Abstract
Background
Calcium (Ca2+) serves as a ubiquitous second messenger and plays a pivotal role in signal transduction. Calcineurin B-like proteins (CBLs) are plant-specific Ca2+ sensors that interact with CBL-interacting protein kinases (CIPKs) to transmit Ca2+ signals. CBL-CIPK complexes have been reported to play pivotal roles in plant development and response to drought stress; however, limited information is available about the CBL and CIPK genes in pecan, an important nut crop.
Results
In the present study, a total of 9 CBL and 30 CIPK genes were identified from the pecan genome and divided into four and five clades based on phylogeny, respectively. Gene structure and distribution of conserved sequence motif analysis suggested that family members in the same clade commonly exhibited similar exon-intron structures and motif compositions. The segmental duplication events contributed largely to the expansion of pecan CBL and CIPK gene families, and Ka/Ks values revealed that all of them experienced strong negative selection. Phylogenetic analysis of CIPK proteins from 14 plant species revealed that CIPKs in the intron-poor clade originated in seed plants. Tissue-specific expression profiles of CiCBLs and CiCIPKs were analysed, presenting functional diversity. Expression profiles derived from RNA-Seq revealed distinct expression patterns of CiCBLs and CiCIPKs under drought treatment in pecan. Moreover, coexpression network analysis helped to elucidate the relationships between these genes and identify potential candidates for the regulation of drought response, which were verified by qRT–PCR analysis.
Conclusions
The characterization and analysis of CBL and CIPK genes in pecan genome could provide a basis for further functional analysis of CiCBLs and CiCIPKs in the drought stress response of pecan.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03601-0.
Keywords: Carya illinoinensis, CBL-CIPK, Drought, Evolution, Gene expression
Background
Calcium ions (Ca2+) serve as a secondary messenger that is involved in various signal transduction processes, including growth, development, and response to environmental stimuli in plants [1]. Ca2+ signals are commonly perceived by Ca2+ sensors, and four Ca2+ sensor families have been identified in high plants, such as calcineurin B-like proteins (CBLs), calmodulins (CaMs), calmodulin-like proteins (CMLs), and calcium-dependent protein kinases (CDPKs) [2]. Unlike CDPKs, the CBL, CaM, and CML proteins do not contain any enzymatic domain but transmit Ca2+ signals to their downstream targets via their interactors [3].
The CBL protein generally contains an important structural component consisting of four elongation factor hand (EF-hand) motifs for calcium binding. CBL proteins function in various biological processes by activating their downstream targets, CBL-interacting protein kinases (CIPKs), also called (SNF)-related kinases (SnRK3s) [4]. CBL and CIPK form the CBL-CIPK complex to function in different signalling pathways in plants [5]. CIPK proteins are composed of a serine/threonine kinase domain at the N-terminus and a NAF/FISL domain at the C-terminus [6]. The autoinhibitory NAF domain is conserved with 24 amino acid residues, and the activation of plant CIPKs is mediated via the interaction of this domain with CBL proteins [7].
The CBL and CIPK families are two plant-specific gene families, and family members have been identified in numerous species. For example, 10 CBL and 26 CIPK family members were identified in Arabidopsis, and 10 CBL and 33 CIPK members were found in rice [1, 8]. Ten CBL and 27 CIPK family members were found in Populus trichocarpa, a model woody plant [9, 10]. This phenomenon reveals that CBL may interact with one or more CIPK proteins [11].
Drought is one of the major abiotic stresses that affect plant growth, development, and productivity [12]. The increase in the frequency of drought, with the decrease in soil moisture, is one of the future challenges that affect our society [13]. Understanding the mechanism of how plants respond to drought has been reported at different levels, including epigenetic regulation, metabolic changes, and molecular mechanisms [14]. Numerous genes were upregulated or downregulated under drought, and some of them have been functionally analysed in model plants, such as Arabidopsis and rice [15]. The CBL-CIPK signalling pathways were reported to play important roles in responding to various environmental stresses, including drought stress. Multiple CBL and CIPK genes could be induced under drought stress in grapevine and tea plants [16, 17]. AtCIPK1 participates in the defence response to drought stress by interacting with both AtCBL1 and AtCBL9, and cbl1 and cipk1 mutant plants are hypersensitive to drought stress [18].
Pecan (Carya illinoinensis [Wangenh.] K. Koch) is an economically important nut tree of the Carya genus that is native to the United States and Mexico, and is now cultivated on six continents [19]. Pecan nuts, the seeds of C. illinoinensis, are a rich source of unsaturated fatty acids, vitamins, and numerous bioactive constituents and are commonly consumed snack [20]. The production of pecan nuts was approximately 300 million pounds in the United States in 2020, with a value of approximately $400 million (https://www.nass.usda.gov/). Pecan nut productivity is commonly affected by various biotic and abiotic stresses. Recently, the availability of chromosome-level genome assembly of pecan has allowed us to characterize the pecan CBL and CIPK gene families, and transcriptome data have helped to analyse their expression patterns in different tissues and under drought stress [21]. In the current work, nine CBL and 30 CIPK genes in pecan were identified, and the evolutionary relationships and duplication events were also analysed. The expression patterns of the two gene family members in various tissues and in response to drought stress were investigated. The precise annotation of the two gene families is the first step to fully understand the roles in pecan drought response.
Materials and methods
Identification of the CBL and CIPK gene families in pecan
To identify the CBL and CIPK proteins, all pecan (Carya illinoinensis) cv. Pawnee protein sequences were downloaded from the Phytozome database v13 (https://phytozome-next.jgi.doe.gov/) [22]. Ten AtCBL and 26 AtCIPK protein sequences from Arabidopsis were retrieved from The Arabidopsis Information Resource (TAIR) database (https://www.arabidopsis.org/). The full-length AtCBL and AtCIPK sequences were aligned with MEGA 7 software (https://www.megasoftware.net) [23]. Then, the alignments were selected to build hidden Markov model (HMM) profiles using the hmmbuild program in HMMER v3.3.2 (http://www.hmmer.org/), and we searched the two HMM profiles against the pecan genome using HMMER with an E-value <1e-10 [24]. The candidate proteins were further examined using Pfam (http://pfam.xfam.org/) and SMART software (http://smart.embl-heidelberg.de/) to confirm the presence of key domains [25]. The EF-hand motif was used for verification of CiCBL family members, while both the kinase and NAF domains were selected for verification of CiCIPK proteins.
The molecular weights (MWs) and isoelectric points (pIs) of CiCBL and CiCIPK proteins were calculated using the ExPASY program (https://web.expasy.org).
Multiple sequence alignment and phylogenetic analysis
Protein sequences of Botryococcus braunii were collected from Botryococcus braunii Showa v2.1 DOE-JGI (http://phytozome.jgi.doe.gov/) [26]. Sequences of Marchantia polymorpha, Physcomitrium patens, Selaginella moellendorffii, Amborella trichopoda, Oryza sativa, Ananas comosus, Betula platyphylla, Populus trichocarpa, and Salix purpurea were downloaded from Phytozome 13, common walnut (Juglans regia) protein sequences were downloaded from the Genomic Data of Juglans (http://xhhuanglab.cn/data/juglans.html), and Chinese hickory (Carya cathayensis) proteins were retrieved from the GigaScience database [27]. The protein sequences of all identified CBL and CIPKs from pecan and the other 13 plant species were used for multiple sequence alignment by MAFFT version 7 (https://mafft.cbrc.jp/alignment/software/) with the G-INS-I program [28]. Phylogenetic trees were then constructed using FastTree software (http://www.microbesonline.org/fasttree/) with the maximum likelihood model [29].
Conserved CBL protein sequences were viewed and edited using GeneDoc software (http://nrbsc.org/gfx/genedoc/). Weblogo (https://weblogo.berkeley.edu/logo.cgi) was then applied to show the features of the NAF domains of CIPK proteins [30].
Analysis of gene structure and conserved motifs
The coding sequences and genomic DNA sequences of CiCBL and CiCIPK genes were collected to analyse structural features using TBtools v1.09832 [31].
The amino acid sequences of CiCBLs and CiCIPKs were used to predict the conserved motifs with the MEME online tool (http://meme-suite.org/tools/meme) [32].
Chromosomal locations of CBL and CIPK genes in pecan
The chromosomal locations of CiCBL and CiCIPK genes were retrieved from the phytozome database, and the chromosomal images were visualized using the Circos program in Tbtools software [31].
Gene duplication and selection pressure analysis
All of the CBL and CIPK protein sequences from the pecan genome were searched against themselves using NCBI-BLAST 2.7.1+ [33]. Collinearity analyses of the CBL and CIPK gene families were performed using MCScanX (Multiple Collinearity Scan toolkit) software (http://chibba.pgml.uga.edu/mcscan2/) [34].
To detect the selection pressure of the duplication events, the CDSs of the CiCBL and CiCIPK genes were aligned using ClustalW v2.0 software [35]. Then, the synonymous substitution (Ks) and nonsynonymous substitution (Ka) of tandem and segmental duplication events were calculated using TBtools software, and the selection pressure was evaluated by the Ka/Ks ratios.
Plant materials, growth conditions and sample collection
Leaf, mature pistillate and staminate flower, young fruit, and seed samples were collected from nine randomly selected nine-year-old healthy pecan trees of the ‘Pawnee’ cultivar at the experimental station of Nanjing Forestry University, which located in Jurong City, China (119°9′6″E, 31°52′45″N). Ten-month-old seedlings propagated by pecan seeds (collected from ‘Pawnee’ trees) were served as rootstocks. Mature staminate flowers were collected in April, leaves and mature pistillate flowers were harvested in May, young fruits were sampled at 60 days after flowering in July, and seeds were collected in October 2019. Root samples were collected from three-month-old pecan seedlings that were propagated by seeds, and harvested from ‘Pawnee’ trees. All the samples were harvested on sunny days (8:00 to 10:00 am).
For drought treatment, ‘Pawnee’ grafted seedlings were used in this study. The commercial pecan cultivar ‘Pawnee’ was selected as scion, and patch budding was applied for grafting at the experimental station of Nanjing Forestry University in August 2019. Pecan seedlings that propagated by seeds (harvested from ‘Pawnee’ trees in October 2018) were used as rootstocks. The grafted plants were placed in 12-L plastic containers containing a soil mixture of peat, vermiculite, and perlite (5: 3: 2 by volume). After 12 months, the grafted pecan seedlings were then moved to a climate chamber with a photoperiod of 14 h of light at 24 °C/ 10 h of dark at 22 °C, and 60–70% relative humidity. Distilled water was irrigated twice every week. The seedlings were grown in well-watered conditions for 1 month, then they were moved to a growth chamber at 24/22 °C day/night temperatures with a 14/10-h photoperiod, and water was withheld for 15 days. Pecan leaves were collected at 0, 3, 6, 9, 12, and 15 d during drought treatment.
The harvested tissue samples were frozen in liquid nitrogen and stored at − 70 °C until RNA was isolated. Each sample was collected from at least three plants, and three biological repetitions were carried out for each treatment.
Measurement of proline content and SOD activity
The free proline content was detected according to the ninhydrin method, and the absorbance was measured at 520 nm [36]. Superoxide dismutase (SOD) activity was measured using a Total SOD Assay Kit (A001–1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
RNA isolation and qRT–PCR analyses
Harvested tissue samples were ground to powder in liquid nitrogen. Total RNA was extracted from various tissues using TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. Genomic DNA was removed using a DNase I kit (Qiagen, Hilden, Germany), and RNA quality was detected with an Agilent 2100 bioanalyzer (Agilent Technologies, CA, USA) and quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, USA).
For qRT–PCR (quantitative real-time PCR) analysis, 1 μg of total RNA was used to synthesize first-strand cDNA using the Prime-Script RT Reagent Kit (Takara, Dalian, China). qRT–PCR was performed on an ABI 7500 Real Time PCR system (Applied Biosystems™, Foster City, USA) using TB Green™ Premix Ex Taq™ II (TaKaRa, Shiga, Japan). Specific primers for the CBL and CIPK genes were designed using the IDT PrimerQuest online tool (https://sg.idtdna.com/PrimerQuest/Home/Index). An actin gene (CiPaw.03G124400) that used in previous studies was applied as an internal control for normalization [37]. Relative quantification of CBL and CIPK genes was determined by the 2-∆∆Ct method [38]. The PCR cycling conditions were as follows: initial denaturation at 95 °C for 30 s, then 40 cycles of 95 °C for 5 s and 60 °C for 15 s.
Transcriptome analysis
cDNA libraries were constructed and sequenced using an Illumina NovaSeq 6000 platform by Gene Denovo Biotechnology Co. (Guangzhou, China). The FPKM (fragments per kilobase per million of reads mapped) values of CBL and CIPK genes were calculated by RSEM software to quantify gene expression levels [39]. Sequence data have been uploaded to the NCBI (National Center for Biotechnology Information) database (https://www.ncbi.nlm.nih.gov/) under the accession number GSE179336 and PRJNA799663.
Coexpression analysis
The Pearson correlation coefficient (PCC) values between different gene pairs of the two family members were calculated with SPSS Statistics 24 based on the expression data of CiCBLs and CiCIPKs during pecan response to drought treatment. A coexpression network was then built to investigate the relationship between CiCBL and CiCIPK genes based on PCCs, and the network was then visualized with Cytoscape version 3.8.2 software [40].
Subcellular localization assays
To investigate the localization of selected CiCIPKs, the coding regions of CiPaw.01G129000, CiPaw.07G161900 and CiPaw.13G065400 were amplified using Pfu DNA polymerase (TransGen, Beijing, China), and the PCR products were cloned upstream of a GFP (green fluorescent protein) gene into the pBWA(V)HS vector driven by the 35S promoter (BioRun, Wuhan, China) to generate the constructs. The protoplasts were extracted from 3-week-old Arabidopsis seedlings and transformed according to the polyethylene glycol (PEG) method [41]. The transformed protoplasts were incubated at 24 °C for 15–18 h, and then the fluorescence signal was determined under a Nikon C2-ER confocal scanning microscope (Nikon, Kyoto, Japan). Each construct was tested and imaged in at least four protoplasts.
Statistical analysis
Statistical analyses were carried out using SPSS 24 software. The results were shown as the means ± SE (standard errors) of three biological replicates. One-way ANOVA and Duncan’s multiple range test were selected to compare the significance of differences (P < 0.05).
Results
Genome-wide investigation of CiCBL and CiCIPK genes
After screening the pecan genome by domain confirmation, a total of 9 CBLs and 30 CIPKs were identified (Additional file 1: Table S1). Sequence analyses of the two gene family members showed that the full-length CiCBL genes varied from 2129 to 13,131 base pairs (bp), and CiCIPK genes varied from 1296 to 24,147 bp. The CiCBL and CiCIPK proteins consisted of 179–252/398–492 amino acids (aa), with the relative molecular weights (MWs) of CBLs ranging from 21,121.18 to 29,077.27 Da (Da), while the MWs of CIPKs ranged from 45,423.42 to 55,346.56 Da. Interestingly, the isoelectric points (pIs) of CBL proteins were conserved in pecan, which varied from 4.6 to 4.88, while the pIs of CIPKs varied from 5.67 to 9.39, and 86.67% (26/30) of them had high pIs (pI > 7). All 9 CBL and 30 CIPK proteins were hydrophilic with negative grand average of hydropathicity (GRAVY) values. Detailed information for the two gene family members is listed (Additional file 1: Table S1).
Gene structural and conserved domain analysis of the pecan CBL and CIPK families
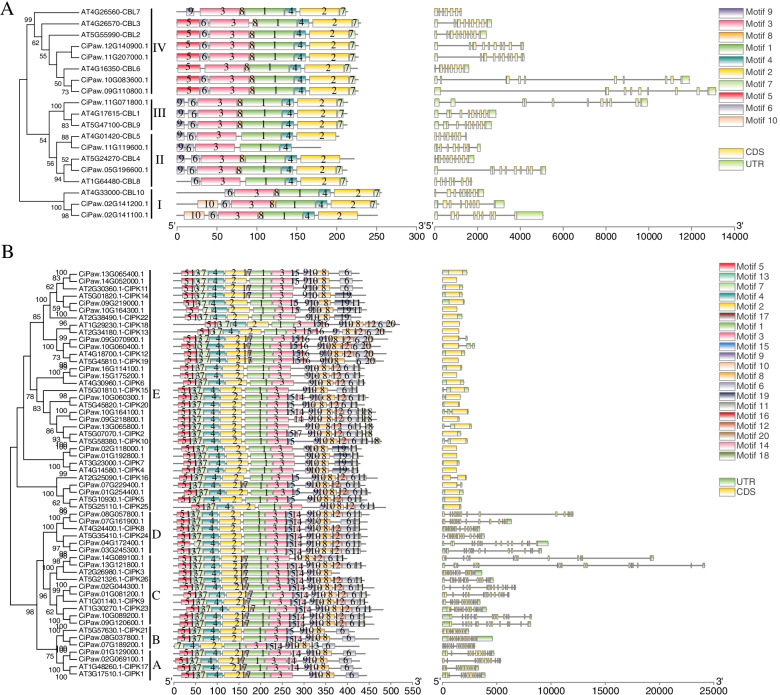
The evolutionary relationships of pecan CBLs and CIPKs were investigated according to phylogenetic trees, which were built using pecan and Arabidopsis protein sequences. Nine CiCBLs were divided into four clades (I–V), and Clade IV was composed of the maximum number of 4 members. Thirty CiCIPK proteins were also divided into five clades (A–E), and Clade E contained 16 CIPKs (Fig. 1). The classification result was consistent with previous reports in Arabidopsis [17, 42].
Fig. 1.
Phylogenetic relationship, gene structure and motif organization analyses of CBLs (A) and CIPKs (B) from pecan and Arabidopsis. The phylogenetic trees on the left included 19 CBL and 56 CIPK proteins from pecan and Arabidopsis and are divided into different clades. The conserved motifs are represented by different coloured boxes. Exon/intron structures of CBL and CIPK genes from pecan are also shown. Green boxes: untranslated regions; yellow boxes: exon regions; black lines: introns
Furthermore, the conserved motifs of CBL and CIPK proteins from pecan and Arabidopsis were visualized (Fig. 1). Ten different motifs with variable lengths were identified in CBLs from pecan and Arabidopsis, and all these proteins contained motifs 1, 3, and 4. Most genes in the same clade exhibited similar motif patterns; for example, CiPaw.02G141100 and CiPaw.02G141200 in clade IV possessed motif 10. Four CiCBLs in clade IV possessed motif 5 (Fig. 1A). The detailed sequence information of the 10 motifs is provided in Additional file 1: Table S2. Twenty conserved motifs were identified in CIPK proteins, and a similar phenomenon also occurred in the pecan CIPK gene family (Fig. 1B). Motifs 5, 13, 7, 4, 2, 1, and 3 at the N-terminus were composed of the catalytic kinase domain. Additionally, motif 9 was the NAF motif, which played a key role in interacting with CBL proteins (Additional file 1: Table S3) [4].
The gene structure analyses showed that each CiCBL gene contained multiple introns, which ranged from 7 to 9, while the intron numbers of AtCBLs ranged from 6 to 9, suggesting that the intron numbers were relatively conserved (Fig. 1A). However, we found that pecan CIPK genes could be divided into two main clusters: an intron-poor cluster (< 4 introns per gene) and an intron-rich cluster (> 10 introns per gene) [43, 44]. All the intron-poor CiCIPK genes that contained 0–2 introns were clustered to clade E; however, the intron-rich members containing 11–16 introns were gathered to the other four clades (A–D) (Fig. 1B).
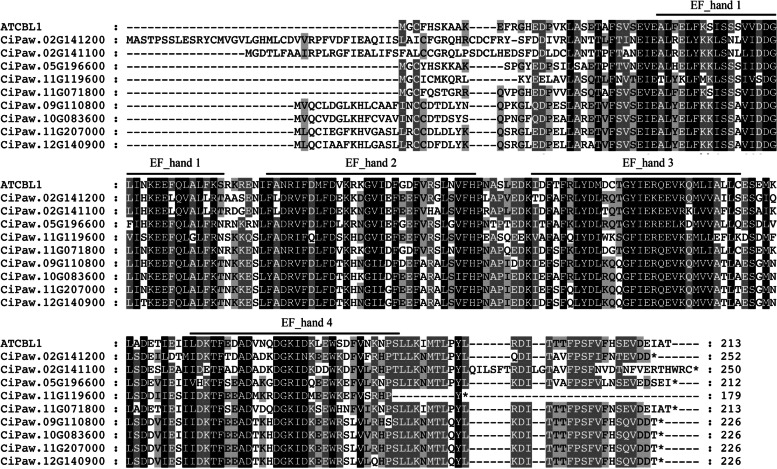
CBLs are able to bind Ca2+ by EF-hands. After comparing the EF-hand motifs of AtCBL1, we also found that four EF-hand motifs existed in all 9 pecan CBL proteins (Fig. 2). Two CiCBLs (CiPaw.05G196600 and CiPaw.11G071800) contained a conserved N-terminal myristoylation site (MGCXXS/T), which might play roles in protein stability and aggregation [45]. CIPK proteins contain an N-terminal protein kinase domain and a NAF domain at the C-terminus. The CBL-CIPK signalling network is mediated by the conserved NAF domain, and our results revealed that the NAF domains in CIPK proteins were highly conserved with many identical residues between Arabidopsis and pecan (Additional file 2: Fig. S1).
Fig. 2.
Domain analysis of pecan CBL proteins. Multiple sequence alignment was performed by ClustalW and illustrated by GeneDoc. The four EF-hand motifs were marked by overbars
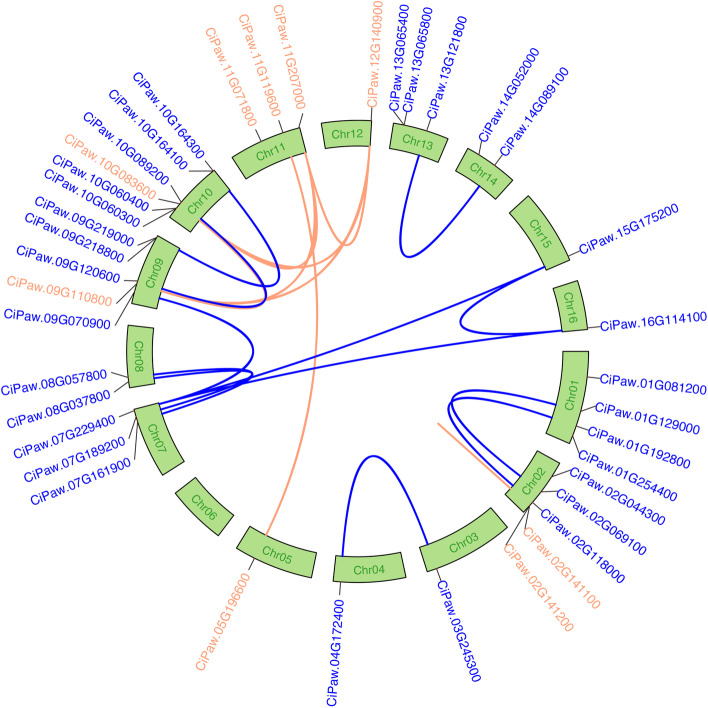
Chromosomal location and duplication events among pecan CBL and CIPK genes
Genome chromosomal location analyses revealed that 9 CiCBLs were distributed on six out of the sixteen chromosomes. Chromosome 11 contained the largest number of CiCBL genes, with three, chromosome 2 had two, and the other four were located on chromosomes 5, 9, 10, and 12 (Fig. 3). The 30 CiCIPK genes were unevenly mapped on 12 pecan chromosomes, except chromosomes 5, 6, 11, and 12. Specifically, chromosome 10 contained the maximum of 5 CiCIPK genes, followed by chromosomes 1 and 9, which both contained 4. In contrast, chromosomes 3, 4, 15 and 16 had only one CiCIPK gene (Fig. 3). These results suggested that genetic variations occurred during the evolutionary process of pecan.
Fig. 3.
Circos figure for chromosomal locations and interchromosomal relationships of CBL and CIPK genes from pecan. The CIPK genes are marked in blue, and the CBL genes are marked in orange. The blue lines in the background represent the gene pairs in the CIPK family, and the orange lines indicate the gene pairs in the CBL family
Furthermore, MCScanX was applied to analyse the duplication events in the pecan CBL and CIPK gene families. In the pecan CBL gene family, seven segmental duplication events with 8 genes and one tandem duplication event were found, suggesting that most CiCBL genes were generated from segmental duplication (Additional file 1: Table S4). In addition, twelve segmental duplication events with 20 CiCIPKs were identified (Additional file 1: Table S4). The above results showed that segmental duplication events played a central role in the evolution of CiCBL and CiCIPK genes.
The synonymous (Ks) and nonsynonymous (Ka) duplication events were further calculated, and the ratio of Ka/Ks could be used to explore the selection pressures influencing sequence divergence (Additional file 1: Table S4). A value of Ka/Ks < 1 indicates negative selection, Ka/Ks > 1 indicates positive selection, and Ka/Ks = 1 indicates neutral selection. Amino acid replacements that increased fitness were retained by positive selection, whereas replacements that reduced fitness were removed by negative selection [37]. The Ka/Ks values of the segmental and tandem duplication events from pecan CBL and CIPK gene families ranged from 0.05 to 0.45, showing that these genes might experience strong negative selection (Additional file 1: Table S4).
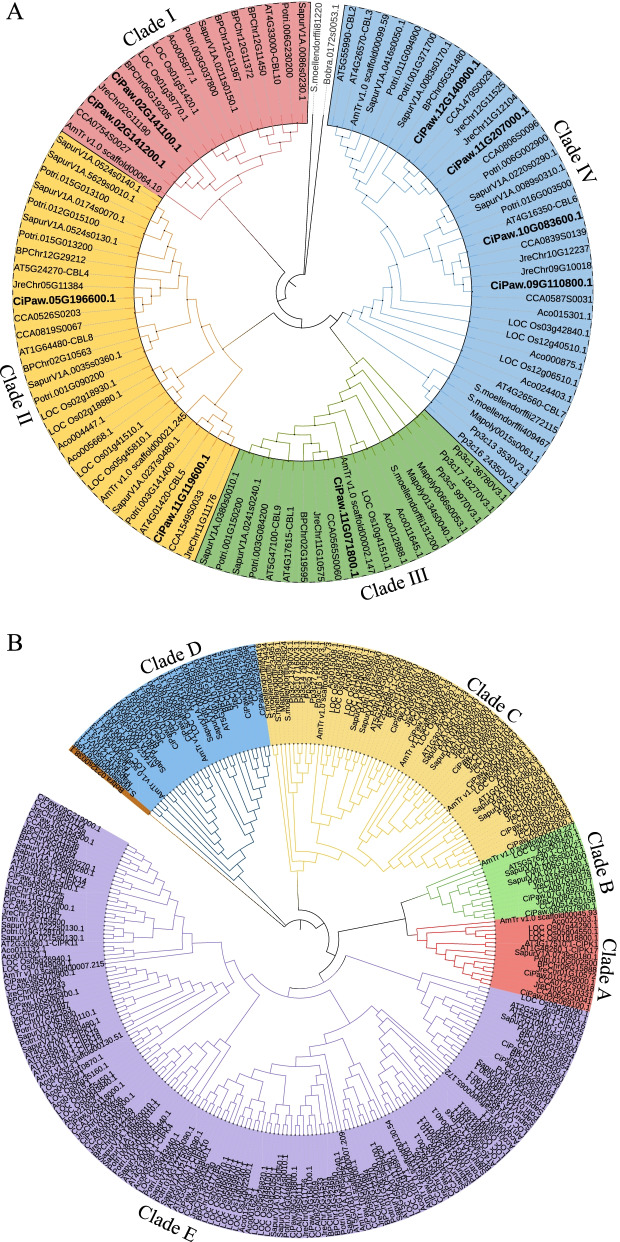
Comparative phylogenetic analyses of the CBL and CIPK families in different species
To elucidate the evolutionary relationships among the CBL and CIPK gene families in green algae and land plants, we retrieved candidates of family members from 14 plant genomes, including green algae (B. braunii), mosses (P. patens), liverworts (M. polymorpha), lycophytes (S. moellendorffii), basal angiosperms (A. trichopoda), monocots (O. sativa, A. comosus), and eudicots (A. thaliana, B. platyphylla, C. illinoinensis, C. cathayensis, J. regia, P. trichocarpa, and S. purpurea). In total, 106 CBL and 283 CIPK candidate proteins were identified from the 14 genomes and applied to construct phylogenetic trees of the CBL and CIPK families (Fig. 4). The numbers of CBLs in the 14 detected species ranged from 1 (B. braunii) to 14 (S. purpurea), while the numbers of CIPKs varied from 1 (B. braunii) to 35 (O. sativa, S. purpurea). We found that the number of CBLs was commonly smaller than that of CIPKs among these species, except M. polymorpha, which contained 3 CBL and 2 CIPK members (Additional file 1: Table S5).
Fig. 4.
Phylogenetic relationships of the CBL (A) and CIPK (B) proteins of pecan with those of 13 plant species. The full-length CBL and CIPK protein sequences from C. illinoinensis, C. cathayensis, J. regia, A. thaliana, B. braunii, M. polymorpha, P. patens, S. moellendorffii, A. trichopoda, O. sativa, A. comosus, B. platyphylla, P. trichocarpa, and S. purpurea were used to build the phylogenetic tree with FastTree. The algae CBL and CIPK proteins were applied as outgroups. The clades are highlighted in different colours
Phylogenetic trees of CBLs and CIPKs were built after sequence alignment analysis. For the phylogeny of the CBL gene family (Fig. 4A), the phylogenetic tree was clearly divided into four clades, which was consistent with our previous results in Fig. 1A. Interestingly, CBL members in Clades I and II were all from seed plants, and CBLs in the moss and liverwort were all grouped in Clades III and IV. For the CIPK gene family (Fig. 4B), the phylogenetic tree was classified into five clades, which was also consistent with previous results (Fig. 1B). The intron-poor CIPK genes were all categorized in Clade E and first appeared in A. trichopoda, the basal angiosperm (Fig. 4B). However, CIPK genes in the moss, liverwort, and lycophyte all contained multiple introns, which were grouped in Clades C and D.
Expression profiling of CiCBL and CiCIPK genes in different pecan tissues
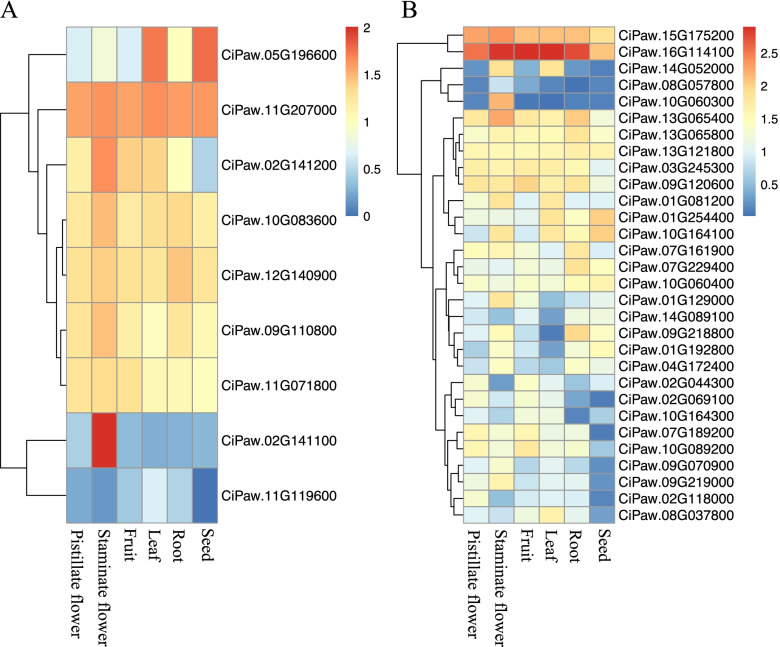
The expression profile of a gene might help to elucidate its biological function. To determine the temporal and spatial expression levels of the pecan CBL and CIPK genes, we investigated the expression profiles of CiCBLs and CiCIPKs using RNA-Seq data of six different tissues of pecan, including seeds, roots, leaves, young fruits, staminate flowers, and pistillate flowers (Fig. 5). Among the nine CiCBL genes, three Clade IV members, CiPaw.09G110800, CiPaw.10G083600 and CiPaw.12G140900, exhibited similar expression patterns in different tissues, and the remaining member, CiPaw.11G207000, was highly expressed in all six tissues (Fig. 5A). Other CiCBLs showed tissue-specific expression patterns. Surprisingly, CiPaw.02G141100 and CiPaw.02G141200 in Clade I both showed high expression levels in the staminate flower, indicating that they might function in the development of staminate flowers in pecan. CiPaw.05G196600 had high expression levels in leaf and seed tissues; however, CiPaw.11G119600 was expressed at low levels in all detected tissues.
Fig. 5.
Hierarchical clustering of expression levels of pecan CBL and CIPK genes in different tissues. A Expression patterns of CBL genes in various pecan tissues. B Expression patterns of CIPK genes in various pecan tissues. Heatmaps of CiCIPK and CiCBL genes were built based on log 10 (FPKM+ 1) values using R language
The expression data of thirty CiCIPK genes in six tissues showed that different genes exhibited different expression patterns (Fig. 5B). Two CIPK genes (CiPaw.15G175200 and CiPaw.16G114100) generated from segmental duplication both exhibited high expression levels in all tissues. The majority of CIPK genes exhibited tissue-specific expression patterns, suggesting that they play roles in different biological processes. For example, CiPaw.10G060300 was highly expressed only in staminate flowers, and CiPaw.08G037800 exhibited high expression levels in leaves. CiPaw.09G218800, CiPaw.01G192800, and CiPaw.04G172400 showed relatively high expression levels in the root, seed, and staminate flower tissues.
Expression patterns and coexpression networks of CiCBL and CiCIPK genes in response to drought
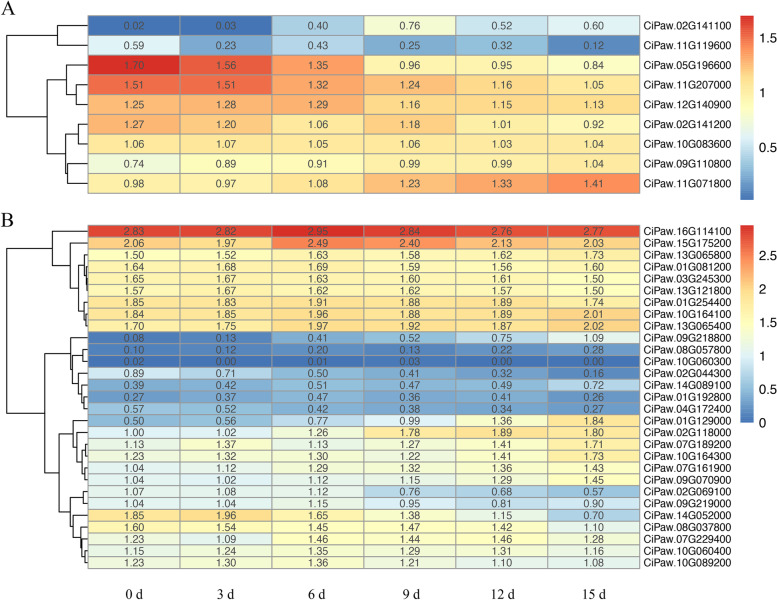
Drought is one of the most important environmental stress problems that inhibit plant growth [14]. As a positive role in plants response to drought, proline was significantly accumulated in pecan seedlings under drought, especially after treatment for 6 d (Additional file 2: Fig. S2A). The accumulation of reactive oxygen species (ROS) has been found to be stimulated in plants under drought stress, resulting in oxidative stress. Plants were protected against the negative effects of ROS by a complex antioxidant system including SODs, which play a crucial role in the removal of ROS [46]. To investigate the SODs responsible for the scavenging of ROS, the activity of SOD was tested in pecan seedlings after drought treatment, and we found the SOD activity also increased significantly (Additional file 2: Fig. S2B).
The CBL-CIPK module has been reported to be involved in the response to environmental stresses, especially drought stress [42, 47]. To investigate the roles of CiCBLs and CiCIPKs in response to drought, their expression profiles were analysed using RNA-Seq datasets. In total, 9 available CBL and 29 CIPK genes showed their expression patterns under drought stress (Fig. 6). Two CiCBL genes (CiPaw.02G141100 and CiPaw.11G119600) showed low expression levels under drought treatment, and CiPaw.05G196600 and CiPaw.11G207000 gradually decreased and showed the lowest expression levels after 15 days of drought application (Fig. 6A). In contrast, CiPaw.09G110800 and CiPaw.11G071800 were gradually upregulated by drought at different time points. Twenty-nine CiCIPK genes showed various expression patterns in pecan subjected to drought stress (Fig. 6B). Six CiCIPKs were expressed at low levels, while three genes were downregulated. Most of the remaining CIPK genes were upregulated, ten of which gradually increased and peaked at 15 days of drought application.
Fig. 6.
Hierarchical clustering of expression levels of pecan CBL and CIPK genes in response to drought. A Expression patterns of CBL genes under drought. B Expression patterns of CIPK genes under drought. Heatmaps of CiCIPK and CiCBL genes were built based on log 10 (FPKM+ 1) values using R language
A coexpression network was further built to explore the mutual relationships of pecan CBL and CIPK genes by analysing their transcript levels under drought. Within the network, 34 nodes (9 CBLs and 25 CIPKs) and 172 edges were found, and 17 nodes contained more than ten edges, suggesting that these nodes were tightly correlated (Additional file 2: Fig. S3). CiPaw.11G071800 contained the maximum numbers of edges, which included 5 CBLs and 15 CIPKs. According to the 172 coexpression events, 105 exhibited significantly positive correlations, and the remaining 67 exhibited significantly negative correlations.
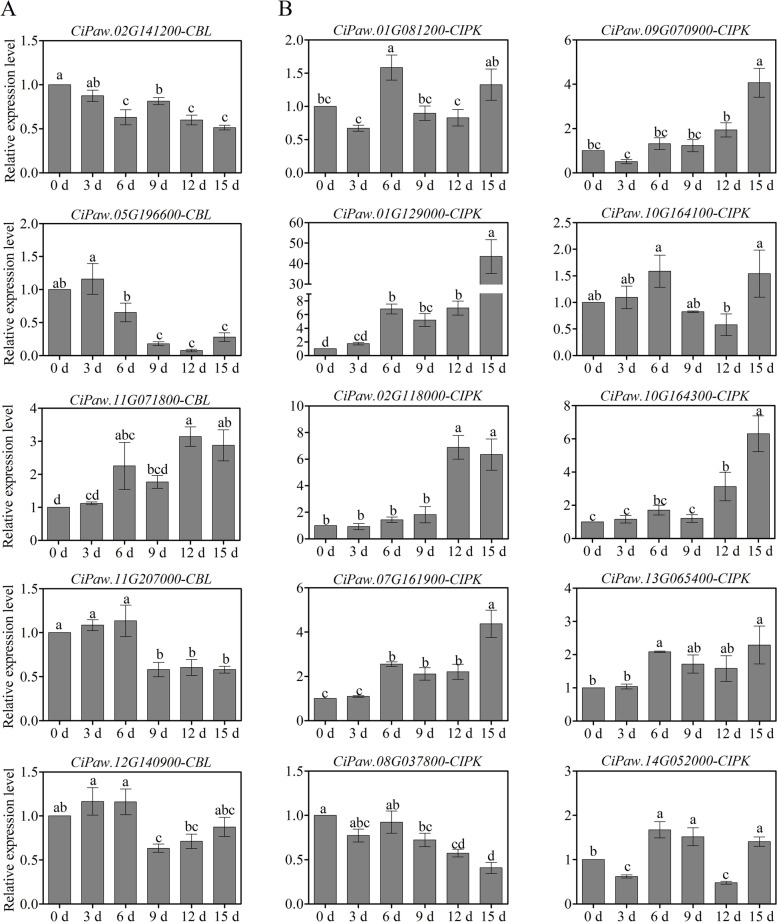
To validate the reliability of the RNA-Seq data, qRT–PCR was applied to analyse the expression levels of pecan CBL and CIPK genes under drought stress. Based on the RNA-Seq and coexpression results, 5 CiCBL and 10 CiCIPK genes were selected for further confirmation after drought stress was imposed (Fig. 7). The specific primers used in the study were listed in Additional file 1: Table S6. As shown in Fig. 7A, the expression patterns of five CiCBL genes were consistent with the previous RNA-Seq results (Fig. 6). Four CiCBLs were downregulated, while CiPaw.11G071800 was significantly induced after 12 and 15 d of drought treatment. The expression patterns of 90% CiCIPKs were consistent between RNA-Seq and qRT–PCR results, except for CiPaw.14G052000, which exhibited different expression patterns (Fig. 7B). Unlike CiCBLs, most CIPK genes were enhanced by drought treatment, especially after 15 d of treatment, indicating that they might play roles in the response to drought. For example, CiPaw.01G129000 was gradually upregulated under drought stress and displayed the highest expression level at 15 days after treatment, which was more than 40-fold higher than that of the control (0 d).
Fig. 7.
Expression analysis of CiCBL and CiCIPK genes in pecan exposed to drought stress. Expression patterns of 5 CiCBL (A) and 10 CiCIPK (B) genes were analysed using qRT–PCR. The actin gene (CiPaw.03G124400) was selected as the reference gene. Lowercase letters represent significant differences (P < 0.05) according to Duncan’s multiple range test. Error bars indicate the means ± SE obtained from three biological replicates
Subcellular localization of CiCIPKs
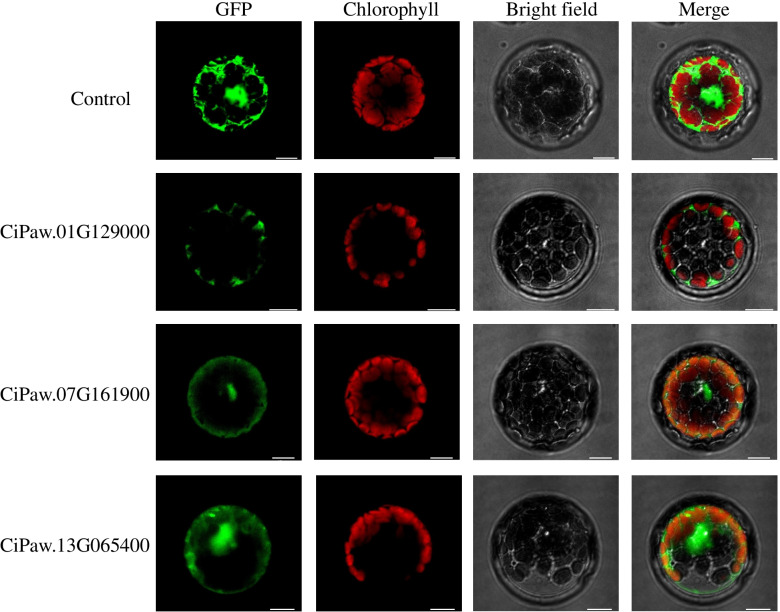
The subcellular localization of a protein might help to reveal its function. According to the expression (Figs. 6 and 7) and coexpression analysis (Additional file 2: Fig. S3), three CiCIPK genes (CiPaw.01G129000, CiPaw.07G161900, and CiPaw.13G065400) that coexpressed with more than ten genes were induced gradually by drought treatment, and showed the highest expression levels after 15 d of drought. These CiCIPKs were selected as candidate genes, and the coding regions of the three genes were fused to the green fluorescent protein (GFP) reporter gene in the binary vectors. Then, the recombinant constructs were transiently expressed in Arabidopsis protoplasts. The results revealed that the control GFP was distributed throughout the cell, whereas the CiPaw.01G129000-GFP fluorescence signal was only found in the cytoplasm, suggesting that the CiPaw.01G129000 protein was localized to the cytoplasm. Moreover, the CiPaw.07G161900-GFP and CiPaw.13G065400-GFP fusion proteins were detected in both the cytoplasm and nucleus (Fig. 8).
Fig. 8.
Subcellular localization of three CiCIPKs based on the transient expression of fused GFPs. Control, GFP alone. Bar = 10 μm
Discussion
The role of calcium in stress signal transduction has been well established in plants [48]. CBLs are plant-specific Ca2+ sensors that interact with and activate CIPKs to transduce Ca2+ signals [4]. To date, CBL and CIPK family members have been identified in model plants and major crops, including Arabidopsis, canola, rice, and poplar [8, 9, 49]. However, very little is known in pecan.
In the current study, 9 CiCBL and 30 CiCIPK members were found in the pecan genome (Fig. 1). Similar to those CBL proteins in Arabidopsis and canola [49], four EF-hand motifs were found in the nine CiCBL proteins (Fig. 2). As a type of Ca2+ sensor, CBL proteins are able to bind Ca2+ through EF-hand motifs [10]. In addition, two CiCBL proteins started with a conserved N-terminal myristoylation site, which might play a role in membrane association of the CBL-CIPK complex by switching calcium with myristoyl [50]. CIPK proteins commonly possess an N-terminal kinase domain and a conserved C-terminal NAF domain, and all CiCIPKs contained these two domains (Fig. 1B). CBLs bind to the short NAF domains to release from the kinase domain and switch the kinase into an active conformation [51].
Comparison of the numbers of CBL and CIPK genes in pecan with other sequenced plant genomes revealed that the two gene families have expanded multiple times in evolutionary history (Additional file 1: Table S5) [52]. In the green algae Botryococcus braunii, both CBL and CIPK contained only one family member, and the family sizes increased quickly following evolution to seed plants. Gene duplication commonly plays a key role in the expansion of a gene family during evolution and functions in adaptation to environments by obtaining new gene functions in plants [53]. Segmental/whole-genome duplications and tandem duplications contribute to the evolutionary expansion of gene families [54]. In pecan, segmental duplication occurred in both CiCBLs and CiCIPKs, and contributed largely to the expansion of the two families, while only one tandem duplication event was detected in the CBL family (Fig. 3). CIPKs resulting from both segmental and tandem duplications were found in Arabidopsis, rice, maize, and grape [16, 43]. In contrast, only tandem duplication events were detected in grape CBLs, and tandem duplications played major roles in Medicago CBL and CIPK families [16, 55]. Negative selection functions in the process of removing deleterious mutations to prevent functional divergence; however, positive selection plays a role in new advantageous mutation collection and spreading throughout the population [56]. The Ka/Ks ratios of all the segmental and tandem duplication events in pecan CBLs and CIPKs showed that they were driven by negative selection (Additional file 1: Table S4). Gene duplication can lead to accumulate degenerative mutations, and negative selection may result in stabilizing selection via removing deleterious variations that arise, indicating these CiCBLs and CiCIPKs under negative selection were functionally conserved, respectively [57]. The CBL and CIPK genes of Medicago have also undergone strong negative selection pressure during evolution [55].
According to the phylogenetic analysis, pecan CBLs and CIPKs were classified into four and five clades, which was consistent with previous findings in grape and pepper (Fig. 4) [16, 58]. Gene structures such as exon-intron organizations and intron numbers are imprints of evolution in several gene families [59]. Eukaryotic genes could be divided into intron-rich, intron-poor (less than four introns), or intronless (no introns), and genes of early-diverging plant species were mostly intron-rich [60]. The intron numbers in CiCBLs were very conserved, ranging from 7 to 9; however, the CiCIPKs were clearly divided into an intron-rich cluster (clades A, B, C, and D) and an intron-poor cluster (clade E) (Fig. 1). This phenomenon of gene structure in CIPK genes widely exists in Arabidopsis, canola, grape, and soybean [1, 16, 42, 49]. Interestingly, the intron-poor cluster members in the CIPK gene family first appeared in seed plants, while CIPKs in algae, moss, spikemoss, and fern all possessed multiple introns (Fig. 4) [5, 42]. These results suggested that intron loss and gain events functioned in the evolution of plant CIPK genes. Interestingly, clustering expression results suggested that Arabidopsis CIPK genes could be induced by environmental stresses, and some CIPK genes in the two clusters shared similar expression patterns in response to environmental stresses [43]. For example, three CIPKs (CIPK8, CIPK21 and CIPK24) in intron-rich cluster and two (CIPK6 and CIPK13) in intron-poor cluster were all involved in the regulation of salt stress [61–65]. For CBLs, proteins in the same clade have high sequence similarities (Fig. 2), when fused with GFP, three CBLs including CBL2, CBL3 and CBL6 in clade IV were localized at the tonoplast, while CBL1 and CBL9 in Clade III were both detected at the plasma membrane [66]. Moreover, CBL1 and CBL9 both functioned in K+ homeostasis under low K+ condition via interacting with CIPK23 in Arabidopsis [67]. These two CBLs also play a crucial role in pollen germination and tube growth [68]. CBL2 and CBL3 coexpressed with CIPK21 to respond to salt stress by regulating ion and water homeostasis [63]. Although genes in the same clade were considered to be functionally identical, CIPKs and CBLs with high sequence similarities may show distinct functions, such as CBL1 showed distinctively different functions from CBL9 in response to ABA treatment [69].
Drought is a major environmental stress that profoundly affects the growth, development, and productivity of plants. CBL-CIPK complexes have been previously proven to play important roles in drought stress signalling pathways [42]. Our RNA-Seq data under drought treatment revealed that the expression levels of some CiCBLs and CiCIPKs were drastically changed (Fig. 6). Most CiCBL genes were downregulated in response to drought, except CiPaw.11G071800, which was significantly induced (Fig. 7A). Orthologous AtCBL9 from Arabidopsis has been shown to be essential for the drought stress response. AtCBL9 was involved in the drought-induced abscisic acid (ABA) production process, which was highly inducible by drought and treatments with the plant hormone ABA, and the cbl9 mutant seedlings were more sensitive to drought [70]. Unlike CiCBLs, the majority of CiCIPK genes were upregulated under drought stress (Fig. 6B), which was further validated by qRT–PCR (Fig. 7B). As protein kinases, CIPKs play central roles in the response to drought in plants, and RNA-Seq results of drought treatment in grapevine revealed that the expression levels of many kinase genes changed, including CIPKs [71]. Interestingly, most CIPKs in soybean and cassava were drought-responsive genes, especially family members belonging to the intron-poor clade [42, 72]. AcCIPK18, an intronless CIPK gene in pineapple, is a positive regulator of drought stress, and the overexpression lines showed significantly stronger drought tolerance than wild-type plants [73]. CaCIPK3 is an intronless gene in pepper that participates in the response to various stresses, including drought. Knockdown of CaCIPK3 improves sensitivity to drought, while overexpression of CaCIPK3 increases drought tolerance by enhancing the activities of antioxidant defence systems [47]. The CcCBL1-CcCIPK14 complex positively regulates drought tolerance through modulation of flavonoid biosynthesis in pigeon pea. CcCIPK14-overexpressing (CcCIPK14-OE) plants had enhanced drought tolerance, but this phenomenon was reversed in CcCBL1-RNAi CcCIPK14-OE double transgenic plants [74].
In conclusion, a total of 9 CBLs and 30 CIPKs were annotated in pecan at the genome scale and were classified into four and five clades, respectively. The gene structure and conserved domains, chromosome locations, duplication events, evolutionary relationships, and expression patterns of CiCBL and CiCIPK genes were investigated. The results indicated that CiCBLs and CiCIPKs might function in development and drought response in pecan. Overall, this research provides important information concerning pecan CBLs and CIPKs, and will be helpful for further functional investigation.
Supplementary Information
Additional file 1: Table S1. CBL and CIPK genes identified in pecan and their sequence characteristics. Table S2. Motif sequences of pecan CBLs. Table S3. Motif sequences of pecan CIPKs. Table S4. Duplication events and related Ka/Ks values of pecan CBLs and CIPKs. Table S5. The CBL and CIPK protein numbers in different plant species. Table S6. Specific primers of pecan CBL and CIPK genes for qRT-PCR analysis.
Additional file 2: Figure S1. Highly conserved NAF domain across CIPK proteins in pecan (A) and Arabidopsis (B). Multiple alignment analysis of CIPK domains was presented by ClustalW and sequence logos were generated by Weblogo. Figure S2. Analysis of proline content (A) and SOD activity (B) of pecan seedlings in response to drought. Lowercase letters represent significant differences (P < 0.05) according to Duncan’s multiple range test. Error bars indicate the means ± SE obtained from three biological replicates. Figure S3. The coexpression network of CBL and CIPK genes in response to drought in pecan. The nodes indicate different genes, and the edges between nodes indicate coexpression correlations of gene pairs (P < 0.05). Edge line colours indicate either positive (red, PCC ≥ 0.6) or negative (blue, PCC ≤ − 0.6) correlations.
Acknowledgements
We would like to thank GENE DENOVO Biotechnology Co., Ltd. (Guangzhou, China) for RNA sequencing support.
Abbreviations
- CBL
Calcineurin B-like protein
- CIPK
CBL-interacting protein kinase
- EF-hand
Elongation factor hand
- FPKM
Fragments per kilobase per million of reads mapped
- GFP
Green fluorescent protein
- GRAVY
Grand average of hydropathicity
- HMM
Hidden Markov model
- Ka
Nonsynonymous substitution
- Ks
Synonymous substitution
- MW
Molecular weight
- PCC
Pearson correlation coefficient
- PEG
Polyethylene glycol
- pI
Isoelectric point
- qRT–PCR
Quantitative real-time PCR
- RNA-Seq
RNA sequencing
- SOD
superoxide dismutase
Authors’ contributions
KZ and FP conceived this research. KZ, PF, PT, and JZ did the experiments. HL, GC, ZM, and WM analysed the data. KZ and PF wrote the paper. FP modified the manuscript. The authors read and approved the final manuscript.
Funding
This research was financially supported by the National Key Research and Development Program of China (grant number 2018YFD1000604), the Natural Science Foundation of Jiangsu Province (grant number BK20190749), the China Postdoctoral Science Foundation (grant number 2020 M681628), Postdoctoral Research Funding Program of Jiangsu Province (grant number 013010428), and the Startup Foundation of Nanjing Forestry University (grant number 163010226).
Availability of data and materials
All the datasets used for this research are available on reasonable request from the corresponding author. The RNA sequencing raw data can be found in the NCBI BioProject (PRJNA799663) and Gene Expression Omnibus (GEO) database (GSE179336), respectively.
Declarations
Ethics approval and consent to participate
The experimental research and field studies on pecans (either cultivated or wild), including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kaikai Zhu, Email: kkzhu@njfu.edu.cn.
Pinghua Fan, Email: 15295537732@163.com.
Hui Liu, Email: liuhui@njau.edu.cn.
Pengpeng Tan, Email: tanpengpeng2002@163.com.
Wenjuan Ma, Email: mabuer1998@163.com.
Zhenghai Mo, Email: mozhenghai@yeah.net.
Juan Zhao, Email: cxyl@njfu.edu.cn.
Guolin Chu, Email: cglluck66@163.com.
Fangren Peng, Email: frpeng@njfu.edu.cn.
References
- 1.Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. Calcium sensorsand their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004;134(1):43–58. doi: 10.1104/pp.103.033068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou LM, Lan WZ, Chen BQ, Fang W, Luan S. A calcium sensor-regulated protein kinase, calcineurin B-like protein-interacting protein kinase 19, is required for pollen tube growth and polarity. Plant Physiol. 2015;167(4):1351–1395. doi: 10.1104/pp.114.256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack E, Tsai YC, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10(8):383–389. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14(1):37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XX, Li XX, Zhao R, Zhou Y, Jiao YN. Evolutionary strategies drive a balance of the interacting gene products for the CBL and CIPK gene families. New Phytol. 2020;226(5):1506–1516. doi: 10.1111/nph.16445. [DOI] [PubMed] [Google Scholar]
- 6.Chaves-Sanjuan A, Sanchez-Barrena MJ, Gonzalez-Rubio JM, Moreno M, Ragel P, Jimenez M, et al. Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc Natl Acad Sci U S A. 2014;111(42):E4532–E4541. doi: 10.1073/pnas.1407610111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht V, Ritz O, Linder S, Harter K, Kudla J. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001;20(5):1051–1063. doi: 10.1093/emboj/20.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwar P, Sanyal SK, Tokas I, Yadav AK, Pandey A, Kapoor S, et al. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice. Cell Calcium. 2014;56(2):81–95. doi: 10.1016/j.ceca.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Yu YH, Xia XL, Yin WL, Zhang HC. Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus. Plant Growth Regul. 2007;52(2):101–110. doi: 10.1007/s10725-007-9165-3. [DOI] [Google Scholar]
- 10.Zhang HC, Yin WL, Xia XL. Calcineurin B-like family in Populus: comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul. 2008;56(2):129–140. doi: 10.1007/s10725-008-9293-4. [DOI] [Google Scholar]
- 11.Weinl S, Kudla J. The CBL-CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol. 2009;184(3):517–528. doi: 10.1111/j.1469-8137.2009.02938.x. [DOI] [PubMed] [Google Scholar]
- 12.Fracasso A, Trindade LM, Amaducci S. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 2016;16(1):115. doi: 10.1186/s12870-016-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Cushman JC, Borland AM, Edwards EJ, Wullschleger SD, Tuskan GA, et al. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 2015;207(3):491–504. doi: 10.1111/nph.13393. [DOI] [PubMed] [Google Scholar]
- 14.Singh D, Laxmi A. Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Front Plant Sci. 2015;6:895. doi: 10.3389/fpls.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci. 2015;6:84. doi: 10.3389/fpls.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi Y, Liu J, Dong C, Cheng ZM. The CBL and CIPK gene family in grapevine (Vitis vinifera): genome-wide analysis and expression profiles in response to various abiotic stresses. Front Plant Sci. 2017;8:978. doi: 10.3389/fpls.2017.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Feng X, Yao L, Ding C, Lei L, Hao X, et al. Characterization of CBL-CIPK signaling complexes and their involvement in cold response in tea plant. Plant Physiol Biochem. 2020;154:195–203. doi: 10.1016/j.plaphy.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelo C, Weinl S, Batistic O, Pandey GK, Cheong YH, Schultke S, et al. Alternative complex formation of the Ca-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J. 2006;48(6):857–872. doi: 10.1111/j.1365-313X.2006.02921.x. [DOI] [PubMed] [Google Scholar]
- 19.Grauke LJ, Wood BW, Harris MK. Crop vulnerability: Carya. Hortscience. 2016;51(6):653–663. doi: 10.21273/HORTSCI.51.6.653. [DOI] [Google Scholar]
- 20.Atanasov AG, Sabharanjak SM, Zengin G, Mollica A, Szostak A, Simirgiotis M, et al. Pecan nuts: a review of reported bioactivities and health effects. Trends Food Sci Technol. 2018;71:246–257. doi: 10.1016/j.tifs.2017.10.019. [DOI] [Google Scholar]
- 21.Lovell JT, Bentley NB, Bhattarai G, Jenkins JW, Sreedasyam A, Alarcon Y, et al. Four chromosome scale genomes and a pan-genome annotation to accelerate pecan tree breeding. Nat Commun. 2021;12(1):4125. doi: 10.1038/s41467-021-24328-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodstein DM, Shu SQ, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(D1):D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46(W1):W200–W204. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46(D1):D493–D496. doi: 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne DR, Jenkins J, Schmutz J, Shu S, Barry K, Grimwood J, et al. Draft nuclear genome sequence of the liquid hydrocarbon-accumulating green microalga Botryococcus braunii race B (Showa) Genome Announc. 2017;5(16):e00215–e00217. doi: 10.1128/genomeA.00215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YJ, Xiao LH, Zhang ZR, Zhang R, Wang ZJ, Huang CY, et al. The genomes of pecan and Chinese hickory provide insights into Carya evolution and nut nutrition. Gigascience. 2019;8(5):giz036. doi: 10.1093/gigascience/giz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Li J, Paterson AH. MCScanX-transposed: detecting transposed gene duplications based on multiple colinearity scans. Bioinformatics. 2013;29(11):1458–1460. doi: 10.1093/bioinformatics/btt150. [DOI] [PubMed] [Google Scholar]
- 35.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 36.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 37.Zhu KK, Fan PH, Mo ZH, Tan PP, Feng G, Li FD, et al. Identification, expression and co-expression analysis of R2R3-MYB family genes involved in graft union formation in pecan (Carya illinoinensis) Forests. 2020;11(9):917. doi: 10.3390/f11090917. [DOI] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 42.Zhu KK, Chen F, Liu JY, Chen XL, Hewezi T, Cheng ZM. Evolution of an intron-poor cluster of the CIPK gene family and expression in response to drought stress in soybean. Sci Rep. 2016;6:28225. doi: 10.1038/srep28225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye CY, Xia XL, Yin WL. Evolutionary analysis of CBL-interacting protein kinase gene family in plants. Plant Growth Regul. 2013;71(1):49–56. doi: 10.1007/s10725-013-9808-5. [DOI] [Google Scholar]
- 44.Wan X, Zou LH, Zheng BQ, Wang Y. Circadian regulation of alternative splicing of drought-associated CIPK genes in Dendrobium catenatum (Orchidaceae) Int J Mol Sci. 2019;20(3):688. doi: 10.3390/ijms20030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohanta TK, Mohanta N, Mohanta YK, Parida P, Bae H. Genome-wide identification of Calcineurin B-like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015;15:189. doi: 10.1186/s12870-015-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005;46(3):209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- 47.Ma X, Li Y, Gai WX, Li C, Gong ZH. The CaCIPK3 gene positively regulates drought tolerance in pepper. Hortic Res. 2021;8(1):216. doi: 10.1038/s41438-021-00651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinhorst L, Kudla J. Signaling in cells and organisms-calcium holds the line. Curr Opin Plant Biol. 2014;22:14–21. doi: 10.1016/j.pbi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang HF, Yang B, Liu WZ, Li HW, Wang L, Wang BY, et al. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L.) BMC Plant Biol. 2014;14:8. doi: 10.1186/1471-2229-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batistic O, Kudla J. Plant calcineurin B-like proteins and their interacting protein kinases. BBA-Mol Cell Res. 2009;1793(6):985–992. doi: 10.1016/j.bbamcr.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Zhu S, Zhou XP, Wu XM, Jiang ZH. Structure and function of the CBL-CIPK Ca2+-decoding system in plant calcium signaling. Plant Mol Biol Report. 2013;31(6):1193–1202. doi: 10.1007/s11105-013-0631-y. [DOI] [Google Scholar]
- 52.Kleist TJ, Spencley AL, Luan S. Comparative phylogenomics of the CBL-CIPK calcium-decoding network in the moss Physcomitrella, Arabidopsis, and other green lineages. Front Plant Sci. 2014;5:187. doi: 10.3389/fpls.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanada K, Zou C, Lehti-Shiu MD, Shinozaki K, Shiu SH. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol. 2008;148(2):993–1003. doi: 10.1104/pp.108.122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du W, Yang J, Ma L, Su Q, Pang Y. Identification and characterization of abiotic stress responsive CBL-CIPK family genes in Medicago. Int J Mol Sci. 2021;22(9):4634. doi: 10.3390/ijms22094634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 57.Hou J, Wei S, Pan H, Zhuge Q, Yin T. Uneven selection pressure accelerating divergence of Populus and Salix. Hortic Res. 2019;6:37. doi: 10.1038/s41438-019-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma X, Gai WX, Qiao YM, Ali M, Wei AM, Luo DX, et al. Identification of CBL and CIPK gene families and functional characterization of CaCIPK1 under Phytophthora capsici in pepper (Capsicum annuum L.) BMC Genomics. 2019;20(1):775. doi: 10.1186/s12864-019-6125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boudet N, Aubourg S, Toffano-Nioche C, Kreis M, Lecharny A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 2001;11(12):2101–2114. doi: 10.1101/gr.200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H, Lyu HM, Zhu K, Van de Peer Y, Max Cheng ZM. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021;105(4):1072–1082. doi: 10.1111/tpj.15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Ding Y, Yang Y, Song C, Wang B, Yang S, et al. Protein kinases in plant responses to drought, salt, and cold stress. J Integr Plant Biol. 2021;63(1):531–578. doi: 10.1111/jipb.13061. [DOI] [PubMed] [Google Scholar]
- 62.Yin X, Xia Y, Xie Q, Cao Y, Wang Z, Hao G, et al. The protein kinase complex CBL10–CIPK8–SOS1 functions in Arabidopsis to regulate salt tolerance. J Exp Bot. 2020;71(6):1801–1814. doi: 10.1093/jxb/erz549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandey GK, Kanwar P, Singh A, Steinhorst L, Pandey A, Yadav AK, et al. Calcineurin B-like protein-interacting protein kinase CIPK21 regulates osmotic and salt stress responses in Arabidopsis. Plant Physiol. 2015;169(1):780–792. doi: 10.1104/pp.15.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A. 2002;99(12):8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang H, Ma QJ, Zhong MS, Gao HN, Li YY, Hao YJ. The apple palmitoyltransferase MdPAT16 influences sugar content and salt tolerance via an MdCBL1–MdCIPK13–MdSUT2. 2 pathway. Plant J. 2021;106(3):689–705. doi: 10.1111/tpj.15191. [DOI] [PubMed] [Google Scholar]
- 66.Batistič O, Waadt R, Steinhorst L, Held K, Kudla J. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J. 2010;61(2):211–222. doi: 10.1111/j.1365-313X.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125(7):1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Mähs A, Steinhorst L, Han JP, Shen LK, Wang Y, Kudla J. The calcineurin B-like Ca2+ sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol Plant. 2013;6(4):1149–1162. doi: 10.1093/mp/sst095. [DOI] [PubMed] [Google Scholar]
- 69.Mao J, Manik SM, Shi S, Chao J, Jin Y, Wang Q, Liu H. Mechanisms and physiological roles of the CBL-CIPK networking system in Arabidopsis thaliana. Genes. 2016;7(9):62. doi: 10.3390/genes7090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey GK, Cheong YH, Kim KN, Grant JJ, Li L, Hung W, et al. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell. 2004;16(7):1912–1924. doi: 10.1105/tpc.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu KK, Wang XL, Liu JY, Tang J, Cheng QK, Chen JG, et al. The grapevine kinome: annotation, classification and expression patterns in developmental processes and stress responses. Hortic Res. 2018;5:19. doi: 10.1038/s41438-018-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu W, Xia ZQ, Yan Y, Ding ZH, Tie WW, Wang LZ, et al. Genome-wide gene phylogeny of CIPK family in cassava and expression analysis of partial drought-induced genes. Front Plant Sci. 2015;6:914. doi: 10.3389/fpls.2015.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aslam M, Fakher B, Greaves JG, Jakada BH, Qin R, Qin Y. A CBL-interacting protein kinase, AcCIPK18, from Ananas comosus regulates tolerance to salt, drought, heat stress and Sclerotinia sclerotiorum infection in Arabidopsis. Environ Exp Bot. 2021;194:104728. doi: 10.1016/j.envexpbot.2021.104728. [DOI] [Google Scholar]
- 74.Meng D, Dong B, Niu L, Song Z, Wang L, Amin R, et al. The pigeon pea CcCIPK14-CcCBL1 pair positively modulates drought tolerance by enhancing flavonoid biosynthesis. Plant J. 2021;106(5):1278–1297. doi: 10.1111/tpj.15234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. CBL and CIPK genes identified in pecan and their sequence characteristics. Table S2. Motif sequences of pecan CBLs. Table S3. Motif sequences of pecan CIPKs. Table S4. Duplication events and related Ka/Ks values of pecan CBLs and CIPKs. Table S5. The CBL and CIPK protein numbers in different plant species. Table S6. Specific primers of pecan CBL and CIPK genes for qRT-PCR analysis.
Additional file 2: Figure S1. Highly conserved NAF domain across CIPK proteins in pecan (A) and Arabidopsis (B). Multiple alignment analysis of CIPK domains was presented by ClustalW and sequence logos were generated by Weblogo. Figure S2. Analysis of proline content (A) and SOD activity (B) of pecan seedlings in response to drought. Lowercase letters represent significant differences (P < 0.05) according to Duncan’s multiple range test. Error bars indicate the means ± SE obtained from three biological replicates. Figure S3. The coexpression network of CBL and CIPK genes in response to drought in pecan. The nodes indicate different genes, and the edges between nodes indicate coexpression correlations of gene pairs (P < 0.05). Edge line colours indicate either positive (red, PCC ≥ 0.6) or negative (blue, PCC ≤ − 0.6) correlations.
Data Availability Statement
All the datasets used for this research are available on reasonable request from the corresponding author. The RNA sequencing raw data can be found in the NCBI BioProject (PRJNA799663) and Gene Expression Omnibus (GEO) database (GSE179336), respectively.