Abstract
This secondary analysis of the phase 3 ENSEMBLE trial (NCT04505722) assessed the impact of preexisting humoral immunity to adenovirus 26 (Ad26) on the immunogenicity of Ad26.COV2.S-elicited severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibody levels in 380 participants in Brazil, South Africa, and the United States. Among those vaccinated in Brazil and South Africa, 31% and 66%, respectively, had prevaccination serum-neutralizing activity against Ad26, with little preexisting immunity detected in the United States. Vaccine recipients in each country had similar postvaccination spike (S) protein–binding antibody levels, indicating that baseline immunity to Ad26 has no clear impact on vaccine-induced immune responses.
Keywords: Ad26.COV2.S, COVID-19, preexisting immunity, SARS-CoV-2, vaccine
Adenovirus 26 (Ad26)–based vaccines have been evaluated in multiple clinical vaccine programs, including for coronavirus disease 2019 (COVID-19). This study assessed the potential impact of preexisting humoral immunity against Ad26 on humoral immunogenicity of the COVID-19 vaccine Ad26.COV2.S.
Ad26.COV2.S is a recombinant, replication-incompetent, human adenovirus type 26 (Ad26)–vector-based vaccine encoding a prefusion-stabilized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein, shown to be efficacious against coronavirus disease 2019 (COVID-19) [1].
The question has arisen whether preexisting immunity against Ad26, the virus from which the nonreplicating vector in Ad26.COV2.S is derived, may decrease the immunogenicity of its transgene-expressed immunogens [2]. Reduced immunogenicity of adenovirus type 5–vectored transgene-expressed immunogens has been reported in individuals who were adenovirus 5 seropositive at baseline [3]. The Ad26 vector from the Janssen Pharmaceuticals AdVac platform has been used to develop an approved vaccine against Ebola, a vaccine against COVID-19 disease approved or authorized for emergency use in >73 countries/regions/territories (as of 19 August 2021), and experimental vaccines against human immunodeficiency virus (HIV) and respiratory syncytial virus that are currently in clinical efficacy trials.
Initial findings from clinical trials of Ad26-based vaccines for Ebola, HIV, and COVID-19 do not indicate any impact of preexisting Ad26 neutralizing antibody on immune responses to the transgene-expressed immunogens in these vaccines [4–6]. Although the proportion of participants with baseline Ad26 seropositivity was small in some previous studies [4, 7], potentially limiting the conclusions about impact on vaccine response, recent Ebola vaccine trials in which the majority of participants had preexisting Ad26 antibodies showed no effect on vaccine-induced immune response [6, 8]. Similarly, there was no impact of Ad26-specific antibody titers on vaccine response in a study of the HIV-1 vaccine that included a high proportion of participants with Ad26-specific neutralizing antibodies at baseline [9].
Here, we report an analysis of preexisting anti-Ad26 immunity and postvaccination SARS-CoV-2 S protein–specific antibody titers elicited by the Ad26.COV2.S vaccine in participants from Brazil, South Africa, and the United States, representing a more geographically and racially diverse population than previously evaluated for Ad26.COV2.S [5].
METHODS
We conducted a secondary analysis of data from 380 randomly selected participants in the ongoing phase 3 ENSEMBLE trial (ClinicalTrials.gov NCT04505722) from Brazil, South Africa, and the United States [1, 10]. The trial began enrollment on 21 September 2020, and the data cutoff date for the present analysis was 22 January 2021. The trial was conducted in accordance with the Declaration of Helsinki and International Council on Harmonization’s guidelines on Good Clinical Practice; the protocol and amendments were approved by institutional review boards per local regulations. Written informed consent was obtained from all participants before trial enrollment.
SARS-CoV-2 S protein–specific antibodies at baseline (day 1) and 28 days after vaccination (day 29) were measured using an enzyme-linked immunosorbent assay (S-ELISA). Preexisting anti-vector immunity was assessed before vaccination (day 1) in a virus neutralization assay (VNA) for Ad26 [11].
RESULTS
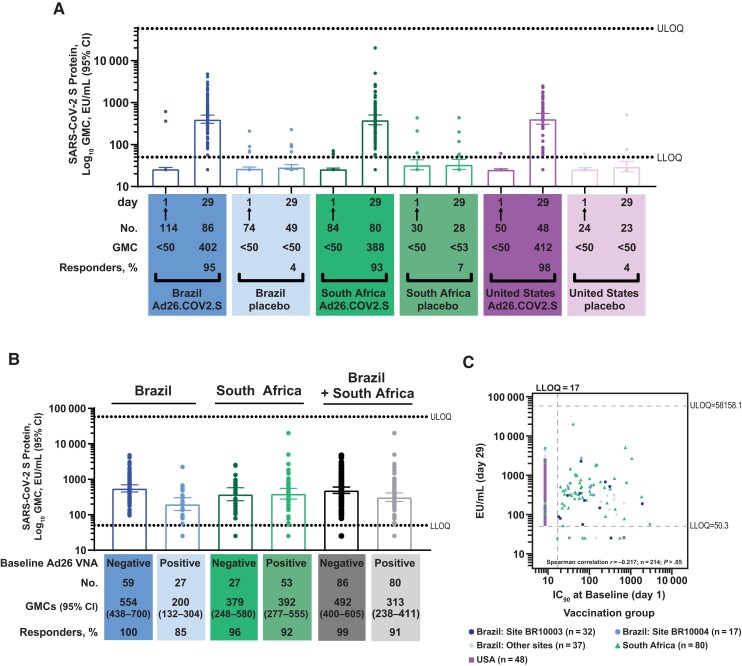
Among the vaccine groups for whom day 29 S-ELISA data were available, 27 of 86 prevaccination serum samples (31%) had neutralizing activity against Ad26 in Brazil, while in South Africa 53 of 80 serum samples tested (66%) had Ad26 neutralizing activity (Supplementary Table 1 and Supplementary Figure 1). Of the 48 participants tested from the United States, only 1 had detectable neutralizing antibody against Ad26 in the assay used. Within the vaccine groups, 2, 3, and 1 participant(s) in Brazil, South Africa, and the United States, respectively, had detectable S-ELISA titers at baseline. By day 29, the vaccine groups demonstrated a strong response to Ad26.COV2.S (Figure 1A). Geometric mean concentrations (GMCs) of S protein antibody increased to 402 (95% confidence interval [CI], 321–505), 388 (95% CI, 297–506), and 412 (95% CI, 306–554) ELISA units (EU) per milliliter (mL) in the Brazil, South Africa, and United States groups, respectively. These results included the few participants seropositive for S-binding antibodies at baseline. The corresponding S antibody seroconversion rates (Supplementary Methods) were 95%, 93%, and 98%.
Figure 1.
A, Country-specific humoral immunogenicity was measured using enzyme-linked immunosorbent assay (ELISA) to assess severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (S)–specific antibodies (S-ELISA) at baseline (day 1) and 28 days after vaccination with Ad26.COV2.S at a dose level of 5 × 1010 viral particles. Response is described using geometric mean concentrations (GMCs) of SARS-CoV-2 S protein–binding antibodies (in ELISA units [EU] per milliliter) and percentages of participants with a response in Brazil, South Africa, and the United States. Arrows indicate the day of vaccination (Ad26.COV2.S recipients) or placebo injection. B, Response by preexisting anti–adenovirus 26 (Ad26) immunity at day 29 is shown stratified by preexisting anti-Ad26 immunity in Brazil, South Africa, and Brazil and South Africa combined; minimal preexisting immunity was observed in the United States. C, Correlation between preexisting anti-Ad26 antibody titers and postvaccination S-ELISA response was low to negligible (P > .05). Abbreviations: CI, confidence interval; IC50, half-maximal inhibitory concentration; LLOQ, lower limit of quantitation; ULOQ, upper limit of quantitation; VNA, virus neutralization assay.
Seroconversion rates for S antibodies were generally similar when stratified by preexisting anti-Ad26 immunity in South Africa (96% vs 92%, respectively, for baseline-negative vs baseline-positive anti-Ad26 VNA), and tended to be somewhat lower in baseline-positive participants in Brazil (100% vs 85%) (Figure 1B). Only 1 participant in the United States had detectable Ad26 VNA titers at baseline, thus precluding any conclusions for this region. The day 29 S antibody levels for South African participants with preexisting anti-Ad26 immunity were higher than those of the Brazilian participants (GMC, 392 vs 200 EU/mL, respectively) despite 2-fold higher Ad26 VNA titers at baseline in South African participants versus Brazilian participants (geometric mean titer, 42 vs 20, respectively; Supplementary Table 1 and Supplementary Figure 1). Although anti-S immunity appeared to be lower in participants with preexisting anti-Ad26 immunity in Brazil than in those who were Ad26 negative at baseline (with no overlap of the 95% CIs), slightly higher titers were observed in participants with preexisting anti-Ad26 immunity in South Africa than in those who were Ad26 negative at baseline (Figure 1B).
Across all participants for whom immunogenicity data were available in the 3 countries evaluated, the correlation between preexisting Ad26 VNA titers and postvaccination S-ELISA titers was low to negligible (Figure 1C).
DISCUSSION
These findings, together with data from previous trials, show that prevaccination baseline immunity to Ad26 has no clear impact on humoral immune responses against immunogens expressed from transgenes delivered by Ad26-based vaccines [1, 5]. Robust vaccine efficacy against severe–critical COVID-19 demonstrated in ENSEMBLE was similar across countries where preexisting immunity against the Ad26 vector was observed, especially in South Africa and Brazil, compared with the United States, where very little preexisting anti-Ad26 immunity was detected [1, 10]. Inconsistent trends in anti-S immunity among participants with preexisting anti-Ad26 immunity in South Africa versus Brazil may be explained by the small sample size of these country-specific analyses. Efficacy against symptomatic infection was highest in the United States in the ENSEMBLE study [1, 10], which could be due to differences in the circulating variants at the time of the study or potential differences in preexisting anti-Ad26 immunity in different countries; this question will be evaluated in a correlate of protection analysis. S-ELISA GMCs and responder rates in ENSEMBLE were comparable to those in the previous phase 1/2a study of Ad26.COV2.S [5].
A limitation of the current study was that we did not evaluate the impact of preexisting anti-Ad26 immunity on cellular immune responses, which may be an important contributor to protection mediated by Ad26.COV2.S. The study results suggest that neutralizing antibodies against Ad26 induced by immunization with Ad26-vectored vaccines will have little effect on subsequent doses of the same vaccine, consistent with the previously reported 3–4-fold increased immune responses after a second dose given 2 months after the first dose [5] and a 9-fold increase in S-binding antibodies after a booster dose given 6 months after a single dose [12].
Supplementary Material
Contributor Information
Mathieu Le Gars, Janssen Vaccines and Prevention, Leiden, the Netherlands.
Jerald Sadoff, Janssen Vaccines and Prevention, Leiden, the Netherlands.
Frank Struyf, Janssen Research and Development, Beerse, Belgium.
Dirk Heerwegh, Janssen Research and Development, Beerse, Belgium.
Carla Truyers, Janssen Research and Development, Beerse, Belgium.
Jenny Hendriks, Janssen Vaccines and Prevention, Leiden, the Netherlands.
Glenda Gray, South African Research Council, Cape Town, South Africa.
Beatriz Grinsztejn, Evandro Chagas National Institute of Infectious Diseases–Fiocruz, Rio de Janeiro, Brazil.
Paul A Goepfert, Department of Medicine, University of Alabama, Birmingham, Alabama, USA.
Hanneke Schuitemaker, Janssen Vaccines and Prevention, Leiden, the Netherlands.
Macaya Douoguih, Janssen Vaccines and Prevention, Leiden, the Netherlands.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank trial participants, staff members, and investigators at the study sites, the COV3001 study team and the Nexelis team, including Helen Diamantakis, Steven Phay Tran, and Hossein Koohsari, for providing laboratory testing services (severe acute respiratory syndrome coronavirus 2 spike protein–specific enzyme-linked immunosorbent assay). Medical writing and editorial assistance were provided by Kurt Kunz, MD, MPH, and Jill E. Kolesar, PhD, of Cello Health Communications/MedErgy, and were funded by Janssen Global Services, LLC.
Financial support. This work was supported by Janssen Research and Development, an affiliate of Janssen Vaccines and Prevention and part of the Janssen pharmaceutical companies of Johnson & Johnson, and in whole or in part by federal funds from the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services (under other transaction agreement HHSO100201700018C), and from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The National Institute of Allergy and Infectious Diseases provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI68614), the HVTN Statistics and Data Management Center (UM1 AI68635), the HVTN Laboratory Center (UM1 AI68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI68636), the Infectious Diseases Clinical Research Consortium Leadership Group (UM1 AI148684), and Vaccine and Therapeutic Evaluation Units (UM1 AI148576, UM1 AI148373, UM1 AI148685, and UM1 AI148452). Funding to pay the Open Access publication charges for this article was provided by Janssen Global Services, LLC.
References
- 1. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Custers J, Kim D, Leyssen M, et al. Vaccines based on replication incompetent Ad26 viral vectors: standardized template with key considerations for a risk/benefit assessment. Vaccine 2021; 39:3081–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020; 396:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, Stieh DJ, Sarnecki M, et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV 2020; 7:e688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med 2021; 384:1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barry H, Mutua G, Kibuuka H, et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: a randomised, placebo-controlled phase II clinical trial in Africa. PLoS Med 2021; 18:e1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salisch NC, Stephenson KE, Williams K, et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti-zika virus vaccine. Ann Intern Med 2021; 174:585–94. [DOI] [PubMed] [Google Scholar]
- 8. Anywaine Z, Barry H, Anzala O, et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: a randomised, placebo-controlled, multicentre phase II clinical trial. PLoS Med 2022; 19:e1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018; 392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sadoff J, Gray G, Vandebosch A, et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med 2022; 386:847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol 2003; 41:5046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadoff J, Le Gars M, Cardenas V, et al. Durability of antibody responses elicited by a single dose of Ad26.COV2.S and substantial increase following late boosting. medRxiv [Preprint: not peer reviewed]. 26 August 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.08.25.21262569v1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.