Abstract
Background
Magnesium ion is one of the essential mineral elements for plant growth and development, which participates in a variety of physiological and biochemical processes. Since there is no report on the research of magnesium ion transporter in grape, the study of the structure and function of magnesium ion transporters (MGT) is helpful to understand the dynamic balance mechanism of intracellular magnesium ions and their inter- or intra-cellular activities.
Result
In this study, we identified the members of MGT protein family in grape and performed the phylogenetic and expression analysis. We have identified nine VvMGT genes in grape genome, which are distributed on eight different chromosomes. Phylogenetic analysis showed that MGT family members of grapes were divided into five subfamilies and had obvious homology with Arabidopsis, maize, and pear. Based on transcriptome data from the web databases, we analyzed the expression patterns of VvMGTs at different development stages and in response to abiotic stresses including waterlogging, drought, salinity, and copper. Using qRT-PCR method, we tested the expression of grape VvMGTs under magnesium and aluminum treatments and found significant changes in VvMGTs expression. In addition, four of the MGT proteins in grape were located in the nucleus.
Conclusion
Overall, in this study we investigated the structural characteristics, evolution pattern, and expression analysis of VvMGTs in depth, which laid the foundation for further revealing the function of VvMGT genes in grape.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03599-5.
Keywords: MGTs, Grapes, Phylogenetic analysis, Gene expression
Background
Magnesium ion is the most abundant divalent cation in plant cells, which plays an important role in plant growth and development [1, 2]. As a coenzyme of ATP, a magnesium ion is the activator of many enzymes, especially phosphorylases and kinases [3, 4]. Magnesium ions can stabilize chromatin and DNA structure and regulate the synthesis of ribosomes. Furthermore, it is an indispensable element in the process of DNA replication, RNA transcription, and translation [5]. Magnesium ion participates in the establishment of transmembrane electron gradient [6], maintains intracellular osmotic pressure, and regulates the activity of various intracellular enzymes [7]. As an important component of chlorophyll, magnesium ion participates in plant photosynthesis [8, 9]. Recent studies demonstrated that magnesium ions also play an important role in reducing the toxicity of aluminum salt in plants [10–12]. Therefore, the transport of magnesium ions in the plasma membrane can activate the different mechanisms inside and outside of the cell.
In total, five families of magnesium ion transporters discovered in bacteria, fungi, animals, and higher plants, which included CorA class, Mg2+ / H+ exchanger, ion channel, P-type phosphatase, and MgtE gene family [13–17]. Among them, first, the CorA protein family was determined as magnesium ion transporter and also deeply studied [4]. There are two hydrophobic transmembrane regions in the C-terminal of these proteins, which are important regions for the transmembrane transport of magnesium ions [18]. There is a completely conserved GMN (Gly-Met-Asn) tripeptide motif near the end of the first transmembrane region [4]. Mutation analysis showed that CorA protein lost the function of magnesium ion transport when an amino acid mutation occurred on GMN motif [19].
In plants, the MGT protein family have been identified in Arabidopsis, Rice, Maize, Dendrobium officinale, Brazilian rubber, and Pear [13, 14, 16, 17, 19, 20]. Ten AtMGTs genes were identified in Arabidopsis [16] and were divided into five subfamilies according to the gene structure and evolutionary relationship. Meanwhile, MGT protein family are homologous to CorA proteins [21]. The protein encoded by AtMGTs gene was located on the cell membrane, vacuole membrane, chloroplast membrane, and mitochondrial membrane [15, 16]. AtMGT1 is involved in the absorption of magnesium ions by roots [16]; although, AtMGT4, AtMGT5, and AtMGT9 play a role in pollen development in Arabidopsis [22, 23]. Furthermore, AtMGT6 and AtMGT7 are involved in resistance to low magnesium stress in Arabidopsis [23, 24]; while AtMGT2 and AtMGT3 can be involved in the regulation of magnesium ion in mesophyll cells [25–28]. AtMGT10 plays an important role in the magnesium ion transport system of chloroplast [15]. In rice, OsMGT1 was identified as a magnesium transporter gene, which participates in the absorption of magnesium ions by roots under aluminum stress, thereby enhancing the resistance to aluminum stress [13]. Subsequently, Saito et al. analyzed the expression and function of all members of the gene family and identified nine OsMGTs genes in rice [19]. Li et al. also identified twelve ZmMGTs in maize genome, but only 5 members had the ability to transport magnesium ions [29]. It was proved that ZmMGT12 with a circadian rhythm pattern was able to transport magnesium iron and its expression can be induced by light [30]. And ZmMGT10 was specifically expressed in maize roots [31]. Zhang et al. identified DoMGT1 in Dendrobium officinale [32], which had tissue-specific expression and only expressed in root, stem, and leaf. Yang et al. cloned the HbMGT10 gene in Hevea brasiliensis and found that it functions magnesium ion transport [33]. It is highly expressed in leaves and participates in the transport of magnesium ions across the chloroplast membrane of rubber leaves. Zhao et al. identified sixteen PbrMGTs in Pear and verified the function of PbrMGT7, which PbrMGT7 is involved in the regulation of magnesium ion transport between cytoplasm and mitochondria [34, 35]. And it also participates in the regulation of dynamic balance of magnesium ion in pollen tube growth [35, 36]. MGT genes were also identified in Solanum lycopersicum and Brassica napus [17, 20]. With the publication of genome-wide sequencing data of more plants, more and more MGT gene families will be identified and discovered.
Magnesium ion transporters play an important role in the absorption, transport, and maintenance of magnesium homeostasis [2, 8]. Studies on Arabidopsis, rice, maize, and other plants have shown that members of the MGTs family are involved in regulating various activities of biological processes. However, no related research on MGT genes has been seen in grapes. In recent years, the phenomenon of magnesium deficiency in grapes in southern China’s vineyards has become more and more serious, and the lack of magnesium nutrition in grapes led to poor growth and development of grapes and reduced fruit quality [37]. In order to solve the above problems, it is necessary to conduct a bioinformatics analysis of the grape magnesium ion transporter gene family and understand the mechanism of magnesium ion transport and distribution.
In this study, we used bioinformatics methods to identify the MGT gene family members and analyze its development process based on the grape genome data. Furthermore, the gene structure, promoter sequence, and physicochemical properties of the protein were also analyzed. In addition, we also studied the expression of VvMGTs in different tissues and organs in response to abiotic stresses. These comprehensive results help us to further understand the potential role of Mg2+ transporters in grape and solve the problems related to magnesium nutrition in grape cultivation and production.
Result
Identification of MGTs in grape
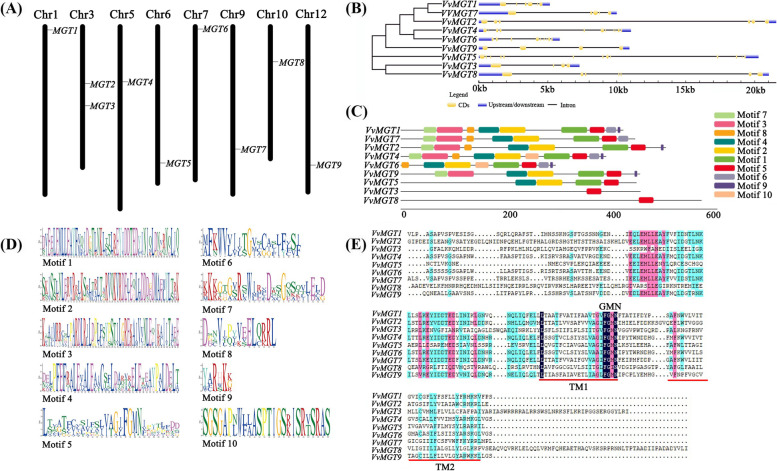
We searched and downloaded the Hidden Markov model file of MGT gene family through Pfam protein family database (Pfam number: PF01544). Based on the V2.1 Grape Genome Database, nine genes of magnesium ion transporter were identified and located on the eight chromosomes (Chr1, 3, 5, 6, 7, 9, 10, and 12) (Table 1). VvMGT1 and VvMGT6 were located near the top of different chromosomes and VvMGT4 and VvMGT8 were distributed in the upper part of chromosome. Meanwhile VvMGT2 and VvMGT3 were located in the middle of chromosome 3 but VvMGT5, VvMGT7 and VvMGT9 were located in the lower part of different chromosomes (Fig. 1A). The length of VvMGT1, VvMGT3, and VvMGT6 was 5153 to 7300 bp, while the other genes were longer, ranging from 10,002 to 21,561 bp. However, there was no significant difference in the length of translation coding region, the length of CDS was between 882 to 1713 bp. Molecular analysis of the full-length deduced polypeptides indicated that the putative proteins of these VvMGT genes contain 293 to 570 amino acids (predicted 32.01 to 64.65 kDa in molecular weight) with their pI calculated ranging from 4.57 to 8.92. According to web-based prediction of VvMGTs location in cells, different members of the MGT gene family were found in the cell membrane, cytoplasm, nucleus, endoplasmic reticulum, chloroplast, mitochondria, golgi, vacuole, and catalase.
Table 1.
Characteristics of MGT family members in grape
| Gene name | Gene ID | Gene size (bp) | CDS (bp) | Number of amino acids(aa) | MW (kDa) | pI | Protein formula | Chr location | Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
| VvMGT1 | VIT_201s0011g00450 | 5153 | 1266 | 421 | 47,449.35 | 5.42 | C2115H3341N567O633S19 | Chr1:453195–458,647 | Nucleus |
| VvMGT2 | VIT_203s0091g01190 | 21,561 | 1509 | 502 | 55,485.66 | 4.95 | C2426H3869N683O767S19 | Chr3:7831679–7,853,239 | Chloroplast, Nucleus |
| VvMGT3 | VIT_203s0097g00520 | 7300 | 1365 | 454 | 51,460.32 | 8.92 | C2308H3651N653O650S16 | Chr3:10861262–10,868,561 | Chloroplast |
| VvMGT4 | VIT_205s0020g04720 | 10,359 | 1170 | 389 | 43,470.61 | 4.97 | C1921H3067N525O590S16 | Chr5:6575685–6,586,713 | Chloroplast |
| VvMGT5 | VIT_206s0061g01060 | 20,176 | 1341 | 446 | 49,799.66 | 6.18 | C2201H3574N610O655S23 | Chr6:18720855–18,741,404 | Nucles |
| VvMGT6 | VIT_207s0141g00590 | 5868 | 882 | 293 | 32,901.06 | 4.57 | C1441H2292N392O465S11 | Chr7:335891–341,775 | Nucles |
| VvMGT7 | VIT_209s0018g00600 | 10,002 | 1335 | 444 | 50,492.17 | 5.03 | C2251H3590N604O669S21 | Chr9:16816304–16,826,350 | Nucles |
| VvMGT8 | VIT_210s0003g02750 | 21,027 | 1713 | 570 | 64,365.38 | 6.59 | C2858H4528N826O837S16 | Chr10:4824556–4,845,582 | Chloroplast |
| VvMGT9 | VIT_212s0034g02550 | 10,920 | 1362 | 453 | 50,457.91 | 5.47 | C2238H3584N620O669S18 | Chr12:19013210–19,024,129 | Nucleus |
Fig. 1.
Chromosome location, phylogenetic tree, structural gene features and conserved protein motif of MGT genes in grape. A Chromosomal location of nine VvMGT genes in grape. B Structure and phylogenetic analysis of VvMGT genes. The blue boxes, black lines and yellow boxes in the gene structure diagram represent untranslated regions(UTRs), introns and coding sequence (CDS), respectively. Gene models are drawn to scale as indicated on the x-axis. C Conserved motif in MGT proteins. Ten motifs (motif1 to motif 10) were identified with MEME tool and representation of each motif was illustrated with a different color. The lengths and positions of the colored blocks correspond to the lengths and positions of the motifs in the individual protein sequence, respectively. D MEME-identified sequence motifs present in the protein sequence of VvMGT genes. E Multiple sequence alignment of VvMGT genes showing their conserved regions and GMN motif, similar amino acids are highlighted in same color, respectively. Predicted C-terminal transmembrane domains (TM1 and TM2) and the conserved GMN motif are indicated
Structural cluster analysis of VvMGTs genes
The clustering of gene families and the analysis of gene structure can provide important clues for revealing the evolution of gene families. We used MEGA7.0 software to analyze nine VvMGTs genes (Fig. 1B), which is divided into five categories (Class I, II, III, IV, and V). We found that VvMGT1, VvMGT7, and VvMGT2 belonged to class I, VvMGT4 and VvMGT6 belonged to class II, VvMGT3 and VvMGT8 belonged to class V, and finally VvMGT5 and VvMGT9 belonged to classes III and IV respectively. Using the full-length gene sequences and CDS sequences of all genes, the VvMGT genes structure was analyzed on the GSDS. The results showed that all gene sequences of the MGT gene family have untranslated regions at both 3’ends and 5’end. The number of exons is between four to thirteen, the length is relatively short, and the intron sequence is long. Combined with gene cluster analysis, it found that the number of exons and introns in the same category are relatively close.
Analysis of conservative domains
The conserved domain of VvMGTs genes was identified by MEME. The specific information is shown in Fig. 1C, D. We found that the types of clustered motifs among a group of genes were basically similar, and the arrangement of motifs was also very similar. For example, the number of motifs in VvMGT1, VvMGT2, and VvMGT7 was ranged from eight to nine. VvMGT3 and VvMGT8 have only one motif5. The most frequently occurring of all members is motif5 and amino acid sequence contains Gly-Met-Asn, which is a conserved motif of the transmembrane region of the MGT family. Through DNAMAN comparison analysis, it was found that the grape MGT gene family contains two typical transmembrane regions (Fig. 1E). Among them, the GMN motif in the VvMGT8 sequence was mutated, and methionine replaced with isoleucine, forming a variant GIN motif.
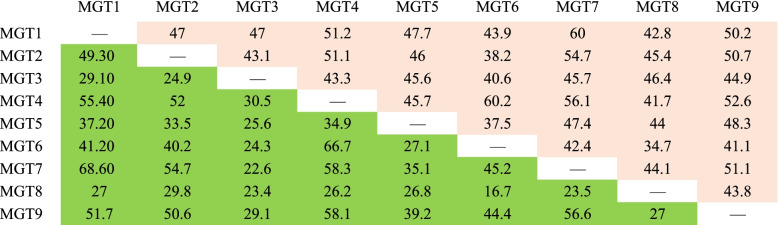
In order to investigate the sequence similarity between VvMGTs genes, we used the EMBOSS website to compare the nucleotide and amino acid sequences of grape MGT family members (Table 2). Sequence comparison revealed that the nucleotide sequence homology of these genes in the coding region is 34.7 to 60.2%, while the amino acid sequence homology is 16.7 to 68.60%, and there are only nine gene pairs above 50%. Although the sequence homology of the MGT gene family is not high, all members have GMN marker sequences.
Table 2.
Coding reging nucleotide and amino acid sequence pairwise comparisons(% similarty) between grape MGT gene family
Protein structure analysis of MGT gene family members
The secondary structure of a protein mainly refers to how the protein itself folds and coils. We found (Table 3) that there are mainly four elements in the secondary structure of the protein in grape MGT gene family including α-helix, β-turn, extended chain, and random coil. Except for VvMGT2, α-helix has the highest proportion among other family members, followed by random coils and extended chains, and the least proportion is β-turn. The proportion of α-helix in each member is about 50%, of which the highest is 59.39% and the lowest is 42.03%. Meanwhile, the proportion of random coils is about 35%, of which VvMGT2 has the highest proportion of irregular curling, up to 45.22%. Furthermore, the proportion of extension chains in each member is about 10%, of which the highest accounts for 15.00% and the lowest reaches 8.33%. The β-turn with the smallest proportion among the 4 elements accounts for 1.90–5.29% of each member.
Table 3.
Secondary structure of nine VvMGT proteins
| H(%) | T(%) | E(%) | RC(%) | |
|---|---|---|---|---|
| VvMGT1 | 54.16 | 1.90 | 9.50 | 34.44 |
| VvMGT2 | 42.03 | 3.59 | 9.16 | 45.22 |
| VvMGT3 | 46.92 | 5.29 | 12.56 | 35.24 |
| VvMGT4 | 54.24 | 3.08 | 9.77 | 32.90 |
| VvMGT5 | 51.79 | 3.14 | 9.64 | 35.43 |
| VvMGT6 | 59.39 | 2.39 | 6.83 | 31.40 |
| VvMGT7 | 51.13 | 3.15 | 8.33 | 37.39 |
| VvMGT8 | 45.44 | 3.86 | 15.00 | 35.61 |
| VvMGT9 | 50.77 | 2.21 | 10.38 | 36.64 |
H Alpha helix (α-helix), T Beta turn (β-turn), E Extended strand, R Random coil
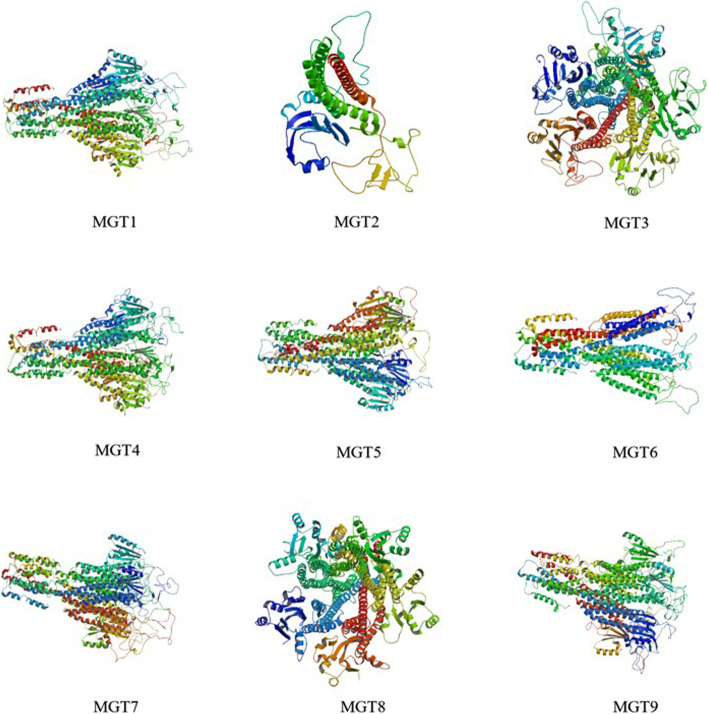
As shown in Fig. 2, the protein tertiary structure related VvMGTs was constructed by Swiss-Model homology modeling. It can be seen that the protein structures of the same group of members are similar to each other. For example, VvMGT3 and VvMGT8 belong to the same group with a similar structure. Besides, the number and types of secondary elements between members of VvMGTs including VvMGT1, VvMGT4, VvMGT5, VvMGT6, VvMGT7, and VvMGT9 are not much different and made a similarity in their tertiary structures. Noticeably, VvMGT2, its protein tertiary structure is different from other family members, and there may be differences in function with other members.
Fig. 2.
Predicted tertiary structure of nine MGT protein in grape. The protein structures all have the same domain color schemes. Structures reveal a high degree of structural homology in most gene members
Analysis of cis-elements on promoter sequences of VvMGTs
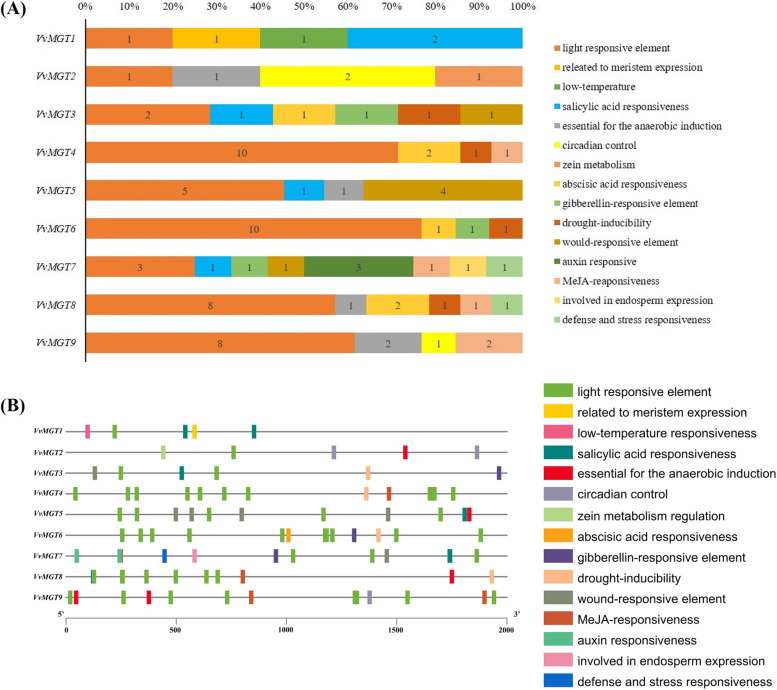
In order to understand the gene function and transcriptional regulation mechanism of VvMGTs, we obtained 2000 bp upstream of the start site of each gene from the Grape Genome Database and analyzed the cis-elements in the promoter regions. In addition to the core components CAAT-box and TATA-box, we found a total of fifteen cis- regulatory elements that respond to hormones, light, circadian, biotic and abiotic stresses. Among them, the promoter region of each gene contains a large number of light-responsive elements, accounting for more than 20%. The types of cis-elements in promoter region of VvMGT are different (Fig. 3A). For example, VvMGT7 has the most cis-element types in promoter region. VvMGT3 and VvMGT8 contain six different kinds of cis-elements; while VvMGT1, VvMGT2, VvMGT4, VvMGT5, VvMGT6, and VvMGT9 also have four different kinds of cis-elements. In addition, the promoter sequence of each gene has a different number of each type of cis-element.
Fig. 3.
Comparism of VvMGT genes promoter sequence. A Number and percentage of cis-elements occurrence in 9 VvMGTs of upstream sequence in grape. The number in bars indicates the number of cis-element. The x-axis of the upper histogram indicates the percentage frequency of each cis-element. B Distribution of cis-elements on the promoter of VvMGTs. Different color bars represent different cis-elements. The x-axis indicates the length of promoter sequence
We also used TBtools software to make the distribution map of promoter sequence according to the number and positions of cis-elements (Fig. 3B). The total number of cis-elements in VvMGTs promoter region was ranged from five to fourteen. The promoter regions of VvMGT4 and VvMGT8 have a high content of cis-elements, each containing 14 cis-elements, while VvMGT1 and VvMGT2 contain only five cis-elements. In addition, the promoter distribution map shows that almost all the cis-elements are distributed throughout the promoter sequence. However, the cis-elements of VvMGT1 are near to start position of promoter sequences.
Phylogenetic analysis of MGT protein
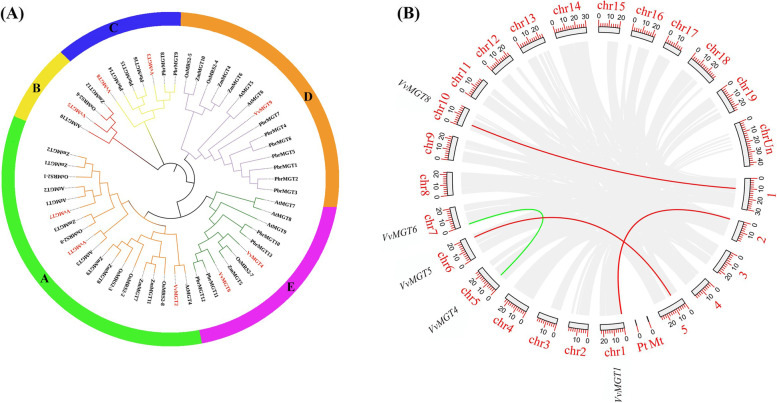
To explore and compare the evolutionary relationship of MGTs in grape with other plants, we constructed a phylogenetic tree based on the maximum likelihood method with fifty-six protein sequences for Arabidopsis (10), Rice (9), Maize (12), Pear (16), and Grape (9) (Fig. 4A). Our results indicated five subfamilies with different members including A (19), B (4), C (7), D (15), and E (11). In subfamily A and B, the members of MGT genes mainly originated from Arabidopsis, Maize, Rice and Grape. In subfamily C, the members mainly belonged to Pear and Grape, while subfamily D and E contain MGT genes from five different species. In order to further understand the evolutionary behavior of grape MGT gene family between species and within species, we analyze gene collinearity in grape and Arabidopsis to figure out the expansion process of MGT family members (See Fig. 4B). Some genes in the Arabidopsis genome have a collinear relationship with the grape MGT genes, such as VvMGT1, VvMGT5, and VvMGT8. In addition, we found that there is no tandem replication event in the grape MGT family, only one fragment repeat, VvMGT4 and VvMGT6.
Fig. 4.
Evolutionary analysis of VvMGTs. A Phylogenetic tree constructed using 56 full-length MGT proteins from Arabidopsis, rice, maize, pear and grape. The phylogenetic tree was constructed using the ML method in MEGA7.0 software with 1000 bootstrap replications. All the MGT proteins are divided into five group (A, B, C, D, E). Branch of different colors to clarify subfamily identification easier. B Segmental duplication of grape MGT genes and syntenic analysis of grape and Arabidopsis MGT genes. The segmental duplicated gene pairs are connected by red lines between Arabidopsis and grape, and green line connected segmental duplicated gene pairs in grape
Expression analysis of VvMGT genes in different organs and tissues
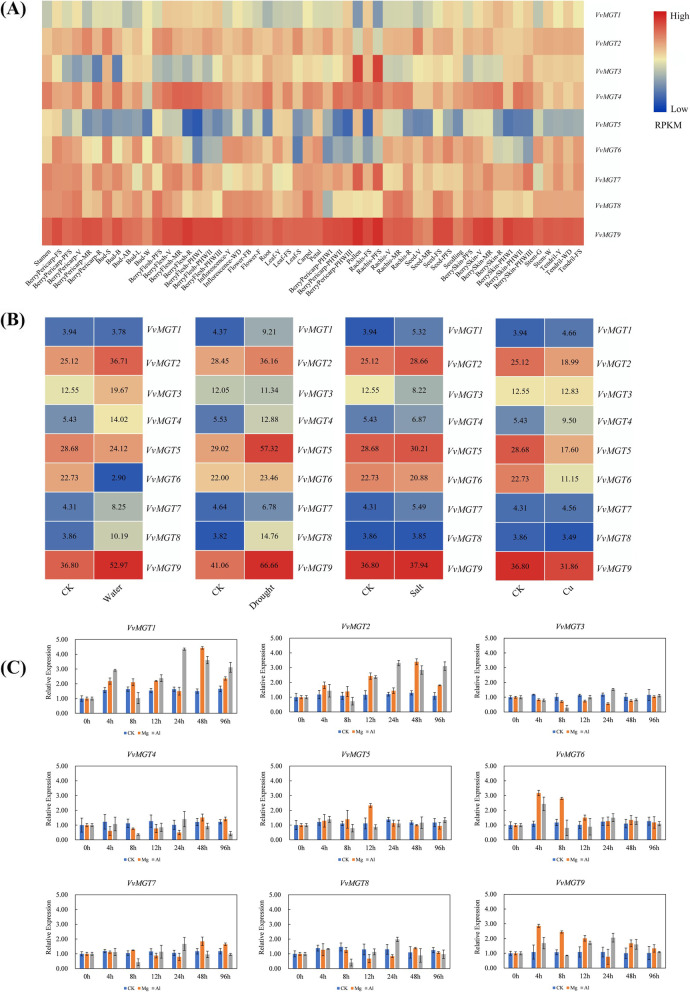
In order to obtain more information related to MGT genes, we used the transcriptome data of GEO database (GSE36128) containing the expression data of fifty-four tissues or organs during grape development. We used TBtools software to map the expression of VvMGTs genes in different developmental stages of grape and different organs (Fig. 5A). The results showed that members of the same subfamily differently expressed in grapes. For example, VvMGT1, VvMGT2, and VvMGT7 belonged to the same subfamily, however, the expression level of VvMGT1 during the entire development process is lower than others. VvMGT1 and VvMGT2 were lowly expressed in Pollen and Rachis-PFS but the expression of VvMGT7 was high in both. This indicates that the expression of MGTs genes alters in different tissues during the development of grapes and is dependent on the characteristics of tissue specificity and spatiotemporal. For example, VvMGT3 is only highly expressed in Pollen and Rachis-PFS and may participate in the signal transduction process of pollen and peel during growth and development. The expression of VvMGT6 was low in leaf senescence and fruit. According to the gene expression profile, VvMGT9 was highly expressed in all tissues and organs durin g the whole growth and development period, although, the expression of VvMGT5 was low in different tissues at different growth stages.
Fig. 5.
Expression pattern of VvMGT genes in grape. A Expression profiles of VvMGT genes in different grape organs, tissues and developmental stages. B Expression profiles of genes in response to water, drought, salt and Cu stresses. C The grapevine leaves were collected at 0, 4, 8, 12, 24, 48, and 96 h after treatment and gene expression was analysed by qRT-PCR The data are expressed as mean ± SD (n = 3)
Expression of VvMGTs gene under abiotic stress
There are four common abiotic stress including waterlogging, drought, salinity, and copper in vineyards, which VvMGTs expression can be involved in different pathways of resistance or adaptation. Here, we used transcriptome data related to VvMGTs to analyze the changes in gene expression under abiotic stresses (Fig. 5B). Under waterlogging stress, VvMGT6 expression was significantly down regulated. Meanwhile, the expression of VvMGT2, VvMGT5, VvMGT6, and VvMGT9 was higher in response to salt stress but the expression of VvMGT6 was down regulated under salinity. Under copper stress, VvMGT2, VvMGT5, VvMGT6, VvMGT8, and VvMGT9 were down regulated. According to the gene expression profile in Fig. 5B, we also found that VvMGT9 was highly expressed under three different abiotic stresses including waterlogging, drought, and salt stress.
We also performed in vivo experiment under 2% MgSO4 and 0.2%AlCl3 solution that was sprayed on grape seedlings and the expression of VvMGTs genes was detected by qRT-PCR (Fig. 5C). After magnesium treatment, the expression level of VvMGT1, VvMGT6, and VvMGT9 increased within 4 till 8 h than 0 h, while the expression of VvMGT2 and VvMGT3 decreased and reached at the lowest level after 24 h, and then gradually returned to normal expression level. After 96 h of magnesium treatment, the expression levels of VvMGT2, VvMGT3, VvMGT5, VvMGT6, VvMGT8 and VvMGT9 returned to normal levels. Under aluminum treatment, the gene expression of VvMGT1, VvMGT2, VvMGT4, VvMGT5, VvMGT6, VvMGT7, VvMGT8 and VvMGT9 increased after 4 h, decreased after 8 h, and then gradually increased during 24 h. These genes decreased slowly and tended to be flat after 24 h. Meanwhile, VvMGT1 and VvMGT2 genes remained at a high level after 96 h of aluminum ion treatment but the expression level of other members was similar to 0 h.
Subcellular localization of VvMGT proteins in grape
To further investigate the function of VvMGT genes in grape, four VvMGT genes were chosen as candidate genes with which to explore subcellular localization. Based on the presence of targeted sequences in the protein, the predictive result from plant-mPLoc website showed that most of the MGT proteins were predicted to be located in the nucleus and chloroplast (Table 1). Four MGT genes from different subfamilies were selected to test the results of subcellular localization prediction experimentally, using transient expression assays of fusion proteins between the MGT and the reporter green fluorescent protein (GFP). The recombinant plasmid of 35 s::VvMGT-GFP was transformed into Nicotiana benthamiana leaves by the Agrobacterium-mediated method. The results showed that the experiment data are basically identical with the plant-mPLoc website predicted ones. Using laser confocal microscopy, for tobacco cells transformed with the recombinant plasmids, however, the green fluorescence of VvMGT1-GFP, VvMGT4-GFP, VvMGT5-GFP, and VvMGT9-GFP were observed in the nucleus (Fig. 6).
Fig. 6.
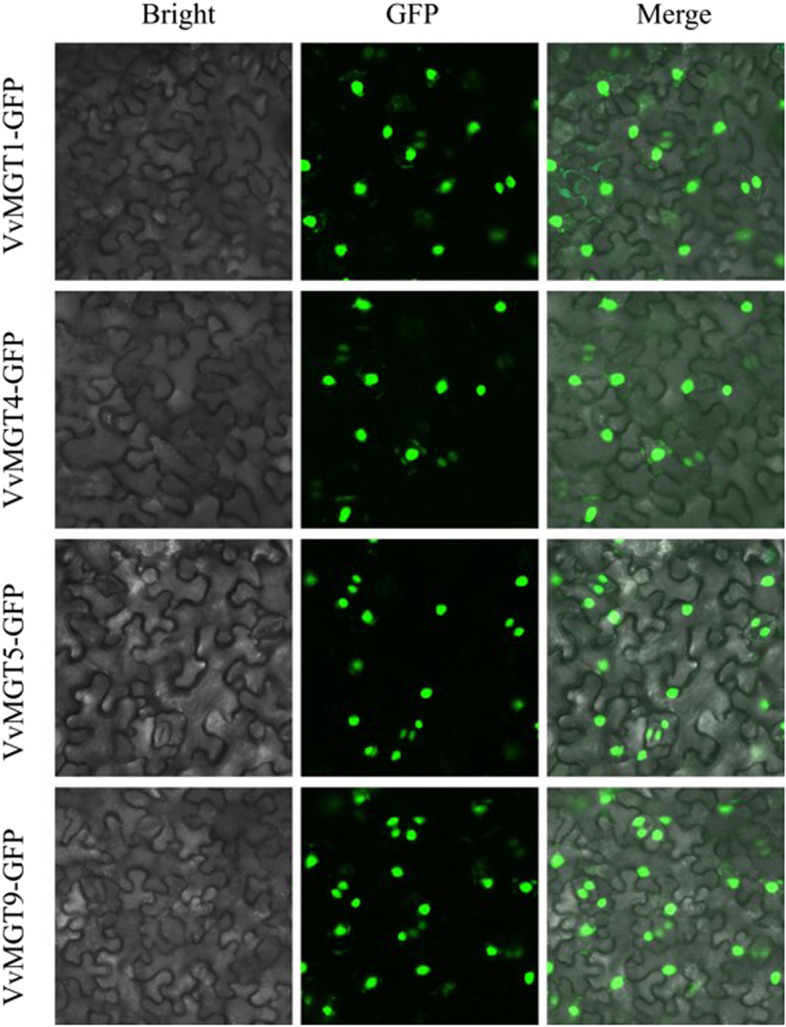
Subcellular localization of four MGT proteins. Four VvMGT-GFP fision proteins were expressed transiently in tobacco leaves in an independent manner, and the results were visualized by laser confocal microscopy. The brightfield images are shown in the first panels. The green fluorescence channel are shown in the second panel. The third are the merged images. Bar = 20um
Discussion
Magnesium ion is an essential nutrient for plant growth and development. As an important magnesium transporter, magnesium ion transporters play an important role in the biological processes of plants [2, 8]. In this study, we investigated the MGT gene family of grape and analyzed chromosomal localization, gene evolution, protein structure, physical and chemical properties. Furthermore, we performed the gene expression analysis of VvMGTs during grape development and various abiotic stresses according to transcriptomics datasets. The detailed analysis of MGT gene family in grape provides important information for understanding its molecular mechanism and possible functions in grape growth and development.
MGT gene family and its evolutionary analysis in grape
We have identified nine MGT family members in the whole grape genome, which is similar to that in Arabidopsis and rice [16, 19]. The length of VvMGTs gene sequence and the structure of intron and exon in grape MGT family members were different. The results of gene and protein sequence alignment show that the sequence consistency of MGT family members is not high (Table 3). Also, the pI range from 4.57 to 8.92 implies that different MGT proteins might be active in different microenvironments. Although the sequence consistency of the family members is low, GMN motifs were determined and were necessary for magnesium ion recognition in each family member. It seems that the function of VvMGT8 gene alters with the variation of GMN motif and needs further experimental verification. According to cluster analysis of grape MGT members and other plants including Arabidopsis(10), Rice(9), Maize(12) and Pear(16) proved that there are differences in the evolution of MGT genes among difference. We also found that VvMGTs genes were distributed in five subfamilies. This is consistent with the research results of Arabidopsis and rice [16, 19], indicating that the evolution of grape MGT gene family is very conservative. Gene replication is considered to be one of the main drivers of the evolution of the genome and genetic systems. Segment repeats and tandem repeats are the two main reasons for the expansion of plant gene families [38–40]. In this study, the results of collinearity analysis of VvMGTs gene in grape genome and Arabidopsis genome further illustrated the conservation of VvMGTs gene family. The above research results indicate that the grape MGT gene family is a slowly conserved gene family, and fragment duplication is the main driving force for the expansion of members of this gene family. In addition, the analysis of promoter sequences of VvMGTs showed that there are many types of cis-elements in response to different stimuli, which may be closely related to the multiple functions of VvMGTs gene in plant species, especially VvMGT7 may play a role in different metabolism.
Functional analysis of MGT genes in grape
To better understand the transport mechanism of MGTs in plants, we performed the functional and expression analysis of MGTs genes. The expression patterns of ten AtMGTs genes in Arabidopsis were different in pod, flower, root, stem, and leaf. AtMGT5 was expressed only in flowers and young pods, while AtMGT8 gene was not expressed in stem [41]. AtMGT10 gene is expressed in tender leaves or mature microtubule tissues, which mediates the transport of magnesium ions in chloroplasts and affects chlorophyll metabolism [16, 42]. In rice, nine OsMGTs genes were low expressed in undeveloped yellow-green leaves, and expression levels increased in mature leaves [11]. These results indicate that different family members participate in the regulation of plant growth and physiological metabolism through different modes of action. In this study, we found that VvMGT3 was highly expressed only in pollen and rachis PFS, while VvMGT6 expression was low in tissue senescence and organs. This indicates that the expression of VvMGTs genes in grape growth and development is also tissue-specific and spatiotemporal expression. In addition, the subcellular localization analysis showed that MGT protein in grape may be play a important role in nucleus.
A previous study showed that AtMGT10 gene is regulated by light and there are binding sites of transcription factors in the promoter region of AtMGT10, which it led to a significant increase in the transcriptional level of AtMGT10 under circadian rhythms [42]. In order to understand more clearly the regulatory network of VvMGTs in grape, the cis-elements of promoter sequence were analyzed. In VvMGT2 and VvMGT9, we observed the cis-element responsive to circadian rhythm, which it suggests the regulation of two genes requires circadian clock-responsive factors in grape. In addition, we found a large number of light- and hormone-responsive elements in the promoter region of MGTs gene, as well as drought and low temperature-responsive elements. This suggests that VvMGTs play a role in abiotic stress.
In order to investigate the expression of VvMGTs genes under environmental stresses, we candidated four abiotic stress including; drought, waterlogging, salinity, and copper damage and analyzed the gene expression patterns using transcriptome data. Under waterlogging, the expression of VvMGT9 gene was significantly up-regulated, indicating that the gene may be closely related to waterlogging resistance of grape. Under drought stress, VvMGT5 and VvMGT9 were significantly up-regulated and the expression level of VvMGT5 was almost twice as much as that of the control group, indicating that these two genes may play an important role in drought tolerance. The expression of VvMGTs also changed under salt and copper stresses but the degree of change was not as obvious as the drought and waterlogging stresses. However, VvMGT9 highly expressed in waterlogging, drought, and salt stress, we speculated that VvMGT9 may play an important role in response to abiotic stress of grape. Furthermore, in order to evaluate the expression of VvMGTs under ionic stresses, we studied the gene expression changes under magnesium and aluminum in grape. We found that the response mode of VvMGTs was different under magnesium and aluminum stresses. Under magnesium treatment, seven genes including VvMGT1, VvMGT2, VvMGT3, VvMGT4, VvMGT7, VvMGT8, and VvMGT9 reached at the highest level after 24 h. After aluminum exposure, the expression levels of all VvMGTs genes were up-regulated in a time-dependent behaviour, except VvMGT2 that its expression decreased. This suggests that the response mechanism of VvMGT2 gene in response to aluminum may be different from other members. In conclusion, the expression levels of VvMGTs genes are in a time-dependent behavior under magnesium and aluminum ion treatment that play different roles in the regulation of biological processes. Deng et al. showed that overexpression of AtMGT1 in tobacco could improve the resistance to aluminum toxicity [43]. In Arabidopsis thaliana, AtMGT2 and AtMGT3 located on vacuole membrane are closely related to the distribution of magnesium and respond to the high concentration of magnesium ion [25, 44]. They can transfer the excessive magnesium ions from the cytoplasm to the vacuole under the condition of magnesium toxicity, reduce the magnesium stress in the cytoplasm, and regulate the dynamic balance of magnesium ions in the cell. AtMGT6 and AtMGT10 were highly expressed in magnesium deficient roots [45, 46]. ZmMGT10 in maize can promote plant growth under magnesium deficiency [29]. The results showed that there were some similarities in function and regulation of MGT gene family members in grape when compared to other species.
It was found that magnesium could alleviate the toxicity of aluminum ion in Arabidopsis [47]. Furthermore, Silva et al. found that magnesium can effectively alleviate the aluminum toxicity of soybean roots [48]. MacDiarmid et al. found that under aluminum stress, yeast’s absorption of magnesium was hindered and the growth of cells inhibited [49]. However, after the addition of magnesium, it was found that the yeast recovered the absorption and transport of magnesium. Meanwhile, yeast cells also grew again and the transporter gene in yeast began to express, which alleviated the effect of aluminum toxicity. In our study, the expression of MGTs genes in grape was up-regulated under aluminum salt stress, which may be related to the regulation of magnesium ion absorption, transport, and distribution to alleviate aluminum salt stress in grape. The subcellular localization experiments further demonstrated that MGT protein may play an important role in the nucleus.
At present, the transport mechanism of plant magnesium ion is only the tip of the iceberg [12]. There are no systematic studies on the characteristics, expression patterns, and biological functions of magnesium ion transporters in different plants. The molecular mechanism of how magnesium ions are absorbed, transported, unloaded and distributed is unclear. Therefore, it is necessary to study the mechanism of MGTs in plants. The identification and expression analysis of MGTs genes in grape will lay a foundation for the study of magnesium ion in grape quality improvement and genetic regulation mechanism.
Conclusion
In this study, we identified nine VvMGTs genes based on the genomic dataset of grape for the first time and divided them into five subfamilies. The gene structure, chromosomal localization, conserved domain, protein structure, evolutionary relationship, and functional elements of the promoter region were analyzed. We observed that the expansion of members of the grape MGT gene family is dependent on the fragment duplication as the main driving force and is a slowly conserved gene family. At the same time, the expression profile of VvMGTs genes in the whole development period of grape was also analyzed, and the expression of VvMGTs gene under abiotic stress was studied. The results indicated that all VvMGTs genes could alter their expression in a time-dependent behavior in grape. Furthermore, determination of cis-acting element involved in light, hormone, and environmental stimuli suggest a critical role in the regulation of transcriptional levels of VvMGT family memebers for tolerance strategy in grape. Furthermore, the regulation of magnesium ion in grape plays an important role in solving ionic toxicity that can lay a theoretical foundation for the functional research of VvMGTs.
Methods
Identification of MGT family members and chromosome distribution in grape
To identity a complete list of grapevine MGT genes, we downloaded the annotated grapevine proteins from three public databases: the National Centre for Biotechnology Information (https://www.ncbi.nlm.nih.gov), the Grapevine Genome Browser (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/), and the Grape Genome Database (http://genomes.cribi.unipd.it/grape/, V2.1). We used the HMMER (http://hmmer.janelia.org/) Hidden Markov model (PF01544) as a probe to screen all the candidate protein with E-values of less than 2.40e-05. Then, the search results of the protein sequences were further confirmed using SMART (http://smart.embl-heidelberg.de) and Inter ProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan5/) to ensure their reliability. Finally, all identified VvMGT genes were utilized to analyze the amino acid length, molecular weight (MW), and isoelectric point (pI) by ExPASY (http://expasy.org). We used the plant-mPLoc website (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) for subcellular localization analysis of the VvMGTs genes [50]. The map of chromosome distribution was made by MapChart software and named by their chromosomal location [51].
Analysis of gene structure and protein classification
Based on VvMGT genes coding sequence (CDS) and the correspondent full-length gene sequences in NCBI, we used Gene Structure Display Server Software (GSDS.v2.0) (http://gsds.cbi.pku.edu.cn/,2.0) to analyze the structure of the VvMGT genes. In order to detect the classification of all VvMGTs, the protein sequences of them were determined by Clustal W [52]. The phylogenetic tree was constructed using MEGA7.0 with the Maximum Likelihood (ML) method and the bootstrap test carried out with 1000 replicates [53].
Conserved motifs analysis and comparative sequence identity of MGTs genes
To identify the conserved motif of grape MGT proteins, we submitted the protein sequence of VvMGTs to the MEME website (http://meme.nbcr.net/meme) and set the motif number as 10 [54]. We used TBtools software to visualize the results of protein conserved sequences [55]. Two transmembrane (TM) domains and the Gly-Met-Asn (GMN) motifs were marked according to the TMHMM analysis and the sequence alignment using the DNAMAN software. We also used the EMBOSS online website (http://emboss.sourceforge.net/) to analyze the similarity of VvMGTs gene and protein sequences.
Structural prediction and modeling of VvMGT proteins
To identify the structural composition of various Mg2+ transporter genes, we use SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred sopma.pl) prediction protein secondary structure. Then, the protein tertiary structure was constructed by Swiss-Model website (https://swissmodel.expasy.org/) using homology modeling.
Finding of cis-regulatory elements in promoter regions
We obtained the 2000 bp sequence upstream of the VvMGT genes as promoter regions and submitted to the PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Cis- acting elements on the promoter sequences were determined in VvMGT genes.
Phylogenetic analysis
First, we downloaded the protein sequences of grapes, Arabidopsis, Rice, Maize, and Pears on the Grape Genome and Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) websites. Phylogenetic analysis of 56 amino acid sequences of five species was performed using MEGA7.0 software. We used TBtools software to perform collinear analysis of the Grape genome, and Arabidopsis genomes.
Expression analysis of VvMGT genes using transcriptome data
The transcriptome data of different organs were downloaded from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) in different development stages of grape (Date code number: GSE36128).
In order to study the expression profiles of VvMGTs in response to various abiotic stresses (Cu, salt, waterlogging, and drought stress), the grapevine transcriptome data in response to waterlogging (SRA accession no. SRP070475) and drought stress (SRA accession no. SRP074162) were retrieved from NCBI database (https://www.ncbi.nlm.nih.gov/sra/SRP070475 and https://www.ncbi.nlm.nih.gov/sra/term=SRP074162, respectively) [56, 57] (Haider et al., 2017; Zhu et al., 2018). Transcriptome data for expression profiles in response to copper (Cu) and salinity were retrieved from published data sets by Guan et al. [58].
The analysis of transcriptome data was based on the Leng et al. method [59], and the RPKM (Reads Per Kilobase per Million mapped reads) values were used to estimate the gene expression level. Expression data was also mapped by TBtools and presented in the heat map format.
Plant materials and experimental treatment
The two-year-old grape cultivar ‘Kyoho’ was planted in the greenhouse of Baima Teaching Base of Nanjing Agricultural University. We set up one control group and two experimental groups in the test. Control group was sprayed with dionized water and the treatment groups were sprayed with AlCl3 and MgSO4 solutions. One treatment group was sprayed using 0.2% AlCl3 solution, and the other treatment was spraying using 2% MgSO4 solution [12, 60, 61]. We collected leaves in a time-course experiment after solution exposure (0, 4, 8, 12, 24, 48, and 96 h). The collected leaves were immediately frozen in liquid nitrogen and stored at − 80 °C for RNA extraction.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using a kit (Vazyme,Beijing,China) and the reverse-transcribed process performed with a HifairII® 1st Strand cDNA Synthesis SuperMix for qPCR kit (Yeasen,Shanghai,China). All primers were designed by Primer Primer5.0 software for qRT-PCR, which were listed in Table S1. Total volume of reaction mixtures was 10 μL, which included 5 μL of SYBR Green Supermix (Bio-Rad), 2 μL of diluted cDNA, 0.2 μL of each primer, and 2.6 μL double-distilled water. All reactions were performed in three biological replicates. qRT-PCR was carried out using the CFX96 Real-Time PCR Detection system (Bio-Rad, Hercules, CA, USA). The following steps were carried out in PCR: predenaturation at 94 °C for 30 s, followed by 40 cycles of denaturation at 94 °C for 5 s, primer annealing at 60 °C for 15 s, and extension at 72 °C for 10 s. Optical data were acquired after the extension step, and the PCR reactions were subjected to a melting curve analysis beginning from 65 °C to 95 °C at 0.1 °C s− 1. VvUBI (Gene code LOC100259511) was used as an internal reference gene, and the expression data were calculated by 2-∆∆CT method [62].
Subcellular localization assays
The coding sequence (CDS) of the genes VvMGT1, VvMGT4, VvMGT5 and VvMGT9 were amplified from ‘Shine Muscat’ grape leaf by PCR, using the corresponding primers, which are listed in Table S1. The amplified PCR products were cloned into the modified pCAMBIA1302-GFP vector carrying the CaMV35s promoter (Clontech, Beijing, China). Subsequently, the fusion plasmids 35 s::VvMGT-GFP were independently transferred into Agrobacterium tumefaciens cells (Weidi, Shanghai, China). Agrobacterium cells transformed with the respective fusion plasmid were then injected into tobacco (Nicotiana benthamiana) leaves, and the green fluorescence signals were visualized with a Zeiss LSM800 Image Browser (ZEISS, Germany) 48 h after transformation. Three independent experiments were performed for each gene.
Supplementary Information
Acknowledgements
We acknowledge central laboratory platform of college of horticulture, Nanjing Agricultural University.
Authors’ contributions
MQG and RZ designed and carried out the experiments, MQG and XX performed all bioinformatic analyses and wrote the manuscript. ES, AH, PPW and JGF directed and revised the manuscript. All authors read, reviewed and approved the final manuscript.
Funding
This work has been supported by the Jiangsu modern agricultural industry technology system construction project (JATS[2019]422); Jiangsu province agricultural science and technology independent innovation project (CX(18)2008); Jiangsu major species creation project (PZCZ201724).
Availability of data and materials
The data presented in this study are available on request from the corresponding author. All databases used in this study are open for public access. The references of these databases are as follow: the Grapevine Genome Browser (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/), the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), the grapevine transcriptome data in response to waterlogging (SRA accession no. SRP070475) and drought stress (SRA accession no. SRP074162) were retrieved from NCBI database (https://www.ncbi.nlm.nih.gov/sra/SRP070475 and https://www.ncbi.nlm.nih.gov/sra/term=SRP074162, respectively).
Declarations
Ethics approval and consent to participate
All the materials of this study are given by Baima Teaching Base of Nanjing Agricultural University (Nanjing, China). The collection of plant material comply with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaul O. Magnesium transport and function in plants: the tip of the iceberg. Biometals. 2002;15:309–323. doi: 10.1023/A:1016091118585. [DOI] [PubMed] [Google Scholar]
- 2.Waters BM. Moving magnesium in plant cells. New Phytol. 2011;190:510–513. doi: 10.1111/j.1469-8137.2011.03724.x. [DOI] [PubMed] [Google Scholar]
- 3.Cowan JA. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15:225–235. doi: 10.1023/A:1016022730880. [DOI] [PubMed] [Google Scholar]
- 4.Smith RL, Banks JL, Snavely MD, Maguire ME. Sequence and opology of the CorA magnesium transport systems of salmonella typhimurium and Escherichia Coli identification of a new class of transport protein. J Biol Chem. 1993;268:14071–14080. doi: 10.1016/S0021-9258(19)85210-9. [DOI] [PubMed] [Google Scholar]
- 5.Martin RB. Bioinorganic chemistry of magnesium. Met Ions Biol Syst. 1990;26:1–13. [Google Scholar]
- 6.Donald P, Ronald JP. Role of magnesium in the plasma membrane ATPase of red beet. Plant Physiol. 1983;71:969–971. doi: 10.1104/pp.71.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters JS, Berkowitz GA. Studies on the system regulating proton movement across the chloroplast envelope: effects of ATPase inhibitors, mg, and an amine anesthetic on stromal pH and photosynthesis. Plant Physiol. 1991;95:1229–1236. doi: 10.1104/pp.95.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horlitz M, Klaff P. Gene-specific trans-regulatory functions of magnesium for chloroplast mRNA stability in higher plants. J Biol Chem. 2000;275:35638–35645. doi: 10.1074/jbc.M005622200. [DOI] [PubMed] [Google Scholar]
- 9.Williams L, Salt DE. The plant ionome coming into focus. Curr Opin Plant Biol. 2009;12:247–249. doi: 10.1016/j.pbi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose J, Babourina O, Rengel Z. Role of magnesium in alleviation of aluminium toxicity in plants. J Exp Bot. 2011;62:2251–2264. doi: 10.1093/jxb/erq456. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZC, Ma JF. Magnesium transporters and their role in Al tolerance in plants. Plant Soil. 2012;368:51–56. doi: 10.1007/s11104-012-1433-y. [DOI] [Google Scholar]
- 12.Guo WL, Chen SN, Hussain N, Cong YX, Liang ZS, Chen KM. Magnesium stress signaling in plant: just a beginning. Plant Signal Behav. 2015. 10.4161/15592324.2014.992287. [DOI] [PMC free article] [PubMed]
- 13.Chen ZC, Yamaji N, Motoyama R, Nagamura Y, Ma JF. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012;159:1624–1633. doi: 10.1104/pp.112.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebert M, Meschenmoser K, Svidova S, Weghuber J, Schweyen R, Eifler K, Lenz H, Weyand K, Knoop V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell. 2009;21:4018–4030. doi: 10.1105/tpc.109.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schock I, Juraj G, Siegfried S, Rudolf AB, Knoop V. A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intro-splicing mutant. Plant J. 2000;24:489–501. doi: 10.1046/j.1365-313x.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Tutone AF, Drummond RS, Gardner RC, Luan S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell. 2001;13:2761–2775. doi: 10.1105/tpc.010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Wen AN, Wu XQ, Pan XL, Wu N, Chen X, Chen Y, Mao DD, Chen LB, Luan S. Molecular identification of the magnesium transport gene family in Brassica napus. Plant Physiol Biochem. 2019;136:204–214. doi: 10.1016/j.plaphy.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Lunin VV, Dobrovetsky E, Khutoreskaya G, Zhang R, Joachimiak A, Doyle DA, Bochkarev A, Maguire ME, Edwards AM, Koth CM. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito T, Kobayashi NI, Tanoi K, Iwata N, Suzuki H, Iwata R, Nakanishi TM. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 2013;54:1673–1683. doi: 10.1093/pcp/pct112. [DOI] [PubMed] [Google Scholar]
- 20.Regon P, Chowra U, Awasthi JP, Borgohain P, Panda SK. Genome-wide analysis of magnesium transporter genes in Solanum lycopersicum. Comput Biol Chem. 2019;80:498–511. doi: 10.1016/j.compbiolchem.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Kehres DG, Maguire ME. Structure, properties and regulation of magnesium transport proteins. Biometals. 2002;15:261–270. doi: 10.1023/A:1016078832697. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Li LG, Liu ZH, Yuan YJ, Guo LL, Mao DD, Tian LF, Chen LB, Luan S, Li DP. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009;19:887–898. doi: 10.1038/cr.2009.58. [DOI] [PubMed] [Google Scholar]
- 23.Li LG, Sokolov LN, Yang YH, Li DP, Ting J, Pandy GK, Luan S. A mitochondrial magnesium transporter functions in Arabidopsis pollen development. Mol Plant. 2008;1:675–685. doi: 10.1093/mp/ssn031. [DOI] [PubMed] [Google Scholar]
- 24.Szegedy MA, Maguire ME. The CorA Mg2+ transport protein of salmonella typhimurium. J Biol Chem. 1999;274:36973–36979. doi: 10.1074/jbc.274.52.36973. [DOI] [PubMed] [Google Scholar]
- 25.Alexandersson E, Saalbach G, Larsson C, Kjellbom P. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 2004;45:1543–1556. doi: 10.1093/pcp/pch209. [DOI] [PubMed] [Google Scholar]
- 26.Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng PY, Tian LF, Chen J, Luan S, Li DP. Functional analysis of AtMGT3 transport in Arabidopsis. Life Sci. 2007;11:328–333. [Google Scholar]
- 28.Whiteman SA, Serazetdinova L, Jones AM, Sanders D, Rathjen J, Peck SC, Maathuis FJ. Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana. Proteomics. 2008;8:3536–3547. doi: 10.1002/pmic.200701104. [DOI] [PubMed] [Google Scholar]
- 29.Li HT, Du HM, Huang KF, Chen X, Liu TY, Gao SB, Liu HL, Tang QL, Rong TZ, Zhang SZ. Identification, and functional and expression analyses of the CorA/MRS2/MGT-type magnesium transporter family in maize. Plant and Cell Physiol. 2016;57(6):1153–1168. doi: 10.1093/pcp/pcw064. [DOI] [PubMed] [Google Scholar]
- 30.Li HY, Zhang SZ, Chen QF. Expression analysis of maize ZmMGT12 gene and genetic transformation of Arabidopsis. Mol Plant Breed. 2018;16(18):5940–5946. [Google Scholar]
- 31.Li HY, Wang N, Ding JZ, Liu C, Du HM, Huang KF, Cao MJ, Lu YL, Gao SB, Zhang SZ. The maize CorA/MRS2/MGT-type mg transporter, ZmMGT10, responses to magnesium deficiency and confers low magnesium tolerance in transgenic Arabidopsis. Plant Mol Biol. 2017;95:269–278. doi: 10.1007/s11103-017-0645-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Zhai QH, Zhang DW, Hu BX, Guo SX. Cloning and expression analysis of a magnesium transporter gene in Dendrobium officinale. Zhongcaoyao. 2014;23:3443–3448. [Google Scholar]
- 33.Yang JH, Qin YX, Fang YJ, Tang CR. Molecular cloning and expression analysis of HbMGT10 from Hevea brasiliensis. Chinese J Tropical Crops. 2016;37:2353–2358. [Google Scholar]
- 34.Zhao ZF. Family analysis of magnesium transpoter in pear and function verification of PbrMGT7. 2016. [Google Scholar]
- 35.Chen JQ, Li XY, Wang DQ, Li LT, Zhou HS, Liu Z, Wu J, Wang P, Jiang XT, Fabrice MR, Zhang SL, Wu JY. Identification and testing of reference genes for gene expression analysis in pollen of Pyrus bretschneideri. Sci Hortic. 2015;190:43–56. doi: 10.1016/j.scienta.2015.04.010. [DOI] [Google Scholar]
- 36.Zhou HL, Yin H, Chen JQ, Liu X, Gao YB, Wu JY, Zhang SL. Gene-expression profile of developing pollen tube of Pyrus bretschneideri. Gene Expr Patterns. 2016;20:11–21. doi: 10.1016/j.gep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Usuda H, Shimogawara K. Phosphate deficiency in maize III. Change in enzyme activities during the course of phosphate deprivation. Plant Physiol. 1992;99:1680–1685. doi: 10.1104/pp.99.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Causier B, Castillo R, Zhou J, Ingram R, Xue Y, Schwarz-Sommer Z, Davies B. Evolution in action: following function in duplicated floral homeotic genes. Curr Biol. 2005;15:1508–1512. doi: 10.1016/j.cub.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 39.Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–124. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- 40.Marques AC, Vinckenbosch N, Brawand D, Kaessmann H. Functional diversification of duplicate genes through subcellular adaptation of encoded proteins. Genome Biol. 2008;9:R54. doi: 10.1186/gb-2008-9-3-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 42.Drummond RSM, Tutone A, Li YC, Gardner RC. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006;170:78–89. doi: 10.1016/j.plantsci.2005.08.018. [DOI] [Google Scholar]
- 43.Deng W, Luo KM, Li DM, Zheng XL, Wei XY, Smith W, Thammina C, Lu LT, Li Y, Pei Y. Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J Exp Bot. 2006;57:4235–4243. doi: 10.1093/jxb/erl201. [DOI] [PubMed] [Google Scholar]
- 44.Gao C, Zhao Q, Jiang L. Vacuoles protect plants from high magnesium stress. Proc Natl Acad Sci U S A. 2015;112:2931–2932. doi: 10.1073/pnas.1501318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao DD, Chen J, Tian LF, Liu ZH, Yang L, Tang RJ, Li J, Lu CQ, Yang YH, Shi JS, Chen LB, Li DP, Luan S. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell. 2014;26:2234–2248. doi: 10.1105/tpc.114.124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherman F. Getting started with yeast methods in enzymology. 1991. [DOI] [PubMed] [Google Scholar]
- 47.Ishijima S, Manabe Y, Shinkawa Y, Hotta A, Tokumasu A, Ida M, Sagami I. The homologous Arabidopsis MRS2/MGT/CorA-type Mg2+ channels, AtMRS2-10 and AtMRS2-1 exhibit different aluminum transport activity. Biochim Biophys Acta Biomembr. 2018;1860:2184–2191. doi: 10.1016/j.bbamem.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Silva IR, Ferrufino A, Sanzonowicz C, Smyth TJ, Israel DW, Junior TEC. Interactions between magnesium, calcium, and aluminum on soybean root elongation. Revista Brasileira De Ciencia Do Solo. 2005;29:747–754. doi: 10.1590/S0100-06832005000500010. [DOI] [Google Scholar]
- 49.MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- 50.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 52.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002. Chapter 2, Unit 2.3. 10.1002/0471250953.bi0203s00. [DOI] [PubMed]
- 53.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME suite: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R. TBtools - an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Haider MS, Zhang C, Kurjogi MM, Pervaiz T, Zheng T, Zhang CB, et al. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci Rep. 2017. 10.1038/s41598-017-13464-3. [DOI] [PMC free article] [PubMed]
- 57.Zhu XD, Li XP, Jiu ST, Zhang KK, Wang C, Fang JG. Analysis of the regulation networks in grapevine reveals response to waterlogging stress and candidate gene-marker selection for damage severity. Roy Soc Open Sci. 2018;5:172–253. doi: 10.1098/rsos.172253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan L, Haider MS, Khan N, Nasim M, Jiu ST, Fiaz M, Zhu XD, Zhang KK, Fang JG. Transcriptome sequence analysis elaborates a complex defensive mechanism of grapevine (Vitis vinifera L.) in response to salt stress. Int J Mol Sci. 2018;19:4019. doi: 10.3390/ijms19124019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leng XP, Jia HF, Sun X, Shangguan LF, Mu Q, Wang BJ, Fang JG. Comparative transcriptome analysis of grapevine in response to copper stress. Sci Rep. 2015;5:17749. doi: 10.1038/srep17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borai IH, Ezz MK, Rizk MZ, Aly HF, EI-Sherbiny M, Matloub AA, Fouad GI. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl(3)-induced Alzheimer’s disease. Biomed Pharmacother. 2017;93:837–851. doi: 10.1016/j.biopha.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 61.Singh RK, Afonso J, Nogueira M, Oliveira AA, Cosme F, Falco V. Slicates of potassium and aluminium (kaolin); comparative foliar mitigation treatments and biochemical insight on grape berry quality in Vitis vinifera L. (cv Touriga National and Touriga Franca) Biology. 2020;9:1–17. doi: 10.3390/biology9030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. All databases used in this study are open for public access. The references of these databases are as follow: the Grapevine Genome Browser (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/), the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), the grapevine transcriptome data in response to waterlogging (SRA accession no. SRP070475) and drought stress (SRA accession no. SRP074162) were retrieved from NCBI database (https://www.ncbi.nlm.nih.gov/sra/SRP070475 and https://www.ncbi.nlm.nih.gov/sra/term=SRP074162, respectively).