Abstract
Background
Seroprevalence studies are important for quantifying the burden of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in resource-constrained countries.
Methods
We conducted a cross-sectional household survey spanning the second pandemic wave (November 2020 to April 2021) in 3 communities. Blood was collected for SARS-CoV-2 antibody (2 enzyme-linked immunosorbent assays targeting spike and nucleocapsid) and human immunodeficiency virus (HIV) testing. An individual was considered seropositive if testing positive on ≥1 assay. Factors associated with infection, and the age-standardized infection case detection rate, infection hospitalization rate, and infection fatality rate were calculated.
Results
Overall, 7959 participants were enrolled, with a median age of 34 years and an HIV prevalence of 22.7%. SARS-CoV-2 seroprevalence was 45.2% (95% confidence interval 43.7%–46.7%) and increased from 26.9% among individuals enrolled in December 2020 to 47.1% among those enrolled in April 2021. On multivariable analysis, seropositivity was associated with age, sex, race, being overweight/obese, having respiratory symptoms, and low socioeconomic status. Persons living with HIV with high viral load were less likely to be seropositive than HIV-uninfected individuals. The site-specific infection case detection rate, infection hospitalization rate, and infection fatality rate ranged across sites from 4.4% to 8.2%, 1.2% to 2.5%, and 0.3% to 0.6%, respectively.
Conclusions
South Africa has experienced a large burden of SARS-CoV-2 infections, with <10% of infections diagnosed. Lower seroprevalence among persons living with HIV who are not virally suppressed, likely as a result of inadequate antibody production, highlights the need to prioritize this group for intervention.
Keywords: SARS-CoV-2, COVID-19, seroprevalence, HIV, South Africa
In South Africa, 47% of individuals were SARS-CoV-2 seropositive following the second wave. PLWHIV that were not virally suppressed were less likely to be seropositive compared to HIV-uninfected individuals. Less than 10% of SARS-CoV-2 infections were diagnosed.
By April 2021, South Africa had experienced 2 epidemic waves peaking in July 2020 and January 2021. A new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lineage 501Y.V2 (Beta variant), associated with increased transmissibility and immune escape, was the predominant lineage during the second wave [1, 2]. As of the end of April 2021, South Africa had reported nearly 1.6 million cases and >54 000 associated deaths.
The extent of the pandemic in Africa is not well understood and the reported burden of disease and deaths has been lower than expected [3]. This is thought to be due to a high proportion of asymptomatic infections, as well as limited access to diagnostic testing. Seroprevalence studies quantify the burden of SARS-CoV-2 infections and are important to improve modeling predictions and public health response planning. South Africa has experienced the highest recorded burden of coronavirus disease 2019 (COVID-19) cases and deaths in sub-Saharan Africa. Human immunodeficiency virus (HIV) infection has been associated with an increased risk of severe illness and in-hospital mortality associated with SARS-CoV-2 [4] however, the influence of HIV on the risk of SARS-CoV-2 infection and antibody response to infection is not yet clear. The Healthcare Utilisation and Seroprevalence (HUTS) study aimed to estimate SARS-CoV-2 seroprevalence by HIV status and identify epidemiologic characteristics associated with seropositivity.
METHODS
Study Design and Population
We conducted a cross-sectional seroprevalence survey, nested in a healthcare utilization survey, in households in 3 communities serviced by facilities where severe respiratory illness and influenzalike illness surveillance is conducted [5], namely, Mitchell’s Plain (Western Cape Province), Pietermaritzburg (KwaZulu-Natal Province), and Klerksdorp (North West Province) (Supplementary Figure 1), using a 1-stage cluster design.
Selection and Enrollment of Households
Survey households were identified using randomly selected global positioning system (GPS) coordinates, and one-third of households were randomly selected for the seroprevalence survey. The boundaries of each catchment area were delineated on aerial maps available from Google Earth or the local municipality. No-residential areas (eg, parks, industrial areas, and sports complexes) were excluded, and GPS coordinates were randomly selected. The household closest (within 30 m) to each coordinate was approached. Additional GPS coordinates were generated at the study start, and households were replaced according to the order on the list. Fieldworker teams visited each household up to 3 times on separate days or times. All household members were invited to enroll.
Sample Size
The sample size was calculated for a 1-stage cluster sampling design, 95% confidence intervals (CIs), 5% desired absolute precision, 30% expected SARS-CoV-2 seroprevalence, and 1.5 design (household cluster) effect within 3 age groups: 0–18, 19–39 and ≥40 years. The required sample size (484 individuals) was applied to the age strata least represented in the target communities (55% for individuals aged 0–18 years, 24% for those aged 19–39 years, and 21% for those aged ≥40 years). The total target sample size was 2304 individuals (ie, 484/0.21) in each community. Assuming an average household size of 3 members, 770 households in each community were randomly selected. We accounted for a 30% household refusal rate.
Data and Specimen Collection
Field workers administered structured questionnaires using Research Electronic Data Capture (REDCap; Vanderbilt University) with the primary caregiver of the household for household demographic information and screening of household members for symptoms for severe respiratory illness (either sudden onset or worsening fever with cough and difficulty breathing lasting 2–30 days or a pneumonia diagnosis) or influenzalike illness (sudden onset or worsening fever with cough) since the beginning of March 2020. Information on underlying illnesses—including tuberculosis (current and previous), asthma, diabetes, chronic heart disease, chronic lung disease, hypertension, and cancer—was collected from participants, and height and weight were measured. If the household member was aged <18 years, information was obtained from the child’s parent/guardian, and assent was obtained for individuals aged 7–17 years. Venous whole blood specimens (serum and plasma samples) were collected.
Laboratory Testing
SARS-CoV-2 antibodies were detected using 2 enzyme-linked immunosorbent assay (ELISA) kits: (1) Wantai SARS-CoV-2 Ab ELISA (Beijing Wantai Biological Pharmacy Enterprise), which measures total antibodies (immunoglobulin [Ig] M, IgG and IgA) against the receptor binding domain in the spike protein [6], and (2) Elecsys Anti-SARS-CoV-2 ELISA (Roche Diagnostics), which measures total antibodies to the nucleocapsid protein [7]. A participant was considered to have had SARS-CoV-2 infection if testing positive on ≥1 assay.
HIV testing was performed on plasma specimens using polymerase chain reaction (PCR) for individuals aged <18 months (Roche Cobas Ampliprep/Cobas Taqman HIV-1 Qualitative Test; version 2.0) and ELISA for individuals aged ≥18 months using the Abbott ARCHITECT HIV Ag/Ab Combo kit (Abbott) for screening and the Bio-Rad Genscreen Ultra HIV Ag-Ab test (Bio-Rad) for confirmation of positive results. Viral load testing by quantitative PCR (Roche Cobas Ampliprep/Cobas Taqman HIV-1 test; version 2.0) was performed in HIV-positive individuals.
Data Analysis
Analysis was performed using Stata 14.1 software (StataCorp). Continuous variables were summarized using median values with interquartile ranges (IQRs). Categorical variables were summarized using frequency distributions and compared using Pearson χ2 test.
Body mass index was calculated (as weight in kilograms divided by height in meters squared) and categorized using World Health Organization standards [8, 9]. Socioeconomic status (SES) was measured by asking primary caregivers “Which of the following items does your household have in the house?” There were 28 answer options, for example, “hot running water.” Items were summed and scores created and categorized as low, medium, or high SES. Household crowding was defined as a mean of >2 individuals per sleeping room. Individuals were categorized as HIV uninfected, persons living with HIV (PLWHIV) with a low viral load (≤1000 copies/mL), and PLWHIV with a high viral load (>1000 copies/mL) [10]. Seroprevalence was calculated as the number of individuals positive for SARS-CoV-2 antibodies (with either the Wantai or Roche Elecsys ELISA) divided by the number of individuals tested, adjusted for household clustering; 95% CIs were also calculated, accounting for clustering by site and household.
We did not adjust for the performance characteristics of each ELISA kit. Agreement between assays was determined using the Cohen κ statistic, which ranges from 0 (poor agreement) to 1 (almost perfect agreement). Using the Roche Elecsys ELISA kit as the reference, we calculated the sensitivity and specificity of the Wantai kit. Mixed effects hierarchical multivariable logistic regression, controlling for random effect of site and household (within site) clustering, was used to identify factors associated with SARS-CoV-2 seropositivity, starting with all variables that were significant at P<.2 at univariate analysis and dropping nonsignificant factors with stepwise backward selection. All 2-way interactions were evaluated. Differences were considered significant at P<.05 (2 sided). We performed a sensitivity analysis in which participants were considered seropositive only if they tested positive on both ELISAs.
For each of the study site districts (uMgungundlovu, Dr Kenneth Kaunda, and City of Cape Town), the number of laboratory-confirmed cases reported from 1 March 2020 through 30 April 2021 was obtained from the Notifiable Medical Conditions Surveillance System [11], and the number of hospitalizations and in-hospital deaths from COVID-19 National Hospital Surveillance [12]. Provincial excess deaths per 100 000 population (based on death trends for 2014–2019) were extracted from the South African Medical Research Council report and applied to district denominators [13]. The age-standardized (to the South African 2020 mid-year population estimate) infection case detection rate (ICR), infection hospitalization rate (IHR), and infection fatality rate (IFR) were calculated by dividing the number of laboratory-confirmed cases, hospitalizations, or deaths (in-hospital or excess deaths), respectively, by the age-adjusted number of infections estimated from seroprevalence during March and April 2021. The 95% CIs were calculated by dividing the number of cases, hospitalizations, or deaths by the lower and upper CIs of the age-adjusted number of infections.
Ethics
This study was approved by the University of the Witwatersrand (no. M200861) and by community and provincial research committees.
RESULTS
Demographic characteristics of participants
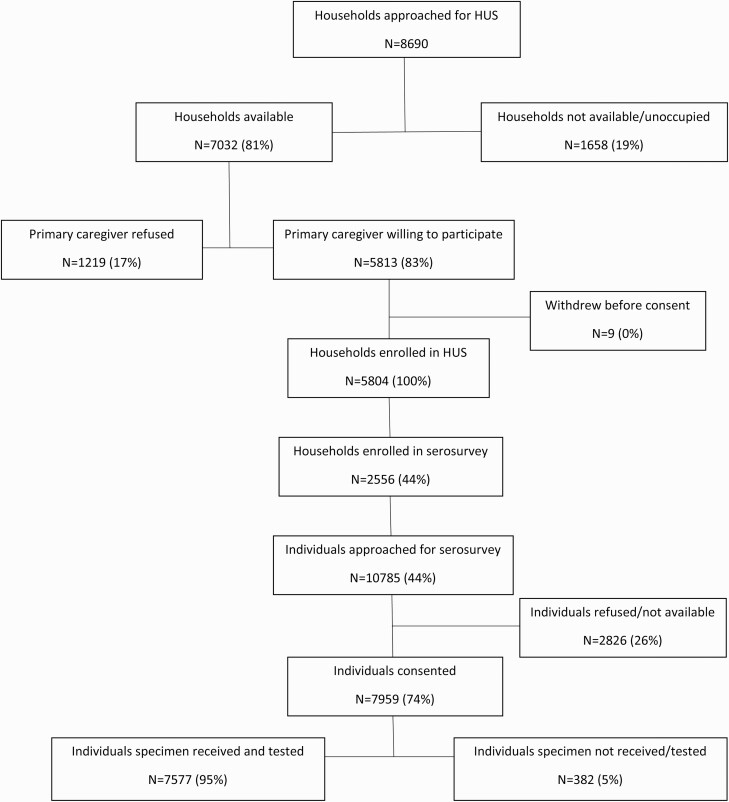
From November 2020 through April 2021, 5804 households were enrolled in the healthcare utilization survey, of which 2556 (44%) were enrolled in the seroprevalence survey (Figure 1). The median number of household members was 4 (IQR, 2–6), and median number of rooms was 5 (4–6) (Table 1). Overall, 29.3% (749 of 2554) of households were considered to have crowding, and 28.1% (718 of 2556) were classified as low SES.
Figure 1.
Flowchart of healthcare utilization survey (HUS) and seroprevalence survey household and participant enrollment and testing in 3 communities in South Africa (Healthcare Utilisation and Seroprevalence study, November 2020 to April 2021).
Table 1.
Demographic Characteristics of Households and Participants by Site—Healthcare Utilisation and Seroprevalence Study, South Africa, November 2020 to April 2021
| Characteristic | Households or Participants, No. (%)a | P Valueb | |||
|---|---|---|---|---|---|
| Overall | Pietermaritzburg | Klerksdorp | Mitchell’s Plain | ||
| Household-level characteristics | N=2556 | n=954 | n=906 | n=696 | |
| Month of enrollment | <.001 | ||||
| Nov 2020 | 31 (1.2) | 23 (2.4) | 8 (0.9) | 0 (0.0) | |
| Dec 2020 | 137 (5.4) | 52 (5.5) | 37 (4.1) | 48 (6.9) | |
| Jan 2021 | 217 (8.5) | 139 (14.6) | 21 (2.3) | 57 (8.2) | |
| Feb 2021 | 357 (14.0) | 103 (10.8) | 124 (13.7) | 130 (18.7) | |
| Mar 2021 | 1183 (46.3) | 375 (39.3) | 436 (48.1) | 372 (53.5) | |
| Apr 2021 | 631 (24.7) | 262 (27.5) | 280 (30.9) | 89 (12.8) | |
| No. of household members, median (IQR) | 4 (2–6) | 4 (3–6) | 3 (2–5) | 5 (3–7) | … |
| No. of household members | n=2554 | n=954 | n=904 | n=696 | <.001 |
| <3 | 695 (27.2) | 237 (24.8) | 325 (36.0) | 133 (19.1) | |
| 3–5 | 1078 (42.2) | 409 (72.9) | 411 (45.5) | 258 (37.1) | |
| 6–10 | 677 (26.5) | 263 (27.6) | 157 (17.4) | 257 (36.9) | |
| >10 | 104 (4.1) | 45 (4.7) | 11 (1.2) | 48 (6.9) | |
| No. of rooms, median (IQR) | 5 (4–6) | 5 (4–7) | 4 (3–5) | 6 (5–6) | … |
| No. of rooms | n=2554 | n=954 | n=904 | n=696 | <.001 |
| 1–4 | 1113 (43.6) | 355 (37.2) | 595 (65.8) | 163 (23.4) | |
| 5–9 | 4367 (53.5) | 558 (54.5) | 294 (32.5) | 515 (74.0) | |
| ≥10 | 74 (2.9) | 41 (4.3) | 15 (1.2) | 18 (2.6) | |
| No. of rooms for sleeping, median (IQR) | 2 (2–3) | 3 (2–4) | 2 (2–2) | 3 (2–3) | … |
| No. of rooms for sleeping | n=2554 | n=954 | n=904 | n=696 | <.001 |
| 1–2 | 1366 (53.5) | 419 (43.9) | 726 (80.3) | 221 (31.8) | |
| 3–4 | 1035 (40.5) | 431 (45.2) | 175 (19.4) | 429 (61.6) | |
| >4 | 153 (6.0) | 104 (10.9) | 3 (0.3) | 46 (6.6) | |
| Crowdingc | 749 (29.3) | 246 (25.8) | 257 (28.4) | 246 (35.3) | <.001 |
| SES | <.001 | ||||
| High | 1143 (44.7) | 389 (40.8) | 499 (55.1) | 255 (36.6) | |
| Medium | 695 (27.2) | 296 (31.0) | 231 (25.5) | 168 (24.1) | |
| Low | 718 (28.1) | 269 (28.2) | 176 (19.4) | 273 (39.2) | |
| Handwashing place with water in house | 2386 (93.6) (n=2549) |
879 (92.3) (n =952) |
821 (91.0) (n =902) |
686 (98.7) (n =695) |
<.001 |
| Main fuel for cooking | n=2549 | n=953 | n=902 | n=694 | <.001 |
| Electricity | 2315 (90.8) | 943 (99.0) | 756 (83.8) | 616 (88.8) | |
| Wood, gas, or paraffin | 234 (9.2) | 10 (1.0) | 146 (16.2) | 78 (11.2) | |
| Individual-level characteristics | N=7959 | n=2686 | n=2409 | n=2864 | |
| Month of enrollment | |||||
| Nov 2020 | 47 (0.6) | 37 (1.4) | 10 (0.4) | 0 (0.0) | <.001 |
| Dec 2020 | 327 (4.1) | 101 (3.8) | 86 (3.6) | 140 (4.9) | |
| Jan 2021 | 555 (7.0) | 303 (11.3) | 43 (1.8) | 209 (7.3) | |
| Feb 2021 | 928 (11.7) | 256 (9.5) | 256 (10.6) | 416 (14.5) | |
| Mar 2021 | 4051 (50.9) | 1072 (39.9) | 1247 (51.8) | 1732 (60.5) | |
| Apr 2021 | 2051 (25.8) | 917 (34.1) | 767 (31.8) | 367 (12.8) | |
| Age, median (IQR), y | 34 (19–50) | 32 (18–48) | 32 (15–49) | 37 (24–52) | … |
| Age group, y | n=7947 | n=2680 | n=2406 | n=2861 | <.001 |
| <5 | 139 (1.8) | 1 (0.0) | 130 (5.4) | 8 (0.3) | |
| 5–12 | 907 (11.4) | 366 (13.7) | 347 (14.4) | 194 (6.8) | |
| 13–18 | 856 (10.8) | 335 (12.5) | 271 (11.3) | 250 (8.7) | |
| 19–24 | 734 (9.2) | 276 (10.3) | 174 (7.2) | 284 (9.9) | |
| 25–39 | 2156 (27.1) | 709 (26.5) | 592 (24.6) | 855 (29.9) | |
| 40–59 | 2059 (25.9) | 618 (23.1) | 573 (23.8) | 868 (30.3) | |
| ≥60 | 1096 (13.8) | 375 (14.0) | 319 (13.3) | 402 (14.1) | |
| Female sex | 4782 (60.2) (n=7946) | 1639 (61.2) (n=2680) | 1389 (57.7) (n=2406) | 1754 (61.3) (n=2860) | .01 |
| Race | n=7725 | n=2621 | n=2338 | n=2766 | <.001 |
| Black African | 5550 (71.8) | 2620 (100.0) | 2317 (99.1) | 613 (22.2) | |
| Mixed | 2169 (28.1) | 1 (0.0) | 19 (0.8) | 2149 (77.7) | |
| Other | 6 (0.1) | 0 (0.0) | 2 (0.1) | 4 (0.1) | |
| HIV infected | 1655 (22.7) (n=7305) | 744 (29.5) (n=2526) | 655 (29.9) (n=2190) | 256 (9.9) (n=2589) | <.001 |
| HIV viral load, copies/mL | n=1622 | n=730 | n=639 | n=253 | |
| ≤1000 | 1145 (70.6) | 551 (75.5) | 435 (68.1) | 159 (62.9) | <.001 |
| >1000 | 477 (29.4) | 179 (24.5) | 204 (31.9) | 94 (37.2) | |
| BMId | n=7676 | n=2672 | n=2275 | n=2729 | |
| Underweight | 547 (7.1) | 69 (2.6) | 332 (14.6) | 146 (5.4) | <.001 |
| Normal weight | 2722 (35.5) | 599 (22.4) | 98 (43.4) | 1135 (41.6) | |
| Overweight | 1874 (24.4) | 635 (23.8) | 481 (21.1) | 758 (27.8) | |
| Obese | 2533 (33.0) | 1369 (51.2) | 474 (20.8) | 690 (25.3) | |
| Other underlying illnesse | 1151 (14.6) (n =7898) |
333 (12.5) (n =2671) |
430 (18.1) (n =2379) |
388 (13.6) (n =2848) |
<.001 |
| Reported respiratory symptoms since March 2020 | 229 (2.9) (n =7955) |
46 (1.7) (n =2686) |
107 (4.4) (n =2409) |
76 (2.7) (n =2860) |
<.001 |
| SARS-CoV-2 vaccinationf | 2 (0.0) (n=6639) | 0 (0.0) (n=1991) | 2 (0.1) (n=2207) | 0 (0.0) (n=2441) | .13 |
| Previously tested for SARS-CoV-2 | 357 (4.5) (n=7946) |
111 (4.1) (n=2685) |
92 (3.8) (n=2407) |
154 (5.4) (n=2854) |
.01 |
| Laboratory-confirmed SARS-CoV-2 infection | 71 (20.8) (n=342) |
17 (16.5) (n=103) |
17 (19.5) (n=87) |
37 (24.3) (n=152) | .30 |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; SARS, CoV-2, severe acute respiratory syndrome coronavirus 2; SES, socioeconomic status.
Data represent no. (%) of households or participants unless identified as median (IQR). Samples sizes (overall and by site) are provided where they differ from the total sample sizes listed for household and individual characteristics.
P values based on Pearson χ2 test.
Crowding was defined as >2 household members per sleeping room.
BMI calculated for individuals aged ≥5 years.
Underlying illness includes current or previous tuberculosis, asthma, diabetes, chronic heart disease, chronic lung disease, hypertension, and and cancer.
Vaccination of healthcare workers (phase 1 of the vaccine program) with a single dose of the Johnson & Johnson vaccine started on 17 February 2021. By 30 April 2021, 0.54% of the population (n=317 656) had been vaccinated. Phase 2, including the general public, started on 17 May 2021.
Of 10 785 individuals living in the households, 7959 (74%) were enrolled (Figure 1). The majority of participants (6102 of 7959 [76.7%]) were enrolled in March–April 2021 (Table 1). The median age of participants was 34 years (IQR, 19–50 years), with 60.2% (4782 of 7946) female, and 71.8% (5550 of 7725) of black African race. The HIV prevalence was 22.7% (1655 of 7305) and differed by site (29.5% in Pietermaritzburg, 29.9% in Klerksdorp, and 9.9% in Mitchell’s Plain) and age group (5.2%, 5.0%, 5.6%, 12.9%, 30.5%, 37.3%, and 14.4%, respectively, in those aged <5, 6–12, 13–18, 19–24, 25–39, 40–59, or ≥60 years respectively). Among participants with available data, 14.6% (1151 of 7898) reported an underlying illness, of which the most common were hypertension (10.4% [827 of 7935]), diabetes (3.3% [259 of 7943]), asthma (1.9% [151 of 7947]), and tuberculosis (1.5% [121 of 7927]). Only 2 individuals had received SARS-CoV-2 vaccination.
SARS-CoV-2 Seroprevalence
Of 7959 participants, SARS-CoV-2 antibody results were available for ≥1 assay for 7577 (95.2%). Results were not available for 382 participants owing to samples being insufficient, grossly hemolyzed, or unable to be linked to a participant. Wantai assay results were available for 99.8% of individuals (7562 of 7577), Roche Elecsys for 98.9% (7494 of 7577), and both assays for 98.7% (7479 of 7577).
Seroprevalence was 43.8% (3283 of 7494) with the Roche Elecsys assay and 41.3% (3126 of 7562) with the Wantai assay. Among individuals testing positive with the Roche Elecsys assay, 91.0% (2982 of 3277) tested positive with the Wantai assay, and 9.0% (295 of 3277) tested negative (Supplementary Table 1). For individuals who tested negative with the Roche Elecsys assay, 97.2% (4083 of 4202) tested negative with the Wantai assay, and 2.8% (119 of 4202) tested positive. Assay agreement was 94.5%, with a Cohen κ statistic of 0.89 (almost perfect agreement). The Wantai assay, compared with the Roche Elecsys assay, had a sensitivity of 91.0% and a specificity of 97.2%.
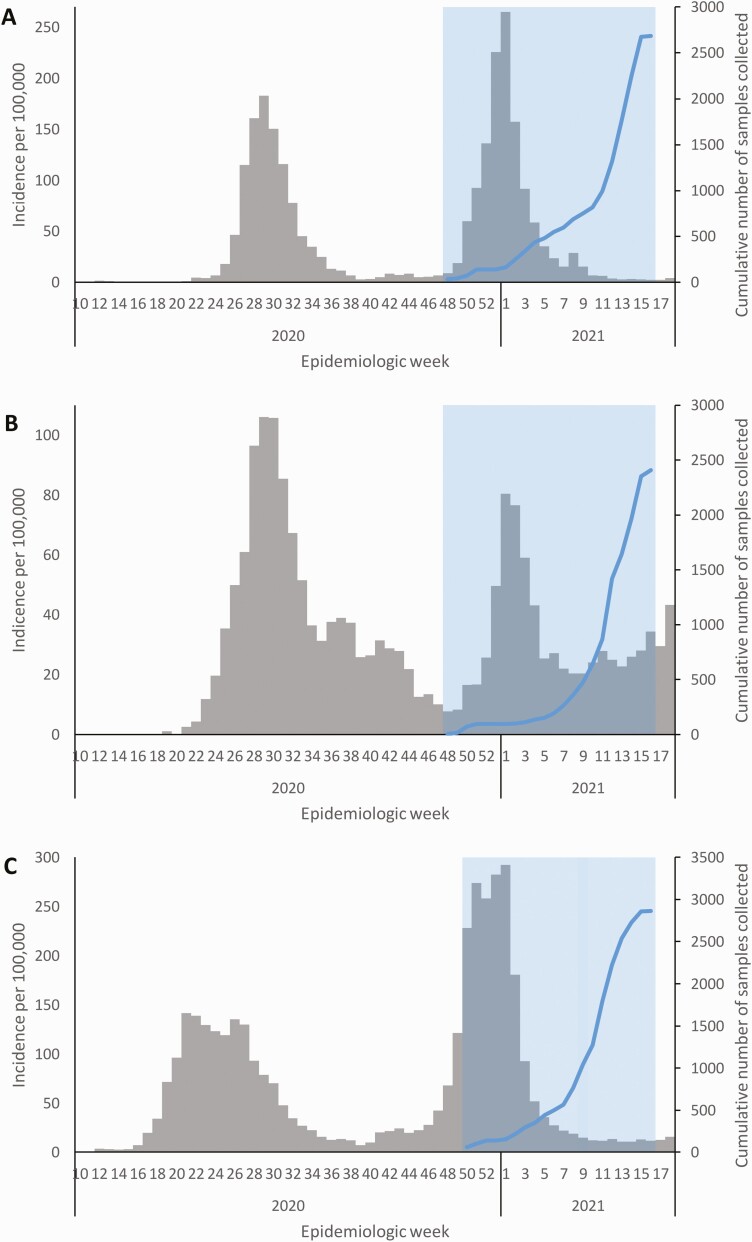
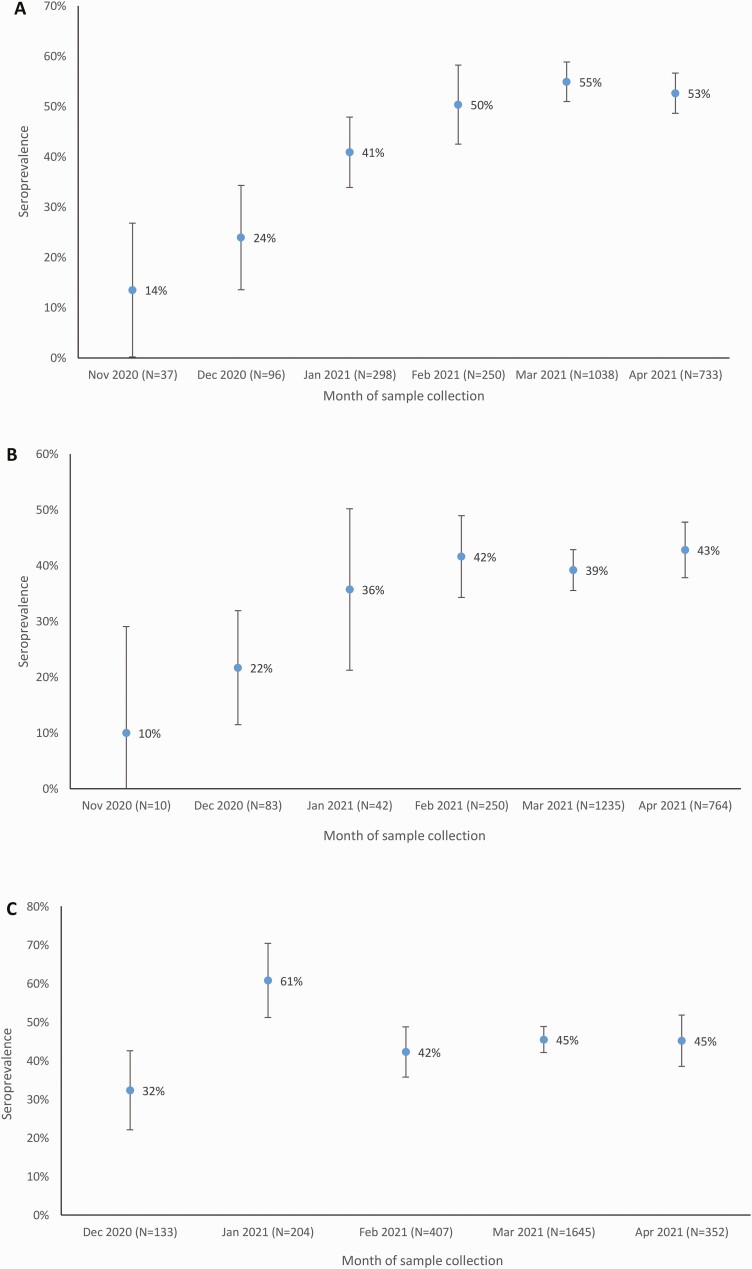
For the criterion of a seropositivity based on testing positive with ≥1 assay, seroprevalence over the study period was 45.2% (3427 of 7577 [95% CI, 43.7%–46.7%]). Over the period of enrollment, seroprevalence increased from 26.9% (84 of 312) among individuals enrolled in December to 47.2% (872 of 1849) among those enrolled in April 2021 (Table 2). Samples were collected during and after the second COVID-19 wave (Figure 2), and seroprevalence increased at each of the 3 sites (from 24.0% to 52.7% in Pietermaritzburg, 21.7% to 42.8% in Klerksdorp, and 32.3% to 45.2% in Mitchell’s Plain) (Figure 3).
Table 2.
Factors Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Seropositivity—Healthcare Utilisation and Seroprevalence Study, South Africa, November 2020 to April 2021
| Variable | Subgroup | SARS-CoV-2 Seropositive, No./Total (%) |
Univariate Analysisa | Multivariable Analysisa | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | aOR (95% CI) | P Value | |||
| Site | Pietermaritzburg | 1232/2452 (50.2) | 1.8 (1.4–2.1) | <.001 | 1.5 (1.2–1.8) | <.001 |
| Klerksdorp | 949/2384 (39.8) | Reference | … | Reference | … | |
| Mitchell’s Plain | 1246/2741 (45.5) | 1.3 (1.1–1.6) | .003 | 4.1 (3.0–5.6) | <.001 | |
| Month of enrollment | November 2020 | 6/47 (12.8) | 0.3 (.1–1.0) | .046 | 0.4 (.1–1.1) | .08 |
| December 2020 | 84/312 (26.9) | Reference | … | Reference | … | |
| January 2021 | 261/544 (48.0) | 2.8 (1.8–4.5) | <.001 | 2.2 (1.4–3.5) | .001 | |
| February 2021 | 402/907 (44.3) | 2.8 (1.8–4.4) | <.001 | 3.0 (1.9–4.6) | <.001 | |
| March 2021 | 1802/3918 (46.0) | 3.2 (2.2–4.8) | <.001 | 3.4 (2.3–5.1) | <.001 | |
| April 2021 | 872/1849 (47.2) | 3.4 (2.2–5.1) | <.001 | 3.7 (2.5–5.5) | <.001 | |
| Age group, y | <5 | 32/130 (24.6) | 0.6 (.4–1.0) | .047 | 0.9 (.4–2.2) | .76 |
| 5–12 | 348/865 (40.2) | Reference | … | Reference | … | |
| 13–18 | 400/810 (49.4) | 1.6 (1.3–2.0) | <.001 | 1.6 (1.3–2.1) | <.001 | |
| 19–24 | 274/695 (53.8) | 1.9 (1.5–2.5) | <.001 | 2.0 (1.5–2.6) | <.001 | |
| 25–39 | 1013/2050 (49.4) | 1.6 (1.3–2.0) | <.001 | 1.5 (1.2–1.9) | <.001 | |
| 40–59 | 873/1981 (44.1) | 1.3 (1.0–1.6) | .02 | 1.2 (1.0–1.5) | .07 | |
| ≥60 | 387/1046 (37.0) | 1.0 (.8–1.2) | .74 | 0.9 (.7–1.1) | .39 | |
| Sex | Male | 1210/2993 (40.4) | Reference | … | Reference | … |
| Female | 2216/4583 (48.4) | 1.5 (1.3–1.7) | <.001 | 1.4 (1.3–1.6) | <.001 | |
| Race | Black African | 2563/5317 (48.2) | 4.7 (3.5–6.4) | <.001 | 5.0 (3.7–6.9) | <.001 |
| Mixed | 808/2138 (37.8) | Reference | … | Reference | … | |
| Other | 1/6 (16.7) | 0.4 (.0–4.3) | .44 | 0.3 (.0–3.5) | .33 | |
| Unknown | 55/116 (47.4) | 4.3 (2.5–7.5) | <.001 | 4.2 (2.4–7.5) | <.001 | |
| HIV status | Uninfected | 2450/5476 (44.7) | Reference | … | Reference | … |
| Infected with viral load ≤1000 copies/mL | 592/1113 (53.2) | 1.4 (1.2–1.7) | <.001 | 1.2 (1.0–1.4) | .09 | |
| Infected with viral load >1000 copies/mL | 166/463 (35.9) | 0.6 (.4–.7) | <.001 | 0.5 (.4–.6) | <.001 | |
| Infected with viral load unknown | 17/32 (53.1) | 1.5 (.6–3.6) | .36 | 1.4 (.6–3.3) | .46 | |
| BMIb | Underweight | 179/536 (33.4) | 0.8 (.6–1.0) | .02 | 0.8 (.6–1.0) | .04 |
| Normal weight | 1115/2593 (43.0) | Reference | … | Reference | … | |
| Overweight | 863/1791 (48.2) | 1.2 (1.1–1.4) | .007 | 1.3 (1.1–1.5) | .002 | |
| Obese | 1185/2387 (49.6) | 1.3 (1.1–1.5) | <.001 | 1.3 (1.1–1.5) | .001 | |
| Unknown | 85/270 (31.5) | 0.6 (.4–.8) | .001 | 0.8 (.5–1.3) | .32 | |
| Other underlying illnessc | No | 2940/6406 (45.9) | Reference | … | … | … |
| Yes | 464/1111 (41.8) | 0.9 (.7–1.0) | .13 | … | … | |
| Reported respiratory symptoms since March 2020 | No | 3307/7350 (45.0) | Reference | … | Reference | … |
| Yes | 118/223 (52.9) | 1.5 (1.0–2.1) | .03 | 1.8 (1.2–2.6) | .003 | |
| Socioeconomic status | High | 1339/3196 (41.9) | Reference | … | Reference | … |
| Medium | 962/2054 (46.8) | 1.2 (1.0–1.5) | .04 | 1.2 (1.0–1.4) | .11 | |
| Low | 1126/2327 (48.4) | 1.4 (1.1–1.7) | .001 | 1.3 (1.1–1.5) | .006 | |
| Number of household members | <3 | 394/972 (40.5) | Reference | … | … | … |
| 3–5 | 1310/2957 (44.3) | 1.2 (1.0–1.5) | .07 | … | … | |
| 6–10 | 1383/2953 (46.8) | 1.4 (1.1–1.7) | .005 | … | … | |
| >10 | 335/688 (48.7) | 1.4 (.9–2.0) | .10 | … | … | |
| Crowdingd | No | 1995/4361 (45.8) | Reference | … | … | … |
| Yes | 1427/3209 (44.5) | 1.0 (.8–1.1) | .59 | … | … | |
| Handwashing place in house | No | 174/410 (42.4) | Reference | … | … | … |
| Yes | 3245/7142 (45.4) | 1.2 (.8–1.6) | .34 | … | … | |
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Mixed effects univariate and multivariable logistic regression analysis, adjusted for clustering by site and household.
BMI calculated for individuals aged ≥5 years.
Underlying illness includes current/previous tuberculosis, asthma, diabetes, chronic heart disease, chronic lung disease, hypertension and cancer.
Crowding was defined as >2 household members per sleeping room.
Figure 2.
Epidemic curves of laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases showing the timing of the first and second waves (gray), the timing of the seroprevalence surveys (blue shading), and the cumulative numbers of samples collected (blue lines) in 3 districts in South Africa where the Healthcare Utilisation and Seroprevalence study was conducted, from March 2020 to April 2021: uMgungundlovu district, KwaZulu-Natal Province (Pietermaritzburg site) (A), Dr Kenneth Kaunda District, North West Province (Klerksdorp site) (B), and City of Cape Town Metropolitan District, Western Cape Province (Mitchell’s Plain site) (C).
Figure 3.
Seroprevalence by site and month in the Healthcare Utilisation and Seroprevalence study, South Africa, November 2020 to April 2021. A, Pietermaritzburg site. B, Klerksdorp site. C, Mitchell’s Plain site.
Seroprevalence was highest among individuals aged 19–24 years (53.8% [274 of 695]), female participants (48.4% [2216 of 4583]), and those of black African race (48.2% [2563 of 5317]) (Table 2). Seroprevalence was higher among PLWHIV with viral load ≤1000 copies/mL (53.2% [592 of 1113]; P<.001) and lower among PLWHIV with viral load >1000 copies/mL (35.9% [166 of 463]; P<.001), compared with HIV-uninfected individuals (44.7% [2450 of 5476]). Among seropositive individuals, only 3.4% (118 of 3425) reported experiencing either mild (fever and cough) or severe (fever, cough, and difficulty breathing) respiratory symptoms. When an individual was considered seropositive only if testing positive with both assays, the seroprevalence was 39.9% (2982 of 7479 [95% CI, 38.4%–41.3%), with an increase from 25.8% (77 of 311) in December 2020 to 41.4% (756 of 1827) in April 2021 (Supplementary Table 2).
Factors Associated With SARS-CoV-2 Infection
With multivariable analysis (Table 2), controlling for clustering by site and household, an increased odds of being seropositive was associated with site, month of enrollment, age group, female sex, black African race, being overweight or obese, reporting respiratory symptoms, and having lower SES. A reduced odds of being seropositive for SARS-CoV-2 was associated with being HIV infected with viral load >1000 copies/mL and being underweight. In the sensitivity analysis, in which an individual was considered SARS-CoV-2 seropositive only if they tested positive on both assays, the same characteristics were found to be associated with seroprevalence (Supplementary Table 2).
Study ICRs, IHRs, and IFRs
Based on the estimated number of infections from the seroprevalence results, the ICRs ranged from 4.4% (95% CI, 3.8%–5.2%) to 8.2% (6.9%–10.3%) (Table 3). The IHRs ranged from 1.2% (95% CI, 1.1%–1.4%) to 2.5% (2.2%–3.0%). Based on the minimum estimate of in-hospital deaths, the IFR was 0.3% at all 3 sites. Using the maximum estimate of excess deaths, the IFRs ranged from 0.3% (95% CI, .3%–.3%) to 0.6% (.5%–.6%).
Table 3.
Age-Standardized Severe Acute Respiratory Syndrome Coronavirus 2 Infection Case Detection Rate, Infection Hospitalization Rate, and Infection Fatality Rate—Healthcare Utilisation and Seroprevalence Study, South Africa, March–April 2021
| Pietermaritzburga | Klerksdorpa | Mitchell’s Plaina | ||||
|---|---|---|---|---|---|---|
| Incidence Risk per 100 000 Population | Rate, % (95% CI) | Incidence Risk per 100 000 Population | Rate, % (95% CI) | Incidence Risk per 100 000 Population | Rate % (95% CI) | |
| Cases detected (ICR)b | 2442 | 5.0 (4.5–5.6) | 1766 | 4.4 (3.8–5.2) | 3515 | 8.2 (6.9–10.3) |
| Hospitalizations (IHR)c | 592 | 1.2 (1.1–1.4) | 1023 | 2.5 (2.2–3.0) | 818 | 1.9 (1.6–2.4) |
| In-hospital deaths (IFR)c | 146 | 0.3 (.3–.3) | 113 | 0.3 (.2–.3) | 129 | 0.3 (.3–.4) |
| Excess deaths (IFR)d | 280 | 0.6 (.5–.6) | 119 | 0.3 (.3–.3) | 199 | 0.5 (.4–.6) |
Abbreviations: CI, confidence interval; ICR, infection case detection rate; IFR, infection fatality rate; IHR, infection hospitalization rate.
The age-adjusted number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections estimated from seroprevalence in March and April 2021 was 48 942 (95% CI, 43 656–54 228) in Pietermaritzburg, 40 310 (34 231–46 389) in Klerksdorp, and 42 671 (34 207–51 134) in Mitchell’s Plain.
Laboratory-confirmed SARS-CoV-2 cases in the site districts reported to the national Notifiable Medical Conditions Surveillance System from March 2020 through April 2021.
Hospitalizations and in-hospital deaths from COVID-19 National Hospital Surveillance in the site districts from March 2020 through April 2021.
Excess deaths from the South African Medical Research Council in the site districts from March 2020 through April 2021.
DISCUSSION
In our household survey, we found that SARS-CoV-2 seroprevalence increased over the study period, reflecting the increasing number of infections during the second wave, reaching 47% by the end of the second wave. Differences in seroprevalence were observed across the sites and were highest among teens and younger adults.
Our findings are similar to those of other South African studies performed after the second wave. Among adult blood donors, national seroprevalence was 47%: 52% in KwaZulu-Natal, 49% in the North West, and 38% in the Western Cape [14, 15]. This study also found higher seroprevalence among black African donors. In a household community cohort study in South Africa, the post–second wave seroprevalence was 26% in a rural community in Mpumalanga Province and 41% in an urban community in North West Province [16].
Seroprevalence was significantly lower among PLWHIV with high viral loads than among HIV-uninfected individuals. This is likely owing to an inability to produce detectable antibodies in response to SARS-CoV-2 infection among those with a suppressed immune response. PLWHIV have been shown to have lower SARS-CoV-2 IgG concentrations and neutralization titers than HIV-uninfected individuals in a case-control study in the United States [17]. Similarly, immunocompromised PLWHIV were found to have a reduced anti–receptor binding domain IgG response to a messenger RNA SARS-CoV-2 vaccine, compared with HIV-uninfected individuals and PLWHIV with CD4 cell counts >250/µL [18]. While the origin of the Omicron strain is unknown, given the high HIV burden in South Africa and documented cases of prolonged SARS-CoV-2 infection in PLWHIV leading to rapid viral escape [19], immunocompromised individuals are a potential source for future immune escape variants [20].
We found that higher seroprevalence was associated with lower SES. Other studies in South Africa found higher seroprevalence to be associated with low SES and areas with high population densities [21, 22], and the association has also been described elsewhere [23].
We found that only 4.4%–8.2% of infections were detected through diagnostic testing. This indicates a substantially larger burden of COVID-19 than identified by laboratory-confirmed cases. The underascertainment of cases may be due to a large proportion of asymptomatic infections (only 3.4% of seropositive individuals reported respiratory symptoms), for which individuals do not seek medical care. A household cohort study in South Africa found that 83% of laboratory-confirmed infections were asymptomatic [24]. However, it may also be a result of limited access to testing in some areas or a reluctance to be tested due to potential negative consequences associated with testing positive. This low case detection rate has likely played a role in the spread of infections. A Kenyan serosurvey conducted in November 2020 showed an adjusted seroprevalence of 34.7% and estimated that only 2.4% of cases were detected [25]. In Mali, the adjusted seroprevalence in December 2020 to January 2021 was 54.7%, and had increased from 10.9% in July–October 2020 [26].
The IFR estimates from the United States of 2.0% [27] were similar to our study findings. Using a modeling framework based on 10 serosurveys, the SARS-CoV-2 IFR was estimated to be 0.23% in low-income countries and 1.15% in high-income countries [28]. Similarly, the estimated IFR across the first and second wave in India was 0.25% [29]. The IFR obtained from our study was comparable, although slightly higher, with a minimum estimate of 0.3%. The IFR in a serosurvey conducted in Kenya was 0.04% [25], lower than observed in our study.
The 2 ELISA kits used had almost perfect agreement, although some differences were observed. This was expected as the assays have different protein targets (spike vs nucleocapsid), and there is heterogeneity in the sensitivity and durability of antibody detection with different ELISAs. A comparison of SARS-CoV-2 serology assays showed differences in performance, particularly in individuals with asymptomatic or mild infection, who have lower antibody responses [30]. In addition, although antinucleocapsid antibodies have been shown to wane faster after infection than antispike antibodies [31], total immunoglobulin direct antigen-sandwich format assays, like the Roche anti-N assay, have been found to have stable antibody detection [7, 30]. The use of 2 assays in our study increased sensitivity to detect prior SARS-CoV-2 infection at different stages of convalescence. However, using the more stringent criterion of seropositivity with both assays, the same factors associated with seroprevalence were identified.
Our study had a number of limitations. First, it was conducted in periurban sites, and findings may not be generalizable to rural areas or other settings. Second, individuals reporting symptoms may have been underestimated as a result of recall bias of symptoms over a long time period. In addition, we collected information only on fever, cough, and difficulty breathing and did not include other COVID-19 symptoms. such as loss of taste or smell and gastrointestinal symptoms. Third, calculation of IFR is dependent on full ascertainment of COVID-19–related deaths and may therefore have been underestimated. Fourth, seroprevalence is likely underestimated owing to individual variation in the production and persistence of antibodies after SARS-CoV-2 infection, particularly because the majority of the seropositive individuals in our study had asymptomatic infection.
Our study showed that by the end of the second wave, just under half of the population had prior infection with SARS-CoV-2, a much larger burden of infection than indicated by enumeration of laboratory-confirmed cases. We have identified risk groups with higher seroprevalence that should be targeted for interventions. Non–virally suppressed PLWHIV have a reduced serologic response to SARS-CoV-2 infection and should be prioritized in COVID-19 prevention programs, such as vaccination and early referral and treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Contributors to the Healthcare Utilisation and Seroprevalence (HUTS) study are thanked for their inputs. These include participants who agreed to be part of the HUTS study; the Department of Health and district counsellors for supporting this research; the Epicentre fieldwork teams; Adrian Puren, Beverley Singh, and Zinhle Brukwe of the Centre for HIV and STIs, National Institute for Communicable Diseases (NICD) for managing logistics surrounding receiving of collected bloods and for HIV testing; Nokuthula Linda and Cayla Reddy of the Centre for Respiratory Diseases and Meningitis, NICD, and Doreen Janse van Rensburg of Ampath Pathology for laboratory testing; Nevashan Govender, Genevie Ntshoe, Andronica Moipone Shonhiwa, Darren Muganhiri, Itumeleng Matiea, Eva Mathatha, Fhatuwani Gavhi, Teresa Mashudu Lamola, Matimba Makhubele, Mmaborwa Matjokotja, Simbulele Mdleleni, and Masingita Makhubela from the national severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) NICD surveillance team; and Fazil Mckenna, Trevor Graham Bell, Ndivhuwo Munava, Muzammil Raza Bano, and Jimmy Khosa from NICD Information Technology (IT) for Notifiable Medical Conditions Surveillance System case data.
Author Contributions. Conceptualization of study: N. W., S. T., A. v. G., SW, JM, and C. Cohen. Study design: N. W., S. T., A. v. G., J. N. B., S. W., J. M., M. L. M., S. A., S. M., C. Cawood, N. M., L. L., and C. Cohen. Data collection: S. A., S. M., J. Y., T. F., T. M., R. W., and W. J. Laboratory testing: J. N. B., A. B., and M. B. Data analysis: N. W., S. T., A. v. G., J. N. B., S. W., J. K., S. A., and C. Cohen. Accessed and verified the underlying data: N. W., S. A., J. Y., T. F., and T. M. Manuscript writing: N. W. and C. Cohen. Manuscript review: S. T., A. v. G., J. N. B., S. W., J. K., J. M., A. B., M. L. M., S. A., S. M., J. Y., T. F., T. M., R. W., C. Cawood, N. M., L. L., W. J., M. B., and C. Cohen.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. This work was supported by the South African Medical Research Council (reference no. SHIPNCD 76756), The Wellcome Trust and the United Kingdom Foreign, Commonwealth and Development Office (grant 221003/Z/20/Z); the US Centers for Disease Control and Prevention (grant 5U01IP001048-05-00). It was also supported through the supply of Wantai enzyme-linked immunosorbent assay kits by WHO Unity Studies, a global seroepidemiologic standardization initiative, with funding from the COVID-19 Solidarity Response Fund and the German Federal Ministry of Health COVID-19 Research and Development Fund.
Potential conflicts of interest. N. W., A. v. G., and J. M. have received grant support from Sanofi Pasteur. N. W. also reports grants or contracts from the Bill & Melinda Gates Foundation. S. M. reports consulting fees from the NICD (which subcontracted Genesis Analytics to manage and support this study). N. M. reports a research grant paid to his institution by Pfizer and unpaid participation on a data and safety monitoring board or advisory board for the New Strat TB Trial by the University of Cape Town. L. L. reports a leadership or fiduciary role with the TB Think Tank, South African Department of Health. C. Cohen has received grant support from Sanofi Pasteur, Advanced Vaccine Initiative, and the South African Medical Research Council and payment of travel costs from Parexel. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Nicole Wolter, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Stefano Tempia, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Anne von Gottberg, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Jinal N Bhiman, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Sibongile Walaza, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Jackie Kleynhans, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Jocelyn Moyes, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Amelia Buys, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa.
Meredith L McMorrow, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Sue Aitken, Genesis Analytics, Johannesburg, South Africa.
Sarah Magni, School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Genesis Analytics, Johannesburg, South Africa.
Jessica Yun, Genesis Analytics, Johannesburg, South Africa.
Tamika Fellows, Genesis Analytics, Johannesburg, South Africa.
Tetelo Maakamedi, Genesis Analytics, Johannesburg, South Africa.
Renay Weiner, School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Cherie Cawood, Epicentre Health Research, Durban, South Africa.
Neil Martinson, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, South Africa; DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, University of the Witwatersrand, Johannesburg, South Africa; Johns Hopkins University Center for TB Research, Baltimore, Maryland, USA.
Limakatso Lebina, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, South Africa.
Waasila Jassat, Division of Public Health Surveillance and Response, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africaand.
Marieke Brauer, Immunology Department, National Reference Laboratory, Ampath Pathology, Pretoria, South Africa.
Cheryl Cohen, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
References
- 1. Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021; 592:438–43. [DOI] [PubMed] [Google Scholar]
- 2. Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021; 27:622–5. [DOI] [PubMed] [Google Scholar]
- 3. Maeda JM, Nkengasong JN.. The puzzle of the COVID-19 pandemic in Africa. Science 2021; 371:27–8. [DOI] [PubMed] [Google Scholar]
- 4. Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV 2021; 8:e554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tempia S, Walaza S, Moyes J, et al. Risk factors for influenza-associated severe acute respiratory illness hospitalization in South Africa, 2012-2015. Open Forum Infect Dis 2017; 4:2012–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020; 11:3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Germanio C, Simmons G, Kelly K, et al. SARS-CoV-2 antibody persistence in COVID-19 convalescent plasma donors: dependency on assay format and applicability to serosurveillance. Transfusion 2021; 61:2677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 20 September 2021.
- 9. World Health Organization. Growth reference data for 5-19 years. Available at: https://www.who.int/toolkits/growth-reference-data-for-5to19-years/indicators/bmi-for-age. Accessed 20 September 2021.
- 10. World Health Organization. Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring: March 2021. Available at: https://www.who.int/publications/i/item/9789240022232. Accessed 24 August 2021. [PubMed]
- 11. National Institute for Communicable Diseases. COVID-19 weekly epidemiology brief. Week 17 2021. Available at: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/weekly-epidemiological-brief/. Accessed 28 March 2022. .
- 12. National Institute for Communicable Diseases. COVID-19 hospital surveillance update, week 18. 2021. Available at: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/weekly-hospital-surveillance-datcov-update/. Accessed 28 March 2022.
- 13. South African Medical Research Council. Report on weekly deaths in South Africa. 2021. Available at: https://www.samrc.ac.za/reports/report-weekly-deaths-south-africa. Accessed 4 June 2021.
- 14. Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq [Preprint: not peer reviewed]. 12 February 2021. Available from: https://www.researchsquare.com/article/rs-233375/v1. [Google Scholar]
- 15. South African National Blood Service. Updated estimates of the prevalence of SARS-CoV-2 antibodies among blood donors in South Africa. 2021. Available at: https://sanbs.org.za/wp-content/uploads/2016/09/UPDATED-ESTIMATES-OF-THE-PREVALENCE-OF-SARS-COV-2-ANTIBODIES-AMONG-BLOOD-DONORS-IN-SOUTH-AFRICA.pdf. Accessed 4 August 2021.
- 16. Kleynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 seroprevalence in a rural and urban household cohort during first and second waves of infections, South Africa, July 2020-March 2021. Emerg Infect Dis 2021; 27:3020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spinelli MA, Lynch KL, Yun C, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV 2021; 8:e334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nault L, Marchitto L, Goyette G, et al. Covid-19 vaccine immunogenicity in people living with HIV-1. bioRxiv [Preprint: not peer reviewed]. 13 August 2021. Available from: https://www.biorxiv.org/content/10.1101/2021.08.13.456258v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cele S, Karim F, Lustig G, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022; 30:154–162.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M.. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shaw JA, Meiring M, Cummins T, et al. Higher SARS-CoV-2 seroprevalence in workers with lower socioeconomic status in Cape Town, South Africa. PLoS One 2021; 16:e02478521–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. George JA, Khoza S, Mayne E, et al. Sentinel seroprevalence of SARS-CoV-2 in Gauteng Province, South Africa, August - October 2020. South African Med J 2021; 111:1078–83. [DOI] [PubMed] [Google Scholar]
- 23. Karmakar M, Lantz PM, Tipirneni R.. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open 2021; 4:e2036462–e2036462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen C, Kleynhans J, von Gottberg A, et al. SARS-CoV-2 incidence, transmission and reinfection in a rural and an urban setting: results of the PHIRST-C cohort study, South Africa, 2020-2021. medRxiv 20 [Preprint: not peer reviewed]. 4 December 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.07.20.21260855v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ngere I, Dawa J, Hunsperger E, et al. High seroprevalence of SARS-CoV-2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. Int J Infect Dis 2021; 112:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagara I, Woodford J, Kone M, et al. Rapidly increasing severe acute respiratory syndrome coronavirus 2 seroprevalence and limited clinical disease in 3 Malian communities: a prospective cohort study. Clin Infect Dis. 2022; 74:1030–8. doi: 10.1093/cid/ciab589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Angulo FJ, Finelli L, Swerdlow DL.. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. 2021; 4:e2033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brazeau N, Verity R, Jenks S, et al. Report 34—COVID-19 infection fatality ratio estimates from seroprevalence. Faculty of Medicine, Imperial College London. Available at: https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-34-ifr/. Accessed 18 August 2021. [Google Scholar]
- 29. Purkayastha S, Kundu R, Bhaduri R, et al. Estimating the wave 1 and wave 2 infection fatality rates from SARS-CoV-2 in India. BMC Res Notes 2021; 14:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peluso MJ, Takahashi S, Hakim J, et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci Adv 2021; 7:eabh3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 2021; 95:e01828–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.