Abstract
Dietary fiber has become increasingly recognized as a key factor in maintaining gastrointestinal health. Dietary fiber sources are often comprised of several different fiber fractions, each with unique physicochemical properties. These properties can have varying physiological effects on the gastrointestinal tract that include modulation of microbiota, production of fermentation-derived metabolites, and laxation. The objectives of this study were 1) to determine the effects of a novel dietary fiber source, miscanthus grass fiber (MF), and prebiotic and fiber blends on gastrointestinal tolerance, apparent total tract digestibility, fecal metabolites, and fecal microbiota and 2) to evaluate the palatability of extruded diets containing MF in comparison to traditional dietary fiber sources. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee. Six dietary treatments were formulated to meet or exceed the AAFCO nutrient profile of 2018 and included either cellulose (CO), beet pulp (BP), MF, or a blend of MF and tomato pomace, MF and resistant starch, or MF and fructooligosaccharide. A total of 12 adult neutered female beagles (mean age 5.8 ± 1.1 yr; mean body weight 10.9 ± 1.0 kg; mean body condition score 5.7 ± 0.7) were randomly assigned to one of the six treatment diets in a replicated 6 × 6 Latin square design. Each dog was fed their assigned diet for a treatment period of 21 d with 17 d of diet adaptation followed by 4 d of total and fresh fecal collection. All diets were well accepted and digested by the dogs. Dogs fed BP had greater fecal total short-chain fatty acid concentration than the CO treatment (P < 0.05), while the dogs fed diets containing MF were intermediate. In a two-bowl palatability trial, no significant preference was observed between the extruded diets containing MF and CO (P > 0.05). However, a significant preference for the extruded diet containing BP over the diet containing only MF was observed (P < 0.05). The α-diversity of fecal microbial communities was not impacted by treatment (P > 0.05), but β-diversity indicated that dogs fed the BP diet differed from the other treatment groups (P < 0.05). The data from this study suggest that miscanthus grass can be successfully utilized in fiber blends in extruded diets for adult dogs, with modulatory effects similar to the traditional dietary fiber source, cellulose.
Keywords: dietary fiber, dogs, fecal metabolites, fecal microbiota, nutrient digestibility, palatability
Lay Summary
There are many ingredients utilized in dog foods that provide a source of dietary fiber. However, new ingredient sources can help to add variety to diet formulas and may provide benefits to pet food processing and animal health. Miscanthus grass is a novel ingredient for dog food that provides an excellent source of dietary fiber. In a dog feeding trial and palatability test, diets containing miscanthus grass had similar results to diets containing cellulose, a traditionally used dietary fiber ingredient. The inclusion of this novel ingredient did not produce any observed negative effects on digestion, stool quality, diet palatability, or overall animal health. This indicates that miscanthus grass is a viable ingredient for use in commercial dog foods as a source of dietary fiber.
Miscanthus grass is a novel ingredient in extruded canine diets that can serve as a functional source of dietary fiber. Results found it comparable to the traditional fiber source, cellulose.
Introduction
The inclusion of dietary fiber in complete and balanced canine diets provides gastrointestinal health advantages and adds flexibility to the formulation. In addition to supporting saccharolytic fermentation and the production of carbohydrate-derived short-chain fatty acids (cSCFA), dietary fiber blends can help to modulate the gut environment to maintain a healthy and balanced microbiome.
With over half (55.8%) of the canine population being considered overweight or obese according to a survey taken by the Association of Pet Obesity (2018), the demand for diets that aid in weight loss or weight control has increased. Ingredients that provide high levels of dietary fiber are common tools that formulators use to help develop diets that have lower caloric density while still promoting satiety. Wood cellulose (CO) and beet pulp (BP) are some examples of the most commonly utilized dietary fiber sources in pet foods. However, the inclusion of other novel dietary fiber ingredients that offer complementary chemical composition, physicochemical, and physiological properties can assist in the development of a variety of functional pet food platforms to support animal health and wellbeing.
Miscanthus (Miscanthus giganteus), a perennial grass that grows in temperate climates, possesses many desirable qualities as a novel dietary fiber source in regards to its composition as well as a variety of positive marketing attributes. Being primarily composed of insoluble fiber (IDF), miscanthus grass fiber (MF) is comparable in composition to the common fiber source, CO (Bauer and Ibanez, 2014). The composition of MF also includes naturally occurring xylooligosaccharides, which may provide a prebiotic-like effect in helping to promote gastrointestinal health (Chen et al., 2014; Tungland, 2018). Additionally, MF is a fiber source that can be marketed as “natural” and “non-GMO” which can be appealing to certain pet food niches and consumers. Limited research is available regarding MF as a dietary fiber ingredient in canine diets. The objective of this study was to compare traditional dietary fiber sources, CO and BP, with the novel fiber source, MF, and miscanthus fiber blends, and evaluate their effects on gastrointestinal intolerance, diet palatability, apparent total tract digestibility (ATTD), fecal fermentative end-products, and microbiota in healthy adult dogs. It was hypothesized that the diet containing MF would have similar results to the diet containing CO, with the addition of resistant starch (RS), fructooligosaccharide (FOS), or tomato pomace (TP) to the miscanthus fiber blend resulting in augmented saccharolytic fermentation.
Materials and Methods
Animals
All animal care procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to animal experimentation. All methods were performed in accordance with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. A total of 12 female, neutered, adult beagles (mean age 5.8 ± 1.08 yr; mean body weight 10.9 ± 1.02 kg; mean body condition score 5.7 ± 0.59) were used in a replicated 6 × 6 Latin square design. Dogs were randomly assigned to one of six experimental diets in each period so that all dogs received all dietary treatments once during the study. Dogs were housed individually in pens that allowed for nose–nose contact between dogs in adjacent pens and visual contact with all dogs in the room. They were housed in an environmentally controlled room with a 14 h light and 10 h dark cycle. They were fed twice a day at 0800 and 1500 hours. After each feeding, the individual food refusals were measured, if present. Dogs had free access to water at all times and were fed to maintain body weight, and body condition scores were evaluated on a weekly basis.
Diets
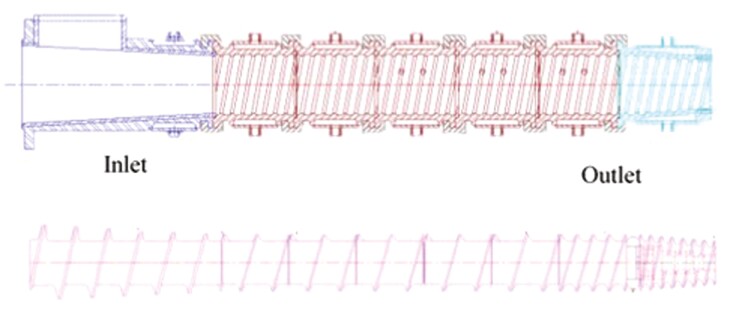
Six diets containing either 7% CO, 9% MF, 7% MF plus 2% TP (MF + TP), 8% MF plus 1% FOS (MF + FOS), 8% MF plus 1% RS (MF + RS), or 11% BP were formulated to meet or exceed the AAFCO (2018) nutritional requirements for adult dogs. They were also formulated to be isocaloric, isonitrogenous, and contain equal total dietary fiber (TDF) content. BP, powdered cellulose, and dried TP were supplied by Fairview Mills (Seneca, KS). Ground miscanthus grass was supplied by M-Fiber (Aurora, MO). The RS source was HI-MAIZE 260 (Ingredion, Westchester, IL), and the FOS source, bioSecure FOS, contained 95% pure FOS polymers with molecular weights less than 5,000 (BioMatrix International, Princeton, MN). The diets were processed using a model E525 extrusion system (Extru-Tech, Inc., Sabetha, KS). This system included an Aseptic Dual Preconditioner, model 145, with two counter-rotating shafts with paddles 16″ in diameter and 72″ long. The E525 extruder consisted of seven-jacketed head segments (Figure 1) with equipment parameters and die configuration set to target a final product that was round in shape and 9 mm in diameter. After extrusion, an Airflow I single-pass dryer was utilized to achieve a final product moisture content of less than 8.0%. Finally, an aseptic dual coater was used to apply a topical palatant.
Figure 1.
Extruder heads and rotating element configuration of Extruder Model E525 (Source: Extru-Tech, Inc.).
Sample collection
At the beginning of each period, dogs were acclimated to their assigned diet for 17 d. On days 18 to 21 of each experimental period, all feces eliminated were collected for each dog. Fecal samples were scored, weighed, and composited by animal and experimental period. Fecal samples were scored using a 5-point scale (1 = hard, dry pellets, small hard mass; 2 = hard formed, remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool, assumes the shape of the container; 5 = watery, liquid that can be poured), and then stored at −20 °C until later chemical analysis.
During the 4-d collection phase, a fresh fecal sample was collected from each dog within 15 min of defecation for dry matter (DM) and fecal metabolite analysis. The pH, as-is weight, and fecal score were measured for each sample. The fresh samples were aliquoted for DM, phenols and indoles, cSCFA, protein-derived SCFA (pSCFA), and ammonia concentration analysis and stored at −20 °C until later analysis. A separate fecal aliquot was placed into sterile cryovials and stored at −80 °C for analysis of fecal microbiota.
A fasted blood sample was collected from each dog at the end of each experimental period to evaluate blood parameters and health status. About 5 mL of blood was collected via jugular or cephalic venipuncture. About 1 mL of blood was aliquoted for complete blood cell count analysis (BD Vacutainer, K2 EDTA 3.6 mg, Franklin Lakes, NJ) and 4 mL were aliquoted for serum chemistry analysis (BD Vacutainer, SST). Serum samples were obtained after blood centrifugation (1,300 × g at 4 °C) and recovery of supernatants. Serum chemistry and complete blood count analyses were completed by the Clinical Pathology staff at the University of Illinois College of Veterinary Medicine (Urbana, IL). All dogs remained healthy for the duration of the study.
Chemical analysis
Experimental diets were subsampled and ground to 2 mm particle size using a Wiley Mill (model 4; Thomas Scientific, Swedesboro, NJ). All fecal samples from the collection phase were composited for each dog and dried at 55 °C in a forced-air oven before being ground through a 2 mm screen using the same Wiley Mill that was used to grind the experimental diets. Ground samples of the experimental diets and composited dried feces were evaluated for DM and ash according to AOAC (2006; methods 934.01 and 942.05) with organic matter (OM) calculated by difference. The methods of the American Association of Cereal Chemists (1983) and Budde (1952) were used to evaluate the acid-hydrolyzed fat (AHF) content in both the diet and fecal samples. A measure of total nitrogen was completed following AOAC (2006; method 992.15) via LECO (TruMac N, Leco Corporation, St. Joseph, MI) and used to calculate crude protein (CP) content according to the Official Method of AOAC International (2006). A Parr 6200 calorimeter (Parr Instruments Co., Moline, IL) was used to evaluate the gross energy of the diets and feces. Analysis of fecal and diet TDF content, as well as soluble dietary fiber (SDF) and IDF content of the diets, were accomplished according to Prosky et al. (1992) and the Official Method of AOAC International (2006; Methods 985.29 and 991.43).
Fecal cSCFA and pSCFA concentrations were determined using gas chromatography according to a modified method of Sunvold et al. (1995a). A Hewlett-Packard (Hewlett-Packard, Avondale, PA) Model 5890A gas chromatograph equipped with a flame ionization detector on a column (1.8 m × 4 mm i.d.) packed with GP 10% SP-1200/1% H3PO4 on 80/100 chromosorb W AW (Supelco, Bellefonte, PA) was used to evaluate the diluted fecal samples for SCFA concentration using nitrogen at a flow rate of 45 mL/min as the carrier gas. The temperatures were 125, 175, and 180 °C for the oven, injection port, and detector port, respectively. Gas chromatography was also used to determine the fecal phenol and indole values following the modified method of Flickinger et al. (2003). A Thermo Scientific TRACE 1300 Gas Chromatograph coupled with a flame ionization detector was used for this analysis, and a 1 µL sample was injected at 220 °C at splitless mode. The phenolic compounds were separated using a Nukol Supelcol column (60 m length, 0.32 mm diameter) with a film thickness of 0.25 µm. For 1 min, the oven temperature was held at 15 °C, and then increased at 25 °C/min to 200 °C. The temperature was then held constant for 35 min. 5-methylindole was used as the internal standard, and samples were analyzed in duplicate. Ammonia concentration was evaluated using the method of Chaney and Marbach (1962).
Calculations
The ATTD of individual macronutrients was calculated using the following equation:
DNA extraction, amplification, sequencing, and bioinformatics
A Mo-Bio PowerSoil kit (MO BIO Laboratories, Inc., Carlsbad, CA) was used to extract the total DNA from the fresh fecal samples, and the extracted DNA concentration was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). A Roche High Fidelity Fast Start Kit (Roche, Indianapolis, IN) and a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) were used to amplify the 16S rRNA gene. Following the PacBio protocol, full-length 16S PacBio (Pacific Biology, Menlo Park, CA) primers, forward (AGRGTTYGATYMTGGCTCAG) and reverse (RGYTACCTTGTTACGACTT), were added. A Fragment Analyzer (Advanced Analytics, Ames, IA) was used to evaluate the quality of the amplicons’ regions and sizes, and equimolar amounts of amplicons from each sample were used to create a DNA pool. A 2% agarose E-gel (Life Technologies) was used to select pooled samples according to size. A Qiagen gel purification kit (Qiagen, Valencia, CA) was then used to extract the samples. The remaining products were evaluated using an Agilent Bioanalyzer to determine the profile and mean size. PacBio sequencing was completed by The Roy J. Carver Biotechnology Center at the University of Illinois. The 2x Roche KAPA HiFi Hot Start Ready Mix (Roche, Willmington, MA) and full-length 16S primers with barcodes from PacBio were used to create the 16S amplicons that were then pooled and entered into a library using the SMRTBell Express Template Prep kit 2.0 (Pacific Biology). Sequencing was performed on 1 SMRT cell 8M in the Sequel II using a 10hs movie time and the circular–consensus–sequencing (CCS) mode. SMRT Link V8.0 was used to evaluate CCS with the following parameters: minimum length 1,200, maximum length 2,000, minimum passes 3, minimum rq 0.99.
The obtained sequences were analyzed using DADA2 (version 1.14; Callahan et al., 2016), and 2,091 taxa were entered into the phyloseq R package (McMurdie and Holmes, 2013). Mitochondrial DNA and taxa with no assigned phylum or zero counts were removed from the analysis in addition to the phyla Campilobacterota, Deferribacterota, and Spirochaetota due to their low prevalence (<0.01% of total reads). Agglomeration of the sequences was completed using a 0.03 threshold by the distribution of inter-taxa phylogenetic distances, independent of the reference database, in order to form clusters of taxa at the ends of the phylogenetic branches. These clusters were assigned as a total of 201 operational taxonomic units (OTUs). Prevalence and singleton filtering was then performed so that OTUs that were reported in less than two samples were discarded. A total of 20 OTUs were observed in only a single sample, reducing the total number of OTUs to 181 after prevalence filtering.
OTUs were converted from abundances to proportions and evaluated for Bray–Curtis dissimilarity (Bray and Curtis, 1957). Observed OTU, Chao1, Shannon entropy, Simpson, and Inverse Simpson indexes were used to evaluate α-diversity. The R package, DESeq2 (Love et al., 2014), was used to evaluate the differential abundance of taxa between treatments. Statistical significance was stated at a false discovery rate (FDR; Benjamini and Hochberg, 1995) less than 0.05. The vegan R package was used to perform Canonical Correspondence Analysis (Oksanen et al., 2019) with fecal acetate, propionate, butyrate, phenols, and indoles as the constraining variables.
Palatability trial
The palatability of the diets containing miscanthus grass and fiber blends was tested against diets containing traditional fiber sources. The palatability comparisons were evaluated at Kennelwood Inc. (Champaign, IL) using a panel of 20 beagles (mean body weight 17.5 ± 6.69 kg). Testing for each comparison was completed in a 2-d trial period. On the first day, 400 g of each tested diet was presented to each dog. On the second trial day, the same test was repeated with reversed bowl position to eliminate any individual “left–right” bias. Total consumption (g) was measured for each diet. The first diet approached and the first diet consumed were also observed each day. The following diet comparisons were tested: CO and MF, CO and MF + FOS, CO and MF + RS, CO and MF + TP, as well as MF and BP.
Statistical analysis
A mixed model of SAS, version 9.4, was used to evaluate data obtained for ATTD of macronutrients, fecal, and blood metabolites. The statistical model included the fixed effect of the diet and the random effect of the animal. The univariate procedure was used to check the normality of the data (residual), and all treatment least-square means were compared. Tukey adjustment was used to control for experiment-wise error. The significance level was set at a P-value of less than 0.05. The results of the palatability tests were evaluated using a paired t-test for means of daily consumption. Preference was considered significant at a P-value of less than 0.05.
Results
The extrusion and processing of the experimental diet matrices were completed without requiring any major changes to system parameters (Table 1). Minor adjustments were made to the extruder speed during the processing of the MF + FOS and BP diets, as well as the steam incorporated at the extruder for the MF + RS and BP diets. The preconditioner speed (180 rpm) and knife speed (2,000 rpm) were held constant during the processing of all treatments.
Table 1.
Processing conditions of treatments containing traditional and novel fiber sources for adult canines
| Parameter | Treatments1 | |||||
|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | |
| Dry bulk density, g/L | 599.1 | 594.3 | 602.3 | 600.7 | 597.5 | 595.9 |
| Dry feed rate, kg/h | 362.9 | 362.8 | 362.8 | 362.8 | 362.8 | 362.8 |
| Specific mechanical energy, kW*h/ton | 64.6 | 66.1 | 67.6 | 71.0 | 72.0 | 74.1 |
| Total mass flow rate, kg/h | 527.5 | 519.5 | 509.6 | 503.4 | 489.6 | 492.2 |
| Preconditioner | ||||||
| Shaft speed, rpm | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| Water, kg/h | 65.9 | 66.3 | 66.2 | 66.1 | 67.0 | 66.5 |
| Steam, kg/h | 95.0 | 83.4 | 72.4 | 66.6 | 74.8 | 78.6 |
| Discharge temperature, °C | 88.9 | 88.8 | 87.8 | 87.4 | 86.4 | 88.3 |
| Extruder | ||||||
| Speed, rpm | 370.7 | 375.0 | 375.0 | 400.0 | 375.0 | 425.0 |
| Steam, kg/h | 28.5 | 29.7 | 28.4 | 26.5 | 0.0 | 0.0 |
| Die | ||||||
| Temperature, °C | 101.9 | 103.2 | 102.4 | 102.0 | 102.1 | 103.6 |
| Pressure, psi | 405.7 | 370.7 | 360.0 | 258.3 | 317.5 | 437.5 |
| Knife speed, rpm | 2,000.0 | 2,000.0 | 2,000.0 | 2,000.0 | 2,000.0 | 2,000.0 |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
The six experimental diets were formulated to be similar in regards to nutrient profiles (Table 2). The chemical analysis of these diets confirmed that nutrient compositions were similar across all treatments (Table 3), except for, the BP diet which contained a larger portion of SDF and lower IDF when compared with the other diets. However, overall TDF concentration was similar across all treatments with only the MF + RS diet being slightly higher.
Table 2.
Ingredient composition of treatments containing traditional and novel fiber sources for adult canines
| Ingredient, % as-is | Treatments1 | |||||
|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | |
| Poultry by-product meal | 40.30 | 40.00 | 38.30 | 40.00 | 40.00 | 37.80 |
| Brewers rice | 32.00 | 30.00 | 32.00 | 30.00 | 30.00 | 30.00 |
| Poultry fat | 8.50 | 8.80 | 8.50 | 8.80 | 8.80 | 9.00 |
| Yellow corn | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Corn gluten meal 60% | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| AFB palatant | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Salt | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline chloride | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Potassium chloride | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| BHT antioxidant | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Mineral premix | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Vitamin premix | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Cellulose | 7.00 | – | – | – | – | – |
| Miscanthus fiber | – | 9.00 | 7.00 | 8.00 | 8.00 | – |
| Beet pulp | – | – | – | – | – | 11.00 |
| Tomato pomace | – | – | 2.00 | – | – | – |
| Fructooligosaccharide | – | – | – | 1.00 | – | – |
| Resistant starch | – | – | – | – | 1.00 | – |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + Fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
Table 3.
Chemical composition of treatments containing traditional and novel fiber sources for adult canines
| Item | Treatments1 | |||||
|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | |
| Dry matter, % | 94.20 | 93.11 | 92.42 | 93.89 | 93.40 | 91.90 |
| % DM basis | ||||||
| Organic matter | 93.88 | 93.54 | 93.80 | 93.74 | 93.65 | 93.08 |
| Ash | 6.12 | 6.46 | 6.20 | 6.26 | 6.35 | 6.92 |
| Acid-hydrolyzed fat | 17.64 | 16.74 | 16.21 | 16.56 | 15.59 | 16.24 |
| Crude protein | 31.32 | 30.88 | 29.63 | 31.62 | 31.56 | 30.34 |
| Total dietary fiber | 15.06 | 14.98 | 15.72 | 16.59 | 18.29 | 15.70 |
| Soluble dietary fiber | 3.41 | 3.78 | 3.22 | 3.15 | 4.14 | 6.10 |
| Insoluble dietary fiber | 11.65 | 11.20 | 12.50 | 13.44 | 14.15 | 9.60 |
| Gross energy, kcal/g | 4.71 | 4.59 | 4.56 | 4.64 | 4.59 | 4.47 |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
No significant difference was observed (P > 0.05) among treatments in regards to daily food intake (as-is) or fecal score (Table 4). The CO treatment had the highest daily food intake (DM basis) of all treatments (P < 0.05) followed by MF + FOS and MF + RS which had a higher intake (DM basis) than MF (P < 0.05). Additionally, MF + TP had lower food intake (DM basis) than MF but greater than BP which had the lowest (P < 0.05). Dogs fed the BP diet had significantly higher fecal output gram per day than all other treatments on an as-is basis (P > 0.05). However, dogs fed the MF + FOS had a significantly higher fecal output gram per day on a DM basis than the BP treatment group (P < 0.05) with all other treatments being intermediate and similar to both MF + FOS and BP (P > 0.05; Table 4). The opposite relationship was observed for ATTD of DM with the BP group being significantly higher than MF + FOS (P < 0.05) with all other treatments being intermediate and similar to both BP and MF + FOS (P > 0.05; Table 4). Dogs fed the BP diet had higher ATTD of OM than all other treatment groups (P < 0.05). In contrast, dogs fed the CO, MF, and MF + RS diets had greater CP digestibility than dogs fed the BP diet (P < 0.05) with MF + FOS and MF + TP groups being intermediate and similar to all other treatments (P > 0.05). The ATTD of AHF was significantly higher for dogs fed the CO diet than all other treatments (P < 0.05). Dogs fed the BP diet had the highest TDF digestibility when compared with the other treatments (P < 0.05) followed by MF + RS which was significantly greater than the CO, MF, and MF + TP groups (P < 0.05). The TDF digestibility of the MF + FOS was intermediate and similar to MF + RS, CO, MF, and MF + TP groups (P > 0.05; Table 4).
Table 4.
Food intake, fecal characteristics, and total tract apparent macronutrient digestibility of adult canines fed dietary treatments containing traditional and novel fiber sources
| Item | Treatments1 | ||||||
|---|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | SEM2 | |
| Food intake, as-is | 154.50 | 154.50 | 154.83 | 154.30 | 154.83 | 154.83 | 3.553 |
| Dry matter, g/d | 145.54a | 143.85c | 143.09d | 144.87b | 144.62b | 142.30e | 3.310 |
| Fecal output, g/d (as is) | 69.06b | 70.33b | 70.58b | 73.93b | 72.58b | 90.63a | 4.403 |
| Fecal output, g/d (DMB) | 28.12ab | 28.11ab | 28.09ab | 29.64a | 28.54ab | 25.42b | 1.247 |
| Fecal score | 2.24 | 2.20 | 2.20 | 2.22 | 2.26 | 2.30 | 0.073 |
| Fecal pH | 7.38ab | 7.44a | 7.33ab | 7.19ab | 7.23ab | 6.85b | 0.144 |
| Digestibility, % | |||||||
| Dry matter | 80.71ab | 80.52ab | 80.3ab | 79.5b | 80.31ab | 82.18a | 0.674 |
| % DM basis | |||||||
| Organic matter | 83.93b | 83.70b | 83.66b | 83.07b | 83.63b | 86.04a | 0.565 |
| Acid-hydrolyzed fat | 94.86a | 93.69b | 93.08b | 93.13b | 93.17b | 93.00b | 0.243 |
| Crude protein | 85.24a | 85.67a | 84.49ab | 83.68ab | 85.26a | 82.90b | 0.621 |
| Total dietary fiber | 28.64c | 27.88c | 30.84c | 34.13bc | 41.79b | 52.15a | 2.471 |
| Gross Energy | 85.44ab | 84.38ab | 83.78b | 83.72b | 84.24b | 86.20a | 0.552 |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
Standard error of the mean.
Superscripts with different letters in a row represent statistical differences (P < 0.05).
Fecal pH was highest for the MF treatment group at 7.44 and lowest for the BP treatment group at 6.85 with CO, MF + TP, MF + FOS, and MF + RS being intermediate (P < 0.05; Table 4). No difference was observed in fecal ammonia concentration among treatments (P < 0.05; Table 5). Dogs fed BP had higher fecal propionate and butyrate concentrations than all other treatments (P < 0.05). The BP group also had higher fecal acetate and total cSCFA concentrations than all other treatments with MF + FOS, MF, MF + RS, and MF + TP being intermediate, and the CO group having the lowest acetate and total cSCFA levels (P < 0.05; Table 5).
Table 5.
Fecal fermentative-end products for adult canines fed treatments containing traditional and novel fiber sources
| Item, µmole/g DM basis | Treatments1 | ||||||
|---|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | SEM2 | |
| Total phenols/indoles | 2.93bc | 3.94ab | 4.44a | 3.46ab | 4.00ab | 2.06c | 0.368 |
| Phenols | 0.70 | 0.64 | 0.98 | 0.75 | 0.79 | 0.26 | 0.222 |
| Indoles | 2.23b | 3.30a | 3.46a | 2.69ab | 3.39a | 1.80b | 0.293 |
| Total cSCFA | 301.65c | 371.05b | 379.80b | 393.18b | 394.05b | 763.32a | 23.943 |
| Acetate | 189.30c | 262.85b | 266.77b | 268.54b | 278.50b | 533.70a | 16.991 |
| Propionate | 79.69b | 73.86b | 77.84b | 87.63b | 78.81b | 182.52a | 6.921 |
| Butyrate | 32.66b | 34.34b | 35.19b | 36.03b | 36.73b | 59.30a | 4.303 |
| Total pSCFA | 19.93 | 19.52 | 20.07 | 21.97 | 21.59 | 20.45 | 1.800 |
| Isobutyrate | 7.68 | 7.71 | 7.95 | 8.53 | 8.31 | 8.26 | 0.715 |
| Isovalerate | 11.87 | 11.32 | 11.59 | 12.51 | 12.32 | 11.19 | 1.023 |
| Valerate | 0.38b | 0.52ab | 0.54ab | 0.95ab | 0.51ab | 1.00a | 0.251 |
| Ammonia, mg/g DM | 2.37 | 2.36 | 2.34 | 2.44 | 2.43 | 2.16 | 0.175 |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
Standard error of the mean.
Superscripts with different letters in a row represent statistical differences (P < 0.05).
Treatment did not have a significant effect on fecal total pSCFA, isobutyrate, or isovalerate concentrations (P > 0.05). However, fecal valerate concentrations were highest in dogs fed the BP diet and lowest in dogs fed the CO diet (P < 0.05) with MF + FOS, MF, MF + RS, and MF + TP being intermediate and not differing from both BP and CO (P > 0.05; Table 5). No significant difference in fecal phenol concentration was observed among treatments (P > 0.05). Indole concentration was highest for dogs fed the MF, MF + TP, and MF + RS diets, and lowest for CO and BP, with MF + FOS being intermediate. Total phenol and indole concentration followed a similar pattern, with dogs fed the MF + TP diet having the highest total fecal concentration, and dogs fed the BP diet having the lowest, while CO, MF, MF + FOS, and MF + RS were intermediate (P < 0.05; Table 5).
The serum chemistry parameters (Table 6) analyzed were within the reference ranges provided by the Clinical Pathology Laboratory at the University of Illinois College of Veterinary Medicine for healthy adult dogs for all treatments, except for globulin concentration. Globulin concentration was observed to be slightly lower in all treatment groups (2.42 to 2.54 g/dL) than the reported reference range (2.7 to 4.4 g/dL). Possible reasons for this finding are unknown, since the dogs remained healthy throughout the experimental period, without any clinical symptoms of the disease. Reference ranges for total globin in the blood of healthy adult dogs have also been reported to vary from 1.6 to 4.0 g/dL depending on the laboratory source (i.e, Cornell University Animal Health Diagnostic Center, 2017; Antech Diagnostics, 2021; and Idexx Laboratory, nd).
Table 6.
Serum metabolites for adult canines fed treatments containing traditional and novel fiber sources
| Item | Reference range2 | Treatments1 | ||||||
|---|---|---|---|---|---|---|---|---|
| CO | MF | MF + TP | MF + FOS | MF + RS | BP | SEM3 | ||
| Creatinine, mg/dL | 0.5 to 1.5 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.06 |
| BUN,4 mg/dL | 6.0 to 30.0 | 16.8 | 19.1 | 18.0 | 17.3 | 17.6 | 18.9 | 1.61 |
| Total protein, g/dL | 5.1 to 7.0 | 5.7 | 5.7 | 5.7 | 5.6 | 5.7 | 5.7 | 0.11 |
| Albumin, g/dL | 2.5 to 3.8 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 0.06 |
| Globulin, g/dL | 2.7 to 4.4 | 2.5 | 2.5 | 2.5 | 2.4 | 2.5 | 2.5 | 0.09 |
| Ca, mg/dL | 7.6 to 11.4 | 9.9 | 9.9 | 9.9 | 9.9 | 9.9 | 9.9 | 0.12 |
| P, mg/dL | 2.7 to 5.2 | 3.7 | 4.0 | 3.8 | 3.8 | 3.8 | 4.0 | 0.17 |
| Na, mmol/L | 141 to 152 | 143.7 | 143.6 | 143.8 | 143.6 | 143.9 | 144.3 | 0.41 |
| K, mmol/L | 3.9 to 5.5 | 4.1 | 4.1 | 4.1 | 4.1 | 4.2 | 4.2 | 0.08 |
| Na:K ratio | 28 to 36 | 35.5 | 34.9 | 35.2 | 35.0 | 34.4 | 34.6 | 0.66 |
| Cl, mmol/L | 107 to 118 | 110.9 | 111.0 | 110.7 | 110.5 | 110.7 | 111.7 | 0.56 |
| Glucose, mg/dL | 68 to 126 | 89.0 | 90.5 | 90.1 | 91.3 | 88.7 | 89.7 | 2.35 |
| Total bilirubin, mg/dL | 0.1 to 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.01 |
| Cholesterol, mg/dL | 129 to 297 | 215.0 | 208.6 | 190.4 | 190.8 | 205.1 | 204.4 | 13.98 |
| Triglycerides, mg/dL | 32 to 154 | 65.6 | 64.4 | 64.9 | 63.0 | 71.1 | 58.3 | 3.45 |
| Bicarbonate, mmol/L | 16 to 24 | 21.2 | 20.8 | 21.0 | 21.7 | 20.8 | 20.9 | 0.43 |
CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp.
Reference ranges were provided by the University of Illinois Veterinary Diagnostics Laboratory.
Standard error of the mean.
BUN, blood urea nitrogen.
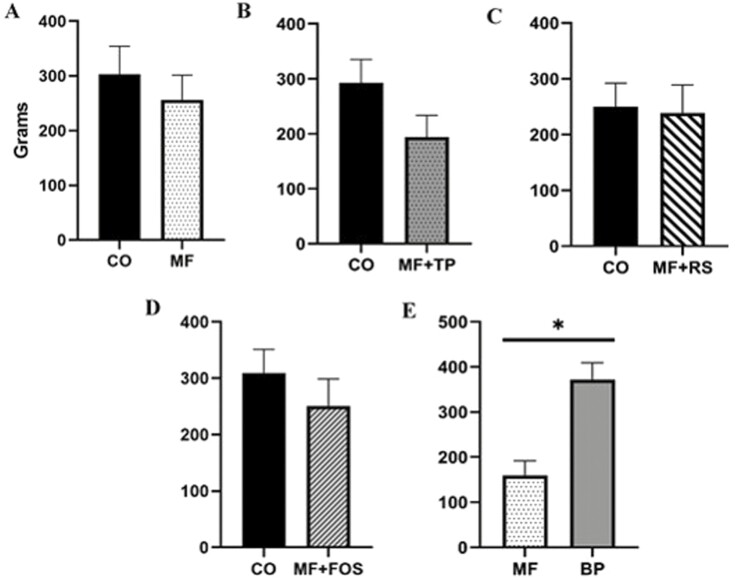
In the palatability trial, the comparison of CO and MF resulted in a total consumption ratio of 1.18:1. The bowl containing CO was approached first on 21 out of 40 occasions over the trial period and consumed first on 18 out of 40 occasions. No preference was observed between the CO and MF diets (P > 0.05). In the palatability comparison of CO and MF + FOS, the total consumption ratio was 1.24:1.00. The CO diet was approached first on 20 out of 40 occasions and consumed first on 21 out of 40 occasions. No significant preference was observed between the CO and MF + FOS diets (P > 0.05). When comparing CO with MF + RS, the total consumption ratio was 1.05:1. The CO diet was approached first on 19 out of 40 occasions and consumed first on 18 out of 40 occasions. No significant preference was observed between the CO and MF + RS diets (P > 0.05). Similarly, when comparing CO and MF + TP, the total consumption ratio was 1.51:1. The CO diet was approached first on 24 out of 40 occasions and consumed first on 23 out of 40 occasions. No significant preference was observed between the CO and MF + TP diets (P > 0.05). However, in the comparison of the BP and MF diets, the total consumption ratio was 2.34:1.0. The MF diet was approached first on 19 out of 40 occasions but was only consumed first on 8 out of 40 occasions. The BP diet was consumed first on 32 out of 40 occasions and was preferred, based on individual consumption, by an average of 45% by 18 out of 20-panel individuals. Results show a significant preference for the BP over the MF diet (P < 0.05; Figure 2).
Figure 2.
Average total consumption of diets (g) containing traditional and novel fiber sources in two-bowl palatability trial (A–E). CO, control; MF, M-Fiber; MF + TP, M-Fiber + tomato pomace; MF + FOS, M-Fiber + fructooligosaccharide; MF + RS, M-Fiber + resistant starch; BP, beet pulp. Asterisk denotes a significant difference (P < 0.05) between diets.
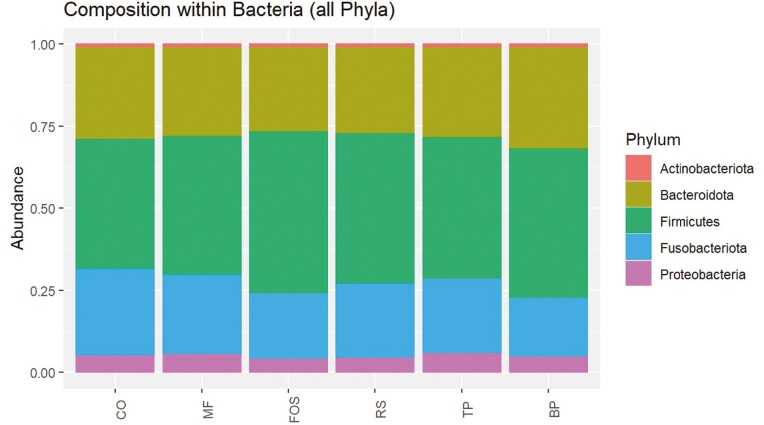
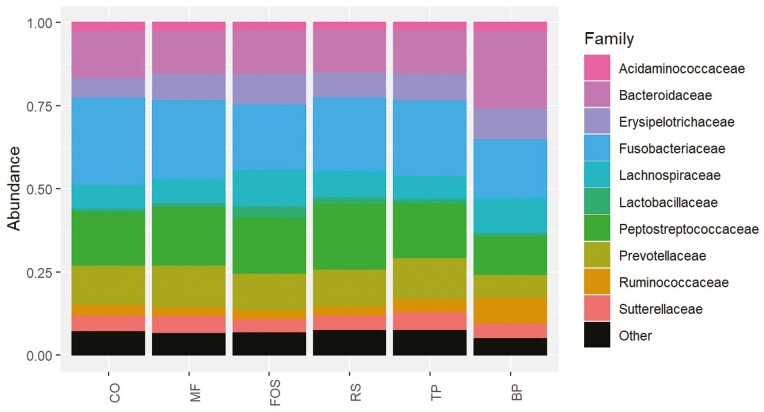
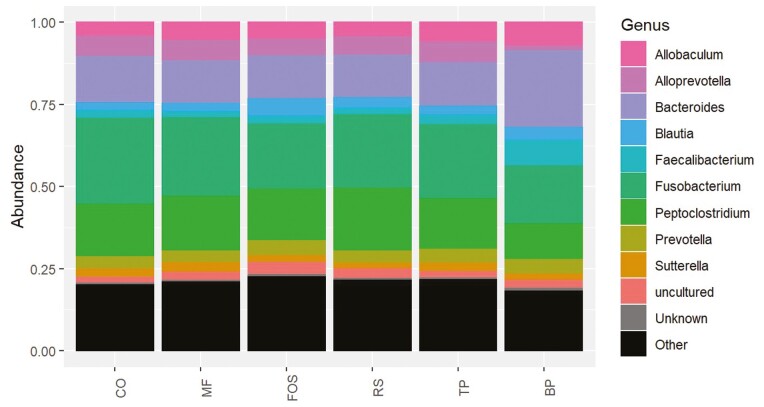
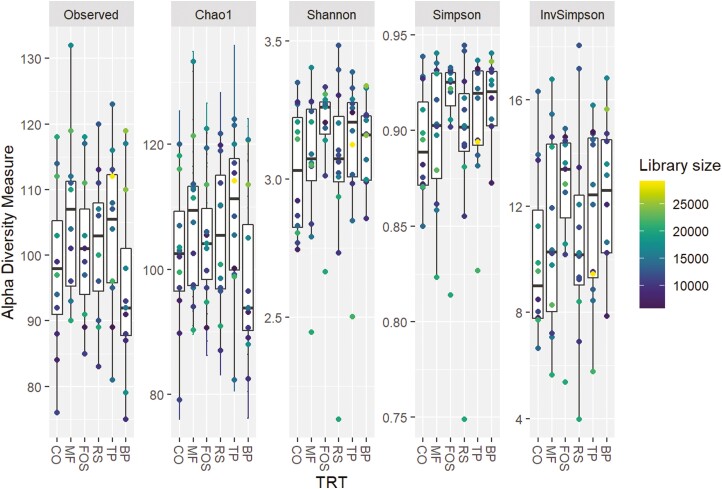
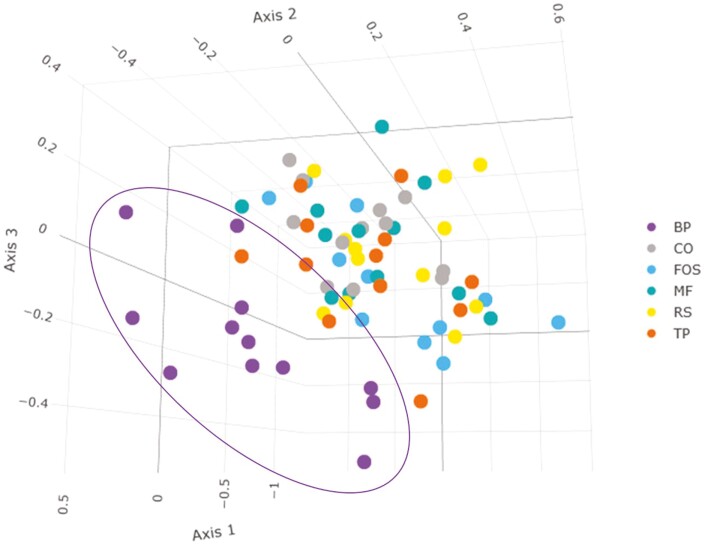
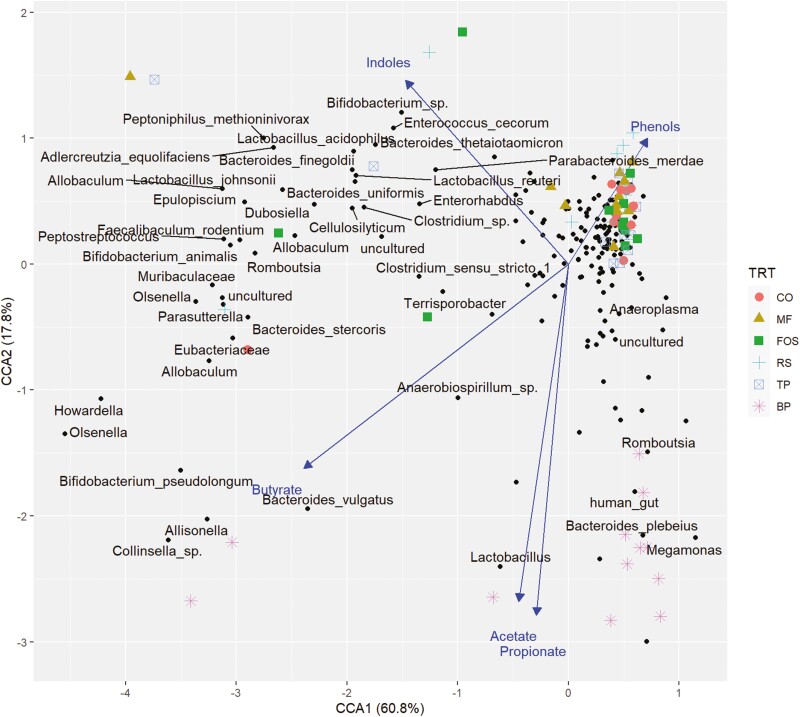
The predominant phyla that comprised the fecal microbial community (Figure 3) were Actinobacteriota, Bacteroidota, Firmicutes, Fusobacteriota, and Proteobacteria, with over 95% of microbial relative abundance being comprised of Bacteroidota, Firmicutes, and Fusobacteriota among all treatment groups. At the family level (Figure 4), dogs fed CO, MF, MF + TP, MF + RS, and MF + FOS had relatively similar relative abundance, whereas the microbial composition of dogs fed BP diet was characterized by a greater relative abundance of Bacteroidaceae and Ruminococcaceae families and lower relative abundance in the Prevotellaceae and Peptostreptococcaceae families. Similarly, at the genus level (Figure 5), the fecal microbial composition of dogs fed CO, MF, MF + TP, MF + RS, and MF + FOS were similar, in contrast with dogs fed the BP diet, which had a higher relative abundance of Bacteroides, Faecalibacterium, and Peptoclostridium, and lower Alloprevotella. Differential abundance of the microbial communities (Table 7), represented as a log 2-fold change with a P-value and FDR value less than 0.05, indicated that 20 taxa increased and 29 taxa decreased between the BP and CO treatment groups (P < 0.05). For both the MF and MF + RS groups, no taxa increased, but one taxon decreased when compared with the CO group (P < 0.05). It was also observed that five taxa increased and three taxa decreased between the MF + FOS and CO treatment groups (P < 0.05), but when comparing the MF + TP and CO groups, no change in the differential abundance of any taxa was observed (P > 0.05). The α-diversity of the fecal microbial community was evaluated using observed OTU, Chao1, Shannon Entropy, Simpson, and Inverse Simpson indexes (Figure 6), and no significant difference was observed among treatments (P > 0.05). The β-diversity of dogs fed BP diet represented as Bray–Curtis distance (Figure 7) differed from the fecal microbial communities of dogs fed MF-containing or CO diet. Canonical correspondence analysis (CCA) of taxa abundance constrained by fecal cSCFA, phenols, and indoles as metabolic variables are shown in Figure 8. The first two axes of the CCA plot represented 60.8% and 17.8%, respectively, of the variance in the metabolic variables. Dogs fed BP were more strongly correlated with fecal cSCFA concentrations and bacterial taxa including Bifidobacterium pseudolongum, Bacteroides vulgatus, Lactobacillus spp., Bacteroides plebeius, and Megamonas. In contrast, dogs fed MF-containing or CO diets were more strongly correlated to fecal indole and phenol concentrations, with Bifidobacterium spp., Enterococcus cecorum, Bacteroides thetaiotaomicron, and Parabacteroides merdae as corresponding taxa, among others.
Figure 3.
Fecal microbial composition at the phyla level of adult canines fed treatments containing traditional and novel fiber sources. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-Fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Figure 4.
Fecal microbial composition at the family level of adult canines fed treatments containing traditional and novel fiber sources. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-Fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Figure 5.
Fecal microbial composition at the genus level of adult canines fed treatments containing traditional and novel fiber sources. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-Fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Table 7.
Differential abundance of microbial communities in adult canines fed treatments containing traditional and novel fiber sources with a minimum of 3-fold change
| Phylum | Family | Genus | Species | Fold Change1 | P-value | FDR2 |
|---|---|---|---|---|---|---|
| BP vs. CO | ||||||
| Bacteroidota | Bacteroidaceae | Bacteroides | Bacteroides plebeius | 4.19 | 0.000 | 0.00 |
| Bacteroides | Bacteroides vulgatus | 4.69 | 0.000 | 0.00 | ||
| Firmicutes | Clostridiaceae | Clostridium sensu stricto 1 | Unclassified | 3.43 | 0.000 | 0.00 |
| Lachnospiraceae | [Ruminococcus] torques group | Uncultured organism | 3.21 | 0.000 | 0.00 | |
| Lachnospira | Uncultured organism | 3.01 | 0.000 | 0.00 | ||
| Unclassified | Unclassified | 4.51 | 0.000 | 0.00 | ||
| Uncultured | Human gut | 3.39 | 0.000 | 0.00 | ||
| Oscillospiraceae | Oscillospira | Uncultured bacterium | −4.33 | 0.000 | 0.00 | |
| UCG-005 | Unclassified | −3.04 | 0.000 | 0.00 | ||
| Selenomonadaceae | Megamonas | Uncultured organism | 3.53 | 0.000 | 0.00 | |
| Veillonellaceae | Allisonella | Uncultured bacterium | 12.60 | 0.012 | 0.04 | |
| MF + RS vs. CO | ||||||
| Firmicutes | Lachnospiraceae | GCA-900066575 | Unclassified | −28.50 | 0.000 | 0.00 |
| MF vs. CO | ||||||
| Firmicutes | Veillonellaceae | Allisonella | Uncultured bacterium | −28.87 | 0.000 | 0.00 |
| MF + FOS vs. CO | ||||||
| Firmicutes | Clostridiaceae | Clostridium sensu stricto 13 | Clostridium_sp. | −19.65 | 0.000 | 0.00 |
| Ruminococcaceae | Anaerofilum | Uncultured bacterium | −26.80 | 0.000 | 0.00 | |
| MF vs. BP | ||||||
| Bacteroidota | Bacteroidaceae | Bacteroides | Bacteroides plebeius | −4.42 | 0.000 | 0.00 |
| Bacteroides | Bacteroides vulgatus | −4.18 | 0.000 | 0.00 | ||
| Firmicutes | Lachnospiraceae | Lachnospira | Uncultured organism | −3.14 | 0.000 | 0.00 |
| Unclassified | Unclassified | −4.68 | 0.000 | 0.00 | ||
| Uncultured | Human gut | −3.14 | 0.000 | 0.00 | ||
| Oscillospiraceae | Oscillospira | Uncultured bacterium | 3.49 | 0.000 | 0.00 | |
| Selenomonadaceae | Megamonas | Uncultured organism | −3.79 | 0.000 | 0.00 | |
| Streptococcaceae | Streptococcus | Streptococcus minor | 16.35 | 0.000 | 0.00 | |
| Uncultured | Uncultured | Uncultured bacterium | 3.57 | 0.000 | 0.00 | |
| Veillonellaceae | Allisonella | Uncultured bacterium | −29.17 | 0.000 | 0.00 | |
| MF + FOS vs. BP | ||||||
| Bacteroidota | Bacteroidaceae | Bacteroides | Bacteroides plebeius | −3.82 | 0.000 | 0.00 |
| Bacteroides | Bacteroides vulgatus | −4.86 | 0.000 | 0.00 | ||
| Firmicutes | Clostridiaceae | Clostridium sensu stricto 13 | Clostridium sp. | −19.84 | 0.000 | 0.00 |
| Erysipelotrichaceae | Allobaculum | Uncultured bacterium | −3.58 | 0.003 | 0.01 | |
| Holdemanella | Metagenome | 3.03 | 0.000 | 0.00 | ||
| Lachnospiraceae | Blautia | Unclassified | −3.05 | 0.000 | 0.00 | |
| Lachnospira | Uncultured organism | −3.93 | 0.000 | 0.00 | ||
| [Ruminococcus] torques group | Uncultured organism | −3.31 | 0.000 | 0.00 | ||
| Unclassified | Unclassified | −4.34 | 0.000 | 0.00 | ||
| Uncultured | Human gut | −3.52 | 0.000 | 0.00 | ||
| Oscillospiraceae | Oscillospira | Uncultured bacterium | 4.02 | 0.000 | 0.00 | |
| Ruminococcaceae | Anaerofilum | Uncultured bacterium | −26.15 | 0.000 | 0.00 | |
| Selenomonadaceae | Megamonas | Uncultured organism | −3.08 | 0.000 | 0.00 | |
| MF + RS vs. BP | ||||||
| Bacteroidota | Bacteroidaceae | Bacteroides | Bacteroides plebeius | −4.58 | 0.000 | 0.00 |
| Bacteroides | Bacteroides vulgatus | −3.91 | 0.000 | 0.00 | ||
| Firmicutes | Lachnospiraceae | Lachnospira | Uncultured organism | −5.36 | 0.000 | 0.00 |
| Unclassified | Unclassified | −4.60 | 0.000 | 0.00 | ||
| Uncultured | Human gut | −4.19 | 0.000 | 0.00 | ||
| Oscillospiraceae | Oscillospira | Uncultured bacterium | 3.66 | 0.000 | 0.00 | |
| Selenomonadaceae | Megamonas | Uncultured organism | −3.82 | 0.000 | 0.00 | |
| MF + TP vs. BP | ||||||
| Actinobacteriota | Bifidobacteriaceae | Bifidobacterium | Bifidobacterium_sp. | 17.94 | 0.000 | 0.00 |
| Bacteroidota | Bacteroidaceae | Bacteroides | Bacteroides plebeius | −3.94 | 0.000 | 0.00 |
| Bacteroides | Bacteroides vulgatus | −3.21 | 0.001 | 0.00 | ||
| Firmicutes | Lachnospiraceae | Unclassified | Unclassified | −3.97 | 0.000 | 0.00 |
| Uncultured | Human gut | −3.30 | 0.000 | 0.00 | ||
| Oscillospiraceae | Oscillospira | Uncultured bacterium | 3.60 | 0.000 | 0.00 | |
| Selenomonadaceae | Megamonas | Uncultured organism | −3.95 | 0.000 | 0.00 | |
| Streptococcaceae | Streptococcus | Streptococcus alactolyticus | −21.41 | 0.000 | 0.00 | |
| Veillonellaceae | Allisonella | Uncultured bacterium | −12.59 | 0.006 | 0.03 | |
Log 2 fold change.
FDR, false discovery rate.
Figure 6.
Fecal microbial α-diversity of adult canines fed treatments containing traditional and novel fiber sources. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-Fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Figure 7.
Principal coordinate analyses with nonmetric multidimensional scaling of Bray–Curtis distance of fecal microbial communities of dogs fed extruded diets containing traditional and novel fiber sources. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Figure 8.
Canonical correspondence analysis of taxa abundance constrained by fecal metabolite variables. CO, control; MF, M-Fiber; FOS, M-Fiber + fructooligosaccharide; RS, M-Fiber + resistant starch; TP, M-Fiber + tomato pomace; BP, beet pulp.
Discussion
Diet composition, food intake, and palatability
A few studies have evaluated the effects of fiber sources in dry extruded dog diets on processing parameters and kibble characteristics. Similar to what was observed during the processing of experimental diets utilized in this study, Donadelli et al. (2021) reported that only minor system adjustments were needed during the processing of diets containing a 10% inclusion of CO, BP, or MF. The study by Donadelli et al. (2021) also reported that the BP diet required higher specific mechanical energy during processing compared with the CO and MF diets, which was also observed in the current study. This observation could be contributed to the fiber composition of BP and its viscous properties. Overall, the changes in processing parameters that were necessary to achieve the desired product characteristics were minimal, indicating that MF can be processed similar to traditional fiber ingredients incorporated in extruded canine diets.
The experimental diets were formulated to have comparable ingredient and nutrient compositions, varying only in dietary fiber sources (i.e., CO, BP, MF, MF and TP blend, MF and FOS blend, and MF and RS blend). To obtain, approximately, 15% TDF content (DM basis) among treatments, small differences in dietary fiber source inclusion rates were required. Because factors such as plant maturity, growing conditions, and the processing method can have an effect on the TDF content of the plant materials used as ingredients, small variations in dietary TDF were expected (de Godoy et al., 2013). The higher SDF content and lower IDF content of the BP diet was anticipated, as it has a lower ratio of IDF to SDF with reported ranges from 1.9 to 5.3:1 compared with CO with reported ranges from 27.5 to 42.2:1 (de Godoy et al., 2013). The IDF to SDF ratio of the CO, MF, MF + TP, MF + FOS, and MF + RS diets were similar due to the compositional similarities of CO and the MF that made up the majority of the fiber blends.
All treatment diets were well accepted by the dogs in the feeding trial, and feed refusals were minimal. Food intake on an as-is basis (g/d) was similar among all treatments, while food intake on a DM basis (g/d) differed. A maximum difference of only 3 g, on average, was observed, and such a small difference is not expected to be of physiological relevance. Donadelli and Aldrich (2019) evaluated three experimental diets with a 10% inclusion rate of CO, BP, and MF, and also reported no significant effect of fiber source on food intake. In the two-bowl palatability trial, dogs fed diets containing MF (i.e., MF, MF + TP, MF + FOS, and MF + RS) showed no food preference in comparison with dogs fed the CO diet. In contrast, the BP diet was preferred over the MF diet. These findings indicate that in practical application, the complete substitution of MF at the expense of CO is unlikely to negatively impact diet palatability, however full replacement of BP by MF in extruded diets for adult dogs may compromise palatability. Limited data have been published evaluating the effects of dietary fiber sources or blends on the palatability of extruded diets for dogs. Sabchuk et al. (2017) reported that canine formulated with either soybean hulls, BP, CO, or sugarcane did not affect the palatability of these extruded diets. It is possible that different fiber sources affect not only the taste and/or aroma of extruded diets, but may also influence kibble texture and mouthfeel properties. Additionally, differences in moisture content as small as 2% have been reported to impact the palatability of dry extruded dog food (de Brito et al., 2010). This is an area that warrants further investigation.
Fecal characteristics
The average fecal score for all treatments evaluated on a scale of 1 to 5 with 1 being hard, dry feces and 5 being liquid diarrhea fell within an ideal range of 2.0 to 3.0. Donadelli and Aldrich (2019) also reported favorable fecal scores with the evaluation of dogs fed diets with 10% inclusion rates of CO, MF, and BP. The authors reported that the diet containing BP resulted in softer feces compared with diets containing MF or CO; however, all treatments resulted in fecal scores close to an ideal score. Dogs fed the BP diet had the highest fecal output on an as-is basis (g/d) compared with all other treatments, but the lowest fecal DM output (g/d). This was likely caused by the water-binding properties of the SDF that is found in higher quantities in BP. The fiber matrix draws water into the feces, subsequently increasing fecal mass. Similarly, Fahey et al. (1990) reported increasing wet feces weight (g/d) with increasing inclusion of BP (0 to 12.5%) in the diets of canines.
Apparent total tract macronutrient and energy digestibilities
The effects of dietary fiber source and inclusion rate on diet digestibility have been reported by several studies. Fahey et al. (1990) reported linear decreases in ATTD of OM and DM with increasing inclusion of BP in extruded canine diets. Sunvold et al. (1995b) reported that feeding dogs extruded diets with fibers and fiber blends with higher fermentability resulted in increased ATTD of DM compared with less fermentable fibers. In this study, the BP treatment was found to have the highest ATTD of DM and OM, however, the ranges across all treatments (79.5% to 82.2% and 83.1% to 86.0%, respectively) are narrow and close to a threshold of 80% digestibility for both OM and DM, indicating that all treatments were well digested by the dogs. Donadelli and Aldrich (2019) reported similar values when evaluating canine diets including 10% CO, MF, and BP, with DM digestibility ranging from 77.2% to 81.3%, and OM digestibility ranging from 80.8% to 86.1%.
Donadelli and Aldrich (2019) similarly observed that the BP treatment group had the lowest CP digestibility (84.5%) compared with CO and MF groups in canines. This effect has been reported in the previous literature and is likely due to the moderate level of fiber fermentability of BP compared with fiber sources with higher levels of IDF such as CO and MF. Increased fermentation in the gut can lead to larger quantities of microbial nitrogen in the feces, which, if not distinguished from dietary nitrogen sources during analysis, can lead to underestimation of ATTD of CP (Sunvold et al., 1995b; Rossoni and Fahey, 2013).
The increased AHF digestibility (94.9%) of the CO treatment group followed the same pattern as digestible energy. Sunvold et al. (1995b) also reported that the addition of CO in an experimental diet for canines resulted in the highest AHF digestibility (96.1%) when compared with diets containing more fermentable fiber sources and fiber blends including BP, citrus pulp, pectin, and gums. The lignin content of fiber sources may have an impact on this result. Donadelli and Aldrich (2019) reported the lignin content of CO, BP, and MF as 0.7%, 6.4%, and 13.7%, respectively. Lignin has been reported to have a negative effect on AHF digestibility due to its ability to bind bile acids, subsequently reducing lipid digestion and absorption (Rodriguez-Gutierrez et al., 2014). This characteristic of MF may be beneficial in therapeutic diets, for example, weight loss and/or maintenance pet foods, which typically aim to increase dietary fiber while decreasing dietary lipid and energy content. This combination can lead to decreased diet palatability. Miscanthus grass has the potential to decrease fat digestibility and digestible energy without the reductions in dietary lipid content that can be detrimental to palatability.
As expected, the BP treatment had the highest TDF digestibility (52.2%) due to its higher fermentative potential compared with CO and MF (Sunvold et al., 1995a). Fahey et al. (1990) reported similar TDF digestibility in canines fed diets with an inclusion of 10% and 12.5% BP (49.4% and 57.5%, respectively). Donadelli and Aldrich (2019) reported slightly higher TDF digestibility in canines with the dietary inclusion of 10% CO, BP, and MF (37.5%, 63.0%, and 46.1%, respectively). The MF, MF + TP, and MF + FOS treatments were similar in TDF digestibility to the CO treatment. This was expected due to the higher levels of IDF in these blends, similar to CO. The intermediate TDF digestibility of the MF + FOS and MF + RS treatments indicates that the addition of more fermentable fibers, such as FOS and RS, to the MF may favor saccharolytic fermentation in the hindgut of dogs and modulate fecal microbiota toward the moderate fermentability and higher TDF digestibility observed with the BP treatment. Although statistical differences were observed among treatments for DM, OM, AHF, and CP digestibilities, the numerical differences among these values were relatively small, and they are likely to have little to null physiological effect in adult dogs.
Fecal fermentative end-products
Saccharolytic fermentation by gut microbiota results in the production of cSCFA, with increased fermentation resulting in proportionally higher fecal cSCFA concentrations (Middelbos et al., 2007; Panasevich et al., 2013; Detweiler et al., 2019). Short-chain fatty acids are considered beneficial to gut health as they can provide energy to colonoytes, reduce inflammation, promote the integrity of the gut barrier, and play a role in the prevention of cancers and other gastrointestinal diseases (Zhang and Davies, 2016). The total fecal cSCFA concentrations of dogs fed the BP diet was over twice the amount observed in dogs fed the CO diet (763.3 and 301.7 µmole/g, DM basis, respectively). This follows what was reported by Sunvold et al. (1995a) using an in vitro model with canine fecal inoculum, and the pattern observed by Donadelli et al. (2019) using a similar in vitro model to evaluate the products of CO, BP, and MF fermentation, with BP resulting in the highest cSCFA production after 12 h of fermentation (2.72 mmol/g), followed by MF (0.11 mmol/g) and CO (0.04 mmol/g). Detweiler et al. (2019) evaluated canine diets including 16.6% (17.3% TDF) BP and 10.3% (14.7% TDF) CO and observed lower total fecal cSCFA concentrations (582.5 and 251.1 µmole/g, DM basis, respectively). Fecal pH was observed to be inversely related to total cSCFA concentration.
It was hypothesized that the addition of TP, FOS, and RS fiber to the MF blend would be effective in increasing fecal cSCFA concentrations as they are sources of soluble and more fermentable fibers. Vickers et al. (2001) reported that fermentation of FOS resulted in higher total cSCFA production compared with CO and BP (3.1, 0.1, and 1.5 mmol/g OM, respectively) in an in vitro model using canine fecal inoculum. Similarly, Pinna et al. (2016) reported that FOS-enhanced cSCFA production in an in vitro assay. Swanson et al. (2001) observed that TP fermentation resulted in higher total cSCFA production after 24 h compared with CO (1.7 and 0.1 mmol/g OM, respectively). In contrast, Beloshapka et al. (2014) did not observe a significant change in fecal cSCFA concentrations when feeding dogs up to 5 g/d of RS. Although small numerical increases were observed for total cSCFA, acetate, and butyrate concentrations in fecal samples of dogs fed MF + TP, MF + FOS, and MF + RS, these were not statistically different from the MF treatment group. It is likely that higher inclusion levels of TP, FOS, and RS will be needed to enhance hindgut saccharolytic fermentation. The inclusion levels adopted herein are within the typical inclusion range of these fermentable fibers in commercial diets. Future studies should evaluate additional fiber blends to more closely mimic physiological effects observed when dogs consume BP as the primary fiber source.
While carbohydrate fermentation produces compounds that are considered beneficial to host animal health, the fermentation of protein results in the production of compounds that are often associated with negative health outcomes and undesirable odor such as ammonia, phenol, indole, and pSCFA (O’Neill and Phillips, 1992). Similar to what was observed in this study, Donadelli et al. (2019) reported no difference in total fecal pSCFA production among CO, BP, and MF in an in vitro fermentation model using canine fecal inoculum. Detweiler et al. (2019) reported similar fecal ammonia concentrations of dogs fed extruded diets containing either BP and CO, but observed lower total fecal phenol and indole concentrations (0.9 and 2.2 µmole/g, DM basis, respectively) and total pSCFA (12.1 and 17.1 µmole/g, DM basis, respectively) compared with the fecal concentration of these metabolites observed in this study.
Fecal microbiota
The inclusion of dietary fiber in canine diets has been reported as an effective modulator of the gastrointestinal microbiota (Beloshapka et al., 2013; Wernimont et al., 2020, Pilla and Suchodolski, 2021). As the gut microbiota and their metabolites have become increasingly associated to host health, an importance has been placed on better understanding how dietary fiber sources and their inclusion levels may impact the gastrointestinal milieu, microbial communities, and their metabolic processes (Wernimont et al., 2020). Previous studies assessing the effects of dietary fiber on canine gastrointestinal microbiota have largely focused on prebiotic fibers as well as changes related to weight loss (Swanson et al., 2002; Schmitz and Suchodolski, 2016; Li et al., 2017; Alexander et al., 2018; Salas-Mani et al., 2018). Currently, there is no literature describing the effects of feeding MF and blends on canine gastrointestinal microbial communities.
Firmicutes, Bacteroidetes (Bacteroidota), Proteobacteria, Fusobacteria, and Actinobacteria have been reported as the dominant phyla making up the gut microbiota in canines (Wernimont et al., 2020). Dogs fed the BP diet had significant increases in two species, B. plebeius and B. vulgatus, within the phylum Bacteroidota, a group generally characterized as exhibiting a wide range of abilities to break down plant cell wall glycans (Martens et al., 2011). The CCA of taxa abundance constrained by fecal metabolite variables indicates a correlation between fecal acetate and propionate concentration and abundance of B. plebeius, with the abundance of B. vulgatus being correlated with fecal butyrate concentration. While butyrate production is generally viewed as a positive metabolic outcome, increased abundance of B. vulgatus has been implicated in the development of irritable bowel disease and other gastrointestinal disorders in both dogs and humans (Fujita et al., 2002; Lucke et al., 2006; Maldonado-Contreras et al., 2020).
Dogs fed BP also had significantly increased abundance of several unclassified species within the family Lachnospiraceae, specifically within the genus Lachnospira when compared with the CO, MF, MF + RS, and MF + FOS groups. This family possesses a wide range of species diversity and metabolic functions that promote host health including production of cSCFA, conversion of bile acid, inhibition of pathogen colonization, and promotion of the host immune system (Sorbara et al., 2020). The CCA indicated an association between abundance of Lachnospira and the favorable fecal metabolites, acetate and butyrate. Uncultured species belonging to the genus Megamonas were also found to be higher in dogs fed BP compared with CO, MF, and MF + FOS. Organisms belonging to this group are characterized as major producers of propionate, which was also reflected by their association in the CCA (Polansky et al., 2016). Megamonas also produces enzymes that result in ammonia production, and although no significant difference in fecal ammonia concentration was detected among treatments, this metabolite can influence microenvironment pH, potentially modulating host cell metabolism as well as other microorganisms (Polansky et al., 2016). Beloshapka et al. (2013) reported that supplementing a raw beef and raw chicken diet with 1.4% inulin led to an increased abundance of Megamonas in the feces of dogs. Similar effects were reported with FOS supplementation in an in vitro model of canine microbial communities but were not observed in a parallel in vivo study (Duysburgh et al., 2020).
Uncultured organisms in the genus Allisonella were found in significantly higher abundance in dogs fed BP compared with CO, MF, and MF + TP, with the MF group having significantly lower abundance than both BP and CO groups. Several members of the family Veillonellaceae are considered potential pathogens (Yang et al., 2020). Organisms belonging to the genus Allisonella are believed to be non-fermentative, using the metabolism of histidine as the primary energy source, leading to the production of histamine and carbon dioxide (Garner et al., 2002). Although not a direct metabolic product of this group, fecal butyrate concentration was observed to be correlated with the abundance of Allisonella on the CCA.
Lower abundance of uncultured Oscillospira species was observed in dogs fed BP compared with all other treatments. Some species belonging to this genus are believed to be butyrogenic, and potential utilizers of glucuronate. Oscillospira have been associated with slower colonic transit time due to their slow growth rate in addition to being positively correlated with harder stool in humans (Gophna et al., 2017). These characteristics are also commonly associated with IDF intake, potentially explaining the increase in abundance of this species that was observed in the treatment groups consuming larger fractions of IDF (CO, MF, MF + RS, MF + TP, MF + FOS). In humans, it has also been reported to be positively correlated with leanness, and negatively correlated with inflammatory diseases such as irritable bowel disease (Gophna et al., 2017).
The relative abundance of individual microbial genera and species differs across the literature due to the variation between individual animals as well as collection methods, sequence analysis, and reference databases. However, the results of this study indicate that different dietary fibers can provide a distinct effect on the gastrointestinal microbiota of dogs. Microbial diversity can be used to evaluate gut health, with low diversity often used as an indicator of gut dysbiosis during disease in humans and pet animals (Félix et al., 2022). Changes in microbial species richness, evenness, and presence do not always lead to dysbiosis, however, particularly in nutritional studies using healthy animals. Dietary fiber source did not affect α-diversity, however, dogs fed BP had greater β-diversity using Bray–Curtis distance in contrast with dogs fed MF-containing or CO diets. Thus, shifts in microbial communities should be attributed to the differences in dietary fiber source and inclusion levels and not as an indication of gut dysbiosis since healthy dogs were used in this study, and these animals remained healthy and without any signs of gastrointestinal intolerance or discomfort in response to experimental diets.
Implications
Overall, data obtained in this canine in vivo study corroborated with results from the feline study conducted in our laboratory using MF and fiber blends (Finet et al., 2021). Therefore, MF when included in extruded diets up to 9% (15% TDF) had no negative physiological effects on voluntary food intake, macronutrient digestibility, and fecal scores in adult healthy dogs. In general, MF-containing diets resulted in concentrations of fecal fermentative-end products and microbiota that were more similar to those observed in the CO group than in the BP group. In this study, the inclusion of 1% to 2% of more fermentable and/or prebiotic fiber sources (TP, FOS, and RS) in combination with MF resulted in small numerical increases in fecal cSCFA concentrations, suggesting that fiber blends can be effective strategies to modulate gut environment, metabolites, and microbiota. However, including higher concentrations or a mixture of different fiber sources with varying physiological properties may be needed to elicit a greater response. Differences in β-diversity of fecal microbiota indicated that feeding different dietary fibers was effective in promoting significant shifts in microbial populations, primarily in families belonging to the phyla Bacteriodota and Firmicutes. In conclusion, MF can be utilized by the pet food industry as an economical and environmentally conscious ingredient that can provide flexibility in the formulation of diets that aim to maximize the health benefits of dietary fiber. MF can be effectively used as a base ingredient to develop fiber blends in combination with more soluble and fermentable dietary fiber. Miscanthus grass and fiber blends may serve as nutraceutical ingredients in multiple dietary platforms, including weight management and gut health.
Acknowledgments
We thank MFiber and Renew Biomass for the financial support. M.R.C.G. designed the experiment. S.E.F. and F.H. performed the laboratory procedures. S.E.F. and M.R.C.G. performed the statistical analyses. B. R. S. and S. L. R. performed bioinformatic analysis for fecal microbial analysis. S.E.F. and M.R.C.G. wrote the manuscript. We all authors revised and provided intellectual input on this manuscript.
Glossary
Abbreviations
- AHF
acid-hydrolyzed fat
- ATTD
apparent total tract digestibility
- BP
beet pulp
- CCA
canonical correspondence analysis
- CO
cellulose
- CP
crude protein
- cSCFA
carbohydrate-derived short-chain fatty acid
- DM
dry matter
- FDR
false discovery rate
- FOS
fructooligosaccharide
- IDF
insoluble dietary fiber
- MF
miscanthus grass fiber
- OM
organic matter
- OTU
operational taxonomic unit
- pSCFA
protein-derived short-chain fatty acid
- RS
resistant starch
- SDF
soluble dietary fiber
- TDF
total dietary fiber
- TP
tomato pomace
Conflict of interest statement
The authors have no conflict of interest to disclose.
LITERATURE CITED
- Alexander, C., Cross T. W. L., Ridlon J. M., de Godoy M. R. C., Swanson K. S., Devendran S., Neumer F., Theis S., and Suchodoski J. S.. . 2018. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and faecal microbiota and metabolites in overweight dogs. Br. J. Nutr. 120:711–720. doi: 10.1017/s0007114518001952 [DOI] [PubMed] [Google Scholar]
- American Association of Cereal Chemists (AACC). 1983. Approved methods, 8th ed. St. Paul (MN): AACC. [Google Scholar]
- Antech. 2021. Laboratory diagnostics. [accessed May 28, 2021]. https://www.antechdiagnostics.com/laboratory-diagnostics.
- Association of American Feed Control Officials (AAFCO). 2018. Official publication. Oxford (IN): AAFCO. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). 2006. Official methods of analysis, 17th ed. Gaithersburg (MD): Association of Official Analytical Chemists. [Google Scholar]
- Association of Pet Obesity Prevention (APOP). 2018. U.S. Pet obesity survey. Available from https://petobesityprevention.org/2017.
- Bauer, S., and Ibanez A. B.. . 2014. Rapid determination of cellulose. Biotechnol. Bioeng. 111:2355–2357. doi: 10.1002/bit.25276 [DOI] [PubMed] [Google Scholar]
- Beloshapka, A. N., Alexander L. G., Buff P. R., and Swanson K. S.. . 2014. The effects of feeding resistant starch on apparent total tract macronutrient digestibility, faecal characteristics and faecal fermentative end-products in healthy adult dogs. J. Nutr. Sci. 3:1–5. doi: 10.1017/jns.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloshapka, A. N., Swanson K. S., Dowd S. E., Suchodolski J. S., Steiner J. M., and Duclos L.. . 2013. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 84:532–541. doi: 10.1111/1574-6941.1208 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., and Hochberg Y.. . 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. 57:289–300. doi: 10.2307/2346101 [DOI] [Google Scholar]
- Bray, J. R., and Curtis J. T.. . 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monographs. 27:325–349. doi: 10.2307/1942268 [DOI] [Google Scholar]
- Budde, E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. AOAC. 35:799–805. doi: 10.1093/jaoac/35.3.799 [DOI] [Google Scholar]
- Callahan, B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P.. . 2016. DADA2: high-resolution sample inference from illumina amplicon data. Nat. Methods. 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney, A. L., and Marbach E. P.. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. PMID: 13878063. [PubMed] [Google Scholar]
- Chen, M.-H., Bowman M. J., Dien B. S., Rausch K. D., Tumbleson M. E., and Singh V.. . 2014. Autohydrolysis of miscanthus x giganteus for the production of xylooligosaccharides (XOS): kinetics, characterization and recovery. Bioresour. Technol. 155:359–365. doi: 10.1016/j.biortech.2013.12.050 [DOI] [PubMed] [Google Scholar]
- Cornell University College of Veterinary Medicine. 2017. Animal health diagnostic center reference intervals: chemistry (Cobas). [accessed May 6, 2021]. https://www.vet.cornell.edu/animal-health-diagnostic-center/laboratories/clinical-pathology/reference-intervals/chemistry.
- de Brito, C. B., Félix A. P., de Jesus R. M., de França M. I., de Oliveira S. G., Krabbe E. L., and Maiorka A.. . 2010. Digestibility and palatability of dog foods containing different moisture levels, and the inclusion of a mould inhibitor. Anim. Feed Sci. Tech. 159:150–155. doi: 10.1016/j.anifeedsci.2010.06.001 [DOI] [Google Scholar]
- de Godoy, M. R. C., Kerr K. R., and Fahey G. C. Jr.. 2013. Alternative dietary fiber sources in companion animal nutrition. Nutrients. 5:3099–3117. doi: 10.3390/nu5083099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler, K. B., He F., Mangian H. F., de Godoy M. R. C., and Davenport G. M.. . 2019. Effects of high inclusion of soybean hulls on apparent total tract macronutrient digestibility, fecal quality, and fecal fermentative end-product concentrations in extruded diets of adult dogs. J. Anim. Sci. 97:1027–1035. doi: 10.1093/jas/skz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadelli, R. A., and Aldrich C. G.. . 2019. The effects on nutrient utilization and stool quality of beagle dogs fed diets with beet pulp, cellulose, and Miscanthus grass. J. Anim. Sci. 97:4134–4139. doi: 10.1093/jas/skz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadelli, R. A., Dogan H., and Aldrich C. G.. . 2021. The effects of fiber source on extrusion parameters and kibble structure of dry dog foods. Anim. Feed Sci. Tech. 274:114884. doi: 10.1016/j.anifeedsci.2021.114884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadelli, R. A., Titgemeyer E. C., and Aldrich C. G.. . 2019. Organic matter disappearance and production of short- and branched-chain fatty acids from selected fiber sources used in pet foods by a canine in vitro fermentation model. J. Anim. Sci. 97:4532–4539. doi: 10.1093/jas/skz302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysburgh, C., Ossieur W. P., De Paepe K., Van den Abbeele P., Vichez-Vargas R., Vital M., Pieper D. H., Van de Wiele T., Hesta M., Possemiers S., . et al. 2020. Development and validation of the simulator of the canine intestinal microbial ecosystem (SCIME)1. J. Anim. Sci. 98:skz357. doi: 10.1093/jas/skz357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey, G. C. Jr., Merchen N. R., Corbin J. E., Hamilton A. K., Serbe K. A., Lewis S. M., and Hirakawa D. A.. . 1990. Dietary fiber for dogs: I. Effects of graded levels of dietary beet pulp on nutrient intake, digestibility, metabolizable energy and digesta mean retention time. J. Anim. Sci. 68:4221–4228. doi: 10.2527/1990.68124221x [DOI] [PubMed] [Google Scholar]
- Félix, A. P., Souza C. M. M., and de Oliveira S. G.. . 2022. Biomarkers of gastrointestinal functionality in dogs: a systematic review and meta-analysis. Anim. Feed Sci. Tech. 283:115183. doi: 10.1016/j.anifeedsci.2021.115183 [DOI] [Google Scholar]
- Finet, S. E., Southey B. R., Rodriguez-Zas S. L., He F., and de Godoy M. R. C.. . 2021. Miscanthus grass as a novel functional fiber sources in extruded feline diets. Front. Vet. Sci. 8:668288. doi: 10.3389/fvets.2021.668288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger, E. A., Schreijen E. M. W. C., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C. Jr.. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Fujita, H., Eishi Y., Ishige I., Saitoh K., Takizawa T., Arima T., and Koike M.. . 2002. Quantitative analysis of bacterial DNA from mycobacteria spp., Bacteroides vulgatus, and Escherichia coli in tissue samples from patients with inflammatory bowel diseases. J. Gastroenterol. 37:509–516. doi: 10.1007/s005350200079 [DOI] [PubMed] [Google Scholar]
- Garner, M. R., Flint J. F., and Russell J. B.. . 2002. Allisonella histaminiformans gen. Nov., sp. Nov. A novel bacterium that produces histamine, utilizes histidine as its sole energy source, and could play a role in bovine and equine laminitis. Syst. Appl. Microbiol. 25:498–506. doi: 10.1078/07232020260517625 [DOI] [PubMed] [Google Scholar]
- Gophna, U., Konikoff T., and Nielsen H. B.. . 2017. Oscillospira and related bacteria – from metagenomic species to metabolic features. Environ. Microbiol. 19:835–841. doi: 10.1111/1462-2920.13658 [DOI] [PubMed] [Google Scholar]
- Idexx Laboratories, Inc. nd. Idexx reference laboratories support resources. [accessed May 28, 2021]. https://www.idexx.com/en/veterinary/support/documents-resources/reference-laboratories-resources/.
- Li, Q., Lauber C. L., Czarnecki-Maulden G., Pan Y., and Hannah S. S.. . 2017. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. MBio. 8:e01703–e01716. doi: 10.1128/mBio.01703-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, M. I., Huber W., and Anders S.. . 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Bio. 15:1–21. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke, K., Miehlke S., Jacobs E., and Schuppler M.. . 2006. Prevalence of bacteroides and prevotella spp. in ulcerative colitis. J. Med. Microbiol. 55:617–624. doi: 10.1099/jmm.0.46198-0 [DOI] [PubMed] [Google Scholar]
- Maldonado-Contreras, A., Ferrer L., Cawley C., Crain S., Bhattarai S., Toscano J., Ward D. V., and Hoffman A.. . 2020. Dysbiosis in a canine model of human fistulizing Crohn’s disease. Gut Microbes. 12:1785246. doi: 10.1080/19490976.2020.1785246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, E. C., Lowe E. C., Chiang H., Pudlo N. A., Wu M., McNulty N. P., Abbot W., Henrissat B., Gilbert H. J., Bolam D. N., . et al. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9:e1001221. doi: 10.1371/journal.pbio.1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie, P. J., and Holmes S.. . 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 8:1–11. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbos, I. S., Fastinger N. D., and Fahey G. C. Jr. 2007. Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. J. Anim. Sci. 85:3033–3044. doi: 10.2527/jas.2007-0080 [DOI] [PubMed] [Google Scholar]
- Oksanen, J., Blanchet F. G., Friendly M., Kindt R., Legendre P., cGlinn D. M., Minchin P. R., O’Hara R. B., Simpson G. L., Solymos P., . et al. 2019. vegan: community ecology package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan
- O’Neill, D. H., and Phillips V. R.. . 1992. A review of the control of odour nuisance from livestock buildings: part 3. Properties of the odorous substances which have been identified in livestock wastes or in the air around them. J. Agric. Eng. Res. 53:23–50. doi: 10.1016/0021-8634(92)80072-Z [DOI] [Google Scholar]
- Panasevich, M. R., Rossoni Serao M. C., de Godoy M. R. C., Swanson K. S., Guerin-Deremaux L., Lynch G. L., Wils D., Fahey G. C. Jr, and Dilger R. N.. . 2013. Potato fiber as a dietary fiber source in dog foods. J. Anim. Sci. 91:5344–5352. doi: 10.2527/jas.2013-6842 [DOI] [PubMed] [Google Scholar]
- Pilla, R., and Suchodolski J. S.. . 2021. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. North Am. Small Anim. Pract. 51:605–621. doi: 10.1016/j.cvsm.2021.01.002 [DOI] [PubMed] [Google Scholar]
- Pinna, C., Giuditta Vecchiato C., Bolduan C., Grandi M., Stefanelli C., Windisch W., Zaghini G., and Biagi G.. . 2016. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 14:1–10. doi: 10.1186/s12917-018-1436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky, O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., and Rychlik I.. . 2016. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Envrion. Microbiol. 82:1569–1576. doi: 10.1128/AEM.03473-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosky, A., Asp N. G., Schweizer T. F., Devries J. W., and Furda I.. . 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J. AOAC. 75:360–367. PMID: 2993226. [Google Scholar]
- Rodriguez-Gutierrez, G., Rubio-Senent F., Lama-Munoz A., Garcia A., and Fernandez-Bolanos J.. . 2014. Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: binding of water, oil, bile acids, and glucose. J. Agric. Food Chem. 62:8973–8981. doi: 10.1021/jf502062b [DOI] [PubMed] [Google Scholar]
- Rossoni Serão, M. C., and Fahey G. C. Jr. 2013. Companion animal nutrition as affected by dietary fibre inclusion. In: Delcour, J. A., and Poutanen K., editors. Fibre-rich and wholegrain foods. Swaston (UK): Woodhead Publishing; p. 407–420. [Google Scholar]
- Sabchuk, T. T., Lowndes F. G., Scheraiber M., Silva L. P., Félix A. P., Maiorka A., and Oliveira S. G.. . 2017. Effect of soya hulls on diet digestibility, palatability, and intestinal gas production in dogs. Anim. Feed Sci. Technol. 225:134–142. doi: 10.1016/j.anifeedsci.2017.01.011 [DOI] [Google Scholar]
- Salas-Mani, A., Jeusette I., Iraculis N., Sanchez N., Vilaseca L., Torre C., Castillo I., Manuelian C. L., Lionnet C., and Fernandez S.. . 2018. Fecal microbiota composition changes after a BW loss diet in beagle dogs. J. Anim. Sci. 96:3102–3111. doi: 10.1093/jas/sky193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, S., and Suchodolski J. S.. . 2016. Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics – what is the evidence? Vet. Med. Sci. 2:71–94. doi: 10.1002/vms3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara, M. T., Littmann E. R., Fontana E., Moody T. U., Kohout C. E., Gjonbalaj M., Eaton V., Seok R., Leiner I. M., and Pamer E. G.. . 2020. Functional and genomic variation between human-derived isolates of lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe. 28:134–146.e4. doi: 10.1016/j.chom.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunvold, G. D., Fahey G. C. Jr, Merchen N. R., and Reinhart G. A.. . 1995a. In vitro fermentation of selected fibrous substrates by dog and cat fecal inoculum: influence of diet composition on substrate organic matter disappearance and short-chain fatty acid production. J. Anim. Sci. 73:1110–1122. doi: 10.2527/1995.7341110x [DOI] [PubMed] [Google Scholar]
- Sunvold, G. D., Fahey G. C. Jr, Merchen N. R., Titgemeyer E. C., Bourquin L. D., Bauer L. L., and Reinhart G. A.. . 1995b. Dietary fiber for dogs: IV. In vitro fermentation of selected fiber sources by dog fecal inoculum and in vivo digestion and metabolism of fiber-supplemented diets. J. Anim. Sci. 73:1099–1109. doi: 10.2527/1995.7341099x [DOI] [PubMed] [Google Scholar]
- Swanson, K. S., Grieshop C. M., Clapper G. M., Shields R. G. Jr., Belay T., Merchen N. R., and Fahey G. C. Jr. 2001. Fruit and vegetable fiber fermentation by gut microflora from canines. J. Anim. Sci. 79:919–926. doi: 10.2527/2001.794919x [DOI] [PubMed] [Google Scholar]
- Swanson, K. S., Grieshop C. M., Flickinger E. A., Merchen N. R., and Fahey G. C. Jr. 2002. Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine. J. Nutr. 132:1717S–1719S. doi: 10.1093/jn/132.6.1717s [DOI] [PubMed] [Google Scholar]
- Tungland, B. 2018. Overview of prebiotics: membership, physiological effects and their health attributes. In: Tungland, B. editor. Human microbiota in health and disease. London (UK): Academic Press; p. 289–384. doi: 10.1016/B978-0-12-814649-1.00016-8 [DOI] [Google Scholar]
- Vickers, R. J., Sunvold G. D., Kelley R. L., and Reinhart G. A.. . 2001. Comparison of fermentation of selected fructooligosaccharides and other fiber substrates by canine colonic microflora. Am. J. Vet. Res. 62:609–615. doi: 10.2460/ajvr.2001.62.609 [DOI] [PubMed] [Google Scholar]
- Wernimont, S. M., Radosevich J., Jackson M. I., Ephraim E., Badri D. V., Macleay J. M., Jewell D. E., and Suchodolski J. S.. . 2020. The effects of nutrition on the gastrointestinal microbiome of cats and dogs: impact on health and disease. Front. Microbiol. 11:1–24. doi: 10.3389/fmicb.2020.01266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q., Liang Q., Balakrishnan B., Belobrajdic D. P., Feng Q.-J., and Zhang W.. . 2020. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 12:381. doi: 10.3390/nu12020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. S., and Davies S. S.. . 2016. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8:1–18. doi: 10.1186/s13073-016-0296-x [DOI] [PMC free article] [PubMed] [Google Scholar]