Abstract
Antispike monoclonal antibody treatment of 180 B-cell-depleted patients with mild-to-moderate coronavirus disease 2019 (COVID-19) resulted in good outcomes overall, with only 12.2% progressing to severe disease, 9.4% requiring hospitalization, 0.6% requiring mechanical ventilation, no deaths within 30 days, and 1.8% developing persistent COVID-19. Antispike monoclonal antibodies appear effective in this immunocompromised population.
Keywords: anti-CD20, COVID-19, monoclonal antibody, rituximab, SARS-CoV-2
Anti-CD20 therapies, commonly used to treat B-cell malignancies, multiple sclerosis, and other autoimmune disorders, result in severe humoral immunodeficiency that increases the risk for infectious complications. During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, B-cell-depleted patients have been observed to be at high risk for complications from coronavirus disease 2019 (COVID-19), including severe or persistent infection [1–3]. Due to their impaired humoral immune response, there has been interest in antibody-based therapies for management of COVID-19 in this population, including the use of high-titer convalescent plasma (ConvP) [4–6].
Anti-spike monoclonal antibodies (mAbs) have been the standard outpatient therapy for mild-to-moderate COVID-19 in patients with high-risk characteristics, including immunocompromised populations [7, 8]. However, there are little data regarding outcomes following these therapies in B-cell-depleted patients. We sought to analyze our cohort of patients who received anti-CD20 therapies and were treated early with mAbs for mild-to-moderate COVID-19.
METHODS
Study Design
We conducted a multicenter, retrospective review of all B-cell-depleted patients diagnosed with mild-to-moderate COVID-19 who received mAb therapy. Patients were ascertained from our internal COVID-19 database for recipients of an anti-CD20 antibody and mAb from 3 Mayo Clinic sites in Arizona, Florida, and Minnesota from November 9, 2020 through February 15, 2022.
Inclusion criteria were age ≥18 years and receipt of an anti-CD20 antibody within 12 months before COVID-19 diagnosis. Exclusion criteria were last anti-CD20 infusion ≥12 months before COVID-19 diagnosis, lack of COVID-19 confirmation by SARS-CoV-2 polymerase chain reaction (PCR) or antigen testing, or lack of research authorization. Data were manually extracted from the electronic medical record, including demographics, anti-CD20 therapy, COVID-19 diagnosis and therapies, and outcome measures.
The primary outcome of this study was progression to severe COVID-19, defined as hypoxia (oxygen saturation ≤94% or supplemental oxygen requirement above baseline) or hospitalization for COVID-19. High-risk outpatients with COVID-19 are routinely offered remote monitoring during their isolation period, which includes medical equipment to monitor vital signs and oxygen saturation and dedicated nursing teams to perform phone visits with patients. Secondary outcomes were emergency department (ED) visits, intensive care unit (ICU) admission, and persistent COVID-19. Persistent COVID-19 was defined as a positive SARS-CoV-2 PCR or antigen test 30–90 days after index diagnosis with compatible clinical symptoms and lack of an alternative explanation [9]. Date of COVID-19 diagnosis was defined as the date a positive SARS-CoV-2 assay was collected. Outcomes were also compared based on the dominant variant of concern (VOC) at the time of COVID-19 diagnosis. Those diagnosed through May 2021 were considered pre-Delta period, those diagnosed July through December 15, 2021 were considered Delta-predominant period, and those diagnosed January 1 through February 15, 2022 were considered Omicron-predominant period. June 2021 and December 16–31, 2021 were periods of overlap between VOC, and the patients diagnosed in these intervals were excluded from the analysis of clinical outcomes according to the dominant circulating VOC.
Statistical Analysis
Continuous variables were summarized as either median with interquartile range or mean with standard deviation. Fisher’s exact test was used for categorical variables and Mann-Whitney test was for continuous variables. Statistical significance was defined as P < .05 from 2-sided tests. All analyses were performed using BlueSky Statistics version 7.40 software (BlueSky Statistics LLC, Chicago, IL).
Patient Consent Statement
This study was approved by our local institutional review board and granted an exempt status (no. 20-012919).
RESULTS
Population and Clinical Outcomes
One hundred eighty patients met inclusion criteria; 163 patients were receiving rituximab and 17 obinutuzumab (Table 1). The most common mAb was casirivimab-imdevimab (45.0%), followed by sotrovimab (33.3%). All patients had at least 30 days of follow-up after COVID-19 diagnosis. Twenty-two (12.2%) patients developed severe COVID-19, including 17 (9.4%) who required an ED visit and 17 (9.4%) who required hospitalization. Among 17 patients who were hospitalized, 16 were treated with remdesivir, 13 received corticosteroids, and 1 received ConvP. No patients received baricitinib or tocilizumab. One patient (0.6%) required ICU admission and mechanical ventilation. No other patients required noninvasive ventilatory support or high-flow oxygen support. No patients died within 30 days of COVID-19 diagnosis.
Table 1.
Characteristics of 180 B-Cell-Depleted Patients With COVID-19, Stratified by Progression to Severe COVID-19
| Characteristics | Mild-Moderate (N = 158) | Severe (N = 22) | P Value |
|---|---|---|---|
| Age, years, mean (SD) | 60.2 (14.8) | 61.4 (16.5) | .748 |
| Female sex | 79 (50.0) | 12 (54.5) | .821 |
| Race | .226 | ||
| Asian | 6 (3.8) | 0 (0.0) | |
| Black or African American | 5 (3.2) | 2 (9.1) | |
| White | 143 (90.5) | 19 (86.4) | |
| Other | 1 (0.6) | 1 (4.5) | |
| Unknown | 1 (1.9) | 0 (0.0) | |
| Ethnicity | .108 | ||
| Hispanic or Latino | 3 (1.9) | 2 (9.1) | |
| Not Hispanic or Latino | 151 (95.6) | 19 (86.4) | |
| Unknown | 4 (2.5) | 1 (4.5) | |
| BMI, kg/m2, median (IQR) | 28.5 (25.0– 32.9) | 30.6 (24.4– 37.0) | .522 |
| Diabetes mellitus | 25 (15.8) | 5 (22.7) | .376 |
| Chronic pulmonary disease | 25 (15.8) | 7 (31.8) | .077 |
| Chronic kidney diseasea | 34 (21.7) | 5 (22.7) | 1 |
| Dialysis dependency | 2 (1.3) | 1 (4.5) | .325 |
| Solid organ transplant recipientb | 7 (4.4) | 3 (13.6) | .108 |
| Charlson comorbidity index, mean (SD) | 1.9 (1.2) | 2.1 (1.4) | .331 |
| MASS, mean (SD) | 5.4 (3.1) | 5.9 (3.5) | .506 |
| Received any COVID-19 vaccine | 110 (69.6) | 13 (59.1) | .335 |
| Received at least 2 mRNA or 1 adenovirus-vector SARS-CoV-2 vaccine dose | 106 (67.1) | 12 (66.7) | .475 |
| Received third SARS-CoV-2 vaccine dosec | 60 (38.0) | 6 (27.3) | .459 |
| Received fourth SARS-CoV-2 vaccine dose | 2 (1.3) | 0 (0.0) | 1 |
| Vaccine brand | .797 | ||
| Johnson & Johnson | 6 (5.5) | 1 (7.7) | |
| Moderna | 39 (35.5) | 4 (30.8) | |
| Pfizer-BioNTech | 65 (59.1) | 8 (61.5) | |
| Anti-CD20 antibody | .699 | ||
| Obinutuzumab | 16 (10.1) | 1 (4.5) | |
| Rituximab | 142 (89.9) | 21 (95.5) | |
| Number of anti-CD20 doses, median (IQR) | 6.0 (4.0–9.0) | 7.0 (4.0–10.0) | .533 |
| Time from last anti-CD20 dose to COVID-19 diagnosis, m, median (IQR) | 4.1 (1.5–7.1) | 4.8 (2.2–5.8) | .709 |
| Indication for anti-CD20 therapy | .100 | ||
| Hematologic malignancy | 92 (58.2) | 10 (45.5) | |
| Neurologic disorder | 8 (5.1) | 0 (0.0) | |
| Rheumatologic disorder | 53 (33.5) | 9 (40.9) | |
| Otherd | 5 (3.2) | 3 (13.6) | |
| Other immunosuppressive drugs | 78 (49.4) | 13 (59.1) | .496 |
| Cytotoxic chemotherapy | 16 (10.1) | 3 (13.6) | .709 |
| BTK inhibitor | 12 (7.6) | 1 (4.5) | 1 |
| Corticosteroid | 37 (23.4) | 8 (36.4) | .196 |
| Antimetabolites | 22 (13.9) | 4 (18.2) | .531 |
| Calcineurin inhibitors | 7 (4.4) | 3 (13.6) | .108 |
| Antispike monoclonal antibody | .307 | ||
| Bamlanivimab | 20 (12.7) | 6 (27.3) | |
| Bamlanivimab-etesevimab | 12 (7.6) | 1 (4.5) | |
| Casirivimab-imdevimab | 71 (44.9) | 10 (45.5) | |
| Sotrovimab | 55 (34.8) | 5 (22.7) | |
| Dominant circulating VOCe | .417 | ||
| Pre-Delta | 28 (19.2) | 6 (28.6) | |
| Delta | 72 (49.3) | 11 (52.4) | |
| Omicron | 46 (31.5) | 4 (19.0) | |
| Time from symptom onset to monoclonal antibody administration, days, median (IQR) | 4.0 (3.0–6.0) | 4.0 (2.0–5.75) | .522 |
| Time from COVID-19 diagnosis to monoclonal antibody administration, days, median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–2.75) | .984 |
| COVID-19 symptoms | |||
| Chills | 31 (19.6) | 4 (18.2) | 1 |
| Cough | 110 (69.6) | 17 (77.3) | .619 |
| Diarrhea | 16 (10.1) | 3 (13.6) | .709 |
| Dyspnea | 23 (14.6) | 10 (45.5) | .002 |
| Fever | 55 (34.8) | 7 (31.8) | 1 |
Abbreviations: BMI, body mass index; BTK, Bruton’s tyrosine kinase; COVID-19, coronavirus disease 2019; IQR, interquartile range; JAK, Janus kinase; m, months; MASS, monoclonal antibody screening score; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; VOC, variant of concern.
NOTE: Data are n (%). Bold values indicate P < .05.
Defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 as calculated by the 2021 CKD-EPI equation. N = 179, excluding 1 patient who did not have renal function testing.
Includes 4 kidney transplant recipients, 2 heart transplant recipients, 2 liver transplant recipients, 1 lung transplant recipient, and 1 kidney-pancreas transplant recipient.
Third SARS-Cov-2 vaccine dose defined as receipt of an mRNA vaccine dose after either 2 prior mRNA vaccine doses or 1 adenovirus-vector dose.
Other indications for anti-CD20 therapy included pemphigus vulgaris (2), membranous nephropathy (2), antibody-mediated allograft rejection (2), focal segmental glomerulosclerosis recurrence after kidney transplantation (1), and desensitization before heart transplantation (1).
N = 167, excluding 13 patients who were diagnosed with COVID-19 during a VOC overlap period (4 in June 2021; 9 in late December 2021).
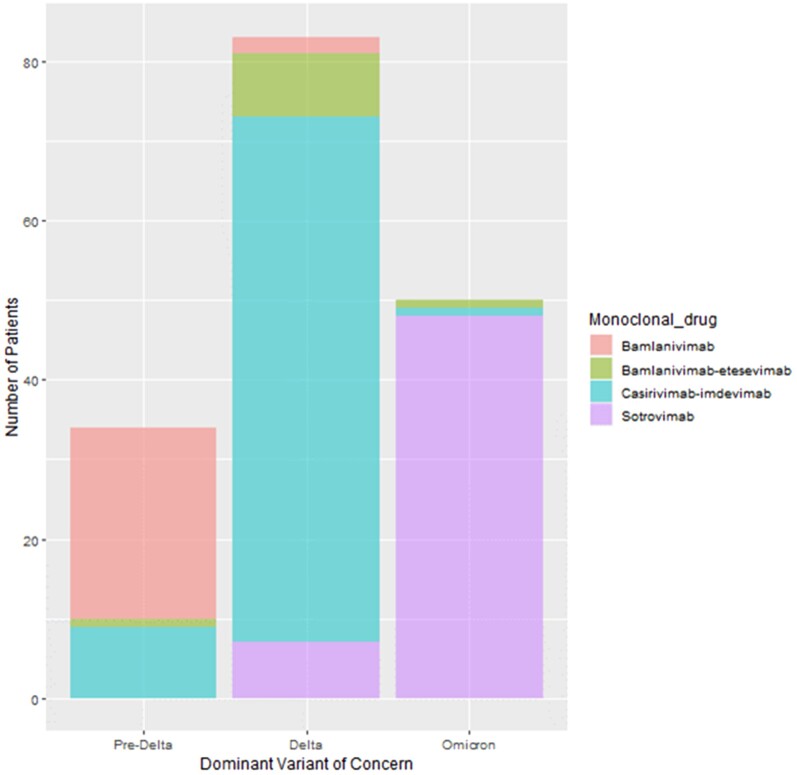
Only dyspnea at diagnosis was associated with progression to severe COVID-19 (P = .002). Monoclonal Antibody Screening Score and Charlson comorbidity index were similar between patients who developed severe COVOD-19 and those who remained with mild-to-moderate COVID-19 (Supplementary Table 1). There was no significant difference in the rates of severe COVID-19 progression based on the type of mAb. The mAbs used based on dominant VOC is presented in Figure 1. The rate of progression to severe COVID-19 was highest in the pre-Delta era (17.6%), followed by Delta (13.3%), and Omicron (8.0%), but these were not significantly different (P = .417). Eleven patients had antispike antibody titers measured before mAb receipt and 7 were positive.
Figure 1.
Bar chart demonstrating antispike monoclonal antibody usage during 3 periods divided by dominant circulating severe acute respiratory syndrome coronavirus 2 variant of concern. Pre-Delta period is November 2020-May 2021, Delta period is July 1–December 15, 2021, and Omicron period is January 1–February 15, 2022. This chart includes 167 patients, excluding 13 patients who were diagnosed during periods of overlap between variants of concern (excluded time: June 2021 and December 1–15, 2021).
Persistent Coronavirus Disease 2019
Of 111 patients with at least 90 days of follow-up, 2 (1.8%) developed persistent COVID-19. Both patients had not received any dose of SARS-CoV-2 vaccine. The first patient was an 81-year-old female with diffuse large B-cell lymphoma who was receiving rituximab, cyclophosphamide, daunorubicin, vincristine, and prednisone. She initially presented with cough, fever, and chills and received bamlanivimab 2 days after onset. Her symptoms were initially mild and improved without other therapies. However, she was admitted 36 days after initial diagnosis with escalating dyspnea. Computed tomography (CT) of the chest showed multifocal peripheral ground-glass and tree-in-bud opacifications. Despite initiation of remdesivir, dexamethasone, and broad-spectrum empiric antibiotics, she experienced progressive respiratory failure. Microbiologic testing from a bronchoalveolar lavage (BAL) was uniformly negative for microbial pathogens except for positive SARS-CoV-2 PCR. After 19 days of hospitalization, she was transitioned to comfort care and died thereafter.
The second patient was a 72-year-old female with a history of kidney transplantation 6 months prior, maintained on tacrolimus, mycophenolate mofetil, and prednisone, who was receiving rituximab for allograft membranous nephropathy. She tested positive for SARS-CoV-2 when she presented with cough and dyspnea. She received casirivimab-imdevimab 4 days after symptom onset. Twelve days later, she was hospitalized due to hypoxia and received remdesivir. After initial clinical improvement, she was readmitted 29 days later due to hypoxia where chest CT showed peripherally based ground-glass and consolidative opacifications. She underwent BAL, where SARS-CoV-2 PCR was positive while all other microbiologic testing was negative. She did not receive further COVID-19 therapy. She was re-admitted again 57 days after diagnosis where BAL sampling was again positive only for SARS-CoV-2. She developed progressive hypoxia and died after a 4-day hospitalization.
DISCUSSION
We describe the clinical outcomes of COVID-19 in B-cell-depleted patients with initial mild-to-moderate infection who were treated with mAb. Overall, most patients experienced favorable outcomes within the first 30 days after infection. In contrast to prior reports, only 12.2% of our cohort progressed to severe disease and 9.4% required hospitalization. Dyspnea on initial presentation was associated with progression to severe disease, which may be reflective of lower respiratory involvement that is already too advanced for mAb therapy. Despite 9.4% requiring hospitalization, the outcomes remained favorable at 30 days, with only 1 patient requiring ICU admission or invasive ventilation. No patient died within 30 days of treatment.
Antispike mAbs have consistently shown efficacy in many other cohorts of high-risk patients [7, 10, 11]. In a single-center study of immunocompromised patients with cancer, primarily hematologic malignancies, 43 patients who received mAb therapy had a hospitalization rate of 12% and only 1 patient required ICU care or died of COVID-19 [12]. Comparable outcomes were shown in solid organ transplant recipients and those with other high-risk comorbidities [7, 8, 10, 11]. Our findings are consistent with these studies, and the outcomes are improved compared with historical studies of COVID-19 in B-cell-depleted patients who did not receive mAb therapies [1, 2]. A recent study of 57 B-cell-depleted patients with COVID-19 who did not receive mAb showed high rates of hospitalization and death, 43.9% and 8.8%, respectively [1]. From patients who survived their acute COVID-19 episode, 17.3% presented again with persistent infection, which is higher than our cohort’s rate of only 1.8%. In a separate study of patients with lymphoid malignancies, 25% of those who survived hospitalization developed persistent infection requiring rehospitalization [2]. Our study suggests that mAb therapy may be efficacious in reducing rates of hospitalization, mortality, and persistent infection in this B-cell-depleted population.
Our findings are similar to prior studies on ConvP therapy in B-cell-depleted patients. A cohort of 17 patients with persistent COVID-19 uniformly recovered after ConvP administration [5]. Another report described that 21 of 25 B-cell-depleted patients recovered after receiving early ConvP infusion [4]. Collectively, early administration of antibody-based therapy is the recommended approach for B-cell-depleted patients with mild-to-moderate COVID-19. It is notable that our cohort was infused with mAbs at a median of 4 days after the onset of symptoms. Providing mAbs early in the disease course may partly account for the favorable outcomes in our cohort. Designing systems to rapidly diagnose and treat those at highest risk is key to optimizing clinical outcomes.
The rates of progression to severe COVID-19 seemed to numerically decline over time with the evolution of SARS-CoV-2 VOC. However, this was not a statistically significant observation. Although this analysis of outcomes may have been underpowered, there were also important advances in management of COVID-19 through time, including the opportunity for primary SARS-CoV-2 vaccination.
The 30-day outcomes in our cohort were improved compared with historical cohorts, but it should also be noted that 2 patients who developed persistent COVID-19 ultimately died from their infection within 60 days of initial diagnosis. Although mAbs seem to be beneficial in the early phases of disease, the optimal management of persistent infection remains unclear, and the role of mAbs in this setting requires further study. Knowledge of the specific SARS-CoV-2 VOC in patients with persistent infection may be an important strategy to determine whether there is a VOC-mAb mismatch that accounts for the poor clinical outcome.
A limitation of this study is its retrospective observational design with sources of bias inherent to this study type. There were very little data on baseline serostatus, limiting our ability to examine association of outcomes with pre-existing antispike antibody titers. The study was conducted through February 15, 2022, before the emergence of the BA.2 Omicron variant. Although mAbs used were effective at the time of the study, many are no longer effective against the currently surging BA.2 Omicron VOC. The rise of BA.2 Omicron has paused the clinical use of sotrovimab. Our program currently only utilizes bebtelovimab for B-cell-depleted patients with mild-to-moderate COVID-19 because it remains effective against Omicron. Finally, we can only assume, but not confirm, effectiveness because our study does not have a comparator group. The high uptake of mAb among our immunocompromised populations made it impossible to identify a comparable contemporary group of untreated B-cell-depleted patients. Thus, although clinical outcomes of our cohort were improved compared with outcomes reported in prior studies, these indirect comparisons should be interpreted with caution.
CONCLUSIONS
In conclusion, this study of 180 immunocompromised B-cell-depleted patients with COVID-19 demonstrated low rates of progression to severe disease, hospitalization, ICU admission, mortality, and persistent infection when treated early with mAb. The use of mAb should be encouraged in this highly vulnerable population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was funded by the National Institutes of Health (UL1TR002377). This work was supported by research funding from the Mayo Clinic (to R. R. R.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Zachary A Yetmar, Division of Infectious Diseases, Mayo Clinic, Rochester, Minnesota, USA.
Ryan B Khodadadi, Division of Infectious Diseases, Mayo Clinic, Rochester, Minnesota, USA.
Maria Teresa Seville, Division of Infectious Diseases, Mayo Clinic, Scottsdale, Arizona, USA.
Lisa Brumble, Division of Infectious Diseases, Mayo Clinic, Jacksonville, Florida, USA.
John C O’Horo, Division of Infectious Diseases, Mayo Clinic, Rochester, Minnesota, USA; Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Ravindra Ganesh, Division of General Internal Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Raymund R Razonable, Division of Infectious Diseases, Mayo Clinic, Rochester, Minnesota, USA; William J. von Liebig Center for Transplantation and Clinical Regeneration, Mayo Clinic, Rochester, Minnesota, USA.
References
- 1. Calderón-Parra J, Múñez-Rubio E, Fernández-Cruz A, et al. Incidence, clinical presentation, relapses and outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients treated with anti-CD20 monoclonal antibodies. Clin Infect Dis 2021; ciab700. [DOI] [PubMed] [Google Scholar]
- 2. Lee CY, Shah MK, Hoyos D, et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov 2022; 12:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gharbharan A, GeurtsvanKessel CH, Jordans CCE, et al. Effects of treatment of coronavirus disease 2019 with convalescent plasma in 25 B-cell–depleted patients. Clin Infect Dis 2022;. 74:1271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19. Blood 2020; 136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson MA, Henderson JP, Shah PK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol 2021; 7:1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Razonable RR, Pawlowski C, O’Horo JC, et al. Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021; 40:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yetmar ZA, Beam E, O’Horo JC, et al. Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients. Open Forum Infect Dis 2021; 8:ofab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yahav D, Yelin D, Eckerle I, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect 2021; 27:315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganesh R, Philpot LM, Bierle DM, et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis 2021; 224:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganesh R, Pawlowski CF, O’Horo JC, et al. Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19. J Clin Invest 2021; 131:e151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puing AG, Ho S, Frankel P, et al. Severe acute respiratory syndrome coronavirus 2–specific monoclonal antibody for the treatment of mild to moderate coronavirus disease 2019 in cancer patients: a single-center experience. J Infect Dis 2022; 225:352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.