Abstract
Background
There is a lack of data regarding how the Delta variant of coronavirus disease 2019 (COVID-19) has impacted the effectiveness of the BNT162b2 (Pfizer–BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Johnson & Johnson–Janssen) vaccines at preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and COVID-19 hospitalization.
Methods
We compared the effectiveness of the three vaccines during the pre- and post-Delta variant period (before and after 1 July 2021) in a large cohort of vaccinated and unvaccinated patients in the Michigan Medicine healthcare system. We assessed vaccine effectiveness (VE) using 2 analyses: an inverse propensity weighted (IPW) Kaplan-Meier (KM) analysis based on time from vaccination, and a Cox model based on calendar time with vaccination as a time-varying covariate.
Results
Compared to Ad26.COV2.S recipients, the risk of hospitalization for COVID-19 in the post-Delta variant period was lower for BNT162b2 recipients (hazard ratio [HR] = 0.37; 95% confidence interval [CI]: [.14–.98]; P = .05) and mRNA-1273 recipients (HR = 0.21; 95% CI: [.07–.64]; P = .006). Recipients of the mRNA-1273 vaccine had a lower risk of SARS-CoV-2 infection than Ad26.COV2.S recipients (HR = 0.6; 95% CI: [.43–.83]; P = .003) and BNT162b2 recipients (HR = 0.64; 95% CI: [.54–.76]; P < .001). After 1 July, efficacy against SARS-CoV-2 infection declined for Ad26.COV2.S recipients (VE = 76% before; VE = 49% after; P = .02), BNT162b2 recipients (VE = 87% before; VE = 52% after; P < .001), and mRNA-1273 recipients (VE = 92% before; VE = 70% after; P < .001). Waning immunity and the Delta variant contributed independently and significantly to this decline.
Conclusions
Although there is a substantial decline in effectiveness, the approved COVID-19 vaccines remain effective against infection and hospitalization due to the Delta variant. The mRNA-based vaccines are more effective than the Ad26.COV2.S vaccine.
Keywords: COVID-19, COVID-19 vaccines, waning immunity, comparative effectiveness, Delta variant
The COVID-19 vaccines have reduced effectiveness against the Delta variant for SARS-CoV-2 infection and COVID-19 hospitalization. The effectiveness of mRNA-based vaccines against Delta hospitalization is substantially greater than the effectiveness of the Ad26.COV2.S (Johnson & Johnson–Janssen) vaccine.
The BNT162b2 (Pfizer–BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Johnson & Johnson–Janssen) vaccines are currently the only coronavirus disease 2019 (COVID-19) vaccines with full approval or emergency use authorization (EUA) from the US Food and Drug Administration (FDA). The Center for Disease Control and Prevention (CDC) defines an individual to be fully vaccinated 2 weeks after their second dose of the BNT162b2 or mRNA-1273 vaccine or 2 weeks after a single dose of the Ad26.COV2.S vaccine. As of 18 November 2021, all adult recipients of the 3 approved vaccines are eligible for an additional dose [1]. Due to differing clinical endpoints, geographic regions, and time frames, the effectiveness of the 3 vaccines cannot be directly compared based on clinical trials [2]. Previously published CDC research and suggested that the Ad26.COV2.S vaccine was less effective than the BNT162b2 and mRNA-1273 vaccines, but this research was limited to data collected from older adults (≥50 years of age) in inpatient settings and did not consider the effect of the Delta variant [3]. A large meta-analysis suggested the same result but did not have baseline patient data to control for confounding factors [4].
A major limitation in existing studies that address vaccine effectiveness (VE) over time is that they are not robust to potential confounding from differential date of vaccination. As the vaccines were approved at different times, the background prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the variants in circulation throughout the follow-up period are different between vaccine groups. One way of addressing this problem is to complement the traditional time-from-vaccination analysis with an analysis based on calendar time. Our objective was to assess the relative and overall effectiveness of the approved COVID-19 vaccines at preventing SARS-CoV-2 infection and COVID-19 hospitalization in fully vaccinated individuals using electronic health records (EHR) at Michigan Medicine from 1 January to 20 October 2021, conducting analyses using both time from vaccination and calendar time.
METHODS
Data
We used EHR data at Michigan Medicine, among patients who were registered with a primary care physician at Michigan Medicine and had at least 1 visit in the past 18 months. We excluded patients who were under 18, had a prior history of COVID-19, or had received a different vaccine than the 3 approved vaccines. Comorbidities and number of visits prior to baseline were assessed based on diagnosis codes 12 months before first dose for vaccinated subjects and before 1 January 2021, for unvaccinated subjects. Patients were defined as immunosuppressed if they took traditional disease-modifying antirheumatic drugs (tDMARDs), biologic DMARDs (bDMARDs), or glucocorticoids (medication list is provided in Supplementary Table 1) within 6 months prior to the baseline date. We identified SARS-CoV-2 infection based on the 10th version International Classification of Diseases (ICD-10) code U07.1 and laboratory test results. Date of infection was identified using test collection date where possible, and diagnosis date where test date was not available. As U07.1 was often used multiple times, we identified only the first instance of U07.1 as indicating a SARS-CoV-2 infection and excluded patients with an instance of U07.1 or positive test prior to the study period. COVID-19 hospitalization was identified using chart review where data was available and using ICD-10 codes J12.82, M35.81, and J18 where chart review data was not available. Detailed criteria are provided in Supplementary Materials.
Inclusion Criteria
We included patients who were over 18, did not have a prior history of COVID-19, and received 1 of the 3 approved vaccines.
Time-From-Vaccination Analysis (Model I)
Only fully vaccinated patients were included in the time-from-vaccination analysis. We used data from 1 January 2021 to 20 October 2021. The baseline date was defined as the date of full vaccination using the CDC definition [1]. We examined the incidence of SARS-CoV-2 infection and COVID-19 hospitalization in separate analyses. Patients were censored at date of receiving an additional dose, date of death, or study end date, whichever occurred first. To balance patient covariates, including age, gender, race, immunosuppression, number of visits, and Charlson comorbidity index [5], the inverse propensity weighted (IPW) [6] Kaplan-Meier method was used to estimate the cumulative incidence curves, and the weighted log-rank test was used to compare between vaccines. Propensity scores were computed using multinomial logistic regression and balance of the covariates was checked using standardized mean differences [7].
Calendar Date Analysis (Models II–IV)
All patients who met the inclusion criteria were included in the calendar date analysis. We used data from 1 April 2021 to 20 October 2021. The later start date was necessary as the Ad26.COV2.S vaccine was not approved for use until late February. For all models based on calendar time, we used a Cox model controlling for patient covariates with a time-varying covariate for vaccination. Vaccinated patients were included in the unvaccinated group before they received their first dose, and we computed vaccine efficacy as 1−hazard ratio (HR). This estimate represents average vaccine efficacy for the vaccine group and portion of the study period being considered.
Model II
We assumed that vaccine efficacy was constant across the study period and fit the model using a single time-varying covariate for vaccine product (Ad26.COV2.S, BNT162b2, mRNA-1273, and Unvaccinated).
Model III
To assess the impact of the Delta variant we conducted another analysis including an interaction term between vaccine product and calendar date (before/after 1 July). This date corresponds to when the Delta variant became the primary circulating variant in the United States [8]. We also used this model to estimate the cumulative incidence of hospitalization by vaccine product and age group. To control for covariates in these estimations, we created a pseudo-population of identical population characteristics as the study subjects for each vaccine and age group and then averaged the predicted values of cumulative incidence.
Model IV
To assess both the impact of Delta variant and waning immunity, we further included vaccination time in Model III as a time-varying covariate indicating whether patients were under 180 days or more than 180 days from full vaccination. In this model, we included 2 interaction terms: between vaccine product and calendar time (before/after 1 July) and between vaccine product and time from full vaccination (≥180 days, <180 days). For each vaccine, this allows us to estimate vaccine efficacy for 4 different groups: ≥180 days from full vaccination pre-Delta, <180 days from vaccination pre-Delta, ≥180 days from vaccination post-Delta, and < 180 days from vaccination post-Delta.
All statistical analyses were performed using R v4.0.2 (R Core Team, Vienna).
RESULTS
Study Population
The total number of patients in the EHR data who had a primary care physician at Michigan Medicine and had at least 1 visit in the past 18 months was 195 581 including 140 731 vaccinated patients, and 54 850 unvaccinated patients. We excluded 36 526 patients who were under 18, partially vaccinated, had a prior history of COVID-19, or had received a different vaccine than the 3 approved vaccines. The final number of patients in the analysis was 159 055. The study population had a median age of 49, was majority female (58%), and predominantly White (77%). Vaccinated patients were older, with a median age of 53 compared to 39 for unvaccinated patients. Due to later approval and an FDA pause, most Ad26.COV2.S recipients became fully vaccinated in mid-April 2021, although BNT162b2 and mRNA-1273 recipients became vaccinated across a wider range of dates from March to May 2021. Patient covariates by vaccine are described in Table 1.
Table 1.
Characteristics of the Study Population by Vaccine Product Received
| Ad26.COV2.S (N = 5796) | BNT162b2 (N = 73 666) | mRNA-1273 (N = 34 474) | Unvaccinated (N = 45 119) | All (N = 159 055) | |
|---|---|---|---|---|---|
| Age, median (IQR) | 46 (32–60) | 50 (35–63) | 59 (43–70) | 39 (28–55) | 49 (33–63) |
| ≤30 | 1325 (23) | 13 634 (19) | 3921 (11) | 14 116 (31) | 32 996 (21) |
| 31–50 | 2062 (36) | 23 961 (33) | 8445 (24) | 16 796 (37) | 51 264 (32) |
| 51–64 | 1581 (27) | 19 513 (26) | 9057 (26) | 8762 (19) | 38 914 (24) |
| ≥65 | 828 (14) | 16 558 (22) | 13 051 (38) | 5444 (12) | 35 881 (23) |
| Length of follow-up, median days (IQR) | 183 (179–197) | 180 (161–226) | 181 (165–203) | N/A | N/A |
| Date of full vaccination, median date (IQR) | 20 April (6–24 April) | 22 April (4 March–12 May) | 22 April (31 March–7 May) | N/A | N/A |
| Gender, no. (%) | |||||
| Male | 2740 (47) | 30 375 (41) | 14 285 (41) | 19 134 (42) | 66 534 (42) |
| Female | 3056 (53) | 43 291 (59) | 20 189 (59) | 25 985 (58) | 92 521 (58) |
| Race, no. (%) | |||||
| White | 4731 (82) | 56 040 (76) | 28 446 (83) | 32 518 (72) | 121 735 (77) |
| Black | 341 (6) | 5843 (8) | 2043 (6) | 6210 (14) | 14 437 (9) |
| Other | 724 (12) | 11 783 (16) | 3985 (12) | 6391 (14) | 22 883 (14) |
| Immunosuppressed,a no. (%) | 197 (3) | 2323 (3) | 1430 (4) | 1319 (3) | 5269 (3) |
| Weighted comorbidity index,b no. (%) | |||||
| 0 | 4511 (78) | 59 094 (80) | 23 705 (69) | 37 063 (82) | 124 373 (78) |
| 1–2 | 893 (15) | 10 381 (14) | 7170 (21) | 5828 (13) | 24 272 (15) |
| 3–4 | 226 (4) | 2522 (3) | 2096 (6) | 1302 (3) | 6146 (4) |
| ≥5 | 166 (3) | 1669 (2) | 1503 (4) | 926 (2) | 4264 (3) |
| No. of visits,c median (IQR) | 2 (1–6) | 3 (1–7) | 3 (1–8) | 2 (0–5) | 3 (1–7) |
| SARS-CoV-2 infections, no. (%) | 55 (1.0) | 599 (0.8) | 189 (0.6) | 1210 (2.7) | 2053 (1.2) |
| COVID-19 hospitalizations, No. (%) | 7 (0.12) | 25 (0.03) | 8 (0.02) | 113 (0.25) | 153 (0.10) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Immunosuppressant status was defined as being on an immunosuppressant medication for at least six months prior to baseline date. See list of medications in Supplementary Materials.
Comorbidity index was calculated using diagnosis codes reported within 12 months before the first vaccine dose and 12 months before 1 January, 2021 for unvaccinated patients.
Number of visits was defined as the number of in-person visits for the patient within 12 months before the first vaccine dose and 12 months before 1 January 2021 for unvaccinated patients.
Comparative Effectiveness Based on Time From Vaccination
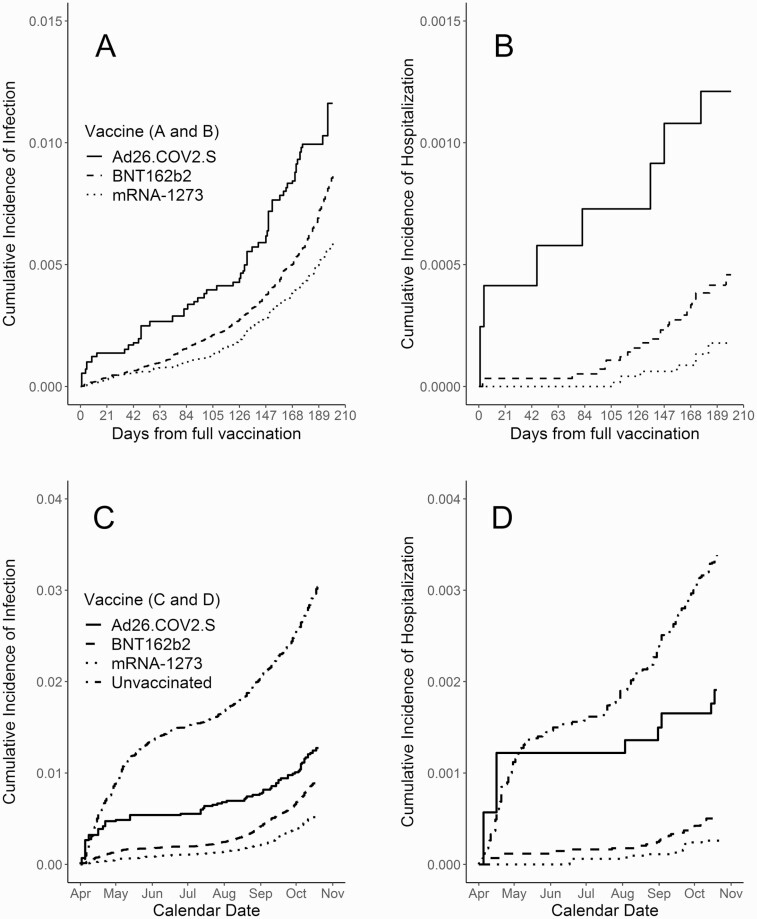
Based on an IPW time from vaccination analysis among fully vaccinated patients (Model I), Ad26.COV2.S vaccine recipients had the highest incidence of SARS-CoV-2 infection and COVID-19 hospitalization, BNT162b2 recipients had the second highest incidence, and mRNA-1273 recipients had the lowest incidence (see Figure 1A and 1B). The advantage of the mRNA-1273 vaccine over each of the other 2 vaccines was highly significant (P < .001) based on the adjusted log-rank test. There was less statistical certainty when comparing the rates of hospitalization due to low sample size. After weighting (IPW) was applied, patient covariates were well balanced between the vaccine groups based on standardized mean differences.
Figure 1.
Cumulative incidence of SARS-CoV-2 infection and COVID-19 hospitalization for each vaccine product by time since vaccination (A, B), and by calendar time (C, D), adjusted for age, immunosuppression, gender, race, number of visits, and Charlson comorbidity index, using inverse propensity score weighting. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Vaccine Effectiveness Based on Calendar Time
In the analysis based on calendar time including the entire study period (Model II), we found that all 3 vaccines were highly effective at preventing SARS-CoV-2 infection and COVID-19 hospitalization (see Figure 1C and 1D). There was significant protection against infection for Ad26.COV2.S recipients (average VE = 61%; 95% confidence interval [CI]: [48–70%]; P < .001), BNT162b2 recipients (VE = 67%; 95% CI: [63–70%]; P < .001), and mRNA-1273 recipients (VE = 79%; 95% CI: [75–82%]; P < .001). Similarly, there was significant protection against hospitalization for Ad26.COV2.S recipients (VE = 55%; 95% CI: [4–79%]; P = .04), BNT162b2 recipients (VE = 87%; 95% CI: [81–92%]; P < .001), and mRNA-1273 recipients (VE = 93%; 95% CI: [86–97%]; P < .001).
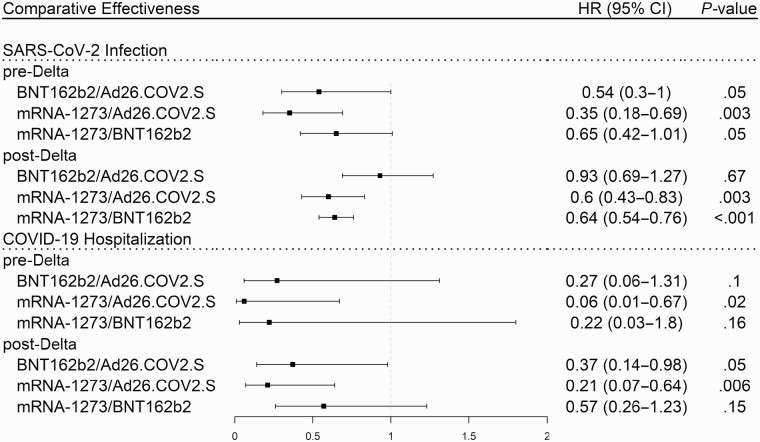
Compared to Ad26.COV2.S recipients, we found that BNT162b2 recipients (HR = 0.28; 95% CI: [.12–.65]; P = .003) and mRNA-1273 recipients (HR = 0.15; 95% CI: [.06–.42]; P < .001) were at lower risk of hospitalization. For infection, mRNA-1273 recipients were at lower risk than BNT162b2 recipients (HR = 0.64; 95% CI: [.54–.76]; P = .003) and Ad26.COV2.S recipients (HR = 0.54; 95% CI: [.40–.73]; P < .001).
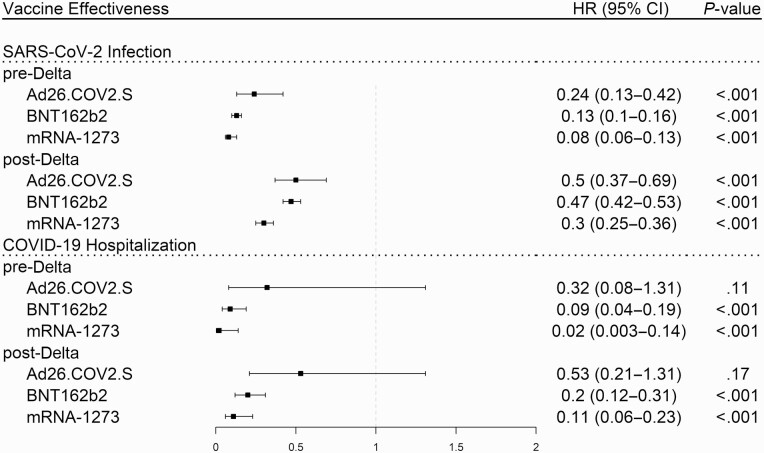
Vaccine Effectiveness Before and After the Delta Variant
Based on the model assuming a change in vaccine effectiveness at 1 July (Model III), all 3 vaccines remained effective after the Delta variant became dominant across the United States [8]. After 1 July , recipients of all vaccine products remained protected against SARS-CoV-2 infection and COVID-19 hospitalization (see Figure 2).
Figure 2.
Protection against SARS-CoV-2 infection and COVID-19 hospitalization for each vaccine product during the pre- and post-Delta variant periods adjusted by Cox regression for age, immunosuppression, gender, race, number of visits, and Charlson comorbidity index. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
However, average efficacy against infection declined for Ad26.COV2.S recipients (VE = 76% before; VE = 49% after; P = .02), BNT162b2 recipients (VE = 87% before; VE = 52% after; P < .001), and mRNA-1273 recipients (VE = 92% before; VE = 70% after; P < .001). Average efficacy against COVID-19 hospitalization declined for BNT162b2 recipients (VE = 95% before; VE = 82% after; P = .03). Due to lower sample size, there was less statistical certainty when comparing the pre- and post-Delta risk of hospitalization for the other 2 vaccines.
The differences in efficacy against infection and hospitalization between the 3 vaccines remained similar before and after Delta variant dominance (see Figure 3). From 1 July to 20 October, the mRNA-1273 vaccine was the most effective at preventing SARS-CoV-2 infection, and the Ad26.COV2.S vaccine was the least effective at preventing COVID-19 hospitalization.
Figure 3.
Comparison between vaccine products for protection against SARS-CoV-2 infection and COVID-19 hospitalization during the pre- and post-Delta variant periods adjusted by Cox regression for age, immunosuppression, gender, race, number of visits, and Charlson comorbidity index. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
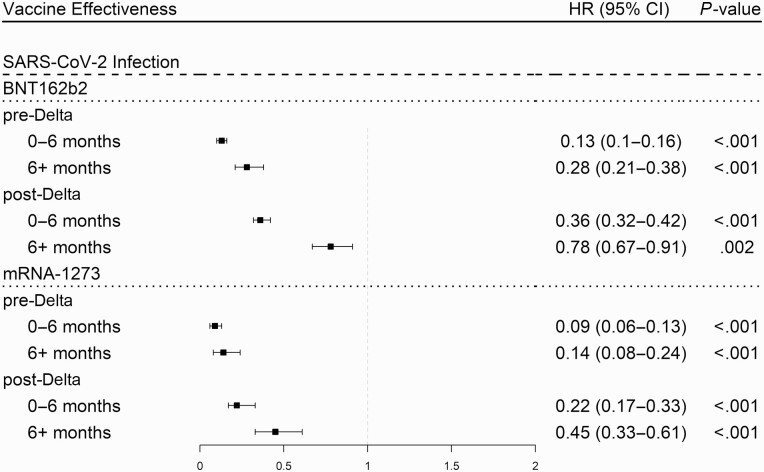
Waning of Vaccine Effectiveness Over Time
In the model accounting for both vaccination time and Delta variant (Model IV), the changes in vaccine protection against SARS-CoV-2 infection due to the Delta variant and due to waning immunity were each highly significant (P < .001) for both the BNT162b2 and mRNA-1273 vaccines. For these vaccines, we saw a substantial drop in effectiveness due to both factors (see Figure 5) and this drop was comparatively larger for the BNT162b2 vaccine compared to the mRNA-1273 vaccine. We did not have sufficient data in all parts of the study period to give robust estimates for the Ad26.COV2.S vaccine or for any vaccines regarding COVID-19 hospitalizations. Prior to the advent of the Delta variant and during the first 6 months after full vaccination, we estimated BNT162b2 vaccine efficacy against SARS-CoV-2 infection at 87%. We observed a decline in efficacy for patients more than 6 months after full vaccination (87% to 72%), a decline in efficacy after the Delta variant (87% to 64%), and a larger decline due to both factors combined (87% to 22%). Similarly, we estimated the initial mRNA-1273 vaccine efficacy against SARS-CoV-2 infection at 91%. We observed a decline in efficacy for patients more than 6 months after full vaccination (91% to 86%), a decline in efficacy after the Delta variant (91% to 78%), and a larger decline due to both factors combined (91% to 55%).
Figure 5.
Protection against SARS-CoV-2 infection for the mRNA-1273 and BNT162b2 vaccines during the pre- and post-Delta variant periods by months from vaccination adjusted by Cox regression for age, immunosuppression, gender, race, number of visits, and Charlson comorbidity index. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Within our data, 33% of SARS-CoV-2 infections and 18% of COVID-19 hospitalizations identified were not associated with a positive polymerase chain reaction (PCR) test result. This is likely a result of shortages in testing materials and testing conducted outside Michigan Medicine system. To assess the possibility of bias due to miscoding, we repeated all the analysis above using only cases with positive PCR results or chart review confirmation of infection, and all conclusions remained the same although there was greater uncertainty due to reduced sample sizes (data not shown).
Risk of Hospitalization Based on Vaccine Type and Age Group
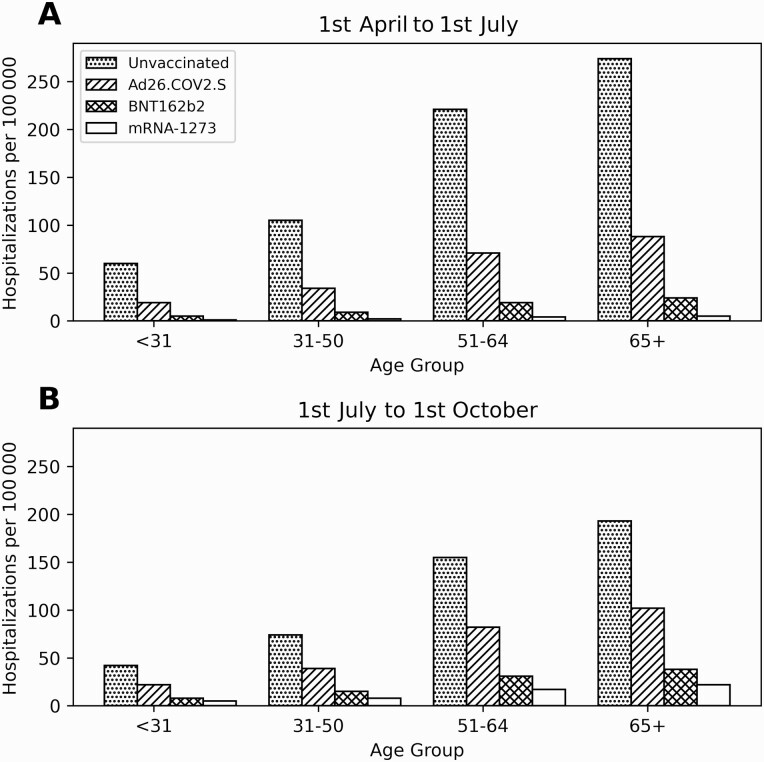
In the pre-Delta variant period (based on Model III) we estimated the covariate-adjusted 3-month cumulative incidence of hospitalizations per 100 000 as 180 for the unvaccinated patients, 60 for the Ad26.COV2.S recipients, 9 for the BNT162b2 recipients, and 4 for the mRNA-1273 recipients. In the post-Delta variant period these estimates were 123 for the unvaccinated patients, 68 for the Ad26.COV2.S recipients, 22 for the BNT162b2 recipients, and 13 for the mRNA-1273 recipients. We also estimated cumulative incidence for each age group (see Figure 4).
Figure 4.
COVID-19 hospitalization rates by vaccine type and age group for 1 April to 1 July (A) and 1 July to 1 October (B) adjusted by Cox regression for immunosuppression, gender, race, number of visits, and Charlson comorbidity index. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
We found that all 3 approved vaccines were effective at preventing SARS-CoV-2 infection and hospitalization due to COVID-19. Based on the time-from-vaccination analysis, the mRNA-1273 vaccine was the most effective of the 3 vaccines at preventing SARS-CoV-2 infection, the BNT162b2 vaccine was the second-most effective, and the Ad26.COV2.S vaccine was the least effective. The calendar date analysis indicated that the mRNA-1273 vaccine was the most effective vaccine at preventing SARS-CoV-2 infection and that the Ad26.COV2.S vaccine was the least effective vaccine at preventing COVID-19 hospitalization. Within the post-Delta variant period, we found that all 3 vaccines had reduced efficacy against both SARS-CoV-2 infection and hospitalization due to COVID-19. For the BNT162b2 and mRNA-1273 vaccines, we found that waning immunity and the Delta variant were independent and important contributors to reduced efficacy against SARS-CoV-2 infection.
It is well known that older patients are at much higher risk of severe COVID-19 [9], but the gap in efficacy by vaccine product was large enough that even the youngest Ad26.COV2.S recipients were at comparable risk to older recipients of the other two vaccines. We estimated a similar cumulative incidence of hospitalization in the post-Delta variant period for mRNA-1273 recipients aged 65 years or older (19 per 100 000) and Ad26.COV2.S recipients aged 30 years or younger (22 per 100 000). We estimate large relative increases in hospitalization risk for BNT162b2 and mRNA-1273 recipients in the post-Delta variant period but these patients remained well-protected against COVID-19 hospitalization. However, there was a concerning rise in hospitalizations in older age groups in the post-Delta variant period, and we would expect vaccine protection to erode further given our findings regarding waning immunity. Ad26.COV2.S recipients had a larger absolute risk of COVID-19 hospitalization, especially in the post-Delta variant period, although they remained at a lower risk than unvaccinated patients.
Using calendar time and time from vaccination to compare vaccine efficacy has important advantages over previous studies. The traditional time from vaccination analysis is important because it allows us to study waning immunity over time. However, using the calendar time scale accounts for the severity of the pandemic over time. This means that we can be confident that results where the 2 analyses agree are robust to potential biases. Both analyses indicate that the mRNA-1273 vaccine is more effective than the other 2 vaccines at preventing SARS-CoV-2 infection. We also find that the Ad26.COV2.S vaccine is less effective than the other 2 vaccines at preventing COVID-19 hospitalization.
In the calendar date analysis, we found that vaccine efficacy against SARS-CoV-2 infection and hospitalization for COVID-19 was lower after 1 July 2021, for all 3 vaccines. The 2 most likely explanations for this are waning immunity and increased Delta variant prevalence. By including time from vaccination and before/after 1 July as covariates in the calendar time analysis, we were able to separate the effect of the Delta variant from the effect of waning immunity for the mRNA-1273 and BNT162b2 vaccines. For these 2 vaccines, we found that both waning immunity and increased Delta variant presence played a major role in declining efficacy. Immunity waned faster for BNT162b2 vaccine recipients compared to mRNA-1273 recipients. Previous studies have found that the Ad26.COV2.S [10], BNT162b2 [11], and Ad26.COV2.S [12] vaccines have reduced serum neutralizing activity against the Delta variant compared to previous circulating variants, and our results confirm that the Delta variant had a negative impact on vaccine protection. Despite the decline in efficacy, BNT162b2 and mRNA-1273 recipients remain at a low risk of COVID-19 hospitalization compared to Ad26.COV2.S recipients and unvaccinated individuals.
The FDA recently approved boosters for adult recipients of all 3 vaccines [2]. Our findings indicate a strong justification for boosters, both in terms of waning immunity and lower efficacy against the Delta variant. We also believe that our findings underscore the importance of acting quickly when a new and highly transmissible variant is identified, as there can be an immediate and substantial reduction in vaccine efficacy. The rise of the Omicron variant makes it critical for individuals to get boosters to ensure robust protection, and boosters are particularly important for recipients of the Ad26.COV2.S vaccine.
Limitations of this study include only having data from a single health system and underestimation of SARS-CoV-2 infection. Michigan Medicine has 15 primary care sites across a 4-county area, and the catchment area for these clinics crosses several more counties. Patients may have sought care at other facilities located within these counties, and incomplete data from these facilities limits our ability to capture all infections. The study sample size was too small to perform the most sophisticated analysis (Model IV) for protection against COVID-19 hospitalizations for any vaccine or protection against infection for the Ad26.COV2.S vaccine.
The study population was predominantly White (77%) reflecting the demographics of Michigan, so we did not have sufficient data to consider differences in efficacy or absolute risk by racial group. Due to irregular updates from state and national databases, we did not have reliable mortality data to explore differences in mortality between vaccinated and unvaccinated patients.
Despite these limitations, our study still provides valuable information regarding the comparative effectiveness of COVID-19 vaccines, the relative impacts of waning immunity and the Delta variant on effectiveness, and the risk differences between unvaccinated and vaccinated individuals. Although there is a substantial decline in efficacy, the approved COVID-19 vaccines remain effective against SARS-CoV-2 infection and hospitalization due to the Delta variant. The mRNA-based vaccines are comparatively more effective than the Ad26.COV2.S vaccine. The Delta variant and waning immunity over time independently and substantially reduce vaccine protection against infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . The authors thank the University of Michigan Data Office for assistance with data extraction from electronic medical records.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI158543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We used de-identified EHR data, the use of which was approved by the Institutional Review Board (IRB).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Malcolm Risk, Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
Chen Shen, Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
Salim S Hayek, Division of Cardiology, Department of Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Lynn Holevinski, Data Office for Clinical and Translation Research, Office of Research, Ann Arbor, Michigan, USA.
Elena Schiopu, Rheumatology, Internal Medicine, Ann Arbor, Michigan, USA.
Gary Freed, Department of Pediatrics and Department of Health Management and Policy, University of Michigan, Ann Arbor, Michigan, USAand.
Cem Akin, Division of Allergy, University of Michigan, Ann Arbor, Michigan, USA.
Lili Zhao, Department of Biostatistics, University of Michigan, Ann Arbor, Michigan, USA.
References
- 1. FDA. FDA expands eligibility for COVID-19 vaccine boosters. 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters. Accessed 19 November 2021.
- 2. CDC. Science brief: COVID-19 vaccines and vaccination. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html. Accessed 25 August 2021.
- 3. Thompson M, et al. . Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med 2021; 385:1355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naranbhai V, et al. . Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J Infect Dis 2021; 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 6. Xie J, Liu C.. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005; 24:3089–110. [DOI] [PubMed] [Google Scholar]
- 7. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC. Monitoring variant proportions. 2021. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed 13 September 2021.
- 9. CDC. COVID-19 hospitalization and death by age. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed 19 November 2021.
- 10. Barouch D, et al. . Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021; 385:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wall EC, Wu M, Harvey R, et al. . Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021; 397:2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi A, et al. . Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol 2021; 95:e01313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.