Abstract
Background
Waning of protection against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) conferred by 2 doses of the BNT162b2 vaccine begins shortly after inoculation and becomes substantial within 4 months. With that, the impact of prior infection on incident SARS-CoV-2 reinfection is unclear. Therefore, we examined the long-term protection of naturally acquired immunity (protection conferred by previous infection) compared to vaccine-induced immunity.
Methods
A retrospective observational study of 124 500 persons, compared 2 groups: (1) SARS-CoV-2-naive individuals who received a 2-dose regimen of the BioNTech/Pfizer mRNA BNT162b2 vaccine, and (2) previously infected individuals who have not been vaccinated. Two multivariate logistic regression models were applied, evaluating four SARS-CoV-2-related outcomes—infection, symptomatic disease (coronavirus disease 2019 [COVID-19]), hospitalization, and death—between 1 June and 14 August 2021, when the Delta variant was dominant in Israel.
Results
SARS-CoV-2-naive vaccinees had a 13.06-fold (95% confidence interval [CI], 8.08–21.11) increased risk for breakthrough infection with the Delta variant compared to unvaccinated-previously-infected individuals, when the first event (infection or vaccination) occurred during January and February of 2021. The increased risk was significant for symptomatic disease as well. When allowing the infection to occur at any time between March 2020 and February 2021, evidence of waning naturally acquired immunity was demonstrated, although SARS-CoV-2 naive vaccinees still had a 5.96-fold (95% CI: 4.85–7.33) increased risk for breakthrough infection and a 7.13-fold (95% CI: 5.51–9.21) increased risk for symptomatic disease.
Conclusions
Naturally acquired immunity confers stronger protection against infection and symptomatic disease caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 2-dose vaccine-indued immunity.
Keywords: COVID-19, SARS-CoV-2, vaccination, naturally acquired immunity, vaccine, induced immunity
The first real-world analysis of naturally acquired immunity versus vaccine induced immunity against SARS-CoV-2. Our findings illustrate that naturally acquired immunity confers stronger protection against infection and symptomatic disease caused by SARS-CoV-2, compared to the BNT162b2 two-dose vaccine-induced immunity.
The heavy toll that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been taking on global health and healthcare resources created an urgent need to estimate which part of the population is protected against coronavirus disease 2019 (COVID-19) at a given time in order to set healthcare policies such as lockdowns and to assess the possibility of herd immunity.
Although antibody levels might be useful to assess short-term protection on a population level, to date, there is still no consensus on an evidence-based, long-term measurement to assess immune correlate of protection [1]. This lack of correlate of protection has led to different approaches in terms of vaccine resource allocation, such as the need for vaccine administration in recovered patients.
With that, evidence of waning vaccine-induced immunity against coronavirus disease 2019 (COVID-19) have surfaced [2–7], although research has demonstrated that this reduction is milder against severe disease, meaning that vaccinated individuals are more protected against severe disease than unvaccinated ones, even if a breakthrough infection (infection after vaccination) occurs [8]. Alongside the question of long-term protection against infection provided by the vaccine, the degree and duration to which previous infection with SARS-CoV-2 affords protection against repeated infection also remains unclear.
Apart from the paucity of studies examining long-term protection against reinfection [9, 10], there is a challenge in defining reinfection as opposed to prolonged viral shedding [11]. Although clear-cut cases exist, namely, 2 separate clinical events with 2 distinct sequenced viruses, relying solely on these cases will likely result in an under-estimation of the incidence of reinfection. Different criteria based on more widely-available information have been suggested [12], as, for example, the Centers for Disease Control and Prevention’s (CDC) guidelines refer to 2 positive SARS-CoV-2 polymerase chain reaction (PCR) test results at least 90 days apart [13].
These challenges and the CDC’s suggested solution to tackle them, require long-term follow-up and free and available access to testing, facilitated largely by integrated healthcare organizations, though this does not eliminate the risk of underestimation. Using similar criteria to the CDC’s, population-based studies demonstrated naturally acquired immunity [14, 15] with no signs of waning immunity for at least 7 months, although protection was lower for those aged 65 or older [9].
Now, when sufficient time has passed since both the beginning of the pandemic and the deployment of the vaccine, we can examine the long-term protection of naturally acquired immunity compared to that afforded by the vaccine. To this end, we compared the incidence rates of breakthrough infections to the incidence rates of reinfection, leveraging the centralized computerized database of Maccabi Healthcare Services (MHS), Israel’s second largest Health Maintenance Organization.
METHODS
Study Design and Population
A retrospective cohort study was conducted. The study population included MHS members aged 16 or older who were twice vaccinated prior to 28 February 2021 or who had a documented SARS-CoV-2 infection by 28 February 2021. The study only included persons who received the BioNTech/Pfizer mRNA BNT162b2 vaccine, as this was given to the vast majority of the Israeli population.
Exposure Variable: Study Groups
The eligible study population was divided into 2 groups: (1) fully vaccinated and SARS-CoV-2-naive individuals, namely, MHS members who received 2 doses of the BioNTech/Pfizer mRNA BNT162b2 vaccine by 28 February 2021 did not receive the third dose by the end of the study period and did not have a positive polymerase chain reaction (PCR) test result by 1 June 2021; and (2) unvaccinated previously infected individuals, namely, MHS members who had a positive SARS-CoV-2 PCR test recorded by 28 February 2021 and who had not been vaccinated by the end of the study period. The fully vaccinated group was the comparison (reference) group in our study.
Dependent Variables
We evaluated 4 SARS-CoV-2-related outcomes: documented PCR confirmed SARS-CoV-2 infection, COVID-19, COVID-19-related hospitalization, and death. Outcomes were evaluated during the follow-up period of 1 June to 14 August 2021, corresponding to the time in which the Delta (B.1.617.2) variant became dominant in Israel [16], before the spread of the Omicron variant.
Statistical Analysis
Two models were applied to evaluate 4 SARS-CoV-2-related outcomes as dependent variables, whereas the study groups were the main independent variables. In both models, we estimated naturally acquired immunity versus vaccine-induced immunity for each outcome, by applying logistic regression to calculate the odds ratio (OR) between the 2 groups with associated 95% confidence intervals (CIs). Results were then adjusted for underlying comorbidities, including obesity, cardiovascular diseases, diabetes, hypertension, chronic kidney disease, cancer, and immunosuppression conditions. Additionally, for each models, in order, to assess the potential robustness of an unmeasured confounder, we conducted a sensitivity analysis using the E-value metric [17]. The E-value is defined as the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away a specific exposure-outcome association, conditional on the measured covariates [18].
Model 1: Previously Infected vs Vaccinated Individuals, With Matching for Time of First Event
In model 1, we examined naturally acquired immunity and vaccine-induced immunity by comparing the likelihood of SARS-CoV-2-related outcomes between previously infected individuals who have never been vaccinated to fully vaccinated SARS-CoV-2-naive individuals. These groups were matched in a 1:1 ratio by age, sex, GSA and time of first event. The first event (the preliminary exposure) was either the time of administration of the second dose of the vaccine or the time of documented infection with SARS-CoV-2 (a positive PCR test result), both occurring between 1 January 2021 and 28 February 2021. Thereby, we matched the “immune activation” time of both groups, examining the long-term protection conferred when vaccination or infection occurred within the same period. The 3-month interval between the exposure and the outcome was implemented to capture reinfections (as opposed to prolonged viral shedding) by following the 90-day guideline of the CDC.
Model 2: Previously Infected vs Vaccinated Individuals, Without Matching for Time of First Event
In model 2, we compared the SARS-CoV-2 naive vaccinees to unvaccinated and previously infected individuals while intentionally not matching the time of the first event (exposure) (i.e., either vaccination or infection), in order to compare vaccine-induced immunity to naturally acquired immunity, regardless of time of infection. Therefore, matching was done in a 1:1 ratio based on age, sex and GSA alone. Similar to model 1, either event (vaccination or infection) had to occur by 28 February to allow for the 90-day interval. The 4 SARS-CoV-2 study outcomes were the same for this model, evaluated during the same follow-up period.
Additionally, we included a sensitivity analysis that addressed the timing of vaccination. As individuals with chronic illness were primarily vaccinated between December and February, we conducted the same design of model 2, this time with those vaccinated later, between March and April 2021, therefore comparing the SARS-CoV-2 naive March and April vaccinees to those unvaccinated and previously infected at any time until 28 February 2021 (to allow for the 90-day interval).
Finally, we performed an alternative model of analysis to address the possible selection bias of mandating previously infected individuals to be unvaccinated until the end of the follow-up period as well as vaccinated individuals not to have received the booster (third) dose by that time, as the booster vaccination campaign began on 31 July 2021. Therefore, we applied a Cox proportional hazards regression to calculate the hazard ratio (HR) of SARS-CoV-2 infections and symptomatic SARS-CoV-2 infections between the groups with associated 95% confidence intervals (CIs). Participants’ vaccination status was determined on 1 June (the start of the follow-up period), and for each person the follow-up ended at the earliest of these events: the tested-outcome (infection or symptomatic infection), vaccination (either a first dose for members of the previously infected group or a third dose for those in the vaccinated group), or the end of the follow-up period. The same matching was applied, as well as adjustment for the same variables.
Analyses were performed using Python version 3.73 with the statsmodels package. P < .05 was considered statistically significant.
Ethics Declaration
This study was approved by the MHS (Maccabi Healthcare Services) institutional review board (IRB). Due to the retrospective design of the study, informed consent was waived by the IRB, and all identifying details of the participants were removed before computational analysis.
RESULTS
Overall, 673 676 MHS members 16 years and older were eligible for the study group of fully vaccinated SARS-CoV-2-naive individuals, and 62 883 were eligible for the study group of unvaccinated previously infected individuals (Supplementary Figure 1). Of those previously infected from the beginning of the pandemic and up to February 2021, who could have potentially been eligible for the study group of the unvaccinated and previously infected individuals, 693 COVID-19-related deaths were recorded. Mean age of death was 78 (SD 12), 90% of deaths were among those 60 years old and over.
Model 1:Previously Infected vs Vaccinated Individuals, With Matching for Time of First Event
In model 1, we matched 16 215 persons in each group. Overall, demographic characteristics were similar between the groups, with some differences in their comorbidity profile (Table 1, model 1).
Table 1.
Characteristics of Study Population, by Model 1 and 2.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Characteristics | Previously Infected (n = 16 215) |
Vaccinated Individuals (n = 16 215) |
Previously Infected (n = 46 035) |
Vaccinated Individuals (n = 46 035) |
| Age, years, mean (SD) | 36.1 (13.9) | 36.1 (13.9) | 36.1 (14.7) | 36.1 (14.7) |
| Age group, no. (%) | ||||
| 16 to 39 yr | 9889 (61.0) | 9889 (61.0) | 28 157 (61.2) | 28 157 (61.2) |
| 40 to 59 yr | 5536 (34.1) | 5536 (34.1) | 14 973 (32.5) | 14 973 (32.5) |
| ≥60 yr | 790 (4.9) | 790 (4.9) | 2905 (6.3) | 2905 (6.3) |
| Sex, no. (%) | ||||
| Female | 7428 (45.8) | 7428 (45.8) | 22 661 (49.2) | 22 661 (49.2) |
| Male | 8,787 (54.2) | 8787 (54.2) | 23 374 (50.8) | 23 374 (50.8) |
| SES, mean (SD) | 5.5 (1.9) | 5.5 (1.9) | 5.3 (1.9) | 5.3 (1.9) |
| Comorbidities, no. (%) | ||||
| Hypertension | 1276 (7.9) | 1569 (9.7) | 4009 (8.7) | 4301 (9.3) |
| CVD | 551 (3.4) | 647 (4.0) | 1,875 (4.1) | 1830 (4.0) |
| DM | 635 (3.9) | 877 (5.4) | 2207 (4.8) | 2300 (5.0) |
| Immunocompromised | 164 (1.0) | 420 (2.6) | 527 (1.1) | 849 (1.8) |
| Obesity (BMI ≥30) | 3076 (19.0) | 3073 (19.0) | 9117 (19.8) | 8610 (18.7) |
| CKD | 196 (1.2) | 271 (1.7) | 659 (1.4) | 814 (1.8) |
| COPD | 65 (0.4) | 97 (0.6) | 218 (0.5) | 292 (0.6) |
| Cancer | 324 (2.0) | 636 (3.9) | 1044 (2.3) | 1364 (3.0) |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; DM, diabetes mellitus; SD, standard deviation; SES, socioeconomic status on a scale from 1 (lowest) to 10.
During the follow-up period, 257 cases of SARS-CoV-2 infection were recorded, of which 238 occurred in the vaccinated group (breakthrough infections) and 19 in the previously infected group (reinfections) (Supplementary Figure 2). After adjusting for comorbidities, we found a statistically significant 13.06-fold (95% CI: 8.08 to 21.11) increased risk for breakthrough infection as opposed to reinfection (P < .001). Apart from age ≥ 60 years, there was no statistical evidence that any of the assessed comorbidities significantly affected the risk of an infection during the follow-up period (Table 2). To further characterize the association with older age, we added an interaction analysis which yielded a non-statistically significant (P = .79) interaction term of age ≥60 years, vaccination and risk for incidence infection.
Table 2.
OR for SARS-CoV-2 Infection, Model 1, Previously Infected vs Vaccinated
| Variable | Category | ß | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Induced immunity | |||||
| Previously infected | Ref | ||||
| Vaccinated | 2.57 | 13.06 | 8.08–21.11 | <.001 | |
| SES | 0.04 | 1.04 | .97–1.11 | .251 | |
| Age group, yr | |||||
| 16−39 | Ref | ||||
| 40−59 | 0.05 | 1.05 | .78–1.4 | .751 | |
| ≥60 | 0.99 | 2.7 | 1.68–4.34 | <.001 | |
| Sex | |||||
| Female | Ref | ||||
| Male | −0.03 | 0.97 | .76–1.25 | .841 | |
| Comorbidities | |||||
| Obesity (BMI ≥30) | 0.01 | 1.01 | .73–1.39 | .967 | |
| Diabetes mellitus | −0.36 | 0.7 | .39–1.25 | .229 | |
| Hypertension | 0.1 | 1.11 | .72–1.72 | .641 | |
| Cancer | 0.37 | 1.44 | .85–2.44 | .171 | |
| CKD | 0.53 | 1.7 | .83–3.46 | .146 | |
| COPD | −0.46 | 0.63 | .15–2.66 | .529 | |
| Immunosuppression | −0.1 | 0.91 | .42–1.97 | .803 | |
| Cardiovascular diseases | 0.26 | 1.3 | .75–2.25 | .343 | |
Abbreviations: BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SES, socioeconomic status on a scale from 1 (lowest) to 10.
The E-value for breakthrough infection was 25.61 (and 15.64 for the lower bound of the CI). Thus, an unmeasured confounder not included in the regression model associated with both a 2-dose vaccination and with a breakthrough infection outcome by an OR of 25.61 each could explain away the lower confidence limit, though a weaker confounder would not.
As for symptomatic SARS-COV-2 infections during the follow-up period, 199 cases were recorded, 191 of which were in the vaccinated group and 8 in the previously infected group. Symptoms for all analyses were recorded in the central database within 5 days of the positive reverse transcription polymerase chain reaction (RT-PCR) test for 90% of the patients and included chiefly fever, cough, breathing difficulties, diarrhea, loss of taste or smell, myalgia, weakness, headache, and sore throat. After adjusting for comorbidities, we found a 27.02-fold risk (95% CI: 12.7 to 57.5) for symptomatic breakthrough infection as opposed to symptomatic reinfection (P < .001) (Supplementary Table 1). None of the covariates were significant, except for age ≥60 years. The sensitivity analyses that adjusted for individuals’ test frequency as a proxy for healthcare seeking behavior did alter results (Supplementary Data).
Eight cases of COVID-19-related hospitalizations were recorded, all of which were in the vaccinated group, and no COVID-19-related deaths were recorded in our cohorts.
Model 2: Previously Infected vs Vaccinated Individuals, Without Matching for Time of First Event
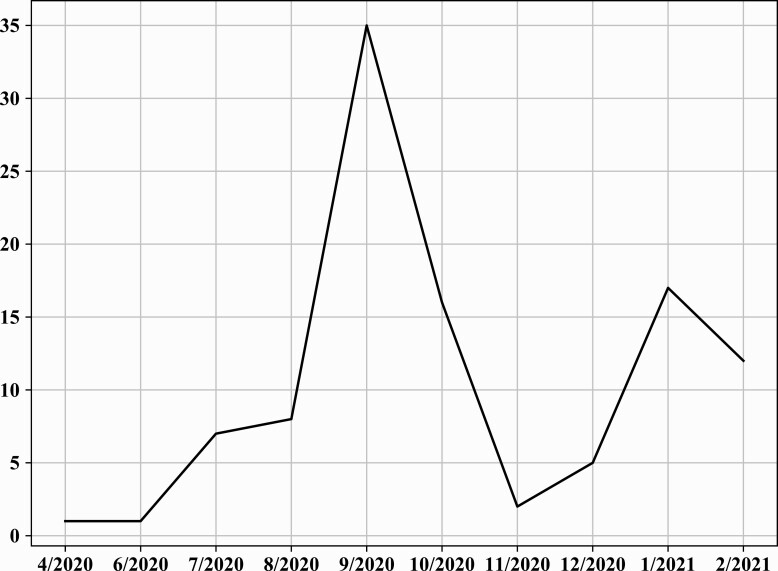
In model 2, we matched 46 035 persons in each of the groups (previously infected vs vaccinated) (Table 1). Figure 1 demonstrates the timely distribution of the first infection in reinfected individuals.
Figure 1.
Time of first infection in those reinfected between June and August 2021, model 2.
When comparing the vaccinated individuals to those previously infected at any time (including during 2020), we found that throughout the follow-up period, 748 cases of SARS-CoV-2 infection were recorded, 640 of which were in the vaccinated group (breakthrough infections) and 108 in the previously infected group (reinfections). After adjusting for comorbidities, a 5.96-fold increased risk (95% CI: 4.85 to 7.33) increased risk for breakthrough infection as opposed to reinfection could be observed (P < .001) (Table 3). Apart from SES level and age ≥ 60, that remained significant in this model as well, there was no statistical evidence that any of the comorbidities significantly affected the risk of an infection. The E-value for breakthrough infection was 11.4 (and 9.17 for the lower bound of the CI).
Table 3.
OR for SARS-CoV-2 Infection, Model 2, Previously Infected vs Vaccinated
| Variable | Category | ß | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Induced immunity | |||||
| Previously infected | Ref | ||||
| Vaccinated | 1.78 | 5.96 | 4.85–7.33 | <.001 | |
| SES | 0.07 | 1.07 | 1.03–1.11 | <.001 | |
| Age group, yr | |||||
| 16–39 | Ref | ||||
| 40–59 | 0.06 | 1.06 | .9–1.26 | .481 | |
| ≥60 | 0.79 | 2.2 | 1.66–2.92 | <.001 | |
| Sex | |||||
| Female | Ref | ||||
| Male | −0.01 | 0.99 | .85–1.14 | .842 | |
| Comorbidities | |||||
| Obesity (BMI ≥30) | 0.12 | 1.13 | .94–1.36 | .202 | |
| Diabetes mellitus | −0.15 | 0.86 | .61–1.22 | .4 | |
| Hypertension | −0.12 | 0.89 | .67–1.17 | .402 | |
| Cancer | 0.2 | 1.22 | .85–1.76 | .283 | |
| CKD | 0.3 | 1.35 | .85–2.14 | .207 | |
| COPD | 0.48 | 1.62 | .88–2.97 | .121 | |
| Immunosuppression | −0.03 | 0.98 | .57–1.66 | .925 | |
| Cardiovascular diseases | 0.08 | 1.09 | .77–1.53 | .638 | |
Abbreviations: BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SES, socioeconomic status on a scale from 1 (lowest) to 10.
Overall, 552 symptomatic cases of SARS-CoV-2 were recorded, 484 in the vaccinated group and 68 in the previously infected group. There was a 7.13-fold (95% CI: 5.51 to 9.21) increased risk for symptomatic breakthrough infection than symptomatic reinfection (Supplementary Table 2). COVID-19 related hospitalizations occurred in 1 and 19 of the reinfection and breakthrough infection groups, respectively. No COVID-19-related deaths were recorded. Similarly to model 1, a sensitivity analysis adjusting for the frequency of testing did not materially alter the OR for infection or symptomatic infection (Supplementary Data).
A second sensitivity analysis accounted for the timing of vaccination. We matched 46 818 persons in each group (previously infected vs later vaccinees, namely those vaccinated between March and April 2021) (Supplementary Table 7). When comparing the later vaccinees to those previously infected at any time (from 2020), 570 cases of SARS-CoV-2 infection were recorded, 463 of which were in the March–April vaccinated group (breakthrough infections) and 107 in the previously infected group (reinfections). After adjusting for comorbidities, a 4.63-fold increased risk (95% CI: 3.53 to 5.38) for breakthrough infection as opposed to reinfection could be observed (Supplementary Table 8). As for symptomatic cases, there was a 6.67-fold (95% CI: 4.9 to 9.06) increased risk for symptomatic breakthrough infection than symptomatic reinfection (Supplementary Table 9). There were 7 cases of COVID-19 related hospitalizations, 4 of which among the April–March vaccinees and 3 among the previously infected. Lastly, the sensitivity analysis that included an alternative model (Cox proportional hazards regression) yielded similar results (Supplementary Data).
DISCUSSION
This is the largest real-world observational study comparing naturally acquired immunity, gained through previous SARS-CoV-2 infection, to vaccine-induced immunity, afforded by the BNT162b2 mRNA vaccine. Our large cohort, enabled by Israel’s rapid rollout of the mass-vaccination campaign, allowed us to investigate the risk for additional infection—either a breakthrough infection in vaccinated individuals or reinfection in previously infected ones—over a longer period than thus far described.
Our analysis demonstrates that SARS-CoV-2-naïve vaccinees had a 13.06-fold increased risk for breakthrough infection with the Delta variant compared to those previously infected, when the first event (infection or vaccination) occurred during January and February of 2021. The increased risk was significant for a symptomatic disease as well.
Broadening the research question to examine the extent of the phenomenon, we allowed the first infection to occur at any time between March 2020 to February 2021 (when different variants were dominant in Israel), compared to vaccination only in January and February 2021. Although the results could suggest waning naturally acquired immunity against the Delta variant, those vaccinated are still at a 5.96-fold increased risk for breakthrough infection and at a 7.13-fold increased risk for symptomatic disease compared to those previously infected. SARS-CoV-2-naive vaccinees had more COVID-19-related-hospitalization compared to those who were previously infected, although the numbers are too small to determine statistical significance. Importantly, in neither group no COVID-19-related deaths were recorded.
The advantageous protection afforded by naturally acquired immunity that this analysis demonstrates could be explained by the more extensive immune response to the SARS-CoV-2 proteins than that generated by the anti-spike protein immune activation conferred by the vaccine [19, 20]. However, as a correlate of protection is yet to be proven [1, 21], including the role of B-Cell [22] and T-cell immunity [23, 24], this remains a hypothesis. Our study matches the CDC report [10], examining cohorts in California and New York, demonstrating that infection-induced protection was more substantial than vaccine induced immunity during the Delta period. The report demonstrates an opposite trend during the previous Alpha dominant period; however, a significant limitation, addressed as such by the researchers of this report as well, pertains to the lack of addressing the varying times-since-vaccination, which could bias the result, especially in the early stages of the follow-up.
Our study has several limitations. First, as the Delta variant was the dominant strain in Israel during the outcome period, the decreased long-term protection of the vaccine compared to that afforded by previous infection cannot be ascertained against other strains, including the Omicron variant. Second, our analysis addressed protection afforded solely by the BioNTech/Pfizer mRNA BNT162b2 vaccine and therefore does not address other vaccines or long-term protection following a third dose, an assessment that might require more data before carrying out. Additionally, as this is an observational real-world study, where PCR screening was not performed by a pre-set protocol, we might be underestimating asymptomatic infections, as these individuals often do not get tested. A related concern is that the frequency of PCR testing differed between groups, meaning that 1 group manifested different health seeking behavior during the pandemic and therefore is potentially more diagnosed rather than more infected. To address that potential detection bias, we conducted a sensitivity analysis where the number of PCR tests undertaken throughout the pandemic was adjusted for, as a proxy for COVID-19-related health seeking behavior. The findings demonstrated that this adjustment did not change the results. Furthermore, the analysis merits addressing the potential survivorship bias, which might have accounted for the stronger protection of the unvaccinated previously infected group. As reported in the results, COVID-19 related mortality in this group (prior to the outcome period) was evaluated at approximately 1% with mean age of 78 years. Therefore, it does not seem to overall account for the significant protection conferred by natural infection across the different age groups. Moreover, as individuals with chronic illness were primarily vaccinated between December and February, confounding by indication needs to be considered; though the groups somewhat differ in their comorbidity profile, adjusting for obesity, cardiovascular disease, diabetes, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, cancer, and immunosuppression had only a small impact on the estimated effect as compared to the unadjusted OR. Therefore, residual confounding by unmeasured factors is unlikely. Nonetheless, to assess whether the association between previous infection or vaccination and a following infection (breakthrough- or re-infection) could be attributed to unmeasured confounding, for example, by differential groups behavior (such as social distancing and mask wearing), we calculated the E-value for an unmeasured confounding. The E-value for both models suggested that only a highly strong association between both the group (vaccinated vs previously infected individuals) and healthcare seeking behavior, and healthcare seeking behavior and the outcome of a subsequent infection (breakthrough- or reinfection) would account for all the observed association between vaccinating convalescent patients and their reduced risk for reinfection.
To further address this issue, we conducted a different sensitivity analysis, where we implemented the same design of model 2, comparing those previously infected at any time to later vaccinees, namely those who completed the second dose between March and April 2021. This time, the latter group had slightly more comorbidities than those previously infected, though again these were not found to affect significantly. The results suggest waning of vaccine-induced immunity against the Delta variant and still point to an increased risk of those vaccinated. Those later vaccinees are at a 4.63-fold increased risk for breakthrough infection and at a 6.67-fold increased risk for symptomatic disease compared to those previously infected. Lastly, as per Israeli regulations the second dose was administered within 21–28 days of the first dose, we could not assess whether an extended interval between the doses affects effectiveness. This analysis demonstrated that naturally acquired immunity affords longer lasting and stronger protection against infection and symptomatic disease due to the Delta variant of SARS-CoV-2, compared to the BNT162b2 2-dose vaccine-induced immunity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
NOTES
Financial support. There was no external funding for the project.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med 2021 277 2021; 27:1147–48. [DOI] [PubMed] [Google Scholar]
- 2. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021; 12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ChemaiteAlly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 Infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 Months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med 2021; 2021:1–3. [DOI] [PubMed] [Google Scholar]
- 7. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. medRxiv 2021:1237. Available at: https://www.medrxiv.org/content/10.1101/2021.12.27.21268424v1. Accessed 5 January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults — United States, March–July 2021. Morb Mortal Wkly Rep 2021; 70:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S.. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet 2021; 397:1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. León TM, Dorabawila V, Nelson L, et al. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis — California and New York, May–November 2021. Centers for Disease Control MMWR Office 2022; 71:4. Available at: https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e1.htm. Accessed 9 February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis 2021; 21:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW.. Setting the criteria for SARS-CoV-2 reinfection: six possible cases. J Infect; 82:282–327. Available at: https://pmc/articles/PMC7422822/. Accessed 15 January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reinfection. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html. Accessed 28 December 2021.
- 14. Perez G, Banon T, Gazit S, et al. A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report. 2021. Available at: http://medrxiv.org/content/early/2021/03/08/2021.03.06.21253051.abstract. Accessed 28 December 2021.
- 15. Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021; 384:533–40. Available at: http://www.nejm.org/doi/10.1056/NEJMoa2034545. Accessed 15 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. SARS-CoV-2 variants in analyzed sequences, Israel. Available at: https://ourworldindata.org/grapher/covid-variants-area?country=~ISR. Accessed 30 December 2021. [Google Scholar]
- 17. VanderWeele TJ. Are Greenland, Ioannidis, and Poole opposed to the Cornfield conditions? A defence of the E-value. Int J Epidemiol 2021; dyab218:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Der Weele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
- 19. Bettini E, Locci M.. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines 2021; 9:147. Available at: pmc/articles/PMC7918810/. Accessed 15 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sette A, Crotty S.. Leading Edge Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184:861–80. Available at: 10.1016/j.cell.2021.01.007. Accessed 21 August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2021; 2:e240–9. Available at: http://www.thelancet.com/article/S2666524721000252/fulltext. Accessed 14 August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho A, Muecksch F, Schaefer-Babajew D, et al. Antibody evolution after SARS-CoV-2 mRNA vaccination. Nature 2021; 600:517–22. Available at: 10.1038/s41586-021-04060-7. Accessed 21 August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Med 2021; 2:100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Z, Laing ED, Pena-Damata J, et al. Durability of SARS-CoV-2-specific T cell responses at 12-months post-infection. The Journal of Infectious Diseases 2021; 224:2010–19. Available at: 10.1093/infdis/jiab543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.