Abstract
Background
Understanding the dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission is important for adequate infection control measures in this ongoing pandemic.
Methods
Households were enrolled upon a polymerase chain reaction–confirmed index case between October and December 2020, prior to the coronavirus disease 2019 vaccination program. Saliva samples were obtained by self-sampling at days 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42 from study inclusion. Nasopharyngeal swabs (NPS) and oropharyngeal swabs (OPS) were collected by the research team at day 7 and capillary blood samples at day 42. Household secondary attack rate (SAR) and per-person SAR were calculated based on at least 1 positive saliva, NPS, OPS, or serum sample. Whole genome sequencing was performed to investigate the possibility of multiple independent SARS-CoV-2 introductions within a household.
Results
Eighty-five households were included consisting of 326 (unvaccinated) individuals. Comparable numbers of secondary cases were identified by saliva (133/241 [55.2%]) and serum (127/213 [59.6%]). The household SAR was 88.2%. The per-person SAR was 64.3%. The majority of the secondary cases tested positive in saliva at day 1 (103/150 [68.7%]). Transmission from index case to household member was not affected by age or the nature of their relationship. Phylogenetic analyses suggested a single introduction for the investigated households.
Conclusions
Households have a pivotal role in SARS-CoV-2 transmission. By repeated saliva self-sampling combined with NPS, OPS, and serology, we found the highest SARS-CoV-2 household transmission rates reported to date. Salivary (self-) sampling of adults and children is suitable and attractive for near real-time monitoring of SARS-CoV-2 transmission in this setting.
Keywords: SARS-CoV-2, COVID-19, household transmission, saliva
By repeated saliva self-sampling combined with nasopharyngeal and oropharyngeal swabs and serology in 85 households, we report the highest SARS-CoV-2 household transmission rates to date. Households are pivotal in SARS-CoV-2 transmission and salivary sampling may assist in infection control in this setting.
Since the first identification in December 2019, coronavirus disease 2019 (COVID-19) numbers continue to increase and have passed 245 million cases globally [1]. Insight into transmission dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is of major importance for infection control measures.
While the current standard testing method for SARS-CoV-2 detection is mainly via reverse-transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swabs (NPS) [2], other specimens like saliva may also offer a good source for detection by RT-PCR [3, 4]. Multiple studies have demonstrated a comparable or higher sensitivity of detecting SARS-CoV-2 using saliva compared to NPS, both in adults and children [5–9]. Furthermore, RT-PCR on saliva effectively identifies SARS-CoV-2 even before the period of infectiousness [10], and particularly high sensitivity rates of RT-PCR on saliva have been found among studies involving asymptomatic individuals [11, 12]. Compared to NPS, salivary testing is less invasive and samples can be easily collected by individuals themselves (including children with assistance of their parents) without need of qualified personnel, allowing frequent sampling. Moreover, salivary self-sampling has been shown to be more sensitive than self-administered nasal swabs [5]. Salivary self-sampling could therefore contribute to improved monitoring and lower costs [13].
As not only symptomatic, but also asymptomatic and presymptomatic individuals are considered to be potential sources of new infections [14, 15], high-density salivary sampling (ie, frequent sampling in a short time period) for RT-PCR testing of SARS-CoV-2 can be applied to investigate SARS-CoV-2 transmission in households. Households are considered to be one of the most frequent settings of SARS-CoV-2 transmission with close contact between members over a long time within confined spaces without use of personal protective equipment [16].
The reported secondary attack rates (SARs) of previous household studies show a wide range of transmission from 6% to 50%, depending on the study setting, study period, frequency of testing, testing method, and specimen types analyzed [17–24]. Because the majority of these studies have been performed in adults, uncertainty still exists about the SARs in children and their role as index cases in household transmission.
Upcoming variants associated with higher transmissibility [25] contribute to the persistence of the SARS-CoV-2 pandemic. A better understanding of SARS-CoV-2 transmission dynamics, including the role of children, will support the rationale behind public health policies, like school closure and other infection control measures. The aim of this study is to assess household transmission dynamics of SARS-CoV-2 by frequent saliva sampling in combination with NPS, OPS, and serology, to determine factors associated with transmission and to investigate the suitability of salivary (self-) sampling to monitor SARS-CoV-2 household transmission.
MATERIALS AND METHODS
Study Design
In this prospective cohort study, SARSLIVA (SARS-CoV-2 in saLIVA), households were eligible in case of an index case aged <65 years with RT-PCR–confirmed SARS-CoV-2 on a combined NPS and oropharyngeal swab (OPS) during the previous 72 hours (index case) and with at least 2 additional household members willing to participate in the study. Between October and December 2020, prior to the COVID-19 vaccination program, participants were recruited by the Public Health Services Kennemerland, The Netherlands or, in case of employees of the Spaarne Gasthuis hospital, Haarlem/Hoofddorp, The Netherlands, by the hospital’s Infection Control Department. Index cases could be either symptomatic or asymptomatic.
This study was reviewed and approved by the Medical Ethical Committee of the Vrije Universiteit University Medical Centre, The Netherlands (reference number 2020.436).
Sample Collection
Saliva samples were obtained by participants themselves at home at days 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42 (with day of inclusion as day 0). At day 7, NPS and OPS were collected from all participants by the research team during a home visit. At day 42, capillary blood samples were collected by the research team during a second home visit for serological analyses. See Supplementary Materials for details.
Questionnaires
An online baseline questionnaire was obtained at day 0. This questionnaire contained questions on household composition, household characteristics, smoking, medical history, self-reported ethnicity, and educational level. Each night before the predefined time points for saliva collection, online COVID-19 symptomatology questionnaires were sent to the participants, consisting of self-perceived severity scores (“no,” “mild,” “moderate,” “severe”) per symptom. Medical records were obtained if participants were admitted to a hospital or visited a general practitioner during the study period.
Case Definitions
The first member of each household with an RT-PCR–confirmed SARS-CoV-2 infection included in the study was defined as the index case. As this index case was not necessarily the primary case (the first SARS-CoV-2 infection) in a household, we performed a sensitivity analysis with a more stringent index case definition (see Statistical Methods below). SARS-CoV-2 infection during follow-up was defined as either at least 1 positive SARS-CoV-2 RT-PCR result on 1 of the saliva samples, on NPS or OPS at day 7, or detection of serum antibodies at day 42, regardless of the presence of symptoms.
COVID-19 disease severity was classified with a 4-degree scale per time point, derived from national and international guidelines [26, 27]: (1) no coronavirus-related symptoms; (2) mild symptoms (rhinitis, pharyngitis, mild dyspnea, mild or moderate coughing, olfactory dysfunction, or gustatory dysfunction); (3) moderate symptoms (moderate or severe dyspnea, severe coughing, temperature >38°C, or a pneumonia diagnosed by a physician); and (4) hospital admission due to coronavirus-related symptoms. A maximum severity score over all time points was calculated per participant.
Molecular Diagnostics and Serology
Initial combined NPS/OPS of the index cases as well as NPS and OPS obtained at day 7 were analyzed for the presence of SARS-CoV-2 by the Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands, as described previously. SARS-CoV-2 viral loads in saliva samples were analyzed by the laboratory of the National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands, as described before [28, 29]. See Supplementary Materials for details.
Amplicon-based SARS-CoV-2 sequencing was performed on the positive saliva sample with the highest viral load for each individual using the Nanopore protocol “PCR tiling of COVID-19 virus (version: PTC_9096_v109_revE_06FEB2020),” which is based on the ARTIC version 3 amplicon sequencing protocol [30, 31]. See Supplementary Materials for details.
Sera were tested for the presence of immunoglobulin G antibodies reactive with the SARS-CoV-2 spike trimer, S1, and N antigens in a protein microarray, in duplicate 2-fold serial dilutions starting at 1:20, essentially as described previously [32]. For each antigen, a 4-parameter log logistic calibration curve was generated and effective concentration 50, mid-point antibody titers were calculated. Raw data were processed with R version 4.04 statistical software [33].
Statistical Methods
The household SAR was calculated by dividing households with secondary transmission by the total number of households. Per-person SAR was calculated by dividing the number of secondary cases by the number of participating household members. Logistic regression models were used to compare characteristics of index cases and household members and to assess the relation between household SAR and per-person SAR and the characteristics of households, index cases, and household members. Statistical analyses were performed with R version 3.6.2 software (R Core Team, Vienna, Austria). P values <.05 were considered significant.
To account for the influence of our index case definition on the (per-person) SARs, a sensitivity analysis was performed in which household and per-person SARs were calculated after excluding households in which it was uncertain whether the index case was the primary case. Households were excluded when a household member had (1) an RT-PCR–confirmed SARS-CoV-2 infection 1–14 days prior to the index case or (2) reported symptoms 1–14 days prior to the index case and tested positive in either saliva, NPS, OPS, or serum during follow-up.
RESULTS
Baseline Characteristics
In total, 390 index cases were approached, of whom 91 were included. Six dropped out, resulting in a total of 85 households consisting of 326 (unvaccinated) participants (85 index cases and 241 household members). For all participants (n = 326), protocol adherence for collection of the specimens was 92.4% (94.8% saliva, 92.9% NPS, 93.6% OPS, and 88.4% serum).
The median age of the index cases was 40.0 (interquartile range [IQR], 22.0–48.0) years, and 17 of 85 (20.0%) index cases were <18 years old (Table 1). The median age of household members was 20.0 (IQR, 12.0–45.0) years and 106 of 241 (44.0%) household members were <18 years old. The majority of the index cases were female (56 [65.9%]). Fifty-five (64.7%) index cases had mild symptoms, 22 had (25.9%) moderate symptoms, and 7 (8.2%) were asymptomatic during the study period. One (1.2%) index case had severe symptoms leading to hospital admission. Roughly half of the participating household members were asymptomatic (121/241 [50.2%]), regardless of their test results. Ninety-four (39.0%) had mild symptoms, 25 (10.4%) had moderate symptoms, and 1 (0.4%) had severe symptoms leading to hospital admission. The median time between index symptom onset and a positive test result (in symptomatic cases) was 1 day (IQR, 1.0–2.0 days) and the median study enrollment (day 0) was 4 (IQR, 3.0–4.0) days after symptom onset.
Table 1.
Baseline Characteristics of Study Participants (N = 326)
| Characteristic | Total No. of Participants (N = 326)a | Index Cases (n = 85) | Household Members (n = 241) | P Value | OR (95% CI) |
|---|---|---|---|---|---|
| Child (<18 y) | 123 (37.7) | 17 (20.0) | 106 (44.0) | <.001 | 0.32 (.18–.57) |
| Adult | 203 (62.3) | 68 (80.0) | 135 (56.0) | <.001 | 3.14 (1.74–5.66) |
| Age, y, median (IQR) | 28.5 (13.0–46.0) | 40.0 (22.0–48.0) | 20.0 (12.0–45.0) | <.001 | 1.03 (1.02–1.05) |
| Age group, y | |||||
| <12 | 59 (18.1) | 1 (1.2) | 58 (24.1) | 1 (ref) | |
| 12–17 | 64 (19.6) | 16 (18.8) | 48 (19.9) | .005 | 19.33 (2.47–151.11) |
| 18–39 | 74 (22.7) | 25 (29.4) | 49 (20.3) | <.001 | 29.59 (3.89–226.36) |
| 40–49 | 78 (23.9) | 25 (29.4) | 53 (22.0) | <.001 | 27.36 (3.58–208.97) |
| 50–65 | 51 (15.6) | 18 (21.2) | 33 (13.7) | <.001 | 31.64 (4.04–247.85) |
| Sex, female | 157 (48.2) | 56 (65.9) | 101 (41.9) | <.001 | 2.68 (1.60–4.49) |
| BMI classb | |||||
| Normal weight | 195 (59.8) | 45 (52.9) | 150 (66.1) | 1 (ref) | |
| Obesity | 23 (7.1) | 10 (11.8) | 13 (5.7) | .038 | 2.56 (1.05–6.24) |
| Overweight | 87 (26.7) | 28 (32.9) | 59 (26.0) | .108 | 1.58 (.90–2.77) |
| Underweight | 7 (2.1) | 2 (2.4) | 5 (2.2) | .736 | 1.33 (.25–7.11) |
| Underlying medical condition | 37 (11.3) | 10 (11.8) | 27 (11.2) | .888 | 1.06 (.49–2.29) |
| Cardiovascular disease | 10 (3.1) | 2 (2.4) | 8 (3.3) | … | |
| Lung disease | 1 (0.3) | 1 (1.2) | 0 (0.0) | … | |
| Immune disorder | 1 (0.3) | 1 (1.2) | 0 (0.0) | … | |
| Diabetes | 3 (0.9) | 1 (1.2) | 2 (0.8) | … | |
| Rheumatic disorder | 2 (0.6) | 1 (1.2) | 1 (0.4) | … | |
| Other | 24 (7.4) | 5 (5.9) | 19 (7.9) | … | |
| Smoking (yes) | 11 (3.4) | 4 (4.7) | 7 (2.9) | .433 | 1.65 (.47–5.79) |
| Nationality (other)c | 4 (1.2) | 2 (2.4) | 2 (0.8) | .298 | 2.86 (.40–20.60) |
| Positive saliva day 1d | 176 (54.0) | 73 (85.9) | 103 (42.7) | <.001 | 13.37 (5.90–30.26) |
| Symptom statuse | |||||
| Severe symptomsf | 2 (0.6) | 1 (1.2) | 1 (0.4) | .052 | 17.29 (.98–306.28) |
| Moderate symptoms | 47 (14.4) | 22 (25.9) | 25 (10.4) | <.001 | 15.21 (5.86–39.46) |
| Mild symptoms | 149 (45.7) | 55 (64.7) | 94 (39.0) | <.001 | 10.11 (4.40–23.23) |
| Asymptomatic | 128 (39.2) | 7 (8.2) | 121 (50.2) | 1 (ref) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Some numbers might not add up to 326 due to missing values.
BMI categories for index cases and household members 2 to <18 years of age were defined as BMI z-score < –2, underweight; –2 to 1, normal weight; 1–2, overweight; >2, obesity (source: Centers for Disease Control and Prevention, https://www.cdc.gov/growthcharts/zscore.htm, accessed 28 September 2021). BMI categories for index cases and household members ≥18 years of age were defined as <18.5 kg/m2, underweight; 18.5–24.9 kg/m2, normal weight; 25.0–29.9 kg/m2, overweight; ≥30.0 kg/m2, obesity.
Other than Dutch.
Saliva at day 1 available for 315 participants.
Maximum over 10 time points.
Hospital admission due to coronavirus-related symptoms.
The median size of the households was 4 (IQR, 3.0–4.0) participating household members (Table 2).
Table 2.
Household Characteristics (n = 85)
| Characteristics | Total Households (n = 85)a | Households With Secondary Transmission (n = 75) | Households Without Secondary Transmission (n = 10) | P Value | OR (95% CI) |
|---|---|---|---|---|---|
| Median household size (IQR) | 4.0 (3.0–4.0) | 4.0 (3.0–4.0) | 3.5 (3.0–4.0) | .274 | 1.76 (.64–4.87) |
| Median household size, No. of persons | |||||
| 3 | 29 (34.1) | 24 (32.0) | 5 (50.0) | 1 (ref) | |
| 4 | 42 (49.4) | 38 (50.7) | 4 (40.0) | .343 | 1.98 (.48–8.11) |
| 5 | 13 (15.3) | 12 (16.0) | 1 (10.0) | .426 | 2.50 (.26–23.86) |
| 6 | 1 (1.2) | 1 (1.3) | 0 (0.0) | … | |
| Educational levelb | |||||
| High | 57 (70.4) | 51 (70.8) | 6 (66.7) | 1 (ref) | |
| Middle/low | 24 (29.6) | 21 (29.2) | 3 (33.3) | .797 | 0.82 (.19–3.60) |
| Median No. of bedrooms per household (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 3.5 (2.3–4.8) | .627 | 1.17 (.62–2.19) |
| No. of bedrooms | |||||
| 2 | 5 (5.9) | 2 (2.7) | 3 (30.0) | 1 (ref) | |
| 3 | 34 (40.0) | 32 (42.7) | 2 (20.0) | .007 | 24.00 (2.43–236.89) |
| 4 | 21 (24.7) | 19 (25.3) | 2 (20.0) | .024 | 14.25 (1.42–143.19) |
| 5 | 20 (23.5) | 18 (24.0) | 2 (20.0) | .027 | 13.5 (1.34–135.98) |
| ≥6 | 5 (5.9) | 4 (5.3) | 1 (10.0) | .214 | 6.00 (.35–101.57) |
| Median No. of toilets per household (IQR) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | .799 | 0.88 (.32–2.40) |
| No. of toilets | |||||
| 1 | 6 (7.1) | 5 (6.7) | 1 (10.0) | 1 (ref) | |
| 2 | 64 (75.3) | 57 (76.0) | 7 (70.0) | .676 | 1.63 (.17–16.02) |
| 3 | 12 (14.1) | 11 (14.7) | 1 (10.0) | .602 | 2.20 (.11-42.74) |
| ≥4 | 3 (3.5) | 2 (2.6) | 1 (10.0) | .577 | 0.40 (.02–10.02) |
| Has pets in the household | 48 (56.5) | 41 (54.7) | 7 (70.0) | .365 | 0.52 (.12–2.15) |
Data are presented as No. (%) unless otherwise indicated. The household secondary attack rate was 88.2%.
Abbreviations: CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Some numbers might not add up to 85 due to missing values.
Educational level was categorized as high if at least 1 household member aged ≥21 years had completed at least vocational or university education, and middle/low for all others.
SARS-CoV-2 Detection in Different Specimens and Phylogenetic Results
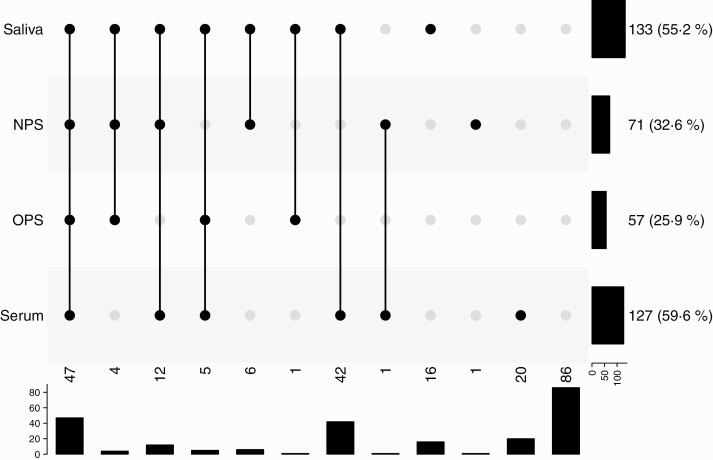
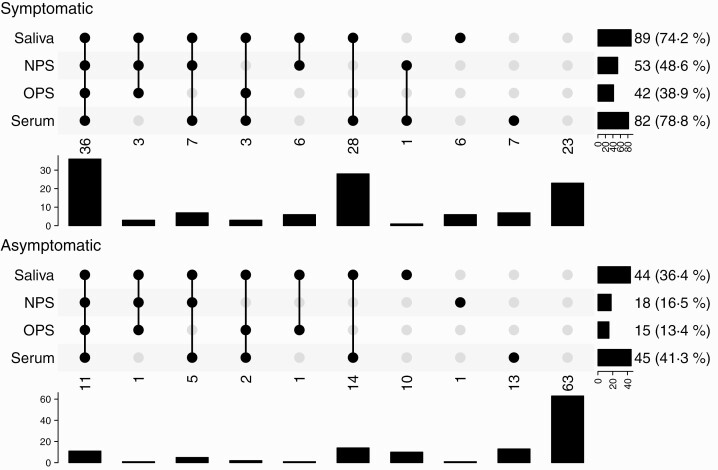
Of the household members, 64.3% tested positive in at least 1 saliva, NPS, OPS, or serum specimen (155/241 secondary cases; Figure 1). Household members tested positive for SARS-CoV-2 in saliva in 55.2% (133/241) and for SARS-CoV-2 antibodies in serum at day 42 in 59.6% (127/213). Only 32.6% (71/218) and 25.9% (57/220) were positive in NPS and OPS at day 7, respectively. Sixteen household members tested positive for SARS-CoV-2 in saliva only, resulting in a decline to 57.7% (139/241) in the proportion of secondary cases if saliva would not have been obtained. Of these household members, 9 tested negative for SARS-CoV-2 antibodies in serum and in 7 household members serum was not collected. Twenty household members tested positive for SARS-CoV-2 antibodies in serum only. Of the secondary cases, 58 (37.4%) were asymptomatic (Figure 2). The highest proportion of asymptomatic secondary cases tested positive in serum (45/109 [41.3%]), followed by saliva (44/121 [36.4%]).
Figure 1.
Secondary transmission in household members (n = 241) defined with different sample type results. In this “upset plot,” each column is a pattern of co-occurrences of positivity (filled and connected dots indicate a positive test, gray dots indicate a negative or missing test result). The rows indicate the different tests, with the bar chart to the right showing the number of occurrences of positivity of each test. Below each column is a bar chart indicating the number of occurrences of the pattern. Data on saliva, nasopharyngeal swabs (NPS), oropharyngeal swabs (OPS), and serology were available for 241, 218, 220, and 213 household members, respectively; 155 household members were positive in either saliva, NPS, OPS, or serology (secondary cases); saliva positivity was defined as ≥1 reverse-transcription polymerase chain reaction–positive saliva sample at days 1–42; serology positivity was defined as immunoglobulin G antibody positivity for ≥1 antigen (severe acute respiratory syndrome coronavirus 2 spike trimer, S1, or N).
Figure 2.
Secondary transmission in household members (n = 241) defined with different sample type results and symptom status. In this “upset plot,” each column is a pattern of co-occurrences of positivity (filled and connected dots indicate a positive test, gray dots indicate a negative or missing test result). The rows indicate the different tests, with the bar chart to the right showing the number of occurrences of positivity of each test. Below each column is a bar chart indicating the number of occurrences of the pattern. One hundred twenty household members were symptomatic and 121 household members were asymptomatic; data on saliva, nasopharyngeal swabs (NPS), oropharyngeal swabs (OPS), and serology were available for 120, 109, 108, and 104 symptomatic household members, respectively; data on saliva, NPS, OPS and serology were available for 121, 109, 112, and 109 asymptomatic household members, respectively. Ninety-seven symptomatic and 58 asymptomatic household members were positive in either saliva, NPS, OPS, or serology (secondary cases); saliva positivity was defined as ≥1 reverse-transcription polymerase chain reaction–positive saliva sample at days 1–42; serology positivity was defined as immunoglobulin G antibody positivity for ≥1 antigen (severe acute respiratory syndrome coronavirus 2 spike trimer, S1, or N); asymptomatic household members were defined as not reporting symptoms on any of the examinations (day 1–42).
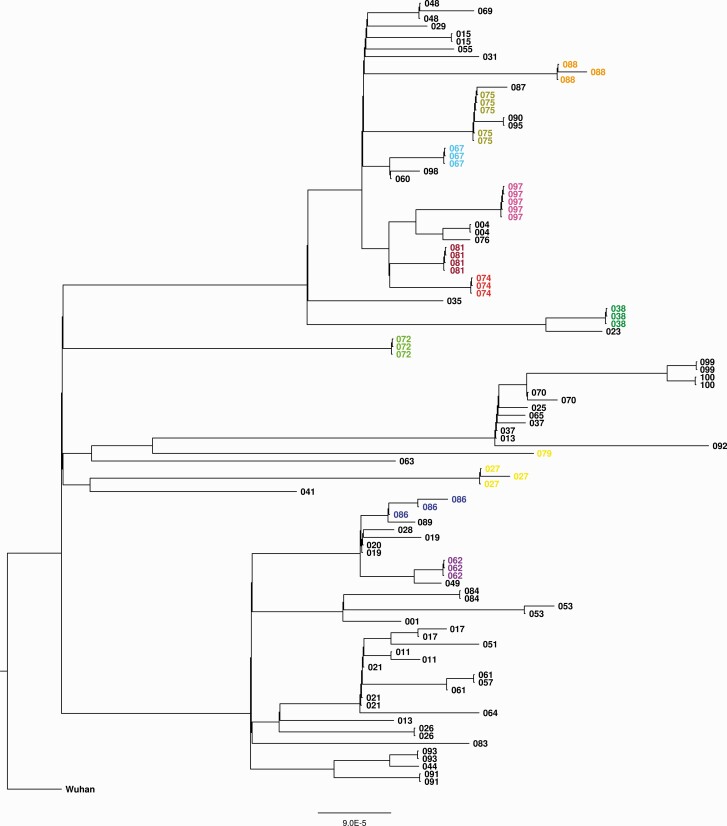
To investigate the possibility of multiple independent SARS-CoV-2 introductions within a household, SARS-CoV-2 genomes were analyzed by whole genome sequencing. For 103 individuals originating from 60 households, successful sequence analyses was possible. Each household shows a distinct cluster in phylogenetic analyses with minimal sequence differences (Figure 3), indicative of a single introduction within each household. However, in certain cases very similar sequences were observed between different households, representing infections in those households with closely related variants. For certain households only a single genome could be determined, for which no conclusions could be drawn.
Figure 3.
Phylogenetic analysis of severe acute respiratory syndrome coronavirus 2 sequences within households (60 households, 103 individuals). Sequences were obtained from saliva samples with the highest viral load and are labeled per household. Households with 3 or more available sequences are indicated in color.
Secondary Attack Rates
Secondary infection (based on saliva, NPS, and OPS RT-PCR results and serological analyses) was detected in 75 of 85 households, leading to a household SAR of 88.2% (Table 2). The median household size did not significantly differ between households with and without secondary transmission (4.0 [IQR, 3.0–4.0] vs 3.5 [IQR, 3.0–4.0] persons; P = .274).
Secondary transmission was detected in households of 16 of 17 index cases under the age of 18 (Table 3). No significant differences in secondary transmission were observed between the age groups of the index cases. Median Cp values of the index case initial NPS/OPS were not significantly different between households with and without secondary transmission (25.0 [IQR, 22.1–29.6] vs 23.9 [IQR, 22.8–26.4]; P = .837).
Table 3.
Index Case Characteristics (n = 85)
| Characteristic | Total Index Cases (n = 85)a | Households With Secondary Transmission (n = 75) | Households Without Secondary Transmission (n = 10) | P Value | OR (95% CI) |
|---|---|---|---|---|---|
| Child (<18 y) | 17 (20.0) | 16 (21.3) | 1 (10.0) | .413 | 2.44 (.29–20.72) |
| Adult | 68 (80.0) | 59 (78.7) | 9 (90.0) | .413 | 0.41 (.05–3.48) |
| Age, y, median (IQR) | 40.0 (22.0–48.0) | 41.0 (24.0–48.0) | 33.0 (21.5–42.8) | .388 | 1.02 (.98–1.07) |
| Age group, y | |||||
| <12 | 1 (1.2) | 1 (1.3) | 0 (0.0) | … | |
| 12–17 | 16 (18.8) | 15 (20.0) | 1 (10.0) | 1 (ref) | |
| 18–39 | 25 (29.4) | 19 (25.3) | 6 (60.0) | .170 | 0.21 (.02–1.95) |
| 40–49 | 25 (29.4) | 23 (30.7) | 2 (20.0) | .834 | 0.77 (.06–9.22) |
| 50–65 | 18 (21.2) | 17 (22.7) | 1 (10.0) | .932 | 1.13 (.07–19.74) |
| Sex (female) | 56 (65.9) | 50 (66.7) | 6 (60.0) | .677 | 1.33 (.35–5.16) |
| BMI classb | |||||
| Normal weight | 45 (52.9) | 39 (52.0) | 6 (60.0) | 1 (ref) | |
| Obesity | 10 (11.8) | 10 (13.3) | 0 (0.0) | … | |
| Overweight | 28 (32.9) | 25 (33.3) | 3 (30.0) | .741 | 1.28 (.29–5.60) |
| Underweight | 2 (2.4) | 1 (1.3) | 1 (10.0) | .206 | 0.15 (.01–2.80) |
| Underlying medical conditionc | 10 (11.8) | 8 (10.7) | 2 (20.0) | .478 | 0.48 (.09–2.65) |
| Smoking (yes) | 4 (4.7) | 4 (5.3) | 0 (0.0) | ||
| Nationality (other)d | 2 (2.4) | 2 (2.7) | 0 (0.0) | … | |
| Symptom statuse | .639 | ||||
| Severe symptomsf | 1 (1.2) | 1 (1.3) | 0 (0.0) | … | |
| Moderate symptoms | 22 (25.9) | 21 (28.0) | 1 (10.0) | .400 | 3.50 (.19–64.67) |
| Mild symptoms | 55 (64.7) | 47 (62.7) | 8 (80.0) | .979 | 0.98 (.10–9.25) |
| Asymptomatic | 7 (8.2) | 6 (8.0) | 1 (10.0) | 1 (ref) | |
| Cp value, initial combined NPS/OPS, median (IQR) | 24.9 (22.2–29.3) | 25.0 (22.1–29.6) | 23.9 (22.8–26.4) | .837 | 1.01 (.89–1.16) |
| Days of symptoms before test, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.7) | .482 | 0.92 (.71–1.17) |
Data are presented as No. (%) unless otherwise indicated. The household secondary attack rate was 88.2%.
Abbreviations: BMI, body mass index; CI, confidence interval; IQR, interquartile range; NPS, nasopharyngeal swab; OPS, oropharyngeal swab; OR, odds ratio.
Some numbers might not add up to 85 due to missing values.
BMI categories for index cases and household members 2 to <18 years of age were defined as BMI z-score < –2, underweight; –2 to 1, normal weight; 1–2, overweight; >2, obesity (source: Centers for Disease Control and Prevention, https://www.cdc.gov/growthcharts/zscore.htm, accessed 28 September 2021). BMI categories for index cases and household members ≥18 years of age were defined as <18.5 kg/m2, underweight; 18.5–24.9 kg/m2, normal weight; 25.0–29.9 kg/m2, overweight; ≥30.0 kg/m2, obesity.
Cardiovascular disease, lung disease, immune disorder, diabetes, rheumatic disorder, and other disorders.
Other than Dutch.
Maximum over 10 time points.
Hospital admission due to coronavirus-related symptoms.
At the household member level, secondary infection was detected in 155 of 241 individuals, leading to a per-person SAR of 64.3% (Table 4). The majority of the secondary cases already tested positive in saliva at sampling day 1 (103/150 [68.7%]). The median age did not differ between secondary and nonsecondary cases (19.0 [IQR, 12.0–44.0] vs 21.0 [IQR, 10.3–47.8]; P = .678), and no significant differences in secondary transmission were observed between the age groups of the household members. The relationship between index case and household member had no remarkable influence on per-person SAR. The proportion of per-person secondary transmission from index case parent to child and from index case to partner were similar (78/117 children [66.7%] and 39/58 partners [67.2%], respectively) (Table 4).
Table 4.
Household Member Characteristics (n = 241)
| Characteristic | Total No. of Household Members at Risk (n = 241) |
Secondary Case (n = 155) |
No Secondary Case (n = 86) |
P Value |
OR (95% CI) |
|---|---|---|---|---|---|
| Child (<18 y) | 106 (44.0) | 68 (43.9) | 38 (44.2) | .962 | 0.99 (.58–1.68) |
| Adult | 135 (56.0) | 87 (56.1) | 48 (55.8) | .962 | 1.01 (.60–1.72) |
| Age, y, median (IQR) | 20.0 (12.0–45.0) | 19.0 (12.0–44.0) | 21.0 (10.3–47.8) | .678 | 1.00 (.98–1.01) |
| Age group, y | |||||
| <12 | 58 (24.1) | 34 (21.9) | 24 (27.9) | 1 (ref) | |
| 12–17 | 48 (20.0) | 34 (21.9) | 14 (16.3) | .194 | 1.71 (.76–3.86) |
| 18–39 | 49 (20.4) | 35 (21.6) | 14 (16.3) | .170 | 1.77 (.79–3.97) |
| 40–49 | 53 (22.1) | 32 (22.6) | 21 (24.4) | .851 | 1.08 (.50–2.30) |
| 50–65 | 33 (13.8) | 20 (12.9) | 13 (15.1) | .853 | 1.09 (.45–2.60) |
| Sex (female) | 101 (41.9) | 62 (40.0) | 39 (45.3) | .420 | 0.80 (.47–1.37) |
| BMI classb | |||||
| Normal weight | 150 (66.1) | 95 (65.5) | 55 (67.1) | 1 (ref) | |
| Obesity | 13 (5.7) | 9 (6.2) | 4 (4.9) | .672 | 1.30 (.38–4.43) |
| Overweight | 59 (26.0) | 37 (25.5) | 22 (26.8) | .933 | 0.97 (.52–1.82) |
| Underweight | 5 (2.2) | 4 (2.8) | 1 (1.2) | .458 | 2.32 (.25–21.24) |
| Underlying medical conditionc | 27 (11.2) | 14 (9.0) | 13 (15.1) | .155 | 0.56 (.25–1.25) |
| Smoking (yes) | 7 (2.9) | 4 (2.6) | 3 (3.5) | .689 | 0.733 (.16–3.35) |
| Nationality (other)d | 2 (0.8) | 0 (0.0) | 2 (2.4) | ||
| Relationship to index case | |||||
| Childe | 117 (48.5) | 78 (50.3) | 39 (45.3) | 1 (ref) | |
| Spouse | 58 (24.1) | 39 (25.2) | 19 (22.1) | .939 | 1.03 (.53–2.01) |
| Other adult | 3 (1.2) | 3 (1.9) | 0 (0.0) | ||
| Parent | 44 (18.3) | 25 (16.1) | 19 (22.1) | .248 | 0.66 (.32–1.34) |
| Sibling | 19 (7.9) | 10 (6.5) | 9 (10.5) | .239 | 0.56 (.21–1.48) |
Data are presented as No. (%) unless otherwise indicated. The per-person secondary attack rate was 64.3%.
Abbreviations: BMI, body mass index; CI, confidence interval; IQR, interquartile range; OR, odds ratio.
Some numbers might not add up to 241 due to missing values.
BMI categories for index cases and household members 2 to <18 years of age were defined as BMI z-score < –2, underweight; –2 to 1, normal weight; 1–2, overweight; >2, obesity (source: Centers for Disease Control and Prevention, https://www.cdc.gov/growthcharts/zscore.htm, accessed 28 September 2021). BMI categories for index cases and household members ≥18 years of age were defined as <18.5 kg/m2, underweight; 18.5–24.9 kg/m2, normal weight; 25.0–29.9 kg/m2, overweight; ≥30.0 kg/m2, obesity.
Cardiovascular disease, lung disease, immune disorder, diabetes, rheumatic disorder, and other disorders.
Other than Dutch.
Children could be either <18 years old or ≥18 years old if their role was a child within a household.
Sensitivity Analysis
The sensitivity analysis excluding 27 households (27 index cases and their 75 household members) with a possible other primary case than our defined index case resulted in a household SAR of 82.8% (48/58 households) and a per-person SAR of 54.2% (90/166 household members). Characteristics of index cases, households, or household members associated with secondary transmission were not remarkably different between the primary and sensitivity analysis (Supplementary Tables 1–3).
DISCUSSION
In this study we have investigated household transmission dynamics of SARS-CoV-2 by frequent and dense saliva sampling in combination with serology, NPS, and OPS in 85 households with a RT-PCR–confirmed index case. We found a household SAR of 88.2%, the highest rate of SARS-CoV-2 household transmission reported to date. The majority of the secondary cases were identified by saliva only. As approximately two-thirds of the secondary cases were already detected at the first sampling event, our study underlines that household transmission occurs rapidly. Secondary transmission was detected from and to different age groups and relationships within households, indicating that children, as well as adults, are at risk of infection and spreading of SARS-CoV-2 among their household members. Additional phylogenetic analyses suggest a single introduction for the investigated households.
A possible explanation for the high SARs in our study could be that other studies performed repeat sampling only in case of symptomatology, thereby ignoring possible asymptomatic cases within the household [18, 20, 24, 34, 35]. In addition, the high frequency and density of salivary sampling may have contributed to the higher SARs in our study compared to earlier reports [19, 22, 35]. We found the highest sensitivity of SARS-CoV-2 detection by salivary testing combined with serological analyses. However, the performance of salivary testing alone was comparable and its timely results could serve infection control purposes whereas serological analyses could not. Two other prospective household studies used saliva as specimen to detect SARS-CoV-2 transmission [19, 23]. One of these studies [23] reported a lower per-person SAR of 43%, possibly because salivary sampling was performed less frequently than in our study and additional sampling was performed only in case of symptoms. In our study, we found that 58 of the 155 secondary cases were asymptomatic and the per-person SAR would have declined from almost 65% to 40% if only symptomatic individuals would have been included. Saliva identified >75% (44/58) of the asymptomatic secondary infections. This confirms saliva (self-) sampling being highly effective in detecting asymptomatic SARS-CoV-2 infections in adults and older children [11, 12]. In addition, the noninvasive character of salivary (self-) sampling facilitates frequent use for near real-time monitoring in symptomatic as well as asymptomatic individuals. Salivary (self-) sampling of household members in the first week after symptom onset or positive test of a household index case could therefore improve infection control in this setting. However, in the context of high transmission rates, household isolation may be more practical and cost-effective.
Other factors that may have affected SARs are the study period including vaccination status of the participants (the COVID-19 vaccination program had not started yet), quarantine policies, and dominating variants (Nextclade 20A, 20B, 20E [EU1]) associated with different transmission rates [36]. All participants were recruited in the second wave of COVID-19 infections in The Netherlands (approximately from July 2020 to January 2021), in which working at home was encouraged or enforced and school classes generally took place at home, thereby possibly increasing the risk of household transmission [16].
In this study, we aimed to identify factors associated with secondary transmission. We found no apparent association between index case, household, or household member characteristics and secondary transmission. These findings are not fully in line with previous studies. In our study, transmission from index case to household member was not affected by age or the nature of their relationship. In contrast, several studies indicate that children (<18 years) play a minor role in household transmission of SARS-CoV-2, but caution must be applied here as the number of index children in these studies was low [17, 19, 21, 24]. In these studies, as well as in our study, the number of younger index children (<12 years) was too small to draw reliable conclusions regarding their role in transmission. We can, however, conclude that introduction and spreading of SARS-CoV-2 in households appears difficult to prevent as it occurs quickly and involves household members of different age groups.
A limitation of our study is that index cases were defined as the first included household member. However, not all index cases might have been the primary cases within the household and co-primary cases were not considered, which could have caused an overestimation of the per-person SAR. Additional serological analyses at study enrollment could have confirmed seroconversion of individuals for whom no positive saliva or OPS/NPS specimens were available, although due to limited circulation of SARS-CoV-2 prior to our study period, chances of previous infection are considered low. In addition, our sequencing results and phylogenetic results show no evidence of multiple introductions within 1 household. However, sequencing analyses were not successful for every infected individual. Our reported SARs therefore represent the maximum contribution from household transmission, as we cannot fully exclude introductions from outside the households. Last, as no transmission occurred in only 10 of 85 households in our study, comparison of the households with and without transmission was hampered.
In summary, this study reveals that households have a pivotal role in SARS-CoV-2 transmission, as we found the highest household SARS-CoV-2 transmission rates reported so far. Household transmission occurs quickly, hampering timely identification of primary cases and underlining the importance of prompt isolation and rapid testing of all household members, regardless of their age and presence of symptoms. Salivary (self-) sampling of adults and children is suitable and attractive for near real-time monitoring of SARS-CoV-2 transmission in this setting.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A. v. H., K. T., E. A. M. S., A. M., E. M. d. K., and W. R. M. contributed to the conception and design of the study. L. M. K., S. F. L. v. L., M. E. H., J. G. C. S., E. M. d. K., J. C. D. K., and M. A. v. H. participated in acquisition of data. R. M., S. M. E., and D. E. coordinated the laboratory analyses. L. M. K., S. F. L. v. L., J. G. C. S., A. N. S., E. C. C., S. M. E., D. E., and M. A. v. H. were responsible for data analyses and interpretation. L. M. K., J. G. C. S., S. M. E., and D. E. verified the underlying data. L. M. K., S. F. L. v. L., J. G. C. S., S. M. E., D. E., and M. A. v. H. wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgments. We thank the team of the Public Health Services Kennemerland, for providing information to the (potential) participants; the laboratory team of the National Institute for Public Health and the Environment (RIVM), including Sophie van Tol, Gert-Jan Godeke, Coralie Valle, Lisa Wijsman, Bas van der Veer, Annemarie van den Brandt, Jeroen Cremer, Sharon van den Brink, Ryanne Jaarsma, Kim Freriks, Lynn Aarts, Sanne Bos, and Euníce Then; the Regional Public Health Laboratory (Streeklab) Kennemerland for laboratory analyses; and the research team of the Spaarne Gasthuis Academy, particularly Greetje van Asselt, Jacqueline Zonneveld, Sandra Kaamer van Hoegee, and Mara van Roermund for their hard work and Coen Lap for his efforts concerning the continuation of the SARSLIVA study. We thank Martijn van Rooijen (RIVM) for his efforts regarding data management.
Disclaimer. The funder had no role in the study design; the collection, management, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
Financial support. This work was supported by internal funds from the National Institute for Public Health and the Environment (RIVM), and by The Netherlands Organisation for Health Research and Development (ZonMw), grant number 10430012010017, financed in part by The Netherlands Ministry of Health, Welfare and Sport. E. A. M. S. reports employment with RIVM, who supported the research for this work.
Potential conflicts of interest. S. F. L. v. L. reports support from ViiV for conference attendance (paid to author). K. T. reports unrestricted research grants (all payments made to home institution) from Pfizer, GlaxoSmithKline Biologicals SA, and Merck Sharp & Dohme; consultation fees from Pfizer and Merck Sharp & Dohme for participation in an advisory board (paid directly to home institution); and honorarium for a lecture from Pfizer (paid directly to home institution), all unrelated to the present work. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Lisa M Kolodziej, Spaarne Gasthuis Academy, Hoofddorp, The Netherlands.
Steven F L van Lelyveld, Department of Internal Medicine, Spaarne Gasthuis, Haarlem/Hoofddorp, The Netherlands.
Mildred E Haverkort, Public Health Services Kennemerland, Haarlem, The Netherlands.
Rob Mariman, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Judith G C Sluiter-Post, Spaarne Gasthuis Academy, Hoofddorp, The Netherlands.
Paul Badoux, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Emma M de Koff, Spaarne Gasthuis Academy, Hoofddorp, The Netherlands.
Jeffrey C D Koole, Spaarne Gasthuis Academy, Hoofddorp, The Netherlands.
Willem R Miellet, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Paediatric Immunology and Infectious Diseases, Wilhelmina Children’s Hospital, University Medical Centre Utrecht, Utrecht, The Netherlands.
Adriaan N Swart, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Elena C Coipan, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Adam Meijer, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Elisabeth A M Sanders, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Krzysztof Trzciński, Department of Paediatric Immunology and Infectious Diseases, Wilhelmina Children’s Hospital, University Medical Centre Utrecht, Utrecht, The Netherlands.
Sjoerd M Euser, Regional Public Health Laboratory Kennemerland, Haarlem, The Netherlands.
Dirk Eggink, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Marianne A van Houten, Spaarne Gasthuis Academy, Hoofddorp, The Netherlands; Department of Paediatrics, Spaarne Gasthuis, Haarlem/Hoofddorp, The Netherlands.
REFERENCES
- 1. Center for Systems Science and Engineering, Johns Hopkins University. COVID-19 dashboard. Available at: https://coronavirus.jhu.edu/map.html. Accessed 28 October 2021.
- 2. Centers for Disease Control and Prevention. Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed 1 July 2021.
- 3. Azzi L, Carcano G, Gianfagna F, et al. . Saliva is a reliable tool to detect SARS-CoV-2. J Infect 2020; 81:e45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang W, Xu Y, Gao R, et al. . Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teo AKJ, Choudhury Y, Tan IB, et al. . Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci Rep 2021; 11:3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wyllie AL, Fournier J, Casanovas-Massana A, et al. . Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 2020; 383:1283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fougère Y, Schwob JM, Miauton A, et al. . Performance of RT-PCR on saliva specimens compared with nasopharyngeal swabs for the detection of SARS-CoV-2 in children: a prospective comparative clinical trial. Pediatr Infect Dis J 2021; 40:e300–4. [DOI] [PubMed] [Google Scholar]
- 8. Bastos ML, Perlman-Arrow S, Menzies D, Campbell JR.. The sensitivity and costs of testing for SARS-CoV-2 infection with saliva versus nasopharyngeal swabs: a systematic review and meta-analysis. Ann Intern Med 2021; 174:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butler-Laporte G, Lawandi A, Schiller I, et al. . Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med 2021; 181:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith RL, Gibson LL, Martinez PP, et al. . Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. J Infect Dis 2021; 224:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao M, Rashid FA, Sabri FSAH, et al. . Comparing nasopharyngeal swab and early morning saliva for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2021; 72:e352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrera LA, Hidalgo-Miranda A, Reynoso-Noverón N, et al. . Saliva is a reliable and accessible source for the detection of SARS-CoV-2. Int J Infect Dis 2021; 105:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegler AJ, Hall E, Luisi N, et al. . Willingness to seek diagnostic testing for SARS-CoV-2 with home, drive-through, and clinic-based specimen collection locations. Open Forum Infect Dis 2020; 7:ofaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, He Y, Tong J, et al. . Characterization of an asymptomatic cohort of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals outside of Wuhan, China. Clin Infect Dis 2020; 71:2132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rothe C, Schunk M, Sothmann P, et al. . Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun K, Wang W, Gao L, et al. . Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 2021; 371:eabe2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J, Huang Y, Tu C, et al. . Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis 2020; 71:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng HY, Jian SW, Liu DP, et al. . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020; 180:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grijalva CG, Rolfes MA, Zhu Y, et al. . Transmission of SARS-COV-2 infections in households—Tennessee and Wisconsin, April–September 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuwelker K, Zhou F, Blomberg B, et al. . Attack rates amongst household members of outpatients with confirmed COVID-19 in Bergen, Norway: a case-ascertained study. Lancet Reg Health Eur 2021; 3:100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cerami C, Popkin-Hall ZR, Rapp T, et al. . Household transmission of SARS-CoV-2 in the united states: living density, viral load, and disproportionate impact on communities of color [manuscript published online ahead of print 12 August 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li W, Zhang B, Lu J, et al. . Characteristics of household transmission of COVID-19. Clin Infect Dis 2020; 71:1943–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reukers DFM, van Boven M, Meijer A, et al. . High infection secondary attack rates of SARS-CoV-2 in Dutch households revealed by dense sampling. Clin Infect Dis 2022; 74:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verberk J, de Hoog M, Westerhof I, et al. . Transmission of SARS-CoV-2 within households: a prospective cohort study in The Netherlands and Belgium—interim results. medRxiv [Preprint]. Posted online 10 May 2021. doi: 10.1101/2021.04.23.21255846. [DOI] [Google Scholar]
- 25. Brown KA, Tibebu S, Daneman N, Schwartz K, Whelan M, Buchan S.. Comparative household secondary attack rates associated with B.1.1.7, B.1.351, and P.1 SARS-CoV-2 variants. medRxiv [Preprint]. Posted online 4 June 2021. doi: 10.1101/2021.06.03.21258302. [DOI] [Google Scholar]
- 26. National Institute for Health and the Environment of The Netherlands. COVID-19 guideline. Available at: https://lci.rivm.nl/richtlijnen/covid-19. Accessed 1 July 2021.
- 27. US National Institutes of Health. COVID-19 treatment guidelines—clinical spectrum of SARS-CoV-2. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 1 July 2021.
- 28. Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheltinga SA, Templeton KE, Beersma MF, Claas EC.. Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR. J Clin Virol 2005; 33:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Artic Network. Protocols. Available at: https://artic.network/2-protocols.html. Accessed 24 February 2022.
- 31. Github. PCR tiling of COVID-19 virus. Available at: https://github.com/CDCgov/SARS-CoV-2_Sequencing/blob/master/protocols/ONT-COVID-19_Tiling/PCR%20tiling%20of%20COVID-19%20virus-minion.pdf. Accessed 24 February 2022.
- 32. van Tol S, Mögling R, Li W, et al. . Accurate serology for SARS-CoV-2 and common human coronaviruses using a multiplex approach. Emerg Microbes Infect 2020; 9:1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koopmans M, de Bruin E, Godeke GJ, et al. . Profiling of humoral immune responses to influenza viruses by using protein microarray. Clin Microbiol Infect 2012; 18:797–807. [DOI] [PubMed] [Google Scholar]
- 34. Bernal JL, Panagiotopoulos N, Byers C, et al. . Transmission dynamics of COVID-19 in household and community settings in the United Kingdom. medRxiv [Preprint]. Posted online 22 August 2020. doi: 10.1101/2020.08.19.20177188. [DOI] [Google Scholar]
- 35. Lewis NM, Chu VT, Ye D, et al. . Household transmission of severe acute respiratory syndrome coronavirus-2 in the United States. Clin Infect Dis 2021; 73:1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE.. Factors associated with household transmission of SARS-CoV-2: an updated systematic review and meta-analysis. JAMA Netw Open 2021; 4:e2122240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.