Abstract
Background
To summarize modifiable factors for coronavirus disease 2019 (COVID-19) suggested by Mendelian randomization studies.
Methods
In this systematic review, we searched PubMed, EMBASE and MEDLINE, from inception to 15 November 2021, for Mendelian randomization studies in English. We selected studies that assessed associations of genetically predicted exposures with COVID-19-related outcomes (severity, hospitalization and susceptibility). Risk of bias of the included studies was evaluated based on the consideration of the three main assumptions for instrumental variable analyses.
Results
We identified 700 studies through systematic search, of which 50 Mendelian randomization studies were included. Included studies have explored a wide range of socio-demographic factors, lifestyle attributes, anthropometrics and biomarkers, predisposition to diseases and druggable targets in COVID-19 risk. Mendelian randomization studies suggested that increases in smoking, obesity and inflammatory factors were associated with higher risk of COVID-19. Predisposition to ischaemic stroke, combined bipolar disorder and schizophrenia, attention-deficit and hyperactivity disorder, chronic kidney disease and idiopathic pulmonary fibrosis was potentially associated with higher COVID-19 risk. Druggable targets, such as higher protein expression of histo-blood group ABO system transferase (ABO), interleukin (IL)-6 and lower protein expression of 2′-5′ oligoadenylate synthetase 1 (OAS1) were associated with higher risk of COVID-19. There was no strong genetic evidence supporting the role of vitamin D, glycaemic traits and predisposition to cardiometabolic diseases in COVID-19 risk.
Conclusion
This review summarizes modifiable factors for intervention (e.g. smoking, obesity and inflammatory factors) and proteomic signatures (e.g. OAS1 and IL-6) that could help identify drugs for treating COVID-19.
Keywords: Systematic review, Mendelian randomization studies, COVID-19
Key Messages.
This systematic review provides an up-to-date summary of the evidence on modifiable factors that contribute to COVID-19 outcomes (severity, hospitalization, susceptibility) from Mendelian randomization studies.
This systematic review highlights modifiable factors for intervention, such as smoking, which are in favour of the recent call from the World Health Organization regarding the importance of tobacco cessation in reducing severe COVID-19.
Identified druggable targets shall facilitate prioritization of drug targets for assessment of efficacy in COVID-19 treatment in clinical trial settings.
Mendelian randomization studies in other ethnic populations would be valuable in assessing external validity from existing studies that were predominantly in European populations.
Background
The coronavirus disease 2019 (COVID-19) pandemic is a major global health threat. As of 1 December 2021, the number of cases exceeded 261 million and led to 5.2 million deaths.1 COVID-19-related research has increased exponentially, although concerns over quality exist,2 including retraction of studies using questionable data sources.3 Paradoxical protective effects, such as smoking reducing the risk of COVID-19-related deaths,4 could arise from selection bias in an observational setting.5 Given the uncertainty of findings from a single study, in particular observational studies, systematic reviews are increasingly used to consolidate the evidence base for prevention and treatment of COVID-19.6,7
Identifying causes of increased susceptibility, hospitalization and severity to COVID-19 based on less biased epidemiologic designs is important to inform prevention and treatment strategies. Mendelian randomization, a study design less susceptible to confounding than conventional observational studies, can help identify causes and possible therapeutic targets.8 For example, a Mendelian randomization study showed genetic inhibition of the interleukin (IL)-6 receptor may protect against severe COVID-19,9 consistently with subsequent randomized–controlled trials (RCTs).6 However, to date, there is no systematic review of Mendelian randomization studies concerning COVID-19 and hence possible targets of intervention/drug reposition opportunities have not been systematically evaluated. This is particularly important as herd immunity is challenging to achieve in view of newly emerging variants,10 vaccine hesitancy11 and vaccine inequity.12 We conducted this systematic review to evaluate possible factors (e.g. socio-demographic factors, lifestyle attributes, anthropometrics, biomarkers, predisposition to diseases and druggable targets) contributing to the risk of COVID-19 from Mendelian randomization studies.
Methods
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.13 The protocol of the systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) on 30 April 2021 (CRD42021252079).
Sources of information and study selection
We included Mendelian randomization studies that assessed the associations of genetically predicted exposures with COVID-19-related outcomes. COVID-19 outcomes included the severity, hospitalization and susceptibility of COVID-19. Two reviewers (Y.L. and T.H.T.W.) independently searched published studies in PubMed, EMBASE and MEDLINE from inception to 15 November 2021. We used a pre-defined search strategy, with key search terms (‘Mendelian randomization’ OR ‘Mendelian randomisation’) AND (‘COVID19’ OR ‘coronavirus disease 2019’) AND (‘genome-wide association study’ OR ‘GWAS’). We restricted the language to English and human studies using PubMed filters. We also manually searched the reference lists of the retrieved studies to identify additional studies. Detailed search term combinations for each database can be found in the Supplementary material (available as Supplementary data at IJE online).
Eligibility criteria
We excluded studies that (i) were duplicated across databases; (ii) were without sufficient original data (e.g. reviews, commentaries, corrections and abstracts); (iii) were not Mendelian randomization studies, including studies that only reported variant–outcome associations [i.e. a reduced form of instrumental variable (IV) analysis]; or (iv) did not include COVID-19 phenotypes as the outcomes. Two reviewers (Y.L. and T.H.T.W.) independently screened the titles, abstracts and full text if necessary of all retrieved studies, and compared and resolved any possible discrepancies. A third reviewer (S.L.) was consulted if the discrepancies were not resolved. The study selection process was summarized in a PRISMA flowchart.
Data extraction
For each included Mendelian randomization study, one reviewer (Y.L.) extracted key information using a tailored template based on the PRISMA checklist13 and the guidelines for strengthening the reporting of observational studies in epidemiology using Mendelian randomization (STROBE-MR).14 These included the name of the first author, the year of publication, the type of Mendelian randomization study (one-sample or two-sample), the number of genetic variants, the data source(s) and ancestry for the genetic variants and outcomes, the sample size of the outcome, the effect estimate for exposure on outcome and the corresponding 95% CI. For a one-sample Mendelian randomization study, both one-sample methods (e.g. two-stage least square and a genetic risk score) and two-sample methods (e.g. a meta-analysis of Wald estimates, i.e. inverse variance weighted) were considered as the main analysis.15 For a two-sample Mendelian randomization study, inverse variance weighted was normally considered as the main analysis.15 By default, we extracted the effect estimates with 95% CI from the main analysis reported in the main text. In addition, for associations reaching statistical significance (nominal P-value < 0.05, or P-value after correcting for multiple testing, if mentioned in the original studies), we additionally extracted the results from the sensitivity analyses (e.g. different sets of genetic variants or statistical methods). The extracted data were verified by another reviewer (S.L.).
Risk of biases in each study
There is no quality assessment tool available for systematic reviews of Mendelian randomization studies. Mendelian randomization studies rely on three main IV assumptions for a valid causal inference.16 Specifically, the genetic variant is strongly associated with the exposure (IV1: relevance), independent of the (measured and unmeasured) confounding factors of the exposure–outcome association (IV2: independence) and has no effect on the outcome except via the exposure (IV3: exclusion-restriction).16 Reviewers (S.L., Y.L. and S.L.A.Y.) independently evaluated whether the included Mendelian randomization studies assessed the validity of each IV assumption adequately. Any inconsistencies were resolved by discussion until a consensus was reached.
IV1: Relevance
For the relevance assumption, we checked the strength of the instruments such as P-values of the variant–exposure association (usually genome-wide significant, P-value < 5 × 10–8), instrument strength (F-statistic > 10 indicates that weak instrument bias is less likely) and the variance of exposure explained by the variants (R2).17 We rated IV1 as ‘high’ if the selection of variants used genome-wide significance (P-value < 5 × 10–8) and an F-statistic > 10; as ‘moderate’ if the selection of variants used a more lenient significance threshold (P-value range from 0.05 to 5 × 10–8) and an F-statistic > 10; and ‘poor’ if none of above was assessed, reported or met.
IV2: Independence
It is assumed that the variant is largely independent of potential confounders because of the random allocation of genetic variants at conception.18 For the independence assumption, we evaluated whether IV2 was assessed in each study, such as exploring the variant–confounder(s) association using individual-level data or curated databases (e.g. PhenoScanner),19 controlling for population stratification or use of ethnically homogenous populations, or assessing the possibility of confounding by linkage disequilibrium (LD) using Bayesian colocalization analysis (for cis-Mendelian randomization studies only).20 The posterior probability of Hypothesis 4 (two traits share a common variant) > 0.80 from colocalization analysis supports causality, i.e. the putative association by the cis-Mendelian randomization study is less likely confounded by variants in LD.20 We rated IV2 as ‘high’ if this assumption was properly assessed using the above-mentioned approaches, ‘moderate’ if this assumption was only described and ‘poor’ if the assumption was not described.
IV3: Exclusion-restriction
Although the exclusion-restriction assumption cannot be fully verified, this can be partly checked via assessment of horizontal pleiotropy,21 e.g. searching for genetic pleiotropic effects via curated databases or the use of statistical methods that rely on different and alternative IV assumptions, such as weighted median (majority valid), weighted mode (plurality valid) and Mendelian randomization-Egger (instrument strength independent of direct effect).22–24 We rated IV3 as ‘high’ if this assumption was properly assessed using above-mentioned approaches, ‘moderate’ if this assumption was only described and ‘poor’ if the assumption was not described.
Searching preprint servers
Preprints are now increasingly used in the dissemination of COVID-19 research.25 Two reviewers (T.H.T.W. and Y.L.) also searched unpublished studies from preprint servers (medRxiv and bioRxiv) using the same search strategy, which may help anticipate upcoming research findings. Two reviewers (T.H.T.W. and S.L.) identified eligible studies with the same selection criteria. We did not include preprints that were published in peer-reviewed journals as they would have been included in our main search. As these preprints have not been peer-reviewed, we only briefly summarized the findings given the concerns over validity.26
Meta-analysis
Based on a preliminary search, we found that the majority of the Mendelian randomization studies used the COVID-19 Host Genetics Initiative (HGI)27 to obtain genetic associations with COVID-19, although the version and hence the sample size used varied across studies. As such, we did not conduct a meta-analysis to pool estimates from Mendelian randomization studies of the same topic.
Results
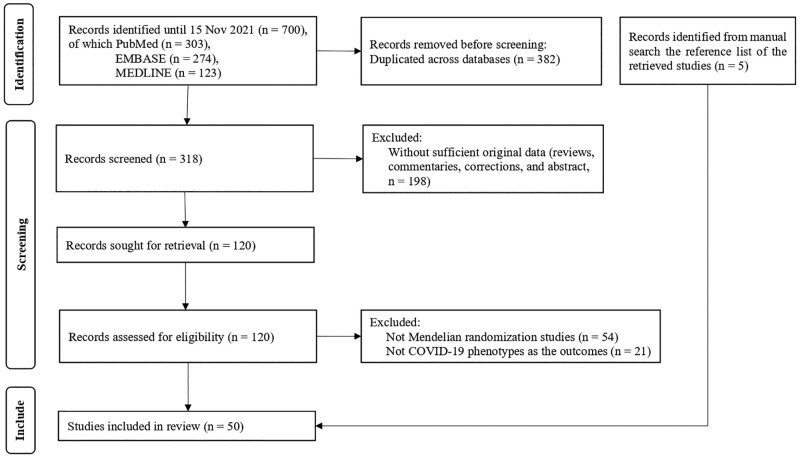
The search returned 700 studies, of which 318 remained after removing 382 duplicates. A further 198 studies were excluded on title and/or abstract as not meeting the eligibility criteria, leaving 120 studies. A further 75 studies were excluded after full text review because they were not Mendelian randomization studies or did not assess COVID-19 outcomes. Five additional studies were identified via the reference list. In total, 50 eligible Mendelian randomization studies were included in this review (Figure 1). The included studies were published between May 2020 and November 2021.
Figure 1.
Flow diagram of the study search and selection process
The characteristics of the included Mendelian randomization studies are presented in Table 1. Of the 50 studies, 46 used a two-sample study design. Forty-one studies used COVID-19 HGI to obtain the genetic associations of outcomes, whilst several studies used the GWAS conducted in the UK Biobank,28 cohorts from Italy and Spain,29 the US Million Veteran Program30 and China Wuhan Union Hospital. Thirty-seven studies focused on the role of socio-demographic factors, lifestyle attributes, anthropometrics, biomarkers and predisposition to disease;27,31–66 and 13 studies focused on druggable targets,9,67–78 amongst which one study also explored the effects of cardiometabolic exposures.77 The findings from these Mendelian randomization studies are summarized in Supplementary Table S1 (available as Supplementary data at IJE online). Eleven of these studies reported the assessment of all three IV assumptions. Most studies were rated as having high validity for IV1 relevance (41/50) and IV3 exclusion-restriction (44/50). Only 13 studies were rated as having high validity for IV2 independence given that these studies conducted analyses to assess the plausibility of IV2 (Supplementary Table S2, available as Supplementary data at IJE online).
Table 1.
The characteristics of the included Mendelian randomization studies of factors on COVID-19 outcomes [(A) severity, (B) hospitalization, (C) susceptibility]
| Study (author, year) | Study design | Category | Exposure (unit) | Outcomes (log odds) [(A) severity, (B) hospitalization, (C) susceptibility] | Source of outcome | Ethnicity of exposure | Ethnicity of outcome |
|---|---|---|---|---|---|---|---|
| Anisul M 2021 | Two-sample | Druggable targets | pQTL (SD) | A, B, C | COVID-19 HGI R4 | European | European |
| Au Yeung SL 2021 | Two-sample | Biomarkers, predisposition to diseases | 2-h glucose (mmol/L), fasting glucose (mmol/L), HbA1c (%), type 2 diabetes (log odds) | A, B, C | COVID-19 HGI R4 | European | Mixed* |
| Aung N 2020 | One-sample and two-sample | Biomarkers | BMI (4.7 kg/m2), BMI-adjusted waist circumference (13.4 cm), SBP (per 20.5 mmHg), fasting glucose (1.2 mmol/L), HbA1c (6.3 mmol/mol), LDL cholesterol (0.87 mmol/L), HDL cholesterol (0.38 mmol/L), triglycerides (1 mmol/L) | C | UKB and COVID-19 HGI R2 | European | European (UKB), mixed* (COVID-19 HGI) |
| Bovijn J 2020 | Two-sample | Biomarkers | IL-6 receptor inhibitor (0.1 SD lower CRP) | A, B, C | COVID-19 HGI R3 | European | Mixed* |
| Bovijn J 2021—Reply | Two-sample | Biomarkers | IL-6 receptor inhibitor (0.1 SD lower CRP) | A, B, C | COVID-19 HGI R3 and 4 | European | Mixed* |
| Butler-Laporte G 2021 | Two-sample | Druggable targets | Angiotensin-converting enzyme (SD decrease) | A, B, C | COVID-19 HGI R3 | Mixed* | European |
| Butler-Laporte G 2021 | Two-sample | Biomarkers | 25OHD (log nmol/L) | A, B, C | COVID-19 HGI R4 | European | European |
| Cai G 2021 | Two-sample | Druggable targets | Protein expression (SD) | A, C | UKB, Ellinghaus et al. GWAS | European | European |
| Clift AK 2021 | One-sample | Lifestyle attributes | Smoking initiation, smoking heaviness (SD) | A, B, C | UKB | European | European |
| Cui Z 2021 | Two-sample | Biomarkers | 25OHD (log nmol/L) | A, B, C | COVID-19 HGI R5 | European | European |
| Fadista J 2021 | Two-sample | Predisposition to disease | Idiopathic pulmonary fibrosis (log odds) | A, B, C | COVID-19 HGI R4 | European | Mixed* |
| Fan X 2021 | One-sample | Lifestyle attributes | Being a frequent drinker (log odds), weekly alcohol consumption | A, B, C | UKB | Mixed* | Mixed* |
| Freuer D 2021 | Two-sample | Biomarkers | BMI (SD), waist circumference (SD), trunk fat ratio (SD) | B, C | COVID-19 HGI R3 | European | Mixed* |
| Gaziano L 2021 | Two-sample | Druggable targets | eQTL (SD), pQTL (SD) | B | COVID-19 HGI R4 and MVP | Mixed | Mixed |
| Gordon DE 2020 | Two-sample | Druggable targets | Soluble interleukin 17 receptor A (SD) | B, C | COVID-19 HGI R3 | Mixed* | Mixed* |
| Hernández Cordero AI 2021 | Two-sample | Druggable targets | eQTL in lung and blood (SD), pQTL in blood (SD) | A, C | COVID-19 HGI R4 | Mixed* (eQTL), European (pQTL) | Mixed* |
| Hilser JR 2021 | One-sample and two-sample | Biomarkers | HDL-cholesterol (mmol/L) | A, B, C | UKB and Ellinghaus et al. GWAS | European | Mixed* |
| Hui LL 2021 | Two-sample | Biomarkers | Vitamin C | A, B, C | COVID-19 HGI R5 | European | European |
| Larsson SC 2021 | Two-sample | Druggable targets | IL-6 receptor inhibitor (0.1 SD lower CRP) | A, B, C | COVID-19 HGI R4 and Ellinghaus et al. GWAS | European | Mixed* |
| Larsson SC 2021 | Two-sample | Predisposition to diseases | Allergic disease (log odds) | B, C | COVID-19 HGI R4 | European | European |
| Leong A 2021 | Two-sample | Biomarkers, predisposition to diseases |
|
A, C | COVID-19 HGI R4 | European or mixed* | European |
| Li GHY 2021 | Two-sample | Socio-demographic factors | Education attainment (year), intelligence (SD) | A, B, C | COVID-19 HGI R5 | European | European |
| Li M 2021 | Two-sample | Biomarkers | Cytokines (SD) | C | COVID-19 HGI R5 | European | European |
| Li S 2021 | Two-sample | Biomarkers, lifestyle attributes | BMI (SD); lifetime smoking index (SD), accelerometer-measured physical activity (SD), alcohol consumption per week (SD) | A, B | COVID-19 HGI R4 | European | Mixed* |
| Li X 2021 | Two-sample | Biomarkers | 25OHD (SD) | C | UKB | European | European |
| Liu D 2021 | Two-sample | Druggable targets | eQTL in blood and lung (SD) | A, B, C | COVID-19 HGI R3 | Mixed* | Mixed* |
| Liu D 2021 | Two-sample | Biomarkers | 25OHD (log nmol/L) | A, B, C | COVID-19 HGI R3 and Ellinghaus et al. GWAS | European | Mixed* |
| Liu N 2021 | Two-sample | Predisposition to disease | ADHD, bipolar disorder, depressive disorder, schizophrenia (log odds) | A, B | COVID-19 HGI R5 | European | European |
| Lorincz-Comi N 2021 | Two-sample | Biomarkers, predisposition to disease |
|
B | COVID-19 HGI R3 and 4 | European | European and mixed* |
| Luykx JJ 2021 | Two-sample | Predisposition to disease | Alzheimer's dementia, bipolar disorder, major depressive disorder, schizophrenia, combined bipolar disorder and schizophrenia (log odds) | A, B, C | COVID-19 HGI R4 and 5 | European | Mixed* |
| Ong JS 2021 | Two-sample | Predisposition to diseases | Gastro-esophageal reflux disease (log odds) | A, B, C | COVID-19 HGI R5 | European | European |
| Pairo-Castineira E 2021 | Two-sample | Druggable targets | eQTL of IFNAR2, IFNAR1, IL6R, JAK1, CTSL, IFNGR2, CSF3 (SD) | A | GenOMICC, COVID-19 HGI R2, 23andMe, UKB | European | European |
| Patchen BK 2021 | Two-sample | Biomarkers | 25OHD (SD of log), vitamin D deficiency (log odds), vitamin D insufficiency (log odds) | A, B, C | COVID-19 HGI R4 | European | Mixed* |
| Ponsford MJ 2020 | Two-sample | Biomarkers, lifestyle attributes, predisposition to diseases | BMI (SD), SBP (SD), LDL cholesterol (mmol/L); lifetime smoking index (SD); type 2 diabetes (log odds) | B, C | Ellinghaus et al. GWAS and COVID-19 HGI R3 | European | European and mixed* |
| Qiu S 2021 | Two-sample | Predisposition to diseases | Alzheimer’s disease (log odds) | C | COVID-19 HGI R5 | European | European |
| Qiu S 2021 | Two-sample | Biomarkers | Blood metabolites | C | COVID-19 HGI R4 | European | European |
| Rao S 2021 | Two-sample | Lifestyle attributes |
|
A, B, C | COVID-19 HGI R5 | European | European |
| Richardson TG 2021 | Two-sample | Biomarkers, lifestyle attributes, druggable targets | BMI, waist–hip ratio adjusted for BMI, childhood adiposity age 10, SBP, DBP, HDL cholesterol, LDL cholesterol, triglycerides, ApoA1, ApoB; liability to lifetime smoking; pQTL (SD) | A | Ellinghaus et al. GWAS | European | European |
| Rosoff DB 2021 | Two-sample | Lifestyle attributes | Lifetime smoking index (SD), cannabis use, cannabis use disorder (log odds), drinks per week, alcohol use disorder (log odds) | A, B, C | COVID-19 HGI R5 | European | European |
| Sun Y 2021 | Two-sample | Biomarkers | Basophil, basophil % of white cells, eosinophil, eosinophil % of white cells, lymphocyte, lymphocyte % of white cells, monocyte, monocyte % of white cells, myeloid white cell count, neutrophil, neutrophil % of white cells, WBC | A, B, C | COVID-19 HGI R4 and R5 | European | European |
| The COVID-19 HGI 2021 | Two-sample | Biomarkers, lifestyle attributes, predisposition to diseases |
|
A, B, C | COVID-19 HGI R5 | European | European |
| Wang K 2021 | Two-sample | Biomarkers | Haematological traits (basophil, eosinophil, hematocrit, haemoglobin, lymphocyte, MCHC, MCH, MCV, monocyte, neutrophil, platelet, RBC, RCDW, WBC), liver function markers (ALT, ALP, AST, GGT, albumin, total bilirubin, direct bilirubin, total protein), renal function marker (serum creatinine), SD | B | COVID-19 HGI R4 | European | European |
| Wang Q 2021 | One-sample | Biomarkers | Leukocyte telomere length, SD shorter | C | UKB | European | European |
| Yoshikawa M 2021 | Two-sample | Societal factors | Educational attainment (SD, 4.2-year) | A | COVID-19 HGI R5 | European | European |
| Zhang K 2021 | Two-sample | Biomarkers, predisposition to diseases |
|
A, C | COVID-19 HGI R4, UKB, Ellinghaus et al. GWAS | European and mixed* | European |
| Zhang X 2020 | One-sample | Lifestyle attributes | Self-reported moderate-to-vigorous physical activity, acceleration vector magnitude physical activity | C | UKB | European | European |
| Zhou S 2021 | Two-sample | Druggable targets | pQTL (SD) | A, B, C | COVID-19 HGI R4 | European or mixed* | European |
| Zhou Y 2021 | Two-sample | Biomarkers | Coagulation factors (SD) | A, C | COVID-19 HGI R5, and Ellinghaus et al. GWAS | European | Mixed* European |
| Zhu H 2021 | One-sample and two-sample | Biomarkers | Laboratory assessment | A | China Wuhan Union Hospital | East Asian | East Asian |
| Zuber V 2021 | Two-sample | Predisposition to diseases | Stroke subtypes (ischaemic, cardioembolic, large artery, small artery, log odds) | A | COVID-19 HGI R5 | European | European |
ADHD, attention-deficit hyperactivity disorder; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; AST, aspartate aminotransferase; Baso, basophil count; CRP, C-reactive protein; DBil, direct bilirubin; DBP, diastolic blood pressure; Eosino, eosinophil count; eQTL, gene expression; GGT, γ-glutamyl transferase; Hb, haemoglobin; HbA1c, glycated haemoglobin; Ht, hematocrit; Lym, lymphocyte count; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; mixed*, mixed (majority of European ancestry); Mono, monocyte count; MVP, Million Veteran Program; Neutro, neutrophil count; Plt, platelet count; PP, pulse pressure; pQTL, protein expression; RBC, red blood cell count; RDW, red cell distribution width; SBP, systolic blood pressure; sCr, serum creatinine; TBil, total bilirubin; The COVID-19 HGI, The COVID-19 Host Genetics Initiative; TP, total protein; UKB, UK Biobank; WBC, white blood cell count.
The unit of the exposure is per unit increases, unless specified.
Socio-demographic factors
Two Mendelian randomization studies assessed the role of educational attainment in COVID-19, which showed that higher educational attainment was associated with reduced risk of COVID-19 severity and hospitalization but not COVID-19 susceptibility.43,61 One of these studies also showed that higher intelligence was associated with reduced risk of COVID-19 hospitalization (Supplementary Table S1, available as Supplementary data at IJE online).43
Lifestyle attributes
Six Mendelian randomization studies consistently demonstrated strong associations of smoking traits, including smoking initiation, smoking heaviness and lifetime smoking index (which combined smoking initiation, duration, heaviness and cessation), in the risk of COVID-19 severity, hospitalization and mortality.27,34,45,53,56,57 Four Mendelian randomization studies explored the role of alcohol drinking in COVID-19 risk,37,45,56,57 of which two studies found null association.45,56 However, a study in UK Biobank provided suggestive evidence that alcohol drinking traits (being a frequent drinker and weekly alcohol consumption) were associated with higher risk of COVID-19 severity and mortality amongst participants who were obese.37 In addition, a study found that alcohol use disorder and cannabis use were nominally associated with higher risk of COVID-19 susceptibility.57 The role of physical activity in the risk of COVID-19 was inconsistent. Higher accelerometer-measured physical activity was associated with lower risk of COVID-19 severity in COVID-19 HGI.45 However, null associations of self-reported moderate-to-vigorous physical activity and acceleration vector magnitude physical activity in COVID-19 susceptibility were reported using UK Biobank.63 There was no evidence supporting the role of sleep duration in COVID-19 risk (Supplementary Table S1, available as Supplementary data at IJE online).27
Anthropometrics and biomarkers
Mendelian randomization studies have explored the role of a wide range of anthropometrics and biomarkers in COVID-19 risk, including anthropometrics, blood pressure, coagulation factors, cytokines, inflammatory markers, glycaemic traits, haematological traits, lipids, liver functions, renal functions and vitamins.27,31–33,35,38–40,42,44–47,49,52,53,55,58–60,62,64,65 Consistent evidence suggested that obesity [higher body mass index (BMI) and trunk fat ratio] was associated with higher COVID-19 risk27,32,38,42,45,53 and one study also showed that increase in height was associated with higher risk of COVID-19 susceptibility.27 Consistent evidence showed systolic and diastolic blood pressure to have no role in COVID-19 risk,27,32,42,49,53 although one study found that higher pulse pressure was associated with higher risk of COVID-19 hospitalization in people of mixed ancestry but not of European ancestry.49 Coagulation factors [higher von Willebrand factor (VWF) and lower disintegrin and metalloprotease with a thrombospondin type 1 motif member 13 (ADAMTS13)]64 and lower cytokine [macrophage inflammatory protein 1 b (MIP1b)]44 were associated with higher risk of COVID-19. The inflammatory marker C-reactive protein (CRP) was nominally associated with higher risk of COVID-19 hospitalization and susceptibility in one study42 but not another study.27 There is suggestive evidence showed that haematological traits (higher basophil count, basophil percentage of white cells, lymphocyte count, myeloid white cell count, neutrophil count, red blood cell count, white blood cell count and lower mean corpuscular haemoglobin) were associated with reduced risk of COVID-19 severity and hospitalization.27,58,59 Evidence was inconsistent for lipids with risk of COVID-19,27,32,39,42,53,55,62,65 where some suggested that higher low-density lipoprotein (LDL)-cholesterol,32,42 small very LDL cholesterol (VLDL) particles,55 apolipoprotein B,62 total cholesterol and total cholesterol in medium VLDL55,62 and triglycerides27,62 were associated with higher risk of COVID-19 whereas others suggested null association.27,32,39,42,53,62,65 Suggestive evidence showed that lower albumin and higher direct bilirubin were associated with higher risk of COVID-19 hospitalization59 but null associations for other liver function markers [alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), indirect bilirubin, total bilirubin and total protein].59,65 No evidence was found for the role of leukocyte telomere length (a determinant of longevity) in COVID-19.60 Consistent evidence showed that glycaemic traits (HbA1c, fasting insulin, fasting glucose and 2-h glucose),31,32,42 high density lipoprotein (HDL) cholesterol and apolipoprotein A,27,32,39,42,62,65 renal traits [creatinine-based estimated glomerular filtration rate (eGFR) and serum creatinine]27,42,59 and vitamins C and D27,33,35,40,46,47,52 were not associated with risk of COVID-19 (Supplementary Table S1, available as Supplementary data at IJE online).
Predisposition to disease
Thirteen Mendelian randomization studies explored whether predisposition to diseases (including diseases of the circulatory, digestive, nervous, respiratory, genitourinary, musculoskeletal systems and connective tissue, metabolic diseases and mental disorders) increased COVID-19 risk.27,31,36,41,42,48–51,53,54,62,66 Positive association of predisposition to ischaemic stroke in COVID-19 risk was found in one study27 but not in another study.66 There was no evidence supporting the role of other circulatory diseases (coronary artery disease, heart failure, any stroke, cardioembolic stroke, large artery stroke and small vessel stroke) in COVID-10 risk.27,42,66 One study found that predisposition to combined bipolar disorder and schizophrenia was associated with higher risk of COVID-19 (based on self-reported symptoms),50 although other studies suggested null association of predisposition to bipolar disorder and predisposition to schizophrenia and COVID-19 risk.27,48 One study suggested a positive association between predisposition to attention-deficit and hyperactivity disorder (ADHD),48 chronic kidney disease42 and idiopathic pulmonary fibrosis36 with COVID-19 risk but this was not confirmed in another study.27 There was suggestive evidence that predisposition to gastro-esophageal reflux disease was associated with higher risk of COVID-19 hospitalization51 and an inverse association of predisposition to allergic disease with COVID-19 susceptibility.41 There was no strong evidence supporting predisposition to metabolic diseases (diabetes, type 1 diabetes, type 2 diabetes),27,31,42,49,53 mental disorders (autism spectrum disorder, depression, insomnia symptoms),27,48 lupus, rheumatoid arthritis, asthma, risk tolerance and diseases of the nervous system (Alzheimer’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and Parkinson’s disease)27,54 in COVID-19 risk (Supplementary Table S1, available as Supplementary data at IJE online).
Druggable targets
There were 13 cis-Mendelian randomization studies that explored potential druggable targets on COVID-19 risk,9,67–78 of which 4 studies performed Bayesian colocalization analysis to assess whether the putative association was confounded by LD.67,71,73,78 Both cis-Mendelian randomization studies and colocalization analyses (PPH4 > 0.80) suggested that higher circulating protein histo-blood group ABO system transferase (ABO)67,73,78 and lower circulating 2′-5′ oligoadenylate synthetase 1 (OAS1)73,78 were associated with higher risk of COVID-19, regardless of severity. Inhibition of IL6 receptor (proxied by lower CRP)9,67,68,74 and its common signal-transducing receptor subunit, glycoprotein 130 (gp130),77 higher IL10 receptor beta subunit (IL10RB)78 and higher IL17 receptor A (IL17RA)72 were associated with lower risk of COVID-19. There was no evidence supporting the role of protein angiotensin-converting enzyme (ACE), ACE2 and liver/lymph node-specific intercellular adhesion molecule-3-grabbing non-integrin (L-SIGN) in COVID-19 risk,69,70 although positive association of dendritic cell (DC)-SIGN (also known as cluster of differentiation, CD209) in COVID-19 susceptibility and severity was found.67,70 Higher protein expression of family with sequence similarity 3, member D (FAM3D), intercellular adhesion molecule 1 (ICAM1) and sulfhydryl oxidase 2 (QSOX2) and lower protein expression of e-selectin (SELE) were associated with higher COVID-19 risk.67 Extensive tissue-specific gene expression of druggable targets has also been explored71,73,75,76,78 in which gene expression of OAS1,78 ACE2,71 IL10RB71 and interferon (IFN) alpha receptor 2 (IFNAR2)71,73,75,76 across several tissues was related to COVID-19 risk (Supplementary Table S1, available as Supplementary data at IJE online).
Works to come
There were 24 studies identified from the preprint servers that explored a wide range of socio-demographic factors, lifestyle attributes, anthropometrics, biomarkers, predisposition to diseases and druggable targets79–102 (Supplementary Table S3, available as Supplementary data at IJE online). Some studies corroborated previous findings, such as the detrimental role of smoking and obesity.79,100 There is one Mendelian randomization study in an East Asian population that also showed a detrimental effect of obesity in severe COVID-19.88 Others identified new determinants including increased testosterone,96 and reduced carnitine and acetyl-carnitine84 was associated with higher risk of COVID-19 severity and hospitalization, respectively. The updated GWAS of COVID-19 HGI included assessment of a wide range of exposures in COVID-19 risk and highlighted increased liability of type 2 diabetes associated with higher risk of COVID-19 susceptibility and hospitalization, although the association was abolished after adjusting for BMI.82 Another study that was published in a peer-reviewed journal showed accelerated ageing was associated with higher COVID-19 risk, although the publication was not indexed in PubMed.102 However, there were methodological concerns in some studies, such as interpreting liability as the effect of disease diagnosis,87 the lack of comprehensive assessment of IV assumptions96 and insufficient details for replication.100
Discussion
To the best of our knowledge, this is the first systematic review focusing on evidence concerning factors contributing to COVID-19 from Mendelian randomization studies. This systematic review summarizes modifiable risk factors for intervention (e.g. obesity and smoking), as well as proteomic signatures (e.g. OAS1 and IL6) that could help identify relevant drugs for treatment of COVID-19.
Possible factors contributing to increased risk of COVID-19
Socio-demographic factors and lifestyle attributes
Our review found two Mendelian randomization investigations of the role of socio-demographic factors (education attainment and intelligence) in COVID-19. This is consistent with previous observational studies103 and highlights the issue of inequalities in the COVID-19 pandemic. Possible pathways may include unhealthy lifestyles associated with lower socio-economic position such as smoking, and possibly physical inactivity and alcohol drinking, where our review suggested a possible link with increasing risk of COVID-19 severity and hospitalization,27,34,37,45,53,56,57 and are consistent with conventional observational studies.104,105 A popular mechanism related to increased risk of COVID-19 concerning smoking was an increased expression of ACE2, a receptor for SARS-CoV-2 in the airway epithelium.106 This is also consistent with the cis-Mendelian randomization study that showed high gene expression of ACE2 at the Brodmann area 9 (BA9) at the brain frontal cortex was associated with higher risk of COVID-19 hospitalization.71 This evidence supports the recent call from the World Health Organization (WHO) regarding the importance of tobacco cessation in reducing severe COVID-19.107 Whilst obesity and hence subsequent pro-inflammatory response may explain why people who were physically inactive have higher risk of COVID-19,108 whether alcohol use is relevant to COVID-19 remains unclear where only a possible positive association amongst participants who were obese was observed.37
Anthropometrics and biomarkers
Our review highlights that CRP, MIP1b, VWF, ADAMTS13, height, obesity and some haematological traits have a role in COVID-19 risk. Age is a key host factor determining disease severity and progression,109 and age-related immunosenescence and age-related decrease in physiological reserve can contribute to vulnerability to COVID-19 in older adults.110 Furthermore, ageing may induce a more pro-inflammatory environment (i.e. inflammaging);111 the same phenomenon may also explain the potential harmful effects of obesity in COVID-19 risk,112 alongside downstream factors such as reduced ADAMTS13 activity and increased VWF.113 The finding that basophil count was inversely associated with the hospitalization and severity of COVID-1958 is consistent with a small immune-monitoring study that observed a positive correlation between basophil count and the level of IgG antibody against SARS-CoV-2.114 This may then enhance the adaptive immune response to SARS-CoV-2 infection.115
Predisposition to diseases
Our review highlights the role of genetic predisposition to ischaemic stroke, idiopathic pulmonary fibrosis, ADHD and combined bipolar disorder and schizophrenia in COVID-19 risk. These findings are consistent with some but not all studies.116 Possible biological mechanisms included differing profile of individuals with bipolar disorders and schizophrenia117 and their genetic variability across human leukocyte antigens that could contribute to differences in immune responses to SARS-CoV-2 infections.118 Alternatively, relevant societal factors may include socio-economic deprivation, social isolation119 and barriers to accessing healthcare services for COVID-19 in individuals with mental health disorders and neurodevelopmental disorders.120 Possible mechanisms for the link of ischaemic stroke and COVID-19 risk may again be due to the pro-inflammatory environment and increased coagulation factors.121 Although genetic predisposition to chronic kidney disease was related to higher risk of hospitalized COVID-19, renal traits (creatinine-based eGFR and creatinine) were not related to COVID-19 and the underlying mechanisms linking kidney function and COVID-19 have yet to be established.122 One limitation with Mendelian randomization studies using binary disease exposures is the interpretation, as the effect may represent the consequence of liability to disease instead of having the disease.123
Protein targets for potential drug reposition
The cis-Mendelian randomization studies integrated with colocalization analyses provided strong genetic evidence for several druggable targets, which can help prioritize possible targets to be tested in an RCT.124 For example, some cis-Mendelian randomization studies suggested that IL6-related pathways (e.g. IL6 receptor and soluble gp130) are related to the risk of COVID-19.9,77 These are generally consistent with suggestive evidence from RCTs that evaluated the efficacy of IL-6 antagonists (tocilizumab, sarilumab and siltuximab) for COVID-19 severity.125 Whether relevant medications such as soluble gp130Fc (Olamkicept) can be repurposed to reduce the severity of COVID-19 should be explored.126,127 Strong genetic evidence suggested a positive association of protein ABO with the risk of COVID-19.73,78 Nevertheless, ABO is highly pleiotropic and has been linked to various phenotypes,78 suggesting that these cis-Mendelian randomization studies may still suffer from horizontal pleiotropy and should be interpreted with caution. OAS1 variants (rs10735079, rs6489867 and rs4767027, in high LD r2 > 0.80) were associated with susceptibility to SARS-CoV-1 and SARS-CoV-2 in populations of different ancestries.76,128,129 The protective effects of protein OAS1 in COVID-19 risk may be due to increased levels of p46 isoform,73,78 which was later verified in a human genetic analysis.130 ACE2 is a functional receptor for SARS-CoV-1 and SARS-CoV-2.131,132 Human recombinant soluble ACE2 (hrsACE2) reduced SARS-CoV-2 viral load in infected Vero-E6 cells133 and its role as a therapeutic target for COVID-19 is currently being explored in a phase II clinical trial (NCT04335136).134 DC-SIGN was considered as a potential receptor for SARS-CoV-2.135 However, the testing of hydroxychloroquine (a modulator of the dendritic cell)136 has been halted due to increased mortality risk reported in RCTs.137
Strengths and limitations
This is the first systematic review on COVID-19-related Mendelian randomization studies to have summarized potentially more credible evidence concerning causes of COVID-19 risk. Nevertheless, there are some limitations in this review. First, Mendelian randomization studies rely on IV assumptions (e.g. relevance, independence and exclusion-restriction),16 although most studies have addressed/acknowledged these assumptions when conducting respective Mendelian randomization studies (Supplementary Table S2, available as Supplementary data at IJE online). It is not generally acknowledged that Mendelian randomization studies, like all observational studies, are open to selection bias from inevitably only including survivors of the genetic predictors of the exposure and competing risk of COVID-19.138 As a result, Mendelian randomization studies may not fully identify effects of harmful exposures on COVID-19 because people who have died from a harmful exposure or from a competing risk of COVID-19 are not available for recruitment. Second, Mendelian randomization studies based on an earlier release of COVID-19 HGI databases (with small number of cases) and the cis-Mendelian randomization studies (with single genetic variant) have relatively lower statistical power and hence null findings from these studies should be interpreted with caution. Third, most (82%) studies used COVID-19 HGI to obtain the variant–outcome associations, given it is the largest available GWAS available, implying that meta-analysis of studies addressing the same question was technically impossible to increase statistical power. Fourth, most studies pertained to populations of predominantly European ancestry and might not be generalizable to other populations, although causes are usually consistent but not always relevant. Genetic data from diverse ancestral populations can better evaluate whether determinants of COVID-19 risk and its severity vary across ethnicities or possibly identify causes of COVID-19 not evident in European populations.139 Fifth, we only included published studies in this review, given the varying quality of preprints, although such issues also applied to published studies (Supplementary Table S2, available as Supplementary data at IJE online). However, given that preprints may foreshadow upcoming publications, we summarized the findings from the preprints briefly although interpretation of these findings should be with caution (Supplementary Table S3, available as Supplementary data at IJE online). Sixth, the factors chosen for investigation may represent those that are best understood rather than those most relevant to equitably protecting the population against the effects of COVID-19. For example, the greater vulnerability of disadvantaged populations and of men to COVID-19 has not yet been investigated using Mendelian randomization. Lastly, whether these factors contribute to milder forms of COVID-19, which comprises most cases, requires additional investigation in larger studies. This is of particular relevance given a recent prospective study indicated that young, home-isolated adults with mild to moderate COVID-19 suffer from long-term complications from COVID-19.140
This systematic review highlighted increases in smoking, obesity and inflammatory factors as causes of increased risk of COVID-19, whilst other factors that were thought to be relevant to COVID-19, such as vitamin D, were unlikely causal in increased susceptibility to COVID-19. Our study also summarized possible druggable targets that are related to COVID-19 risk, thus providing additional insights regarding the prioritization and repositioning of medications to mitigate the risk of COVID-19. This systematic review reveals factors contributing to COVID-19 risk that would guide subsequent policy interventions to mitigate the global COVID-19 pandemic.
Ethics approval
Not applicable. All the work was developed using published data.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
S.L.A.Y. and S.L. contributed to the study conception and design. Y.L., T.H.T.W. and S.L. contributed to the literature search, study selection and data extraction. S.L., Y.L. and S.L.A.Y. evaluated the quality assessment of the included studies. S.L. and S.L.A.Y. drafted the manuscript with critical feedback and revisions from C.M.S., Y.L. and T.H.T.W. All authors approved the final version of the manuscript. S.L. is the guarantor. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
None.
Conflict of interest
None declared.
Supplementary Material
Contributor Information
Shan Luo, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Ying Liang, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Tommy Hon Ting Wong, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Catherine Mary Schooling, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; Environmental, Occupational, and Geospatial Health Sciences, School of Public Health and Health Policy, City University of New York, New York, USA.
Shiu Lun Au Yeung, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
References
- 1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (1 December 2021, date last accessed).
- 2. Jung RG, Di Santo P, Clifford C et al. Methodological quality of COVID-19 clinical research. Nat Commun 2021;12:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehra MR, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis [Retraction of 10.1016/S0140-6736(20)31180-6, 2020]. Lancet 2020;395:1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffith GJ, Morris TT, Tudball MJ et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 2020;11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rochwerg B, Agarwal A, Siemieniuk RA et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 7. Stroehlein JK, Wallqvist J, Iannizzi C et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev 2021;5:CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schooling CM, Freeman G, Cowling BJ. Mendelian randomization and estimation of treatment efficacy for chronic diseases. Am J Epidemiol 2013;177:1128–33. [DOI] [PubMed] [Google Scholar]
- 9. Bovijn J, Lindgren CM, Holmes MV. Genetic variants mimicking therapeutic inhibition of IL-6 receptor signaling and risk of COVID-19. Lancet Rheumatol 2020;2:e658–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021;385:187–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Razai MS, Chaudhry UAR, Doerholt K, Bauld L, Majeed A. Covid-19 vaccination hesitancy. BMJ 2021;373:n1138. [DOI] [PubMed] [Google Scholar]
- 12. Burki T. Global COVID-19 vaccine inequity. Lancet Infect Dis 2021;21:922–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skrivankova VW, Richmond RC, Woolf BAR et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 2021;326:1614–21. [DOI] [PubMed] [Google Scholar]
- 15. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 2017;26:2333–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burgess S, Thompson SG; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 2011;40:755–64. [DOI] [PubMed] [Google Scholar]
- 18. Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamat MA, Blackshaw JA, Young R et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019;35:4851–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giambartolomei C, Vukcevic D, Schadt EE et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet 2014;10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labrecque J, Swanson SA. Understanding the assumptions underlying instrumental variable analyses: a brief review of falsification strategies and related tools. Curr Epidemiol Rep 2018;5:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 2017;46:1985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraser N, Brierley L, Dey G et al. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biol 2021;19:e3000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopalakrishna G. Preprint advocates must also fight for research integrity. Nature 2021. Sep 13; doi:10.1038/d41586-021-02481-y. [DOI] [PubMed] [Google Scholar]
- 27. Niemi MEK, Karjalainen J, Liao RG et al. ; COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021;600:472–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sudlow C, Gallacher J, Allen N et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellinghaus D, Degenhardt F, Bujanda L et al. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med 2020;383:1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaziano JM, Concato J, Brophy M et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214–23. [DOI] [PubMed] [Google Scholar]
- 31. Au Yeung SL, Zhao JV, Schooling CM. Evaluation of glycemic traits in susceptibility to COVID-19 risk: a Mendelian randomization study. BMC Med 2021;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aung N, Khanji MY, Munroe PB, Petersen SE. Causal inference for genetic obesity, cardiometabolic profile and COVID-19 susceptibility: a Mendelian randomization study. Front Genet 2020;11:586308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butler-Laporte G, Nakanishi T, Mooser V et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: a Mendelian randomization study. PLoS Med 2021;18:e1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clift AK, von Ende A, Tan PS et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax 2022;77:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui Z, Tian Y. Using genetic variants to evaluate the causal effect of serum vitamin D concentration on COVID-19 susceptibility, severity and hospitalization traits: a Mendelian randomization study. J Transl Med 2021;19:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fadista J, Kraven LM, Karjalainen J et al. Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity. EBioMedicine 2021;65:103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan X, Liu Z, Poulsen KL et al. Alcohol consumption is associated with poor prognosis in obese patients with COVID-19: a Mendelian randomization study using UK Biobank. Nutrients 2021;13:1592. 10.3390/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: a two-sample multivariable Mendelian randomization study. Metabolism 2021;118:154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hilser JR, Han Y, Biswas S et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J Lipid Res 2021;62:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hui LL, Nelson EAS, Lin SL, Zhao JV. The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: a Mendelian randomisation study. Eur J Clin Nutr 2022;76:588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsson SC, Gill D. Genetic predisposition to allergic diseases is inversely associated with risk of COVID-19. Allergy 2021;76:1911–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leong A, Cole JB, Brenner LN, Meigs JB, Florez JC, Mercader JM. Cardiometabolic risk factors for COVID-19 susceptibility and severity: a Mendelian randomization analysis. PLoS Med 2021;18:e1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li GHY, Lam SKK, Wong ICK, Chu JKP, Cheung CL. Education attainment, intelligence and Covid-19: a Mendelian randomization study. JCM 2021;10:4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li M, Yeung CHC, Schooling CM. Circulating cytokines and coronavirus disease: a bi-directional Mendelian randomization study. Front Genet 2021;12:680646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li S, Hua X. Modifiable lifestyle factors and severe COVID-19 risk: a Mendelian randomisation study. BMC Med Genomics 2021;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, van Geffen J, van Weele M et al. An observational and Mendelian randomisation study on vitamin D and COVID-19 risk in UK Biobank. Sci Rep 2021;11:18262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu D, Tian QY, Zhang J et al. Association between 25 Hydroxyvitamin D concentrations and the risk of COVID-19: a Mendelian randomization study. Biomed Environ Sci 2021;34:750–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu N, Tan JS, Liu L, Wang Y, Hua L, Qian Q. Genetic predisposition between COVID-19 and four mental illnesses: a bidirectional, two-sample Mendelian randomization study. Front Psychiatry 2021;12:746276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lorincz-Comi N, Zhu X. Cardiometabolic risks of SARS-CoV-2 hospitalization using Mendelian randomization. Sci Rep 2021;11:7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luykx JJ, Lin BD. Are psychiatric disorders risk factors for COVID-19 susceptibility and severity? A two-sample, bidirectional, univariable, and multivariable Mendelian randomization study. Transl Psychiatry 2021;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ong JS, Gharahkhani P, Vaughan TL, Whiteman D, Kendall BJ, MacGregor S. Assessing the genetic relationship between gastro-esophageal reflux disease and risk of COVID-19 infection. Hum Mol Genet 2022;31:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patchen BK, Clark AG, Gaddis N, Hancock DB, Cassano PA. Genetically predicted serum vitamin D and COVID-19: a Mendelian randomisation study. BMJ Nutr Prev Health 2021;4:213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ponsford MJ, Gkatzionis A, Walker VM et al. Cardiometabolic traits, sepsis, and severe COVID-19: a Mendelian randomization investigation. Circulation 2020;142:1791–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiu S, Hu Y, Cheng L. A genome-wide cross-trait analysis highlights the shared genetic structure between COVID-19 and Alzheimer's disease. J Infect 2022;84:e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qiu S, Wang D, Zhang Y, Hu Y. Mendelian randomization reveals potential causal candidates for COVID-19 in 123 blood metabolites. J Infect 2022;84:248–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rao S, Baranova A, Cao H, Chen J, Zhang X, Zhang F. Genetic mechanisms of COVID-19 and its association with smoking and alcohol consumption. Brief Bioinform 2021;22:bbab284. [DOI] [PubMed] [Google Scholar]
- 57. Rosoff DB, Yoo J, Lohoff FW. Smoking is significantly associated with increased risk of COVID-19 and other respiratory infections. Commun Biol 2021;4:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun Y, Zhou J, Ye K. White blood cells and severe covid-19: a Mendelian randomization study. JPM 2021;11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang K, Qu M, Ding L et al. Liver and kidney function biomarkers, blood cell traits and risk of severe COVID-19: a Mendelian randomization study. Front Genet 2021;12:647303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Q, Codd V, Raisi-Estabragh Z et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: a cohort study in UK Biobank. EBioMedicine 2021;70:103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yoshikawa M, Asaba K. Educational attainment decreases the risk of COVID-19 severity in the European population: a two-sample Mendelian randomization study. Front Public Health 2021;9:673451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang K, Dong SS, Guo Y et al. Causal associations between blood lipids and COVID-19 risk: a two-sample Mendelian randomization study. Arterioscler Thromb Vasc Biol 2021;41:2802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang X, Li X, Sun Z et al. Physical activity and COVID-19: an observational and Mendelian randomisation study. J Glob Health 2020;10:020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou Y, Qian X, Liu Z et al. Coagulation factors and the incidence of COVID-19 severity: Mendelian randomization analyses and supporting evidence. Signal Transduct Target Ther 2021;6:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhu H, Zheng F, Li L et al. A Chinese host genetic study discovered IFNs and causality of laboratory traits on COVID-19 severity. iScience 2021;24:103186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zuber V, Cameron A, Myserlis EP et al. Leveraging genetic data to elucidate the relationship between COVID-19 and ischemic stroke. J Am Heart Assoc 2021;10:e022433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anisul M, Shilts J, Schwartzentruber J et al. A proteome-wide genetic investigation identifies several SARS-CoV-2-exploited host targets of clinical relevance. Elife 2021;10:e69719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bovijn J, Lindgren CM, Holmes MV. Genetic IL-6R variants and therapeutic inhibition of IL-6 receptor signalling in COVID-19—authors' reply. Lancet Rheumatol 2021;3:e97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Butler-Laporte G, Nakanishi T, Mooser V et al. The effect of angiotensin-converting enzyme levels on COVID-19 susceptibility and severity: a Mendelian randomization study. Int J Epidemiol 2021;50:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cai G, Du M, Bossé Y et al. SARS-CoV-2 impairs dendritic cells and regulates DC-SIGN gene expression in tissues. IJMS 2021;22:9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gaziano L, Giambartolomei C, Pereira AC et al. ; VA Million Veteran Program COVID-19 Science Initiative. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med 2021;27:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gordon DE, Hiatt J, Bouhaddou M et al. ; QCRG Structural Biology Consortium. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 2020;370:eabe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hernández Cordero AI, Li X, Milne S et al. Multi-omics highlights ABO plasma protein as a causal risk factor for COVID-19. Hum Genet 2021;140:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Larsson SC, Burgess S, Gill D. Genetically proxied interleukin-6 receptor inhibition: opposing associations with COVID-19 and pneumonia. Eur Respir J 2021;57:2003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu D, Yang J, Feng B, Lu W, Zhao C, Li L. Mendelian randomization analysis identified genes pleiotropically associated with the risk and prognosis of COVID-19. J Infect 2021;82:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pairo-Castineira E, Clohisey S, Klaric L et al. ; Gen-COVID Investigators. Genetic mechanisms of critical illness in COVID-19. Nature 2021;591:92–98. [DOI] [PubMed] [Google Scholar]
- 77. Richardson TG, Fang S, Mitchell RE, Holmes MV, Davey Smith G. Evaluating the effects of cardiometabolic exposures on circulating proteins which may contribute to severe SARS-CoV-2. EBioMedicine 2021;64:103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou S, Butler-Laporte G, Nakanishi T et al. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nat Med 2021;27:659–67. [DOI] [PubMed] [Google Scholar]
- 79. Cabrera-Mendoza B, Wendt FR, Pathak GA et al. The effect of obesity-related traits on COVID-19 severe respiratory symptoms is mediated by socioeconomic status: a multivariable Mendelian randomization study. medRxiv; doi:10.1101/2021.06.08.21258587, 12 June 2021, preprint: not peer reviewed. [Google Scholar]
- 80. Degenhardt F, Ellinghaus D, Juzenas S et al. New susceptibility loci for severe COVID-19 by detailed GWAS analysis in European populations. medRxiv; doi:10.1101/2021.07.21.21260624, 21 July 2021, preprint: not peer reviewed. [Google Scholar]
- 81. Huang W, Xiao J, Ji J, Chen L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. Elife 2021;10:e73873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. The COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19: an update. medRxiv; doi:10.1101/2021.11.08.21265944, 11 November 2021, preprint: not peer reviewed. [Google Scholar]
- 83. Junqueira C, Crespo Â, Ranjbar S et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv; doi:10.1101/2021.03.06.21252796, 8 March 2021, preprint: not peer reviewed. [Google Scholar]
- 84. Kazmi N, Smith GD, Lewis SJ. Mendelian randomization analyses show that higher acetyl-carnitine and carnitine levels in blood protect against severe Covid19. medRxiv; doi:10.1101/2021.05.31.21257910, 31 May 2021, preprint: not peer reviewed. [Google Scholar]
- 85. Klaric L, Gisby JS, Papadaki A et al. Mendelian randomisation identifies alternative splicing of the FAS death receptor as a mediator of severe COVID-19. medRxiv; doi:10.1101/2021.04.01.21254789, 7 April 2021, preprint: not peer reviewed. [Google Scholar]
- 86. Klinger JE, Ravarani CNJ, Bannard C et al. Critically ill COVID-19 status associated trait genetics reveals CDK6 inhibitors as potential treatment. medRxiv; doi:10.1101/2021.05.18.21256584, 28 May 2021, preprint: not peer reviewed. [Google Scholar]
- 87. Liu D, Zhang X, Cao W et al. Association between Alzheimer’s disease and COVID-19: A bidirectional Mendelian randomization. medRxiv; doi:10.1101/2020.07.27.20163212, 30 July 2020, preprint: not peer reviewed. [Google Scholar]
- 88. Namkoong H, Edahiro R, Fukunaga K et al. Japan COVID-19 Task Force: a nation-wide consortium to elucidate host genetics of COVID-19 pandemic in Japan. medRxiv; doi:10.1101/2021.05.17.21256513, 18 May 2021, preprint: not peer reviewed. [Google Scholar]
- 89. Palmos AB, Millischer V, Menon DK et al. Proteome-wide Mendelian randomization identifies causal links between blood proteins and severe COVID-19. PLoS Genet 2022;18:e1010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schooling CM, Yeung SLA, Kwok MK, Zhao JV. Genetic validation of the use of tocilizumab, statins and dexamethasone in COVID-19. medRxiv; doi:10.1101/2020.07.09.20149450, 20 July 2020, preprint: not peer reviewed. [Google Scholar]
- 91. Sobczyk MK, Gaunt TR. The effect of circulating zinc, selenium, copper and vitamin K1 on COVID-19 outcomes: a Mendelian randomization study. Nutrients 2022;14:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang A, Liu W, Liu Z. A two-sample robust Bayesian Mendelian Randomization method accounting for linkage disequilibrium and idiosyncratic pleiotropy with applications to the COVID-19 outcomes. Genet Epidemiol 2022. doi:10.1002/gepi.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wu L, Zhu J, Wu C. Mendelian randomization analysis to characterize causal association between coronary artery disease and COVID-19. medRxiv; doi:10.1101/2020.05.29.20117309, 30 May 2020, preprint: not peer reviewed. [Google Scholar]
- 94. Xiang Y, Chau CK-L, Qiu J, Rao S, So H-C. Exploring causal relationships between COVID-19 and cardiometabolic disorders: a bi-directional Mendelian randomization study. medRxiv; doi:10.1101/2021.03.20.21254008, 20 March 2021, preprint: not peer reviewed. [Google Scholar]
- 95. Xiang Y, Qiu J, Zhang R, Chau CK-L, Rao S, So H-C. Neuropsychiatric disorders as risk factors and consequences of COVID-19: a Mendelian randomization study. medRxiv; doi:10.1101/2021.06.29.21259609, 3 July 2021, preprint: not peer reviewed. [Google Scholar]
- 96. Xiong J, Kong X, Ma Z et al. Genetically determined serum testosterone level and Covid-19 illness level: a Mendelian randomization study. medRxiv; doi:10.1101/2021.10.10.21264779, 13 October 2021, preprint: not peer reviewed. [Google Scholar]
- 97. Xu S, Wang P, Fung WK, Liu Z. A novel penalized inverse-variance weighted estimator for Mendelian randomization with applications to COVID-19 outcomes. medRxiv; doi:10.1101/2021.09.25.21264115, 6 October 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang S, Cooper-Knock J, Weimer AK et al. Common and rare variant analyses combined with single-cell multiomics reveal cell-type-specific molecular mechanisms of COVID-19 severity. medRxiv; doi:10.1101/2021.06.15.21258703, 21 June 2021, preprint: not peer reviewed. [Google Scholar]
- 99. Zhang X, Wang B, Geng T et al. Causal associations between COVID-19 and atrial fibrillation: a bidirectional Mendelian randomization study. Nutr Metab Cardiovasc Dis 2022;32:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zheng B-K, Li N. Smoking and COVID-19: a two-sample Mendelian randomization study. medRxiv; doi:10.1101/2021.03.31.21254730, 6 April 2021, preprint: not peer reviewed. [Google Scholar]
- 101. Zheng J, Zhang Y, Liu Y et al. Multi-omics study revealing putative drug targets of COVID-19 severity and other viral infection diseases. medRxiv; doi:10.1101/2020.05.07.20093286, 3 July 2021, preprint: not peer reviewed. [Google Scholar]
- 102. Ying K, Zhai R, Pyrkov TV et al. Genetic and phenotypic analysis of the causal relationship between aging and COVID-19. Commun Med 2021;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open 2021;4:e2036462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hopkinson NS, Rossi N, El-Sayed Moustafa J et al. Current smoking and COVID-19 risk: results from a population symptom app in over 2.4 million people. Thorax 2021;76:714–22. [DOI] [PubMed] [Google Scholar]
- 105. Sallis R, Young DR, Tartof SY et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med 2021;55:1099–105. [DOI] [PubMed] [Google Scholar]
- 106. Leung JM, Sin DD. Smoking, ACE-2 and COVID-19: ongoing controversies. Eur Respir J 2020;56:2001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. World Health Organization. The importance of tobacco cessation in the context of the COVID-19 pandemic. https://www.who.int/news-room/events/detail/2021/03/16/default-calendar/the-importance-of-tobacco-cessation-in-the-context-of-the-COVID-19-pandemic (1 December 2021, date last accessed).
- 108. Gao M, Piernas C, Astbury NM et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol 2021;9:350–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- 110. Akbar AN, Gilroy DW. Aging immunity may exacerbate COVID-19. Science 2020;369:256–57. [DOI] [PubMed] [Google Scholar]
- 111. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Padalia K, O’Hayer PJ, Anderson E et al. Obesity, inflammation, and outcomes in Covid-19. J Am Coll Cardiol 2021;77:3032. [Google Scholar]
- 113. Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 2004;104:100–06. [DOI] [PubMed] [Google Scholar]
- 114. Rodriguez L, Pekkarinen PT, Lakshmikanth T et al. Systems-level immunomonitoring from acute to recovery phase of severe COVID-19. Cell Rep Med 2020;1:100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Denzel A, Maus UAR, Gomez M et al. Basophils enhance immunological memory responses. Nat Immunol 2008;9:733–42. [DOI] [PubMed] [Google Scholar]
- 116. Ng WH, Tipih T, Makoah NA et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. mBio 2021;12; doi:10.1128/mBio.03647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Purcell SM, Wray NR, Stone JL et al. ; International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009;460:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tamouza R, Krishnamoorthy R, Leboyer M. Understanding the genetic contribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain Behav Immun 2021;91:731–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ye J, Wen Y, Sun XF et al. Socioeconomic deprivation index is associated with psychiatric disorders: an observational and genome-wide gene-by-environment interaction analysis in the UK Biobank cohort. Biol Psychiat 2021;89:888–95. [DOI] [PubMed] [Google Scholar]
- 120. Fond G, Nemani K, Etchecopar-Etchart D et al. Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis. JAMA Psychiatry 2021;78:1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McAlpine LS, Zubair AS, Maran I et al. Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke 2021;52:e233–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. D'Marco L, Puchades MJ, Romero-Parra M et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J 2020;13:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 2018;33:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zheng J, Haberland V, Baird D et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet 2020;52:1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Snow TAC, Saleem N, Ambler G, Nastouli E, Singer M, Arulkumaran N. Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med 2021;47:641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen LYC, Biggs CM, Jamal S, Stukas S, Wellington CL, Sekhon MS. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep Med 2021;2:100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19? Nat Rev Immunol 2021;21:337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hamano E, Hijikata M, Itoyama S et al. Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem Biophys Res Commun 2005;329:1234–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. He J, Feng D, de Vlas SJ et al. Association of SARS susceptibility with single nucleic acid polymorphisms of OAS1 and MxA genes: a case-control study. BMC Infect Dis 2006;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Soveg FW, Schwerk J, Gokhale NS et al. Endomembrane targeting of human OAS1 p46 augments antiviral activity. Elife 2021;10:e71047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li W, Moore MJ, Vasilieva N et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Monteil V, Kwon H, Prado P et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020;181:905–13.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xiao T, Lu J, Zhang J et al. A trimeric human angiotensin-converting enzyme 2 as an anti-SARS-CoV-2 agent. Nat Struct Mol Biol 2021;28:202–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Amraei R, Yin W, Napoleon MA et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. ACS Cent Sci 2021;7:1156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:155–66. [DOI] [PubMed] [Google Scholar]
- 137. Axfors C, Schmitt AM, Janiaud P et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 2021;12:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Schooling CM, Zhao JV, Au Yeung SL, Kwok MK. Letter in response to ‘Bias in two-sample Mendelian randomization when using heritable covariable-adjusted summary associations’—‘Interpreting Mendelian randomization studies pre-adjusted for the heritable covariable survival to recruitment’. Int J Epidemiol 2021;50:1744–45. [DOI] [PubMed] [Google Scholar]
- 139. Wojcik GL, Graff M, Nishimura KK et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 2019;570:514–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Blomberg B, Mohn KG, Brokstad KA, Bergen COVID-19 Research Group et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021;27:1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.