Abstract
Billions of doses of coronavirus disease 2019 (COVID-19) vaccines have been administered globally, dramatically reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incidence and severity in some settings. Many studies suggest vaccines provide a high degree of protection against infection and disease, but precise estimates vary and studies differ in design, outcomes measured, dosing regime, location, and circulating virus strains. In this study, we conduct a systematic review of COVID-19 vaccines through February 2022. We included efficacy data from Phase 3 clinical trials for 15 vaccines undergoing World Health Organization Emergency Use Listing evaluation and real-world effectiveness for 8 vaccines with observational studies meeting inclusion criteria. Vaccine metrics collected include protection against asymptomatic infection, any infection, symptomatic COVID-19, and severe outcomes including hospitalization and death, for partial or complete vaccination, and against variants of concern Alpha, Beta, Gamma, Delta, and Omicron. We additionally review the epidemiological principles behind the design and interpretation of vaccine efficacy and effectiveness studies, including important sources of heterogeneity.
Keywords: COVID-19, SARS-CoV-2, systematic review, vaccine effectiveness, vaccine efficacy
We conduct a systematic review of COVID-19 vaccine efficacy and effectiveness through February 2022. This synthesis includes 15 vaccine products and covers protection against infection and disease for multiple SARS-CoV-2 variants, for partial and complete primary vaccination series.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)’s rapid global spread and alarming clinical severity have accelerated demand for vaccines that safely and effectively prevent disease or reduce severity. To date, 30 coronavirus disease 2019 (COVID-19) vaccines have received emergency use authorization in at least 1 country, and approximately 5 billion people have been vaccinated [1, 2].
Evidence from clinical trials and observational studies overwhelmingly supports the safety and efficacy and/or effectiveness of numerous COVID-19 vaccines, especially against severe disease and death in fully vaccinated individuals. However, precise estimates of vaccine efficacy and effectiveness have varied across studies due to a variety of factors. For example, efficacy against symptomatic COVID-19 for AstraZeneca’s 2-dose viral vector vaccine (AZD1222) ranged from 62% to 90% in Phase 3 clinical trials [3], which is attributed to differences in dosing schedules. Observational studies in a variety of settings also produced a wide range of effectiveness estimates (50%–100%) against different clinical outcomes [4–14]. The emergence of SARS-CoV-2 “variants of concern” (VOC), including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529), further confounds interpretation of estimates obtained from comparably designed vaccine studies. Some variants are associated with higher viral load [15–19], evasion of neutralizing antibodies ex vivo [20–25], and lower vaccine effectiveness [13, 19, 26–29]. However, separating diminished protection against a variant from the effects of waning immunity, study methodology, or other contextual factors (eg, lower use of nonpharmaceutical interventions) is complicated by their co-occurrence and endogeneity.
Previous reviews of COVID-19 vaccines have focused mainly on the developmental pathway and early results from preclinical and clinical trials, the immunological basis of vaccine-induced protection, a narrow subset of vaccines or clinical outcomes, or have been rapid in nature and limited to earlier studies [30–47]. There remains an urgent need for comprehensive, up-to-date review of COVID-19 vaccine effects, including real-world evidence. In this study, we systematically reviewed COVID-19 vaccine efficacy and effectiveness data against multiple clinical outcomes, for both full and partial immunization courses, by circulating SARS-CoV-2 variants of concern. We focused on studies conducted in primarily adult populations, for the primary vaccine course only (ie, excluding “booster” doses), and outcomes measured within the first 6 months after the final vaccine dose.
METHODS
We reviewed studies reporting vaccine “efficacy” (from randomized clinical trials) or “effectiveness” (from observational studies) [48, 49] for vaccines that had received or submitted applications for Emergency Use Listing from the World Health Organization (WHO) as of February 1, 2022 [50] and had at minimum publicly-released data from completed Phase 3 trials (Table 1). We searched for clinical trial results published in peer-reviewed scientific journals (via PubMed) and preprint servers (medRxiv, bioRxiv, SSRN), government public health, regulatory agency, and vaccine manufacturers’ websites, and in news articles (Google). Searches were conducted using the vaccine’s brand, trade, or research name. For observational studies, we applied detailed queries to multiple databases (Supplementary Methods), and we only included results that appeared in at least a detailed report and/or preprint. Study title and abstract were screened before progressing to full-text review (Supplementary Figure S1). We extracted vaccine efficacy and/or effectiveness against disease endpoints, specifically asymptomatic infection, any infection, symptomatic disease, severe disease (including “hospitalization”), and death (Supplementary Table S1).
Table 1.
Status of COVID-19 Vaccines Within the World Health Organization Emergency Use Listing Evaluation Processa
| Countries | Phase 3 Efficacy Trial | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine Name | Vaccine Type | WHO Status | Developed in | No. Approved | Data? | CIs? | Peer Reviewed? | Ref | |
| AstraZeneca/ Oxford |
AZD1222 ChAdOx1- nCoV-19 Vaxzevria Covishield |
Viral vector | EUL Authorized | UK | 162 | Yes | Yes | Yes | [3, 68, 82, 86] |
| Pfizer/ BioNTech |
BNT162b2 Comirnaty Tozinameran |
mRNA | EUL Authorized | Germany | 134 | Yes | Yes | Yes | [64, 71, 88] |
| Janssen/ Johnson & Johnson | Ad26.COV2.S | Viral vector | EUL Authorized | USA | 105 | Yes | Yes | Yes | [66] |
| Sinopharm- Beijing |
BBIBP-CorV Covilo |
Inactivated virus | EUL Authorized | China | 87 | Yes | Yes | Yes | [89] |
| Moderna | mRNA-1273 Spikevax |
mRNA | EUL Authorized | USA | 85 | Yes | Yes | Yes | [67] |
| Gamaleya Institute | Sputnik V Gam-COVID-Vac |
Viral vector | Submission in Progress |
Russia | 74 | Yes | Yes | Yes | [72] |
| Sinovac | CoronaVac | Inactivated virus | EUL Authorized | China | 52 | Yes | Yes | Yes | [73, 74, 90] |
| Novavax | NVX-CoV2373 Nuvaxovid Covovax |
Protein subunit | EUL Authorized | USA | 31 | Yes | Yes | Yes | [70, 83, 223] |
| Bharat Biotech | BBV152 Covaxin |
Inactivated virus | EUL Authorized | India | 13 | Yes | Yes | Yes | [65] |
| CanSinoBIO | Ad5-nCoV Convidecia |
Viral vector | EOI Accepted | China | 10 | Yes | Yes | Yes | [75] |
| BioCubaFarma | Abdala CIGB-66 |
Protein subunit | Submission in progress |
Cuba | 6 | Yes | Yes | No | [91] |
| BioCubaFarma | Soberana 02 FINLAY-FR-2 |
Conjugate | Submission in progress |
Cuba | 4 | Yes | Yes | No | [92] |
| Vector Institute | EpiVacCorona | Protein subunit | Submission in Progress |
Russia | 4 | No | No | No | |
| Anhui Zhifei Longcom | ZF2001 ZIFIVAX |
Protein subunit | EOI accepted | China | 3 | Yes | No | No | |
| Sinopharm- Wuhan |
WIBP-CorV | Inactivated virus | EOI accepted | China | 2 | Yes | Yes | Yes | [89] |
| IMBCAMS | Covidful | Inactivated virus | Submission in progress |
China | 1 | No | No | No | |
| BioCubaFarma | Soberana 02+ FINLAY-FR-1A |
Protein subunit | Submission in progress |
Cuba | 1 | Yes | Yes | No | [92] |
| Clover | SCB-2019 | Protein subunit | EOI accepted | China | 0 | Yes | Yes | Yes | [76] |
| CureVac | CVnCoV | mRNA | EOI accepted/ withdrawn |
Germany | 0 | Yes | Yes | Yes | [224] |
| Sanofi | CoV2 preS dTM-AS03 Vidprevtyn |
Protein subunit | EOI accepted | France | 0 | No | No | No | |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; EOI, expression of interest; EUL, emergency use listing; WHO, World Health Organization.
Vaccine products are listed in descending order based on the number of countries in which the vaccine is approved for emergency use as of February 2022. Vaccines included in the current study—based on availability of efficacy data from Phase 3 clinical trials—are highlighted in gray. Vaccine details were obtained from McGill University’s COVID-19 Vaccine Tracker: https://covid19.trackvaccines.org/, which aggregates data from multiple sources. Original source for the WHO. EUL status: https://extranet.who.int/pqweb/key-resources/documents/status-covid-19-vaccines-within-who-eulpq-evaluation-process.
Observational studies were only eligible if the comparison group included concurrent individuals (eg, modeled or historic controls were excluded), outcomes were laboratory-confirmed, the study design attempted to account for confounding, vaccination status was determined by self-report for <10% of participants, confidence intervals were reported, no significant bias was present as determined by expert opinion, and controls were unvaccinated (eg, excluded if “unvaccinated” included days 0–12 postvaccination). Only studies comparing persons with and without the clinical outcome under investigation and with and without vaccination were included. Impact studies and studies evaluating progression to severe disease among SARS-CoV-2-positive individuals were excluded. For full immunization (1 or 2 doses, depending on the vaccine) results were included if at least 1 week had passed between the final dose and case detection; for partial immunization, cases must have occurred at least 2 weeks after dose 1 but before dose 2. We excluded efficacy and/or effectiveness values for which (1) follow-up period after final dose was >6 months, (2) doses beyond the primary series were given (ie, boosters), or (3) multiple vaccines were combined. We classified efficacy and/or effectiveness against specific SARS-CoV-2 variants if sequencing (or other molecular methods) either (1) confirmed the variant in all cases contributing to the estimate or (2) confirmed the variant caused the vast majority of cases in sample of study participants or of the larger population from which they came. Our data are available on VIEW-hub, a weekly updated resource developed by Johns Hopkins’ International Vaccine Access Center (https://view-hub.org/resources), and in Supplementary Table S1.
EPIDEMIOLOGICAL PRINCIPLES
Measuring How Well a Vaccine Prevents Infection and Disease
Vaccine efficacy is evaluated in randomized controlled trials (RCTs) and is defined as the relative reduction in the probability of developing disease in a particular time period in vaccinated individuals compared with unvaccinated individuals. In RCTs, subjects are randomly assigned to receive the vaccine or not (instead usually receiving a placebo or another vaccine). Vaccine efficacy (VE) is calculated using the formula:
where sometimes the denominator “# vaccinated” (“# unvaccinated”) is replaced with the sum of the total time enrolled in the study among vaccinated (unvaccinated) subjects (ie, the “person time”) [51, 52]. Although RCTs are the gold standard for vaccine studies [53], they are costly and generally too small to evaluate rare outcomes (eg, death).
Vaccine efficacy describes the “relative”, as opposed to “absolute”, risk of disease. This is a desired feature of a metric for vaccine strength, because absolute risk may change over time as the background disease incidence changes during an epidemic due to factors such as seasonality and behavior change. For example, if the absolute risk of disease in one setting is reduced from 50% to 10% through vaccination, then the vaccine efficacy (80%) is the same as in another setting where the absolute risk was reduced from 5% to 1%. The value of vaccine efficacy also does not tell us whether vaccine failure occurs in a “leaky” or “all-or-nothing” way [52, 54, 55].
Once a vaccine is shown to be safe and efficacious in clinical trials and is authorized for general use, further RCTs to evaluate efficacy under new conditions or in special populations are often considered unethical or impractical, especially in the setting of a wide-spread epidemic, because it necessitates withholding vaccines from people who might benefit from them. Instead, observational studies evaluating real-world effectiveness are used to augment efficacy trials, and they include designs such as case-control studies (including test-negative designs) and cohort studies (prospective or retrospective) [52, 56, 57]. Although observational studies must carefully address biases due to differences in those who chose to or were eligible to receive vaccines compared with those who did not, they may provide a more realistic picture of population heterogeneity and include more high-risk groups compared with RCTs. During massive vaccination campaigns like those occurring for COVID-19, observational studies can have much larger sample sizes.
To measure vaccine efficacy and/or effectiveness, the specific clinical outcome that the vaccine is meant to prevent must be carefully defined. The ideal goal of vaccination is to completely prevent infection (and thus disease and transmission), meaning that vaccine-induced immunity blocks the earliest attempts of the pathogen to replicate within the body. This “sterilizing immunity” is rare, and vaccine efficacy and/or effectiveness against infection is difficult to measure [58, 59] for short-lived and commonly asymptomatic infections like SARS-CoV-2 because it requires frequent testing of the study cohort. To reduce the public health impact of an infectious disease, prevention of severe disease (including hospitalization and death) is desired, even if infection still occurs [53, 59, 60]. However, because most individuals with COVID-19 recover completely with only mild or moderate symptoms [61–63], studies of severe outcomes can require hundreds of thousands of participants or months to years of observation time. For COVID-19, the primary endpoint for most clinical trials was symptomatic, laboratory-confirmed COVID-19 disease [53], defined as the occurrence of COVID-19-associated symptoms (eg, cough, shortness of breath, fever) in the presence of detectable SARS-CoV-2. This outcome choice represents a trade-off between public health importance and practicality. Symptomatic disease is on the spectrum leading to severe disease but much more common, and testing can be restricted to those self-reporting symptoms. Although some trials reported efficacy against severe disease as secondary outcomes despite small numbers [64–76], large observational studies provide more precise estimates.
Sources of Heterogeneity Across Studies
The COVID-19 vaccine studies were conducted by many independent research teams in diverse epidemic settings around the world (Table 1, Table 2, Supplementary Table S1, Figure 1). As a result, there are several potential sources of heterogeneity between studies that make comparing vaccine efficacy and/or effectiveness estimates difficult [57, 77]:
Table 2.
Summary of Vaccine Effectiveness Studiesa
| Vaccine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 (Pfizer- BioNTech) |
mRNA- 1273 (Moderna) |
AZD1222 (AstraZeneca-Oxford) |
Ad26.COV2.S (Janssen/ Johnson & Johnson) |
Sputnik V (Gamaleya) | CoronaVac (Sinovac) | BBV152 (Bharat) |
BBIBP-CorV (Sinopharm- Beijing) |
All | |
| Total | 86 | 39 | 30 | 16 | 1 | 6 | 1 | 2 | 107 |
| Outcome Type | |||||||||
| Death | 12 | 6 | 5 | 6 | 1 | 4 | 0 | 1 | 21 |
| Severe disease | 38 | 19 | 12 | 10 | 0 | 3 | 0 | 1 | 50 |
| Symptomatic disease | 28 | 12 | 12 | 4 | 0 | 3 | 1 | 0 | 38 |
| Asymptomatic infection | 5 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 9 |
| Any infection | 58 | 25 | 14 | 7 | 1 | 2 | 0 | 1 | 67 |
| Population Type | |||||||||
| General population | 40 | 26 | 15 | 9 | 1 | 2 | 0 | 2 | 45 |
| Health care workers | 18 | 4 | 1 | 1 | 0 | 0 | 1 | 0 | 20 |
| Older adults (≥60 years) | 8 | 0 | 8 | 1 | 0 | 2 | 0 | 0 | 11 |
| Other age groups | 6 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 7 |
| Contacts of cases | 6 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 6 |
| LTCF/SNF residents | 5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Other groups | 6 | 3 | 1 | 1 | 0 | 2 | 0 | 0 | 9 |
| Study Design | |||||||||
| Test-negative case control | 30 | 21 | 13 | 7 | 0 | 3 | 1 | 1 | 42 |
| Traditional case-control | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Prospective cohort | 17 | 4 | 7 | 1 | 0 | 1 | 0 | 0 | 21 |
| Retrospective cohort | 36 | 13 | 9 | 8 | 1 | 2 | 0 | 1 | 41 |
| No. of Doses | |||||||||
| Complete | 76 | 36 | 19 | 16 | 1 | 6 | 1 | 2 | 96 |
| Partial | 44 | 14 | 25 | N/A | 0 | 3 | 1 | 0 | 53 |
| Variants of Concern | |||||||||
| Alpha | 33 | 8 | 17 | 2 | 1 | 0 | 1 | 0 | 35 |
| Beta | 4 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 6 |
| Gamma | 2 | 2 | 3 | 1 | 0 | 1 | 0 | 0 | 5 |
| Delta | 22 | 13 | 9 | 5 | 0 | 1 | 1 | 0 | 29 |
| Omicron | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
Abbreviations: COVID-19, coronavirus disease 2019; LTCF, long-term care facility; N/A, not applicable; SNF, skilled nursing facility.
Number of included studies by COVID-19 vaccine and by outcome, population, study design, number of doses, and variant of concern. No studies reported for other vaccine candidates met our inclusion criteria (see Methods). Details of all the individual studies are included in Supplementary Table S1.
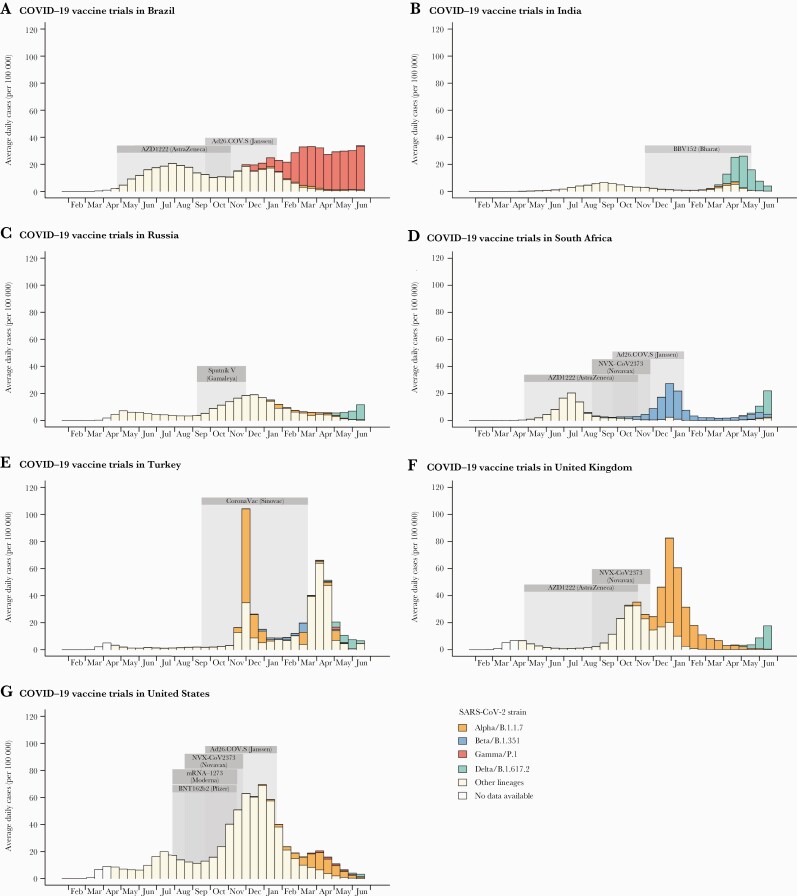
Figure 1.
Local context of Phase 3 clinical trials of coronavirus disease 2019 (COVID-19) vaccines. For each country, the time period (2020-2021) during which outcomes were observed during each vaccine trial is shaded gray. For each 2-week period, the average daily incidence of reported cases is shown (height of bars) [225]. The contribution of each major variant of concern to total case counts is estimated from the reported fraction of sequenced severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) samples belonging to that strain (fill color) [226]. Figure includes only vaccine trials described in published or preprint reports, trial sites with at least 10 000 individuals from the general adult population, and countries regularly reporting SARS-CoV-2 lineages to the GISAID database.
Study population: Efficacy/effectiveness can be lower in studies including more participants at high risk of disease (eg, comorbid individuals) or with reduced immune function (eg, people with human immunodeficiency virus).
Outcome and case definition: Vaccine efficacy and/or effectiveness differs between disease outcomes with varying severity. Even when studies have the same stated outcome, the case definition can vary substantially. For example, most clinical trials used “symptomatic COVID-19 disease” as the primary outcome, but included anywhere from 5 (for AstraZeneca/AZD1222 [3]) to 16 (Janssen/Ad26.COV2.S [66]) different potential symptoms, and varied in requiring 1 or 2 to be present. These differences are exacerbated in effectiveness studies that often rely on passive surveillance by health systems. In addition, different definitions in the timing of the disease outcome (eg, death within 30 days after diagnosis) can lead to heterogeneities.
Follow-up period: Because developing an adaptive immune response after vaccination takes time [59, 78–80], studies that begin the follow-up period sooner could observe lower efficacy and/or effectiveness (eg, 7+ days for Novavax/NVX-CoV2373 [70] vs 28+ days for Janssen/Ad26.COV2.S [66]). In addition, if immune protection wanes, studies with longer follow-up could have lower estimates.
Predominant variants: Some SARS-CoV-2 variants of concern have been observed to exhibit immune-escape properties [21, 81] (eg, Beta [28, 82–84], Delta [13, 19, 65], Omicron [26, 27, 29]). Studies conducted when such variants account for a large proportion of infections could have lower efficacy and/or effectiveness (Figure 1).
Study design and analysis: To make up for lack of randomization, vaccine effectiveness studies can attempt to reduce confounding through study design (eg, the test-negative design) and during analyses (eg, controlling for potential confounding variables in regression models). Some studies, especially those that use administrative data, may not collect and therefore cannot control for such possible confounders.
Other reviews of COVID-19 vaccine efficacy and/or effectiveness have tended to summarize results from studies with different designs, participant pools, and settings with a single effectiveness value or range, sometimes via formal meta-analysis [36, 37, 39, 41, 42, 44, 47, 85]. In this study, we have taken a different approach and show individual efficacy and/or effectiveness measures and their sources, and we discuss reasons for the observed heterogeneity, while also highlighting the consensus that emerges among them.
RESULTS OF CORONAVIRUS DISEASE 2019 VACCINE STUDIES
Data Available From Clinical Trials of Vaccine Efficacy
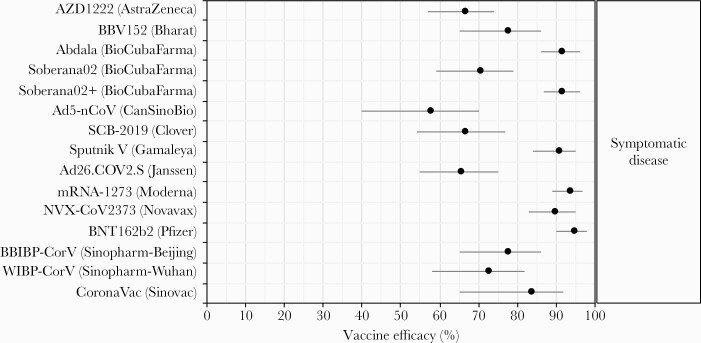
As of February 3, 2022, 20 unique vaccine products were in the WHO Emergency Use Listing (EUL) evaluation process, including messenger ribonucleic acid (mRNA), viral vector, inactivated virus, protein subunit, and conjugate vaccine platforms, and were developed by a mix of pharmaceutical companies, nonprofit research institutes, and government agencies (Table 1). Eight vaccines representing 4 platforms had received authorization. Efficacy estimates with uncertainty intervals from Phase 3 clinical trials were publicly available for 15 of the 20 vaccine candidates in 22 separate reports [3, 64–70, 72–76, 82, 83, 86–92] (Figure 2, Supplementary Figure S1). Vaccine efficacy against symptomatic COVID-19 was available for all 15 vaccines, ranging from 58% to 95%, with highest efficacy (>90%) from 6 products representing mRNA, protein subunit, and viral vector platforms. Efficacy against any infection was available for 5 EUL-authorized vaccines and against severe disease for 6, although confidence intervals (CIs) for severe disease tended to be wide due to limited sample sizes and trial durations (Figures 3–7, Supplementary Table S1).
Figure 2.
Vaccine efficacy against symptomatic coronavirus disease 2019 (COVID-19), from Phase 3 clinical trials. Each efficacy value is for the complete vaccine course (1 dose for Ad26.COV2.S/Janssen and Ad5-nCoV/CanSinoBio, 3 doses for BioCubaFarma/Abdala and Soberana02+/BioCubaFarma, and 2 doses for all others). Two vaccines which withdrew from the World Health Organization Emergency Use Listing evaluation process but completed Phase 3 trials were not included (CureVac’s mRNA vaccine CVnCoV, reporting 48% efficacy [224], and Kazakhstan RIBSP’s inactivated virus vaccine QazCovid-in, reporting 82% efficacy [227]).
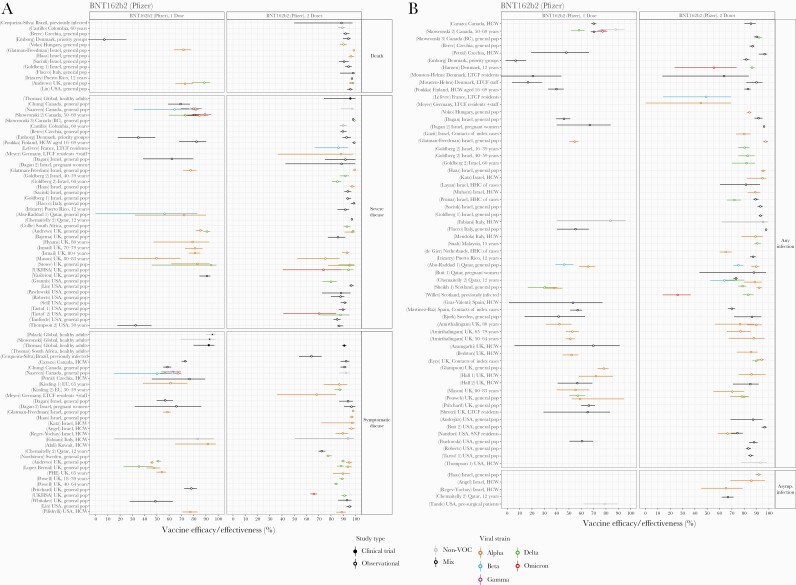
Figure 3.
Vaccine efficacy and effectiveness estimates for BNT162b2, a 2-dose mRNA vaccine developed by Pfizer/BioNTech. (A) Efficacy and/or effectiveness against death, severe disease, or symptomatic disease. (B) Efficacy and/or effectiveness against any or asymptomatic infection. Estimates are colored by the viral variant against which the vaccine efficacy or effectiveness value was measured. Solid markers are estimates from randomized clinical trials (efficacy values), and open markers are estimates from observational studies (effectiveness values). The source of each estimate is given by the labels on the left side (“(reference number) Country, population”). Within each disease severity level, estimates are ordered alphabetically by country and then by population.
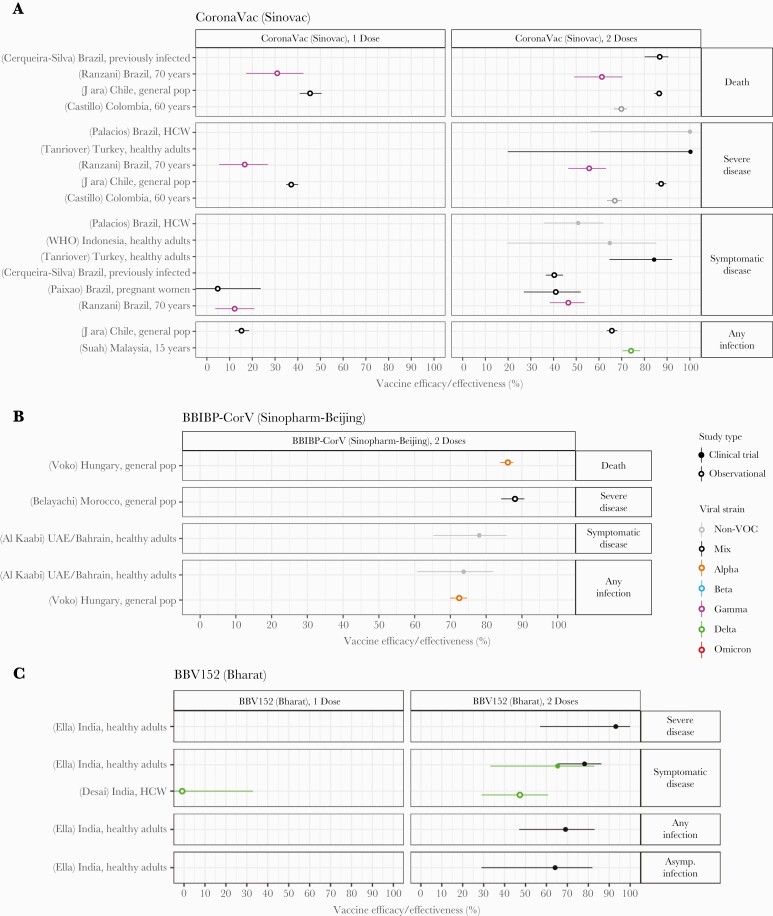
Figure 7.
Vaccine efficacy and effectiveness estimates for three 2-dose inactivated virus vaccines: (A) CoronaVac, developed by Sinovac (B) BBIBP-CorV, developed by Sinopharm Beijing, and (C) BBV152, developed by Bharat Biotech. Estimates are colored by the viral variant against which the efficacy or effectiveness value was measured. Solid markers are estimates from randomized clinical trials (efficacy values), and open markers are estimates from observational studies (effectiveness values). The source of each estimate is given by the labels on the left side (“(reference number) Country, population”). Within each disease severity level, estimates are ordered alphabetically by country and then by population.
Data Available From Observational Studies of Vaccine Effectiveness
We identified 107 real-world vaccine effectiveness studies that met our inclusion criteria [4–14, 19, 26–29, 38, 84, 93–179]. These studies covered 8 vaccines: 7 with WHO EUL authorization (all but Novavax’s NVX-CoV2373) and 1 with EUL submission in progress (Gamaleya’s Sputnik V) (Table 2, Supplementary Figure S1, Supplementary Table S1). These observational studies provided metrics not reported in clinical trials for some vaccines, including effectiveness against severe outcomes, asymptomatic infection, any infection, specific circulating variants, and effectiveness of a single dose of 2-dose vaccines (Figures 3–7). In general, effectiveness estimates overlapped with efficacy estimates and were high for full vaccine courses. Vaccines were more effective at preventing severe infection or death compared with symptomatic COVID-19. The degree to which vaccines prevented any infection, and the degree to which partial courses prevented infection or disease, varied widely by product. Data were sparse for effectiveness against death or asymptomatic infection and the Gamma variant.
Efficacy and/or Effectiveness of Messenger Ribonucleic Acid Vaccines
The most data were available for BNT162b2, the 2-dose mRNA vaccine developed by Pfizer/BioNTech, mainly due to its early 2021 use in Israel, the United Kingdom, and the United States (Figure 3). Efficacy and/or effectiveness estimates after 2 doses in the general population, pre-Omicron, ranged from 90% to 99% for death, 80% to 100% for severe infection, 70% to 100% for symptomatic disease, 65% to 98% for any infection, and 65% to 90% for asymptomatic infection. These values were lower after only a single dose: 70%–90% for death, 55%–95% for severe infection, 35%–93% for symptomatic disease, and 30%–80% for any infection. Some studies found lower effectiveness in special populations, including residents of long-term care facilities [84, 149, 150], priority groups [122], the elderly [147], and those previously infected [113, 179]. Heterogeneities between studies made direct comparisons of efficacy and/or effectiveness for variants difficult. A few studies with head-to-head comparisons suggested slightly reduced effectiveness for BTN162b2 against Beta and Delta compared with Alpha or non-VOC, for symptomatic cases or any infection [13, 28, 96, 123, 145, 162]. Evidence for reduced effectiveness against Omicron was more pronounced, with protection dropping to ~70% for severe disease, ~65% for symptomatic disease, and ~55% for any infection [26, 27, 136].
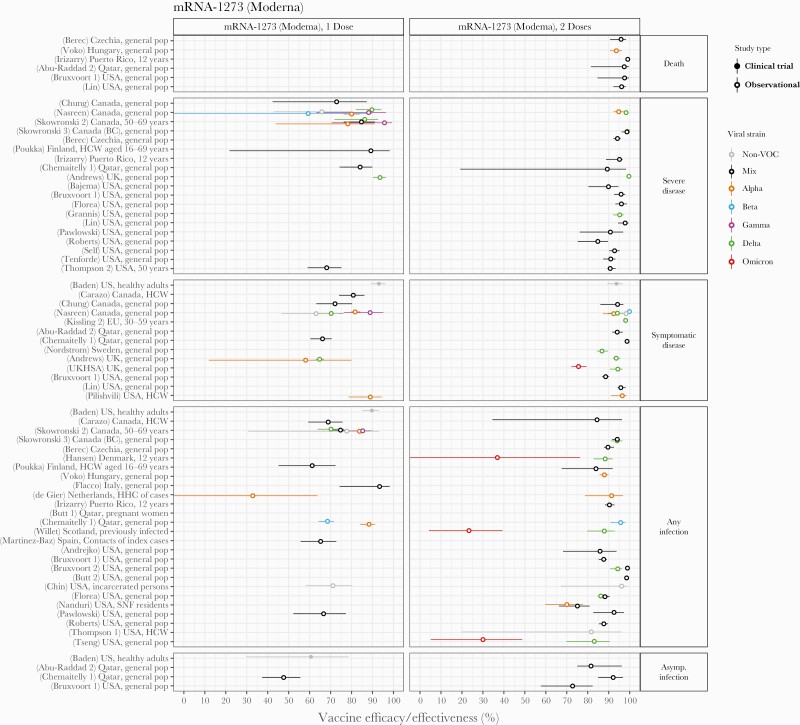
The other authorized 2-dose mRNA vaccine, Moderna’s mRNA-1273, was also relatively well studied, largely using data from the United States, Canada, and Qatar (Figure 4). After dose 2, efficacy and/or effectiveness estimates in the general population (pre-Omicron) were between 94% and 99% for death, 85% and 100% for severe disease, 87% and 100% for symptomatic disease, 83% and 100% for any infection, and 73% and 93% for asymptomatic infection. With only a single dose, values were 60%–95% for severe disease, 60%–95% for symptomatic disease, 65%–95% for any infection, and 45%–60% for asymptomatic infection. Studies in the United Kingdom, United States, and Denmark [27, 29, 136] estimated effectiveness against the Omicron variant after 2 doses was reduced to ~75% for symptomatic infection and to 30%–40% for any infection. Some evidence for reduction in effectiveness against infection or severe disease with Beta after only 1 dose was observed in studies in Canada and Qatar [12, 114]. Significant reductions were not consistently reported for effectiveness against other VOC.
Figure 4.
Vaccine efficacy and effectiveness estimates for mRNA-1273, a 2-dose mRNA vaccine developed by Moderna. Estimates are colored by the viral variant against which the vaccine efficacy or effectiveness value was measured. Solid markers are estimates from randomized clinical trials (efficacy values), and open markers are estimates from observational studies (effectiveness values). The source of each estimate is given by the labels on the left side (“(reference number) Country, population”). Within each disease severity level, estimates are ordered alphabetically by country and then by population.
Efficacy and/or Effectiveness of Viral-Vector Vaccines
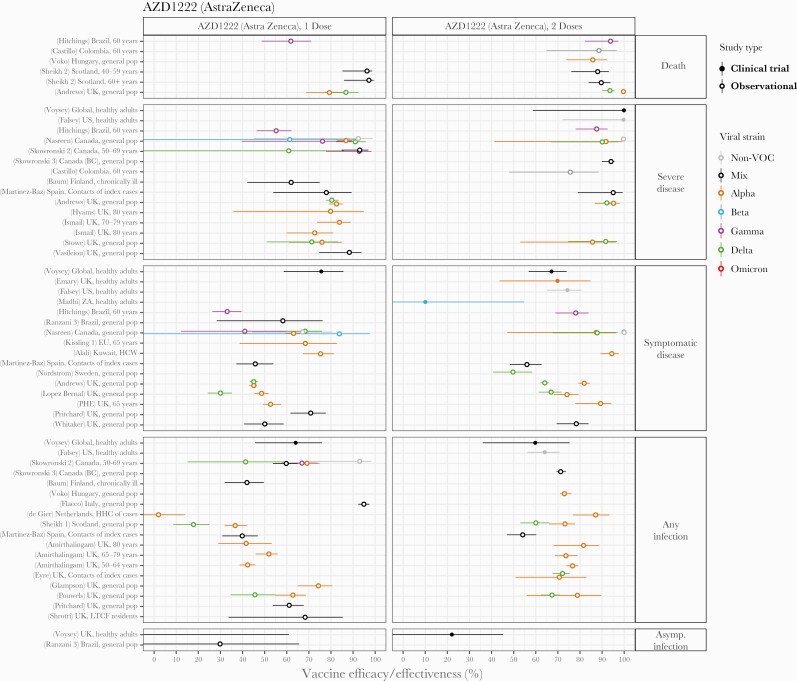
AstraZeneca’s 2-dose viral vector vaccine (AZD1222) was also frequently studied, especially after only 1 dose, likely because the recommended interval between doses is longer than other vaccines (12 weeks) and some countries including the United Kingdom and Canada adopted the strategy of prioritizing first doses over second doses in early 2021 (Figure 5). Efficacy and/or effectiveness against non-VOC strains, the Alpha variant (predominant during most studies), or mixes of strains after 2 doses was 85%–100% for death or severe disease, 65%–100% for symptomatic disease, 60%–80% for any infection, and 22% for asymptomatic infection in the general population. After a single dose, efficacy and/or effectiveness was 80%–96% for death, 75%–92% for severe disease, 45%–75% for symptomatic disease, and 35%–95% for any infection. The Delta variant appeared to reduce effectiveness against symptomatic disease or infection compared with the earlier Alpha variant by 10%–15% after 2 doses and ~20%–30% after a single dose in some [13, 19, 96, 145, 171] but not all [12, 123] studies. Effectiveness against the Gamma variant was within the range of earlier strains. Clinical trial evidence from the South Africa site [82] suggested loss of efficacy against symptomatic infection with the Beta variant but with much uncertainty (efficacy 10% CI, <0–55). No studies meeting our inclusion criteria reported on the protection that AZD1222 alone provided against the Omicron variant.
Figure 5.
Vaccine efficacy and effectiveness estimates for AZD1222, a 2-dose viral vector vaccine developed by AstraZeneca. Estimates are colored by the viral variant against which the efficacy or effectiveness value was measured. Solid markers are estimates from randomized clinical trials (efficacy values), and open markers are estimates from observational studies (effectiveness values). The source of each estimate is given by the labels on the left side (“(reference number) Country, population”). Within each disease severity level, estimates are ordered alphabetically by country and then by population.
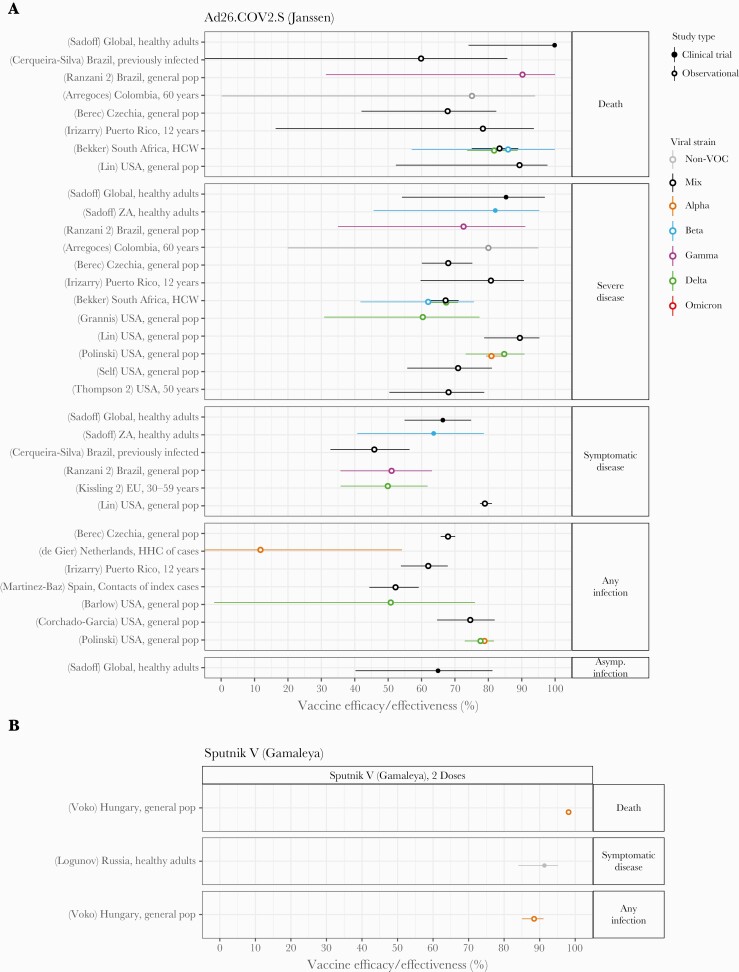
Janssen’s Ad26.COV2.S single-dose viral vector vaccine was studied in a variety of settings around the world (Figure 6), with efficacy and/or effectiveness estimates of 70%–100% against death, 60%–90% for severe disease, 50%–80% for symptomatic disease, 50%–80% for any infection, and 65% for asymptomatic infection. Protection against infection or severe outcomes appeared to be preserved with SARS-CoV-2 variants of concern, although confidence intervals were extremely wide and no data for Omicron were available meeting inclusion criteria.
Figure 6.
Vaccine efficacy and effectiveness estimates for 2 viral vector vaccines: (A) Ad26.COV2.S, a single-dose vaccine developed by Janssen/Johnson & Johnson, and (B) Sputnik V, a 2-dose vaccine developed by Gamaleya Institute. Estimates are colored by the viral variant against which the efficacy or effectiveness value was measured. Solid markers are estimates from randomized clinical trials (efficacy values), and open markers are estimates from observational studies (effectiveness values). The source of each estimate is given by the labels on the left side (“(reference number) Country, population”). Within each disease severity level, estimates are ordered alphabetically by country and then by population.
Data were sparse for Gamaleya Institute’s 2-dose viral vector vaccine Sputnik V (Figure 6), despite its use in dozens of countries (Table 1). A single clinical trial estimated efficacy against symptomatic disease with non-VOC virus of 91% [72], whereas an observational study in Hungary in the context of the Alpha variant reported effectiveness of 98% against death and 88% against any infection [178]. No studies measuring protection against the Beta, Delta, or Omicron variant met our inclusion criteria.
Efficacy and/or Effectiveness of Inactivated Virus Vaccines
Estimates of vaccine efficacy and effectiveness were available for 3 different 2-dose inactivated virus vaccines: CoronaVac (Sinovac), BBIBP-CorV (Sinopharm-Beijing), and BBV152 (Bharat) (Figure 7). Effectiveness studies of Sinovac’s inactivated virus vaccine CoronaVac were available from only 6 studies, despite being one of the most widely used vaccines worldwide. These complemented results from 3 separate clinical trials, including one restricted to healthcare workers. After 2 doses, in the general population, efficacy and/or effectiveness was 86% against death, 88%–100% against severe disease, 65%–85% against symptomatic disease, and 65%–75% against any infection. With only 1 dose, these values were significantly reduced, to 46% for death, 37% for severe disease, and 16% for any infection. Lower values were reported in studies in older adults, including one study conducted in adults over 70 years old in Brazil during a time when Gamma was predominant [163]. The only other variant-specific study suggested effectiveness against any infection was preserved against the Delta variant [180]. Sinopharm-Beijing’s BBIBP-CorV could only be assessed after both doses and using 2 observational studies [104, 178] and a single clinical trial [89]. Efficacy and/or effectiveness was 86% for death, 88% for severe disease, 78% for symptomatic disease, and 74% for any infection. The only information on variant-specific protection came from the study from Hungary, which was conducted during a time when the Alpha variant dominated, and suggested that protection against any infection was similar to non-VOC strains. Bharat’s BBV152 was evaluated in only a single effectiveness study in healthcare workers [121] in addition to the initial Phase 3 trial [65]. Efficacy was 93% for severe disease, 78% for symptomatic disease, 69% for any infection, and 64% for asymptomatic infection. The RCT suggested that protection against symptomatic disease with the Delta variant was reduced by 10-15 percentage points, whereas the observational study suggested <50% effectiveness against Delta after 2 doses and none after only 1 dose.
Efficacy and/or Effectiveness of Other Vaccines
Although at the time of writing there were at least 10 other COVID-19 vaccines that had received emergency authorization for widespread use in at least 1 country, we did not identify effectiveness studies that met our inclusion criteria.
DISCUSSION
The development of COVID-19 vaccines has been an astounding feat of science. Within 1 year of detecting the first outbreak and isolating the SARS-CoV-2 virus, multiple vaccines were deployed around the world. In this review, we systematically collected efficacy and effectiveness values by vaccine platform, disease outcome, number of doses, and SARS-CoV-2 variant. These findings demonstrate robust evidence for the high efficacy of COVID-19 vaccines in clinical trials and high effectiveness in real-world settings. We found that across all vaccine platforms, protection against severe infection or death in the general population was at least 60% and most often close to 100%. Efficacy and/or effectiveness against symptomatic disease was heterogeneous between vaccine products and studies but was almost always greater than 50% and often greater than 90%. The vast majority of studies showed that vaccines provided protection against infection—not just disease—demonstrating the potential for indirect protective effects (“herd immunity”) via reduced transmission. The degree of protection offered by only a single dose of 2-dose vaccine courses varied by product. Most vaccines retained high levels of protection for most SARS-CoV-2 variants, especially against severe outcomes. Some studies provided evidence of slight reductions in efficacy and/or effectiveness for infection or mild disease with the Beta and Delta strains for some vaccines. Although few studies of the Omicron variant were available at publication time, the available evidence supports larger reductions in protection against both mild and severe infection.
There are several important components of COVID-19 vaccine efficacy and/or effectiveness not addressed here. Vaccine-induced protection can wane over months or years [59, 60, 78, 79, 181]. Although all estimates reviewed here were restricted to within 6 months of vaccination, waning is a critical issue to monitor for COVID-19 vaccines. A recent meta-analysis estimated efficacy and/or effectiveness drops ~10% for severe disease and ~20% for infection over 5 months [85]. These drops appear to be more extreme for the Omicron variant; nevertheless, evidence suggests booster doses—administered in many countries during Fall/Winter 2021—enhance immunogenicity and effectiveness [20, 26, 27, 29, 136, 182, 183]. However, the WHO stresses prioritization of primary doses globally [184].
Unvaccinated individuals previously infected with SARS-CoV-2 have some protection against reinfection, which is estimated in recent study to be ~90% [185, 186]. As a result, including previously infected persons in the unvaccinated group of studies could bias estimates of effectiveness downward. As an alternative, if prior immunity synergizes with vaccine-induced immunity to enhance protection, this bias could be in the opposite direction. Of 107 observational studies included in this review, 33 are known to have included persons with previous SARS-CoV-2 infection. For studies that stratified efficacy and/or effectiveness estimates by prior infection status, results were mixed, finding higher, lower, or unchanged values in previously infected individuals [107, 187].
For multidose vaccine regimens, the time interval between doses may affect protection. Efficacy of AZD1222 (AstraZeneca) increased from 55% with <6 weeks between doses to 81% with >12 weeks [3]. In real-world settings, dose interval sometimes varied due to policies of delaying second doses in favor of universal partial vaccination [188, 189], allowing for evaluation of its impact on neutralizing antibody levels and effectiveness [6, 19, 190].
Although most vaccine manufacturers and international advisory committees (including WHO [191]) initially recommended all doses be with the same product, many countries allowed for “mixing and matching” of doses [192–194]. Effectiveness studies have shown that boosting AZD1222 with either mRNA vaccine increases neutralizing antibody levels compared with 2 doses of AZD1222 alone [195–199], and this is also more effective against preventing infection [153, 171, 200]. Further evaluation is needed to understand which COVID-19 vaccine combinations are safe and effective.
For an individual, the goal of vaccination against COVID-19 is to prevent disease, but from a population perspective, there is an additional goal to reduce transmission, which can eliminate the need for complete vaccine coverage. Some studies have observed a reduction in transmission in vaccinated individuals infected with SARS-CoV-2 [201] by testing close contacts of cases [10, 123, 143, 149, 162, 202–204]. Others have reported reductions in viral load in the respiratory tract—expected to be a determinant of infectiousness—in vaccinated (versus unvaccinated) cases [19, 166, 176, 205].
At this time, approval of new COVID-19 vaccines typically requires Phase 3 trials directly measuring vaccine efficacy. However, as vaccine availability increases in the face of an ongoing epidemic, further placebo-controlled trials may be considered impractical or unethical. Instead, new vaccines or immunization schedules will likely be evaluated using “immunobridging”: indirectly inferring vaccine efficacy by measuring immunological biomarkers (like neutralizing antibody levels) identified as correlates of protection [53, 55, 206–208]. Such study designs—not reviewed here—have already been used for early assessment of waning rates [207, 209], identifying potential loses in protection against new variants [24, 25, 210], promoting the need for booster doses [24, 25, 211, 212], justifying heterologous vaccine courses [212, 213], expanding eligible age groups to children [214, 215], and to grant initial emergency approval to some vaccines (eg, Medigen’s MVC-COV1901 in Taiwan [216]). As preapproval efficacy trials become rarer, postauthorization vaccine effectiveness studies will become even more important.
Although our review focused on studies conducted in adults, some countries have now expanded vaccination campaigns to include children as young as 2 years old [217, 218]. Limited studies suggest that comparable safety, immunogenicity, efficacy, and effectiveness to adults can be achieved with adjusted dosing [219–222]. Although children are at lower risk for severe disease, they are susceptible to infection, contribute to transmission, and—because they are more likely to have mild symptoms—may be less likely to be tested or reported as cases, and to isolate during infection. Thus, the ability of COVID-19 vaccines to prevent asymptomatic infection and reduce transmission is especially relevant to the population-level impact of pediatric vaccination.
CONCLUSIONS
In conclusion, data from a wide variety of study types and settings demonstrate that COVID-19 vaccines provide high levels of protection against severe disease, and they additionally protect against infection and mild disease, even those caused by most SARS-CoV-2 variants of concern. Preliminary evidence supports the idea that the spread of the Omicron variant beginning late 2021 is at least partially driven by reductions in vaccine effectiveness.
Supplementary Material
Acknowledgments
We thank M. Kate Grabowski and the Johns Hopkins University Novel Coronavirus Research Compendium for bringing the study authors together to work on this paper.
Author contributions. A. L. H. proposed the Review with input from B. W., C. B. J., J. G. R., M. D. K, M. M. H., and S. A. T. A. A. N., A. L. H. , B. W., C. B. J., and J. G. R. and conducted the search for clinical trials of coronavirus disease 2019 (COVID-19) vaccines and extracted efficacy values. A. B., P. A. S.Z., K. K. W., and M. M. H. conducted the search for observational studies of COVID-19 vaccines and extracted effectiveness values. M. D. K., D. R. F., and M. K. P. supervised the collection of vaccine effectiveness studies including designing the search strategy, choosing the inclusion criteria, and evaluating studies against these criteria. A. L. H. and M. M. H. synthesized and interpreted the data on efficacy and effectiveness. B. W. assembled data on clinical trial timing, location, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant prevalence. A. L. H. , B. W., and M. M. H. created the tables and figures. A. L. H., C. B. J., B. W., J. G. R., and M. M. H. drafted the manuscript. All authors revised the manuscript and approved it for submission.
Disclaimer. The study sponsors had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication. The views represented in this article do not necessarily reflect the views of the World Health Organization or the National Institutes of Health (NIH).
Financial support. M. M. H., A. B., P. A. S.Z., K. K. W., and M. D. K. received funding to collect the data used in work through a contract from the Center for Epidemic Preparedness Innovations (CEPI) to the International Vaccine Access Center at Johns Hopkins University. C. B. J. and J. G. R. received funding from the Novel Coronavirus Research Compendium at Johns Hopkins to conduct reviews of COVID-19 vaccine papers for other purposes. A. L. H. and A. A. N. received support from the US National Institutes of Health (NIH DP5OD019851).
Potential conflicts of interests. M. M. H. and M. D. K. have previously received support from a grant from Pfizer Inc. to Johns Hopkins University for a non-COVID-19 vaccine. B. W. provided unpaid technical support to Bharat Biotech related to the clinical development of the BBV152 vaccine candidate. D. R. F. previously served on an independent data monitoring committee for GlaxoSmithKline for a non-COVID-19 vaccine candidate. S. A. T. served as an expert consultant for Milliman, Inc. on future COVID-19 trajectories. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Melissa M Higdon, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Brian Wahl, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Carli B Jones, Department of Pathology Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Joseph G Rosen, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Shaun A Truelove, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Anurima Baidya, International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Anjalika A Nande, Institute for Computational Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Parisa A ShamaeiZadeh, International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Karoline K Walter, International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Daniel R Feikin, Department of Immunization, Vaccines, and Biologicals, World Health Organization, Geneva, Switzerland.
Minal K Patel, Department of Immunization, Vaccines, and Biologicals, World Health Organization, Geneva, Switzerland.
Maria Deloria Knoll, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Alison L Hill, Institute for Computational Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
References
- 1. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav 2021; 5:947–53. [DOI] [PubMed] [Google Scholar]
- 2. Basta N, Moodie E; McGill University COVID19 Vaccine Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. COVID19 Vaccine Tracker. Available at: https://covid19.trackvaccines.org/. Accessed 26 August 2021.
- 3. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stowe J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. Available at: https://khub.net/documents/135939561/479607266/Effectiveness+of+COVID-19+vaccines+against+hospital+admission+with+the+Delta+%28B.1.617.2%29+variant.pdf/1c213463-3997-ed16-2a6f-14e5deb0b997?t=1623689315431. Accessed 22 June 2021.
- 5. Whitaker HJ, Tsang RS, Byford R, et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J Infect 2022; 0. doi: 10.1016/j.jinf.2021.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun 2021; 12:7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Public Health England. COVID-19 vaccine surveillance report - week 20. Public Health England, 2021. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990089/Vaccine_surveillance_report_-_week_20.pdf. Accessed 11 April 2022. [Google Scholar]
- 8. Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021; 397:1646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hitchings MDT, Ranzani OT, Dorion M, et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat Commun 2021; 12:6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Gier B, Andeweg S, Joosten R, et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill 2021; 26:2100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ismail SA, Vilaplana TG, Elgohari S, et al. Effectiveness of BNT162b2 mRNA and ChAdOx1 adenovirus vector COVID-19 vaccines on risk of hospitalisation among older adults in England: an observational study using surveillance data [preprint]. Public Health Engl 2021:18. [Google Scholar]
- 12. Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada [preprint]. medRxiv 2021. doi: 10.1101/2021.06.28.21259420. [DOI] [Google Scholar]
- 13. Sheikh1 A, McMenamin J, Taylor B, Robertson C.. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021; 397(10293):2461–2. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheikh2 A, Robertson C, Taylor B.. BNT162b2 and ChAdOx1 nCoV-19 vaccine effectiveness against death from the delta variant. N Engl J Med 2021; 385:2195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kissler SM, Fauver JR, Mack C, et al. Viral Dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N Engl J Med 2021; 385:2489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang M, Xin H, Yuan J, et al. Transmission dynamics and epidemiological characteristics of Delta variant infections in China [preprint ]. medRxiv 2021. doi: 10.1101/2021.08.12.21261991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li B, Deng A, Li K, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat Commun 2022; 13:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo CH, Morris CP, Sachithanandham J, et al. Infection with the SARS-CoV-2 delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin Infect Dis 2021:ciab986. doi: 10.1093/cid/ciab986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Weekly epidemiological update on COVID-19 - 8 February 2022. Geneva, Switzerland; World Health Organization; 2022. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---8-february-2022. [Google Scholar]
- 21. Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed 7 February 2022.
- 22. Garcia-Beltran WF, Lam EC, Denis KS, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021; 184:2372–83.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2021; 602:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edara V-V, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med 2022; 3(2):100529. doi: 10.1016/j.xcrm.2022.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobsen H, Strengert M, Maaß H, et al. Diminished neutralization responses towards SARS-CoV-2 Omicron VoC after mRNA or vector-based COVID-19 vaccinations [preprint ]. medRxiv 2021. doi: 10.1101/2021.12.21.21267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tartof2 SY, Slezak JM, Puzniak L, et al. BNT162b2 (Pfizer–Biontech) mRNA COVID-19 vaccine against omicron-related hospital and emergency department admission in a large US health system: a test-negative design [preprint ]. SSRN 2022. doi: 10.2139/ssrn.4011905. [DOI] [Google Scholar]
- 27. UK Health Security Agency. COVID-19 vaccine surveillance report - week 4. UK Health Secur Agency 2022; 59. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf. Accessed 11 April 2022. [Google Scholar]
- 28. Abu-Raddad1 LJ, Chemaitelly H, Butt AA.. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021; 385:187–9. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med 2022. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fathizadeh H, Afshar S, Masoudi MR, et al. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: a review. Int J Biol Macromol 2021; 188:740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ.. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021; 6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO.. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 2021; 6:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. García-Montero C, Fraile-Martínez O, Bravo C, et al. An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines 2021; 9:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng H, Peng Z, Luo W, et al. Efficacy and safety of COVID-19 vaccines in phase III trials: a meta-analysis. Vaccines 2021; 9:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF.. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021; 21:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadarangani M, Marchant A, Kollmann TR.. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol 2021; 21:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bok K, Sitar S, Graham BS, Mascola JR.. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity 2021; 54:1636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng C-J, Lu C-Y, Chang Y-H, et al. Effectiveness of the WHO-authorized COVID-19 vaccines: a rapid review of global reports till 30 June 2021. Vaccines 2021; 9:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meggiolaro A, Schepisi MS, Nikolaidis G, Mipatrini D, Siddu A, Rezza G.. Effectiveness of vaccination against symptomatic and asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis [preprint ]. medRxiv 2021. doi: 10.1101/2021.08.25.21262529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harder T, Koch J, Vygen-Bonnet S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill 2021; 26:2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shapiro J, Dean NE, Madewell ZJ, Yang Y, Halloran ME, Longini I.. Efficacy estimates for various COVID-19 vaccines: what we know from the literature and reports [preprint ]. medRxiv 2021. doi: 10.1101/2021.05.20.21257461. [DOI] [Google Scholar]
- 42. Kow CS, Hasan SS.. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology 2021:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Azevedo TCP, de Freitas PV, da Cunha PHP, et al. Efficacy and landscape of Covid-19 vaccines: a review article. Rev Assoc Médica Bras 2021; 67:474–8. [DOI] [PubMed] [Google Scholar]
- 44. Kow CS, Ramachandram DS, Hasan SS.. The effectiveness of mRNA-1273 vaccine against COVID-19 caused by Delta variant: a systematic review and meta-analysis. J Med Virol 2022; 94(5): 2269–74. doi: 10.1002/jmv.27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N.. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022; 28:202–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Fusco M, Lin J, Vaghela S, et al. COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies. Expert Rev Vaccines 2022; 21(4):435–51. doi: 10.1080/14760584.2022.2035222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marra AR, Kobayashi T, Suzuki H, et al. Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis. J Infect 2021; 84(3):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weinberg GA, Szilagyi PG.. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis 2010; 201:1607–10. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization. Vaccine efficacy, effectiveness and protection. Available at: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection. Accessed 27 August 2021.
- 50. World Health Organization. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. Available at: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_23Dec2021.pdf. Accessed 15 February 2022.
- 51. Halloran ME, Longini IM Jr, Struchiner CJ.. Design and interpretation of vaccine field studies. Epidemiol Rev 1999; 21:73–88. [DOI] [PubMed] [Google Scholar]
- 52. Crowcroft NS, Klein NP.. A framework for research on vaccine effectiveness. Vaccine 2018; 36:7286–93. [DOI] [PubMed] [Google Scholar]
- 53. Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ.. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021; 21:e26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith PG, Rodrigues LC, Fine PEM.. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int J Epidemiol 1984; 13:87–93. [DOI] [PubMed] [Google Scholar]
- 55. World Health Organization. Correlates of vaccine-induced protection: methods and implications. Geneva, Switzerland; World Health Organization; 2013. Available at: https://apps.who.int/iris/handle/10665/84288. [Google Scholar]
- 56. Patel MK, Bergeri I, Bresee JS, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine 2021; 39:4013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. World Health Organization. Evaluation of COVID-19 vaccine effectiveness: Interm Guidance. Geneva, Switzerland; World Health Organization; 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1. [Google Scholar]
- 58. Leshem E, Lopman BA.. Population immunity and vaccine protection against infection. Lancet 2021; 397:1685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim DS, Rowland-Jones S, Gea-Mallorquí E.. Will SARS-CoV-2 infection elicit long-lasting protective or sterilising immunity? Implications for vaccine strategies (2020). Front Immunol 2020; 11:3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pollard AJ, Bijker EM.. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021; 21:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G.. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol 2020; 35:1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palmer S, Cunniffe N, Donnelly R.. COVID-19 hospitalization rates rise exponentially with age, inversely proportional to thymic T-cell production. J R Soc Interface 2021; 18(176). doi: 10.1098/rsif.2020.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O’Driscoll M, Ribeiro D, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021; 590:140–5 [DOI] [PubMed] [Google Scholar]
- 64. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021; 398:2173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med 2021; 385:2348–60. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med 2021; 0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021; 385:1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thomas SJ, Edson D, Moreira J, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021; 385:1761–73. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021; 398:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. Rochester, NY: [preprint]. SSRN. 2021. doi: 10.2139/ssrn.3822780. [DOI] [Google Scholar]
- 75. Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022; 399:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bravo L, Smolenov I, Han HH, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2022; 399(10323):461–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rapaka RR, Hammershaimb EA, Neuzil KM.. Are some COVID vaccines better than others? Interpreting and comparing estimates of efficacy in trials of COVID-19 vaccines. Clin Infect Dis 2021; 74(2):352–8. doi: 10.1093/cid/ciab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Murphy KM, Weaver C.. Janeway’s Immunobiology, 9th ed. New York, NY; W. W. Norton & Company; 2016. [Google Scholar]
- 79. Poland GA, Ovsyannikova IG, Kennedy RB.. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396:1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wei J, Stoesser N, Matthews PC, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol 2021; 6:1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. World Health Organization. Weekly epidemiological update on COVID-19 - 24 August 2021. Geneva, Switzerland; World Health Organization; 2021. [Google Scholar]
- 82. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 2021; 384:1885–98. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lefèvre B, Tondeur L, Madec Y, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Healthy Longev 2021; 2:e685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feikin D, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022; 399(10328):924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021; 397:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thomas SJ, Moreira ED, Kitchin N, et al. Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. bioRxiv 2021. doi: 10.1101/2021.07.28.21261159. [DOI] [Google Scholar]
- 88. Skowronski DM, De Serres G.. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2021; 384:1576–78. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 89. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. World Health Organization. Evidence Assessment: Sinovac/CoronaVac COVID-19 vaccine. Geneva, Switzerland; World Health Organization; 2021. [Google Scholar]
- 91. Caraballosa E. How was the efficacy of the Cuban COVID-19 vaccine candidates calculated? OnCuba News Engl. Available at: https://oncubanews.com/en/cuba/how-was-the-efficacy-of-the-cuban-covid-19-vaccine-candidates-calculated/. Accessed. 2 August 2021.
- 92. Toledo-Romani ME, Garcia-Carmenate M, Silva CV, et al. Efficacy and safety of SOBERANA 02, a COVID-19 conjugate vaccine in heterologous three-dose combination [preprint ]. medRxiv 2021. doi: 10.1101/2021.10.31.21265703. [DOI] [Google Scholar]
- 93. Abu-Raddad2 LJ, Chemaitelly H, Bertollini R.. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med 2022; 386:1091–93. doi: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alali WQ, Ali LA, AlSeaidan M, Al-Rashidi M.. Effectiveness of BNT162b2 and ChAdOx1 vaccines against symptomatic COVID-19 among healthcare workers in Kuwait: a retrospective cohort study. Healthcare 2021; 9:1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Andrejko KL, Pry J, Myers JF, et al. Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis 2021. doi: 10.1093/cid/ciab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 2022; 386:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 2021; 325:2457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Azamgarhi T, Hodgkinson M, Shah A, et al. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers – a single centre cohort study. Nat Commun 2021; 12:3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bajema KL. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five veterans affairs medical centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1700–05. doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Barlow RS, Jian K, Larson L.. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection during a delta variant epidemic surge in Multnomah County, Oregon, July 2021 [preprint ]. medRxiv 2021. doi: 10.1101/2021.08.30.21262446. [DOI] [Google Scholar]
- 101. Baum U, Poukka E, Palmu AA, Salo H, Lehtonen TO, Leino T.. Effectiveness of vaccination against SARS-CoV-2 infection and Covid-19 hospitalisation among Finnish elderly and chronically ill—An interim analysis of a nationwide cohort study. PLoS One 2021; 16:e0258704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bedston S, Akbari A, Jarvis CI, et al. COVID-19 vaccine uptake, effectiveness, and waning in 82,959 health care workers: a national prospective cohort study in Wales. Vaccine 2022; 40:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bekker L-G, Garrett N, Goga A, et al. Effectiveness of the Ad26.Cov2.S vaccine in health care workers in South Africa [preprint]. SSRN 2021. doi: 10.2139/ssrn.3979291. [DOI] [Google Scholar]
- 104. Belayachi J, Obtel M, Razine R, Abouqal R.. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco [preprint ]. medRxiv 2022. doi: 10.1101/2022.01.25.22269822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Berec L, Šmíd M, Přibylová L, et al. Real-life protection provided by vaccination, booster doses and previous infection against covid-19 infection, hospitalisation or death over time in the Czech Republic: a whole country retrospective view [preprint]. medRxiv 2021:2021.12.10.21267590. [Google Scholar]
- 106. Björk J, Inghammar M, Moghaddassi M, Rasmussen M, Malmqvist U, Kahn F.. High level of protection against COVID-19 after two doses of BNT162b2 vaccine in the working age population – first results from a cohort study in Southern Sweden. Infect Dis 2022; 54:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bruxvoort1 KJ, Sy LS, Qian L, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study. Lancet Reg Health - Am 2022; 6:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bruxvoort2 KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ 2021; 375:e068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Butt1 AA, Chemaitelly H, Khal AA, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest 2021; 131(23):e153662. doi: 10.1172/JCI153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Butt2 AA, Omer SB, Yan P, Shaikh OS, Mayr FB.. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann Intern Med 2021. doi: 10.7326/M21-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Carazo S, Talbot D, Boulianne N, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2 in healthcare workers extending 16 weeks post-vaccination: a test-negative design from Quebec, Canada. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Castillo LA, Fernández-Niño JA, Rojas-Botero ML, et al. Effectiveness of COVID-19 vaccines in preventing hospitalizations and deaths in Colombia: a pair-matched, national-wide cohort study in older adults [preprint]. SSRN 2021. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3944059. [Google Scholar]
- 113. Cerqueira-Silva T, Andrews JR, Boaventura VS, et al. Effectiveness of CoronaVac, ChAdOx1, BNT162b2 and Ad26.COV2.S among individuals with prior SARS-CoV-2 infection in Brazil [preprint ]. medRxiv 2021. doi: 10.1101/2021.12.21.21268058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021; 27:1614–21. [DOI] [PubMed] [Google Scholar]
- 115. Chemaitelly2 H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chin ET, Leidner D, Zhang Y, et al. Effectiveness of coronavirus disease 2019 (COVID-19) vaccines among incarcerated people in California state prisons: retrospective cohort study. Clin Infect Dis 2022. doi: 10.1093/cid/ciab1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Collie S, Champion J, Moultrie H, Bekker L-G, Gray G.. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med 2022; 386:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Corchado-Garcia J, Zemmour D, Hughes T, et al. Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for Preventing COVID-19. JAMA Netw Open 2021; 4:e2132540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27:1693–95. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 121. Desai D, Khan AR, Soneja M, et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study. Lancet Infect Dis 2021; 22(3):349–56. doi: 10.1016/S1473-3099(21)00674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Emborg H-D, Valentiner-Branth P, Schelde AB, et al. Vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine against RT-PCR confirmed SARS-CoV-2 infections, hospitalisations and mortality in prioritised risk groups [preprint ]. medRxiv 2021. doi: 10.1101/2021.05.27.21257583. [DOI] [Google Scholar]
- 123. Eyre DW, Taylor D, Purver M, et al. Effect of covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med 2022; 386:744–56. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Flacco ME, Soldato G, Acuti Martellucci C, et al. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. Vaccines 2021; 9:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Florea A, Sy LS, Luo Y, et al. Durability of mRNA-1273 against COVID-19 in the time of Delta: Interim results from an observational cohort study [preprint ]. medRxiv 2021. doi: 10.1101/2021.12.13.21267620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gazit S, Mizrahi B, Kalkstein N, et al. BNT162b2 mRNA vaccine effectiveness given confirmed exposure: analysis of household members of coronavirus disease 2019 patients. Clin Infect Dis 2021.. doi: 10.1093/cid/ciab973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Glampson B, Brittain J, Kaura A, et al. Assessing COVID-19 vaccine uptake and effectiveness through the North West London vaccination program: retrospective cohort study. JMIR Public Health Surveill 2021; 7(9):e30010. doi: 10.2196/30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Glatman-Freedman A, Bromberg M, Dichtiar R, Hershkovitz Y, Keinan-Boker L.. The BNT162b2 vaccine effectiveness against new COVID-19 cases and complications of breakthrough cases: a nation-wide retrospective longitudinal multiple cohort analysis using individualised data. eBioMedicine 2021; 72:103574. doi: 10.1016/j.ebiom.2021.103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Goldberg1 Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Goldberg2 Y, Mandel M, Woodbridge Y, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: a three-month nationwide experience from Israel [preprint ]. medRxiv 2021. doi: 10.1101/2021.04.20.21255670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grannis SJ. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance — nine states, June–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70(37):1291–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gras-Valentí P, Chico-Sánchez P, Algado-Sellés N, et al. Effectiveness of the first dose of BNT162b2 vaccine to preventing covid-19 in healthcare personnel. Rev Esp Salud Publica 2021; 95:e202104070. [PubMed] [Google Scholar]
- 133. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hall H, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–44. [DOI] [PubMed] [Google Scholar]
- 135. Hall2 V, Foulkes S, Insalata F, et al. Effectiveness and durability of protection against future SARS-CoV-2 infection conferred by COVID-19 vaccination and previous infection; findings from the UK SIREN prospective cohort study of healthcare workers March 2020 to September 2021 [preprint ]. medRxiv 2021. doi: 10.1101/2021.11.29.21267006. [DOI] [Google Scholar]
- 136. Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study [preprint]. medRxiv 2021. doi: 10.1101/2021.12.20.21267966. [DOI] [Google Scholar]
- 137. Hyams C, Marlow R, Maseko Z, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis 2021; 21(11):1539–48. doi: 10.1016/S1473-3099(21)00330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Irizarry RA, Robles-Fontán MM, Nieves EG, Cardona-Gerena I.. Time-varying effectiveness of three COVID-19 vaccines in Puerto Rico [preprint]. SSRN 2021. doi: 10.1101/2021.10.17.21265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021; 385:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Katz MA, Harlev EB, Chazan B, et al. Early effectiveness of BNT162b2 Covid-19 vaccine in preventing SARS-CoV-2 infection in healthcare personnel in six Israeli hospitals (CoVEHPI). Vaccine 2022; 40:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kissling1 E, Hooiveld M, Martín VS, et al. Vaccine effectiveness against symptomatic SARS-CoV-2 infection in adults aged 65 years and older in primary care: I-MOVE-COVID-19 project, Europe, December 2020 to May 2021. Euro Surveill 2021; 26:2100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kissling2 E, Hooiveld M, Martínez-Baz I, et al. Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant Delta circulation in Europe: multicentre study analysis by age-group, vaccine brand and time since vaccination, I-MOVE-COVID-19 and ECDC networks, July–August 2021 [pre-print]. Open Sci Found 2021. doi: 10.31219/osf.io/3nhps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Layan M, Gilboa M, Gonen T, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study [preprint ]. medRxiv 2021. doi: 10.1101/2021.07.12.21260377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lin D-Y, Gu Y, Wheeler B, et al. Effectiveness of covid-19 vaccines in the United States over 9 months: surveillance data from the state of North Carolina [preprint]. medRxiv 2021. doi: 10.1101/2021.12.20.21267966. [DOI] [Google Scholar]
- 145. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Martínez-Baz I, Miqueleiz A, Casado I, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill 2021; 26:2100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mason TFD, Whitston M, Hodgson J, et al. Effects of BNT162b2 mRNA vaccine on COVID-19 infection and hospitalisation amongst older people: matched case control study for England. BMC Med 2021; 19:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mendola M, Tonelli F, Garletti FS, et al. COVID-19 impact and vaccine effectiveness among healthcare workers of a large University Hospital in Lombardy, Italy. Med Lav Work Environ Health 2021; 112:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Meyer ED, Sandfort M, Bender J, et al. Two doses of the mRNA BNT162b2 vaccine reduce severe outcomes, viral load and secondary attack rate: evidence from a SARS-CoV-2 Alpha outbreak in a nursing home in Germany, January-March 2021 [preprint ]. medRxiv 2021. doi: 10.1101/2021.09.13.21262519. [DOI] [Google Scholar]
- 150. Moustsen-Helms IR, Emborg H-D, Nielsen J, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 vaccine in long-term care facility residents and healthcare workers – a Danish cohort study [preprint]. medRxiv 2021. doi: 10.1101/2021.03.08.21252200. [DOI] [Google Scholar]
- 151. Muhsen K, Maimon N, Mizrahi A, et al. Effectiveness of BNT162b2 mRNA coronavirus disease 2019 (covid-19) vaccine against acquisition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among healthcare workers in long-term care facilities: a prospective cohort study. Clin Infect Dis 2021. doi: 10.1093/cid/ciab918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nanduri S. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1163–66. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nordström P, Ballin M, Nordströöm A.. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur 2021; 11:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Paixão ES, Wong KL, Alves FJO, et al. Effectiveness of the CoronaVac vaccine in prevention of symptomatic and progression to severe COVID-19 in pregnant women in Brazil [preprint ]. SSRN 2021. doi: 10.2139/ssrn.3962119. [DOI] [Google Scholar]
- 155. Pawlowski C, Lenehan P, Puranik A, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med 2021; 2:979–92.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Petráš M, Lesná IK, Večeřová L, et al. The effectiveness of post-vaccination and post-infection protection in the hospital staff of three Prague hospitals: a cohort study of 8-month follow-up from the start of the COVID-19 Vaccination Campaign (COVANESS). Vaccines 2022; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pilishvili T, Gierke R, Fleming-Dutra KE, et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. N Engl J Med 2021; 385:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Polinski JM, Weckstein AR, Batech M, et al. Effectiveness of the single-dose Ad26.COV2.S COVID vaccine [preprint ]. medRxiv 2021. doi: 10.1101/2021.09.10.21263385. [DOI] [Google Scholar]
- 159. Poukka E, Baum U, Palmu AA, et al. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020 - October 2021. Vaccine 2022; 40:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Powell AA, Kirsebom F, Stowe J, et al. Adolescent vaccination with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine and effectiveness of the first dose against COVID-19: national test-negative case-control study, England [preprint ]. medRxiv 2021. doi: 10.1101/2021.12.10.21267408. [DOI] [Google Scholar]
- 161. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science; 375(6585):1151–54. doi: 10.1126/science.abl4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ 2021; 374:n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ranzani2 OT, Leite R dos S, Castilho LD, et al. Vaccine effectiveness of Ad26.COV2.S against symptomatic COVID-19 and clinical outcomes in Brazil: a test-negative study design [preprint ]. medRxiv 2021. doi: 10.1101/2021.10.15.21265006. [DOI] [Google Scholar]
- 165. Ranzani3 OT, Silva AAB, Peres IT, et al. One-dose ChAdOx1 nCoV-19 vaccine effectiveness against symptomatic COVID-19 in a vulnerable community in Rio de Janeiro, Brazil: test-negative design study [preprint]. medRxiv 2021. doi: 10.1101/2021.10.16.21265095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Regev-Yochay G, Amit S, Bergwerk M, et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg Health Eur 2021; 7:100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Roberts EK, Gu T, Mukherjee B, Fritsche LG.. Estimating COVID-19 vaccination effectiveness using electronic health records of an academic medical center in Michigan [preprint ]. medRxiv 2022. doi: 10.1101/2022.01.29.22269971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Saciuk Y, Kertes J, Mandel M, Hemo B, Shamir Stein N, Ekka Zohar A.. Pfizer-BioNTech vaccine effectiveness against Sars-Cov-2 infection: findings from a large observational study in Israel. Prev Med 2022; 155:106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Self WH. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis 2021; 21(11):1529–38. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Skowronski3 DM, Setayeshgar S, Febriani Y, et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada [preprint ]. medRxiv 2021. doi: 10.1101/2021.10.26.21265397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Skowronski2 DM, Setayeshgar S, Zou M, et al. Comparative single-dose mRNA and ChAdOx1 vaccine effectiveness against SARS-CoV-2, including variants of concern: test-negative design, British Columbia, Canada. J Infect Dis 2022. doi: 10.1093/infdis/jiac023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tande AJ, Pollock BD, Shah ND, et al. Impact of the coronavirus disease 2019 (COVID-19) vaccine on asymptomatic infection among patients undergoing preprocedural COVID-19 molecular screening. Clin Infect Dis 2021; 74(1):59–65. doi: 10.1093/cid/ciab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tartof1 SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health 2022. doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tenforde MW, Billig R, Lindsell CJ, et al. Characteristics of adult outpatients and inpatients with COVID-19 — 11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep 2020; 69:841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. M, G, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med 2021; 385:320–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Thompson2 MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med 2021; 385:1355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vokó Z, Kiss Z, Surján G, et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary—the HUN-VE study. Clin Microbiol Infect 2021; 28(3):398–404. doi: 10.1016/j.cmi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Willett BJ, Grove J, MacLean OA, et al. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism [preprint ]. medRxiv 2022. doi: 10.1101/2022.01.03.21268111. [DOI] [Google Scholar]
- 180. Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study [preprint]. medRxiv 2022. doi: 10.1101/2022.01.15.22269326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Andeweg SP, de Gier B, Eggink D, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1 and Delta SARS-CoV-2 infections, the Netherlands, 22 November 2021- 19 January 2022 [preprint ]. medRxiv 2022. doi: 10.1101/2022.02.06.22270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of protection of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 Omicron infection in Qatar [preprint]. medRxiv 2022. doi: 10.1101/2022.02.07.22270568. [DOI] [Google Scholar]
- 184. World Health Organization. Interim statement on booster doses for COVID-19 vaccination. Available at: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination---update-22-december-2021. Accessed 14 February 2022.
- 185. Kojima N, Shrestha NK, Klausner JD.. A Systematic review of the protective effect of Prior SARS-CoV-2 infection on repeat infection. Eval Health Prof 2021; 44:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Leidi A, Koegler F, Dumont R, et al. Risk of reinfection after seroconversion to SARS-CoV-2: a population-based propensity-score matched cohort study. Clin Infect Dis 2021;74(4):622–9. doi: 10.1093/cid/ciab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA 2021; 326(19):1930–39. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Fay Cortez M. Delayed Second Dose Turns Into a Win for Vaccine-Starved Places. Available at: https://www.bloomberg.com/news/articles/2021-05-20/delayed-second-dose-turns-into-a-win-for-vaccine-starved-places. Accessed 26 August 2021.
- 189. Wu KJ, Robbins R.. In Europe, more countries delay second vaccine doses or mull plans to do so. New York Times. Available at: https://www.nytimes.com/2021/01/04/world/second-covid-vaccine-delay.html. Accessed 26 August 2021.
- 190. Payne RP, Longet S, Austin JA, et al. Sustained T Cell Immunity, Protection and Boosting Using Extended Dosing Intervals of BNT162b2 mRNA Vaccine [preprint]. SSRN 2021. doi: 10.2139/ssrn.3891065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. World Health Organization. Interim statement on heterologous priming for COVID-19 vaccines. Available at: https://www.who.int/news/item/10-08-2021-interim-statement-on-heterologous-priming-for-covid-19-vaccines. Accessed 26 August 2021.
- 192. Lewis D. Mix-and-match COVID vaccines: the case is growing, but questions remain. Nature 2021; 595:344–5. [DOI] [PubMed] [Google Scholar]
- 193. Cunningham E. Covid-19 global updates: Some countries defend mixing vaccines after WHO suggests booster strategy is ‘chaotic’. Washington Post. Available at: https://www.washingtonpost.com/world/2021/07/14/coronavirus-latest-updates/. Accessed 26 August 2021.
- 194. Anthes E. Why More People Are Getting Two Different Coronavirus Vaccines. New York Times. Available at: https://www.nytimes.com/2021/06/24/world/europe/covid-vaccine-mix-and-match-pfizer-moderna.html. Accessed 26 August 2021.
- 195. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med 2021;27:1525–29. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med 2021; 9(11):1255–65. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021; 398(10303):856–69. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 2021; 27:1530–35. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med 2021; 385:1049–51. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: a nationwide population-based cohort study. PLoS Med 2021; 18:e1003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lipsitch M, Kahn R.. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine 2021; 39:4082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G.. Effect of vaccination on household transmission of SARS-CoV-2 in England0 qqqqN Engl J Med 2021; 385:759–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med 2021; 385:1718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE.. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis [preprint ]. medRxiv 2022. doi: 10.1101/2022.01.09.22268984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021; 27:790–2. [DOI] [PubMed] [Google Scholar]
- 206. Access Consortium. Access Consortium: Alignment with ICMRA consensus on immunobridging for authorising new COVID-19 vaccines. Available at: https://www.gov.uk/government/publications/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines. Accessed 16 February 2022.
- 207. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 208. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med 2020; 383:1085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Gardner BJ, Kilpatrick AM.. Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS-CoV-2 variant, Omicron (B.1.1.529), using neutralizing antibody titers [preprint]. medRxiv 2021. doi: 10.1101/2021.12.10.21267594. [DOI] [Google Scholar]
- 211. Gardner BJ, Kilpatrick AM.. Third doses of COVID-19 vaccines reduce infection and transmission of SARS-CoV-2 and could prevent future surges in some populations: a modeling study [preprint ]. medRxiv 2021.. doi: 10.1101/2021.10.25.21265500. [DOI] [Google Scholar]
- 212. Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021; 398(10318):2258–76. doi: 10.1016/S0140-6736(21)02785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Chiu N-C, Chi H, Tu Y-K, et al. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev Vaccines 2021; 20:1211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med 2022; 386:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. US Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Available at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age. Accessed 16 February 2022.
- 216. Silver A. Why Taiwan approved its own vaccine before phase III trials. BMJ 2021; 374:n2104. doi: 10.1136/bmj.n2104. [DOI] [PubMed] [Google Scholar]
- 217. Reuters. Factbox: Countries vaccinating children against COVID-19. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/countries-vaccinating-children-against-covid-19-2021-06-29/. Accessed 14 February 2022.
- 218. World Health Organization. Interim statement on COVID-19 vaccination for children and adolescents. Available at: https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-vaccination-for-children-and-adolescents. Accessed 14 February 2022.
- 219. Vadrevu KM, Reddy S, Jogdand H, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (BBV152) in children from 2 to 18 years of age: an open-label, age-de-escalation phase 2/3 study [preprint ]. medRxiv 2021. doi: 10.1101/2021.12.28.21268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis 2022; 22:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Moderna. Moderna Announces Positive Top Line Data from Phase 2/3 Study of COVID-19 Vaccine in Children 6 to 11 Years of Age. Available at: https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Positive-Top-Line-Data-from-Phase-23-Study-of-COVID-19-Vaccine-in-Children-6-to-11-Years-of-Age-10-25-2021/default.aspx. Accessed 8 February 2022.
- 222. Robert W, Frenck J, Klein NP, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239–50. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Novavax. Novavax COVID-19 Vaccine Demonstrates 90% Overall Efficacy and 100% Protection Against Moderate and Severe Disease in PREVENT-19 Phase 3 Trial - Jun 14, 2021. Available at: https://ir.novavax.com/2021-06-14-Novavax-COVID-19-Vaccine-Demonstrates-90-Overall-Efficacy-and-100-Protection-Against-Moderate-and-Severe-Disease-in-PREVENT-19-Phase-3-Trial. Accessed 20 July 2021.
- 224. Kremsner PG, Guerrero RAA, Arana-Arri E, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2021; 22(3):329–40. doi: 10.1016/S1473-3099(21)00677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20(5):533–4. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shu Y, McCauley J.. GISAID: Global initiative on sharing all influenza data – from vision to reality. Euro Surveill 2017; 22:30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Khairullin B, Zakarya K, Orynbayev M, et al. Efficacy and safety of an inactivated whole-virion vaccine against COVID-19, QazCovid-in®, in healthy adults: a multicentre, randomised, single-blind, placebo-controlled phase 3 clinical trial with a 6-month follow-up [preprint]. SSRN 2022. doi: 10.2139/ssrn.4016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.