Abstract
Background
The study objective was to evaluate 2- and 3-dose coronavirus disease 2019 (COVID-19) mRNA vaccine effectiveness (VE) in preventing COVID-19 hospitalization among adult solid organ transplant (SOT) recipients.
Methods
We conducted a 21-site case-control analysis of 10 425 adults hospitalized in March to December 2021. Cases were hospitalized with COVID-19; controls were hospitalized for an alternative diagnosis (severe acute respiratory syndrome coronavirus 2-negative). Participants were classified as follows: SOT recipient (n = 440), other immunocompromising condition (n = 1684), or immunocompetent (n = 8301). The VE against COVID-19-associated hospitalization was calculated as 1-adjusted odds ratio of prior vaccination among cases compared with controls.
Results
Among SOT recipients, VE was 29% (95% confidence interval [CI], −19% to 58%) for 2 doses and 77% (95% CI, 48% to 90%) for 3 doses. Among patients with other immunocompromising conditions, VE was 72% (95% CI, 64% to 79%) for 2 doses and 92% (95% CI, 85% to 95%) for 3 doses. Among immunocompetent patients, VE was 88% (95% CI, 87% to 90%) for 2 doses and 96% (95% CI, 83% to 99%) for 3 doses.
Conclusions
Effectiveness of COVID-19 mRNA vaccines was lower for SOT recipients than immunocompetent adults and those with other immunocompromising conditions. Among SOT recipients, vaccination with 3 doses of an mRNA vaccine led to substantially greater protection than 2 doses.
Keywords: COVID-19, immunocompromised, SARS-CoV-2, solid organ transplantation, vaccine effectiveness
Effectiveness of COVID-19 mRNA vaccines against COVID-19 hospitalization is lower for solid organ transplant (SOT) recipients compared with immunocompetent people and those with other immunocompromising conditions. Among SOT recipients, 3 vaccine doses lead to substantially greater protection than 2.
Postmarketing observational vaccine effectiveness (VE) studies have demonstrated excellent effectiveness of mRNA vaccines (including BNT-162b2 from Pfizer-BioNTech and mRNA-1273 from Moderna) for the prevention of coronavirus disease 2019 (COVID-19)-associated hospitalization among immunocompetent people, generally with estimates of 85% or higher [1–6]. However, lower estimates of VE against hospitalization have been reported among people with immunocompromising conditions [7, 8]. This is an area of concern, because immunocompromised people, especially those severely immunocompromised such as solid organ transplant (SOT) recipients, are at increased risk of severe illness with COVID-19 [9].
Immunogenicity studies suggest that COVID-19 mRNA vaccines produce less robust immune responses among people who are immunocompromised [9–13]. Early clinical studies suggest this translates into lower VE for the prevention of severe COVID-19 among adults with immunocompromising conditions [7, 8, 14–16]. However, a greater understanding of VE for COVID-19 vaccines among immunocompromised populations is needed. Prior studies have largely pooled all patients with immunocompromising conditions together without consideration for the intensity of immunosuppression, producing VE results that may overestimate VE for the most severely immunocompromised people [8, 16].
In the United States, initial recommendations for people with moderate-to-severe immunocompromising conditions were 2 doses of an mRNA COVID-19 vaccine; this recommendation was updated to 3 doses on August 12, 2021, with a fourth dose currently recommended at least 3 months after the third dose as a vaccine booster [17]. Understanding the clinical protection provided by mRNA vaccines in immunocompromised populations is critically important for several reasons, including the following: helping guide decisions on the prophylactic use of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) monoclonal antibody therapies for vaccinated immunocompromised patients; informing recommendations regarding continued use of nonpharmaceutical preventative strategies, such as mask wearing, after vaccination; and informing recommendations for additional vaccine booster doses [9, 14, 18]. In this analysis, we estimated VE against COVID-19 hospitalization for 2-dose and 3-dose mRNA vaccine series among SOT recipients, a population that is typically severely immunocompromised and, therefore, potentially susceptible to severe COVID-19 despite prior vaccination. We also estimated 2-dose and 3-dose mRNA VE among adults with other types of immunocompromising conditions.
METHODS
Project Design
This work was conducted as part of an ongoing prospective COVID-19 surveillance program by the Influenza and Other Viruses in the Acutely Ill (IVY) Network (a full list of investigators and collaborators in the Influenza and other Viruses in the Acutely Ill (IVY) Network is available in Supplementary Appendix A) [8, 15], a collaboration among 21 United States hospitals in 18 states and the US Centers for Disease Control and Prevention (CDC) coordinated from Vanderbilt University Medical Center. The IVY Network initiated participant enrollment for COVID-19 VE assessments on March 11, 2021 and has been iteratively publishing VE results (Supplementary Table S1). The current analysis evaluated participants enrolled from March 11, 2021 through December 15, 2021 and focused on SOT recipients. This period largely predated Omicron variant circulation in the United States. We utilized a test-negative case-control design to assess VE for mRNA vaccines to prevent COVID-19 hospitalization.
Patient Population and Data Collection
This work was determined to be a public health surveillance activity by all enrolling sites, Vanderbilt University Medical Center (coordinating center), and CDC (funder and government sponsor). Adult patients aged 18 years and older hospitalized at 21 IVY Network hospitals between March 11 and December 15, 2021 were included. As previously described [8, 16], and detailed in Supplementary, Appendix B, Section 1, case patients had COVID-19-like illness and a positive SARS-CoV-2 molecular (eg, reverse-transcription polymerase chain reaction [RT-PCR]) or antigen test result in the clinical setting [8, 16]. COVID-19-like illness was defined as having 1 or more of the following: fever, cough, shortness of breath, loss of taste, loss of smell, use of respiratory support for the acute illness, or new pulmonary findings on chest imaging consistent with pneumonia. Control patients included (1) test-negative controls who were admitted with a COVID-19-like illness and tested negative for SARS-CoV-2 by a molecular assay and (2) syndrome-negative controls who were admitted without COVID-19-like illness and tested negative for SARS-CoV-2 by a molecular assay. In addition to clinical testing, nasal specimens were also collected from patients and tested for SARS-CoV-2 by RT-PCR at a central laboratory at Vanderbilt University Medical Center using standardized methods developed by CDC. Final case and control status was determined using both clinical testing and central laboratory results [8]. Thus, cases were patients hospitalized with laboratory-confirmed, symptomatic COVID-19, and controls were patients hospitalized without COVID-19. Severe acute respiratory syndrome coronavirus 2 detected in the central laboratory with a cycle threshold <32 also underwent viral whole-genome sequencing at University of Michigan for lineage determination [8, 16]. Patients or their proxies were interviewed to obtain information about demographic characteristics, clinical history, and COVID-19 vaccination status, and structured medical record abstraction was used to collect information on chronic medical conditions and clinical outcomes [8, 16].
Classification of COVID-19 Vaccination Status
Receipt of COVID-19 vaccine doses was ascertained by participant interview, hospital medical records, state immunization registries, vaccination record cards, and provider and pharmacy records. A vaccine dose was classified as received if verified by source documentation or plausible self-report including dates and locations of vaccination. In this analysis, we evaluated VE of 2-dose and 3-dose mRNA vaccine regimens (BNT-162b2 from Pfizer-BioNTech or mRNA-1273 from Moderna). Thus, patients were included if they had received 2 mRNA vaccine doses, 3 prior doses, or were unvaccinated. Patients were classified as 2-dose recipients if they received 2 mRNA vaccine doses with the second dose received ≥14 days before illness onset in cases and test-negative controls or ≥19 days before admission in syndrome-negative controls. Patients were classified as 3-dose recipients if they received 3 mRNA vaccine doses with the third dose received ≥7 days before illness onset in cases and test-negative controls or ≥12 days before admission in syndrome-negative controls and after authorization of third doses by the US Food and Drug Administration (FDA), which was August 12, 2021 for immunocompromised patients and September 22, 2021 for immunocompetent patients. Patients were classified as unvaccinated if they had never received any COVID-19 vaccine in their lifetimes. Patients were excluded from the analyses if they received 1 mRNA vaccine dose, 2 mRNA vaccine doses with the second dose <14 days before illness onset for cases and test-negative controls or <19 days before admission for syndrome-negative controls, a third mRNA vaccine dose before FDA authorization, or a COVID-19 vaccine not evaluated in this analysis (eg, Ad26.SOV.2 vaccine).
Classification of Immune Status
Data on immunocompromising conditions were collected via standardized medical record review. Using these data, patients were categorized into 1 of 3 mutually exclusive categories of immune status at the time of hospital admission: Group 1, SOT recipient; Group 2, other immunocompromising condition [8]; or Group 3, immunocompetent (details provided in Supplementary, Appendix B, Section 2). Older age was not considered an immunocompromising condition in this analysis. For SOT recipients, detailed information on type and date of organ transplant, organ transplanted (heart, lung, liver, pancreas, intestine, or mixed organs), immunosuppressive regimen at the time of hospital admission, and history of transplant rejection in the previous year were collected through detailed physician-level medical record review. The SOT recipients were excluded from the analysis if they were not on immunosuppressive medications (eg, due to renal graft failure) or the date of transplant was found to be after the illness onset date. Patients with other immunocompromising conditions besides solid organ transplantation and immunocompetent patients were used as comparator groups to show VE among SOT patients in the context of other, concurrently enrolled populations.
Outcomes
Our goals were to estimate mRNA VE against COVID-19 hospitalizations for SOT recipients and to describe in-hospital clinical outcomes for SOT recipients hospitalized with COVID-19. For VE calculations, hospitalization for COVID-19 (case status) versus hospitalization for another reason (control status) was the outcome of interest. For description of clinical course, outcomes included the following variables, ascertained during the index hospitalization through hospital day 28: in-hospital death, invasive mechanical ventilation, noninvasive ventilation, vasopressor use, new renal replacement therapy, intensive care unit (ICU) admission, and hospital length of stay.
Statistical Analysis
Vaccine effectiveness against COVID-19 hospitalization was calculated using multivariable logistic regression. The dependent variable was case versus control status, with the control group including both test-negative and syndrome-negative controls. The primary independent variable was vaccination status classified into 3 groups, including unvaccinated (the reference group), vaccinated with 2 doses, or vaccinated with 3 doses. Models used to estimate VE included the following prespecified covariables: admission date (categorized in biweekly intervals), US Department of Health and Human Services geographic region, age, sex, and race/ethnicity [8, 16]. Vaccine effectiveness was calculated as follows: (1 – adjusted odds ratio [aOR]) × 100), with the aOR representing the odds of vaccination (2 doses versus unvaccinated or 3 doses versus unvaccinated) among cases compared with controls derived from the logistic regression model. Separate VE estimates were calculated for each of the 3 immune status groups (SOT recipients, other immunocompromising conditions, immunocompetent). For other non-SOT immunocompromising conditions, stratified VE estimates were calculated for common immunocompromised subgroups, including patients with active hematologic malignancy, active solid organ malignancy, and either rheumatologic conditions or inflammatory bowel disease (IBD). As a sensitivity analysis to account for the potential of SOT recipients being hospitalized for less severe COVID-19 than immunocompetent patients, VE estimates were calculated after restricting cases to those with hypoxemia at hospital admission, defined as SpO2 <92% or treated with supplemental oxygen within 24 hours of admission. All VE results were presented as pooled analyses including both COVID-19 mRNA vaccines available in the United States (BNT-162b2 and mRNA-1273). Two-dose and 3-dose VE estimates were compared using the pwcompare function in Stata, with a 2-sided P < .05 considered statistically significant.
In-hospital clinical outcomes among COVID-19 case patients were compared across the 3 immune status groups, stratified by vaccination status. Analyses were conducted using STATA (release 16; StataCorp) and RStudio (version 1.2).
RESULTS
Patients
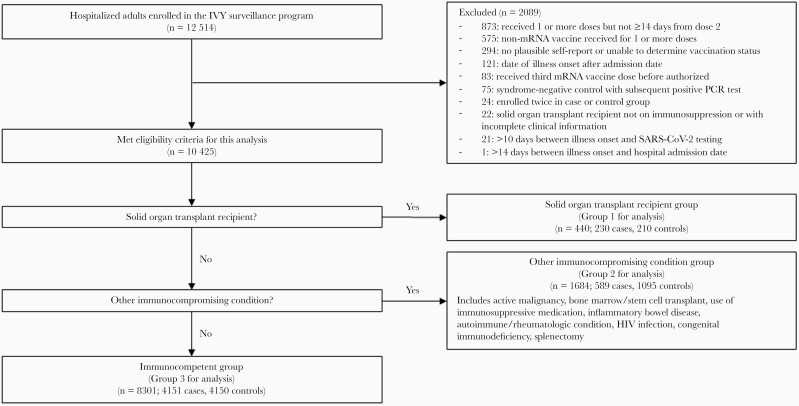
Between March 11, 2021 and December 15, 2021, 12 514 patients were enrolled across 21 hospitals. After 2089 patients were excluded (575 for receiving a COVID-19 vaccine other than an mRNA vaccine or unknown vaccine product, 873 for not being in an included vaccination group, and 641 for other reasons), 10 425 patients were included in this analysis (Figure 1). The analytical population included 440 patients in Group 1 (SOT recipients), 1684 patients in Group 2 (other immunocompromising condition), and 8301 patients in Group 3 (immunocompetent) (Table 1). Among 4970 COVID-19 cases, 2263 (46%) had a SARS-CoV-2 lineage identified; the most common lineages were the Delta variant (n = 1856, 82%) and the Alpha variant (n = 247, 11%).
Figure 1.
Flow diagram of patient participation.
Table 1.
Participant Characteristics
| Characteristic | Group 1: Solid Organ Transplant Recipients | Group 2: Other Immunocompromising Condition | Group 3: Immunocompetent | |||
|---|---|---|---|---|---|---|
| Cases (n = 230) | Controls (n = 210) | Cases (n = 589) | Controls (n = 1095) | Cases (n = 4151) | Controls (n = 4150) | |
| Age in Years, No. (%) | ||||||
| 18–49 | 49 (21) | 55 (26) | 137 (23) | 229 (21) | 1574 (38) | 1040 (25) |
| 50–64 | 95 (41) | 81 (39) | 214 (36) | 371 (34) | 1295 (31) | 1225 (30) |
| ≥65 | 86 (37) | 74 (35) | 238 (40) | 495 (45) | 1282 (31) | 1885 (45) |
| Female sex, No. (%) | 87 (38) | 98 (47) | 305 (52) | 597 (55) | 1919 (46) | 2037 (49) |
| Race/ethnicity, No. (%) | ||||||
| Non-Hispanic White | 132 (57) | 125 (60) | 360 (61) | 690 (63) | 2139 (52) | 2496 (60) |
| Non-Hispanic Black | 53 (23) | 38 (18) | 133 (23) | 221 (20) | 907 (22) | 885 (21) |
| Hispanic, any race | 27 (12) | 33 (16) | 75 (13) | 135 (12) | 814 (20) | 530 (13) |
| Other | 18 (8) | 14 (7) | 21 (4) | 49 (4) | 291 (7) | 239 (6) |
| Census region, No. (%) | ||||||
| Northeast | 18 (8) | 17 (8) | 79 (13) | 146 (13) | 705 (17) | 625 (15) |
| South | 69 (30) | 67 (32) | 235 (40) | 411 (38) | 1623 (39) | 1667 (40) |
| Midwest | 79 (34) | 60 (29) | 191 (32) | 293 (27) | 954 (23) | 943 (23) |
| West | 64 (28) | 66 (31) | 84 (14) | 245 (22) | 869 (21) | 915 (22) |
| Hypoxemic at hospital admissiona | 147/221 (67) | 87/168 (52) | 352/550 (64) | 450/830 (54) | 3014/3957 (76) | 1717/2878 (60) |
| Vaccinated with 2 or 3 doses of mRNA vaccine, No. (%) | 164 (71) | 160 (76) | 254 (43) | 756 (69) | 766 (18) | 2596 (63) |
| 2 doses | 140 (85) | 118 (74) | 235 (93) | 646 (85) | 744 (97) | 2455 (95) |
| 3 doses | 24 (15) | 42 (26) | 19 (7) | 110 (15) | 22 (3) | 141 (5) |
| Among Vaccinated, Product Received | ||||||
| BNT162b2 (Pfizer-BioNTech) | 98 (60) | 95 (59) | 164 (65) | 441 (58) | 506 (66) | 1498 (58) |
| mRNA-1273 (Moderna) | 65 (40) | 63 (39) | 89 (35) | 313 (41) | 257 (34) | 1091 (42) |
| Mixed products | 1 (0.6) | 2 (1) | 1 (0.4) | 2 (0.3) | 3 (0.4) | 7 (0.3) |
| Among vaccinated with 2 doses, days between dose 2 and illness onset, median (IQR) | 134.5 (93.5–179) | 113.5 (64–155) | 163 (114–208) | 127 (71–182) | 170 (125–210) | 128 (77–184) |
| Among vaccinated with 3 doses, days between dose 3 and illness onset, median (IQR) | 49 (22–71) | 64.5 (33–83) | 34 (23–55) | 36 (17–56) | 29 (12–54) | 27 (14–38) |
| Immunocompromising Conditions | ||||||
| Solid organ transplantb | 230 (100) | 210 (100) | --- | --- | --- | --- |
| Bone marrow or stem cell transplant | 0 (0) | 1 (0.5) | 10 (2) | 26 (2) | --- | --- |
| Hematologic malignancy | 2 (0.9) | 6 (3) | 118 (20) | 144 (13) | --- | --- |
| Solid organ malignancy | 12 (5) | 15 (7) | 212 (36) | 497 (45) | --- | --- |
| Congenital immunodeficiency | 0 (0) | 2 (1) | 4 (0.7) | 5 (0.5) | --- | --- |
| Immunosuppressive medications | 230 (100) | 210 (100) | 128 (22) | 179 (16) | --- | --- |
| IBD/rheumatologic condition | 14 (6) | 16 (8) | 163 (28) | 330 (30) | --- | --- |
| HIV infection | 0 (0) | 2 (1) | 54 (9) | 97 (9) | --- | --- |
| Prior splenectomy | 1 (0.4) | 0 (0) | 2 (0.3) | 9 (0.8) | --- | --- |
Abbreviations: HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; IQR, interquartile range; mRNA, messenger ribonucleic acid.
Hypoxemia at hospital admission was defined as an SpO2 <92% or use of supplemental oxygen within 24 hours of hospital admission. This information was collected for COVID-19 case patients and test-negative controls but not syndrome-negative controls.
Additional details about solid organ transplantation, including organ(s) transplanted, are in Table 2.
Among 440 SOT recipients (Group 1), 214 had a kidney transplant, 93 had a lung transplant, 48 had a liver transplant, 45 had a heart transplant, 2 had a pancreas transplant, and 38 had multiple organs transplanted (Table 2). The median time between organ transplantation and hospital admission resulting in enrollment was 4.4 years (interquartile range [IQR], 1.6–9.5 years; 5 missing date); 48 (11%) of 435 SOT patients with known date of transplant were admitted within 6 months after the date of transplant. Among 439 SOT recipients (1 missing), 76 (17%) patients had evidence of organ rejection in the prior year; all included SOT patients were taking immunosuppressive medications at the time of admission. Among the 440 SOT recipients, 230 (52%) patients were COVID-19 cases and 210 (48%) patients were controls (Table 1). Two vaccine doses had been received by 140 of 230 (61%) SOT cases and 118 of 210 (56%) SOT controls. Three doses had been received by 24 of 230 (10%) SOT cases and 42 of 210 (20%) SOT controls.
Table 2.
Additional Information on Transplant History and Immunosuppressive Drugs in Solid Organ Transplant Recipients (Immune Status Group 1) (See Separate Upload per Format per Guidelines)
| Characteristic | Overall (N = 440) | Case (N = 230) | Control (N = 210) | P Value |
|---|---|---|---|---|
| Organ Transplanted, No. (%) | ||||
| Kidney | 214 (49) | 119 (52) | 95 (45) | .17 |
| Liver | 48 (11) | 24 (10) | 24 (11) | .74 |
| Heart | 45 (10) | 22 (10) | 23 (11) | .63 |
| Pancreas | 2 (0.5) | 2 (0.9) | 0 (0) | .18 |
| Intestine | 0 (0) | 0 (0) | 0 (0) | --- |
| Lung | 93 (21) | 43 (19) | 50 (24) | .19 |
| Multiple organs | 38 (9) | 20 (9) | 18 (9) | .96 |
| On immunosuppressive medications at time of admission, No./Total (%) | 440 (100) | 230 (100) | 210 (100) | --- |
| Years since most recent transplant, median (IQR) | 4.4 (1.6–9.5) | 4.1 (1.7–8.4) | 4.8 (1.6–10.6) | .42 |
| History of rejection in previous year, No./Total (%) | 76/439 (17) | 33/229 (14) | 43/210 (20) | .09 |
Abbreviations: IQR, interquartile range.
Among 1684 patients with other immunocompromising conditions (Group 2), the most common conditions included active solid organ cancer (n = 709, 42%), IBD or rheumatologic conditions (n = 493, 29%), and active hematologic malignancy (n = 262, 16%) (Table 1). Within Group 2, 2 mRNA vaccine doses had been received by 235 of 589 (40%) cases and 646 of 1095 (59%) controls. Three doses had been received by 19 of 589 (3%) cases and 110 of 1095 (10%) controls.
Among 8301 immunocompetent patients (Group 3), 2 mRNA vaccine doses had been received by 744 of 4151 (14%) cases and 2455 of 4150 (57%) controls and 3 vaccine doses by 22 of 4151 (0.5%) cases and 141 of 4150 (3%) controls.
Vaccine Effectiveness Against Coronavirus Disease 2019 Hospitalization
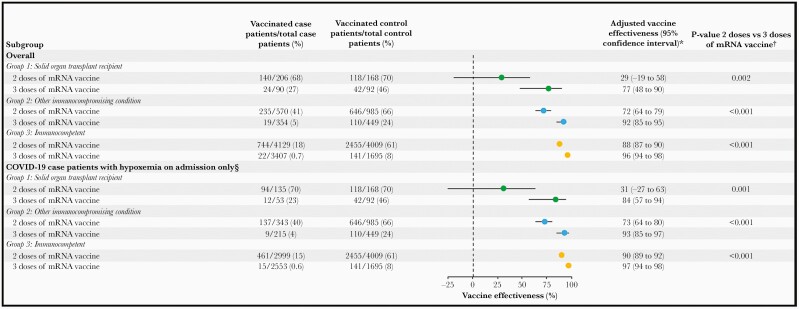
Among SOT recipients (Group 1), VE against COVID-19 hospitalization for 2 mRNA vaccine doses was 29% (95% confidence interval [CI], –19% to 58%), and for 3 mRNA vaccine doses it was 77% (95% CI, 48% to 90%), with a significant difference in VE between 2 doses and 3 doses (P = .002) (Figure 2). Among patients with other immunocompromising conditions (Group 2), VE against COVID-19 hospitalization for 2 mRNA vaccine doses was 72% (95% CI, 64% to 79%) and, for 3 doses it was 92% (95% CI, 85% to 95%) (P < .001 for 2 doses vs 3 doses). Among subgroups within Group 2, VE for 2 mRNA vaccine doses was 75% (95% CI, 62% to 84%) and for 3 vaccine doses it was 91% (95% CI, 78% to 97%) for those with active solid organ cancer (P = .02 for 2 doses vs 3 doses); VE for 2 vaccine doses was 74% (95% CI, 57% to 85%) and for 3 vaccine doses it was 97% (95% CI, 83% to 99%) for those with rheumatologic conditions or IBD (P = .012 for 2 doses vs 3 doses); and VE for 2 doses was 61% (95% CI, 14% to 83%) and for 3 doses it was 94% (95% CI, 72% to 98%) for those with active hematologic malignancies (P = .013 for 2 doses vs 3 doses). Among patients without immunocompromising conditions, VE for 2 vaccine doses was 88% (95% CI, 87% to 90%) and for 3 doses it was 96% (95% CI, 83% to 99%) (P < .001 for 2 doses vs 3 doses). Vaccine effectiveness results were similar after restricting COVID-19 cases to those with evidence of hypoxemia within 24 hours of hospital admission (Figure 2). Findings were also consistent in a post hoc analysis restricted to patients admitted after third-dose authorization.
Figure 2.
Vaccine effectiveness of mRNA vaccination against coronavirus disease 2019 (COVID-19) hospitalization by immune status group. Separate estimates were calculated for 2 doses of an mRNA vaccine and 3 doses of an mRNA vaccine. *Models were adjusted for calendar time of admission (in biweekly intervals), US Health and Human Services region, age group (18–49, 50–64, or ≥65 years), sex, and race and Hispanic ethnicity. †Post hoc P value comparisons of vaccine effectiveness for 2 doses vs 3 doses of an mRNA vaccine were obtained using the pwcompare function in Stata §Defined as receiving supplemental oxygen support or having a documented oxygen saturation <92% within 24 hours of admission. This analysis was restricted to cases who met criteria for hypoxemia within 24 hours of admission and all control patients (ie, including those with or without hypoxemia).
In-Hospital Clinical Outcomes of Coronavirus Disease 2019 Cases
Among 227 SOT recipients hospitalized with COVID-19 with outcome data (3 missing), 38 (17%) patients died in the hospital before day 28, including 30 of 163 (18%) who were vaccinated (with 2 or 3 doses) and 8 of 64 (13%) who were unvaccinated (P = .28). Among vaccinated cases, the severity of illness was generally greater for the SOT recipients and those with other immunocompromising conditions compared with immunocompetent patients, including for ICU admission (45% vs 32% vs 27%), invasive mechanical ventilation (21% vs 15% vs 13%), and in-hospital death within 28 days (18% vs 15% vs 10%) (Table 3). Among immunocompetent patients hospitalized with COVID-19, severe in-hospital outcomes were more common in unvaccinated patients compared with vaccinated patients. However, among patients with immunocompromising conditions hospitalized with COVID-19, the proportion of patients who experienced severe outcomes was similar for those unvaccinated and vaccinated (Table 3).
Table 3.
In-Hospital Clinical Outcomes Among COVID-19 Cases by Vaccination Status Within Each of the 3 Immune Status Groups (See Separate Upload per Format per Guidelines)
| Outcome, No./ Total No. (%)a | Group 1: Solid Organ Transplant Recipients | Group 2: Other Immunocompromising Condition | Group 3: Immunocompetent | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated With 2 or 3 Doses (n = 163) | Unvaccinated (n = 64) | P Valueb | Vaccinated With 2 or 3 Doses (n = 253) | Unvaccinated (n = 333) | P Value | Vaccinated With 2 or 3 doses (n = 757) | Unvaccinated (n = 3362) | P Value | |
| In-hospital death (within 28 days) | 30 (18) | 8 (13) | .28 | 37 (15) | 44 (13) | .62 | 79 (10) | 349 (10) | .96 |
| Invasive mechanical ventilation | 35 (21) | 11 (17) | .47 | 38 (15) | 58 (17) | .44 | 98 (13) | 847 (25) | <.001 |
| Noninvasive ventilation | 30 (18) | 8 (13) | .28 | 47 (19) | 58 (17) | .72 | 101 (13) | 583 (17) | .008 |
| Vasopressor use | 36 (22) | 12 (19) | .58 | 41 (16) | 63 (19) | .39 | 93 (12) | 790 (23) | <.001 |
| New renal replacement therapy | 14 (9) | 6 (9) | .85 | 9 (4) | 17 (5) | .37 | 39 (5) | 194 (6) | .51 |
| ICU admission | 74 (45) | 19 (30) | .03 | 81 (32) | 119 (36) | .35 | 203 (27) | 1441 (43) | <.001 |
| Hospital length of stay, median (IQR) | 6 (4–11) | 6 (4–8) | .60 | 5.5 (3–10) | 6 (3–11) | .29 | 5 (3–9) | 6 (3–10) | <.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Results provided for COVID-19 case patients who had information available on clinical outcomes (outcomes were not available for 38 of 4970 [0.8%] COVID-19 case patients); outcomes were censored at 28 days from the date of hospital admission.
P value comparisons between vaccinated and unvaccinated cases were calculated using the Pearson’s χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables.
DISCUSSION
In this multicenter, observational study of adults hospitalized in the United States between March and December 2021, VE for mRNA COVID-19 vaccines against COVID-19-associated hospitalization was significantly lower for SOT recipients than for immunocompetent people and for those with other immunocompromising conditions. These results highlight that people with moderate-to-severe immunosuppression are not a homogeneous group with regards to vaccine responses, and those with the greatest immunosuppression seem to have lower vaccine effectiveness than those with less severe immunosuppression. More importantly, VE against COVID-19 hospitalization for SOT recipients was substantially higher after 3 mRNA vaccine doses (77%) than after 2 doses (29%), demonstrating the importance of additional vaccine doses for this population. However, once hospitalized for COVID-19, vaccinated and unvaccinated SOT recipients had similar clinical outcomes, suggesting that vaccine-associated attenuation of disease severity previously observed in the immunocompetent population [16] may be less pronounced or even absent in the SOT population.
These results highlight that SOT recipients remain at risk for severe COVID-19 despite vaccination and support the need for continued efforts to mitigate the risk of COVID-19 in this population. Fourth doses of mRNA COVID-19 vaccines are now recommended for people with moderate-to-severe immunocompromising conditions, including solid organ transplantation. Future analyses will be important to understand the effectiveness of fourth doses, the residual risk of severe COVID-19 among SOT recipients after 4 vaccine doses, and the durability of protection. In addition, these results highlight that other measures to reduce the risk of COVID-19 among SOT recipients should be considered, including vaccination of close contacts [19], individual immune monitoring [20], and infection prevention strategies such as face masking in public spaces and physical distancing.
Several studies have indicated that COVID-19 mRNA vaccination results in reduced immune response among SOT recipients on immunosuppressive medication regimens compared with immunocompetent patients [11, 21–27]. Results of the current study suggest that reduced immunogenicity observed in the SOT population does translate into lower protection against severe COVID-19. However, there is hope vaccination can provide important protection against severe COVID-19 for SOT recipients because mRNA vaccine doses elicit cellular and humoral responses in many SOT recipients, including kidney [28], heart, and liver transplant recipients [29]. Increased VE against COVID-19 hospitalization with a third mRNA vaccine dose among SOT recipients in this study is encouraging.
Impaired immune responses in immunosuppressed patients can often be overcome to provide benefit from vaccination through strategies such as additional priming doses, boosters, and modifying vaccines (eg, addition of adjuvants; increasing antigens) [30, 31]. However, immunosuppressive states are a heterogeneous range of conditions and treatments. Thus, the effects of vaccines likely vary by the severity and type of immunosuppression, and greater understanding of vaccine responses in the face of specific immunocompromising conditions is needed. For example, as shown in this study, SOT recipients appear to garner less protection from COVID-19 mRNA vaccine doses than people with some other immunocompromising conditions. From a vaccination policy perspective, immunosuppression has generally been considered binary—immunosuppressed or not. As our understanding of vaccination in immunosuppressed hosts improves, a better approach may be to consider the mechanistic effect that a specific condition has on the immune system and clinical effectiveness data for mechanistically rational subgroups of immunocompromising conditions [30].
Prior studies evaluating vaccine performance in immunocompromised hosts have generally taken 2 approaches. One approach has been to focus on evaluating immunogenicity (without clinical outcomes) in specific immunocompromising conditions [32, 33]. These studies are valuable initial steps but must be paired with clinical effectiveness data. The second approach has been to utilize electronic health records to identify enough patients with electronic codes for immunocompromising conditions to complete vaccine effectiveness analyses [7, 34]. These analyses are limited by lack of specificity for immunocompromising conditions and potential misclassification, because immunocompromising states are often transient and difficult pinpoint with electronic codes alone.
The current study identified patients with a prior SOT and currently taking antirejection immunosuppressive medications as one specific subgroup of immunosuppression. We did this by leveraging the strengths of trained clinician investigators and a large hospital-based active surveillance program. Trained personnel collected accurate information on immunocompromising conditions, including the timing of immunosuppression in relation to vaccine receipt and onset of illness. We then applied modified immunosuppression algorithms developed by previous investigators to classify patients into groups relevant for VE evaluation [35, 36]. We then assessed for effect modification of VE across these groups and observed that lower VE in the large group traditionally labeled as “moderately-to-severely immunocompromised” was a weighted average of VE from many separate smaller groups, and VE for SOT recipients was substantially lower than for patients with many other immunocompromising conditions.
This study has limitations to consider. First, although this was a multicenter study that enrolled over 10 000 participants, the number of SOT recipients hospitalized with COVID-19 was modest (n = 230), which prevented robust analyses evaluating factors that may be contributing determinants of VE for SOT patients, such as immunosuppressive medication regimen and time from organ transplant to vaccination. Second, the group of patients in this study classified as having other immunocompromising conditions (Group 2) included a heterogeneous array of medical conditions associated with varying degrees of immunosuppression; this group, along with the immunocompetent group, provided a comparator for interpretation of the VE estimates for SOT recipients. Third, this study included only hospitalized patients; thus, we are unable to provide data on VE against less severe COVID-19, such as symptomatic disease not resulting in hospitalization. Fourth, although potential confounders were included in multivariable models, residual confounding is possible in this observational study.
CONCLUSIONS
In conclusion, vaccine effectiveness of mRNA COVID-19 vaccines to prevent COVID-19-associated hospitalization was lower for SOT recipients than immunocompetent people. Three doses of an mRNA COVID-19 vaccine provided substantially greater protection than 2 doses for SOT recipients. Despite vaccination, SOT recipients remain at risk for severe COVID-19 and should take additional precautions to mitigate the risk of SARS-CoV-2 exposure.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. The Centers for Disease Control and Prevention (CDC) funded this program, and coauthors from the CDC took part in the project design, conduct, analysis, and manuscript preparation.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. Primary funding was provided by the Centers for Disease Control and Prevention (CDC Award 75D30121F00002; to W. H. S.). The REDCap data tool used in this program was funded by Clinical and Translational Science Award UL1 TR002243 from the National Center for Advancing Translational Sciences.
Potential conflicts of interest. All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. J. H. K. reports grant support from National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (1K23 AI137321-01A1). M. G. reports grant support from CDC, CDC-Abt, CDC-Westat, and Janssen and a role as the Co-Chair of the Infectious Diseases and Immunization Committee, Texas Pediatric Society, Texas Chapter of American Academy of Pediatrics (AAP). H. K. T. reports a grant from CDC. A. A. G. reports grant support from CDC, NIH, US Department of Defense (DOD), AbbVie, and Faron Pharmaceuticals. T. M. reports payments as a panelist for the Society of Hospital Medicine Updates in Heart Failure (August 25, 2021) and as a board member of the Scott & White Clinic Physicians Board of Directors. D. J. D. reports grant support from NIH/National Institute of General Medical Sciences (T32GM135169). J. D. C. reports grants from the NIH and DOD. K. W. G. reports grant support as a NECTAR Executive Committee member ACTIV-4HT. D. C. F. reports consultant fees from Cytovale and membership on a Medpace Data Safety Monitoring Board (DSMB). D. N. H. reports a contract from CDC and grant support from National Heart, Lung, and Blood Institute (NHLBI) for participation in the ACTIV4d-Host Tissue Trial. M. C. E. reports talks on nutrition in COVID pneumonia at ASPEN conference sponsored by Abbott Laboratories. M. N. G. reports grant support from the CDC, funding from NHLBI and Agency for Healthcare Research and Quality (AHRQ), and fees from Endpoint for participating on a scientific advisory panel, payment for Medicine Grand Rounds at Westchester Medical Center, travel support for attending the ATS Board of Directors meeting, and participation on a DSMB for Regeneron and REPLINISH trial. N. J. J. reports grants from the NIH, DOD, and Medic One Foundation, payment for expert testimony from the Washington Department of Health, participation on DSMBs for Opticyte Inc., Multi-Center, Randomized Control Trial to Study the Effectiveness of Hyperbaric Oxygen for COVID-19 patients with Moderate to Severe Hypoxemia (NCT04619719), and Remote Ischemic Conditioning to Enhance Resuscitation (RICE) Pilot (NCT04265807), and participation as Chair of the ACEP Critical Care Section. I. D. P. reports grants from the CDC, NIH, Janssen Pharmaceuticals, and Intermountain Research & Medical Foundation, institutional fees from Asahi Kasei Pharma and from Regeneron Pharmaceuticals. S. M. B. reports grants from the CDC, NIH, and DOD, fees from Hamilton ventilators for chairing a DSMB, and personal fees from New York University for service on a DSMB. E. T. M. reports a grant from Merck outside the submitted work. A. K. reports grants from the NIH, Ely Lily, United Therapeutics, Johnson & Johnson, BOA Medical, and 4D Medical. A. D. reports funding as the Center PI for PETAL network through NHLBI grant and for participation on a steering committee for ALung technologies. J. G. W. reports grant support from NIH/NHLBI for ACTIV-3 and ACTIV-4 trials and membership on the American Board of Internal Medicine Critical Care Exam Committee. S. Y. C. reports payment as a speaker for La Jolla Pharmaceuticals and consultant fees for PureTech Health and Kiniska Pharmaceuticals. H. M. B. reports grants from the CDC. A. S. L. reports grants from the CDC, NIH, and Burroughs Wellcome Fund, consultant fees from Sanofi, and fees from Roche for membership on a trial steering committee. N. H. reports grants from the CDC, NIH, Sanofi, and Quidel and honoraria for speaking at Continuing Medical Education event at AAP. J. D. C. reports grant support from the CDC. C. G. G. reports consultant fees from Pfizer, Merck, and Sanofi-Pasteur and grants from Campbell Alliance/Syneos Health, CDC, NIH, US Food and Drug Administration, AHRQ, and Sanofi. T. W. R. reports grants from the NIH, DOD, and AbbVie, personal fees from Cumberland Pharmaceuticals as the Director of Medical Affairs, consultant fees from Cytovale, Inc., personal fees from Sanofi, Inc. for serving as a DSMB board member, a role as the Immediate Past President of the American Society of Parenteral and Enteral Nutrition (ASPEN), and stock from Cumberland Pharmaceuticals for consulting work. C J. L. reports grants/contracts from the CDC, NIH, DOD, bioMerieux, Endpoint LLC, Entegrion, Inc., and AbbVie, a patent issued to Cincinnati Children’s Hospital Medical Center for risk stratification in sepsis and septic shock, participation on DSMBS for Study Principal Investigators, roles on the Executive Committee, Immediate Past President, Member, Board of Directors for the Association of Clinical and Translational Science, and stock options in Bioscape Digita. W. H. S. reports grant funding from the CDC for this work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Jennie H Kwon, Department of Medicine, Washington University, St. Louis, Missouri, USA.
Mark W Tenforde, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Manjusha Gaglani, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
H Keipp Talbot, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Tresa McNeal, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
Shekhar Ghamande, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
David J Douin, Department of Anesthesiology, University of Colorado School of Medicine, Aurora, Colorado, USA.
Jonathan D Casey, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Nicholas M Mohr, Department of Emergency Medicine, University of Iowa, Iowa City, Iowa, USA.
Anne Zepeski, Department of Emergency Medicine, University of Iowa, Iowa City, Iowa, USA.
Nathan I Shapiro, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Kevin W Gibbs, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
D Clark Files, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
David N Hager, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Arber Shehu, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Matthew E Prekker, Department of Emergency Medicine and Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Sean D Caspers, Department of Emergency Medicine and Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Matthew C Exline, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Mena Botros, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Michelle N Gong, Department of Medicine, Montefiore Health System, Albert Einstein College of Medicine, Bronx, New York, USA.
Alex Li, Department of Medicine, Montefiore Health System, Albert Einstein College of Medicine, Bronx, New York, USA.
Amira Mohamed, Department of Medicine, Montefiore Health System, Albert Einstein College of Medicine, Bronx, New York, USA.
Nicholas J Johnson, Department of Emergency Medicine and Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, Seattle, Washington, USA.
Vasisht Srinivasan, Department of Emergency Medicine, University of Washington, Seattle, Washington, USA.
Jay S Steingrub, Department of Medicine, Baystate Medical Center, Springfield, Massachusetts, USA.
Ithan D Peltan, Department of Medicine, Intermountain Medical Center, Murray, Utah and University of Utah, Salt Lake City, Utah, USA.
Samuel M Brown, Department of Medicine, Intermountain Medical Center, Murray, Utah and University of Utah, Salt Lake City, Utah, USA.
Emily T Martin, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Akram Khan, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Catherine L Hough, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Laurence W Busse, Department of Medicine, Emory University, Atlanta, Georgia, USA.
Abhijit Duggal, Department of Medicine, Cleveland Clinic, Cleveland, Ohio, USA.
Jennifer G Wilson, Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California, USA.
Cynthia Perez, Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California, USA.
Steven Y Chang, Department of Medicine, University of California-Los Angeles, Los Angeles, California, USA.
Christopher Mallow, Department of Medicine, University of Miami and Jackson Memorial Health System, Miami, Florida, USA.
Randal Rovinski, Department of Medicine, University of Miami and Jackson Memorial Health System, Miami, Florida, USA.
Hilary M Babcock, Department of Medicine, Washington University, St. Louis, Missouri, USA.
Adam S Lauring, Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan, USA.
Laura Felley, Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan, USA.
Natasha Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
James D Chappell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Todd W Rice, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kelsey N Womack, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Christopher J Lindsell, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kimberly W Hart, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adrienne Baughman, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Samantha M Olson, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Stephanie Schrag, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Miwako Kobayashi, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Jennifer R Verani, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Manish M Patel, CDC COVID-19 Response Team, Atlanta, Georgia, USA.
Wesley H Self, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med 2021; 27:2136–43. [DOI] [PubMed] [Google Scholar]
- 3. Young-Xu Y, Korves C, Roberts J, et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw Open 2021; 4:e2128391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥65 years - COVID-NET, 13 states, February-April 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022; 376:e069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing covid-19 hospitalizations in the United States. Clin Infect Dis 2021; doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 9. Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med 2021; 174:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021; 3:e778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021; 7:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021; 22:765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maneikis K, Sablauskas K, Ringeleviciute U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol 2021; 8:e583–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021; 326:2043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed 18 November 2021.
- 18. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021; 80:1330–8. [DOI] [PubMed] [Google Scholar]
- 19. Eberhardt CS, Balletto E, Cornberg M, Mikulska M. Coronavirus disease 2019 vaccination in transplant recipients. Curr Opin Infect Dis 2021; 34:275–87. [DOI] [PubMed] [Google Scholar]
- 20. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest 2021; 6:eabj1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guarino M, Cossiga V, Esposito I, Furno A, Morisco F. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: the debate is open! J Hepatol 2021; 76:237–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021; 13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant 2021; 21:2913–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med 2021; 174:1336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miele M, Busà R, Russelli G, et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant 2021; 21:2919–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prendecki M, Thomson T, Clarke CL, et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet 2021; 398:1482–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant 2021; 21:2727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant 2021; 21:3971–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health 2022; 10:e326–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caldera F, Mercer M, Samson SI, Pitt JM, Hayney MS. Influenza vaccination in immunocompromised populations: strategies to improve immunogenicity. Vaccine 2021; 39:A15–a23. [DOI] [PubMed] [Google Scholar]
- 32. Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beck CR, McKenzie BC, Hashim AB, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis 2012; 206:1250–9. [DOI] [PubMed] [Google Scholar]
- 34. Blanchette PS, Chung H, Pritchard KI, et al. Influenza vaccine effectiveness among patients with cancer: a population-based study using health administrative and laboratory testing data from Ontario, Canada. J Clin Oncol 2019; 37:2795–804. [DOI] [PubMed] [Google Scholar]
- 35. Patel M, Chen J, Kim S, et al. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg Infect Dis 2020; 26:1720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenberg JA, Hohmann SF, Hall JB, Kress JP, David MZ. Validation of a method to identify immunocompromised patients with severe sepsis in administrative databases. Ann Am Thorac Soc 2016; 13:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.