Abstract
Background
While the number of cases of multisystem inflammatory syndrome in children (MIS-C) is increasing, reported cases in Asian countries are still low, particularly in Indonesia. This study aimed to describe the characteristics of patients with MIS-C in a tertiary referral hospital in Indonesia.
Methods
This is a cross-sectional study with collected data of patients with MIS-C admitted to Dr. Cipto Mangunkusumo from March 2020 to April 2021.
Results
The first case of MIS-C was detected 5 months after the first reported coronavirus disease 2019 case in Indonesia. Thirteen patients out of 158 positive admitted patients for COVID-19 were diagnosed with MIS-C during the study period. Of these 13 patients, 2 patients (15%) had a fatal outcome. Subjects were predominantly male, and the median age was 7.58 years (IQR 12.3) years. Most patients required mechanical ventilation (7 out of 13 patients) and intubation (8 out of 13 patients). Patients who needed intubation usually needed mechanical ventilation. All inflammatory markers, white blood cells, neutrophil counts, and all coagulation factor parameters (except for normal prothrombin time and activated partial prothrombin time) were elevated. The median time to MIS-C diagnosis was 2 days in the survivor group (n = 11) compared to 8.5 days in the non-survivor group (n = 2). Compared to the non-survivor group, those who survived spent more days in the hospital, received vasopressors earlier, and did not require mechanical ventilation as early as the non-survivors.
Conclusions
Our work highlights the differences in MIS-C clinical course, treatment, and clinical outcomes between the two groups.
Keywords: COVID-19, MIS-C, children, Indonesia
Key points.
There were 13 patients with the multisystem inflammatory syndrome in children (MIS-C) with 2 mortalities. Eleven patients were categorized as MIS-C without overlap with Kawasaki disease. Survivors spent more days in the hospital and received vasopressors earlier compared to non-survivors.
BACKGROUND
Since its initial outbreak, cases of coronavirus disease 2019 (COVID-19) in children have persisted, particularly in Indonesia [1, 2]. The pediatric population often presents with milder symptoms and better outcomes. However, all age groups may contract this disease, though recovery is expected within 1 to 2 weeks and deaths are rare [3–5].
However, in mid-April 2020, eight pediatric hyperinflammatory shock cases were seen in South Thames Retrieval Service in London, United Kingdom [6]. Despite having characteristics similar to toxic shock syndrome, no microbial etiologies were found. Patients were severely ill with multi-organ involvement, unlike any other COVID-19 cases observed at that time [7]. The Royal College of Paediatrics and Child Health (RCPCH) was among the first medical organizations to acknowledge this condition and coined the term pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [8]. The Centers for Disease Control and Prevention (CDC), followed by the World Health Organization (WHO), labeled this condition multisystem inflammatory syndrome in children (MIS-C) [9, 10]. From here on, the condition will be referred to as MIS-C.
Several systematic reviews have stated that children of certain ethnicities are particularly prone to MIS-C, namely Afro-Caribbean, Latino/Hispanic and Black children [11–13]. Thus far, there have been only 38 reported cases of MIS-C in Asia [11]. Although Indonesia is severely affected by the COVID-19 pandemic, there is a lack of MIS-C data, apart from a single case report [14]. Therefore, this paper aimed to provide new data on the characteristics and prevalence of patients with MIS-C admitted to a tertiary referral hospital in Jakarta, Indonesia.
METHODS
Data collection
This is a cross-sectional study with secondary data obtained from the pediatric COVID-19 registry at Dr. Cipto Mangunkusumo National Central Hospital, Jakarta, a tertiary referral hospital in Indonesia, covering March 2020, when the first Indonesian COVID-19 case was announced, to April 2021 [15]. In 2020, 31 075 people of all ages presented to the emergency room, with 1373 (4.41%) of them testing positive for COVID-19. A brief description about our hospital has been described elsewhere [16].
We included all pediatric patients (0–18 years old) diagnosed with MIS-C based on the criteria from the RCPCH, WHO and CDC [8–10]. Comparisons between these three sets of criteria were made in our sample. Patients whose clinical presentations did not meet any of the three sets of criteria were excluded. The collected data included demographic data; vital signs upon admission; COVID-19 status (rapid antibody test results, reverse transcriptase-polymerase chain reaction [RT-PCR] results, cycle threshold [Ct] values); signs and symptoms of MIS-C, such as generalized symptoms (fever or lethargy), respiratory symptoms, gastrointestinal symptoms, mucosal changes, and neurological symptoms; comorbidities; intubation and mechanical ventilation status; laboratory and imaging results; cause of death; length of stay; and treatment administered.
The RCPCH criteria for PIMS-TS diagnosis include children with the following [1]: recurrent fever, inflammation (neutrophilia, elevated C-reactive protein [CRP] and lymphopenia), and signs of single- or multi-organ dysfunction (shock, cardiac, respiratory, kidney, gastrointestinal or neurological disorder) with additional characteristics such as conjunctivitis, lymphadenopathy, or rash, including children meeting complete or partial Kawasaki (KD) disease requirements [2]; no other microbial source, including myocarditis-related infections such as enterovirus, bacterial sepsis, and staphylococcal or streptococcal shock syndrome; and [3] either positive or negative polymerase chain reaction testing for SARS-CoV-2 [8].
The case definition of MIS-C based on the WHO guidelines are children and adolescents 0–19 years of age with fever > 3 days and [1] increased inflammation indicators such as erythrocyte sedimentation rate (ESR), procalcitonin or CRP [2]; no other exact microbial cause of inflammation, including bacterial sepsis and staphylococcal or streptococcal shock syndrome [3]; evidence of positive COVID-19 status (RT-PCR, antigen or serologic test) or possible interaction with a COVID-19 patient; and [4] two of the following: (i) rash or non-purulent bilateral conjunctivitis or symptoms of mucocutaneous inflammation (oral, hands or feet), (ii) shock or hypotension, (iii) characteristics of myocardial dysfunction, pericarditis, valvulitis or coronary irregularities (including ECHO echocardiography or elevated troponin/N-terminal-pro hormone BNP [NT-proBNP] findings), (iv) evidence of coagulopathy (based on prothrombin time, partial thromboplastin time or elevated D-dimers), and (v) acute gastrointestinal issues (diarrhea, vomiting or abdominal pain) [9].
The final MIS-C criteria were from the CDC, which include any individual < 21 years of age demonstrating the following [1]: fever, laboratory evidence of inflammation and evidence of hospitalized clinically serious illness, with multi-organ (>2) involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological) [2]; no plausible alternative diagnosis; and [3] positive findings for current or recent infection with SARS-CoV-2 by RT-PCR, serology, or antigen test, or exposure to COVID-19 within 4 weeks before symptom onset [10].
The PEdiatric Logistic Organ Dysfunction-2 (PELOD-2) was used to assess organ dysfunction. This scoring system assesses neurological, cardiovascular, renal, respiratory and hematological dysfunction [17]. Sepsis diagnosis followed the International Consensus on Sepsis, in which sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [18].
Detection of SARS-CoV-2
The N gene quantification cycle value was used as the parameter for the RT-PCR target, and all naso-oropharyngeal and sputum/endotracheal tube aspirate samples were checked for the presence of SARS-CoV-2. The samples were collected using naso-oropharyngeal swabs, with a minimum of two samples taken within a 1-day interval. If the samples were positive, further samples were taken every 5–7 days before the conversion was complete. Ct values greater than 40 (detection limit) were classified as negative, while Ct values less than 37 were classified as positive [19]. In our hospital, a medium load (Ct value of 37 to 40) necessitated confirmation via at least one repeat check. All patients were examined for SARS-CoV-2 antibodies.
Statistical analysis
IBM Statistical Package for the Social Sciences version 26.0 (SPSS, IBM Corp., Armonk, NY, USA) was used for statistical analysis. The Kolmogorov–Smirnov procedure was utilized to evaluate the normality of the data, and p > 0.05 indicated that the data had a natural normal distribution. The data were presented as mean and standard deviation if the distribution was regular and as median and range if it was not. We included only the peak values for all laboratory data due to numerous findings of heightened inflammatory markers correlated with the severity of the disease [20, 21].
RESULTS
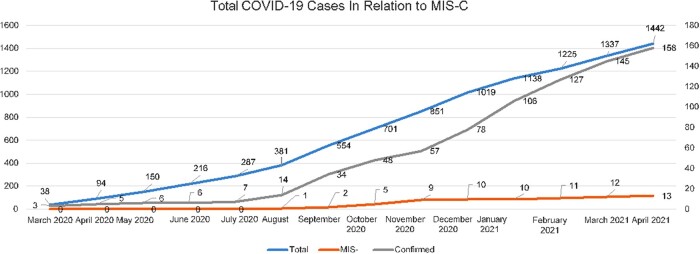
From March 2020 to April 2021, 1442 pediatric cases were classified as suspected or probable COVID-19 cases. Of these, 158 (10.95%) patients tested positive for COVID-19, while 13 (8.2%) were diagnosed as MIS-C amongst these 158 patients. Patients 1 and 5 were in both the COVID-19–positive and MIS-C groups (MIS-C overlapping with severe acute COVID-19). Among the MIS-C cases, two patients had fatal outcomes. The first MIS-C case was reported in August 2020, while the largest increase in MIS-C cases was observed between October and November 2020 (Fig. 1).
Fig. 1.
Total COVID-19 cases in relation to MIS-C cases relative to total COVID-19 cases in Cipto Mangunkusumo Hospital.
The median age of the subjects was 7.58 years with an interquartile range of 12.3 years, and a predominance of male patients (9 of 13 subjects). One patient was severely underweight (Patient 8), while Patient 4 was overweight (Table 1). Pre-existing conditions were found in eight patients (patients 1, 3, 6, 7, 10, 11, 12 and 13). Patient 9 was diagnosed with Guillain-Barré syndrome, which is considered part of the MIS-C/PIMS-TS criteria. Hypovolemic shock was the most common circulatory failure detected, with five patients experiencing this type of shock. Five circulatory failure cases responded to fluid resuscitations. Eight patients required intubation, seven of whom received mechanical ventilation, due to a lack of mechanical ventilators at that time. Most of the patients (10 subjects) presented in critical condition upon admission. Among the 13 patients, 7 patients were positive for anti-SARS-CoV-2 immunoglobulin IgG (IgG), 2 patients were positive for both anti-SARS-CoV-2 immunoglobulin M (IgM) and IgG, 1 patient was positive for anti-SARS-CoV-2 IgM and 4 patients were positive for COVID-19 based on SARS-CoV-2 RT-PCR tests.
Table 1.
Demographics, clinical features, treatment, key initial laboratory and radiographic findings, and outcomes in patients with multisystem inflammatory syndrome in children (MIS-C)
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient | Patient | Patient | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (F) | (M) | (M) | (M) | (M) | (F) | (F) | (M) | (M) | (F) | 11 (M) | 12 (M) | 13 (M) | ||
| Age (years) | 14.17 | 16.5 | 1.17 | 15.11 | 0.92 | 12.5 | 3.01 | 0.33 | 5.58 | 0.75 | 17.75 | 7.58 | 11.33 | |
| Presenting symptoms | Fever, abdominal pain, nausea, vomiting | Fever, lymphadenopathy, headache, diarrhea, nausea, vomiting, rash, pedal edema | Fever, diarrhea, anorexia, dyspnea, pedal edema | Fever, dyspnea | Fever, diarrhea, dyspnea, cough | Fever, cough, rash, minimal pedal edema | Fatigue, dizziness | Diarrhea, vomiting, dyspnea | Fever, limb weakness, conjunctivitis, diarrhea, dyspnea | Fever, seizure, diarrhea, vomiting | Dyspnea, cough | Fever, nausea, abdominal pain, decreased appetite | Fever, fatigue, lymphadenopathy, headache, seizure, vomiting, transient visual impairment | |
| Clinical findings | Days before admission | 0 | 6 | 4 | 7 | 10 | 4 | 1 | 1 | 10 | 3 | 1 | 3 | 7 |
| Comorbidities | Acute appendicitis with generalized peritonitis | None | Spastic cerebral palsy | None | None | End- stage kidney disease on hemodialysis | Total AV block, infective endocarditis | None | None | Intussusception | End- stage kidney disease on hemodialysis, focal segmental glomerulosclerosis | Acute appendicitis | Hypertensive encephalopathy, nephritic syndrome, neuromyelitis optica, optic neuritis | |
| Initial oxygen supplementation therapy | No | No | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | Yes | |
| Presence of shock | Septic shock mimic | None | Hypovolemic | Cardiogenic | Hypovolemic | None | Cardiac, hypovolemic, septic shock mimic | Hypovolemic | None | Hypovolemic | None | Septic shock mimic | None | |
| Mechanical ventilation | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | Yes | No | Yes | |
| Intubation | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Exposure to healthcare facilities or professionals | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | |
| History of contact with suspected or confirmed cases | No | Yes | No | No | Yes | Yes | No | No | No | No | No | No | No | |
| Cause of death | Septic shock mimic | – | – | – | – | – | – | – | – | – | ARDS | – | – | |
| Key initial laboratory and radiographic findings | PCR sample | Naso-oropharyngeal | Naso-oropharyngeal and rectal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal and rectal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal | Naso-oropharyngeal and ETT aspirate |
| PCR results |
|
Negative | Negative | Positive |
|
Negative | Positivea | Negative | Negative | Negative | Negative | Negative | Negative | |
| SARS- CoV-2 antibody test | Negative | IgG (+) | IgG (+) | IgM and IgG (+) | N/A | Negative | IgM (+) | IgG (+) | IgG (+) | IgG (+) | IgG (+) | IgM and IgG (+) | IgG (+) | |
| Chest X-ray | Normal | Lower left lobe infiltrate | Bilateral infiltrate | Cardiomegaly with lung edema, thickening of hilar bilaterally | Infiltrate at right paracardial and bilateral suprahilar and perihilar infiltrate | N/A | Cardiomegaly, inhomogeneous opacity at the apex of right lung, cardiac pacemaker projected in the ventricle | Infiltrate at right upper and lower lobe and left perihilar | Normal | Normal | Inhomogeneous consolidation at both lungs | Normal | Infiltrate at both lungs | |
| Echocardiography | N/A | Depressed LV function with EF 42% | Normal | Poor contractility | N/A | N/A | Vegetation (+) | N/A | N/A | N/A |
|
N/A | N/A | |
Note: N/A, not available; M, male; F, female; ER, emergency room; BPM, beats per minute; L, liter; NC, nasal cannula; NRM, non-rebreathing mask; RA, room air; MV, mechanical ventilation; PCR, polymerase chain reaction; Ab, antibody; IgG, immunoglobulin G; IgM, immunoglobulin M; MAP, mean arterial pressure; CRP, C-reactive protein; LV, left ventricle; EF, ejection fraction; MR, mitral regurgitation; TR, tricuspid regurgitation; NS, normal saline; RL, ringer lactate; FiO2, fraction of inspired oxygen; ETT, endotracheal tube; ARDS, acute respiratory distress syndrome; Ct, cycle threshold; AV, atrio-ventricular.
Positive result was obtained from the referring hospital.
Excluding the PELOD-2 score from Patient 9 due to a missing lactate value, the median PELOD-2 score was 3.5 (0–10). Patient 3 presented with the highest PELOD-2 score of 10, while patient 12 presented with a PELOD-2 score of 0. In the mortality group, Patient 1 had a PELOD-2 score of 6 and Patient 11 had a PELOD-2 score of 9 (not shown in the tables). In terms of comorbidities, Patient 1 had acute appendicitis with generalized appendicitis and septic shock mimic while Patient 11 suffered from appendicitis and also septic shock mimic. Echocardiography was performed for four patients, of which all results were abnormal (Patient 2 presented with depressed left ventricle function, Patient 4 had poor contractility, Patient 7 had vegetations and Patient 11 had minimal pericardial effusion).
All patients received antibiotics, and six patients required fluid resuscitation. Only two patients received antiviral therapy, namely Patient 2, who received favipiravir, and Patient 3, who received a combination of lopinavir and ritonavir. Eight patients received corticosteroids, five patients received vasopressors and six patients received intravenous immunoglobulin (IVIG). The clinical outcomes in our subjects consisted of 2 deaths out of the 13 patients (Patient 1 due to septic shock and Patient 11 due to acute respiratory distress syndrome).
The peak laboratory findings are presented in Table 2. We found elevated median values of white blood cell and neutrophil count, all coagulation parameters except prothrombin time and activated partial prothrombin time, and inflammatory markers. Troponin I and troponin T were assessed in five and three patients, respectively. Median troponin I was elevated, while troponin T was normal. Among the liver and renal tests, aspartate transaminase and urea levels were elevated. Albumin was the only value that was below the reference range.
Table 2.
Peak laboratory features of children with MIS-C
| Parameter | Number of patients | Reference value | Median (range) |
|---|---|---|---|
| Hematology | |||
| Hemoglobin (g/dl) | 13 | 12.0–1.50 | 13.5 (8.6–20.8) |
| White blood cells (103/µl) | 13 | 4.0–10.0 | 28.9 (7.01–53.04) |
| Platelets (103/µl) | 13 | 150–410 | 348 (46–849) |
| Neutrophil count (103/µl) | 13 | 1.7–7.5 | 15.42 (2.59–48.15) |
| Lymphocyte count (103/µl) | 13 | 1.0–3.2 | 2.19 (0.18–8.08) |
| Inflammatory markers | |||
| Erythrocyte sedimentation rate (mm) | 6 | 0–15 | 43 (20–100) |
| C-reactive protein (mg/l) | 13 | <5.0 | 158.4 (0.5–558) |
| Procalcitonin (ng/m) | 13 | <0.05 | 55 (0.14–1168) |
| Ferritin (ng/ml) | 9 | 20–200 | 319.75 (0.9–2397.15) |
| Liver and renal function | |||
| Aspartate transaminase (U/l) | 12 | 10–40 | 45.5 (14–584) |
| Alanine aminotransferase (U/l) | 12 | 5.9–37 | 40 (16–119) |
| Serum creatinine (mg/dl) | 13 | 0.22–0.59 | 0.7 (0.1–12) |
| Urea (mg/dl) | 13 | 11–29 | 48.7 (18.6–1183) |
| Albumin (g/dl) | 13 | 3.8–5.4 | 3.35 (2.04–4.39) |
| Coagulation factors | |||
| D-dimer (µg/l) | 13 | <440 | 4100 (1190–14510) |
| Fibrinogen (mg/dl) | 12 | 200–400 | 547.3 (157.2–981.5) |
| Prothrombin time (s) | 13 | 9.8–12.6 | 12.6 (10.5–180) |
| Activated partial prothrombin time (s) | 13 | 31.0–47.0 | 43.9 (19.4–168.6) |
| Cardiac and other markers | |||
| Lactic acid (mmol/l) | 10 | 0.7–2.5 | 2.65 (1.2–5.2) |
| Troponin I (ng/l) | 5 | ≤15.6 | 140.9 (2.8–277.3) |
| Troponin T (pg/ml) | 3 | 0–50 | 39 (39–762) |
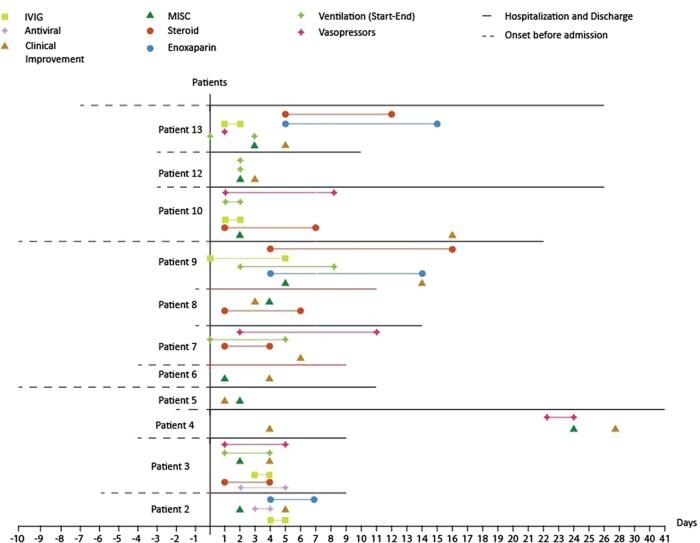
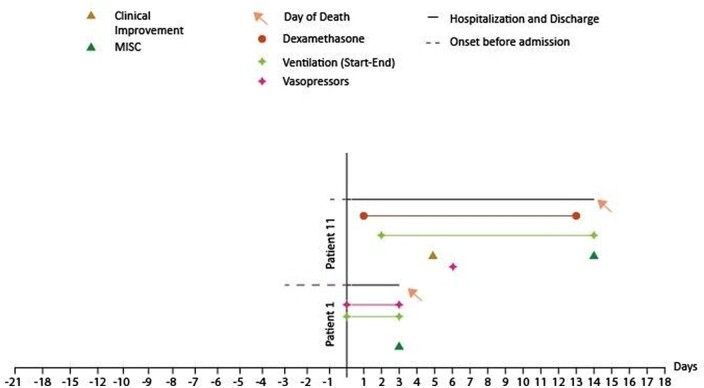
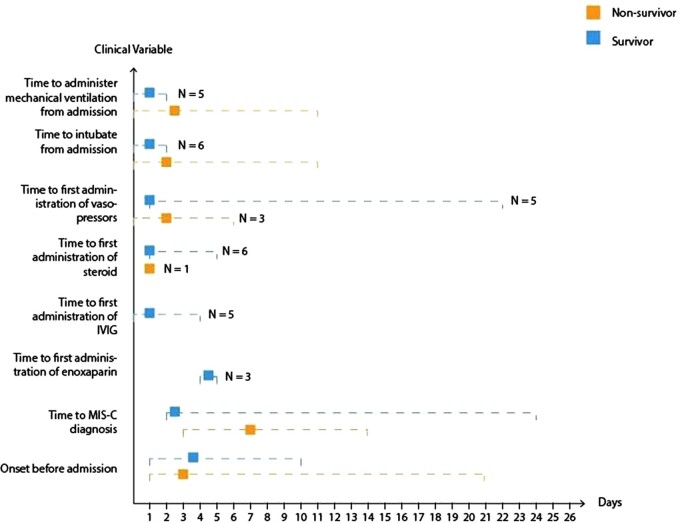
We also examined any discrepancies in diagnosis between the three criteria paradigms employed. All patients fulfilled the criteria from the RCPCH, WHO and CDC. Graphical representations of patients with MIS-C who died and survived are shown in Figs 2 and 3, respectively. Figure 4 shows the comparison of clinical features between the two groups. There was only a slight difference in the median duration of symptoms before admission between the two groups (3 days in the non-survivor group vs. 4 days in the survivor group). However, the median time to MIS-C diagnosis was 2 days in the survivor group, while it took 8.5 days to diagnose MIS-C in the non-survivor group. Patients in the survivor group received the first enoxaparin dose 4.5 days after admission and the first IVIG dose within 1 day after admission. In contrast, the non-survivor group did not receive any doses of enoxaparin or IVIG.
Fig. 2.
Graphical representation of patients with MIS-C who survived. Antivirals used in this study were favipiravir and lopinavir–ritonavir. MIS-C, multisystem inflammatory syndrome in children; IVIG, intravenous immunoglobulin.
Fig. 3.
Graphical representation of patients with MIS-C who did not survive. MIS-C, multisystem inflammatory syndrome in children.
Fig. 4.
Comparison of clinical features between MIS-C survivors and non-survivors. The squared boxes represent medians while the two extremes of the dotted lines represent the range.
In the survivor group, 6 out of 11 patients did not receive steroid treatment, while in the non-survivor group, only 1 out of 2 patients received steroid treatment. Patients in the survivor group spent more days in the hospital (13 days for survivors vs. 8.5 days for non-survivors), received vasopressors earlier (1 day for survivors vs. 3 days for non-survivors), and did not require mechanical ventilation as early (4 days for survivors vs. 1 day for non-survivors) compared to the non-survivor group.
DISCUSSION
The mortality rate of patients with MIS-C was lower in our study compared to other studies. A systematic review found a mortality rate of 1.7% in 662 MIS-C cases [11]. The highest mortality rate was reported by Feldstein et al. [22], with a mortality rate of 2.15%. The lower mortality rate can be attributed to an increasing awareness of MIS-C, reflected in the rapid diagnosis of MIS-C in the patients in this study. With a median of 2 days to diagnosis for all 13 subjects, the patients could receive life-saving treatments, such as vasopressors, IVIG and corticosteroids, in a more timely manner [23, 24].
Godfred-Cato et al. [25] divided MIS-C into three distinct phenotypes: MIS-C without overlap with KD, MIS-C overlapping with KD, and MIS-C overlapping with severe acute COVID-19. In our cohort, 11 of the 13 patients (84.6%) were classified as MIS-C without overlap with KD. These patients have cardiovascular or gastrointestinal involvement with the presence of shock, elevated inflammatory markers, and positive SARS-CoV-2 serology [25]. Patients 1 and 5 were classified as MIS-C overlapping with severe acute COVID-19, as they presented with positive SARS-CoV-2 RT-PCR without antibody seropositivity.
A previous study found a median PELOD-2 score of 10 in a sample of pediatric patients with MIS-C, which is much higher than the median score found in our study [26]. The lower PELOD-2 score can partially explain the reduced mortality rate of the patients with MIS-C in our study. A study conducted in India found that a PELOD-2 score of 0–4 correlated with a 1.6% mortality rate, 5–9 correlated with a 14.8% mortality rate and 10–14 correlated with a 50% mortality rate [27]. In our study, of the two patients who passed away, Patient 1 had a PELOD- 2 score of 6 and Patient 9 had a PELOD-2 score of 9. However, this finding needs to be interpreted with caution as every single patient comes with varying onset and severity of diseases. Also, PELOD-2 is measured after initial life-saving treatments have been administered, which explains the lower scores in our studies compared to other studies. Serial measurements of PELOD-2 score may be a better way to assess their prognosis. Therefore, the low median PELOD-2 score may explain the lower mortality rate in our study.
Abnormal results were found in the majority of patients who underwent echocardiography. This is similar to other findings, although it is notable that a depressed ejection fraction was the most common finding in previous research [11]. In this study, a depressed ejection fraction was only found in one patient with an ejection fraction of 42% (Patient 2). While the pathophysiology of myocardial dysfunction in MIS-C is still not established, possible causes in adults include acute myocarditis, hypoxic injury, microvascular or coronary artery disease, ischemic injury, right heart strain (acute cor pulmonale), cardiomyopathy stress (Takotsubo) and systemic inflammatory response syndrome [28]. Our findings highlight the importance of baseline and serial echocardiography to monitor current and long-term cardiovascular status in patients with MIS-C [11].
Neurological involvement in COVID-19 and MIS-C can be classified as a suspected acute neurological disease (e.g. demyelination, stroke) or acute neurological signs and symptoms (e.g. headache, myalgia, seizures) [29]. In our series, 3 out of 13 patients had severe neurological symptoms as part of their MIS-C presentation. In addition, one patient had an existing neurological disorder (cerebral palsy) but developed no additional neurological signs and symptoms. A case series reported neurological manifestations in 365/1965 COVID-19 patients <21 years old. Of these, 43 had severe neurologic involvement, including 20 patients who met the criteria for MIS-C [29]. Guillain-Barré syndrome and its variants occurring in association with COVID-19 have been increasingly reported and may occur both during and after active infection [30, 31]. Patient 10 had a clinical, laboratory and neurophysiologic features consistent with the acute motor axonal neuropathy (AMAN) variant of GBS. In a systematic review of case reports, AMAN was the least reported variant of GBS, occurring in 8 out of 77 cases [30]. To date, there are only two reports of pediatric optic neuritis associated with COVID-19, one occurring during active infection and one as the presenting symptom of MIS-C in a PCR-negative, seropositive child [32, 33]. Patient 13 presented with acute vision impairment and neurophysiologic evidence of optic nerve demyelination in the face of a negative PCR and positive IgG. In both our patients with demyelinating disease, corticosteroid treatment yielded a good outcome. Acute encephalopathy is the most frequently reported severe neurological manifestation of COVID-19 and MIS-C [29]. However, in our small case series, only one patient had this presentation, which may have been secondary to hypovolemic shock.
Our laboratory findings are similar to other studies except for the normal troponin T values found in our study [12, 34]. One explanation is that troponin T was only tested in three patients. Another plausible cause is that initial testing may not reflect the peak severity of MIS-C, which may cause troponin T values to be mistakenly considered normal. Thus, serial testing should be performed in these patients [34, 35].
All of our patients fulfilled the three MIS-C criteria paradigms defined by the RCPCH, CDC and WHO [8–10]. It is clinically essential to define which criteria are employed, as these three sets of criteria are quite different. For instance, according to the RCPCH guideline [8], RT-PCR can be positive or negative in MIS-C. On the other hand, the CDC and WHO require positive RT-PCR results as one of the MIS-C diagnostic criteria [9, 10]. One study in Spain found that a child could fulfill MIS-C criteria but not PIMS-TS and vice versa [36]. Therefore, clinical researchers need to identify the specific guideline used in the study, as patients may not meet all three sets of criteria. In our study, an initial MIS-C diagnosis was made using the WHO criteria.
However, all subjects met all of the MIS-C criteria from the WHO, CDC and RCPCH, suggesting that no one method is superior to the others [36].
Antibiotics were administered according to our local guidelines [37] as well as the CDC guidelines [10], which state that antibiotics should be given while waiting for bacterial culture and sensitivity results. Once the results show no growth of bacteria, the antibiotics are stopped/discontinued. The two patients in the non-survivor group did not receive IVIG treatment, had a longer time to diagnosis of MIS-C, received vasopressors later, and required mechanical ventilation earlier compared to the patients in the survivor group. The difference in treatment can be attributed to the evolving COVID-19 disease progression. Initially, these two patients were diagnosed with suspected sepsis, a more common diagnosis than MIS-C in our institution. When no specific etiologies were found, MIS-C diagnosis was considered according to clinical manifestations and supporting laboratory findings. This explains the median of 8.5 days required to diagnose MIS-C in this group. Due to the initial sepsis management, potentially life-saving treatment for MIS-C, such as IVIG and corticosteroids, were delayed until sepsis was excluded [38].
There are several limitations to our study. First, as Dr. Cipto Mangunkusumo Hospital is a referral hospital for managing COVID-19 patients, particularly those with comorbidities, the incidence and mortality rates for patients with MIS-C reported in this study cannot be extrapolated to other hospitals, cities or regions in the country. Second, significant associations between the investigated variables and MIS-C were not assessed. Third, we could not determine whether causes of death were attributable to MIS-C or underlying comorbidities. Finally, we did not assess several laboratory panels, such as interleukins and other cytokines, to measure MIS-C severity. Nevertheless, despite these limitations, our study revealed a high mortality rate in MIS-C patients. To our knowledge, this study is the first to describe the clinical characteristics of MIS-C in an Indonesian pediatric population.
CONCLUSIONS
This study described MIS-C cases in a tertiary referral hospital. Compared to the non-survivor group, those who survived were diagnosed with MIS-C earlier and received earlier treatment, including IVIG, vasopressors, enoxaparin and corticosteroids. This is the first study in Indonesia that highlights the clinical characteristics of patients with MIS-C. However, further multicenter studies and intervention and management studies are required to optimize public health measures, particularly for MIS-C cases.
ETHICS APPROVAL
The Ethics Committee approved this study of the Faculty of Medicine, Universitas Indonesia (no. 596/UN2.F1/ETIK/PPM.00.02/2020).
ACKNOWLEDGEMENTS
We would like to thank Cipto Mangunkusumo Hospital Director, all patients and their families, and pediatric residents of Universitas Indonesia, the Kiara Ultimate medical team for their help and support.
FUNDING
This study was supported by a research grant from Cipto Mangunkusumo Hospital.
DATA AVAILABILITY
The datasets used for this study are available from the corresponding author upon a reasonable request.
REFERENCES
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020;145:e20200702.32179660 [Google Scholar]
- 2. World Health Organization. Coronavirus Disease 2019. (COVID-19) Situation Report - 38. 2020. https://www.who.int/docs/default-source/searo/indonesia/covid19/external-situation-report-38_16-december-2020.pdf?Status=Master&sfvrsn=d8d31f7e_5 (30 December 2020, date last accessed).
- 3. Patel NA. Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol 2020;41:102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020;174:882–9. [DOI] [PubMed] [Google Scholar]
- 5. Badal S, Thapa Bajgain K, Badal S, et al. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta- analysis. J Clin Virol 2021;135:104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet (London, England). 2020;395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White M, Tiesman B, Handforth J, Evelina PIMS TS working group, et al. Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS): the Evelina Experience. Arch Dis Child 2020;105:1025–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royal College of Paediatrics and Child Health. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians. 2020. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (30 December 2020, date last accessed).
- 9. World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. 2020. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (30 December 2020, date last accessed).
- 10. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). 2020. https://emergency.cdc.gov/han/2020/han00432.asp (30 December 2020, date last accessed).
- 11. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020;26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr 2020;226:45–54.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoste L, Van Paemel R, Haerynck F.. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr 2021;180:2019–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Somasetia DH, Malahayati TT, Andriyani FM, Setiabudi D, et al. A fatal course of multiple inflammatory syndrome in children coinfection with dengue. A case report from Indonesia. IDCases 2020;22:e01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Coronavirus Disease 2019. (COVID-19) Situation Report - 1. 2020. https://www.who.int/docs/default-source/searo/indonesia/covid19/who-indonesia-situation-report-1.pdf?sfvrsn=6be5b359_0 (30 December 2020, date last accessed).
- 16. Dewi R, Kaswandani N, Karyanti MR, et al. Mortality in children with positive SARS-CoV-2 polymerase chain reaction test: lessons learned from a tertiary referral hospital in Indonesia. Int J Infect Dis 2021;107:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matics TJ, Sanchez-Pinto LN.. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr 2017;171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–5. [DOI] [PubMed] [Google Scholar]
- 20. Torres JP, Izquierdo G, Acuña M, et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis 2020;100:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abrams JY, Oster ME, Godfred-Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health 2021;5:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feldstein LR, Rose EB, Horwitz SM, CDC COVID-19 Response Team, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakra NA, Blumberg DA, Herrera-Guerra A, et al. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children 2020;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harwood R, Allin B, Jones CE, PIMS-TS National Consensus Management Study Group, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2021;5:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Godfred-Cato S, Bryant B, Leung J, California MIS-C Response Team, et al. COVID-19- associated multisystem inflammatory syndrome in children - United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 2020;10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshmukh T, Varma A, Damke S, et al. Predictive efficacy of pediatric logistic organ dysfunction-2 score in pediatric intensive care unit of rural hospital. Indian J Crit Care Med 2020;24:701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021;180:307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LaRovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 2021;78:536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aladawi M, Elfil M, Abu-Esheh B, et al. Guillain Barre syndrome as a complication of COVID-19: a systematic review. Can J Neurol Sci 2021;49:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 2021;268:1133–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parvez Y, AlZarooni F, Khan F.. Optic neuritis in a child with COVID-19: a rare association. Cureus 2021;13:e14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sinha A, Dwivedi D, Dwivedi A, et al. Optic neuritis as a presenting symptom of post-COVID-19 multisystem inflammatory syndrome in children (MIS-C). Indian J Pediatr 2021;88:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021;143:78–88. [DOI] [PubMed] [Google Scholar]
- 35. Nelson C, Ishimine P, Hayden SR, et al. Multisystem inflammatory syndrome in children (MIS-C) in an adolescent that developed coronary aneurysms: a case report and review of the literature. J Emerg Med 2020;59:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernández-Cooke E, Grasa CD, Domínguez-Rodríguez S, KAWA-RACE Study Group, et al. Prevalence and clinical characteristics of SARS-CoV-2 confirmed and negative Kawasaki disease patients during the pandemic in Spain. Front Pediatr 2020;8:617039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kementerian Kesehatan Republik Indonesia. Pedoman Pencegahan Pengedalian Coronavirus Disease (COVID-19). [online]. Kementerian Kesehatan Republik Indonesia. https://covid19.go.id/storage/app/media/Protokol/2020/Juli/REV-05_Pedoman_P2_COVID-19_13_Juli_2020.pdf (4 January 2021, date last accessed).
- 38. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr 2020;224:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for this study are available from the corresponding author upon a reasonable request.