Abstract
Background
One- and two-dose mRNA vaccine effectiveness (VE) estimates against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by dosing interval and time since vaccination were assessed among healthcare workers (HCWs) in publicly funded acute and community (nonresidential) healthcare facilities in British Columbia, Canada.
Methods
A test-negative design was used with controls matched to cases (6:1) on epidemiological week of SARS-CoV-2 test date. mRNA vaccination was defined by receipt of the first dose ≥21 days or second dose ≥14 days before the test date. HCWs ≥18 years old tested for SARS-CoV-2 between epi-weeks 3 and 39 (January 17–October 2, 2021) were included, when varying dosing intervals and a mix of circulating variants of concern contributed, including Delta dominance provincially from epi-week 31 (August 1).
Results
Single- and two-dose analyses included 1265 and 1246 cases, respectively. The median follow-up period (interquartile range) was 49 (34–69) days for single-dose and 89 (61–123) days for two-dose recipients, with 12%, 31%, and 58% of second doses given 3–5, 6, or ≥7 weeks after the first. Adjusted mRNA VE against SARS-CoV-2 was 71% (95% CI, 66%–76%) for one dose and 90% (95% CI, 88%–92%) for two doses, similar to two heterologous mRNA doses (92%; 95% CI, 86%–95%). Two-dose VE remained >80% at ≥28 weeks post–second dose. Two-dose VE was consistently 5%–7% higher with a ≥7-week vs 3–5-week interval between doses, but with overlapping confidence intervals.
Conclusions
Among HCWs, we report substantial single-dose and strong and sustained two-dose mRNA vaccine protection, with the latter maintained for at least 7 months. These findings support a longer interval between doses, with global health and equity implications.
Keywords: vaccine effectiveness, SARS, CoV, 2, healthcare workers, test, negative design
Healthcare workers (HCWs) have been at the front lines of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. With high potential for exposure [1] and to mitigate the risk to themselves and their patients, HCWs in Canada were also among the first prioritized for vaccination. Two mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), were first authorized in Canada on December 9 (epi-week 50) and December 23 (epi-week 52), 2020, respectively, with to a two-dose schedule spaced 3–4 weeks apart [2].
In the province of British Columbia (BC), Canada, HCW vaccination began on December 15, 2020 (epidemiological-week 51) at the manufacturer-recommended interval of 3–4 weeks between doses. In response to elevated COVID-19 activity and scarce vaccine supply, a modified 5-week dosing interval was announced in epi-week 52. A 6-week interval was subsequently recommended in late January (epi-week 4) of 2021 and was maintained until early March (epi-week 9), when Canada’s National Advisory Committee on Immunization (NACI) recommended an even longer 16-week interval between the first and second doses [3]. As vaccine supply improved, the dosing interval was reduced in BC to 8 weeks in late May (epi-week 21), 7 weeks in late July (epi-week 30), and 4 weeks in August (epi-week 32), with a preferred interval of at least 6 weeks still emphasized [4].
Immunogenicity studies among HCWs in British Columbia and Ontario, Canada, have compared antibody response at a 3–4-week interval vs a 6–7-week interval [5] and at 3–6 vs 8–16 weeks [6] between the first and second doses. Both showed improved antibody levels at the longer interval, but antibody thresholds for protection have not been established. In a population-based evaluation of vaccine effectiveness (VE) using the test-negative design (TND) in BC and Quebec, Canada, protection was lower at 3–4-week and 5–6-week intervals compared with a 7–8-week interval between doses, and was stable thereafter [7].
To measure the degree and duration of one- and two-dose mRNA VE against SARS-CoV-2 infection in HCWs and to further explore the effects of extended dosing intervals, we applied the TND to acute care and community-based HCWs in British Columbia, Canada. We also assessed one- and two-dose VE by vaccine type (BNT162b2 and mRNA-1273), age group, sex, and level of patient care.
METHODS
Study Design
We used the TND with incidence density sampling, matching test-negative controls 6:1 to test-positive cases based on epidemiological week (epi-week) of the nucleic acid amplification test date. For HCWs with multiple negative tests throughout the study period, a single negative test per epi-week was randomly selected. Further HCW case or control contribution was censored on the date of the first positive test.
Outcome and Vaccine Status Definition
VE was estimated against any infection. Testing was mostly symptom-based by nasopharyngeal swab, but available data do not support VE stratification by symptom status. Gargle (saliva) specimens were available but not widely or systematically used in HCWs. Rapid antigen tests were not broadly deployed during the analysis period.
SARS-CoV-2 vaccination was publicly funded and encouraged but not mandatory for HCWs in BC during the study period. Vaccination status was defined by the time between the last vaccine dose and the specimen test date, the latter defined by the earliest of test order or result date. Given analyses based upon testing rather than onset date, we incorporated additional lag time, in addition to the expected timeline for vaccine-induced immunity following each dose [8]. For primary VE estimation, single-dose vaccine status was defined by ≥21 days, and two-dose status was defined by ≥14 days between vaccination and test date. Specimens with a first dose <21 days prior or a second dose <14 days prior were excluded. Unvaccinated HCWs were those tested who received no vaccine dose on or before the test date. For single-dose analyses, specimens collected on/after the date of the second dose were excluded. For two-dose analyses, specimens collected on/after the date of receipt of the first dose were excluded.
Study Period
Single- and two-dose VE analyses spanned epi-weeks 3–30 (January 17–July 31, 2021) and epi-weeks 9–39 (February 28–October 2), respectively. However, analyses of VE at longer durations after the second dose were restricted to more recent study periods to enable a sufficient number of vaccinated cases and controls with that amount of follow-up. For example, to assess VE at 16–27 weeks post–second dose, analyses were restricted to epi-weeks 20–39 (May 16–October 2), and at ≥28 weeks postvaccination, analyses were restricted to epi-weeks 35–39 (August 29–October 2).
Although genetic characterization of case viruses among HCWs was not available for the current analysis, a mix of circulating variants of concern (VOC) were detected in the general BC population, including working-age adults age ≥18 years [7, 9]. Alpha and Gamma variants were first detected in January and February 2021, respectively, increasing to unique co-dominance through May and June. The Delta variant appeared started circulating in April, increased through July, and comprised almost sole detection provincially from August 1, 2021 (epi-week 31), through the rest of the analysis period.
Data Sources
The source population was the Workplace Health Incident Tracking and Evaluation (WHITE) database. The WHITE database was introduced in 2004 by the Occupational Health and Safety Agency for Healthcare in British Columbia, in conjunction with health regions, to track occupational exposures, stressors, injuries, and illnesses among HCWs employed by BC’s 5 health authorities (HAs) [10]. These include Fraser (FHA) and Vancouver Coastal (VCHA) in the Lower Mainland, where >60% of the BC population resides [11], as well as the Interior (IHA), Northern (NHA), and Vancouver Island (VIHA) regions.
WHITE captures all HCWs (~140 000) employed by HAs including some long-term care facilities (LTCFs) but excluding any facilities privately managed or not affiliated with HAs. Data from WHITE were extracted on October 7, 2021, along with relevant employee characteristics (eg, age, sex, occupation, HA of residence, type of employment) and SARS-CoV-2 laboratory results (imported from the Provincial Laboratory Information Solution [PLIS]) and COVID-19 immunization details (imported from the Provincial Immunization Registry [PIR]). PLIS contains all SARS-CoV-2 test results from private and public laboratories across BC. The PIR captures all COVID-19 vaccinations for BC residents, including HCWs, regardless of place of delivery, HA, or employee status.
Study Population
All HCWs within the WHITE database aged ≥18 years (at test date), hired before October 1, 2021 (epi-week 39), and tested for SARS-CoV-2 within the analysis period were included. A broad array of HCW occupations were included (eg, physicians, paramedics/emergency responders, nurses, pharmacists, therapists, technicians, clinical, service, research, and administrative/managerial staff) from publicly funded acute (eg, hospitals, emergency departments, urgent care) and community care settings (eg, home or ambulatory care services).
Excluded HCWs were those who tested positive before the analysis period or with missing, inconclusive, or invalid information for vaccination or test details, age, sex, or HA. Also excluded were HCWs tested for a possible workplace exposure/cluster or who worked in LTCF, assisted living, hospice, or correctional facilities (residential facilities), where cluster testing/screening was more likely. Due to smaller numbers, specimens from recipients of non-mRNA vaccines were also excluded.
Statistical Analyses
The odds of vaccination among SARS-CoV-2 test-positive vs test-negative specimens were compared through the odds ratio (OR) using multivariable conditional logistic regression, with the matching epi-week for cases and controls as a stratum. Single- and two-dose mRNA VE was derived as [(1 – ORadjusted) ×100] with 95% CIs. Covariates included age group (18–29, 30–39, 40–49, 50–59, ≥60 years), sex (male, female), and HA of HCW residence (5 categories).
VE was separately estimated by mRNA product, age, sex, and, to compare with estimates previously published for VCHA [12], separately for VCHA, FHA, and other HAs combined provincially. We also assessed two-dose VE among those classified as providing direct patient care (including all categories of nurses) or service providers (eg, food, transportation, cleaning, laundry, maintenance services) based upon the first-mentioned job, recognizing that >90% of HCWs had just 1 job.
VE was explored by time since vaccination, and two-dose VE was further stratified by interval between doses. The choice of intervals took into account the timeline of vaccine schedule adjustments and their impact on second-dose rollout and coverage in BC HCWs. Given early implementation of the 5-week interval in BC, we combined that with the manufacturer-specified 3–4-week schedule. Additionally, since a substantial proportion were immunized according to the 6-week interval, we assessed that intermediate group separately so as not to drive or dilute comparison between the shorter and longer intervals. The third stratum included those who received their second dose ≥7 weeks after the first, as informed by earlier population-based findings [7].
Ethics Statement
Data access was approved by WHITE's steering committee. Vaccine effectiveness evaluations were conducted as legally mandated public health investigations. Data linkages and analyses by the BC Centre for Disease Control were authorized under the Public Health Act as a delegated function of the Provincial Health Officer. The lead investigator (Skowronski) also sought independent research ethics board review which was waived by the University of British Columbia Clinical Research Ethics Board.
Patient Consent
The study does not include factors requiring patient consent.
RESULTS
Study Population: Case and Control Profile
Data extracted from WHITE included 141 465 unique HCWs, among whom a total of 31 858 specimens (23 794 unique HCWs) met inclusion criteria and were available for single-dose VE analyses, and 36 776 specimens (27 602 unique HCWs) met inclusion criteria and were available for two-dose analyses. Following matching, there were 1265 cases and 7590 controls (n = 8855) for single-dose VE, and 1246 cases and 7476 controls (n = 8722) for two-dose VE. In both analyses, HCWs were more often female (>80%), with cases about a decade younger than controls (median, 36–37 vs 46–47 years) (Table 1; Supplementary Table 1). The distribution of cases and controls by HA was similar, with a slightly higher share of cases in FHA for single-dose VE and a lower share in VIHA in both analyses, commensurate with variation in pandemic intensity provincially (Supplementary Table 1) [13].
Table 1.
Profile of Participants in Two-Dose Vaccine Effectiveness Analyses Among HCWs in British Columbia, Epi-Weeks 9–39, 2021
| All Specimens | Distribution by Case Status | Specimens From Vaccinated Health Care Workers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Overall | Cases | Controls | ||||||||
| Characteristics | No. (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Overall | 8722 | 1246 | (14) | 7476 | (86) | 6372 | (73) | 535 | (43) | 5837 | (78) | |
| Age group | ||||||||||||
| 18–29 y | 425 | (5) | 332 | (27) | 93 | (1) | 179 | (42) | 121 | (36) | 58 | (62) |
| 30–39 y | 2280 | (26) | 420 | (34) | 1860 | (25) | 1575 | (69) | 196 | (47) | 1379 | (74) |
| 40–49 y | 2727 | (31) | 249 | (20) | 2478 | (33) | 2108 | (77) | 112 | (45) | 1996 | (81) |
| 50–59 y | 2242 | (26) | 174 | (14) | 2068 | (28) | 1693 | (76) | 72 | (41) | 1621 | (78) |
| 60+ y | 1048 | (12) | 71 | (6) | 977 | (13) | 817 | (78) | 34 | (48) | 783 | (80) |
| Median age (IQR), y | 45 | (38–54) | 36 | (29–47) | 46 | (39–55) | 46 | (39–55) | 37 | (30–47) | 46 | (40–55) |
| Sex | ||||||||||||
| Female | 7594 | (87) | 1031 | (83) | 6563 | (88) | 5561 | (73) | 439 | (43) | 5122 | (78) |
| Male | 1128 | (13) | 215 | (17) | 913 | (12) | 811 | (72) | 96 | (45) | 715 | (78) |
| Epidemiological wk, 2021 | ||||||||||||
| 9–12 (Feb 28–Mar 27) | 1197 | (14) | 171 | (14) | 1026 | (14) | 389 | (32) | 4 | (2) | 385 | (38) |
| 13–16 (Mar 28–Apr 24) | 1400 | (16) | 200 | (16) | 1200 | (16) | 671 | (48) | 17 | (9) | 654 | (55) |
| 17–20 (Apr 25–May 22) | 714 | (8) | 102 | (8) | 612 | (8) | 423 | (59) | 23 | (23) | 400 | (65) |
| 21–24 (May 23–Jun 19) | 189 | (2) | 27 | (2) | 162 | (2) | 144 | (76) | 8 | (30) | 136 | (84) |
| 25–28 (Jun 20–July 17) | 147 | (2) | 21 | (2) | 126 | (2) | 118 | (80) | 7 | (33) | 111 | (88) |
| 29–32 (July 18–Aug 14) | 917 | (11) | 131 | (11) | 786 | (11) | 805 | (88) | 75 | (57) | 730 | (93) |
| 33–36 (Aug 15–Sep 11) | 2268 | (26) | 324 | (26) | 1944 | (26) | 2051 | (90) | 201 | (62) | 1850 | (95) |
| 37–39 (Sep 12–Oct 2) | 1890 | (22) | 270 | (22) | 1620 | (22) | 1771 | (94) | 200 | (74) | 1571 | (97) |
| Employee health authority | ||||||||||||
| Fraser | 4034 | (46) | 578 | (46) | 3456 | (46) | 2953 | (73) | 213 | (37) | 2740 | (79) |
| Interior | 1742 | (20) | 305 | (24) | 1437 | (19) | 1189 | (68) | 131 | (43) | 1058 | (74) |
| Northern | 384 | (4) | 111 | (9) | 273 | (4) | 265 | (69) | 46 | (41) | 219 | (80) |
| Vancouver Coastal | 1541 | (18) | 170 | (14) | 1371 | (18) | 1252 | (81) | 106 | (62) | 1146 | (84) |
| Vancouver Island | 1021 | (12) | 82 | (7) | 939 | (13) | 713 | (70) | 39 | (48) | 674 | (72) |
| Job category | ||||||||||||
| Direct patient care (including nursing) | 3879 | (44) | 476 | (38) | 3403 | (46) | 2981 | (77) | 244 | (51) | 2737 | (80) |
| Service provider | 330 | (4) | 100 | (8) | 230 | (3) | 168 | (51) | 16 | (16) | 152 | (66) |
| Interval between 2 doses | ||||||||||||
| 3 wk | 20 | (0) | 2 | (0) | 18 | (0) | ||||||
| 4 wk | 100 | (2) | 4 | (1) | 96 | (2) | ||||||
| 5 wk | 642 | (10) | 59 | (11) | 583 | (10) | ||||||
| 6 wk | 1963 | (31) | 156 | (29) | 1807 | (31) | ||||||
| 7–8 wk | 278 | (4) | 32 | (6) | 246 | (4) | ||||||
| 9–11 wk | 489 | (8) | 45 | (8) | 444 | (8) | ||||||
| 12–15 wk | 2514 | (40) | 203 | (38) | 2311 | (40) | ||||||
| 16+ wk | 363 | (6) | 34 | (6) | 329 | (6) | ||||||
| Median (IQR), interval between two doses d | 70 | (42–98) | 69 | (43–98) | 70 | (42–98) | ||||||
| Vaccine name | ||||||||||||
| Pfizer | 5303 | (83) | 469 | (88) | 4834 | (83) | ||||||
| Moderna | 883 | (14) | 51 | (10) | 832 | (14) | ||||||
| Mixed mRNA | 186 | (3) | 15 | (3) | 171 | (3) | ||||||
| Time since vaccination, d/wk | ||||||||||||
| 14–20 d (2 wk) | 201 | (3) | 1 | (0) | 200 | (3) | ||||||
| 21–27 d (3 wk) | 175 | (3) | 4 | (1) | 171 | (3) | ||||||
| 28–34 d (4 wk) | 176 | (3) | 9 | (2) | 167 | (3) | ||||||
| 35–41 d (5 wk) | 240 | (4) | 11 | (2) | 229 | (4) | ||||||
| 42–55 d (6–7 wk) | 549 | (9) | 23 | (4) | 526 | (9) | ||||||
| 56–83 d (8–11 wk) | 1531 | (24) | 119 | (22) | 1412 | (24) | ||||||
| 84–111 d (12–15 wk) | 1459 | (23) | 114 | (21) | 1345 | (23) | ||||||
| 112–195 d (16–27 wk) | 1390 | (22) | 151 | (28) | 1239 | (21) | ||||||
| ≥196 d (≥28 wk) | 651 | (10) | 103 | (19) | 548 | (9) | ||||||
| Median (IQR), da | 89 | (61–123) | 108 | (76–180) | 88 | (60–120) | ||||||
| Range, d | 14–253 | 16–248 | 14–253 | |||||||||
Abbreviations: HCW, health care worker; IQR, interquartile range.
Among HCW participants tested ≥14 days after the second dose, with test-negative controls matched in a 6:1 ratio. For two-dose vaccinated HCWs, tested and matched without regard to time since vaccination, the median time since vaccination (IQR, range) was 90 (61–124, 0–253) days; 104 (73–177, 1–248) days for two-dose vaccinated cases; and 90 (60–122, 0–253) days for two-dose vaccinated controls.
Vaccine Coverage
Among vaccinated participants, 92% of single-dose and 83% of two-dose HCWs received BNT162b2. Among all HCWs in the WHITE database (without exclusions), the percentage who had received at least 1 mRNA vaccine dose was 30% in epi-week 3 and 91% in epi-week 30 (end of single-dose study period) (Supplementary Figure 1). The percentage who had received a second mRNA vaccine dose was 2% in epi-week 3; it increased to 24% by epi-week 8 and remained stable at about 25% between epi-weeks 9 and 17, increasing gradually thereafter to 54%, 86%, and 92% by epi-weeks 22, 30, and 39, respectively. Among the matched two-dose recipients for the primary analysis (n = 6372), the interval between doses was 3–5, 6, or ≥7 weeks among 762 (12%), 1963 (31%), and 3644 (58%), respectively (Table 1).
Single-Dose VE
The median (interquartile range [IQR]) duration of available follow-up among single-dose participants was 49 (34–69) days (Supplementary Table 1). Across that period, the adjusted single-dose mRNA VE against any SARS-CoV-2 infection was 71% (95% CI, 66%–76%): 70% (95% CI, 65%–75%) for BNT162b2 and 77% (95% CI, 64%–85%) for mRNA-1273 (Table 2). VE was similar for direct care (70%; 95% CI, 60%–77%) and service providers (68%; 95% CI, 43%–82%), and in VCHA (67%; 95% CI, 50%–78%) and FHA (71%; 95% CI, 65%–76%), but slightly higher in other HA combined (80%; 95% CI, 70%–86%), with overlapping confidence intervals. Adjusted single-dose VE was 79% (95% CI, 69%–85%) at 3 weeks, remaining 69% (95% CI, 59%–76%) at 6–7 weeks and 61% (95% CI, 52%–68%) at ≥8 weeks after the first dose (Supplementary Table 2). High coverage and second-dose rollout limited one-dose analyses at longer durations thereafter.
Table 2.
One- and Two-Dose Vaccine Effectiveness Against Any SARS-CoV-2 Infection
| Cases | Controls | Adjusted (Conditioned) for Matching Week | Fully Adjusteda | |||||
|---|---|---|---|---|---|---|---|---|
| All | Vaccinated | All | Vaccinated | VE, % | (95% CI), % | VE, % | (95% CI), % | |
| Single-dose VE (vaccinated ≥3 wk) | ||||||||
| Any mRNA vaccine | 1265 | 404 | 7590 | 4267 | 74 | (70–78) | 71 | (66–76) |
| By vaccine type | ||||||||
| Pfizer mRNA BNT162b2 | 1243 | 382 | 7458 | 4024 | 73 | (69–77) | 70 | (65–75) |
| Moderna mRNA-1273 | 883 | 22 | 5298 | 574 | 80 | (69–87) | 77 | (64–85) |
| By health authorityb | ||||||||
| Fraser Health | 724 | 240 | 4344 | 2614 | 74 | (68–78) | 71 | (65–76) |
| Vancouver Coastal Health Authority | 242 | 102 | 1452 | 844 | 69 | (55–79) | 67 | (50–78) |
| Other health authoritiesc | 299 | 62 | 1794 | 824 | 79 | (54–90) | 80 | (70–86) |
| By occupation | ||||||||
| Direct patient care (including nursing) | 461 | 176 | 2303 | 1394 | 72 | (64–78) | 70 | (60–77) |
| Service providersd | 101 | 27 | 401 | 175 | 58 | (30–75) | 68 | (43–82) |
| Two-dose VE (vaccinated ≥2 wk) | ||||||||
| Any mRNA vaccine | 1246 | 535 | 7476 | 5837 | 91 | (90–93) | 90 | (88–92) |
| By vaccine type | ||||||||
| Pfizer mRNA BNT162b2 | 1180 | 469 | 7080 | 5230 | 90 | (89–92) | 89 | (87–91) |
| Moderna mRNA-1273 | 762 | 51 | 4510 | 1726 | 93 | (90–95) | 93 | (90–95) |
| Mixed mRNA | 726 | 15 | 4066 | 546 | 91 | (85–95) | 92 | (86–95) |
| By sexe | ||||||||
| Female | 1031 | 439 | 6186 | 4808 | 91 | (89–93) | 90 | (88–92) |
| Male | 215 | 96 | 1290 | 1047 | 92 | (88–95) | 91 | (86–94) |
| By age groupf | ||||||||
| 18–29 y | 332 | 121 | 1992 | 1483 | 90 | (87–93) | 91 | (87–93) |
| 30–39 y | 420 | 196 | 2520 | 1963 | 89 | (85–92) | 89 | (85–92) |
| 40–49 y | 249 | 112 | 1494 | 1205 | 93 | (89–95) | 92 | (88–95) |
| 50–59 y | 174 | 72 | 1044 | 847 | 91 | (87–94) | 91 | (86–94) |
| 60+ y | 71 | 34 | 424 | 304 | 82 | (63–91) | 80 | (58–91) |
| By health authorityb | ||||||||
| Fraser Health Authority | 578 | 213 | 3468 | 2587 | 91 | (89–93) | 90 | (86–92) |
| Vancouver Coastal Health Authority | 170 | 106 | 1020 | 833 | 84 | (73–91) | 84 | (71–91) |
| Other health authoritiesc | 498 | 216 | 2988 | 2448 | 92 | (90–94) | 93 | (90–94) |
| By occupation | ||||||||
| Direct patient care (including nursing) | 476 | 244 | 2856 | 2371 | 88 | (84–90) | 85 | (80–89) |
| Service providersd | 100 | 16 | 566 | 366 | 95 | (90–97) | 96 | (92–98) |
| Two-dose VE, sensitivity analysisg | ||||||||
| Long-term care, assisted living, hospice, or correctional facilities | 287 | 134 | 1722 | 1457 | 87 | (82–90) | 82 | (76–87) |
Abbreviations: HCW, healthcare worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness.
Conditional logistic regression model adjusted for age group (18–29, 30–39, 40–49, 50–59, 60+ years), sex (male or female), and health authority of HCW’s residence (Fraser Health Authority, Interior Health Authority, Northern Health Authority, Vancouver Island Health Authority, and Vancouver Coastal Health Authority) using the matching epidemiological week of testing as strata.
Adjustment variables exclude HCW’s health authority.
Other health authorities include Northern Health Authority, Interior Health Authority, and Vancouver Island Health Authority.
Service providers included employees working in food, transportation, cleaning, laundry, and maintenance services in health care settings.
Adjustment variables exclude sex.
Adjustment variables exclude age group.
Analysis restricted to HCWs in long-term care/assisted living/hospice/correctional facilities.
Two-Dose VE
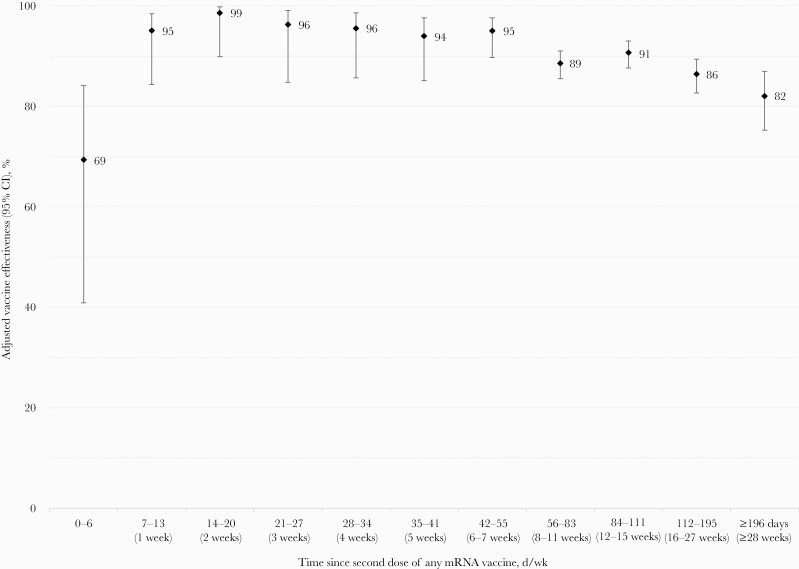
The median (IQR) follow-up for two-dose participants was 89 (61–123) days (Table 1). For specimens sampled and matched between epi-weeks 9 and 39, the adjusted two-dose mRNA VE overall was 90% (95% CI, 88%–92%): 89% (95% CI, 87%–91%) for BNT162b2, 93% (95% CI, 90%–95%) for mRNA-1273, and 92% (95% CI, 86%–95%) for mixed mRNA doses (Table 2). The two-dose VE of 69% (95% CI, 41%–84%) at 0–6 days post–second dose may reflect residual single-dose protection. From 1 week to 6–7 weeks after the second dose, VE remained ≥95% against infection, declining slightly but still ≥85% by 16–27 weeks and ≥80% by ≥28 weeks after the second dose (Figure 1). Two-dose VE did not vary meaningfully by sex or age group, slightly lower but still substantial against any infection in those ≥60 years (80%; 95% CI, 58%–91%). VE estimates were all within 10% (absolute) by HA. Two-dose VE was 85% (95% CI, 80%–89%) for direct care and 96% (95% CI, 92%–98%) for service providers.
Figure 1.
Adjusted two-dose mRNA vaccine effectiveness against any SARS-CoV-2 infection by days since vaccination among healthcare workers in British Columbia, Canada. Displayed are adjusted estimates of vaccine effectiveness against any SARS-CoV-2 infection, with 95% CI, by time since second dose of any mRNA vaccine (in days/weeks) among HCWs in British Columbia, Canada. A conditional logistic regression model was used with adjustment for age group (18–29, 30–39, 40–49, 50–59, 60+ years), sex (male or female), and health authority of HCW’s residence (Fraser Health Authority, Interior Health Authority, Northern Health Authority, Vancouver Island Health Authority, and Vancouver Coastal Health Authority) using the matching epidemiological week of testing as strata. Analysis periods were modified for corresponding DSV to enable sufficient tests among vaccinated individuals to have accrued as follows: for 0–55 DSV, the analysis period spanned epi-weeks 9–39; for 56–83 DSV, the analysis period spanned epi-weeks 11–39; for 84–11 DSV, the analysis period spanned epi-weeks 15–39; for 112–195 DSV, the analysis period spanned epi-weeks 20–39; and for ≥196 DSV, the analysis period spanned epi-weeks 35–39. For VE analysis at 84–111 DSV, the ratio of matched controls to cases was reduced to 5:1 per epi-week of testing due to an insufficient number of controls in some weeks for 6:1 matching. Refer to Supplementary Table 2 for more details. Abbreviations: DSV, days since vaccination; HCW, healthcare worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness.
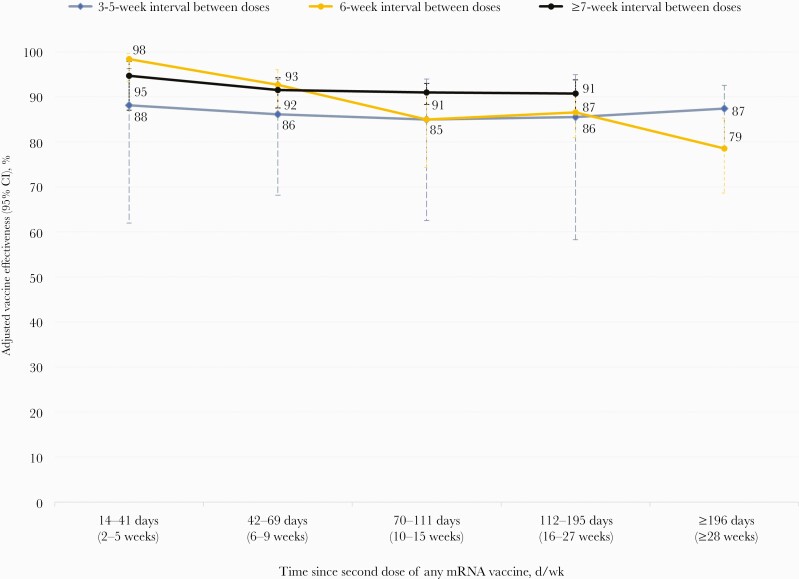
In a simultaneous assessment of VE by interval between doses and time since second dose, point estimates of VE were consistently 5%–7% (absolute) higher among those vaccinated with an interval ≥7 weeks (91%–95%) than among those vaccinated with an interval of 3–5 weeks (85%–88%) between doses, but also with overlapping confidence intervals (Figure 2; Supplementary Table 3). For neither interval did VE decline with time since vaccination out to at least 16 weeks. Among those twice vaccinated at a 6-week schedule, VE initially approximated that of the ≥7-week dosing interval (98%), but aligned more closely with the shorter 3–5-week interval by 10–15 weeks (85%) and 16–27 weeks (86%), declining at ≥28 weeks (79%) postvaccination, accompanied by wide and overlapping confidence intervals.
Figure 2.
Adjusted two-dose mRNA vaccine effectiveness against any SARS-CoV-2 infection, by interval between doses and days since vaccination, among healthcare workers in British Columbia, Canada. Displayed are adjusted estimates of vaccine effectiveness against any SARS-CoV-2 infection, with 95% CI, by interval between first and second doses and time since second dose of any mRNA vaccine (in days/weeks) among HCWs in British Columbia, Canada. A conditional logistic regression model was used with adjustment for age group (18–29, 30–39, 40–49, 50–59, 60+ years), sex (male or female), and health authority of HCW’s residence (Fraser Health Authority, Interior Health Authority, Northern Health Authority, Vancouver Island Health Authority, and Vancouver Coastal Health Authority) using the matching epi-week of testing as strata. Refer to Supplementary Table 3 for more details. Abbreviations: HCW, health care worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Compared with our overall adjusted two-dose mRNA VE of 90% (95% CI, 88%–92%), VE with restriction to HCWs in residential facilities was slightly lower, at 82% (95% CI, 76%–87%). Of these HCWs in residential facilities; however, median follow-up was longer (126 days), with 24% revaccinated at a 3–5-week interval and 75% at a 3–6-week interval following the first dose, representing more at shorter intervals between doses than in our primary analysis (12% and 43%, respectively).
DISCUSSION
In this observational study, 1 dose of mRNA vaccine provided substantial protection, and 2 doses were associated with strong and sustained reduction of SARS-CoV-2 infection risk among HCWs in BC, Canada. Overall, mRNA VE against any SARS-CoV-2 infection was about 70% for a single dose across a median follow-up (IQR) of 7 (~5–10) weeks and 90% for 2 doses across a median follow-up (IQR) of ~13 (~9–18) weeks. VE estimates were similar in sensitivity and subgroup analyses and were slightly higher for mRNA-1273 than BNT162b2 for one-dose (77% vs 70%) and two-dose (93% vs 89%) recipients, but with overlapping confidence intervals. Two-dose VE of ~≥90% against infection was maintained to at least 16 weeks and was ≥80% to ≥28 weeks. VE trended higher among HCWs twice vaccinated at a longer interval of ≥7 weeks vs 3–5 weeks after the first dose, consistent with earlier population-based findings [7]. Importantly, strong protection was sustained across follow-up spanning a mix of circulating VOCs, including earlier Alpha and Gamma, and later Delta, dominance.
Recognizing underlying variability in study conditions when comparing overall estimates, our findings, predicated on generally longer dosing intervals and follow-up periods, nevertheless align well with estimates elsewhere, particularly with studies using similar methods. For instance, in a TND study with epi-week matching from Quebec, Canada, where an extended interval between doses was also applied, VE against SARS-CoV-2 infection at ≥14 days after 1 dose was 70% (95% CI, 68%–73%; 8-week median follow-up), and at ≥7 days after 2 doses it was 86% (95% CI, 81%–89%; shorter 18-day median follow-up) [14]. Similarly, using the TND with matching, a US HCW study reported single-dose mRNA VE (≥14 days postvaccination) of 80% (95% CI, 74%–84%) with second doses administered according to the manufacturer-specified interval (median, 21 days apart); second-dose VE at ≥7 days was 90% (95% CI, 87%–93%) with shorter 41-day median case follow-up, but this was also sustained at >80% through 14 weeks postvaccination [15]. Our findings further align with prospective cohort studies among HCWs from the United States and the United Kingdom [16, 17]. In prospective evaluation against any SARS-CoV-2 infection in the United States [16], mRNA VE at ≥14 days after either dose was 81% (95% CI, 64%–90%) for one-dose recipients and 91% (95% CI, 76%–97%) for two-dose recipients, the latter across a median (IQR) follow-up of 69 (53–81) days. In another prospective study in the United Kingdom [17], VE ≥21 days after 1 BNT162b2 dose was 70% (95% CI, 55%–85%; median, 21-day follow-up) and 85% (95% CI, 74%–96%) at ≥7 days after a second dose (median follow-up, 39 days). Other studies among HCWs predicated upon retrospective cohort design [12, 18, 19] have shown more variability. For example, in an earlier cohort analysis among HCWs in Vancouver, BC, that also used the WHITE database (applying continuous calendar time adjustment rather than categorical or matched analysis), VE at ≥14 days after a single dose of mRNA vaccine was as low as 37% (95% CI, 17%–53%) [12], whereas VE at 14–34 days after 1 BNT162b2 dose ranged as high as 98% (95% CI, 95%–99%) in Italy [18]. Similarly, two-dose VE has ranged in retrospective cohort design from 79% (95% CI, 65%–88%) at ≥7 days in Vancouver, BC [12], to 97% (95% CI, 95%–98%) at >14 days for BNT162b2 [19] and 99% (95% CI, 90%–100%) at >14 days for mRNA-1273 in the United States [19]. Retrospective cohort analyses may be subject to the precarious assumption that absence of a test result equates with absence of infection. Such an assumption may be variably true depending upon access and completeness of testing by jurisdiction but overall introduces greater risk of selection bias compared with more systematic or standardized likelihood of testing among prospective cohort or TND approaches. Other differences in prevailing conditions and methods or context should also be taken into account when comparing VE estimates across studies.
In addition to our generally longer follow-up periods, second-dose scheduling recommendations in Canada facilitated assessment of VE at intervals longer than specified by manufacturers. The findings from this study reassuringly add to other evidence [5–7] in reinforcing policy recommendations to extend the interval between mRNA doses to ≥8 weeks by the NACI in Canada [2] and subsequently by the Centers for Disease Control and Prevention in the United States [20]. We observed higher VE at a longer interval of ≥7 weeks between doses, also consistent with accumulating epidemiological [7, 21] and immunological evidence [5, 6, 22]. Protection was more variable over time among HCWs revaccinated at 6-week vs 3–5-week or ≥7-week intervals. The pattern of decline in two-dose VE among those revaccinated at 6-week but not shorter or longer dosing intervals may reflect differential exposure risk and/or testing behaviors among HCWs prioritized for earlier vaccination and then revaccinated 6 weeks later in January/February 2021 compared with most who received their first and second doses later in the year and with a longer interval between. We also acknowledge our smaller sample size with greater stratification, particularly among those revaccinated at the shortest 3–5-week interval between doses, with broadly overlapping confidence intervals.
With calendar time being among the most important confounders in observational VE studies, an important strength of this study is the matching of cases and controls on the epidemiological week of testing in addition to covariate adjustment for age, sex, and geographic region. However, this study also has limitations, mainly related to its observational design and use of surveillance-based data subject to misclassified, missing, or incomplete information. Vaccine status based on PIR record and case ascertainment based on specific nucleic acid detection mitigated vaccine or outcome misclassification. We were unable to adjust for comorbidities or other potential confounders (such as race/ethnicity) due to data limitations, and our findings were based on any SARS-CoV-2 infection without regard to symptoms or severity. Given the young profile of our study population, comorbidities and severe outcomes are less contributory, but our outcome of any infection may have underestimated VE compared with clinical trials based on symptomatic infection [8, 23, 24]. We attempted to standardize for the testing indication by excluding HCWs screened for workplace exposure or employed by residential care facilities, where both vaccination and test positivity rates were more likely to differ. However, these efforts may have been incomplete and would further limit the generalizability of our findings. Provincial policy did not otherwise include routine screening of HCWs in acute or community care settings (or based on vaccine status), but we cannot rule out such testing. Although our study population was restricted to HCWs and we further stratified based on broad worker categories, differential exposure risk may still exist between vaccinated and unvaccinated workers. The analysis of longer time since vaccination (≥28 weeks) was confounded by VOC circulation with Delta dominance during that period (August 29–October 2); however, in population-based analyses, two-dose VE against Delta did not meaningfully differ from Alpha among adults in BC or Quebec, Canada [7]. We cannot reliably comment upon the extent to which our observations may extend to other variants that have further evolved genetically and antigenically away from the vaccine strain, such as the more recently emergent Omicron VOC. Regardless, understanding the potential duration of two-dose protection under conditions of relatively greater or lesser genetic match between the vaccine and circulating strain may help inform the timing of booster doses if and when the vaccine antigen is updated.
In summary, we show substantial single-dose and strong and sustained two-dose mRNA VE against any infection among a large and diverse population of HCWs in acute and community care, spanning at least 7 months of follow-up. Our findings reassure against marked waning of protection against infection per se, even among HCWs and despite VOC circulation, notably Delta. Such protection may have been facilitated by the Canadian decision to extend the interval between first and second doses, reinforcing that option in other jurisdictions or for future cohorts in the context of vaccine supply constraints and/or where urgent top-up of already substantial 1-dose protection is less pressing. These findings among a potentially highly exposed population may inform booster dose discussions more generally, particularly in considering the health and equity implications for HCWs, their patients, and others globally.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Ms. Catherine Ogden and Mr. Jeffrey Phung for data access and assembly assistance and Dr. Solmaz Setayeshgar for programming assistance related to this work.
Financial support. Funding for this work was provided in part by the BCCDC Foundation for Public Health.
Potential conflicts of interest. M.K. received grants/contracts paid to his institution from Roche, Hologic, and Siemens, unrelated to this work. The other authors have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. S.E.A.: protocol development, data analysis, manuscript draft. D.M.S.: overall conceptualization and supervision. M.Z.: data management. S.K.: literature review. B.H.: data access. All authors: manuscript review and approval.
Data availability. The data are not publicly available.
References
- 1. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Advisory Committee on Immunization (NACI). Recommendations on the use of COVID-19 vaccines. Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html. Accessed 23 December 2021. [Google Scholar]
- 3. National Advisory Committee on Immunization (NACI). NACI rapid response: extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in the context of limited vaccine supply. 2021. Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/extended-dose-intervals-covid-19-vaccines-early-rollout-population-protection.html. Accessed 23 December 2021. [Google Scholar]
- 4. British Columbia Centre for Disease Control (BCCDC). Immunization manual. Part 4: Biological products. COVID-19 vaccines. Available at: http://www.bccdc.ca/health-professionals/clinical-resources/communicable-disease-control-manual/immunization/biological-products. Accessed 23 December 2021. [Google Scholar]
- 5. Grunau B, Asamoah-Boaheng M, Lavoie PM, et al. A higher antibody response is generated with a 6- to 7-week (vs standard) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dosing interval. Clin Infect Dis. 2021; ciab938. doi: 10.1093/cid/ciab938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall VG, Ferreira VH, Wood H, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol 2022; 23:380–5. [DOI] [PubMed] [Google Scholar]
- 7. Skowronski DM, Setayeshgar S, Febriani Y, et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada. Clin Infect Dis 2022:ciac290. doi: 10.1093/cid/ciac290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. British Columbia Centre for Disease Control (BCCDC). COVID-19 VoC report. Available at: http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data#variants. Accessed 23 December 2021. [Google Scholar]
- 10. Gilligan T, Alamgir H.. Bridging the knowledge gap: an innovative surveillance system to monitor the health of British Columbia’s healthcare workforce. Can J Public Health 2008; 99:478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. BC STATS. Population projections. 2021. Available at: https://www2.gov.bc.ca/gov/content/data/statistics/people-population-community/population/population-projections. Accessed 3 November 2021. [Google Scholar]
- 12. Yassi A, Grant JM, Lockhart K, et al. Infection control, occupational and public health measures including mRNA-based vaccination against SARS-CoV-2 infections to protect healthcare workers from variants of concern: a 14-month observational study using surveillance data. PLoS One 2021; 16:e0254920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. British Columbia Centre for Disease Control (BCCDC). British Columbia COVID-19 situation report. Available at: http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data. Accessed 23 December 2021. [Google Scholar]
- 14. Carazo S, Talbot D, Boulianne N, et al. . Single-dose mRNA vaccine effectiveness against SARS-CoV-2 in healthcare workers extending 16 weeks post-vaccination: a test-negative design from Quebec, Canada. Clin Infect Dis. 2021; ciab739. doi: 10.1093/cid/ciab739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pilishvili T, Gierke R, Fleming-Dutra KE, et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. N Engl J Med 2021; 385:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273. Vaccines 2021; 385:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021; 397:1725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bianchi FP, Tafuri S, Migliore G, et al. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection and symptomatic disease in five-month follow-up: a retrospective cohort study. Vaccines 2021; 9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swift MD, Breeher LE, Tande AJ, et al. Effectiveness of messenger RNA coronavirus disease 2019 (COVID-19) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a cohort of healthcare personnel. Clin Infect Dis 2021; 73:e1376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention (CDC). Interim clinical considerations for Use of COVID-19 vaccines currently approved or authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed 10 March 2022. [Google Scholar]
- 21. Amirthalingam G, Bernal JL, Andrews NJ, et al. Higher serological responses and increased vaccine effectiveness demonstrate the value of extended vaccine schedules in combatting COVID-19 in England. Nat Commun 2021; 12:7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tauzin A, Gong SY, Beaudoin-Bussières G, et al. . Strong humoral immune responses against SARS-CoV-2 spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022; 30:97–109.e5. doi: 10.1016/j.chom.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skowronski DM, De Serres G.. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Eng J Med 2021; 384:1576–8. [DOI] [PubMed] [Google Scholar]
- 24. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Eng J Med 2020; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.