Abstract
The potential preventive efficacy of tenofovir/emtricitabine on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was assessed in human immunodeficiency virus preexposure prophylaxis (PrEP) users. Prevalence of SARS-CoV-2 immunoglobulin G between May and October 2020 was similar in PrEP users and in a matched population-based cohort, suggesting that tenofovir/emtricitabine has no role in reducing the risk of SARS-CoV-2 acquisition.
Keywords: HIV, PrEP, SARS-CoV-2
Coronavirus disease 2019 (COVID-2019), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been defined as a pandemic by the World Health Organization. SARS-CoV-2 is a coronavirus, like the severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses, that is a positive-sense single-strand RNA virus with replication mechanism requiring an RNA-dependent RNA polymerase (RdRp). Therefore, at the beginning of the pandemic, known drugs targeting viral RdRp of SARS-CoV or other coronaviruses were repurposed due to the urgent need for an antiviral therapy for the new member of the family, SARS-CoV-2.
Tenofovir disoproxil fumarate (TDF), one of the components of preexposure prophylaxis (PrEP), is transformed intracellularly in its active form, tenofovir triphosphate, which inhibits the activity of viral polymerase in human immunodeficiency virus (HIV) and hepatitis B virus and has also shown an antiviral activity on SARS-CoV-2 in vitro and in a ferret model [1–3].
Different observational studies, performed between February and September 2020, report a matter of debate regarding the potential benefit of TDF to reduce the incidence of SARS-CoV-2 infection or COVID-19–related morbidity and mortality in persons using TDF-based regimens as PrEP or treatment for HIV-infected patients [4–8].
To assess the potential preventive impact of TDF/emtricitabine (FTC) on SARS-CoV-2 acquisition, we compared during the same time period the seroprevalence of SARS-CoV-2 immunoglobulin G (IgG) antibody rates in male participants of the PREVENIR cohort study of PrEP implementation in the Paris area, with participants using intermittent or daily TDF/FTC to that of male participants enrolled in the SAPRIS-Sero study in Paris area, France.
METHODS
Study Population
Between 1 May and 31 October 2020, male participants using either daily or on-demand PrEP with TDF/FTC enrolled in the PREVENIR study conducted in the Paris area, France, and who had a stored serum sample available were included in this study [9]. Participants unexposed to TDF/FTC were male participants living in the Paris area included in the SAPRIS-Sero national survey that estimates SARS-CoV-2 antibody prevalence in the French general population [10].
Based on the factors associated with the risk of SARS-CoV-2 infection in the initial analysis of the SAPRIS cohort [11] and on available data in both studies, the matching criteria were age (±5 years), socio-occupational category, and date of sampling (±1 month). Ethical approval and written or electronic informed consent were obtained from each participant before enrollment in both original cohorts. When PREVENIR participants could not be matched on the 3 criteria, they were matched on age and socio-occupational category, then on age and sampling date, then on age.
Patient Consent Statement
Ethical approval and written or electronic informed consent were obtained from each participant before enrollment in the original cohort. The SAPRIS-Sero study was approved by the Sud-Mediterranée III ethics committee (approval number 20.04.22.74247). The PREVENIR study was approved by the CPP Paris Ile de France IV ethics committee (NCT03113123 and EudraCT 2016-A01577-44).
Virological Tests
A systematic serology was performed in all participants in both cohorts. In the PREVENIR cohort, the SARS-CoV-2 IgG determination was performed using the SARS-CoV-2 IgG II Quantitative antibody enzyme-linked immunosorbent assay (ELISA; Architect, Abbott, France) on frozen serum. It is a chemiluminescent microparticle immunoassay used for the qualitative and quantitative determination of IgG antibodies against the spike receptor-binding domain of SARS-CoV-2. The analytical measurement interval is stated as 21 to 40 000 AU/mL, and positivity cutoff is ≥50 AU/mL (and low positive <100 AU/mL). In the SAPRIS-Sero cohorts, we used the anti–SARS-CoV-2 IgG ELISA (Euroimmun, Germany) that provides semi-quantitative serology results against the S1 domain of the spike protein of SARS-CoV-2 [10]. Samples with an ELISA-S test optical density ratio of <0.8 were considered as negative, ≥0.8 to <1.0 as undetermined, and ≥1.0 as positive. For both tests, the sensitivity (based on 14-day post–positive reverse-transcription polymerase chain reaction [PCR] samples) and specificity were >98% and >99%, respectively [12].
Statistical Methods
Because only age, socioprofessional category, and date of sampling were collected in both studies and used as matching criteria, we did not compare these factors between the 2 study populations.
The primary outcome was the proportion of participants with positive SARS-CoV-2 IgG. The primary analysis considered low-positive results as positive serology and undetermined results as negative serology. Odds ratios of the comparison between studies were calculated using logistic regression, stratified on each matched pair without adjustment because only the matching criteria identified as the main factors associated with the risk of SARS-CoV-2 infection were available in both studies.
Two sensitivity analyses were performed: (1) restricted to fully matched participants and (2) considering undetermined results as positive serology.
RESULTS
In PREVENIR, 844 participants with a median age of 38 (interquartile range [IQR], 31–45) years were matched to 844 participants of SAPRIS-Sero cohort, aged 41 (IQR, 35–48) years. Median month of sampling was August (IQR, July–September) for PREVENIR and August (IQR, July–August) for SAPRIS.
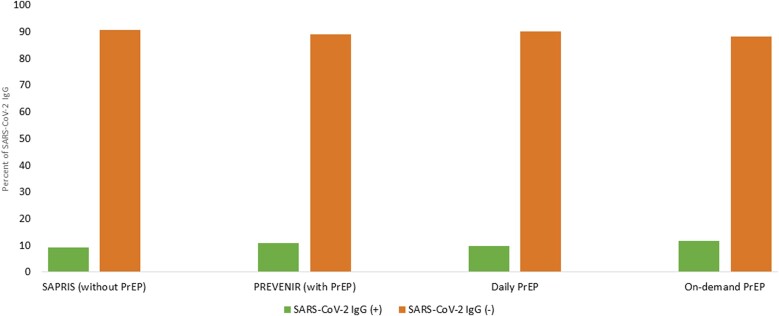
PrEP was on demand in 420 (49.8%) and daily in 424 (50.2%) individuals. Because the matching on 3 criteria concerned 86.5% (n = 729) individuals, the results are presented for the whole and fully matched population. As shown in Table 1 and Figure 1, SARS-CoV-2 IgG was positive in 91 (10.8%) and 78 (9.2%) for the whole population in the PREVENIR and SAPRIS-Sero cohorts, respectively (odds ratio, 1.17 [95% confidence interval, .86–1.60]). The seroprevalence rates were similar among daily or on-demand users (9.9% vs 11.7%, Fisher exact P = .4095). Sensitivity analyses (restricted to fully matched participants or considering undetermined as positive serology) led to similar results.
Table 1.
Proportion of Participants With Positive Serology for Severe Acute Respiratory Syndrome Coronavirus 2
| Cohort | Positive Rate | OR (95% CI) | P Value |
|---|---|---|---|
| Whole population | |||
| SAPRIS (n = 844) | 78 (9.2%) | 1 | |
| PREVENIR (n = 844) | 91 (10.8%) | 1.2 (.862–1.603) | .3061 |
| Fully matched population (on 3 criteria) | |||
| SAPRIS (n = 729) | 66 (12.0%) | 1 | |
| PREVENIR (n = 729) | 78 (10.7%) | 1.1 (.786–1.604) | .5248 |
Abbreviations: CI, confidence interval; OR.
Figure 1.
Presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) in 844 males living in the Paris area, France, with the use of tenofovir/emtricitabine preexposure prophylaxis (PrEP) (PREVENIR cohort), matched to males without PrEP (SAPRIS cohort). In the PREVENIR cohort, PrEP was on demand in 420 (49.8%) and daily in 424 (50.2%) individuals.
DISCUSSION
We evaluated whether the use of tenofovir/emtricitabine as HIV preexposure prophylaxis in HIV-negative males included in the PREVENIR study could reduce the prevalence of SARS-CoV-2 seroconversion as compared to a matched to a control cohort of males from the general population not using PrEP in Paris (from SAPRIS). The SARS-CoV-2 IgG seroprevalence was 10.3% in the PREVENIR study and 9.2% in the SAPRIS survey among 844 male individuals between May to October 2020 in the Paris region. This prevalence of 10% was found for the Paris region corresponding to one of the most affected regions by the first wave of SARS-CoV-2 [10]. Interestingly, we showed that the proportion of participants with a positive SARS-CoV-2 serology was similar whether PrEP was used daily or on demand. Because the SARS CoV-2 serology was performed in all patients in both cohorts, our results were not dependent of the presence or absence of symptoms.
Several publications have associated the use of TDF to a significantly lower risk of SARS-CoV-2 infection, COVID-19–related hospitalization, or death among HIV-infected persons receiving TDF-based antiretroviral regimens than among those receiving other HIV therapies (eg, abacavir or tenofovir alafenamide [TAF]) [4–6], while the difference of effect between TAF and TDF was poorly explained. Baseline characteristic of patients such as age and comorbidities were not accounted for in these studies, while each has been identified as an independent risk factor for poorer outcomes with COVID-19 [13] and can influence the choice of regimen, for instance not using TDF in patients with renal impairment. Recently, an update of the study from del Amo demonstrated that TDF has a protective effect restricted to individuals aged 50 years and older [14]. Moreover, during the first wave, most SARS-CoV-2 PCR tests were done only in symptomatic or even hospitalized individuals, which could lead to bias.
One study from May to June 2020 in Spain assessed the effect of tenofovir-emtricitabine use as HIV PrEP in HIV-negative persons showing a higher seroprevalence to SARS-CoV-2 IgG anti-nucleocapsid than in persons without PrEP (15% vs 9.2%, P = .026) [7]. Interestingly, there were no significant differences in the sex, presence of comorbidities, occupational exposure, or exposure by households with confirmed cases. However, the population of PrEP users and those who did not take PrEP was not adjusted, in contrast to our study in which we selected factors (age, socio-occupational category, and sampling date) that were found to be associated with the risk of SARS-CoV-2 infection in the initial analysis of the SAPRIS cohort [11].
Our study has some limitations. First, we did not collect exhaustive information about positive SARS-CoV-2 PCR and COVID-19–like symptoms to determine the effect TDF/FTC on SARS-CoV-2 infection, since some individuals might become infected without seroconversion. Our study could not explore the effect of TDF on illness but only on SARS-CoV-2 infection.
Second, the factors of exposure to SARS-CoV-2 were not collected. Third, we used 2 serological tests from different manufacturers to compare the IgG detection, but the viral target is the same and sensitivity and specificity were comparable [12]. A lower sensitivity of the test used in the SAPRIS-Sero cohort compared with the test used in the PREVENIR study would not affect our conclusions as it would decrease the difference in measured seroprevalence.
To conclude, the similar seroprevalence of SARS-CoV-2 IgG found in participants using or not using TDF/FTC as HIV PrEP suggests that TDF/FTC has no role in reducing SARS-CoV-2 acquisition.
Contributor Information
Constance Delaugerre, Service de Virologie, Hôpital Saint Louis, Assistance Publique–Hôpitaux de Paris, Paris, France; Université de Paris, Inserm, U944, Paris, France.
Lambert Assoumou, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France.
Sarah Maylin, Service de Virologie, Hôpital Saint Louis, Assistance Publique–Hôpitaux de Paris, Paris, France.
Marine Minier, Service de Virologie, Hôpital Saint Louis, Assistance Publique–Hôpitaux de Paris, Paris, France.
Audrey Gabassi, Service de Virologie, Hôpital Saint Louis, Assistance Publique–Hôpitaux de Paris, Paris, France.
Michèle Genin, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France.
Lydie Beniguel, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France.
Jade Ghosn, Service de Maladies infectieuses, Hôpital Bichat, Assistance Publique–Hôpitaux de Paris, Infection, Antimicrobials, Modelling, Evolution, Inserm, Université mixte de recherche 1137, Université de Paris, Paris, France.
Xavier de Lamballerie, Unité des Virus Emergents, Université Aix Marseille, Institut de recherche pour le développement 190, Inserm U1207, Marseille, France.
Mayssam El Mouhebb, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France.
Dominique Costagliola, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France.
Fabrice Carrat, Sorbonne Université, Inserm, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France; Département de Santé Publique, Hôpital Saint-Antoine, Assistance Publique–Hôpitaux de Paris, Paris, France.
Jean Michel Molina, Université de Paris, Inserm, U944, Paris, France; Service de Maladies Infectieuses, Hôpital Saint Louis, Assistance Publique–Hôpitaux de Paris, Paris, France.
Notes
Financial support. This study was funded by a grant from the ANRS-PREVENIR study.
Potential conflicts of interest. J. M. M. has participated in advisory boards from Gilead, Merck, and ViiV, and has received research grants from Gilead. C. D. has participated in advisory boards for ViiV, Gilead, MSD, and Janssen, and has received grants from ViiV and Gilead. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jockusch S, Tao C, Li X, et al. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res 2020; 180:104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elfiky AA. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn 2021; 39:3204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park S-J, Yu K-M, Kim Y-I, et al. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio 2020; 11:e01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020; 173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenguer J, Díez C, Martín-Vicente M, et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect 2021; 27:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa . Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2021; 73:e2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayerdi O, Puerta T, Clavo P, et al. Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users. Open Forum Infect Dis 2020; 7:ofaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parienti J-J, Prazuck T, Peyro-Saint-Paul L, et al. Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021; 38:100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molina JM, Ghosn J, Delaugerre C, et al. Incidence of HIV infection with daily or on-demand oral PrEP with TDF/FTC in France. In: Conference on Retroviruses and Opportunistic Infections. 2021. https://www.croiconference.org/abstract/incidence-of-hiv-infection-with-daily-or-on-demand-oral-prep-with-tdf-ftc-in-france/. Accessed 18 January 2022. [Google Scholar]
- 10. Carrat F, de Lamballerie X, Rahib D, et al. Antibody status and cumulative incidence of SARS-CoV-2 infection among adults in three regions of France following the first lockdown and associated risk factors: a multicohort study. Int J Epidemiol 2021; 50:1458–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrat F, Touvier M, Severi G, et al. Incidence and risk factors of COVID-19-like symptoms in the French general population during the lockdown period: a multi-cohort study. BMC Infect Dis 2021; 21:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicol T, Lefeuvre C, Serri O, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J Clin Virol 2020; 129:104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li C, Islam N, Gutierrez JP, et al. Diabetes, obesity, hypertension and risk of severe COVID-19: a protocol for systematic review and meta-analysis. BMJ Open 2021; 11:e051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Amo J, Polo R, Moreno S, et al. Tenofovir disoproxil fumarate and severity of COVID-19 in people with HIV infection. In: Abstracts of the 2022 Conference on Retroviruses and Opportunistic Infections. Top Antiv Med 2022; 30(1 suppl):137. [Google Scholar]