Abstract
Background
COVID-19 has disproportionately affected older adults. Frailty has been associated with impaired vaccine response in other vaccine types, but the impact of frailty on mRNA vaccine response is undefined.
Methods
Observational study of adults aged 55 and older from 1 U.S. health care system between January 22, 2021 and September 16, 2021 with self-reported Moderna or Pfizer COVID-19 mRNA vaccine and an electronic frailty index (eFI) score from their medical record (n = 1 677). Participants’ frailty status was compared with positive antibody detection (seroconversion) following full vaccination and subsequent loss of positive antibody detection (seroreversion) using logistic regression models.
Results
Of 1 677 older adults with median (interquartile range) age, 67 (62 and 72) years, and frailty status (nonfrail: 879 [52%], prefrail: 678 [40%], and frail: 120 [7.2%]), seroconversion was not detected in 23 (1.4%) over 60 days following full vaccination. Frail individuals were less likely to seroconvert than nonfrail individuals, adjusted odds ratio (OR) 3.75, 95% confidence interval (CI; 1.04, 13.5). Seroreversion was detected in 50/1 631 individuals (3.1%) over 6 months of median follow-up antibody testing. Frail individuals were more likely to serorevert than nonfrail individuals, adjusted OR 3.02, 95% CI (1.17, 7.33).
Conclusion
Overall antibody response to COVID-19 mRNA vaccination was high across age and frailty categories. While antibody detection is an incomplete descriptor of vaccine response, the high sensitivity of this antibody combined with health-system data reinforce our conclusions that frailty is an independent predictor of impaired antibody response to the COVID-19 mRNA vaccines. Frailty should be considered in vaccine studies and prevention strategies.
Keywords: COVID-19, Frailty, Immune function
Older adults have been disproportionately affected by the COVID-19 pandemic, with higher odds of hospitalization and death even among vaccinated individuals (1,2). Two COVID-19 mRNA vaccines, BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), have demonstrated robust protection from infection and severe disease outcomes, including in older adults, whom health policies prioritized in vaccination strategies (2,3). Positive detection of antibodies has been correlated with a reduced risk for infection (4). However, older adults have lower antibody levels relative to young and middle-aged adults following COVID-19 mRNA vaccination (5,6). Vaccine response in older adults remains incompletely understood, in part due to selection criteria excluding frail older adults from vaccine trials (7). Evidence from prior vaccine studies, including influenza, varicella-zoster, and pneumococcal pneumonia have described frailty as a better predictor than chronological age for impaired vaccine effectiveness in older adults (8–10).
Frailty is a geriatric syndrome characterized by disproportionate vulnerability to adverse health outcomes due to impaired physiological and functional reserve. Additionally, frailty is a reliable predictor for COVID-19 disease severity (11). Frailty can be measured across the population of older adults, with up to 40% of community-dwelling older adults categorized as frail (11,12). The frailty index (FI), which measures the accumulation of health-related deficits to categorize frailty with well-defined cutoffs, has been validated as a tool in vaccine research in older adults (8–10,12,13). At Wake Forest School of Medicine, an electronic frailty index (eFI) score is readily available using electronic health record (EHR) data for patients seen in the health care system (12).
The continuing COVID-19 pandemic in conjunction with waning vaccine-induced immunity emphasizes the importance of understanding the impact of frailty on COVID-19 vaccine responses in older adults (7–9). We hypothesized that frail individuals would have a decreased COVID-19 mRNA vaccine antibody response compared to nonfrail older adults.
Method
This observational study was approved by the Wake Forest School of Medicine institutional review board IRB#64912. Written informed consent was obtained via electronic survey. We examined data, including self-reported vaccination and subsequent test-verified severe acute respiratory coronavirus (SARS-CoV-2) antibodies from an existing cohort the COVID-19 Community Research Partnership (CRP). The CRP is a health system-based, longitudinal serologic surveillance study launched in April 2020. Participants were recruited online via email, internal communications, and social media. A majority of cohort participants were selected for at-home fingerstick dried blood spot cards for antibody testing. Multiple factors including time of study enrollment and rate of return of completed antibody tests influenced the number and frequency of test results per person. Dried blood spot cards were analyzed at Labcorp (Burlington, NC) for IgG antibodies to SARS-CoV-2 spike protein via Euroimmun test (Lübeck, Germany). Positive spike protein antibody results were reflex tested for SARS-CoV-2 Nucleocapsid protein to detect if antibodies were developed from infection via Roche test (Basel, Switzerland). Both tests have reported sensitivity >90% and specificity >99% (14). Antibody test results were reported to the study coordinating site for analysis.
The current analysis is based on data collected between January 22, 2021 and September 16, 2021. CRP cohort participants from 1 North Carolina health care system who were 55-years-old or greater during the study surveillance period and were fully vaccinated with antibody testing at least 2 weeks following their second dose of either Pfizer-BioNTech or Moderna COVID-19 mRNA vaccine were included. Serologic data from the CRP was harmonized with eFI scores extracted from the study participants’ EHR clinical encounters closest in time to vaccination (EPIC Systems, Madison, WI). Frailty was categorized using an EHR-based FI, adapted from the deficit-accumulation model of frailty (13). The eFI leverages routine health data, condensing 54 total age-related deficits into a single proportion present for each individual (12). Domains include diagnosis codes, medications, laboratory studies, biometrics, and functional measurements, with nonfrail (eFI < 0.1), prefrail (0.10 < eFI ≤ 0.21), and frail (eFI > 0.21).
Study outcomes were odds of nonseroconversion and odds of seroreversion. Seroconversion was defined as positive SARS-CoV-2 spike antibody test results within 60 days following full vaccination. Seroreversion was defined as individuals with prior positive spike antibody results, who subsequently developed negative spike antibody test results. To account for differential immune responses following infection, participants with positive SARS-CoV-2 Nucleocapsid antibody detection at the time of seroconversion were excluded from the analysis. Vaccination date was self-reported by participants. EHR records for participants with negative spike antibody tests were reviewed by a study team physician for criteria meeting moderate to severely immunocompromising conditions by Centers for Disease Control and Prevention (CDC) definition (15).
Participant data were analyzed with R statistical software version 4.1.2 (R Foundation, Vienna, Austria). Outcomes were reported using logistic regression with odds ratios for multivariable models including frailty category, age, sex, vaccine manufacturer, and time since vaccination. Adjusted models used Firth’s correction method. Time-dependent seroreversion was also modeled using Cox regression. Two-sided significance was at p = .05.
Results
In a cohort of n = 1 787 adults aged 55 years and older in the CRP with 2 reported mRNA vaccine doses and accessible eFI scores, n = 110 (6.2%) were excluded due to positive nucleocapsid antibodies indicating prior infection. Of the included n = 1 677 participants, 964 (57%) were women, 1 512 (90.2%) were White (non-Hispanic/Latino), and 165 (9.8%) were another race/ethnicity. Individuals were grouped by frailty category (nonfrail: 879 [52%], prefrail: 678 [40%], and frail: 120 [7.2%]), and median age was 67 years, interquartile range (62 and 72). Older individuals were more likely to be categorized as prefrail: median age 69 years and frail: median age 72 years (reference age nonfrail category, both p < .0001). Among vaccine recipients, 654 (39%) received Moderna mRNA-1273 and 1 023 (61%) received Pfizer-BioNTech BNT162b2 (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Total, N = 1,6771 | Non-frail, N = 879 (52%)1 | Pre-frail, N = 678 (40%)1 | Frail, N = 120 (7.2%)1 |
|---|---|---|---|---|
| Age (Years) | 67 (62, 72) | 66 (61, 71) | 69 (64, 73) | 72 (67, 76) |
| Sex | ||||
| Female | 964 (57%) | 511 (58%) | 388 (57%) | 65 (54%) |
| Male | 713 (43%) | 368 (42%) | 290 (43%) | 55 (46%) |
| Race/Ethnicity | ||||
| White (not Hispanic/Latino) | 1,512 (90%) | 791 (90%) | 612 (90%) | 109 (91%) |
| Other | 165 (9.8%) | 88 (10%) | 66 (9.7%) | 11 (9.2%) |
| Vaccine Manufacturer | ||||
| Moderna | 654 (39%) | 341 (39%) | 255 (38%) | 58 (48%) |
| Pfizer | 1,023 (61%) | 538 (61%) | 423 (62%) | 62 (52%) |
1n (%); Median (IQR).
Seroconversion was not detected in 23 individuals (1.4%). Frail individuals were less likely to seroconvert than nonfrail individuals, adjusted odds ratio (OR) 3.75, 95% confidence interval (CI; 1.04, 13.5). Frailty was the only significant variable in the adjusted model for seroconversion (p = .043; Table 2). Median time to positive antibody detection was 7 days following full vaccination. Those without seroconversion received follow up antibody testing for a median of 167 days, and 14 (60.9%) were immunocompromised by CDC criteria (15).
Table 2.
Seroconversion and Seroreversion Status
| Counts | Univariate Regression | Adjusted Odds Ratios | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Antibody Positive* | Antibody Negative* | OR | 95% CI | OR | 95% CI | p Value |
| Seroconversion total | 1 654 (98.6%) | 23 (1.4%) | |||||
| Frailty | |||||||
| Nonfrail | 872 (99%) | 7 (0.8%) | — | — | — | — | |
| Prefrail | 666 (98%) | 12 (1.8%) | 2.24 | 0.90, 6.06 | 2.1 | 0.83, 5.73 | .12 |
| Frail | 116 (97%) | 4 (3.3%) | 4.3 | 1.11, 14.4 | 3.75 | 1.04, 13.5 | .043 |
| Age (years) | 67 (62, 72) | 70 (66, 73) | 1.04 | 0.98, 1.10 | 1.03 | 0.97, 1.09 | .4 |
| Sex | |||||||
| Female | 951 (99%) | 13 (1.3%) | — | — | — | — | |
| Male | 703 (99%) | 10 (1.4%) | 1.04 | 0.44, 2.38 | 0.96 | 0.42, 2.24 | >.9 |
| Race/ethnicity | |||||||
| White (non-Hispanic/Latino) | 1 492 (99%) | 20 (1.3%) | — | — | — | — | |
| Other | 162 (98%) | 3 (1.8%) | 1.38 | 0.32, 4.08 | |||
| Vaccine manufacturer | |||||||
| Moderna | 645 (99%) | 9 (1.4%) | — | — | — | — | |
| Pfizer | 1 009 (99%) | 14 (1.4%) | 0.99 | 0.43, 2.40 | 1.03 | 0.45, 2.49 | >.9 |
| Seroreversion total | 1 581 (96.9%) | 50 (3.1%) | |||||
| Frailty | |||||||
| Nonfrail | 843 (98%) | 16 (1.9%) | — | — | — | — | |
| Prefrail | 629 (96%) | 25 (3.8%) | 2.09 | 1.12, 4.03 | 1.66 | 0.86, 3.25 | .13 |
| Frail | 109 (92%) | 9 (7.6%) | 4.35 | 1.80, 9.90 | 3.02 | 1.17, 7.33 | .017 |
| Age (years) | 67 (62, 72) | 74 (72, 78) | 1.14 | 1.10, 1.19 | 1.12 | 1.07, 1.17 | <.001 |
| Sex | |||||||
| Female | 924 (98%) | 18 (1.9%) | — | — | — | — | |
| Male | 657 (95%) | 32 (4.6%) | 2.5 | 1.41, 4.58 | 1.92 | 1.05, 3.60 | .037 |
| Time since full vaccination (days) | 153 (115, 176) | 156 (127, 174) | 1.01 | 1.00, 1.01 | 1 | 1.00, 1.01 | .8 |
| Vaccine manufacturer | |||||||
| Moderna | 637 (100%) | 3 (0.5%) | — | — | — | — | |
| Pfizer | 944 (95%) | 47 (4.7%) | 10.6 | 3.85, 43.7 | 10.9 | 3.93, 45.4 | <.001 |
Notes: CI = confidence interval; OR = odds ratio.
*n (%); median (interquartile range).
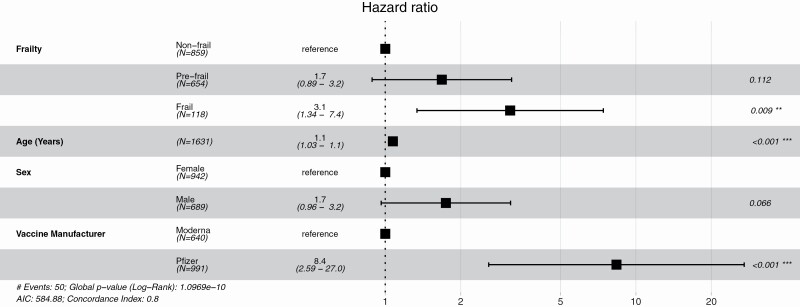
Seroreversion was detected in 50/1 631 individuals (3.1%) over 6 months of median follow-up antibody testing, with no difference in follow-up time by frailty category (Figure 1). Frail individuals were more likely to serorevert than nonfrail individuals, adjusted OR 3.02, 95% CI (1.17, 7.33). Frailty, age, sex, and vaccine manufacturer were significantly associated with seroreversion. Among the 50 individuals who lost antibody detection, 6 (12%) were immunocompromised, and 47 (94%) had received the Pfizer vaccine. Seroreversion occurred earlier in frail individuals than nonfrail individuals, 131 days versus 163 days (p = .049).
Figure 1.
Seroreversion risk factors. Cox proportional-hazards model for risk of seroreversion.
Discussion
In this cohort study, we analyzed serologic results from the COVID-19 CRP with participants’ eFI scores to describe the relationship between mRNA vaccine response and frailty. Overall postvaccine antibody detection was high among all older adults. Frailty alone was significantly associated with nonseroconversion, while frailty and age were both independently associated with seroreversion. Frail individuals experienced seroreversion earlier than nonfrail individuals. Pfizer-BioNTech vaccine had higher odds of seroreversion compared to Moderna after 2 vaccine doses, which is consistent with reports of lower antibody titers in older adults (5,6). Immunocompromising conditions were highly prevalent in the nonseroconversion group, though infrequent in the seroreversion group. This suggests confounding from immunocompromise on frailty and nonseroconversion but not for seroreversion.
The strengths of this study include examining frailty in a large population of community-based older adults using serological surveillance cohort results harmonized with an eFI from health-system data. Although the study outcomes were infrequent, the robust performance characteristics of the antibody tests provided accurate results with a low false-negative detection rate, 95% CI (0%–5%) (14). Furthermore, this study reports novel findings on a relevant topic, as even vaccinated frail older adults remain at risk for adverse COVID-19 disease-related outcomes (1,11). While COVID-19 vaccine response has been examined in frail nursing home populations, these studies included smaller populations where frailty was either unmeasured or examined with simpler scales (6,16,17).
The limitations of this study include underrepresentation of individuals at the oldest age range and frail categories in the population. The majority of participants were in early-old age with their selection influenced by the online methodology of the CRP cohort. We previously described frailty but not age in this cohort was significantly associated with a lower likelihood of completing at home serologic testing, which adversely impacts the inclusion of individuals with frailty (18). While using multivariable regression controlled for age and frailty allowing for precise outcome estimates, the interaction between age and frailty and small frail category population decreased the accuracy of our estimates.
Additionally, it is known that qualitative antibody results to a single antigen are an imperfect assessment of immunity, and comprehensive analysis of vaccine responses includes T-cell responses and antibody binding specificity (4,14,19). When interpreting qualitative antibody test results, a negative result does not confer a complete lack of protection, rather the amount of antibodies was measured below the detection limit. Waning protection after COVID-19 mRNA vaccination in uninfected individuals has been well described (20). Our findings suggest frail older adults are particularly vulnerable to waning vaccine immunity, but further research on the durability of COVID-19 vaccine responses and clinical outcomes in frail populations is needed to test this hypothesis. In addition, we did not study the impact of tertiary vaccine booster doses, which became widely available after the serological study surveillance period. Although impaired vaccine effectiveness in frail individuals has been well described, the immunological effects of frailty remain incompletely understood, warranting further investigation (7–10).
Conclusion
While antibody detection was high across age and frailty categories following vaccination, frailty independently influences impaired antibody response to COVID-19 mRNA vaccines in older adults. Further studies including clinical outcomes are needed to validate whether frailty is predictive of COVID-19 vaccine effectiveness. Automated frailty assessment in vaccine research is needed to better characterize vaccine responses in older adults.
Acknowledgments
We would like to acknowledge the Informatics Department/Translational Data Warehouse (TDW) Team of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420.
Contributor Information
Charles T Semelka, Section on Geriatric Medicine, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Michael E DeWitt, Section on Infectious Diseases, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Kathryn E Callahan, Section on Geriatric Medicine, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
David M Herrington, Section on Cardiovascular Medicine, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Martha A Alexander-Miller, Department of Microbiology and Immunology, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Joshua O Yukich, Department of Tropical Medicine, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, USA.
Iqra Munawar, Section on Infectious Diseases, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Lewis H McCurdy, Section on Infectious Diseases, Department of Internal Medicine, Atrium Health, Charlotte, North Carolina, USA.
Michael A Gibbs, Department of Emergency Medicine, Atrium Health, Charlotte, North Carolina, USA.
William S Weintraub, MedStar Health Research Institute, Washington, District of Columbia, USA; Department of Medicine, Georgetown University, Washington, District of Columbia, USA.
John W Sanders, Section on Infectious Diseases, Department of Internal Medicine, Wake Forest University School of Medicine, Winston Salem, North Carolina, USA.
Funding
This work was supported by a grant from the State of North Carolina funded by the CARES Act, of the U.S. Department of Health and Human Services (HHS). The study received additional funding from the Centers for Disease Control and Prevention (CDC) Contract #75D30120C08405. The sponsors had no role in the developing the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. C.T.S. is supported by the National Institutes of Health 5T32AG033534-12 training grant. K.E.C. is supported by the National Institute of Aging K76-AG059986.
Conflict of Interest
None declared.
References
- 1. CDC. Risk for COVID-19 infection, hospitalization, and death by age group. Centers for Disease Control and Prevention. Accessed December 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html#print
- 2. Thompson MG, Stenehjem E, Grannis S, et al. . Effectiveness of covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santacatterina M, Sanders JW, Weintraub WS, North Carolina COVID-19 Community Research Partnership. Prevention of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(19):1817–1818. doi: 10.1056/NEJMc2113575 [DOI] [PubMed] [Google Scholar]
- 4. Lumley SF, O’Donnell D, Stoesser NE, et al. . Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4(9):e2124331. doi: 10.1001/jamanetworkopen.2021.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canaday DH, Carias L, Oyebanji OA, et al. . Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naive nursing home residents. Clin Infect Dis. 2021;73(11):2112–2115. doi: 10.1093/cid/ciab447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet. 2021;396(10267):1942–1944. doi: 10.1016/S0140-6736(20)32481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrew MK, Shinde V, Ye L, et al. . The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216(4):405–414. doi: 10.1093/infdis/jix282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curran D, Kim JH, Matthews S, et al. . Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc. 2021;69(3):744–752. doi: 10.1111/jgs.16917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macintyre CR, Ridda I, Gao Z, et al. . A randomized clinical trial of the immunogenicity of 7-valent pneumococcal conjugate vaccine compared to 23-valent polysaccharide vaccine in frail, hospitalized elderly. PLoS One. 2014;9(4):e94578. doi: 10.1371/journal.pone.0094578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. 2021;69(9):2419–2429. doi: 10.1111/jgs.17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a medicare accountable care organization. J Gerontol A Biol Sci Med Sci. 2019;74(11):1771–1777. doi: 10.1093/gerona/glz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 14. FDA. EUA authorized serology test performance. Food and Drug Administration. Accessed December 12, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 15. CDC. COVID-19 vaccines for moderately or severely immunocompromised people. Centers for Disease Control and Prevention. Accessed December 11, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 16. Seiffert P, Konka A, Kasperczyk J, et al. . Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in older residents of a long-term care facility: relation with age, frailty and prior infection status. Biogerontology. 2022;23(1):53–64. doi: 10.1007/s10522-021-09944-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salmeron Rios S, Mas Romero M, Cortes Zamora EB, et al. . Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc. 2021;69(6):1441–1447. doi: 10.1111/jgs.17153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Semelka C, Munawar I, Seals A, Callahan K, Sanders JW. Methodology for COVID-19 Community Research Partnership Symptom and Antibody Monitoring in Community Dwelling Older Adults. Poster presented at: 2021 AGS Annual Scientific Meeting; May 2021; Online. [Google Scholar]
- 19. Abbasi J. The flawed science of antibody testing for SARS-CoV-2 immunity. JAMA. 2021;326(18):1781–1782. doi: 10.1001/jama.2021.18919 [DOI] [PubMed] [Google Scholar]
- 20. Hall V, Foulkes S, Insalata F, et al. . Protection against SARS-CoV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691 [DOI] [PMC free article] [PubMed] [Google Scholar]