Abstract
A checkerboard methodology, based on standardized methods proposed by the National Committee for Clinical Laboratory Standards for broth microdilution antifungal susceptibility testing, was applied to study the in vitro interactions of flucytosine (FC) and posaconazole (SCH 56592) (FC-SCH) against 15 isolates of Cryptococcus neoformans. Synergy, defined as a fractional inhibitory concentration (FIC) index of <0.50, was observed for 33% of the isolates tested. When synergy was not achieved, there was still a decrease in the MIC of one or both drugs when they were used in combination. Antagonism, defined as a FIC of >4.0, was not observed. The in vitro efficacy of combined therapy was confirmed by quantitative determination of the CFU of C. neoformans 486, an isolate against which the FC-SCH association yielded a synergistic interaction. To investigate the potential beneficial effects of this combination therapy in vivo, we established two experimental murine models of cryptococcosis by intracranial or intravenous injection of cells of C. neoformans 486. At 1 day postinfection, the mice were randomized into different treatment groups. One group each received each drug alone, and one group received the drugs in combination. While combination therapy was not found to be significantly more effective than each single drug in terms of survival, tissue burden experiments confirmed the potentiation of antifungal activity with the combination. Our study demonstrates that SCH and FC combined are significantly more active than either drug alone against C. neoformans in vitro as well in vivo. These findings suggest that this therapeutic approach could be useful in the treatment of cryptococcal infections.
Although most infections respond satisfactorily to a single antimicrobial agent, there are clinical circumstances in which a combination of antimicrobial agents is advantageous (1, 2, 3, 4, 5, 13, 18). Antifungal combinations may increase the rate of microbial killing, shorten the duration of therapy, avoid the emergence of drug resistance, expand the spectrum of activity, and decrease drug-related toxicities by allowing the use of lower doses of antifungals. Infection due to Cryptococcus neoformans is an example of an infection against which a combination therapy might be useful: this infection is often incurable, mainly in patients with AIDS, despite aggressive antifungal therapy (16, 28). Indeed, amphotericin B and flucytosine (FC) have been shown in clinical trials to be superior to amphotericin B alone in both human immunodeficiency virus (HIV)-infected patients and non-HIV-infected patients (3, 28).
In the last few years, several antifungal combination therapies, other than the classic combination of amphotericin B and FC, have been evaluated against infections due to C. neoformans or other fungi (4, 7, 8, 12, 13, 18, 23). The combination of polyenes and azoles has always been questioned because of the potential for antagonism (1, 2, 9, 23–26).
Data from both studies with animals and human clinical trials have supported use of the combination of FC and fluconazole (4, 12, 13, 18). Recently, Nguyen et al. (18) studied the effects of therapy with the combination of fluconazole and FC against three isolates of C. neoformans using a murine model of cryptococcal meningitis and found that combination therapy was superior to therapy with either drug alone. In addition, Ding et al. (4) showed that this combination would exert the most potent antifungal effect in animals with more severe cryptococcal meningitis.
Data from the literature regarding the interactions of FC and itraconazole against C. neoformans are controversial (10, 22, 27). Polak (22) experimented with several antifungal combination therapies in mouse models of candidosis, cryptococcosis, aspergillosis, and wangiellosis and found that the combination of FC-itraconazole was definitely synergistic or additive against candidosis and aspergillosis but was indifferent against cryptococcosis. Iovanitti et al. (10) studied this combination therapy in a hamster model of cryptococcosis and found that it was less effective than therapy with the drugs separately. On the contrary, Van Custem (27) found that this combination therapy was more efficacious than monotherapy in an experimental model of murine cryptococcosis.
Posaconazole (SCH; SCH 56592) is a new broad-spectrum antifungal triazole currently under development. SCH has been shown to have potent in vitro and in vivo activities against Candida spp., C. neoformans, Aspergillus spp., Fusarium solani, Blastomyces dermatitidis, and Coccidioides immitis (6, 14, 15, 19–21).
The main aim of the study described here was to evaluate the in vitro and in vivo interactions of this new antifungal triazole with FC against C. neoformans.
MATERIALS AND METHODS
Organisms.
Fifteen isolates of C. neoformans were used in the study. They included 14 clinical isolates (each from a single patient) obtained from blood, cerebrospinal fluid, or skin biopsy specimens from AIDS patients and one strain belonging to the American Type Culture Collection (ATCC): C. neoformans ATCC 90112. All isolates were assigned to C. neoformans var. neoformans on the basis of no color change on canavanine-glycine-bromothymol blue agar (11). All the strains were maintained on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, Mich.) slants at 4°C.
Antifungal agents.
For in vitro studies, a stock solution of FC (Sigma, Milan, Italy) was prepared in sterile distilled water. A stock solution of SCH (Schering Ploug Research Institute, Kenilworth, N.J.) was prepared in polyethylene glycol (polyethylene glycol 400; Janssen Chimica, Geel, Belgium). Further dilutions of both drugs were prepared in the test medium. The final concentration of the solvent did not exceed 1% in any well. For in vivo studies, FC was dissolved in the drinking water, while SCH was prepared in carboxymethyl cellulose and suspended by sonication in 0.3% Noble agar (Difco Laboratories).
Antifungal susceptibility testing. (i) MICs.
Drug interactions were assessed by a checkerboard method based on standardized methods proposed by the National Committee for Clinical Laboratory Standards (NCCLS) for broth microdilution antifungal susceptibility testing (17). Briefly, testing was performed in RPMI 1640 medium (Sigma) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Gibco Laboratories, Milan, Italy) buffer. Volumes of 50 μl of each drug at a concentration of four times the targeted final concentration were dispensed in the wells of 96-well microtiter plates (Falcon 3072; Becton Dickinson). Final concentrations of the antifungal agents ranged from 0.0078 to 4.0 μg/ml for SCH and from 0.125 to 8.0 μg/ml for FC. Yeast inocula (100 μl), prepared spectrophotometrically and further diluted in order to obtain a concentration ranging from 1.0 × 103 to 5.0 × 103 CFU/ml (2× inoculum), were added to each well of the microdilution trays. The trays were incubated in air at 35°C and read at 72 h.
Readings were performed spectrophotometrically with an automatic plate reader (model MR 700; Dynatech) set at 490 nm. MIC endpoints were determined as the first concentration of the antifungal agent tested alone and in combination at which the turbidity in the well was 80% less than that in the control well. Both on-scale and off-scale results were included in the analysis. The high off-scale MICs (>4.0 μg/ml for SCH and >8.0 μg/ml for FC) were converted to the next highest concentrations (8.0 and 16 μg/ml, respectively), and the low off-scale MICs (0.0078 μg/ml for SCH and 0.125 μg/ml for FC) were left unchanged.
(ii) Definitions.
Drug interaction was classified as synergistic, additive, indifferent, or antagonistic on the basis of the fractional inhibitory concentration (FIC) index. The FIC index is the sum of the FIC of each of the drugs, which in turn is defined as the MIC of each drug when used it is in combination divided by the MIC of the drug when it is used alone. The interaction was defined as synergistic if the FIC index was <0.50, additive if the FIC index was >0.50 to 1.0, indifferent if the FIC index was between >1.0 and 4.0, and antagonistic if the FIC index was >4.0 (1a).
(iii) Determination of CFU per milliliter.
Determination of the numbers of CFU per milliliter was performed only with C. neoformans 486. After the MICs were recorded, aliquots of 100 μl from each well were serially diluted and spread onto SDA plates to determine the number of colonies per milliliter. The plates were incubated for 48 to 72 h at 35°C, and then the number of CFU per milliliter was counted.
Animal studies.
In vivo experiments were performed only with C. neoformans 486. Outbred male ICR mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used for the model of cryptococcal meningitis. Male BALB/c mice (Charles River Laboratories, Calco, Italy) were used for the model of systemic cryptococcosis. Each mouse weighed 20 g. Groups of mice were housed at five per bonneted cage and had access to food and water ad libitum. One day before infection, C. neoformans 486 was inoculated into brain heart infusion broth and incubated for 24 h at 37°C on a gyratory shaker (200 rpm). Organisms were harvested by low-speed centrifugation (1,500 × g), washed three times in 0.9% saline, counted with a hemacytometer, and suspended in sterile saline to the desired concentrations.
(i) Cryptococcal meningitis.
In the model of experimental cryptococcal meningitis, the mice were briefly anesthetized with methoxyflurane. These studies were performed by challenging the mice with an inoculum given in 0.06 ml of sterile saline administered intracranially. Inoculum sizes were confirmed by quantitative cultures on SDA plates.
(ii) Systemic cryptococcosis.
In the model of experimental systemic cryptococcosis, the mice were challenged with an inoculum given in 0.2 ml of sterile saline administered intravenously. Inoculum sizes were confirmed by quantitative cultures on SDA plates.
(iii) Treatment.
At 24 h postchallenge, the mice were randomized into control and treatment groups. SCH was administered at 0.2 ml orally by gavage once daily. FC was made every 3 days and was given in drinking water, with the volume of water consumption estimated to be 4 ml/mouse/day. The triazole was given at 2 and 20 mg/kg of body weight/day, while FC was given at concentrations that ranged from 10 to 60 mg/kg/day. Controls received 0.2 ml of Noble agar by oral gavage. In survival studies there were 10 mice per group. Therapy was continued for 10 consecutive days, and the mice were observed through day 30 postinfection. The clinical response to antifungal therapy was measured by determining the delay to the time of mortality. In tissue burden studies there were seven mice per group. Therapy was continued for 6 to 10 consecutive days, depending on the experiment (see below). Twenty-four hours after the end of therapy, the mice were euthanatized by CO2-induced asphyxiation, and the number of viable CFU per gram of brain or lung of each animal was determined by quantitative plating of organ homogenates on SDA.
Statistical analysis.
The MIC data were transformed logarithmically to approximate a normal distribution before statistical analysis. Continuous variables were compared by Student's t test or the Mann-Whitney U test. The results of the numbers of CFU per milliliter were obtained as means and standard deviations of at least three repetitions carried out for each compound, alone and in combination. Statistical significance was evaluated by analysis of variance, followed by the Bonferroni t test. The log-rank test was used to determine the difference between survival groups, and the Mann-Whitney U test was used to determine the significance in tissue burden studies. Differences were considered significant if P was less than 0.05.
RESULTS
In vitro studies. (i) MICs.
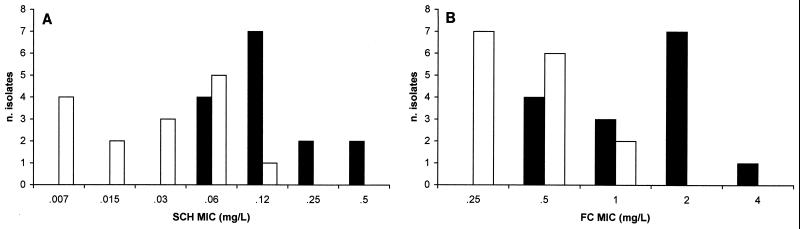
SCH MICs ranged from 0.06 to 0.5 μg/ml, with an MIC at which 50% of the isolates are inhibited (MIC50) and an MIC90 of 0.125 and 0.5 μg/ml, respectively (Fig. 1A). FC MICs ranged from 0.5 to 4.0 μg/ml, with an MIC50 and an MIC90 of 2.0 μg/ml (Fig. 1B). When SCH and FC were used in combination, there were significant reductions in the geometric mean SCH MIC (from 0.13 to 0.02 μg/ml [P = 0.0001]) and FC MIC (from 1.26 to 0.39 μg/ml, [P = 0.0001]) (Fig. 1). For 33% (5 of 15) of the isolates the interactions were synergistic, and for 67% (10 of 15) of the isolates they were additive, while neither indifference nor antagonism was observed.
FIG. 1.
Distribution of SCH (A) and FC (B) MICs for 15 isolates of C. neoformans. Black and white bars, MIC of each drug when used alone or in combination, respectively.
(ii) CFU per milliliter.
To investigate the effects of combinations of subinhibitory concentrations of SCH and FC on the numbers of CFU of C. neoformans, we selected a clinical isolate, C. neoformans 486, against which the combination therapy yielded a synergistic interaction: the SCH MIC was reduced from 0.5 to 0.125 μg/ml upon its combination with FC, while the FC MIC was reduced from 2.0 to 0.5 μg/ml upon its combination with SCH. The results of these experiments are shown in Table 1. Marked reductions in the numbers of CFU were observed when FC (at 0.25, 0.5, and 1.0 μg/ml) was combined with SCH (at 0.06, 0.125, and 0.25 μg/ml). For all combinations, the reduction in the numbers of CFU was significantly greater than that observed with single drugs alone (P = 0.0001).
TABLE 1.
Effects of combination of FC and SCH on numbers of CFU of C. neoformans 486a
| SCH concn (μg/ml) | Mean ± SD CFU/ml (106) for combination therapy with FC used at the following concn (μg/ml):
|
||
|---|---|---|---|
| 0.25 | 0.5 | 1.0 | |
| 0 | 14 ± 0.2 | 11 ± 0.7 | 10 ± 1.4 |
| 0.06 | 3.8 ± 0.6 | 2.4 ± 0.4 | 2.5 ± 0.06 |
| 0.125 | 2.4 ± 0.4 | 1.0 ± 0.1 | 1.3 ± 0.5 |
| 0.25 | 2.5 ± 0.06 | 0.5 ± 0.1 | 0.4 ± 0.2 |
The numbers of CFUs were determined after 72 h of incubation under the conditions recommended by NCCLS (17). Data are the averages of three experiments. Note that for all combinations, the reduction in the numbers of CFU was significantly greater than that observed with FC alone (P = 0.0001).
In vivo studies.
To investigate the effects of SCH-FC combination therapy in vivo, we established two experimental models of murine cryptococcosis with C. neoformans 486. Overall, four studies were performed. In the first three studies the mice were challenged with yeast cells given intracranially (cryptococcal meningitis). In study 4 the mice were challenged with yeast cells given intravenously (systemic cryptococcosis).
(i) Cryptococcal meningitis.
Two survival studies were performed. Treatments were similar in both experiments and consisted of the following regimens: FC at 20 mg/kg/day, FC at 30 mg/kg/day, FC at 50 mg/kg/day, SCH at 2 mg/kg/day, FC at 20 mg/kg/day plus SCH at 2 mg/kg/day, FC at 30 mg/kg/day plus SCH at 2 mg/kg/day, and FC at 50 mg/kg/day plus SCH at 2 mg/kg/day. In study 1, the mice were challenged with 1.5 × 103CFU/mouse, while in study 2, the mice were challenged with 1.8 × 104 CFU/mouse. In study 1, only SCH combined with FC at 20 mg/kg/day prolonged survival significantly compared with that for the control group (P = 0.03), while in study 2, all treatment regimens with the exception of SCH given as a single drug prolonged survival significantly compared with that for the control group (P < 0.01) (data not shown). Study 3 was a tissue burden experiment. The mice were challenged with 1.0 × 104 CFU/mouse. Mice were either treated for 6 days and killed on day 7 or treated for 10 days and killed on day 12. The results are shown in Table 2. Yeast cell counts in the brains of the mice killed on day 7 postinfection showed that FC alone and combination therapy were both effective at reducing the count significantly below that in the brains of the controls (P < 0.05). Although the number of CFU/gram of brain for the mice treated with the combination was lower than that for the mice treated with FC alone, the difference was not statistically significant. The tissue burden on day 12 showed substantially the same results. However, at this time the combination therapy was significantly more effective than each monotherapy (P < 0.05).
TABLE 2.
Yeast cell counts in brains of mice infected intracranially with C. neoformans 486 (study 3)a
| Treatmentb | Median (range) no. of CFU/g (106) of brains of mice killed on the following days postinfection:
|
|
|---|---|---|
| 7 | 12 | |
| None | 8.9 (1.6–52.8) | 18 (6.2–130) |
| SCH | 9.0 (0.8–15.3) | 38 (4.9–97.8) |
| FC | 0.8c (0.02–2.6) | 5.2c (3.8–19.6) |
| Combination | 0.09c (0.05–0.6) | 1.5cd (0.5–3.6) |
Mice were infected intracranially with 1.0 × 104 CFU/mouse. They were either treated for 6 days and killed on day 7 postinfection or treated for 10 days and killed on day 12 postinfection.
There were seven mice per group receiving no therapy (control), SCH at 2 mg/kg/day, FC at 20 mg/kg/day, or SCH at 2 mg/kg/day plus FC at 20 mg/kg/day (combination).
Treatment reduced the count significantly below those for control mice and mice treated with SCH alone.
Combination therapy reduced the count significantly below that in the brains of mice treated with each drug alone.
(ii) Systemic cryptococcosis.
In study 4, the mice were challenged with 1.7 × 104 CFU/mouse. In this experiment SCH was given at 20 mg/kg/day and FC was given at 60 mg/kg/day. Therapy was administered for 7 consecutive days, and the mice were killed on day 8 postinfection for determination of yeast cell counts in lung and brain tissues. The results of this study are shown in Table 3. All treatment regimens were effective in reducing the fungal burdens in both organs compared with the burdens in the organs of the controls (P ranged from <0.05 to <0.01). SCH was more effective than FC in both organs (P < 0.01). Combination therapy was more effective than FC alone (P < 0.01) but was not more effective than SCH alone in reducing the fungal burden in the lung. On the other hand, combination therapy was more effective than each single drug in reducing the fungal burden in the brain (P ranged from <0.05 to <0.01).
TABLE 3.
Yeast cell counts in tissues of mice infected intravenously with C. neoformans 486 (study 4)a
| Treatmentb | Median (range) CFU/g (104) in the following tissues:
|
|
|---|---|---|
| Lung | Brain | |
| None | 828 (550–1,285) | 69 (46–116) |
| SCH | 1.7cd (0.1–2.0) | 2.3cd (0.5–5.0) |
| FC | 74c (48–147) | 14c (3.9–40) |
| Combination | 1.6ce (0.5–2.6) | 0.04cef (0.01–0.1) |
Mice were infected via the lateral teil vein with 1.7 × 104 CFU/mouse. They were treated for 7 days and killed on day 8 postinfection.
There were seven mice per group receiving no therapy (control), SCH at 20 mg/kg/day, FC at 60 mg/kg/day, or SCH at 20 mg/kg/day plus FC at 60 mg/kg/day (combination).
Treatment reduced the count significantly below that for the controls.
SCH reduced the count significantly below that for the group of mice treated with FC alone.
Combination therapy reduced the count significantly below that for the group of mice treated with FC alone.
Combination therapy reduced the count significantly below that for the group of mice treated with each drug alone.
DISCUSSION
In the study described here we evaluated the combination of a new antifungal compound, SCH, with FC against C. neoformans in vitro and in vivo. Our in vitro results underline several important features of the susceptibility of C. neoformans to this triazole. First, we confirmed the excellent activity of this broad-spectrum antifungal agent against isolates of C. neoformans, having found an MIC50 and an MIC90 of 0.125 and 0.5 μg/ml, respectively. These data are in agreement with those previously reported by others (6, 22). Second, we showed a marked enhancement of antifungal activity when the new triazole was associated with FC. In general, the geometric mean MICs of both drugs dropped dramatically when they were given in combination: the geometric mean MIC of FC dropped from 1.26 to 0.39 μg/ml, and the geometric mean MIC of SCH dropped from 0.13 to 0.02 μg/ml. It has been reported that the peak concentration of SCH in serum, as determined by high-performance liquid chromatography (data from Schering Plough Research Institute), is 5 μg/ml after the administration of a single dose of 20 mg/kg. Interestingly, our SCH MICs, upon combination of SCH with FC, were approximately 250-fold lower than the attainable level in serum in vivo. Because of the lipophilicity of SCH, the levels of this drug in tissue would be substantially higher that the corresponding levels in serum. This fact could explain the efficacy of this triazole in a rabbit model of experimental cryptococcal menigitis, despite undetectable concentrations in cerebrospinal fluid (20).
The beneficial interaction between these drugs was also confirmed by counting the number of CFU of a selected clinical isolate, C. neoformans 486, incubated for 72 h under the conditions recommended by NCCLS. Combinations of FC and SCH at subinhibitory concentrations that were as much as eightfold lower than the respective MICs reduced the counts significantly below those observed with each drug alone.
Although in the present study the mechanism by which one drug enhances the activity of the other was not investigated, one can speculate as to potential mechanisms on the basis of the known effects of these drugs: SCH acts by inhibiting incorporation of ergosterol into the fungal cell membrane, which would facilitate the in vitro uptake of FC.
To investigate the effects of this combination therapy in vivo, we used two experimental models of murine cryptococcosis. Two separate experiments of survival were carried out. In study 1, only SCH at 2 mg/kg/day combined with FC at 20 mg/kg/day was effective in prolonging the survival compared with the length of survival for the controls. Combination therapies with higher doses of FC did not show any beneficial effect compared to the effect of the control treatment. Although study 2 clearly confirmed the lack of antagonism between FC and SCH, combination therapies were not superior to therapy with each drug alone. In general, our tissue burden experiments appeared to correlate better with in vitro data than length of survival did. Actually, both experimental models of infection showed that combination therapy for 7 to 10 days reduced the count significantly below that in the brains of mice treated with each drug alone.
It must be noted that our in vivo experiments were performed with one clinical isolate of C. neoformans. Due to the considerable degree of variation among isolates of C. neoformans with respect to genetic background, the effects of such combination therapy in animal models should be further explored with multiple strains.
In conclusion, our study demonstrates that SCH and FC combined are significantly more active than either drug alone against C. neoformans in vitro as well in vivo. These findings suggest that this therapeutic approach could be useful in the treatment of cryptococcal infections. Clinical studies are warranted to elucidate further the potential utility of this combination therapy.
ACKNOWLEDGMENT
This work was supported in part by a grant from Istituto Superiore di Sanità, Rome, Italy (III AIDS project, grant no. 50C.29).
REFERENCES
- 1.Albert M M, Graybill J R, Rinaldi M G. Treatment of murine cryptococcal meningitis with an SCH 39304-amphotericin B combination. Antimicrob Agents Chemother. 1991;35:1721–1725. doi: 10.1128/aac.35.9.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.American Society for Microbiology. Instructions to authors. Antimicrob Agents Chemother. 2001;45:xvii. [Google Scholar]
- 2.Barchiesi F, Schimizzi A M, Caselli F, Novelli A, Fallani S, Giannini D, Arzeni D, Di Cesare S, Falconi Di Francesco L, Fortuna M, Giacometti A, Carle F, Mazzei T, Scalise G. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:2435–2441. doi: 10.1128/aac.44.9.2435-2441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennet J E, Dismukes W E, Duma R J, Medoff G, Sande M A, Gallis H, Leonard F, Fields B T, Bradshaw M, Haywood H, McGee Z A, Cate T R, Cobbs C G, Warner J F, Alling D W. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 4.Ding J C, Bauer M, Diamond D M, Leal M A E, Johnson D, Williams B K, Thomas A M, Najvar L, Graybill J R, Larsen R. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–1593. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzot S P, Casadevall A. Pneumocandin L-743, 872 enhances the activity of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1997;41:331–336. doi: 10.1128/aac.41.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galgiani J N, Lewis M L. In vitro studies of activities of the antifungal triazole SCH 56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other patogenic yeasts. Antimicrob Agents Chemother. 1997;41:180–183. doi: 10.1128/aac.41.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George D, Kordick D, Miniter P, Patterson T F, Andreole V T. Combination therapy in experimental invasive aspergillosis. J Infect Dis. 1993;168:692–698. doi: 10.1093/infdis/168.3.692. [DOI] [PubMed] [Google Scholar]
- 8.Graybill J R, Bocanegra R, Najvar L K, Lowenberg D, Luther M F. Granulocyte colony-stimulating factor and azole antigungal therapy in murine aspergillosis: role of immune suppresion. Antimicrob Agents Chemother. 1998;42:2467–2473. doi: 10.1128/aac.42.10.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horne T J, Hollomon D, Loeffler R S T, Kelly S L. Cross-resistance to polyene and azole drugs in Cryptococcus neoformans. Antmicrob Agents Chemother. 1995;39:1526–1529. doi: 10.1128/aac.39.7.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iovanitti C, Negroni R, Bava J, Finquelievich J, Kral M. Itraconazole and flucytosine plus itraconazole combination in the treatment of experimental cryptococcosis in hamsters. Mycoses. 1995;38:449–452. doi: 10.1111/j.1439-0507.1995.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 11.Kwon-Chung K J, Polacheck I, Bennett J E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C) J Clin Microbiol. 1992;15:535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen R A, Bauer M, Weiner J M, Diamond D M, Leal M E, Ding J C, Rinaldi M G, Graybill J R. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1996;40:2178–2182. doi: 10.1128/aac.40.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen R A, Bozzette S A, Jones B E, Haghighat D, Leal M A, Forthal D, Bauer M, Tilles J G, McCuthan J A, Leedom J M. Fluconazole combined with flucytosine for the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19:741–745. doi: 10.1093/clinids/19.4.741. [DOI] [PubMed] [Google Scholar]
- 14.Lozano-Chiu M, Arikan S, Paetznick L. L, Anaissie E J, Loebenberg D, Rex J H. Treatment of murine fusariosis with SCH 56592. Antimicrob Agents Chemother. 1997;43:589–591. doi: 10.1128/aac.43.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz J E, Clemons K V, Aristizabal B H, Stevens D A. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:1558–1561. doi: 10.1128/aac.41.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 18.Nguyen M H, Najvar L K, Yu Y C, Graybill J R. Combination therapy with fluconazole and flucytosine in murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1120–1123. doi: 10.1128/aac.41.5.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH 56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH 56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller M A, Messer S, Jones R N. Activity of a new triazole, SCH 56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41:233–235. doi: 10.1128/aac.41.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polak A. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy (Basel) 1987;33:381–395. doi: 10.1159/000238524. [DOI] [PubMed] [Google Scholar]
- 23.Sanati H, Ramos C, Bayer A, Ghannoum M. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic mouse and infective endocarditis rabbit models. Antimicrob Agents Chemother. 1997;41:1345–1348. doi: 10.1128/aac.41.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheven M, Schwegler F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother. 1995;38:371–373. doi: 10.1128/aac.39.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugar A M. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob Agents Chemother. 1995;39:1907–1912. doi: 10.1128/aac.39.9.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugar A M, Liu X P. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J Infect Dis. 1998;177:1660–1663. doi: 10.1086/515319. [DOI] [PubMed] [Google Scholar]
- 27.Van Custem J. Therapy of experimental meningeal and disseminated cryptococcosis. Mycoses. 1993;36:357–367. doi: 10.1111/j.1439-0507.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuger A, Louie E, Holzman R S, Simberkoff M S, Rahal J J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]