Abstract
Summary
Retrospective analysis of the post–propensity score (PS)–matched cohort of 8426 outpatients balanced in clinical and demographic covariates showed that treatment with casirivimab-imdevimab monoclonal antibody was effective against the SARS-CoV-2 Delta variant to reduce hospitalization, mortality, and intensive care unit admission rates within 30 days.
Background
Real-world data on the effectiveness of neutralizing casirivimab-imdevimab monoclonal antibody (Cas-Imd mAb) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among high-risk patients may inform the response to future SARS-CoV-2 variants.
Methods
This study covers an observational retrospective data analysis in Banner Health Care System sites, mainly in Arizona. During the study period, the prevalence of SARS-CoV-2 Delta variant was between 95% and 100%. Of 29 635 patients who tested positive for coronavirus disease 2019 (COVID-19) between 1 August 2021 and 30 October 2021, in the Banner Health Care System, the study cohort was split into 4213 adult patients who received Cas-Imd mAb (1200 mg) treatment compared to a PS-matched 4213 untreated patients. The primary outcomes were the incidence of all-cause hospitalization, intensive care unit (ICU) admission, and mortality within 30 days of Cas-Imd mAb administration or Delta variant infection.
Results
Compared to the PS-matched untreated cohort, the Cas-Imd mAb cohort had significantly lower all-cause hospitalization (4.2% vs 17.6%; difference in percentages, −13.4 [95% confidence interval {CI}, −14.7 to −12.0]; P < .001), ICU admission (0.3% vs 2.8%; difference, −2.4 [95% CI, −3.0 to −1.9]; P < .001), and mortality (0.2% vs 2.0%; difference, −1.8 [95% CI, −2.3 to −1.3]; P < .001) within 30 days. The Cas-Imd mAb treatment was associated with lower rate of hospitalization (hazard ratio [HR], 0.22 [95% CI, .19–.26]; P < .001) and mortality (HR, 0.11 [95% CI, .06–.21]; P < .001).
Conclusions
Cas-Imd mAb treatment was associated with a lower hospitalization rate, ICU admission, and mortality within 30 days among patients infected with the SARS-CoV-2 Delta variant.
Keywords: all-cause hospitalization, casirivimab-imdevimab monoclonal antibody, Delta variant, mortality, propensity matching, SARS-CoV-2
The coronavirus disease 2019 (COVID-19) pandemic has caused a significant number of deaths in the United States (US) and globally [1]. With ongoing infections worldwide, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mutations are occurring, and new variants continue to emerge. The SARS-CoV-2 variants of concern, notably the B.1.617.2 (Delta) and most recently B.1.1.529 (Omicron) variants, have reduced antibody neutralization [2].
The COVID-19 vaccines have been instrumental in preventing hospitalizations and deaths [3]. However, there is a risk of breakthrough infections secondary to waning immunity [4–6] and reduced response to vaccination, especially among immunosuppressed patients [7]. Therefore, in addition to the vaccine, therapeutic options are essential in the fight against the COVID-19 pandemic. Researchers have been developing therapies for COVID-19 in the outpatient setting. While oral agents have been studied to treat COVID-19, including fluvoxamine [8] and, recently authorized by the US Food and Drug Administration (FDA) under Emergency Use Authorization (EUA), nirmatrelvir-ritonavir [9] and molnupiravir [10], these drugs were not approved during the period of Delta variant spread. During this time, the only available therapeutics for the outpatient management of COVID-19 were neutralizing monoclonal antibodies (mAbs) targeting the SARS-CoV-2 spike protein. The mAbs used to treat mild to moderate COVID-19 and/or to prevent severe disease include casirivimab-imdevimab (Cas-Imd mAb), bamlanivimab-etesevimab, and sotrovimab [11–15]. Data from clinical trials indicate a significant reduction in hospitalization rates of up to 70% with bamlanivimab, 67% with casirivimab-imdevimab, 87% with bamlanivimab-etesevimab, and 85% with sotrovimab in high-risk patients [16–20]. In addition, mAb showed a role in treating COVID-19 breakthrough infections in vaccinated individuals [21]. Also, pre–Delta variant SARS-CoV-2 real-world data showed promise for Cas-Imd mAb in reducing hospitalizations [22]. Recently, it was shown that Cas-Imd mAb decreased the rate of hospitalization among patients with COVID-19 during the early period of the Delta variant [21]. In vitro data showed that Cas-Imd mAb is possibly effective against the Delta variant [23], which presents Cas-Imd mAb as a viable option in treating the Delta variant. The Cas-Imd treatment was initially dosed at 2400 mg under the authorized FDA EUA; it was later changed to 1200 mg in June 2021 [24, 25]. In this study, we aimed to determine the effectiveness of Cas-Imd mAb (1200 mg) in reducing all-cause hospitalization, intensive care unit (ICU) admission, and all-cause mortality within 30 days of administration of Cas-Imd mAb or COVID-19 infection diagnosis.
METHODS
Patient Consent Statement
This study was approved by the Institutional Review Board of the University of Arizona with a waiver of patient consent given the retrospective nature of the study. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Overview
This study is an observational retrospective electronic health record (Cerner EHR) analysis in the Banner Health Care System (a nonprofit, large healthcare organization) which has 30 hospitals and several associated clinics across the western United States. The Banner Health Care System Monoclonal Antibody Treatment program was established in December 2020 (see Supplementary Document A). A multidisciplinary team reviews patients for eligibility for monoclonal antibody treatment, guided by the FDA EUA.
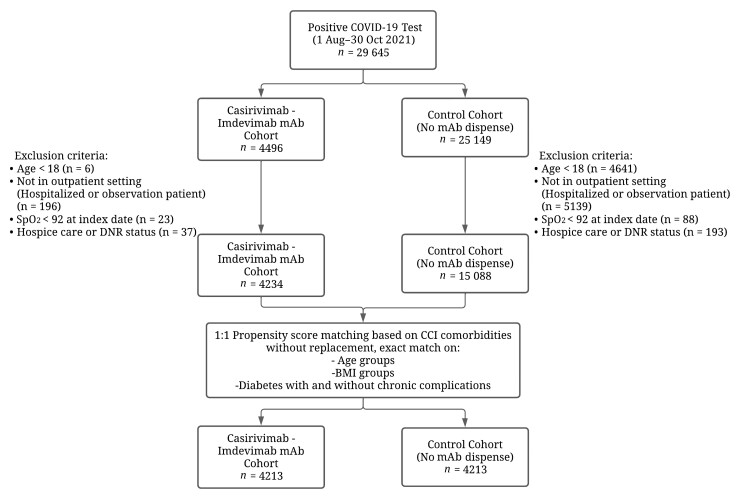
Among 29 635 patients who tested positive for COVID-19 (positive polymerase chain reaction (PCR) or direct antigen test) between 1 August 2021, and 30 October 2021, the study cohort was split into the treatment cohort who received Cas-Imd mAb (1200 mg) and the untreated control cohort (Figure 1). During the study period, there were 22 infusion sites (for the treatment cohort) and 128 testing sites (untreated control cohort) in the Banner Health Care System. The study index date for cohorts was determined as the date of Cas-Imd mAb administration or the date of the first positive COVID-19 test. Index dates were used as an enrollment date for the study. Patients who were <18 years old, were already hospitalized, had a pulse oximetry (SpO2) reading <92% [26], were on hospice care. or had “do not resuscitate” status were excluded from the cohorts. Asymptomatic high-risk patients who received Cas-Imd mAb for postexposure prophylaxis were also excluded. Clinical and demographic covariates were extracted from the Cerner EHR for the remaining patients. The clinical covariates were derived from the Charlson Comorbidity Index [27] and extracted for each patient based on International Classification of Diseases, Tenth Revision (ICD-10) codes documented in the 5 years preceding the patient index date. Then, one-to-one propensity score (PS) matching with no replacement was used to match both cohorts (Figure 2). Postmatch cohort size was determined as 4213 pairs. For additional analysis, the matched cohort was rematched based on the COVID-19 mRNA vaccination subgroups.
Figure 1.
Flowchart for study cohort selection. Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; DNR, do not resuscitate; mAb, monoclonal antibody; SpO2, oxygen saturation.
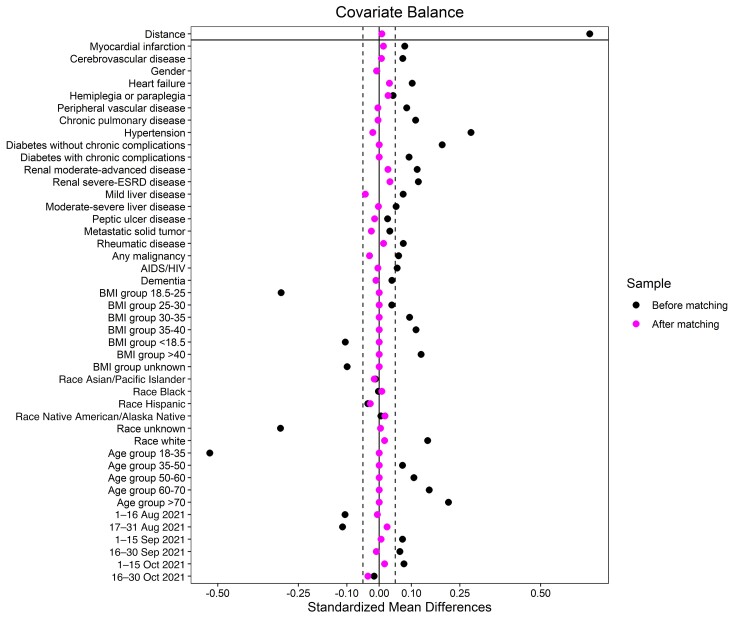
Figure 2.
Covariate balance plot for before and after propensity score matching. Abbreviations: BMI, body mass index; ESRD, end-stage renal disease; HIV, human immunodeficiency virus.
The PS-matched cohort spanned the Banner Health Care System sites in multiple states, with 82.1% from Arizona, 9.4% from Colorado, 4.1% from Wyoming, 2.5% from Nevada, 1.5% from California, and 0.5% from Nebraska. During the study period, the prevalence of the Delta variant among SARS-CoV-2–infected patients was between 95% and 100% in states where the Banner Health Care System sites exist (Supplementary Figure 1A–F). In addition, if vaccination status of a patient residing in Arizona was missing, the vaccination status was imported from the Arizona State Immunization Information System, an online verification resource.
Outcomes
The primary outcomes were the proportion of all-cause hospitalization, ICU admission, and all-cause mortality that was observed within our electronic medical record system, within 30 days of the index date. The secondary outcomes included hospitalization length of stay (LOS) and cumulative ICU LOS, oxygen therapy, and acute kidney injury (AKI) stages during the first hospitalization. AKI is defined according to the Kidney Disease Improving Global Outcomes (KDIGO) classification [28]. The data were right censored on 30 November 2021. In this analysis, death was not used as competing risk to all-cause hospitalization or ICU admission due to small numbers.
Multivariable Propensity Score Matching
One-to-one PS matching with no replacement was performed using an optimal matching algorithm [29] that minimizes the sum of the absolute pairwise distance across the matched sample. The optimal matching algorithm was compared with nearest neighbors and complete matching algorithms and was determined the best per the covariate balance and the number of unmatched individuals. Pairs were matched exactly on age group, body mass index (BMI) group, and diabetes status (with or without complications). These variables along with the remaining demographics, clinical covariates, and time periods (composed of 2-week periods between the study start and end date) were included as predictors in a logistic regression model to estimate the PS. Distance function was determined considering its performance in minimizing the unmatched sample while keeping covariate standardized mean differences (SMDs) to a minimum. Covariate balance was assessed by looking at the SMD and empirical cumulative distribution function statistics for each covariate and by a covariate balance plot (Figure 2) that displays the SMDs before and after matching. The MatchIt package [30] from the statistical computing software R was utilized for building and assessing the PS-matching model.
Statistical Analysis
All statistical analyses were conducted on the paired (matched) dataset. For each primary outcome, the event count and percentage of the event was reported. Ninety-five percent Clopper-Pearson confidence intervals (CIs) for percentages were computed in the R package Exactci. Exact McNemar test was used to compare the difference in percentages between the treatment and control cohorts. Differences in percentages between cohorts and related 95% CIs were reported along with the McNemar test P value. Calculations were performed using the R package exact2x2. In addition, Kaplan-Meier survival analysis was performed to evaluate the difference in time to all-cause hospitalization and mortality rates (using Stata version 17 software, StataCorp, College Station, Texas).
For secondary continuous outcomes, hospital LOS and ICU LOS, mean and standard deviation (SD) of the outcome were reported. Individuals who were not hospitalized were considered as they had zero LOS in both outcomes. A 2-part generalized linear mixed model with random effect for matched pairs was fitted to compare LOS across cohorts among patients who have LOS >0, by evaluating the model’s fixed effects for Cas-Imd mAb use. The estimated coefficient, 95% CI of the coefficient, and the statistical significance of the fixed antibody effect were reported. The R package GLMMadaptive was used to fit the mixed model.
For our categorical secondary outcomes, intensity of oxygen therapy and AKI stages during hospitalization, counts, and percentages were reported. The subcategories for both variables were grouped into 2 clinically meaningful categories due to the small sample size. Wald test of no differences was conducted to compare the distributions for treatment and control cohort cases, given a hospitalized patient.
An additional analysis was conducted to assess how the primary outcomes differ based on COVID-19 messenger RNA (mRNA) vaccination status. An individual was considered fully vaccinated if 14 days had passed after their final dose of the vaccine before the index date. First, the Stuart-Maxwell test for marginal homogeneity was used to compare vaccination status between the treatment and control cohorts. Then, the postmatch treatment and control cohorts were combined and then split into vaccinated and unvaccinated cohorts, excluding the individuals with missing vaccination status. The optimal matching method was used to rematch both vaccinated and unvaccinated cohorts separately for the PS calculation, using the same model in the previous analysis [31]. Following the optimal rematching, 949 pairs (n = 1898) were matched in the vaccinated cohort, and 2732 (n = 5464) were matched in the unvaccinated cohort. Primary outcome counts and percentages with Clopper-Pearson CIs were reported. Finally, the hazard ratio (HR) for the effect of Cas-Imd mAb treatment on time to primary outcomes was calculated using a Cox proportional hazards model adjusted for COVID-19 mRNA vaccination status.
Missing Data
Data were missing in 22 (2.4% of hospitalized) patients for intensity of oxygen therapy, 26 (2.8% hospitalized) patients for serum creatinine and AKI categories, and 461 (5.5% of the study cohort) patients for vaccination status. Observations with missing data on secondary outcomes were considered free of the outcome for their respective statistical tests.
RESULTS
Patient Characteristics
Table 1 shows the characteristics of the Cas-Imd mAb and untreated control cohorts before and after PS matching. All post-PS-matching covariate SMDs were below a 0.05 threshold, indicating an optimal matching (Figure 2). In the post-PS-matched cohort, the median age of patients in the Cas-Imd mAb treatment arm was 50 (interquartile range [IQR], 34–64) years; 55.9% were female, and 67.8% were White race. Some of the high-risk characteristics were age ≥65 years (30.6%), BMI ≥35 kg/m2 (35.3%), diabetes mellitus (17.6%), chronic lung disease (18.2%), kidney disease–any stage (8.5%), and human immunodeficiency virus (HIV)/AIDS (5.6%). The median time from COVID-19 PCR positivity to infusion was 1 day (IQR, 0–2 days) in the Cas-Imd mAb treatment cohort, shown in Supplementary Figure 2. In a subgroup analysis based on number of days from COVID-19 PCR positivity to Cas-Imd mAb infusion, we categorized time to mAb infusion variable as <2 vs ≥2 days and as <3 vs ≥3 days and calculated the proportion of patients who are hospitalized and died in the mAb-treated cohort (Supplementary Table 1). The results show no significant difference regarding the hospitalization and mortality in both categories.
Table 1.
Clinical Covariate Balance Before and After Propensity Score Matching
| Clinical Covariates | After PS Matching | Before PS Matching | ||||
|---|---|---|---|---|---|---|
| Cas-Imd mAb Treatment Cohort | Untreated Control Cohort | SMD | Cas-Imd mAb Treatment Cohort | Untreated Control Cohort | SMD | |
| No. | 4213 | 4213 | 4234 | 15 088 | ||
| Age, y, median (IQR) | 50.0 (38.0–64.0) | 50.0 (38.0–63.0) | 50.0 (38.0–64.0) | 40.0 (29.0–55.0) | ||
| Age group, y | ||||||
| 18–35 | 822 (19.5) | 822 (19.5) | 0.00 | 827 (19.5) | 6085 (40.3) | −0.52 |
| 36–50 | 1330 (31.6) | 1330 (31.6) | 0.00 | 1343 (31.7) | 4278 (28.4) | 0.07 |
| 51–60 | 769 (18.3) | 769 (18.3) | 0.00 | 771 (18.2) | 2121 (14.1) | 0.11 |
| 61–70 | 655 (15.5) | 655 (15.5) | 0.00 | 655 (15.5) | 1489 (9.9) | 0.15 |
| >70 | 637 (15.1) | 637 (15.1) | 0.00 | 638 (15.1) | 1115 (7.4) | 0.21 |
| Sex, male | 1860 (44.1) | 1877 (44.6) | −0.01 | 1872 (44.2) | 6729 (44.6) | −0.01 |
| Race/Ethnicity | ||||||
| White | 2855 (67.8) | 2822 (67.0) | 0.02 | 2869 (67.8) | 9165 (60.7) | 0.15 |
| Black | 233 (5.5) | 225 (5.3) | 0.01 | 234 (5.5) | 842 (5.6) | 0.00 |
| Hispanic | 885 (21.0) | 932 (22.1) | −0.03 | 891 (21.0) | 3392 (22.5) | −0.04 |
| Asian/Pacific Islander | 37 (0.9) | 43 (1.0) | −0.02 | 37 (0.9) | 148 (1.0) | −0.01 |
| Native American/Alaska Native | 62 (1.5) | 53 (1.3) | 0.02 | 62 (1.5) | 211 (1.4) | 0.01 |
| Unknown | 141 (3.3) | 138 (3.3) | 0.00 | 141 (3.3) | 1330 (8.8) | −0.31 |
| BMI, group, kg/m2 | ||||||
| <18.5 | 31 (0.7) | 31 (0.7) | 0.00 | 33 (0.8) | 257 (1.7) | −0.11 |
| 18.5–25 | 569 (13.5) | 569 (13.5) | 0.00 | 572 (13.5) | 3605 (23.9) | −0.30 |
| 26–30 | 1210 (28.7) | 1210 (28.7) | 0.00 | 1214 (28.7) | 4061 (26.9) | 0.04 |
| 31–35 | 1036 (24.6) | 1036 (24.6) | 0.00 | 1045 (24.7) | 3111 (20.6) | 0.09 |
| 36–40 | 600 (14.2) | 600 (14.2) | 0.00 | 600 (14.2) | 1538 (10.2) | 0.11 |
| >40 | 466 (11.1) | 466 (11.1) | 0.00 | 467 (11.0) | 1050 (7.0) | 0.13 |
| Unknown | 301 (7.1) | 301 (7.1) | 0.00 | 303 (7.2) | 1466 (9.7) | −0.1 |
| Myocardial infarction | 118 (2.8) | 109 (2.6) | 0.00 | 118 (2.8) | 224 (1.5) | 0.08 |
| Heart failure | 154 (3.7) | 129 (3.1) | 0.03 | 155 (3.7) | 263 (1.7) | 0.10 |
| Cerebrovascular disease | 126 (3.0) | 121 (2.9) | 0.01 | 127 (3.0) | 264 (1.7) | 0.07 |
| Hemiplegia or paraplegia | 31 (0.7) | 21 (0.5) | 0.03 | 31 (0.7) | 55 (0.4) | 0.04 |
| Peripheral vascular disease | 134 (3.2) | 137 (3.3) | 0.00 | 136 (3.2) | 258 (1.7) | 0.09 |
| Chronic pulmonary disease | 767 (18.2) | 773 (18.3) | 0.00 | 774 (18.3) | 2101 (13.9) | 0.11 |
| Hypertension | 1234 (29.3) | 1272 (30.2) | −0.02 | 1247 (29.5) | 2487 (16.5) | 0.28 |
| Diabetes without chronic complications | 590 (14.0) | 590 (14.0) | 0.00 | 604 (14.3) | 1122 (7.4) | 0.20 |
| Diabetes with chronic complications | 150 (3.6) | 150 (3.6) | 0.00 | 168 (4.0) | 326 (2.2) | 0.09 |
| Renal disease, mild-moderate-advanced (CKD stage 1–4) | 189 (4.5) | 165 (3.9) | 0.03 | 195 (4.6) | 323 (2.1) | 0.12 |
| Renal disease, severe (CKD stage 5 and ESRD) | 168 (4.0) | 140 (3.3) | 0.03 | 174 (4.1) | 257 (1.7) | 0.12 |
| Mild liver disease | 226 (5.4) | 267 (6.3) | −0.04 | 229 (5.4) | 562 (3.7) | 0.07 |
| Moderate to severe liver disease | 44 (1.0) | 45 (1.1) | 0.00 | 45 (1.1) | 79 (0.5) | 0.05 |
| Peptic ulcer disease | 44 (1.0) | 50 (1.2) | −0.01 | 44 (1.0) | 117 (0.8) | 0.03 |
| Rheumatic disease | 110 (2.6) | 101 (2.4) | 0.01 | 111 (2.6) | 215 (1.4) | 0.07 |
| Malignancy including skin cancers and lymphoproliferative disorders | 109 (2.6) | 129 (3.1) | −0.03 | 109 (2.6) | 245 (1.6) | 0.06 |
| Metastatic solid tumor | 26 (0.6) | 34 (0.8) | −0.02 | 26 (0.6) | 54 (0.4) | 0.03 |
| HIV/AIDS | 235 (5.6) | 239 (5.7) | 0.00 | 238 (5.6) | 654 (4.3) | 0.06 |
| Dementia | 39 (0.9) | 43 (1.0) | −0.01 | 39 (0.9) | 82 (0.5) | 0.04 |
| Time period | ||||||
| 1–16 Aug 2021 | 538 (12.8) | 546 (13.0) | −0.01 | 540 (12.8) | 2457 (16.3) | −0.11 |
| 17–31 Aug 2021 | 682 (16.2) | 644 (15.3) | 0.02 | 685 (16.2) | 3071 (20.4) | −0.11 |
| 1–15 Sep 2021 | 848 (20.1) | 838 (19.9) | 0.01 | 850 (20.1) | 2591 (17.2) | 0.07 |
| 16–30 Sep 2021 | 757 (18.0) | 771 (18.3) | −0.01 | 761 (18.0) | 2341 (15.5) | −0.06 |
| 1–15 Oct 2021 | 730 (17.3) | 703 (16.7) | 0.02 | 735 (17.4) | 2180 (14.4) | 0.08 |
| 16–30 Oct 2021 | 658 (15.6) | 711 (16.9) | −0.03 | 663 (15.7) | 2448 (16.2) | −0.02 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; Cas-Imd mAb, casirivimab-imdevimab monoclonal antibody; CKD, chronic kidney disease; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; IQR, interquartile range; PS, propensity score; SMD, standardized mean difference.
Primary and Secondary Outcomes
Table 2 and Supplementary Table 2 show the results of the primary and/or secondary outcomes within 30 days in the post-PS-matched cohorts. Compared to the untreated control cohort, the percentage of patients with all-cause hospitalizations in the Cas-Imd mAb cohort was 4.2% (95% CI, 3.6%–4.8%) vs 17.6% (95% CI, 16.4%–18.7%) (P < .001); the percentage of patients with ICU admission in the Cas-Imd mAb cohort was 0.3% (95% CI, 0.25%–0.5%) vs 2.8% (95% CI, 2.3%–3.3%; P < .001); and the proportion of patients with all-cause mortality was 0.2% (95% CI, .1%–.4%) vs 2.0% (95% CI, 1.6%–2.4%; P < .001). Death rarely occurred (8 of 4213 patients in the Cas-Imd cohort and 83 of 4213 patients in the untreated control cohort) and mostly happened in the ICU (75 of 91). Sixteen patients died without hospitalization to the Banner Healthcare Centers (assumed to have died either at home or other healthcare facilities). Kaplan-Meier survival analysis showed significant differences in time to all-cause hospitalization and mortality between the Cas-Imd mAb treatment and PS-matched untreated cohort (Supplementary Figures 3 and 4). Supplementary Figures 5A, 5B, 6A, and 6B illustrate the substratification of Kaplan-Meier survival analysis according to baseline SpO2 (substratifying the cohort SpO2 92%–95% and ≥96%), demonstrating comparable results. In terms of the secondary outcomes, compared to the untreated control cohort, the mean for hospital LOS was 5.3 (SD, 5.3) days in the Cas-Imd mAb cohort vs 6.9 (SD, 7.9) days (P = .06), and the mean for ICU LOS was 3.6 (SD, 4.8) days in the Cas-Imd mAb cohort vs 3.8 (SD, 5.3) days (P = .85). The generalized linear mixed model that estimates the mean ratio of cohorts showed that upper 95% CIs of both hospital LOS and ICU LOS outcomes include 1. The percentage of the highest-intensity oxygen requirements (including mechanical ventilation/continuous positive airway pressure–bilevel positive airway pressure/high-flow oxygen) were lower in the Cas-Imd mAb cohort (25.8% vs 42.5%; P < .001) compared with the untreated control cohort. The percentage of the KDIGO AKI stage 2–3 in the Cas-Imd mAb cohort (1.4%) was lower than in the untreated cohort (6.1%; P < .001).
Table 2.
Primary and Secondary Outcomes in the Post–Propensity Score–Matched Cohorts
| Outcome | Cas-Imd mAb Treatment Cohort (n = 4213) | Untreated Control Cohort (n = 4213) | Difference, % (95% CI)b | P Value | ||
|---|---|---|---|---|---|---|
| No. (%) | (95% CI)a | No. (%) | (95% CI)a | |||
| Primary outcomes in post-PS-matched cohorts | ||||||
| All-cause hospitalization within 30 d | 176 (4.2) | (3.6–4.8) | 740 (17.6) | (16.4–18.7) | −13.4 (−14.7 to −12.0) | <.001 |
| ICU admission within 30 d | 13 (0.3) | (.2–.5) | 116 (2.8) | (2.3–3.3) | −2.4 (−3.0 to −1.9) | <.001 |
| Mortality in 30 d | 8 (0.2) | (.1–.4) | 83 (2.0) | (1.6–2.4) | −1.8 (−2.3 to −1.3) | <.001 |
| Secondary outcomes in post-PS-matched cohorts | Cas-Imd mAb Treatment Cohort (n = 176) | Untreated Control Cohort (n = 740) | Ratio of Means for Cohortsc | 95% CI for Mean Ratio of Cohortsc | P Value | |
| Hospital LOS, d, mean (SD) | 5.3 (5.3) | 6.9 (7.9) | 0.86 | (.74–1.01) | .06 | |
| ICU LOS, d, mean (SD) | 3.6 (4.8) | 3.8 (5.3) | 0.95 | (.54–1.67) | .85 | |
| CI mAb Treatment Cohort (n = 176) | Untreated Control Cohort (n = 740) | Difference Between Cohorts (Proportions) | 95% CI for Difference (Proportions) | Wald Test of No Differences (P Value) | ||
| Highest intensity oxygen requirement | ||||||
| MV, CPAP-BiPAP, high-flow oxygen | 42 (25.8) | 311 (42.5) | 16.8 (3.9) | (9.1–24.4) | 18.6 (<.001) | |
| Nasal cannula–room air | 121 (74.2) | 420 (57.5) | −16.8 (3.9) | (−24.4 to −9.2) | ||
| AKI in the hospitalization | ||||||
| No AKI, KDIGO stage 1 | 138 (98.6) | 601 (93.9) | −4.7 (1.4) | (−7.4 to −2.0) | 11.5 (<.001) | |
| KDIGO stage 2/3 | 2 (1.4) | 39 (6.1) | 4.7 (1.4) | (2.0–7.4) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AKI, acute kidney injury; BiPAP, bilevel positive airway pressure; Cas-Imd mAb, casirivimab-imdevimab monoclonal antibody; CI, confidence interval; CPAP, continuous positive airway pressure; ICU, intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; LOS, length of stay; MV, mechanical ventilation; PS, propensity score; SD, standard deviation.
The Clopper-Pearson method was used to calculate 95% CIs for the outcome percentages using the R package (Exactci).
Exact McNemar test was used to compare the percentage difference between the treatment and control cohorts.
Comparison between 2 continuous variables in paired data was calculated using R package GLMMadaptive for a fixed treatment effect from a 2-part mixed model.
Subgroup Analysis Stratified Based on COVID-19 Vaccination Status
Table 3 shows the primary outcomes for the PS-rematched Cas-Imd mAb–treated and untreated cohorts, stratified by vaccination status. The study cohort received COVID-19 mRNA vaccines from Pfizer-BioNTech (67.9%) and Moderna (32.1%). The prevalence of COVID-19 mRNA vaccination was lower among study participants (28.3% in the Cas-Imd mAb cohort vs 23.8% in the control untreated cohort; P < .001) as compared to the state and national reported vaccination rates. In terms of the primary outcomes, the lowest all-cause hospitalization (1.7%)/ICU admission (0.0%)/mortality (0.0%) rates were observed in the vaccinated Cas-Imd mAb treatment cohort while the highest all-cause hospitalization (23.3%)/ICU admission (3.7%)/mortality (2.3%) rates were encountered in the unvaccinated untreated cohort. The primary outcomes were similar between the Cas-Imd mAb–treated unvaccinated cohort and the untreated vaccinated cohort. In multivariable Cox proportional hazards models, Cas-Imd mAb treatment and COVID-19 mRNA vaccination were independently associated with lower rate of all-cause hospitalization (HR, 0.22 [95% CI, .19–.26]; P < .001 vs HR, 0.23 [95% CI, .18–.30]; P < .001) and mortality (HR, 0.11 [95% CI, .06–.21]; P < .001 vs HR, 0.37 [95% CI, .20–.70]; P = .02), respectively.
Table 3.
Distribution of Coronavirus Disease 2019 Vaccination Status Among Post–Propensity Score (PS)–Matched Cohort, the Primary Outcomes Stratified by Vaccination Status Among Post-PS-Matched Cohort, Multivariable Cox Proportional Hazard Models for All-Cause Hospitalization, and Mortality Adjusted for Vaccination Status Among Post-PS-Matched Cohort
| Distribution of COVID-19 mRNA Vaccination Status Among Post-PS-Matched Cohort | ||||||
|---|---|---|---|---|---|---|
| Cas-Imd mAb Treatment Cohort (n = 4213) | Untreated Control Cohort (n = 4213) | χ2 Testa | P Value | |||
| Fully vaccinated against COVID-19 | ||||||
| Yes | 1192 (28.3) | 1003 (23.8) | 217.52 | <.001 | ||
| No | 2943 (69.9) | 2827 (67.1) | ||||
| Missing | 78 (1.9) | 383 (9.1) | ||||
| Primary Outcomes Among COVID-19 mRNA Vaccinated Subgroup After PS Matching | ||||||
| Cas-Imd mAb (n = 949) | Control Cohort (n = 949) | |||||
| No. (%) | (95% CI) | No. (%) | (95% CI)b | Difference, % (95% CI)c | P Value | |
| All-cause hospitalization within 30 d | 16 (1.7) | (1.0–2.7) | 60 (6.3) | (4.9–8.1) | −4.6 (−6.5 to −2.8) | <.001 |
| ICU admission within 30 d | 0 (0.0) | (0–.4) | 9 (0.9) | (.4–1.8) | −0.9 (−1.8 to −.2) | .004 |
| Mortality in 30 d | 0 (0.0) | (0–.4) | 8 (0.8) | (.4–1.7) | −0.8 (−1.7 to −.2) | .01 |
| Primary Outcomes Among COVID 19 Unvaccinated Subgroup of Patients After PS Matching | ||||||
| Cas-Imd mAb (n = 2732) | Control Cohort (n = 2732) | |||||
| No. (%) | (95% CI) | No. (%) | (95% CI)b | Difference, % (95% CI)c | P Value | |
| All-cause hospitalization within 30 d | 150 (5.5) | (4.7–6.4) | 637 (23.3) | (21.7–24.9) | −18.3 (−20.1 to −16.4) | <.001 |
| ICU admission within 30 d | 12 (0.4) | (.2–.8) | 101 (3.7) | (3.0–4.5) | −3.3 (−4.1 to −2.6) | <.001 |
| Mortality in 30 d | 8 (0.3) | (.1–.6) | 62 (2.3) | (1.7–2.9) | −2.6 (−3.2 to −1.9) | <.001 |
| Multivariable Cox Proportional Hazards Model for All-Cause Hospitalization Within 30 d of Index Date in the Post-PS-Matched Cohort | ||||||
| HR | (95% CI) | P Value | ||||
| Cas-Imd mAb (Yes) | 0.22 | (.19–.26) | <.001 | |||
| Fully vaccinated against COVID-19 | ||||||
| Yes | 0.23 | (.18–.30) | <.001 | |||
| Missing | 0.16 | (.10–.26) | <.001 | |||
| Multivariable Cox Proportional Hazards Model for Mortality Within 30 d of Index Date in the Post-PS-Matched Cohort | ||||||
| HR | (95% CI) | P Value | ||||
| Cas-Imd mAb (Yes) | 0.11 | (.06–.21) | <.001 | |||
| Fully vaccinated against COVID-19 | ||||||
| Yes | 0.37 | (.20–.70) | .002 | |||
| Missing | 0.50 | (.20–1.23) | .13 | |||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: Cas-Imd mAb, casirivimab-imdevimab monoclonal antibody; CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; ICU, intensive care unit; mRNA, messenger RNA; PS, propensity score.
Stuart-Maxwell Test for marginal homogeneity χ2 test.
The Clopper-Pearson method was used to calculate 95% CIs for the outcome percentages using the R package (Exactci).
The exact McNemar test was used to compare the percentage difference between the treatment and control cohorts.
DISCUSSION
This study is one of the largest real-world studies reporting the use of Cas-Imd mAb in reducing mortality and hospitalization among high-risk populations during the pandemic period when infections were caused predominantly by the SARS-CoV-2 Delta variant [32]. Our study includes a diverse population of adults (approximately 20% Hispanic, 5% Black, and 1.5% American Indian). The untreated cohort was also more predisposed to higher oxygen requirement and mechanical ventilation and developed higher AKI stages than the Cas-Imd mAb group.
Cas-Imd efficacy against SARS-CoV-2 was shown previously in a clinical trial with COVID-19 patients who received Cas-Imd mAb having lower SARS-CoV-2 viral loads and hospitalization [14]. Another recent study from the Mayo Clinic showed that administration of Cas-Imd mAb was associated with a lower risk of hospitalization in high-risk populations [22]; however, that study included a smaller number of patients (N = 1392) during the period predating the Delta variant spread compared to our investigation. In addition, we demonstrate that the rates of 30-day ICU admission and all-cause mortality are significantly lower in the Cas-Imd mAb–treated group compared to previous studies.
In our subgroup analysis based on vaccination status, we found that Cas-Imd mAb retained its efficacy in lowering the rates of hospitalizations, ICU admissions, and all-cause mortality and endured its effect even when the individual was fully vaccinated, but the effect size was smaller compared to the unvaccinated cohort. Our study shows that approximately 25% of the PS-matched population, mainly from Arizona, were fully vaccinated, which is a number lower than that reported by the Arizona Department of Health Services (AZDHS) [33]. Such a difference in the number of vaccinated populations could be either secondary to the underlying characteristics of the population that visited our hospital system or secondary to lacking data of reported vaccinations. Moreover, our cohort had a higher percentage of hospitalizations and deaths than the same period reported by AZDHS [33]. This could be related to the high-risk population included in the study as we matched our controls with the Cas-Imd mAb cohort on our institutional mAb eligibility criteria, or to a lesser extent because of the included population from the states of Montana and Colorado [19, 34]. Similar to previous reports [35], our study shows that fully vaccinated adults (not boosted) had significantly reduced hospitalization, ICU admission, and death compared to the unvaccinated cohort.
For the study’s secondary outcomes, patients receiving Cas-Imd mAb had significantly reduced oxygen requirements and AKI, including the need for renal replacement therapy. The findings of our study corroborate with other randomized placebo-controlled trials which reported that the use of other mAb products reduced the risk of hospitalization and death in high-risk patients with mild to moderate COVID-19 infections [13].
This study has significant implications for treating COVID-19 and preventing its complications, as we show that delivering monoclonal antibodies directed toward a SARS-CoV-2 Delta variant can significantly lower hospitalization rates, ICU stay, and all-cause mortality. The Omicron variant has spread very quickly since December 2021; hence, there exists a pandemic with mainly the Omicron variant coinciding with declining incidence of Delta variant infections among newly infected patients [36]. It is crucial to know predominantly which variants are causing most infections in order to administer appropriate mAbs for patients infected with SARS-CoV-2 (ie, Cas-Imd mAb for the Delta variant and sotrovimab for the Omicron variant). This study highlights the role of mAbs in reducing COVID-19–associated complications; a similar approach can be used to assess the effectiveness of other mAbs or for other SARS-CoV-2 variants. Furthermore, our results show that irrespective of COVID-19 vaccination status, receiving mAbs led to significantly reduced hospitalization rates, ICU stays, and all-cause mortality, which is essential in treating breakthrough infections. Such findings have implications for unvaccinated individuals and immunocompromised individuals who will have attenuated immune responses to vaccines and may benefit from passive immunity for the treatment of COVID-19.
Our study has several strengths, including a large sample size from an extensive hospital network system that evaluated >8400 patients (the largest reported real-world experience) with analysis capturing the Delta variant period exclusively. This includes patients in both rural and urban settings with excellent representation of minority groups. Moreover, the study matched patients according to different risk factors that minimized bias by utilizing optimal PS matching. We also imported the vaccination data from the Arizona State Immunization Information System.
The limitations of our study include the retrospective design, which may be associated with reporting and selection biases, and the lack of information regarding possible hospitalizations and deaths that occurred outside of Banner Health. Lack of information about previous infections and possible missing immunization information limits further interpretation of the benefits of mAb based on previous immune status. In addition, a diverse subgroup of patients with hematopoietic stem-cell or solid organ transplants were not included as a separate category in the propensity analysis due to small sample size; such patients are known to have poorer outcomes and more likely to be referred to mAb treatment. Last, missing information regarding patients’ earliest symptom date may have introduced a selection bias.
In conclusion, Cas-Imd mAb treatment was associated with a lower hospitalization rate, ICU admission, and all-cause mortality within 30 days among those with COVID-19 infections. Both Cas-Imd mAb treatment and COVID-19 mRNA vaccination were independently associated with a lower hospitalization and mortality rate.
Supplementary Material
Contributor Information
Mohanad M Al-Obaidi, Division of Infectious Disease, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Ahmet B Gungor, Division of Nephrology, Banner University Medical Center, Tucson, Arizona, USA.
Saman Nematollahi, Division of Infectious Disease, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Tirdad T Zangeneh, Division of Infectious Disease, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Edward J Bedrick, Department of Epidemiology and Biostatistics, College of Public Health, University of Arizona, Tucson, Arizona, USA.
Katherine M Johnson, Division of Clinical Pharmacy, Banner University Medical Center, Tucson, Arizona, USA.
Nicole E Low-Adegbija, Department of Surgery, Banner University Medical Center, Tucson, Arizona, USA.
Ruhaniyah Alam, Division of Clinical Pharmacy, Banner University Medical Center, Tucson, Arizona, USA.
Pooja Rangan, Department of Medicine, Banner University Medical Center Phoenix, Phoenix, Arizona, USA.
C William Heise, Division of Clinical Data Analytics and Decision Support, Department of Medicine, College of Medicine–Phoenix, University of Arizona, Phoenix, Arizona, USA.
Venkatesh K Ariyamuthu, Division of Nephrology, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Aneesha Shetty, Division of Nephrology, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Abd Assalam Qannus, Division of Nephrology, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Sangeetha Murugapandian, Division of Nephrology, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Mehmet M S Ayvaci, Information Systems, Naveen Jindal School of Management, University of Texas at Dallas, Dallas, Texas, USA.
Prince Mohan Anand, Medical University of South Carolina, Lancaster, South Carolina, USA.
Bekir Tanriover, Division of Nephrology, College of Medicine, University of Arizona, Tucson, Arizona, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Banner University Medical Group–Tucson Chief Executive Officer Chad Whelan, MD, Physician Executive Joshua Lee, MD, and Chief Medical Officer Gordon Carr, MD, for their support. The authors also thank Ms Shreya Bharath and Ms Riva Arian Kaul for their contributions.
Financial support. C. W. H. reports support from the Flinn Foundation, Phoenix, Arizona.
Potential conflicts of interest. M. M. A.-O. reported that he received an honorarium from Shionogi Inc and La Jolla pharmaceuticals for serving in their advisory board meetings. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed 13 December 2021.
- 3. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chemaitelly H, Tang P, Hasan M, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin E, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scobie H, Johnson A, Suthar A, et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status—13 U.S. jurisdictions, April 4–July 17, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reis G, Dos S, Moreira-Silva EA, Silva D, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10:e42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. Accessed 23 December 2021.
- 10. Pfizer . Pfizer announces additional phase 2/3 study results. 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Accessed 22 December 2021.
- 11. RECOVERY Collaborative Group; Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022; 399:665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 13. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinreich D, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure. Accessed 22 December 2021.
- 16. Stosor V, Angarone M. Not all monoclonal antibodies for coronavirus disease 2019 are created equal. J Infect Dis 2021; 224:1275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization (EUA) of bamlanivimab and etesevimab. https://www.fda.gov/media/145802/download. Accessed 16 January 2022.
- 18. US Food and Drug Administration . Fact sheet for health care providers. Emergency use authorization (EUA) of bamlanivimab. https://www.fda.gov/media/143603/download. Accessed 16 January 2022.
- 19. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab). https://www.fda.gov/media/145611/download. Accessed 16 January 2022.
- 20. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization (EUA) of sotrovimab. https://www.fda.gov/media/149534/download. Accessed 16 January 2022.
- 21. Bierle D, Ganesh R, Tulledge-Scheitel S, et al. Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities. J Infect Dis 2022; 225:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Razonable R, Pawlowski C, O’Horo J, et al. Casirivimab-imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021; 40:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021; 596:276–80. [DOI] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed 11 March 2022.
- 25. US Food and Drug Administration . Regeneron fact sheet for health care providers: emergency use authorization (EUA) of REGEN-COV. https://www.fda.gov/media/145611/download. Accessed 11 March 2022.
- 26. National Institutes of Health . Oxygenation and ventilation. 2022. https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/. Accessed 11 March 2022.
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 28. Work Group Membership . Kidney Int Suppl (2011) 2012; 2:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat 2006; 15:609–27. [Google Scholar]
- 30. Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42:1–28. [Google Scholar]
- 31. Wang SV, Jin Y, Fireman B, et al. Relative performance of propensity score matching strategies for subgroup analyses. Am J Epidemiol 2018; 187:1799–807. [DOI] [PubMed] [Google Scholar]
- 32. O’Toole Á, Hill V, Pybus OG, et al. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. Wellcome Open Res 2021; 6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arizona Department of Health Services . COVID-19 vaccine administration data. http://www.azdhs.gov/covid19/data/index.php. Accessed 16 January 2022.
- 34. Petrilli C, Jones S, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg E, Holtgrave D, Dorabawila V, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status—New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention . COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed 16 January 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.