Abstract
Background
The Canadian coronavirus disease 2019 (COVID-19) immunization strategy deferred second doses and allowed mixed schedules. We compared 2-dose vaccine effectiveness (VE) by vaccine type (mRNA and/or ChAdOx1), interval between doses, and time since second dose in 2 of Canada’s larger provinces.
Methods
Two-dose VE against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or hospitalization among adults ≥18 years, including due to Alpha, Gamma, and Delta variants of concern (VOCs), was assessed ≥14 days postvaccination by test-negative design studies separately conducted in British Columbia and Quebec, Canada, between 30 May and 27 November (epi-weeks 22–47) 2021.
Results
In both provinces, all homologous or heterologous mRNA and/or ChAdOx1 2-dose schedules were associated with ≥90% reduction in SARS-CoV-2 hospitalization risk for ≥7 months. With slight decline from a peak of >90%, VE against infection was ≥80% for ≥6 months following homologous mRNA vaccination, lower by ∼10% when both doses were ChAdOx1 but comparably high following heterologous ChAdOx1 + mRNA receipt. Findings were similar by age group, sex, and VOC. VE was significantly higher with longer 7–8-week versus manufacturer-specified 3–4-week intervals between mRNA doses.
Conclusions
Two doses of any mRNA and/or ChAdOx1 combination gave substantial and sustained protection against SARS-CoV-2 hospitalization, spanning Delta-dominant circulation. ChAdOx1 VE against infection was improved by heterologous mRNA series completion. A 7–8-week interval between first and second doses improved mRNA VE and may be the optimal schedule outside periods of intense epidemic surge. Findings support interchangeability and extended intervals between SARS-CoV-2 vaccine doses, with potential global implications for low-coverage areas and, going forward, for children.
Keywords: SARS-CoV-2, vaccine effectiveness, test-negative design, heterologous, waning
Test-negative design studies conducted among adults in British Columbia and Quebec, Canada, show two doses of homologous or heterologous SARS-CoV-2 vaccines provide substantial and sustained protection against hospitalization, and reinforce the use of mixed schedules and longer intervals between doses.
The first severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in Canada were messenger RNA (mRNA) formulations, including BNT162b2 (Pfizer-BioNTech) authorized on 9 December (epi-week 50) 2020 and mRNA-1273 (Moderna) on 23 December (epi-week 52) 2020, both as 2-dose schedules, 3–4 weeks apart. Soon after authorization and given constrained vaccine supplies, experts in the provinces of British Columbia (BC) and Quebec simultaneously (epi-week 51) recommended deferral of second doses until as many prioritized individuals as possible could benefit from substantial single-dose protection [1] (Supplementary Material 1). To facilitate subsequent completion of the 2-dose schedule, both provinces also highlighted the likely interchangeability of available vaccines. Through January 2021, health authorities in BC and Quebec extended the dosing interval (to 5–6 weeks and 6–12 weeks, respectively), in keeping with recommendations elsewhere [2, 3]. On 3 March (epi-week 9), Canada’s National Advisory Committee on Immunization (NACI) endorsed second-dose deferral, recommending an even longer dosing interval of 16 weeks that was immediately adopted by BC and Quebec. A timeline of provincial and national vaccine recommendations and program modifications, with references, is provided in Supplementary Table 1.
On 26 February (epi-week 8) 2021, a chimpanzee adenoviral vectored (ChAdOx1) vaccine (AstraZeneca) was also authorized in Canada as a 2-dose schedule, 4–12 weeks apart. On 29 March (epi-week 13), NACI recommended that ChAdOx1 be restricted to adults aged 55 years and older due to vaccine safety concerns (thrombosis with thrombocytopenia), lowering to 30 years or older on 23 April (epi-week 16). On 1 June (epi-week 22), NACI also recognized the interchangeability of vaccines, recommending that first-dose recipients of ChAdOx1 or mRNA vaccines could (modified in epi-week 24 to should) complete the series with either mRNA product (Supplementary Table 1).
In BC and Quebec, vaccination started with long-term-care-facility residents and healthcare workers. Community-dwelling adults were next sequenced by age with single-dose coverage gradually increasing through spring 2021. In late-May/early-June (epi-weeks 21–22), as vaccine supply improved, BC and Quebec reduced the dosing interval from 16 to 8 weeks (Supplementary Table 1), by which time 70% or more of adults aged 18 years or older had received at least 1 dose and less than 10% had received 2 doses. To maximize 2-dose coverage by autumn, both provinces reduced the dosing interval to 4 weeks, such that by early September (epi-week 35), 80% of adults were considered fully vaccinated. The timeline of 1- and 2-dose vaccine coverage in BC and Quebec is provided in Supplementary Figure 1.
We report 2-dose vaccine effectiveness (VE) against infection and hospitalization among adults 18 years and older in BC and Quebec, spanning 30 May–27 November (epi-weeks 22–47) 2021, including early Alpha/Gamma and later Delta variant of concern (VOC) circulation. Mixed vaccine schedules and modified dosing intervals enabled VE comparison by vaccine type (homologous and heterologous), interval between doses, and time since the second dose.
METHODS
Study Design and Analysis
Two-dose VE was estimated by a test-negative design (TND), using multivariable logistic regression to derive the adjusted odds ratio (aOR) for vaccination among SARS-CoV-2 test-positive cases versus test-negative controls. Vaccine effectiveness and 95% confidence intervals (CIs) were computed as (1 − aOR) × 100%. Adjusted models included age group (18–49, 50–69, 70–79, ≥80 years), sex (men, women), epi-week (categorical), and region. The latter includes the 5 health authorities in BC, with 18 administrative regions of Quebec also regrouped into 5 categories (Greater Montreal, Greater Quebec City, Central Quebec, Northern Quebec, Other).
Case and Control Selection
Specimens collected from adults aged 18 years and older between epi-weeks 22 and 47 and assessed for SARS-CoV-2 by publicly funded nucleic acid amplification test (NAAT) were eligible. In both provinces, publicly funded, foremost symptom-based SAR-CoV-2 NAAT testing was broadly accessible through community-based assessment centers, emergency rooms, hospitals, and other sites. Rapid antigen tests were not broadly deployed in Canada during the analysis period.
Case specimens were SARS-CoV-2 NAAT positive; controls were NAAT negative. Both were sampled from laboratory databases capturing such tests province-wide. Individuals could contribute the first test-positive specimen and were censored thereafter. A single test-negative specimen was randomly selected per individual across the analysis period with the same controls used for VE estimation against infection and hospitalization.
Because symptoms and onset dates were not consistently captured, VE was primarily assessed against any infection timed on specimen collection date. In sensitivity analysis, VE estimates in Quebec were derived with restriction to specimens with the “M7” code indicating collection at designated outpatient screening centers for symptomatic individuals only (not possible in BC). Hospitalized cases were admitted on or within 30 days after specimen collection, identified through linkage with notifiable disease lists, supplemented in Quebec by administrative databases. In variant-specific analyses, cases were categorized as Alpha, Gamma, or Delta as per Supplementary Material 2.
Vaccination Definition
SARS-CoV-2 vaccines were delivered through publicly funded programs primarily at public health clinics and retail pharmacies. Vaccine information was obtained from provincial immunization registries (PIRs). All vaccine providers in both provinces were required to enter SARS-CoV-2 vaccination information into the PIR. Resident vaccinations received outside the province were also entered.
Based upon PIR record on or before the specimen collection date, those who had received 2 doses of BNT162b2, mRNA-1273, or ChAdOx1 were considered vaccinated; those who had received just 1 dose of any vaccine were excluded; and those who received no doses were considered unvaccinated. In overall VE analysis, vaccination was defined by second-dose receipt 14 days or more before specimen collection, excluding those vaccinated 0–13 days prior; however, a range of time since the second dose was explored. Individual-level linkage across databases was achieved through unique personal identifiers.
Exclusions
Specimens with invalid or missing information were excluded as were specimens collected from individuals identified as cases before the analysis period; residents of long-term-care, assisted-living, or independent-living facilities; and those vaccinated with a product other than BNT162b2, mRNA-1273, or ChAdOx1.
Ethics Statement
In both British Columbia (BC) and Quebec, vaccine effectiveness evaluations were conducted as legally mandated public health investigations. Data linkages and analyses by the BC Centre for Disease Control were authorized under the Public Health Act as a delegated function of the Provincial Health Officer and by the Institut national de santé publique du Québec by direct order of the National Director of Public Health. Lead investigators in BC (Skowronski) and Quebec (De Serres) additionally sought independent research ethics board review which was waived by the University of British Columbia Clinical Research Ethics Board and granted by the Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Ethics Board.
RESULTS
Case and Control Contribution
The total number of specimens collected from adults aged 18 years and older between epi-weeks 22 and 47 and tested for SARS-CoV-2 by publicly funded NAAT was 872 440 in BC and 1 973 637 in Quebec, including 59 590 (7%) and 40 145 (2%) test-positive specimens, respectively (data not shown). Of the latter, 44 964 (75%) and 31 718 (79%) cases, respectively, were eligible for VE analyses, among whom 3173 (7%) and 1452 (5%), respectively, were hospitalized (Table 1). Of 812 850 and 1 933 492 test-negative specimens in total, 622 602 (77%) and 1 501 548 (78%), respectively, were eligible for VE analyses. Among contributing adults, 96% in BC and 90% in Quebec provided up to 2 test-negative specimens each. After randomly selecting 1 test-negative specimen per individual, 468 913 (75%) controls in BC and 985 641 (66%) in Quebec were included in VE analyses.
Table 1.
Profile of Participants ≥18 Years Old, by Case and Vaccine Status (Regardless of Time Since Vaccination): British Columbia and Quebec, Canada
| British Columbia | Quebec | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 513 877), n (column %) | Vaccinated (n = 409 195), n (row %)a | Overall (N = 1 017 359), n (column %) | Vaccinated (n = 852 594), n (row %)a | |||||||||
| Cases | Hosp | Controls | Cases | Hosp | Controls | Cases | Hosp | Controls | Cases | Hosp | Controls | |
| Total | 44 964 (9) | 3131 (7)b | 468 913 (91) | 17 835 (40) | 537 (17) | 391 360 (83) | 31 718 (3) | 1452 (5)b | 985 641 (97) | 15 096 (48) | 386 (27) | 837 498 (85) |
| Age group (years) | ||||||||||||
| 18–49 | 31 031 (69) | 1043 (33) | 294 946 (63) | 10 984 (35) | 77 (7) | 236 104 (80) | 22 923 (72) | 527 (36) | 581 213 (59) | 9435 (41) | 65 (12) | 463 887 (80) |
| 50–69 | 10 817 (24) | 1228 (39) | 119 581 (26) | 5111 (47) | 187 (15) | 105 464 (88) | 6802 (21) | 502 (35) | 272 785 (28) | 4123 (61) | 99 (20) | 249 170 (91) |
| 70–79 | 2201 (5) | 511 (16) | 35 608 (8) | 1197 (54) | 128 (25) | 32 817 (92) | 1371 (4) | 222 (15) | 88 922 (9) | 1041 (76) | 93 (42) | 84 744 (95) |
| 80+ | 915 (2) | 349 (11) | 18 778 (4) | 543 (59) | 145 (42) | 16 975 (90) | 622 (2) | 201 (14) | 42 721 (4) | 497 (80) | 129 (64) | 39 697 (93) |
| Median (IQR) years | 39 (29–54) | 59 (43–71) | 42 (31–58) | 43 (33–58) | 70 (59–80) | 43 (32–60) | 40 (29–52) | 58 (43–72) | 44 (32–61) | 44 (35–58) | 73 (60–82) | 46 (34–63) |
| Sex | ||||||||||||
| Female | 22 218 (49) | 1302 (42) | 263 608 (56) | 9520 (43) | 216 (17) | 224 240 (85) | 16 480 (52) | 639 (44) | 574 263 (58) | 8209 (50) | 169 (26) | 490 303 (85) |
| Male | 22 746 (51) | 1829 (58) | 205 305 (44) | 8315 (37) | 321 (18) | 167 120 (81) | 15 238 (48) | 813 (56) | 411 378 (42) | 6887 (45) | 217 (27) | 347 195 (84) |
| Epidemiological week, 2021 | ||||||||||||
| 22–26 (30 May–3 July) | 1603 (4) | 137 (4) | 24 989 (5) | 106 (7) | 5 (4) | 9993 (40) | 1383 (4) | 74 (5) | 78 552 (8) | 108 (8) | 8 (11) | 38 666 (49) |
| 27–30 (4–31 July) | 1490 (3) | 79 (3) | 30 236 (6) | 337 (23) | 12 (13) | 21 443 (71) | 966 (3) | 39 (3) | 83 187 (8) | 211 (22) | 6 (15) | 63 379 (76) |
| 31–34 (1–28 August) | 9515 (21) | 526 (17) | 74 181 (16) | 2556 (27) | 64 (9) | 59 702 (80) | 4513 (14) | 212 (15) | 120 864 (12) | 1376 (30) | 42 (20) | 100 335 (83) |
| 35–39 (29 August–2 October) | 14 827 (33) | 1036 (33) | 133 760 (29) | 5702 (38) | 171 (20) | 115 241 (86) | 10 613 (33) | 599 (41) | 251 348 (26) | 4368 (41) | 134 (22) | 222 203 (88) |
| 40–43 (3–30 October) | 10 227 (23) | 808 (26) | 114 252 (24) | 5022 (49) | 153 (19) | 102 338 (90) | 5958 (19) | 290 (20) | 221 372 (22) | 3316 (56) | 102 (35) | 201 376 (91) |
| 44–47 (31 Oct –27 Nov) | 7302 (16) | 545 (17) | 91 495 (20) | 4112 (56) | 132 (24) | 82 643 (90) | 8285 (26) | 238 (16) | 230 318 (23) | 5717 (69) | 94 (39) | 211 539 (92) |
| Two–dose vaccine statusc,d | ||||||||||||
| Unvaccinated | 27 129 (60) | 2594 (83) | 77 553 (17) | NA | NA | NA | 16 622 (52) | 1066 (73) | 148 143 (15) | NA | NA | NA |
| Twice vaccinated, any vaccine | 17 835 (40) | 537 (17) | 391 360 (83) | 17 835 (100) | 537 (100) | 391 360 (100) | 15 096 (48) | 386 (27) | 837 498 (85) | 15 096 (100) | 386 (100) | 837 498 (100) |
| Two any mRNA vaccines | 15 625 (35) | 497 (16) | 356 578 (76) | 15 625 (88) | 497 (93) | 356 578 (91) | 13 854 (44) | 344 (24) | 771 821 (78) | 13 854 (92) | 344 (89) | 771 821 (92) |
| Two BNT162b2 | 11 411 (25) | 347 (11) | 256 357 (55) | 11 411 (64) | 347 (65) | 256 357 (66) | 10 943 (35) | 275 (19) | 580 186 (59) | 10 943 (72) | 275 (71) | 580 186 (69) |
| Two mRNA–1273 | 3007 (7) | 100 (3) | 71 406 (15) | 3007 (17) | 100 (19) | 71 406 (18) | 2554 (8) | 61 (4) | 170 714 (17) | 2554 (17) | 61 (16) | 170 714 (20) |
| Two mixed mRNA | 1207 (3) | 50 (2) | 28 815 (6) | 1207 (7) | 50 (9) | 28 815 (7) | 357 (1) | 8 (1) | 20 921 (2) | 357 (2) | 8 (2) | 20 921 (2) |
| Two ChAdOx1 | 1246 (3) | 33 (1) | 12 682 (3) | 1246 (7) | 33 (6) | 12 682 (3) | 597 (2) | 30 (2) | 22 740 (2) | 597 (4) | 30 (8) | 22 740 (3) |
| Two mixed ChAdOx1/mRNA | 962 (2) | 6 (<1) | 22 094 (5) | 962 (5) | 6 (<1) | 22 094 (6) | 645 (2) | 12 (1) | 42 937 (4) | 645 (4) | 12 (3) | 42 937 (5) |
| Interval between dosesc | ||||||||||||
| 21–34 days (3–4 weeks) | NA | NA | NA | 659 (4) | 24 (5) | 11 738 (3) | NA | NA | NA | 1151 (8) | 38 (10) | 40 264 (5) |
| 35–48 days (5–6 weeks) | NA | NA | NA | 1611 (9) | 46 (9) | 25 028 (6) | NA | NA | NA | 1158 (8) | 14 (4) | 50 667 (6) |
| 49–62 days (7–8 weeks) | NA | NA | NA | 6473 (36) | 88 (16) | 151 324 (39) | NA | NA | NA | 4346 (29) | 53 (14) | 220 589 (26) |
| 63–83 days (9–11 weeks) | NA | NA | NA | 5787 (32) | 180 (34) | 138 467 (35) | NA | NA | NA | 4926 (33) | 108 (28) | 289 152 (35) |
| 84–111 days (12–15 weeks) | NA | NA | NA | 2805 (16) | 161 (30) | 55 546 (14) | NA | NA | NA | 2242 (15) | 99 (26) | 154 183 (18) |
| 112+ days (16+ weeks) | NA | NA | NA | 500 (3) | 38 (7) | 9257 (2) | NA | NA | NA | 1273 (8) | 74 (19) | 82 643 (10) |
| Median (IQR) days | NA | NA | NA | 63 (55–77) | 76 (60–91) | 63 (56–75) | NA | NA | NA | 65 (56–82) | 80 (59–100) | 69 (57–87) |
| Time since second dosec | ||||||||||||
| 0–13 days (0–1 weeks) | NA | NA | NA | 1206 (7) | 29 (5) | 19 806 (5) | NA | NA | NA | 917 (6) | 17 (4) | 51 377 (6) |
| 14–27 days (2–3 weeks) | NA | NA | NA | 498 (3) | 14 (3) | 24 902 (6) | NA | NA | NA | 425 (3) | 21 (5) | 58 734 (7) |
| 28–55 days (4–7 weeks) | NA | NA | NA | 2016 (11) | 45 (8) | 63 850 (16) | NA | NA | NA | 1640 (11) | 35 (9) | 137 333 (16) |
| 56–83 days (8–11 weeks) | NA | NA | NA | 3361 (19) | 95 (18) | 83 821 (21) | NA | NA | NA | 2821 (19) | 59 (15) | 167 477 (20) |
| 84–111 days (12–15 weeks) | NA | NA | NA | 4051 (23) | 121 (23) | 87 149 (22) | NA | NA | NA | 3300 (22) | 98 (25) | 175 612 (21) |
| 112–139 days (16–19 weeks) | NA | NA | NA | 3750 (21) | 114 (21) | 67 778 (17) | NA | NA | NA | 3428 (23) | 87 (23) | 148 369 (18) |
| 140–167 days (20–23 weeks) | NA | NA | NA | 1739 (10) | 87 (16) | 29 354 (8) | NA | NA | NA | 1847 (12) | 55 (14) | 71 897 (9) |
| 168–195 days (24–27 weeks) | NA | NA | NA | 381 (2) | 12 (2) | 5815 (1) | NA | NA | NA | 455 (3) | 9 (2) | 18 520 (2) |
| 196+ days (28+ weeks) | NA | NA | NA | 833 (5) | 20 (4) | 8885 (2) | NA | NA | NA | 263 (2) | 5 (1) | 8179 (1) |
| Median (IQR) days | NA | NA | NA | 96 (63–127) | 103 (71–133) | 85 (51–116) | NA | NA | NA | 98 (64–129) | 102 (72–127) | 84 (48–118) |
Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine; Hosp, hospitalized cases; IQR, interquartile range; NA, not applicable.
Unless otherwise specified, percentages are cases, hospitalized cases, or controls who received a second vaccine dose on/before specimen collection, by row category, regardless of time since second dose. Excludes specimens from once or more than twice vaccinated on/before specimen collection.
Displayed is the percentage of cases who were hospitalized.
Vaccinated tallies are specimens from individuals twice vaccinated on or before specimen collection, regardless of time since second dose. Unvaccinated received no vaccine doses on or before specimen collection. Excludes specimens from single-dose recipients or those thrice vaccinated on or before specimen collection. All percentages displayed below this row are column %.
In British Columbia, 2 cases (1 hospitalized) and 6 controls from vaccinated individuals had an unspecified vaccine type for the first or second dose.
During epi-weeks 22–34, 90% of case viruses in BC and 59% in Quebec were genetically characterized: Alpha, Gamma, and Delta comprised 7%, 7%, and 86%, respectively, in BC and 41%, 0%, and 59% in Quebec (Supplementary Table 2). In both provinces, more than 70% of cases accrued during the second half of the analysis period. From epi-weeks 35–47, 65% and 42% of BC and Quebec case viruses, respectively, were genetically characterized, of which 99% or more were Delta. Assuming full Delta attribution from epi-week 35 in BC and epi-week 36 in Quebec, 94% (42 143/44 964) and 84% (26 520/31 718), respectively, of case viruses across the analysis period were Delta.
Vaccination Profiles
Compared with provincial coverage estimates, the weekly percentage who were twice-vaccinated among study controls was higher in the early analysis period (as expected given the exclusion of remaining single-dose recipients), becoming similar (within 5%) from epi-week 30 in BC and epi-week 33 in Quebec (Supplementary Figure 1). Among twice-vaccinated controls in both provinces, approximately 90% received 2 mRNA doses: 66–69% BNT162b2 and 18–20% mRNA-1273 (Table 1). ChAdOx1 recipients comprised 3% and were generally older and more often male (Supplementary Tables 3 and 4). Mixed mRNA recipients comprised 7% in BC and 2% in Quebec, with approximately 5% in both provinces receiving mixed ChAdOx1 + mRNA doses.
Among twice-vaccinated controls, the median interval between first and second doses of mRNA or ChAdOx1vaccines was 63 and 62 days, respectively, in BC, and longer at 69 and 73 days in Quebec. In both provinces, few mRNA or ChAdOx1 recipients were revaccinated less than 7 weeks apart (∼10% and 5%, respectively). More ChAdOx1 recipients in Quebec were revaccinated at 9–11-week interval, also with more in Quebec who were revaccinated at 12 weeks or later among recipients of both kinds of vaccine (Supplementary Tables 3 and 4). Among twice-vaccinated controls in both provinces, median follow-up post–second dose was approximately 12 weeks, and slightly longer for ChAdOx1 recipients at approximately 14–15 weeks (Supplementary Tables 3 and 4).
Vaccine Effectiveness
Overall
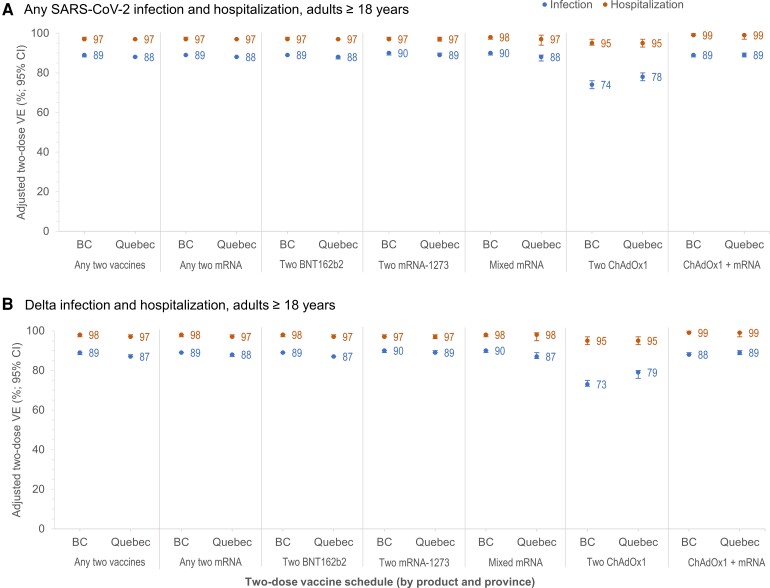
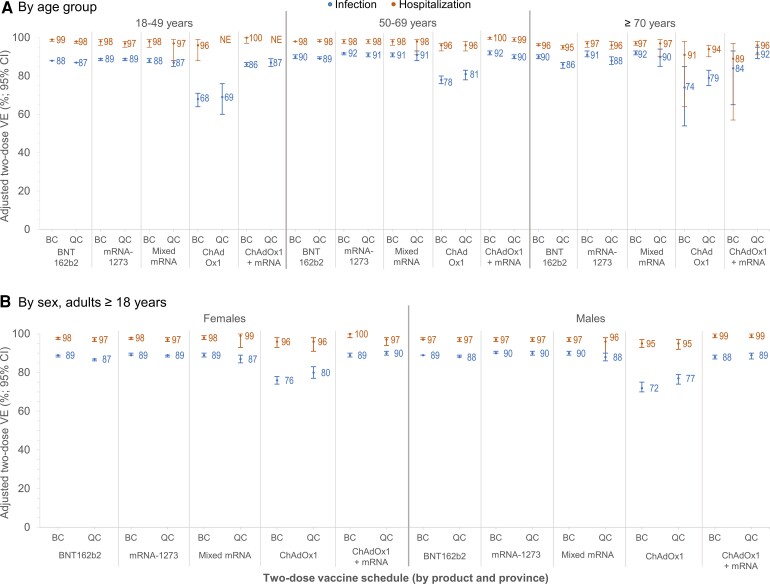
At 14 days or more post–second dose, all schedules of homologous or heterologous mRNA and/or ChAdOx1 vaccines were associated with 95% or greater reduction in SARS-CoV-2 hospitalization risk (Figure 1). Vaccine effectiveness against infection was 88–90% for 2 homologous or heterologous mRNA doses (Figure 1, Supplementary Table 5), significantly lower for 2 homologous ChAdOx1 doses at 74% (95% CI: 72–76%) in BC and 78% (95% CI: 76–80%) in Quebec, but improved significantly with heterologous ChAdOx1 + mRNA vaccination at 89% (95% CI: 88–90%). Findings were similar by age group, sex (Figure 2, Supplementary Table 8), and VOC (Figure 1, Supplementary Tables 9 and 10).
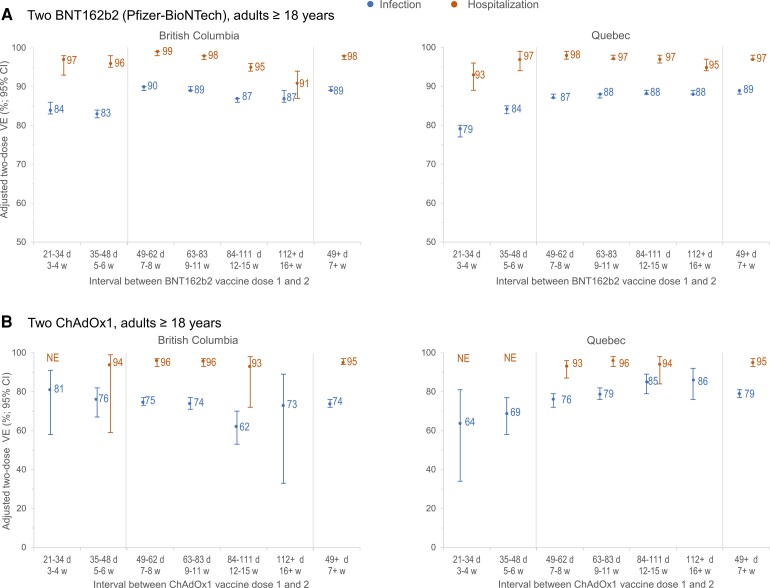
Figure 1.
Adjusted 2-dose VE against infection and hospitalization (overall and Delta-specific), by vaccine type, in adults ≥18 years old: British Columbia and Quebec, Canada, epi-weeks 22–47 (30 May–27 November) of 2021. Shown are adjusted VE and 95% CIs against infection (blue) and hospitalization (orange) ≥14 days after the second dose by vaccine type, overall (A) and for the Delta variant of concern (B) among adults ≥18 years old in the provinces of BC and Quebec, Canada. In Quebec, VE against Delta hospitalization was assessed only between epi-weeks 31 and 47 because no hospitalized Delta variant cases were identified prior to that period. For additional details, including corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Table 5 (overall) and Supplementary Tables 9 and 10 (Alpha, Gamma, and Delta variants of concern). Abbreviations: BC, British Columbia; ChAdOx1, chimpanzee adenoviral vectored vaccine; CI, confidence interval; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness.
Figure 2.
Adjusted 2-dose VE against infection and hospitalization, by age group, sex, and vaccine type in adults ≥18 years old: British Columbia and Quebec, Canada, epi-weeks 22–47 (30 May–27 November) of 2021. Shown are adjusted VE estimates and 95% CIs against infection (blue) and hospitalization (orange) ≥14 days after the second dose by age group (A) and sex (B) and by vaccine type in BC and Quebec, Canada. In Quebec, adjusted VE against hospitalization in ≥70-year-old adults required collapse of epi-week categories (triweekly) owing to sample-size considerations. For additional details, including corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Tables 6–8. Abbreviations: BC, British Columbia; ChAdOx1, chimpanzee adenoviral vectored vaccine; CI, confidence interval; mRNA, messenger RNA; NE, not estimable or total span of CI is ≥100%; QC, Quebec; VE, vaccine effectiveness.
With restriction to outpatient symptom-based testing in Quebec, findings were similar to overall estimates against any infection in both provinces, notably for mRNA recipients overall (within 5% absolute) and by epi-period (within 10% absolute) (Supplementary Table 11). Two-dose ChAdOx1 estimates were more variable, due to reduced sample size and other differences by province (eg, dosing intervals).
By Time Since Vaccination
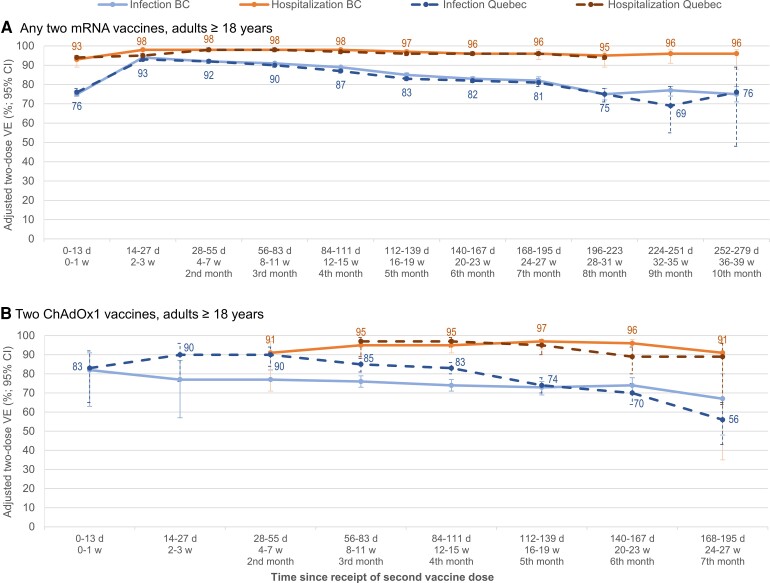
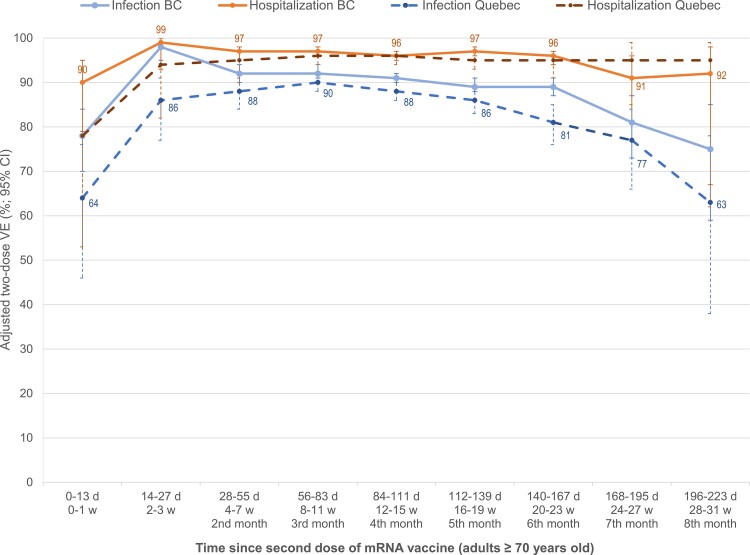
Two-dose VE of 95% or greater against hospitalization lasted at least 8 months for mRNA recipients and 5 months for ChAdOx1, remaining at 90% or greater for at least 7 months for both products (Figure 3, Supplementary Table 12). Against infection, 2-dose mRNA VE was more than 90% through at least the third month, declining slightly but still 80% or higher through 6–7 months postvaccination, including in adults aged 70 years and older (Figures 3 and 4, Supplementary Tables 12 and 13). Findings were similar for schedules including at least 1 mRNA dose (Supplementary Table 12), by age group (Supplementary Table 13), and for Delta-specific outcomes (Supplementary Table 14). In Quebec, 2-dose ChAdOx1 VE against infection was 80% or higher through 4 months postvaccination, while ranging from 74 to 77% in BC and 70% or higher in both provinces through the sixth month (Figure 3, Supplementary Table 12).
Figure 3.
Adjusted 2-dose mRNA and ChAdOx1 VE against infection and hospitalization by time since vaccination, in adults ≥18 years old: British Columbia and Quebec, Canada, epi-weeks 22–47 (30 May–27 November) of 2021. Shown are adjusted VE estimates and 95% CIs against infection (blue) and hospitalization (orange) by time between receipt of the second dose and specimen collection, among adults ≥18 years old in BC (solid lines) and Quebec (dashed lines). Panel A displays estimates for those who received any 2 mRNA vaccines and panel B displays estimates for those who received 2 ChAdOx1 vaccines. Due to sparse data, mRNA estimates are not displayed beyond the eighth month postvaccination against hospitalization in Quebec or beyond the seventh month postvaccination for ChAdOx1 in either province. Displayed are the point estimates against hospitalization for BC and against infection for Quebec. For additional details including all corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Table 12 (including details by mRNA vaccine type and for mixed product schedules). The corresponding information by age subgroups is displayed in Supplementary Table 13 and for Delta-specific VE in Supplementary Table 14 (also by mRNA vaccine type and for mixed products). Abbreviations: BC, British Columbia; ChAdOx1, chimpanzee adenoviral vectored vaccine; CI, confidence interval; d, days; mRNA, messenger RNA; VE, vaccine effectiveness; w, weeks.
Figure 4.
Adjusted 2-dose VE against infection and hospitalization, by time since mRNA vaccination, in adults ≥70 years old: British Columbia and Quebec, Canada. Shown are adjusted VE estimates and 95% CIs against infection (blue) and hospitalization (orange) by time between receipt of the second dose of any mRNA vaccine and specimen collection, among adults ≥70 years old in BC (solid lines) and Quebec (dashed lines). In Quebec, adjusted VE against hospitalization required collapse of epi-week categories (triweekly) owing to sample size considerations. Displayed are the point estimates against hospitalization for BC and against infection for Quebec. For additional details including all corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Table 13 (including details by type of mRNA vaccine). Abbreviations: BC, British Columbia; CI, confidence interval; d, days; mRNA, messenger RNA; VE, vaccine effectiveness; w, weeks.
By Interval Between Doses
As shown in Figure 5, BNT162b2 VE against infection was significantly higher when the interval between doses was extended from 3–4 or 5–6 weeks to 7–8 weeks, without further improvement thereafter. At intervals of 7 weeks or more versus 3–4 weeks between doses, BNT162b2 VE was significantly higher by 5% (absolute) in BC and 10% in Quebec. A similar but less pronounced pattern was observed for mRNA-1273. ChAdOx1 VE gradually increased with a longer dosing interval in Quebec but not in BC where CIs were wider (Figure 5, Supplementary Table 15). Vaccine effectiveness against hospitalization exceeded 90% regardless of dosing interval.
Figure 5.
Adjusted 2-dose VE against infection and hospitalization, by interval between doses, mRNA and ChAdOx1 vaccines, in adults ≥18 years old: British Columbia and Quebec, Canada. Shown are adjusted VE estimates and 95% CIs against infection (blue) and hospitalization (orange) at ≥14 days after the second dose, by interval between the first and second dose among adults ≥18 years old who were vaccinated with any 2 mRNA vaccines (A) or 2 ChAdOx1 vaccines (B) in British Columbia and Quebec. For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Table 15 (including details by type of mRNA vaccine). Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine; CI, confidence interval; d, days; mRNA, messenger RNA; NE, not estimable or total span of CI is ≥100%; VE, vaccine effectiveness; w, weeks.
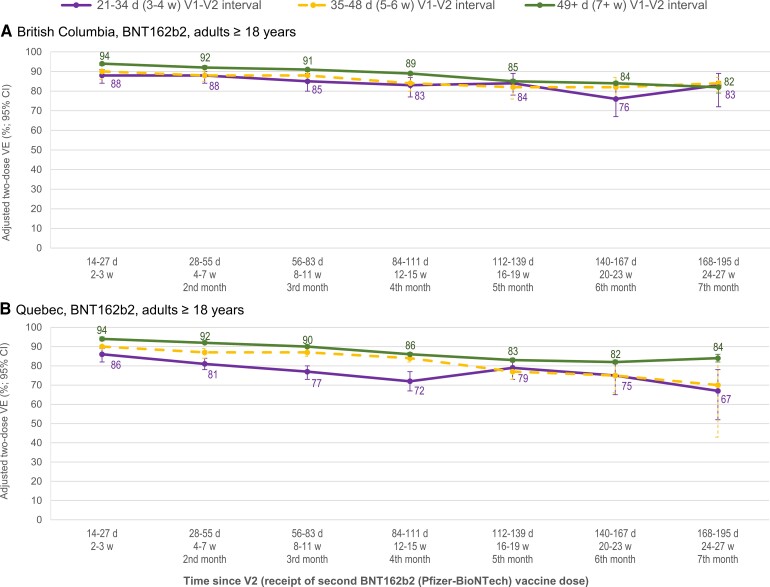
Since shorter dosing interval may have been associated with longer time since second dose, VE was stratified on both conditions. At intervals of 7–8 weeks versus 3–4 weeks between BNT162b2 doses, VE was 4–6% higher in BC and 8–14% higher in Quebec through the first 4 months post–second dose (Figure 6, Supplementary Table 16). Thereafter, estimates had wide CIs. A similar pattern was observed for mRNA-1273, but could not be assessed for ChAdOx1.
Figure 6.
Adjusted 2-dose BNT162b2 VE against infection by interval between doses and time since second dose, in adults ≥18 years old: British Columbia and Quebec, Canada. Shown are adjusted VE estimates and 95% CIs against infection by interval between the first and second BNT162b2 dose (3–4 weeks in purple; 5–6 weeks in dashed gold; 7+ weeks in green) and time since the second dose among adults ≥18 years old in the provinces of British Columbia and Quebec. For additional details including corresponding sample sizes and precise unadjusted and adjusted estimates with 95% CIs (and adjustment covariates specified), see Supplementary Table 16 (including both types of mRNA vaccine and ChAdOx1). Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine; CI, confidence interval; d, days; mRNA, messenger RNA; VE, vaccine effectiveness; V1, dose 1; V2, dose 2; w, weeks.
DISCUSSION
We report similar findings from 2 of Canada’s larger provinces, where mixed SARS-CoV-2 vaccine schedules and extended dosing intervals were adopted in response to urgent public health need during the pandemic. In both provinces, a 2-dose schedule using any combination of available homologous or heterologous vaccines was associated with a 90% or greater reduction in hospitalization risk for at least 7 months, spanning early-Alpha and late-Delta circulation. With a slight decline from a peak of more than 90%, 2-dose VE against infection was still 80% or more for at least 6 months following homologous mRNA vaccination, lower by approximately 10% when both doses were ChAdOx1, but improved and comparably high with heterologous ChAdOx1 + mRNA receipt. Vaccine protection was also improved when first and second mRNA doses were separated by 7–8 weeks rather than the manufacturer-specified 3–4 weeks apart.
Our observations of substantial and sustained mRNA VE align with randomized controlled trial (RCT) findings. In extended follow-up of participants in the BNT162b2 (Pfizer-BioNTech) RCT, 2-dose efficacy against clinical infection peaked at 96% during the first 2 months, remaining greater than 80% from 4 months to the end of follow-up [4, 5]. In the mRNA-1273 (Moderna) RCT, 2-dose efficacy against coronavirus disease 2019 (COVID-19) illness was 93%, without waning across a median of 5.2 months [6, 7]. In a 2-year open-label study initiated after unblinding of the Moderna RCT, COVID-19 incidence during the July/August 2021 Delta surge among earlier mRNA-1273–vaccinated participants (median, 13-month follow-up from first dose) was approximately 1.6 times greater than among the placebo recipients later vaccinated (median, 7.9-month follow-up) [8]. Applying a relative risk (RR) of 1.6 to the COVID-19 incidence among individuals vaccinated during the Moderna RCT corresponds to a minor decrease in efficacy from 93% to 89%, consistent with what we report [7, 8].
Sustained 2-dose VE against hospitalization, including Delta-associated, has been observed elsewhere, but with more variability in the reported duration of protection against infection [9–15]. Studies from Israel have reported greater risk of both infection and hospitalization with time since the second BNT162b2 dose [16]. Conversely, in the United Kingdom, VE against Delta hospitalization remained greater than 90% by 5 months after the second BNT162b2 dose, and lower against symptomatic infection at 70% [13]. Even lower mRNA VE against infection was reported by 5 months post–second dose from California (50%) and Qatar (22%) [14, 15]. Methodological differences should be considered in comparing findings across these studies. Despite limitations, surveillance data may also provide a reality check for some of the more dramatic declines in reported VE. For example, a VE = 50% from California corresponds to RR = 2 for COVID-19 in unvaccinated versus fully vaccinated people. Statewide surveillance instead showed an RR > 7 between 26 September and 2 October 2021 [17], crudely corresponding to a VE = 87% at about 5 months after most fully vaccinated Californians had received their second dose. UK surveillance-based RRs seem more in keeping with their VE estimates [18]. Likewise, in BC and Quebec, surveillance-based (age-adjusted) RRs in November 2021 of 32 and 16, respectively, against hospitalization correspond to VE estimates of greater than 90%, and RRs of 8 and 3.6, respectively, against infection correspond to VE estimates of greater than 70% [19, 20], in keeping with the sustained VE we report.
Pandemic vaccine program modifications in BC and Quebec were informed by ethical and vaccine principles, real-time risk–benefit assessment, and expert committee recommendations, but were implemented outside of regulatory approval. To date, there is still no head-to-head RCT comparison of mixed (heterologous) versus matched (homologous) SARS-CoV-2 vaccine efficacy. Immunogenicity studies show higher antibody titers following heterologous ChAdOx1 + mRNA versus homologous vector-based vaccination, with titers similar to homologous mRNA vaccination [21–23], but antibody thresholds for protection are not established. Our findings are thus important in providing epidemiological evidence in support of SARS-CoV-2 vaccine interchangeability and, moreover, the preferred use of mRNA vaccines to complete the 2-dose series initiated with ChAdOx1. The NACI recommendation in June 2021 enabling mixed schedules in Canada (Supplementary Table 1) removed the requirement to retain half of available doses in reserve for homologous series completion. This decision simplified vaccine logistics and likely improved protection for ChAdOx1 recipients during the ensuing Delta wave.
The lower 2-dose ChAdOx1 versus mRNA VE we report against infection is consistent with indirect comparison of efficacies across product-specific RCTs (∼67% vs ∼90%, respectively) [4, 6, 7, 24]. In pooled RCT meta-analysis, ChAdOx1 efficacy was better at longer intervals between doses: 55% at less than 6 weeks, 60% at 6–8 weeks, 64% at 9–11 weeks, and 81% at 12 weeks or more. Among RCT participants, 82% were revaccinated at the less-than-12-week interval, two-thirds of these at the shortest less-than-6-week spacing [24]. Our ChAdOx1 VE estimates are also weighted by and may reflect this variation by dosing interval. In BC, 94% of controls twice-vaccinated with ChAdOx1 were revaccinated at less than 12 weeks, about half at 7–8 weeks, and 40% at 9–11 weeks, with overall VE = 74%. In Quebec, 86% were revaccinated at less than 12 weeks, about one-third of them at 7–8 weeks, but more (60%) at the longer 9–11-week interval, yielding an overall higher ChAdOx1 VE = 78%, and exceeding 80% at the interval of 12 weeks or more, as per RCT analysis [24].
Improved 2-dose VE with longer spacing between the first and second doses may reflect improved opportunity for immune maturation between prime-boost events, reinforced empirically for mRNA vaccines in recent immunogenicity studies [25–28]. In addition to the population-based findings we report here, we also found 5–7% higher mRNA VE with the 7-week-or-greater compared with the 3–5-week dosing interval in TND analysis restricted to BC healthcare workers [29]. Epidemiological findings from the United Kingdom, however, have been more variable [25, 30, 31]. In a TND study of adults aged 50 years and older, Amirthalingam et al [25] found higher BNT162b2 VE at an authorized schedule of more than 6 weeks versus 3 weeks between doses, whereas longitudinal studies among UK households [30] and healthcare workers [31] found no difference by interval. In dichotomizing dosing intervals, however, the latter 2 studies combined 7–8 weeks with shorter spacing (<9 weeks and ≥6 weeks, respectively), without displaying participant distributions by additional interval categories and potentially obscuring VE differences on that basis.
Ultimately, the optimal interval between first and second doses represents a balance between rapid and enhanced protection. Rapid revaccination may prevent some additional cases in the short term, but with substantial single-dose protection the absolute difference in severe outcomes prevented would be small outside periods of intense epidemic surge. Conversely, with a few added weeks between doses, the more durable immunity and approximately 5–10% increment in VE we report could ultimately prevent more cases and hospitalizations in the long term (depending upon evolving incidence and duration of protection). Informed by these and other considerations, including vaccine safety and routine schedule harmonization, Canada’s NACI articulated 8 weeks as the preferred interval between mRNA doses in October (epi-week 42), 2021 (Supplementary Table 1), as did the World Health Organization in January (epi-week 3), 2022 [32], and the US Centers for Disease Control and Prevention in February (epi-week 8), 2022 [33].
Our study, based on general laboratory submissions and surveillance data, has limitations. Provincial immunization registries and NAAT-specific detection mitigated vaccination or outcome misclassification, but incomplete information remains possible. Exclusion of cases before the analysis period will have been incomplete, recognizing that not all infections were tested. The TND standardizes for the likelihood of being tested, but case ascertainment may vary by testing indication and vaccine status. Testing in both provinces was foremost, but not exclusively, symptom based. With restriction to outpatient symptom-based testing in Quebec, however, findings were similar to overall estimates against any infection in both provinces. We cannot rule out residual confounding such as associated with comorbidity, ethnicity, or socioeconomic status. Healthcare workers or immunocompromised individuals targeted for more rapid second-dose administration may have contributed to lower VE with shorter dosing intervals; however, weighted by their small percentage of the population, such underestimation would be minor. With high vaccine coverage, the subset remaining unvaccinated may differ, with the direction of resulting bias unknown and likely to vary with other public health measures. Reduced sample size affects the stability and precision of VE estimates, especially with greater sub-stratification. Finally, our studies were conducted in community-dwelling adults and may not be generalizable to other groups such as care-facility residents.
In conclusion, 2 doses of homologous or heterologous mRNA and/or ChAdOx1 vaccines provided powerful and persistent protection against hospitalization, spanning the duration of Delta dominance. Our findings support interchangeability and extended intervals between SARS-CoV-2 vaccine doses, with potential global implications for low-coverage areas and/or future cohorts of children. Vaccine effectiveness estimates reflect the prevailing conditions of vaccine-relatedness to predominantly circulating VOCs during the study period. As conditions change (eg, emergence and spread of Omicron or other immunological-escape variants), further vaccine and program adjustments (eg, antigen update and/or additional doses) should be guided by ongoing, real-time, risk–benefit assessment.
Supplementary Material
Contributor Information
Danuta M Skowronski, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada; University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada.
Yossi Febriani, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada.
Manale Ouakki, Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada.
Solmaz Setayeshgar, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada.
Shiraz El Adam, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada.
Macy Zou, BC Centre for Disease Control, Data and Analytics Services, Vancouver, British Columbia, Canada.
Denis Talbot, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Natalie Prystajecky, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada; University of British Columbia, Department of Pathology and Laboratory Medicine, Vancouver, British Columbia, Canada.
John R Tyson, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada.
Rodica Gilca, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Nicholas Brousseau, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Geneviève Deceuninck, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada.
Eleni Galanis, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada; University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada.
Chris D Fjell, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada.
Hind Sbihi, University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada; BC Centre for Disease Control, Data and Analytics Services, Vancouver, British Columbia, Canada.
Elise Fortin, Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada; Université de Montréal, Département de Microbiologie, Infectiologie et Immunologie, Montreal, Quebec, Canada.
Sapha Barkati, McGill University, Department of Medicine, Division of Infectious Diseases, McGill University Health Center, Montreal, Quebec, Canada.
Chantal Sauvageau, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Monika Naus, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada; University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada.
David M Patrick, BC Centre for Disease Control, Communicable Diseases and Immunization Services, Vancouver, British Columbia, Canada; University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada.
Bonnie Henry, University of British Columbia, School of Population and Public Health, Vancouver, British Columbia, Canada; Office of the Provincial Health Officer, Ministry of Health, Victoria, British Columbia, Canada.
Linda M N Hoang, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada; University of British Columbia, Department of Pathology and Laboratory Medicine, Vancouver, British Columbia, Canada.
Philippe De Wals, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Christophe Garenc, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada.
Alex Carignan, Sherbrooke University, Department of Microbiology and Infectious Diseases, Sherbrooke, Quebec, Canada.
Mélanie Drolet, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Agatha N Jassem, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada; University of British Columbia, Department of Pathology and Laboratory Medicine, Vancouver, British Columbia, Canada.
Manish Sadarangani, BC Children’s Hospital Research Institute, Vaccine Evaluation Center, Vancouver, British Columbia, Canada; University of British Columbia, Department of Pediatrics, Vancouver, British Columbia, Canada.
Marc Brisson, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Mel Krajden, BC Centre for Disease Control, Public Health Laboratory, Vancouver, British Columbia, Canada; University of British Columbia, Department of Pathology and Laboratory Medicine, Vancouver, British Columbia, Canada.
Gaston De Serres, Centre Hospitalier Universitaire (CHU) de Québec-Université Laval Research Center, Quebec City, Quebec, Canada; Institut National de Sante Publique du Québec, Biological and Occupational Risks, Quebec City, Quebec, Canada; Laval University, Department of Social and Preventive Medicine, Faculty of Medicine, Quebec City, Quebec, Canada.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Shinhye Kim for support in manuscript assembly and summary tabulation and Erica Chuang for analytical support, both of the British Columbia Centre for Disease Control. They also thank the many frontline, regional, and provincial practitioners, including clinical, laboratory and public health providers, epidemiologists, Medical Health Officers, laboratory staff, vaccinators, participants, and others who contributed to the epidemiological, virological and genetic characterization data underpinning these analyses.
Disclaimer. The funders did not play a role in the design, results, interpretation, or decision to submit the manuscript for publication.
Financial support. This work was supported by provincial health authorities and, in part, by the British Columbia Centre for Disease Control Foundation for Public Health. M. S. acknowledges general salary support provided to him by awards from the British Columbia Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program, and the Michael Smith Foundation for Health Research. D. T. was recipient of a Career Award from the Fond de Recherche du Québec–Santé.
References
- 1. Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Eng J Med 2021; 384:1576–7. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. Interim guidance. Geneva, Switzerland: WHO, 2021. Available at:https://assets.documentcloud.org/documents/20445916/who-2019-ncov-vaccines-sage_recommendation-bnt162b2-20211-eng.pdf. Accessed 21 March 2022. [Google Scholar]
- 3. Joint Committee on Vaccination and Immunisation (JCVI) . Advice on priority groups for COVID-19 vaccination. JCVI, 2020. Available at:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950113/jcvi-advice-on-priority-groups-for-covid-19-vaccination-30-dec-2020-revised.pdf. Accessed 21 March 2022. [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv [preprint], 28 July 2021. doi: 10.1101/2021.07.28.21261159. [DOI]
- 6. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. N Engl J Med 2021; 385:2485–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med 2021; 385:1355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernal JL, Andrews N, Gower C, et al. Effectiveness of Covid-19, vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrejko KL, Pry J, Myers JF, et al. Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis 2022; 74:1382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y) 2021; 2:979–92, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. Knowledgehub [preprint], 15 September 2021. Available at: https://khub.net/documents/135939561/338928724/Vaccine+effectiveness+and+duration+of+protection+of+covid+vaccines+against+mild+and+severe+COVID-19+in+the+UK.pdf/10dcd99c-0441-0403-dfd8-11ba2c6f5801. Accessed 16 October 2021.
- 14. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Government of California . Tracking COVID-19 in California. Unvaccinated and vaccinated data. Updated 15 October 2021. Available at:https://covid19.ca.gov/state-dashboard/#postvax-status. Accessed 16 October 2021.
- 18. UK Health Security Agency . COVID-19 vaccine surveillance report, week 39. Available at:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1022238/Vaccine_surveillance_report_-_week_39.pdf. Accessed 16 October 2021.
- 19. British Columbia Centre for Disease Control (BCCDC) . BC COVID-19 data. December 2 data summary. 2021. Vancouver, Canada: BCCDC. Available at:http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data. Accessed 21 March 2022. [Google Scholar]
- 20. Gouvernement du Québec . Tableau de bord—situation de la COVID-19–29 Novembre 2021. Available at:https://cdn-contenu.quebec.ca/cdn-contenu/sante/documents/Problemes_de_sante/covid-19/20-210-382W_infographie_sommaire-executif.jpg? 1634310027. Accessed 22 March 2022.
- 21. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID- 19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021; 398:856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med 2022; 386:1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med 2021; 27:1525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun 2021; 12:7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021; 184:5699–714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grunau B, Asamoah-Boaheng M, Lavoie PM, et al. A higher antibody response is generated with a 6- to 7-week (vs standard) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dosing interval. Clin Infect Dis 2022; 75:e888–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall VG, Ferreira VH, Wood H, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol 2022; 23:380–5. [DOI] [PubMed] [Google Scholar]
- 29. El Adam Shiraz, Zou Macy, Kim Shinhye, et al. SARS-CoV-2 mRNA vaccine effectiveness in health care workers by dosing interval and time since vaccination: test-negative design, British Columbia, Canada. Open Forum Infect Dis 2022; 9(5):e475. doi: 10.1093/ofid/ofac178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall V, Foulkes S, Insalata F, et al. Effectiveness and durability of protection against future SARS-CoV-2 infection conferred by COVID-19 vaccination and previous infection; findings from the UK SIREN prospective cohort study of healthcare workers March 2020 to September 2021. medRxiv [preprint], 1 December 2021. Available at: https://www.medrxiv.org/content/10.1101/2021.11.29.21267006v1. Accessed 21 March 2022.
- 32. World Health Organization (WHO) . Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing. Geneva, Switzerland: WHO, 2022. Available at:https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1. Accessed 21 March 2022. [Google Scholar]
- 33. Centers for Disease Control and Prevention (CDC) . Use of COVID-19 vaccines in the United States. Interim clinical considerations. Atlanta: CDC, 2022. Available at:https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed 21 March 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.