Abstract
STUDY QUESTION
Does maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the first trimester affect the risk of miscarriage before 13 week’s gestation?
SUMMARY ANSWER
Pregnant women with self-reported diagnosis of SARS-CoV-2 in the first trimester had a higher risk of early miscarriage.
WHAT IS KNOWN ALREADY
Viral infections during pregnancy have a broad spectrum of placental and neonatal pathology. Data on the effects of the SARS-CoV-2 infection in pregnancy are still emerging. Two systematic reviews and meta-analyses reported an increased risk of preterm birth, caesarean delivery, maternal morbidity and stillbirth. Data on the impact of first trimester infection on early pregnancy outcomes are scarce. This is the first study, to our knowledge, to investigate the rates of early pregnancy loss during the SARS-CoV-2 outbreak among women with self-reported infection.
STUDY DESIGN, SIZE, DURATION
This was a nationwide prospective cohort study of pregnant women in the community recruited using social media between 21 May and 31 December 2020. We recruited 3545 women who conceived during the SARS-CoV-2 pandemic who were <13 week’s gestation at the time of recruitment.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The COVID-19 Contraception and Pregnancy Study (CAP-COVID) was an on-line survey study collecting longitudinal data from pregnant women in the UK aged 18 years or older. Women who were pregnant during the pandemic were asked to complete on-line surveys at the end of each trimester. We collected data on current and past pregnancy complications, their medical history and whether they or anyone in their household had symptoms or been diagnosed with SARS-CoV-2 infection during each trimester of their pregnancy. RT-PCR-based SARS-CoV-2 RNA detection from respiratory samples (e.g. nasopharynx) is the standard practice for diagnosis of SARS-CoV-2 in the UK. We compared rate of self-reported miscarriage in three groups: ‘presumed infected’, i.e. those who reported a diagnosis with SARS-CoV-2 infection in the first trimester; ‘uncertain’, i.e. those who did not report a diagnosis but had symptoms/household contacts with symptoms/diagnosis; and ‘presumed uninfected’, i.e. those who did not report any symptoms/diagnosis and had no household contacts with symptoms/diagnosis of SARS-CoV-2.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 3545 women registered for the CAP-COVID study at <13 weeks gestation and were eligible for this analysis. Data for the primary outcome were available from 3041 women (86%). In the overall sample, the rate of self-reported miscarriage was 7.8% (238/3041 [95% CI, 7–9]). The median gestational age (GA) at miscarriage was 9 weeks (interquartile range 8–11). Seventy-seven women were in the ‘presumed infected’ group (77/3041, 2.5% [95% CI 2–3]), 295/3041 were in the uncertain group (9.7% [95% CI 9–11]) and the rest in the ‘presumed uninfected’ (87.8%, 2669/3041 [95% CI 87–89]). The rate of early miscarriage was 14% in the ‘presumed infected’ group, 5% in the ‘uncertain’ and 8% in the ‘presumed uninfected’ (11/77 [95% CI 6–22] versus 15/295 [95% CI 3–8] versus 212/2669 [95% CI 7–9], P = 0.02). After adjusting for age, BMI, ethnicity, smoking status, GA at registration and the number of previous miscarriages, the risk of early miscarriage appears to be higher in the ‘presumed infected’ group (relative rate 1.7, 95% CI 1.0–3.0, P = 0.06).
LIMITATIONS, REASONS FOR CAUTION
We relied on self-reported data on early pregnancy loss and SARS-CoV-2 infection without any means of checking validity. Some women in the ‘presumed uninfected’ and ‘uncertain’ groups may have had asymptomatic infections. The number of ‘presumed infected’ in our study was low and therefore the study was relatively underpowered.
WIDER IMPLICATIONS OF THE FINDINGS
This was a national study from the UK, where infection rates were one of the highest in the world. Based on the evidence presented here, women who are infected with SARS-CoV-2 in their first trimester may be at an increased risk of a miscarriage. However, the overall rate of miscarriage in our study population was 8%. This is reassuring and suggests that if there is an effect of SARS-CoV-2 on the risk of miscarriage, this may be limited to those with symptoms substantial enough to lead to a diagnostic test. Further studies are warranted to evaluate a causal association between SARS-CoV-2 infection in early pregnancy and miscarriage risk. Although we did not see an overall increase in the risk of miscarriage, the observed comparative increase in the presumed infected group reinforces the message that pregnant women should continue to exercise social distancing measures and good hygiene throughout their pregnancy to limit their risk of infection
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by a grant from the Elizabeth Garrett Anderson Hospital Charity (G13-559194). The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. J.A.H. is supported by an NIHR Advanced Fellowship. A.L.D. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support to J.A.H. and A.L.D. as above; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: early miscarriage, pregnancy loss, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, Coronavirus disease 2019, COVID-19
Introduction
Despite more than 300 million cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections worldwide, many important questions remain unanswered on the impact of this infection in pregnancy. There have been numerous publications on SARS-CoV-2 in pregnancy but the focus of research has been on hospitalized pregnant women with severe infections in the late second and third trimesters (Khalil et al., 2020; Knight et al., 2020; Pierce-Williams et al., 2020). Seroprevalence studies and a recent meta-analysis have shown that SARS-CoV-2 is commonly asymptomatic in pregnant women (Allotey et al., 2020; Crovetto et al., 2020); yet little is known about the effect of asymptomatic or mild infections on early pregnancy loss. Vaccine hesitancy amongst young women remains a concern and one of the latest reports from UK Obstetric Surveillance System reveals that 99% of pregnant women admitted to hospital with symptomatic infection are unvaccinated (Vousden et al., 2022). Therefore, information about the potential effect of SARS-COV-2 on pregnancy is pertinent and urgent.
Studies of past outbreaks of viral infections in pregnancy, such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and influenza A/H1N1, show conflicting data. The 2002 SARS coronavirus epidemic was associated with severe maternal illness, maternal death, and miscarriage, but there were no cases of vertical transmission (Schwartz and Graham, 2020). There are only a few documented cases of MERS in pregnancy (n = 11) but the maternal and foetal fatality rates were high in both (27%) (Schwartz and Graham, 2020). During the 2009 influenza A/H1N1 pandemic, pregnant women were found to be at an increased risk of becoming severely ill and increased risk of hospitalization, intensive care unit admission and death (Jamieson et al., 2009; Mosby et al., 2011). Infants of affected mothers were rarely affected but they were more likely to be born preterm (Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007), whilst vertical transmission was not conclusively established (Mosby et al., 2011).
Data on the effects of the SARS-CoV-2 infection in pregnancy are still emerging. Two systematic reviews and meta-analyses reported an increased risk of preterm birth, caesarean delivery, maternal morbidity (Khalil et al., 2020) and stillbirth (Allotey et al., 2020). Infants born to mothers with confirmed SARS-CoV-2 infection were mostly asymptomatic and transmission of the virus was uncommon (Knight et al., 2020; Pierce-Williams et al., 2020) with around 1.9% of infants born to women with confirmed infection testing positive (Khalil et al., 2020). Emerging data show that vertical transmission of the virus is probable (Baud et al., 2020; Farrell et al., 2020; Khalil et al., 2020) but further evidence is still required.
Data on the impact of first trimester infection with SARS-CoV-2 on early pregnancy outcomes are scarce. The majority of published studies are retrospective in design with small sample sizes (Cosma et al., 2021; la Cour Freiesleben et al., 2021; Sacinti et al., 2021). We performed a nationwide prospective cohort study in the United Kingdom (UK) where infection rates are one of the highest in the world and where nasopharyngeal PCR testing was readily available from 28 May 2020 to those with symptoms. We collected prospective data on pregnancy outcomes from women in the community who conceived during the SARS-CoV-2 pandemic. In this paper, we assess the impact of SARS-CoV-2 infection in the first trimester on early miscarriage risk (pregnancy loss before 13 weeks (≤12 + 6) of pregnancy).
Materials and methods
Study design and participants
The COVID-19 Contraception and Pregnancy Study (CAP-COVID) is an on-line survey study collecting longitudinal data from pregnant women in the UK aged 18 years or older.
The purpose of the study was to evaluate the impact of the SARS-CoV-2 pandemic on first trimester outcomes among non-hospitalized women with mild/asymptomatic infection. Women who were pregnant during the pandemic were asked to complete on-line surveys at the end of each trimester. We collected data on current and past pregnancy complications, their medical history and whether or not they or anyone in their household had symptoms or been diagnosed with SARS-CoV-2 infection during each trimester of their pregnancy. RT-PCR-based SARS-CoV-2 RNA detection from respiratory samples (e.g. nasopharynx) is the standard practice for diagnosis of SARS-CoV-2 in the UK.
Women were invited to take part in the study through social media advertising. We used two social media channels, Facebook and Instagram. The CAP-COVID Facebook and Instagram accounts were set up as a business page by the UK-based social media marketing company MCRLF Ltd. All posts, both organic and paid for, were posted under that name and contained the University College London (UCL) logo. No posts were made from a personal account. The participants were targeted by gender (female), age (18–45 years) and location (UK). We also targeted a range of interests that were relevant to women who had recently become pregnant, for example pregnancy tests and childbirth. All social media posts were created and posted by MCRLF Ltd and were assessed for suitability by the UCL Ethics Committee. The ad campaigns ran from 25 May to 2 December 2020. Those interested in participating were directed to the study website (https://cap-covid.uk). Participants were asked to provide informed consent before being given access to the online registration questionnaire (Supplementary Data Files S1 and S2) both of which were hosted on the UCL RedCap server (https://redcap.idhs.ucl.ac.uk/surveys). Survey data were stored on a secure UCL server and password protected.
Demographic variables were recorded for all participants including age, ethnicity and geography (i.e. postcode). Information regarding their current and previous pregnancy outcomes and medical history were also collected. The gestational age (GA) was calculated from the last menstrual period date (LMP) or expected due date where available, or if neither were known we asked for an estimated GA at the time of registration. Women registering for the study before 13 completed weeks of gestation were sent their first trimester survey (T1) at the end of the first trimester of their pregnancy. A total of three reminder emails were sent. Participants were asked if they had an ongoing pregnancy or whether they had experienced a miscarriage; termination of pregnancy for medical or other reasons; or other pregnancy outcomes (e. g. pregnancy of unknown location, ectopic pregnancy or molar pregnancy). Participants were also asked whether they: had symptoms of SARS-CoV-2; had been diagnosed with SARS-CoV-2; and/or had any household contact(s) who were diagnosed or had symptoms of SARS-CoV-2. We recruited participants from May 2020 to December 2020. The study period for this analysis was 3 June 2020 to 25 February 2021 when the first participant completed the first trimester follow-up survey to the last participant to complete the follow-up survey.
Testing capacity for SARS-CoV-2 changed significantly during the study period. Although the UK started testing for SARS-CoV-2 using PCR from March 2020, they had limited capacity for tests, which were reserved for patients admitted to hospital, and for active contract tracing, which was confined to high-risk settings such as care homes. From 18 May 2020, PCR tests were made widely available to anyone with symptoms of SARS-CoV-2; however, laboratory capacity was still limited until June 2020. Antigen testing using lateral flow tests only became widely available in the UK from 9 April 2021 to screen for asymptomatic infection. Antibody testing was only used in research settings in the UK. We did not ask study participants what tests were used to diagnose SARS-CoV-2 but, based on this timeline, it is likely that during our study period anyone reporting a diagnosis of SARS-CoV-2 would have tested positive on PCR testing.
Study participants were grouped based on their presumed infection status. Those who reported a diagnosis of SARS-CoV-2 were allocated to the ‘presumed infected’ group. Those had symptoms and/or household contacts with symptoms/diagnosis of SARS-CoV-2 but did not report a diagnosis were allocated to the ‘uncertain’ group. Those who did not report any symptoms/diagnosis with SARS-CoV-2 and had no household members with symptoms/diagnosis of SARS-CoV-2 were allocated to the ‘presumed uninfected’ group.
Outcome measures
Our primary outcome was the rate of self-reported miscarriage between registration and the conclusion of 13 weeks pregnancy among those who conceived during the SARS-CoV-2 pandemic. The secondary outcomes include the rate of SARS-CoV-2 infection in this population and the clinical manifestations of those reporting a diagnosis of SARS-CoV-2.
Sample size and statistical analysis
A power calculation was performed based on the prevalence of early miscarriage in the general population (20%) (Rossen et al., 2018; Tommys, 2018). We calculated that 1400 women in the first trimester would need to be included to provide 90% power to detect a 5% increase in the rate of early miscarriage (25% versus 20%), at a two-sided alpha level of 0.05. We planned to recruit 1680 women in their first trimester to account for an expected 20% loss to follow-up. At the time of designing the study, the true prevalence of SARS-CoV-2 among the pregnant population was unknown owing to limitations on testing availability. Data published in October 2020 showed that prevalence of SARS-CoV-2 among pregnant women was only 2.9%. We therefore continued to recruit beyond the calculated sample size.
For variables with missing data, we used case wise deletion. A Poisson regression model with a sandwich error term was used to estimate the relative rates and corresponding 95% two-sided CIs. Previous studies have shown this method is appropriate for analysing rare events in cohort studies when subjects are followed for a variable length of time and that it is less prone to convergence issues than other similar methods (Zou, 2004; Coomarasamy et al., 2019). We used Stata version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, USA: StataCorp LLC) and IBM SPSS Statistics for Windows version 27.0. (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY, USA: IBM Corp) for all analyses.
Patient and public involvement
The study survey was distributed among pregnant women undergoing routine antenatal care to assess comprehension and sensitivity of the questions. The feedback received was incorporated in the final version.
Ethics
The University College London (UCL) Ethics Committee approved all data collection (REC ID Number 18251/001). The online surveys and database were hosted on RedCap using the UCL Data Safe Haven platform.
Results
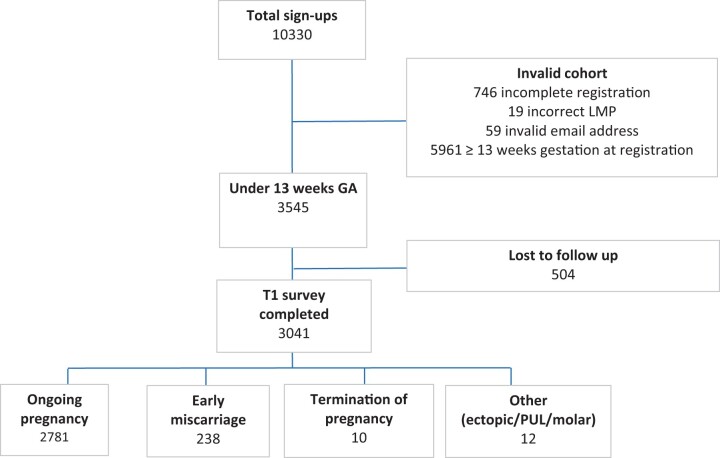
From 24 May 2020 to 31 December 2020, a total of 3545 pregnant women registered for the study and were <13 weeks gestation (Fig. 1). The percentage of women with available data for the primary outcome was 86% (3041/3545); the remaining 14% (504/3545) did not complete their first trimester follow-up survey and were lost to follow-up. The earliest LMP was 25 February 2020 and the latest LMP was 29 November 2020. Demographic characteristics of those who completed the study are presented in Table I.
Figure 1.
Trial flowchart and first trimester pregnancy outcomes. This was a UK population-based prospective cohort study of 3041 pregnancies conceived during pandemic to determine if maternal infection with SARS-CoV-2 in the first trimester affected the risk of miscarriage before 13 week’s gestation. GA, gestational age; LMP, last menstrual period; PUL, pregnancy of unknown location; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T1, first trimester survey.
Table I.
Baseline characteristics and first trimester pregnancy outcomes in the UK in 2020 by SARS-CoV-2 infection status.
| Outcomes | Presumed infected (n=77) | Uncertain (n=295) | Presumed uninfected (n=2669) | P value |
|---|---|---|---|---|
| Median age [IQR] | 31 [27-39] | 32 [29-35] | 34 [30-36] | 0.13 |
| Median GA at recruitment | 8 [6-9] | 7 [5-10] | 7 [5-9] | 0.07 |
| Median BMI (kg/m2) [IQR] | 24 [23-31] | 25 [20-32] | 24 [22-29] | 0.06 |
| Ethnicity | 0.94 | |||
| White - n (%) | 69 (90) | 270 (92) | 2425 (91) | |
| BAME - n (%) | 5 (6) | 14 (5) | 154 (6) | |
| Did not disclose - n (%) | 3 (4) | 11 (4) | 90 (3) | |
| Smokers - n (%) | 1 (1) | 11 (4) | 94 (4) | 0.56 |
| Type of conception | 0.48 | |||
| Natural - n (%) | 71 (92) | 281 (95) | 2501 (94) | |
| Fertility treatment - n (%) | 6 (8) | 14 (5) | 168 (6) | |
| Previous early miscarriages < 13 wk – n (%) | 0.06 | |||
| 0 | 57 (74) | 215 (73) | 2126 (80) | |
| 1 or 2 | 18 (23) | 71 (24) | 467 (17) | |
| ≥ 3 | 2 (3) | 9 (3) | 76 (3) | |
| Ongoing pregnancy at 13 wk - n (%) | 66 (86) | 279 (95) | 2436 (91) | 0.03 |
| Miscarriage < 13 wk - n (%) | 11 (14) | 15 (5) | 212 (8) | 0.02 |
| Median GA at miscarriage diagnosis | 9 [8-11] | 9 [8-12] | 9 [8-11] | 0.95 |
| Termination of pregnancy - n (%) | 0 (0) | 1 (0) | 9 (0) | 0.71 |
| Other (molar/ectopic/PUL) - n (%) | 0 (0) | 0 (0) | 12 (0) | 0.48 |
SARS-CoV-2: severe acute respiratory syndrome coronavirus, IQR: interquartile range, GA: gestational age, BAME: Black, Asian and minority ethnic PUL: pregnancy of unknown location, wk: weeks. We used non-parametric tests to compare medians and chi square test to compare proportions. Bold indicates statistical significance.
First trimester pregnancy outcomes
The median GA at registration was 8 weeks (interquartile range (IQR) 6–10). The rate of self-reported miscarriage before 13 week’s gestation was 8% (238/3041, 95% CI 7–9) and median GA at miscarriage was 9 weeks (IQR 8–11) (Table I).
SARS-COV-2 infection and its clinical manifestations in the first trimester
A total of 77/3041 women were in the ‘presumed infected’ group (2.5% [95% CI 2–3]), 295/3041 were in the ‘uncertain’ group (9.7%, [95% CI 9–11]) and the rest in the ‘presumed uninfected’ (87.8%, 2669/3041, [95% CI 87–89]) (Table I).
The most common symptoms reported in the ‘presumed infected’ group were fatigue (83%), loss of smell/taste (68%) and headaches (66%) (Table II). Five percent of women were asymptomatic (4/77). None of the 77 women in the SARS-CoV-2 positive group were hospitalized.
Table II.
Clinical manifestations of SARS-CoV-2 among pregnant women in the UK who reported a diagnosis with SARS-CoV-2 infection in their first trimester.
| Symptoms | Participants (n=77) n (%, 95% CI) |
|---|---|
| Asymptomatic | 4 (5, 1-13) |
| Fever | 30 (39, 28-51) |
| Persistent cough | 26 (34, 23-45) |
| Fatigue | 64 (83, 73-91) |
| Headache | 51 (66, 55-77) |
| Shortness of breath | 38 (49, 38-61) |
| Sore throat | 26 (34, 23-45) |
| Loss of smell/taste | 52 (68, 56-78) |
| Hoarse voice | 6 (8, 3-16) |
| Abdominal pain | 7 (9, 4-18) |
| Diarrhoea | 17 (22, 13-33) |
| Confusion/disorientation or drowsiness | 9 (12, 5-21) |
| Loss of appetite | 31 (40, 29-52) |
| Muscle pains and/or aches | 43 (56, 44-67) |
SARS-CoV-2: severe acute respiratory syndrome coronavirus
Pregnancy outcomes by SARS-CoV-2 infection status
The rate of early miscarriage was 14% in the ‘presumed infected’ group, 5% in the ‘uncertain’ group and 8% in the ‘presumed uninfected’ group (11/77 [95% CI 6–22] versus 15/295 [95% CI 3–8] versus 212/2669 [95% CI 7–9], P = 0.02) (Table I).
After adjusting for age, BMI, ethnicity, smoking status, GA at registration and the number of previous miscarriages, the risk of early miscarriage appears to be higher among women with presumed SARS-CoV-2 infection in the first trimester compared to those with no infection; however, it does not reach statistical significance (relative rate 1.7, 95% CI 1.0–3.0, P = 0.06) (Table III).
Table III.
Relative risk of early miscarriage.
| Relative Risk | 95% CI |
P Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| SARS-CoV-2 status | ||||
| Presumed uninfected (baseline) | 1.0 | |||
| Presumed infected | 1.7 | 1.0 | 3.0 | 0.06 |
| Age - yrs. | 1.1 | 1.1 | 1.1 | <0.001 |
| BMI | 1.0 | 1.0 | 1.0 | 0.3 |
| Gestational age at recruitment | 0.8 | 0.8 | 0.9 | <0.001 |
| Ethnicity | ||||
| White (baseline) | 1.0 | |||
| BAME | 0.7 | 0.3 | 1.2 | 0.1 |
| Smoking status | ||||
| Non-smoker (baseline) | 1.0 | |||
| Smoker | 0.4 | 0.1 | 1.2 | 0.1 |
| Number of previous miscarriages | ||||
| No previous miscarriages (baseline) | 1.0 | |||
| 1 to 2 previous miscarriages | 1.0 | 0.8 | 1.4 | 0.9 |
| 3 or more previous miscarriages | 1.3 | 0.7 | 2.5 | 0.3 |
SARS-CoV-2: severe acute respiratory syndrome coronavirus, BAME: Black, Asian and minority ethnic.
Discussion
Main findings
We undertook a nationwide (UK) prospective cohort study of women who conceived during the SARS-CoV-2 pandemic and obtained longitudinal data on their pregnancy outcomes at 13 weeks completed pregnancy. The rate of SARS-COV-2 diagnosis among pregnant women in their first trimester was 3%, with predominant symptoms being fatigue, loss of smell/taste and headaches. In our overall study population, the miscarriage rate was of 8%, which is reassuring. However, in those who reported a diagnosis of SARS-CoV-2 infection we observed an increased risk of early pregnancy loss compared to the rest of our sample. While our reported relative risk of 1.7 is a strong indication of an association between ‘presumed infection’ and pregnancy loss, the study was underpowered owing to the low numbers of exposed women and hence it did not reach formal statistical significance (Wasserstein and Lazar, 2016). Nevertheless, women who are hoping to conceive or are in early stages of pregnancy during this time should continue to exercise caution and social distancing as well as consider vaccination until further studies corroborate or refute our finding.
This is the first study to date to investigate the rates of early pregnancy loss during the SARS-CoV-2 outbreak and, to the best of our knowledge, there are no other prospective studies evaluating the impact of SARS-CoV-2 infection on first trimester pregnancy loss in non-hospitalized women. We identified three retrospective studies investigating whether infection in pregnancy is associated with early miscarriage (la Cour Freiesleben et al., 2021; Cosma et al., 2021; Sacinti et al., 2021). All three studies recruited women attending early pregnancy and antenatal clinic appointments.
In a letter to the Editor, Sacinti et al. (2021) compared the incidence of miscarriage between a 13 week period in 2019 and 2020, and found that the incidence of miscarriage was increased by 25% during the SARS-CoV-2 pandemic, reporting an overall rate of 11.8%. This finding is in agreement with our observation that SARS-CoV-2 infection increases the risk of a loss. In contrast, the overall rate of miscarriage for women who conceived during our study period was only 8%, which is lower than expected at a population level. A recent paper, which collected pooled data from nine cohort studies using self-reported pregnancy outcomes, calculated the risk of miscarriage as 15.3% of all recognized pregnancies (95% CI 12.5–18.7%) (Quenby et al., 2021). This disparity may be because the median GA at registration in our study was 8 weeks, thus pregnancies lost before 8 weeks would have been missed. We are unable to determine the GA at registration in the Sacinti et al. (2021) report.
The second study, by Cosma et al. (2021), found no significant difference in the cumulative incidence of SARS-CoV-2 infection among women who experienced a miscarriage to those with ongoing pregnancies. Infection status was confirmed with a positive antibody test or PCR. The third study, by la Cour Freiesleben et al. (2021), compared nuchal translucency thickness in those with SARS-CoV-2 infection and healthy controls, and found no difference. They also reported no significantly increased risk of pregnancy loss in women with antibodies for SARS-CoV-2 (odds ratio = 3.4, 0.08–24.3 95% CI, P = 0.27): in this study, infection status was confirmed using antibody status alone. All three studies were also limited by very low numbers of positive cases for SARS-CoV-2 in their study populations (n = 3, n = 23 and n = 18, respectively).
Viral infections during pregnancy have a broad spectrum of placental and neonatal pathology and can lead to foetal malformation, preterm labour, growth restriction, stillbirth and spontaneous abortion (León-Juárez et al., 2017; Oliveira et al., 2019; Prochaska et al., 2020). Several studies using viral models, such as human cytomegalovirus, herpes virus, HIV-1, influenza and, most recently, ZIKA, have demonstrated that the immune response at the maternal–foetal interface is directed towards a pro-inflammatory state, which can interrupt the structural and functional conditions of the human placenta (León-Juárez et al., 2017). In humans, placental development starts in the endometrium 7 days after fertilization (León-Juárez et al., 2017). We now have indirect evidence of placental involvement with SARS-CoV-2 infection, which could explain the association between early miscarriage and SARS-CoV-2 infection (Prochaska et al., 2020; Shanes et al., 2020; Martinez-Perez et al., 2021). Coronaviruses have been shown to induce a proinflammatory cytokine storm, primarily through the production of IL-6 (Sharma et al., 2020). This is the mechanism thought to be behind the association between SARS-CoV-2 and premature rupture of membranes (Prochaska et al., 2020; Shanes et al., 2020; Martinez-Perez et al., 2021). Cytokines are also thought to play a role in the pathogenesis of recurrent pregnancy loss and thus the pro-inflammatory response triggered by SARS-CoV-2 could also explain the increased risk of early miscarriage among infected women.
The SARS-CoV-2 pandemic has been continuously evolving with different variants, symptom manifestations, virulence and vaccination statuses, and evaluation of pregnancy outcomes needs to be an ongoing exercise.
Limitations
At the time of designing the study, the true prevalence of SARS-CoV-2 among the pregnant population was unknown owing to limitations on testing availability. Our study reported a prevalence close to 3% in this population. Based on this, we would need to recruit 545 pregnant women diagnosed with SARS-CoV-2 to detect a 5% increase in the rate of early miscarriage and thus the study was underpowered. The number of pregnant women in our study with presumed SARS-CoV-2 infection was small (3% (77/3041)). This is most likely a result of limited testing availability and extended measures implemented by the UK government to limit the transmission of the virus, as well as pregnant women taking additional precautionary measures such as self-quarantine and limiting social contacts.
Symptoms of SARS-CoV-2 infection in pregnancy are non-specific. Since testing was not widely available in the early stages of the pandemic and pregnant women are commonly asymptomatic for the infection, it is likely that a number of women classified as ‘presumed uninfected’ may have in fact been infected. We do not know whether those in the ‘presumed uninfected’ group had a negative PCR test or were never tested and we do not have any serological data from either group.
We used self-reported data on pregnancy outcomes and infection status. Previous studies have evaluated self-reported data on spontaneous pregnancy loss, and found good correlation between hospital records and self-reported data in questionnaire studies, with 75–80% of self-reported miscarriages being verified with hospital records (Wilcox and Horney, 1984; Axelsson, 1990).
Our study population was recruited primarily through social media. This has been shown to yield a less demographically diverse sample than hospital-based recruitment; however, hospital-based recruitment would bias the study towards those with pregnancy complications.
Despite a high participant number, we lost 13% to follow-up. This could be a potential source of selection bias if those lost to follow-up were different in terms of rates of SARS-CoV-2 infection and/or pregnancy loss. Although every effort was made to encourage reporting and contact these women sensitively, some women who experienced miscarriage may have not wanted to report it or feel able to continue with the study.
Conclusion
This is a national study from the UK, where infection rates were one of the highest in the world. Based on the evidence presented here, women who are infected with SARS-CoV-2 in their first trimester may be an increased risk of an early miscarriage. Our findings should reinforce the message that pregnant women should continue to exercise social distancing measures and good hygiene throughout their pregnancy to limit their risk of infection.
Several different strains of SARS-CoV-2 have emerged since our study period and infection with these variants may not pose the same risk as the original virus. Further studies are warranted to evaluate a causal association between infection with new variants of SARS-CoV-2 in early pregnancy and miscarriage risk. A prospective longitudinal study of women hoping to conceive should be designed with periodic SARS-CoV-2 screening to identify asymptomatic carriers and robust follow-up with serological testing to reduce the risk of response bias.
Data availability
The data relating to this analysis will be made available from the UCL Discovery database linked to the publication record in the UCL Research Publication Service. The corresponding author attests that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained. Dissemination to participants and related patient and public communities: results of the study will be accessible to participants and the public through the study websites.
Authors’ roles
N.B. conceived and J.A.H., G.B.P., J.M.S., E.Y., D.M., M.C.D., A.L.D. and N.B. designed the study. N.B., E.Y., H.O.N., G.B.P. and D.M. analysed and interpreted the data. N.B., E.Y. and D.M. drafted the first version of the manuscript. All of the authors contributed to, read, and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. D.M. and E.Y. are the guarantors.
Funding
This study was supported by a grant from the Elizabeth Garrett Anderson Hospital Charity (G13-559194). The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. J.A.H. is supported by an NIHR Advanced Fellowship. A.L.D. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support to J.A.H. and A.L.D. as above; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
Contributor Information
Neerujah Balachandren, Reproductive Medicine Unit, University College London Hospital, London, UK.
Melanie C Davies, Reproductive Medicine Unit, University College London Hospital, London, UK.
Jennifer A Hall, Elizabeth Garrett Anderson Institute for Women’s Health, University College London, London, UK.
Judith M Stephenson, Elizabeth Garrett Anderson Institute for Women’s Health, University College London, London, UK.
Anna L David, Elizabeth Garrett Anderson Institute for Women’s Health, University College London, London, UK.
Geraldine Barrett, Elizabeth Garrett Anderson Institute for Women’s Health, University College London, London, UK.
Helen C O’Neill, Elizabeth Garrett Anderson Institute for Women’s Health, University College London, London, UK.
George B Ploubidis, Centre for Longitudinal Studies, Social Research Institute, University College London, London, UK .
Ephia Yasmin, Reproductive Medicine Unit, University College London Hospital, London, UK.
Dimitrios Mavrelos, Reproductive Medicine Unit, University College London Hospital, London, UK.
References
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D et al. ; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson G. Use of questionnaires in a study of spontaneous abortion in a general population. J Epidemiol Community Health 1990;44:202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020;323:2198–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, Williams H, Eapen AK, Roberts T, Ogwulu CC et al. A randomized trial of progesterone in women with bleeding in early pregnancy. N Engl J Med 2019;380:1815–1824. [DOI] [PubMed] [Google Scholar]
- Cosma S, Carosso AR, Cusato J, Borella F, Carosso M, Bovetti M, Filippini C, D'Avolio A, Ghisetti V, Di Perri G et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am J Obstet Gynecol 2021;224:391.e391–391.e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovetto F, Crispi F, Llurba E, Figueras F, Gómez-Roig MD, Gratacós E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet 2020;396:530–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell R, Michie M, Pope R. Pregnant women in trials of Covid-19: a critical time to consider ethical frameworks of inclusion in clinical trials. Ethics Hum Res 2020;42:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. The National Academies Collection: Reports funded by National Institutes of Health. In: Behrman RE, Butler AS (eds). Preterm Birth: Causes, Consequences, and Prevention. Washington (DC: ): National Academies Press, 2007. [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451–458. [DOI] [PubMed] [Google Scholar]
- Khalil A, Kalafat E, Benlioglu C, O'Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Heath P, Ladhani S et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020;25:100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O'Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour Freiesleben N, Egerup P, Hviid KVR, Severinsen ER, Kolte AM, Westergaard D, Fich Olsen L, Prætorius L, Zedeler A, Christiansen AH et al. SARS-CoV-2 in first trimester pregnancy: a cohort study. Hum Reprod 2021;36:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Juárez M, Martínez–Castillo M, González-García LD, Helguera-Repetto AC, Zaga-Clavellina V, García-Cordero J, Flores-Pliego A, Herrera-Salazar A, Vázquez-Martínez ER, Reyes-Muñoz E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog Dis 2017;75:ftx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Perez O, Prats Rodriguez P, Muner Hernandez M, Encinas Pardilla MB, Perez Perez N, Vila Hernandez MR, Villalba Yarza A, Nieto Velasco O, Del Barrio Fernandez PG, Forcen Acebal L et al. ; Spanish Obstetric Emergency Group. The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth 2021;21:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011;205:10–18. [DOI] [PubMed] [Google Scholar]
- Oliveira GM, Pascoal-Xavier MA, Moreira DR, Guimarães VS, Aguiar RALP, Miranda DM, Romanelli RMC. Detection of cytomegalovirus, herpes virus simplex, and parvovirus b19 in spontaneous abortion placentas. J Matern Fetal Neonatal Med 2019;32:768–775. [DOI] [PubMed] [Google Scholar]
- Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, Penfield CA, Roman AS, DeBolt CA, Stone JL et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol 2020;2:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska E, Jang M, Burd I. COVID-19 in pregnancy: placental and neonatal involvement. Am J Reprod Immunol 2020;84:e13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021;397:1658–1667. [DOI] [PubMed] [Google Scholar]
- Rossen LM, Ahrens KA, Branum AM. Trends in risk of pregnancy loss among US women, 1990-2011. Paediatr Perinat Epidemiol 2018;32:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacinti KG, Kalafat E, Sukur YE, Koc A. Increased incidence of first-trimester miscarriage during the COVID-19 pandemic. Ultrasound Obstet Gynecol 2021;57:1013–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol 2020;154:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma BK, Kakker NK, Bhadouriya S, Chhabra R. Effect of TLR agonist on infections bronchitis virus replication and cytokine expression in embryonated chicken eggs. Mol Immunol 2020;120:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommys. How Common is Miscarriage? 2018. https://www.tommys.org/pregnancy-information/im-pregnant/early-pregnancy/how-common-miscarriage#:~:text=The%20estimated%20figure%20is%20that,13%20to%2024%20of%20pregnancy.
- Vousden N, Ramakrishnan R, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, Quigley M, Brocklehurst P, Kurinczuk JJ et al. Severity of maternal infection and perinatal outcomes during periods of SARS-CoV-2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Med 2022;1:e000053. https://doi.org/10.1136/bmjmed-2021-000053. [DOI] [PMC free article] [PubMed]
- Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat 2016;70:129–133. [Google Scholar]
- Wilcox AJ, Horney LF. Accuracy of spontaneous abortion recall. Am J Epidemiol 1984;120:727–733. [DOI] [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data relating to this analysis will be made available from the UCL Discovery database linked to the publication record in the UCL Research Publication Service. The corresponding author attests that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained. Dissemination to participants and related patient and public communities: results of the study will be accessible to participants and the public through the study websites.