Introduction
Vaccination with messenger RNA (mRNA) or vector vaccines is immunogenic for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and protective for the occurrence and severity of coronavirus disease 2019 (COVID-19). Anti-SARS-CoV-2 antibodies dominate protection against initial infection but are diminished in inflammatory bowel disease (IBD) patients receiving anti–tumor necrosis factor (anti-TNF) therapy.1–3 T-cells play a large role in preventing disease progression,4 but the T-cell response to vaccines in IBD patients is poorly understood, as are effects of risk factors on this aspect of the vaccine response. Here, we assess IBD patients for their clonal T-cell vaccine response and their alterations by immunotherapy.
Methods
We studied 303 IBD patients (Table 1) enrolled in an Institutional Review Board–approved prospective registry at Cedars-Sinai between January and June 2021.5 Samples were collected longitudinally at the time of SARS-CoV-2 vaccine dose 1 and dose 2, and at 2 and 8 weeks after dose 2 (after dose 1 for vector vaccine participants). We quantified spike-specific and nucleocapsid SARS-CoV-2 antibody levels using the SARS-CoV-2 Ig (immunoglobin) G-II assay (Abbott Labs). We excluded individuals who had experienced COVID-19 (positive IgG[N] at any time point, or those with a prior clinical COVID-19 diagnosis.
Table 1.
Study cohort.
| Total subjects | n = 303 |
|---|---|
| Race, n (%) | |
| Asian | 7 (2.36) |
| Black or African American | 5 (1.68) |
| Multiple | 4 (1.35) |
| Other | 10 (3.37) |
| Prefer not to answer | 3 (1.01) |
| White | 268 (90.24) |
| Hispanic, n (%) | 15 (5.05) |
| Gender, female, n (%) | 166 (55.89) |
| Vaccine type, n (%) | |
| BNT162 (Pfizer/BioNtech) | 160 (52.81) |
| JNJ-78436725 (Johnson & Johnson) | 15 (4.95) |
| mRNA-1273 (Moderna/NIH) | 128 (42.24) |
| Prior COVID-19 history, n (%) | 15 (5.08) |
| Treatments, n (%) | |
| No immune suppression | 48 (16.22) |
| Anti-TNF | 104 (35.14) |
| Other biologics (anit-IL23, anti-integrin) | 126 (42.57) |
| Immunomodulators | 18 (6.08) |
| Age group, n (%) | |
| ≤30 | 44(14.52) |
| 30–40 | 83(27.39) |
| 40–50 | 71(23.43) |
| 50–60 | 45(14.85) |
| >60 | 60(19.8) |
Abbreviations: COVID-19, coronavirus disease 2019; IL, interleukin; JNJ, Johnson & Johnson; mRNA, messenger RNA; TNF, tumor necrosis factor.
The T-cell clonal response was quantified by T-cell receptor (TCR) β sequencing of blood genomic DNA (ImmunoSEQ, Adaptive Biotechnologies), library-based attribution of TCR sequences to SARS-CoV-2 spike or other nonspike SARS-CoV-2 protein specificities, and the calculation of clonal expansion using a depth metric model.6 SARS-CoV-2-associated TCRβ sequences were identified using a 1-tailed Fisher’s exact test comparing the TCRβ presence in SARS-CoV-2 polymerase chain reaction–positive samples (n = 1954) with those of negative controls (n = 3903). Subsets of these SARS-CoV-2-associated sequences were assigned to spike and nonspike antigens based on data from multiplexed antigen stimulation assays.7,8 A total of 917 TCRs were assigned to the SARS-CoV-2 spike protein and 1564 to nonspike viral proteins. Where studied, cytokine and clonal measures of the T-cell vaccine response are concordant,7–9 but standardized clonal metrics of the T-cell response, as used here, are just emerging and await detailed analytic comparisons to functional T-cell response metrics.
The comparisons of TCR depths to clinical metadata used a Mixed Linear Model across time points and a Generalized Linear Model within time points. Inverse normal transformation on the computed depth metric was performed with age and sex included as covariates. Confidence intervals for binomial probabilities were computed using exact methods.
Results
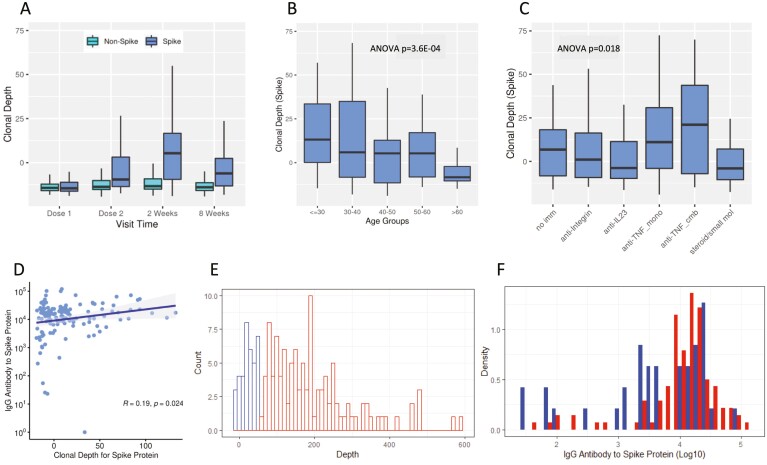
The T-cell response to vaccination across different time points is shown in Figure 1A. At dose 1, spike-specific depths of SARS-CoV-2 clones were low (reflecting their basal level in an individual’s repertoire). Levels peaked 2 weeks after the second vaccination (5-fold and with a P value = 2.42E-25 relative to dose 1). From this peak, levels declined at 8 weeks after the second vaccination but were still significantly elevated (5.30E-14 relative to dose 1). In contrast, no changes were observed in T-cell responses for nonspike clones, demonstrating the specificity of the vaccine responses. The timing of the peak spike-specific T-cell responses corresponded to the peak functional T-cell vaccine response previously reported,9,10 and may relate to postactivation population contraction of effector T-cells and recompartmentalization from blood to lymph nodes.11 As a positive control, spike T-cell response metrics were elevated in COVID-19-experienced versus -naive subjects at basal (dose 1) but not at subsequent postvaccine samplings. Similarly, nonspike T-cell responses were elevated in COVID-19-experienced versus -naive subjects at all time points (Supplemental Figure 1).
Figure 1.
T-cell clonal response to SARS-CoV-2 immunization. (A) Box plots show means, quartiles, and data ranges. Relative to dose 1, P values (mixed-effect model analysis with adjustment for age and sex) for dose 2, at 2 weeks after the second vaccination, and at 8 weeks after the second vaccination were 9.87E-11, 2.42E-25, and 5.30E-14, respectively (n = 88 at dose 1, 148 at dose 2, 136 at 2 weeks, and 163 at 8 weeks). (B) Age. Numbers of subjects by age group are tabulated in Table 1. (C) Immunologic treatment and response at week 2. No Imm (n = 19), anti-integrin (n = 14), anti-IL23 (n = 36), anti-TNF mono (n = 36), anti-TNF cmb (n = 11), steroids/small mol (n = 16). Boxes are mean values, bars are data ranges, and P values were calculated by ANOVA after adjustment for age, sex, vaccine type, and COVID-19 history. (D) T-cell response and anti-spike IgG levels at week 2 (Spearman’s correlation; N = 148). (E) T-cell response at week 2 (blue, lowest quartile; red, remaining quartiles; N = 148). (F) Anti-spike IgG levels at week 2 by subject distribution (blue, lowest T-cell response quartile; red, remaining T-cell response quartiles; N = 148). Abbreviations: anti-TNF cmb, combined therapy with anti-tumor necrosis factor and a thiopurine or methotrexate; ANOVA, analysis of variance; anti-TNF mono, monotherapy with anti-tumor necrosis factor; Ig, immunoglobin; IL, interleukin; JAK, Janus kinase; no Imm, no treatment, 5-aminosalicylates, or rectal steroids; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; steroids/small mol, treatment with systemic corticosteroids or monotherapy with thiopurines, methotrexate, or Janus kinase inhibitors; TNF, tumor necrosis factor.
The spike-specific T-cell response was reduced substantially with age (P = 3.62E-4 for trend test; Figure 1B). There was no statistically significant association between sex and spike T-cell responses at 2 weeks after dose 2, but was a moderate reduction in males versus females at 8 weeks (P = .0077). The IBD disease type (Crohn’s disease vs. ulcerative colitis) did not significantly impact the temporal kinetics or levels of spike T-cell clonal responses to vaccines (data not shown). There was no significant difference in any clonal metrics of the T-cell anti-spike response at either 2 or 8 weeks with respect to the mRNA vaccine type.
The T-cell response was significantly and selectively associated with modes of suppressive immunotherapy (Figure 1C; analysis of variance P = .018). Compared to patients with no treatment, there were no significant effects of anti-IL12/23, anti-integrin, or steroids/small molecular treatments. However, we observed augmentation with anti-TNF (P = .0174) after adjustments for age and sex, with consistent trends in subgroups of patients receiving anti-TNF as monotherapy or in combination with immunomodulators.
Spike-specific T-cell and antibody responses were compared at week 2 after dose 2, which corresponds to the peak of both antibody and T-cell vaccine responses (Figure 1D).9,10 They were significantly but only modestly correlated (R = 0.19). Moreover, when we compared the lowest-quartile T-cell responders to the remaining T-cell responders, there was no difference in their distribution of antibody responses (Figures 1E and F).
Discussion
Few studies have assessed the T-cell response to SARS-CoV-2 vaccines; with a few exceptions,9 they have used methods that enumerate SARS-CoV-2-specific T-cells based on peptide-stimulated cytokine production.10,12,13 Such studies don’t permit assessment of repertoire diversity and clonal size, important factors in protective T-cell immunity.4
Antibody levels were poorly correlated with the T-cell clonal response and did not distinguish individuals even with the lowest T-cell response. This is consistent with findings reported from polyfunctional T-cell assessments.10,12,13 In the context of booster strategies, T-cell assessments may be important to evaluate both the initial vaccine response and the persistence of immunity after vaccination. Limitations of this study include a cohort of only individuals with IBD, a lack of racial diversity, use of a tertiary-center population, and use of a single type of T-cell response assay, which reduce generalizability.
Immune-modifying therapies selectively influenced the T-cell responses. Notably, the T-cell response was preserved with biologic therapies targeting IL12/23 and integrins but augmented by anti-TNF therapy. Paradoxically, our recent study of these same patients documented that anti-TNF therapy reduced the vaccine-induced antibody response,3 as also reported by other investigators.1,2 The biologic basis of this association of the T-cell response with anti-TNF is unclear. One likely mechanism is the selective apoptotic signaling by TNF receptors in activated effector T-cells that, when blocked by anti-TNF therapy, permits a net increase in T-cell clonal expansion.14 Augmentation of the T-cell response by anti-TNF therapy may illuminate the recently reported association of anti-TNF therapy with reduced hospitalization or death from COVID-19.15 Taken together, we speculate that while anti-TNF therapy may blunt antibody responses and thereby reduce vaccine protection from infection, it is associated with a robust T-cell clonal expansion that may enhance vaccine-mediated protection against severe disease once infection ensues.
Supplementary Data
Supplementary data are available at Inflammatory Bowel Diseases online.
Supplemental Figure 1. T-cell clonal response to SARS-CoV-2 immunization in COVID-19-naive and -experienced subjects. Box plots show means, quartiles, and data ranges. SARS-CoV-2 spike, (A) breadth and (B) depth. SARS-CoV-2 nonspike, (C) breadth and (D) depth. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Acknowledgments
We thank all those who have contributed to the CORALE-IBD Vaccine study: James Beekley, Sarah Contreas, Joseph Ebinger, Ergueen Herrera, Amy Hoang, Nathalie Nguyen, Sarah Sternbach, Nancy Sun, Min Wu, Keren Appel, Andrea Banty, Edward Feldman, Christina Ha, Dmitry Karayev, Benjamin Kretzman, Rashmi Kumar, Susie Lee, Shervin Rabizadeh, Theodore Stein, Gaurav Syal, Stephan Targan, Eric Vasiliauskas, David Ziring, Brigid Boland, Mary Hanna, Elizabeth Khanishian, Melissa Hampton, Justina Ibrahim, Ashley Porter, Shane White, and Cindy Zamudio.
Contributor Information
Dalin Li, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Alexander Xu, Cedars Sinai Cancer and Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Emebet Mengesha, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Rebecca Elyanow, Adaptive Biotechnologies, Seattle, WA, USA.
Rachel M Gittelman, Adaptive Biotechnologies, Seattle, WA, USA.
Heidi Chapman, Adaptive Biotechnologies, Seattle, WA, USA.
John C Prostko, Applied Research and Technology, Abbott Diagnostics, Abbott Park, IL.
Edwin C Frias, Applied Research and Technology, Abbott Diagnostics, Abbott Park, IL.
James L Stewart, Applied Research and Technology, Abbott Diagnostics, Abbott Park, IL.
Valeriya Pozdnyakova, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Philip Debbas, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Angela Mujukian, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Arash A Horizon, Center for Rheumatology Medical Group, Los Angeles, CA, USA.
Noah Merin, Cedars Sinai Cancer and Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Sandy Joung, Department of Cardiology, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Gregory J Botwin, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Kimia Sobhani, Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Jane C Figueiredo, Cedars Sinai Cancer and Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Susan Cheng, Department of Cardiology, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Ian M Kaplan, Cedars Sinai Cancer and Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Dermot P B McGovern, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Akil Merchant, Cedars Sinai Cancer and Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Gil Y Melmed, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Jonathan Braun, F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA; Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Funding
This study was supported by the Leona M. and Harry B. Helmsley Charitable Trust, the Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, and the National Institute of Diabetes and Digestive and Kidney Disease (grants P01DK046763 and U01DK062413); and the Cedars-Sinai Precision Health Initiative, the Erika J. Glazer Family Foundation.
Conflicts of Interest
GYM has consulted for AbbVie, Arena Pharmaceuticals, Boehringer-Ingelheim, Bristol-Meyers Squibb/Celgene, Entasis, Janssen, Medtronic, Pfizer, Samsung Bioepis, Shionogi, Takeda, and Techlab, and has received research funding from Pfizer for an unrelated investigator-initiated study. JB has received research funding from Janssen. DPBM has consulted for Takeda, Boehringer-Ingelheim, Palatin Technologies, Bridge Biotherapeutics, Pfizer, and Gilead, and is a consultant/stockholder for Prometheus Biosciences.
Data Availability
Requests for deidentified data may be directed to the corresponding authors (JB, GYM) and will be reviewed by the Office of Research Administration at Cedars-Sinai Medical Center before issuance of data sharing agreements. Data limitations are designed to ensure patient and participant confidentiality.
References
- 1. Edelman-Klapper H, Zittan E, Bar-Gil Shitrit A, et al. Lower serologic response to COVID-19 mRNA vaccine in patients with inflammatory bowel diseases treated with anti-TNFα. Gastroenterology. 2022;162(2):454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70(10):1884–1893. [DOI] [PubMed] [Google Scholar]
- 3. Melmed GY, Botwin GJ, Sobhani K, et al. Antibody responses after SARS-CoV-2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med. 2021;174(12):1768–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. doi: 10.1101/2021.03.30.21254607, 31 March 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Snyder TM, Gittelman RM, Klinger M, et al. Magnitude and dynamics of the T-cell response to SARS-CoV-2 infection at both individual and population levels. medRxiv. doi: 10.1101/2020.07.31.20165647, 17 September 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 8. Klinger M, Pepin F, Wilkins J, et al. Multiplex identification of antigen-specific T cell receptors using a combination of immune assays and immune receptor sequencing. PLoS One. 2015;10(10):e0141561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collier DA, Ferreira I, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3(9):e627–e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agrati C, Castilletti C, Goletti D, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. 2021;9(6):1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta AK, Gracias DT, Croft M.. TNF activity and T cells. Cytokine. 2018;101:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4(10):e2129639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for deidentified data may be directed to the corresponding authors (JB, GYM) and will be reviewed by the Office of Research Administration at Cedars-Sinai Medical Center before issuance of data sharing agreements. Data limitations are designed to ensure patient and participant confidentiality.