.C, Kolvenbach B.A, Nunes O.C, Corvini P.F. Biodegradation of antibiotics:The new resistance determinants-part I. N. Biotechnol. 2020;54:34–51. doi: 10.1016/j.nbt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Schaenzer A.J, Wright G.D. Antibiotic resistance by enzymatic modification of antibiotic targets. Trends. Mol. Med. 2020;26(8):768–782. doi: 10.1016/j.molmed.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Xu L, Zhou Z, Zhu L, Han Y, Lin Z, Feng W, Chen H. Antibiotic resistance genes and microcystins in a drinking water treatment plant. Environ. Pollut. 2020;258:113718. doi: 10.1016/j.envpol.2019.113718. [DOI] [PubMed] [Google Scholar]
- 79.Pollock J, Low A.S, McHugh R.E, Muwonge A, Stevens M.P, Corbishley A, Gally D.L. Alternatives to antibiotics in a one-health context and the role genomics can play in reducing antimicrobial use. Clin. Microbiol. Infect. 2020;26(12):1617–1621. doi: 10.1016/j.cmi.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 80.Beyene T. Veterinary drug residues in food-animal products:Its risk factors and potential effects on public health. J. Vet. Sci. Technol. 2016;7(1):1–7. [Google Scholar]
- 81.Kyuchukova R. Antibiotic residues and human health hazard-review. Bulg. J. Agric. Sci. 2020;26(3):664–668. [Google Scholar]
- 82.Baynes R.E, Dedonder K, Kissell L, Mzyk D, Marmulak T, Smith G, Riviere J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016;88:112–122. doi: 10.1016/j.fct.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Raison-Peyron N, Messaad D, Bousquet J, Demoly P. Anaphylaxis to beef in penicillin-allergic patient. Allergy. 2001;56(8):796–797. doi: 10.1034/j.1398-9995.2001.056008796.x. [DOI] [PubMed] [Google Scholar]
- 84.Donkor E.S, Newman M.J, Tay S.C, Dayie N.T, Bannerman E, Olu-Taiwo M. Investigation into the risk of exposure to antibiotic residues contaminating meat and egg in Ghana. Food Control. 2011;22(6):869–873. [Google Scholar]
- 85.Hautekeete M.L. Hepatotoxicity of antibiotics. Acta Gastroenterol. Belg. 1996;58(3):290–296. [PubMed] [Google Scholar]
- 86.Van Gerven N.M, de Boer Y.S, Mulder C.J, van Nieuwkerk C.M, Bouma G. Auto immune hepatitis. World J. Gastroenterol. 2016;22(19):4651. doi: 10.3748/wjg.v22.i19.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Treiber F.M, Beranek-Knauer H. Antimicrobial residues in food from animal origin a review of the literature focusing on products collected in stores and markets worldwide. Antibiotics (Basel) 2021;10(5):534. doi: 10.3390/antibiotics10050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Botsoglou N, Fletouris D. Drug Residues in Foods Pharmacology:Food Safety and Analysis. Mrcel Dekker, New York. 2001:98–150. [Google Scholar]
- 89.Mbarga M.J.A, Podoprigora I.V, Davares A.K.L, Razan M, Das M.S, Senyagin A.N. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World. 2021;14(5):1330–1341. doi: 10.14202/vetworld.2021.1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevens D.L, Ma Y, Salmi D.B, McIndoo E, Wallace R.J, Bryant A.E. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2007;195(2):202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 91.Dopcea G.N, Dopcea I, Nanu A.E, Diguţă C.F, Matei F. Resistance and cross-resistance in Staphylococcus spp. strains following prolonged exposure to different antiseptics. J. Glob. Antimicrob. Resist. 2020;21:399–404. doi: 10.1016/j.jgar.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 92.Lan S, Li Z, Su A, Peng Y, Liao Y, Liu X, Tan Q. Study on the mechanisms of the cross-resistance to TET, PIP, and GEN in Staphylococcus aureus mediated by the Rhizoma coptidis extracts. J Antibiot (Tokyo) 2021;74(5):330–336. doi: 10.1038/s41429-021-00407-4. [DOI] [PubMed] [Google Scholar]
- 93.Baseri N, Najar-Peerayeh S, Bakhshi B. The effect of subinhibitory concentration of chlorhexidine on the evolution of vancomycin-intermediate Staphylococcus aureus and the induction of mutations in walKR and vraTSR systems. Infect. Genet. Evol. 2021;87:104628. doi: 10.1016/j.meegid.2020.104628. [DOI] [PubMed] [Google Scholar]
- 94.Puangseree J, Jeamsripong S, Prathan R, Pungpian C, Chuanchuen R. Resistance to widely-used disinfectants and heavy metals and cross-resistance to antibiotics in Escherichia coli isolated from pigs, pork and pig carcass. Food Control. 2021;124:107892. [Google Scholar]
- 95.Arsene M.M.J, Viktorovna P.I, Grigorievna V.E, Davares A.K, Sergeevna D.M, Nikolaevna S.I. Prolonged exposure to antimicrobials induces changes in susceptibility to antibiotics, biofilm formation and pathogenicity in Staphylococcus aureus. J. Pharm. Res. Int. 2021;33(34B):140–151. [Google Scholar]
- 96.Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007;2(3):323–334. doi: 10.2217/17460913.2.3.323. [DOI] [PubMed] [Google Scholar]


