Abstract
Background
Despite the proportion of receiving a minimum acceptable diet (minimum meal frequency and minimum dietary diversity) is lower in east Africa, there is limited evidence on minimum acceptable diet. Therefore, this study aimed to investigate the minimum acceptable diet and associated factors among children aged 6–23 months in east Africa.
Methods
A secondary data analysis of the most recent Demographic and Health Survey (DHS) data of 12 east African countries was done. A total weighted sample of 34, 097 children aged 6–23 months were included. A multilevel binary logistic regression model was applied. The Intra-class Correlation Coefficient (ICC) and Median Odds Ratio (MOR) were calculated to assess the clustering effect. Besides, deviance was used for model comparison as the models are nested models. Both crude and adjusted Odds Ratio (OR) with a 95% Confidence Interval (CI) were reported as potential predictors of minimum acceptable diet feeding practice.
Results
The prevalence of minimum acceptable diet feeding practice among children in east Africa was 11.56%; [95%CI; 11.22%, 11.90%]. In the multilevel analysis; child age of 12–17 month (AOR = 1.33: 95%CI; 1.20, 1.48), maternal primary (AOR = 1.21: 95%CI; 1.08, 1.35), secondary (AOR = 1.63: 95%CI; 1.44, 1.86) higher (AOR = 2.97: 95%CI; 2.30, 3.38) education level, media exposure (AOR = 1.38, 95%CI; 1.26, 1.51), household wealth statues (AOR = 1.28, 95%CI; 1.15, 1.42 for middle and AOR = 1.50: 95%CI; 1.42, 1.71 foe rich), employed mother (AOR = 1.27: 95%CI; 1.17, 1.37), maternal age 25–34 (AOR = 1.20: 95%CI; 1.09, 1.32) and 35–49 (AOR = 1.22: 95%; 1.06, 1.40) years, delivery in health facility (AOR = 1.43: 95%CI; 1.29, 1.59) and high community education level (AOR = 1.05: 95%CI; 1.01, 1.17) were positively associated with minimum acceptable diet child feeding practice. Meanwhile, the use of wood (AOR = 0.72: 95%CI; 0.61, 0.86) and animal dug (AOR = 0.34: 95%CI; 0.12, 0.95) as a source of cooking fuel and being from female-headed households (AOR = 0.88: 95%CI; 0.81, 0.96) were negatively associated with minimum acceptable diet feeding practice.
Conclusion
Child age, mother’s educational level, source of cooking fuel, exposure to media, sex of household head, household wealth status, mother working status, age of the mother, place of delivery and community-level education were the significant determinants of minimum acceptable diet feeding practices. Therefore, designing public health interventions targeting higher-risk children such as those from the poorest household and strengthening mothers’ education on acceptable child feed practices are recommended.
Keywords: Minimum acceptable diet, Children, Multilevel analysis, East Africa
Background
Childhood undernutrition is a major public health problem, particularly in developing countries [1]. Globally, around 45% of infant and young child deaths occur due to malnutrition, where two-thirds of these are because of inadequate child feeding and associated infectious disease [1–4]. According to recent studies from low and middle income (LMICs) Countries, the magnitude of minimum acceptable diet among children ranges from 6.1% to 36% [5, 6]. The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) recommends sufficient, safe and adequate complementary foods for children aged 6–23 months to meet their nutritional requirement and developmental needs [7, 8]. However, reports from low and middle-income countries indicated that many infants and young children are not receiving appropriate complementary foods [7, 9, 10]. Continued breastfeeding beyond six months should be supplemented by these complementary foods, as breast milk alone is not sufficient to fulfill their nutritional requirements [11]. As the first two years of life are a crucial window for ensuring optimum child growth and development, nutritional deficiencies during this period often contribute to impaired cognitive development, educational achievement and poor economic performance [10].
Appropriate breastfeeding practices and successful complementary feeding prevent the occurrence of various pathological conditions in the infant including; childhood under-nutrition[12], diarrheal disease[13, 14] and under-five mortality [12, 15]. Despite the demonstrated benefits of complementary feeding practice to the health and development of children, insufficient complementary feeding is still widespread in many developing countries [15, 16]. Hence, considering the minimum acceptable diet, which is a combination of minimum nutritional diversity and minimum meal frequency, as one of the main complementary feeding indicators [5], the WHO has established guidelines for infant and young child feeding practices. Therefore, promoting breastfeeding and appropriate complementary child feeding practices are very crucial for decreasing the above-mentioned consequences [17–19].
According to the finding of previous literature educational status, wealth status, media exposure, occupation, source of cooking fuel, place of delivery, antenatal care (ANC) visit and community-level education are associated with minimum acceptable diet feeding practice among children aged 6–23 months [5, 9, 17, 20–24].
Although identifying the potential determinants of infant and young child feeding practice will help to improve the conditions for child feeding and child nutrition status, there is insufficient updated information on the magnitude and determinants of minimum appropriate diet feeding practices in low-income countries. Therefore, this study aimed to determine the prevalence and associated factors of minimum acceptable feeding practices among children in east Africa.
Methods
Data source
Secondary data analysis was conducted based on the pooled data from the most recent Demographic and Health Surveys of east African countries conducted from 2008 to 2018 (Burundi_2016, Ethiopia_2016, Comoros_2012, Uganda_2016, Rwanda_2014/15, Tanzania_2015/16, Mozambique_2011, Madagascar_2008, Zimbabwe_2013/14, Kenya_2014, Zambia_2018, and Malawi_2015/16). Each country’s DHS survey consists of men, women, children, birth, and household datasets and the kids dataset (KR file) was used for this study. In the KR file, all children aged 6–23 months were considered for the analysis. The DHS used two stages stratified sampling technique to select the study participants. We pooled the most recent DHS surveys done in the 12 east African countries and a total weighted sample of 34, 097 was included in the final analysis. The total weighted sample of children included for each country was presented in Table 1.
Table 1.
The study participants were included in the study by country and year of survey in east Africa
| Country | Year of survey | Frequency (n) | Percentage |
|---|---|---|---|
| Burundi | 2016 | 2,009 | 5.89 |
| Ethiopia | 2016 | 2,985 | 8.75 |
| Kenya | 2014 | 2,128 | 6.24 |
| Comoros | 2012 | 730 | 2.14 |
| Madagascar | 2008 | 1,427 | 4.19 |
| Malawi | 2015/16 | 1,556 | 4.56 |
| Mozambique | 2011 | 3,330 | 9.77 |
| Rwanda | 2014/15 | 2,352 | 6.90 |
| Tanzania | 2015/16 | 6137 | 18.00 |
| Uganda | 2016 | 2,845 | 8.34 |
| Zambia | 2018 | 5,484 | 16.08 |
| Zimbabwe | 2013/2014 | 3,114 | 9.13 |
| Total | 34,097 | ||
Variables of the study
Dependent variable
The minimum acceptable diet feeding practice was the outcome variable. It is a binary outcome variable, which was coded as 0 if the child didn’t feed a minimum acceptable diet and 1 if the child feed a minimum acceptable diet. The child is said to be fed with MAD if he/she had both minimum meal frequency and minimum dietary diversity in both breastfeeding and non-breastfeeding children. These children who received solid, semi-solid or soft foods, two times for breastfed infants 6–8 months, three times for breastfed children 9–23 months and four times for non-breastfed children is said to have minimum meal frequency. These children with 6–23 months of age received foods from four or more food groups of the seven food groups (Cereals, Legumes and nuts, Dairy products, Eggs, Flesh foods, Vitamin A-rich fruits and dark green leafy vegetables and other fruits) are said to have minimum dietary diversity [25].
Independent variables
The independent variables included in this study were respondent’s age, preceding birth interval, birth order, age of the child, sex of household head, family size, number of under-five children, maternal educational level, source of cooking fuel, distance to get water, media exposure, household wealth status, employment status, place of delivery, number of antenatal visits, residence, community poverty level, community educational level and community level of ANC utilization.
Data management and analysis
Data extraction, recoding and analysis were done using STATA version 14 software. To restore the representativeness of the data as well as to get a reliable estimate and standard error, the data were weighted before doing any statistical analysis. The hierarchical nature of the DHS data, which violates the independent assumptions of the standard logistic regression model was handled with a multilevel logistic regression analysis. Children in the same cluster are more likely to be similar to each other than children from another cluster. This implies that there is a need to take into account the cluster variability by using advanced models such as the multilevel logistic regression model. The Interclass Correlation Coefficient (ICC) and Median Odds Ratio (MOR) were checked to assess whether there was clustering or not. Model comparison was done using deviance (-2LL). The null model-a model without explanatory variables, model I-a model with individual-level factors, model II-a model with community-level factors and model III-a model with both individual and community-level factors were fitted. Multicollinearity among the independent variables was checked using VIF and the mean VIF was less than 5, which indicates there is no multicollinearity among the included independent variables. Both bivariable and multivariable multi-level logistic regression were done. At the bivariable analysis variables with a p-value ≤ 0.2 were considered for multivariable analysis. In the multivariable multilevel analysis, the Adjusted Odds Ratio (AOR) with 95% Confidence Interval (CI) was reported to declare the statistical significance of the association.
Results
Individual and community-level characteristics of the study participants
Nearly 35% of children were in the age group of 12–17 months. The majority of the children (88.22%) were born within more than 24 months of pregnancy interval. Regarding media exposure, more than two-thirds (65.82%) of the mothers were exposed to at least one of the media sources (watching television, listening to the radio or reading a newspaper) and nearly half of households (46.29%) travel 30 min or longer round trip to fetch drinking water. Regarding the source of cooking fuel, only 3.26% were used electricity as a source of cooking fuel. More than half (55.96%) of the mothers had at least 4 ANC visits during their last pregnancy. About 46.11% of the children were from poor households and 52.28% of the mother had a primary education level. More than half (50.3%) of the mothers were from a community with a high poverty level. Nearly half of the mothers (50.49%) were from the community with high ANC services utilization (Table 2).
Table 2.
Individual and community-level characteristics of the study participants in east Africa using 2008–2018 demographic health survey data (N = 34, 097)
| Variables | Categories | Frequency | Percentage |
|---|---|---|---|
| Age of the child(months) | 6–8 | 6006 | 17.61 |
| 9–11 | 5829 | 17.10 | |
| 12–17 | 11,911 | 34.93 | |
| 18–23 | 10,351 | 30.36 | |
| Respondents age (years) | 15–24 | 12,253 | 35.984 |
| 25–34 | 15,367 | 45.07 | |
| 35–49 | 6475 | 18.99 | |
| Birth order | First | 8012 | 23.50 |
| 2nd-4th | 16,560 | 48.57 | |
| Fifth and above | 9525 | 27.94 | |
| Preceding birth interval | Less than 24 month | 4018 | 11.78 |
| 24 months and above | 3007 | 88.22 | |
| Number of antenatal care visits | 0 | 2083 | 6.11 |
| 1–3 | 12,935 | 37.94 | |
| ≥ 4 | 19,097 | 55.96 | |
| Family size | < 5 | 16,766 | 49.17 |
| ≥ 5 | 17,331 | 50.83 | |
| Source of cooking fuel | Electricity | 1111 | 3.26 |
| Charcoal | 6130 | 17.98 | |
| Wood | 24,398 | 71.55 | |
| Animal dug | 178 | 0.52 | |
| Other | 2280 | 6.69 | |
| Number of under fifth children | No | 384 | 1.12 |
| One | 12,616 | 37.00 | |
| More than one | 21,098 | 61.88 | |
| Media exposure | No | 11,654 | 34.18 |
| Yes | 22,443 | 65.82 | |
| Wealth status | Poor | 15,723 | 46.11 |
| Middle | 6626 | 19.43 | |
| Rich | 11,747 | 34.5 | |
| Maternal educational | No education | 7393 | 21.68 |
| Primary education | 17,827 | 52.28 | |
| Secondary education | 7865 | 23.07 | |
| Higher education | 1012 | 2.97 | |
| Working statues | No | 20,427 | 59.91 |
| Yes | 13,670 | 40.09 | |
| Residence | Urban | 8182 | 23.99 |
| Rural | 25,916 | 76.01 | |
| Distance to a water source | 30 min and less | 19,744 | 53.29 |
| Greater than 30 min | 17,308 | 46.71 | |
| Sex of household head | Female | 7718 | 22.64 |
| Male | 26,379 | 77.36 | |
| Community level education | Low | 17,182 | 50.39 |
| High | 16,915 | 49.61 | |
| Place of delivery | Home | 8085 | 23.71 |
| Health facility | 26,012 | 76.29 | |
| Community level poverty | Low | 17,371 | 50.95 |
| High | 16,726 | 49.05 | |
| Community level ANC utilization | Low | 17,759 | 52.08 |
| High | 16,338 | 47.92 |
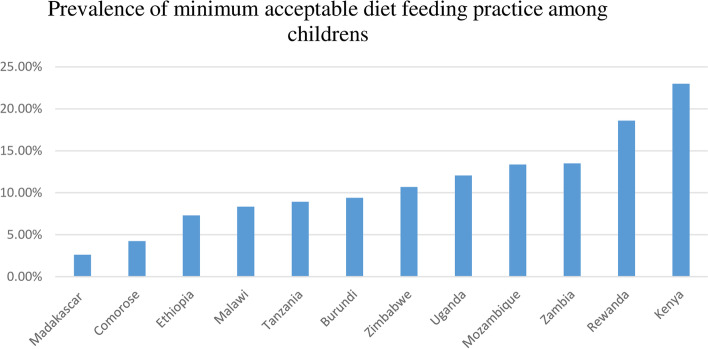
Prevalence of minimum acceptable diet feeding practice
The prevalence of minimum acceptable diet child feeding practice was 11.56% [95%CI: 11.22, 11.90] in east Africa. It was highest in Kenya (23%) and lowest in Madagascar (2.61%) (Fig. 1).
Fig. 1.
Showing the prevalence of minimum acceptable diet feeding practice in east Africa
Random effect model and model fitness
The ICC, MOR, and percentage change in variation (PCV) were used to assess the random-effect model of null model, model I, model II and model III. The ICC value of 0.069 in the null model indicates that a 6.9% variance in minimum acceptable diet feeding practice was due to cluster/community variations. In addition, the highest MOR value of 1.59 suggests a significant clustering of MAD feeding among children. In addition, the highest PCV (0.41%) in the final model (model III) indicated that both individual and community-level variables explained about 41% of the variation in minimum acceptable diet feeding practice. The final model (model III), which incorporates both individual and community level variables was the best-fitted model (it had the lowest deviance) (Table 3).
Table 3.
Multilevel random effect model and model fitness of null model (a model without explanatory variables), model I (a model with individual-level factors), model II (a model with community level factors) and modell III (a model with both individual and community-level factors) for the assessment of minimum acceptable diet feeding practice among children of 6–23 months in eastern Africa using 2008–2018 demographic health survey data
| Parameter | Null model | Model I | Model II | Model III |
|---|---|---|---|---|
| ICC | 0.069 | 0.07 | 0.064 | 0.067 |
| PCV | Reff | 0.042 | 0.083 | 0.41 |
| MOR | 1.59 | 1.23 | 1.21 | 1.57 |
| Model comparison | ||||
| Log likelihood | -12,006.411 | -11,416.813 | -11,838.149 | -11,410.956 |
| Deviance | 24,012.822 | 22,833.626 | 23,676.298 | 22,821.912 |
ICC Intraclass Correlation Coefficient, PCV Percentage Change in Variation, MOR Median Odd Ratio
Factors associated with minimum acceptable diet feeding
To determine the associated factors of MAD feeding practice, those variables with p ≤ 0.2 at bivariable analysis were entered to multivariable multi-level regression analysis. Accordingly, child age, mother’s educational level, source of cooking fuel, media exposure, sex of household head, household wealth status, mother working status, age of respondent, ANC visit and community-level education were independent predictors of MAD feeding (p ≤ 0.05). The odds of feeding a child with MAD was 1.33 times (AOR = 1.33: 95%CI; 1.20, 1.48) higher among children aged between 12–17 months than children aged 6–8 months. The odds of feeding the child with a minimum acceptable diet was 1.27 times (AOR = 1.27: 95%CI; 1.17, 1.37) higher among employed mothers compared with their counterparts. Mothers with media exposure had 1.38 times (AOR = 1.38; 95%CI; 1.26, 1.51) higher odds of feeding a MAD for their children than mothers who had no access to media. Mothers with primary (AOR = 1.21: 95%CI; 1.08, 1.35), secondary (AOR = 1.63: 95%CI; 1.44, 1.86) or higher (AOR = 2.97: 95%CI: 2.30, 3.38) educational level had higher odds to feed their children with MAD compared with mothers with no formal education. Old-aged mothers (AOR = 1.20: 95%CI; 1.09, 1.32 for 25–34 aged mothers and AOR = 1.22: 95%CI; 1.06, 1.40 for 35–49 aged mothers) were more likely to practice minimum acceptable diet feeding compared to young aged mother. Children from the female-headed household were less likely to meet the minimum acceptable diet (AOR = 0.88: 95%CI; 0.81, 0.96). Mothers who have used wood (AOR = 0.72: 95%CI; 0.61, 0.86), animal dug (AOR = 0.34: 95%CI; 0.12, 0.95) and other (AOR = 0.72: 95%CI; 0.59, 0.89) as a source of fuel were less likely to provide minimum acceptable diet to their child compared with those who used electricity as a source of fuel. Mothers who have delivered in the health facility had 1.43 times (AOR = 1.43: 95%CI; 1.29, 1.59) more likely to provide a minimum acceptable diet for their child compared with their counterparts. Regarding community-level education, mothers from the community of higher educational level were 1.05 times (AOR = 1.05: 95% CI; 1.01, 1.17) more likely to complement a minimum acceptable diet for their children (Table 4).
Table 4.
The bi-variable and multivariable multilevel binary logistic regression analysis of factors associated with minimum acceptable diet feeding practice in east Africa using 2008–2018 demographic health survey data
| Variables | Category | Minimum acceptable diet | Crude Odds Ratio(95%CI) | Adjust Odds Ratio (95%CI) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Age of the child (months) | 6–8 | 634 | 5372 | 1 | 1 |
| 9–11 | 609 | 5220 | 1.02(0.90, 1.15) | 1.01(0.89, 1.14) | |
| 12–17 | 1550 | 10,362 | 1.32(1.20, 1.47) | 1.33(1.20, 1.48)* | |
| 18–23 | 1148 | 9202 | 1.11(1.01, 1.23) | 1.07(0.96, 1.20) | |
| Respondents age (years) | 15–24 | 1250 | 11,003 | 1 | 1 |
| 25–34 | 1959 | 13,410 | 1.24(1.15, 1.34) | 1.20(1.09, 1.32)* | |
| 35–49 | 733 | 5743 | 1.12(1.02, 1.24) | 1.22(1.06, 1.40)* | |
| Birth order | 1 | 1021 | 6991 | 1 | 1 |
| 2–4 | 2008 | 14,552 | 0.92(0.85, 1.01) | 0.96(0.87, 1.07) | |
| ≥ 5 | 912 | 8613 | 0.73(0.66, 0.81) | 0.98(0.84, 1.14) | |
| Preceding birth interval (months) | < 24 | 330 | 3689 | 1 | 1 |
| ≥ 24 | 3612 | 26,467 | 1.27(1.13, 1.42) | 1.09(0.97, 1.23) | |
| Number of ANC visit | 0 | 145 | 1938 | 1 | 1 |
| 1–3 | 1479 | 11,456 | 1.99(1.63, 2.43) | 1.16(0.94, 1.44) | |
| ≥ 4 | 2317 | 16,762 | 2.16(1.77, 2.63) | 1.04(0.84, 1.29) | |
| Family size | < 5 | 2037 | 14,729 | 1 | 1 |
| ≥ 5 | 1904 | 15,427 | 0.88(0.82, 0.94) | 0.96(0.88, 1.04) | |
| Source of cooking fuel | Electricity | 284 | 827 | 1 | 1 |
| Charcoal | 1040 | 5090 | 0.62(0.53, 0.72) | 0.86(0.73, 1.01) | |
| Wood | 2264 | 22,134 | 0.30(0.26, 0.35) | 0.72(0.61, 0.86)* | |
| Animal dug | 7 | 171 | 0.12(0.04, 0.33) | 0.34(0.12, 0.95)* | |
| Other | 346 | 1934 | 0.45(0.37, 0.55) | 0.72(0.59, 0.89)* | |
| Number of under fifth children | No | 51 | 333 | 1 | 1 |
| One | 1681 | 10,935 | 1.12(0.82, 1.54) | 1.06(0.75, 1.53) | |
| More than one | 2209 | 18,888 | 0.84 (0.62, 1.16) | 0.98(0.69, 1.40) | |
| Media exposure | No | 815 | 10,839 | 1 | 1 |
| Yes | 3127 | 19,317 | 2.11(1.95, 2.29) | 1.38(1.26, 1.51)* | |
| Wealth status | Poor | 1231 | 14,493 | 1 | 1 |
| Middle | 684 | 5942 | 1.50(1.36, 1.67) | 1.28(1.15, 1.42)* | |
| Rich | 2027 | 9721` | 2.60(2.40, 2.81) | 1.5(1.42, 1.71)* | |
| Maternal education level | No education | 522 | 6871 | 1 | 1 |
| Primary education | 1801 | 16,027 | 1.55(1.39, 1.72) | 1.21(1.08, 1.35)* | |
| Secondary education | 1310 | 6555 | 1.63(2.36, 2.94 | 1.63(1.44, 1.86)* | |
| Higher education | 309 | 703 | 6.31(5.35, 7.44) | 2.97(2.30, 3.38)* | |
| Working statues | No | 2546 | 17,882 | 1 | 1 |
| Yes | 1396 | 12,274 | 1.31(1.22, 1.41) | 1.27(1.17, 1.37)* | |
| Residence | Urban | 1479 | 6702 | 1 | 1 |
| Rural | 2462 | 23,454 | 0.49(0.46, 0.53) | 0.94(0.84, 1.04) | |
| Distance to water source | ≤ 30 min’ | 1898 | 16,415 | 1 | 1 |
| > Greater than 30 min’ | 2043 | 13,741 | 1.33(1.24, 1.43) | 1.06(0.98, 1.14) | |
| Sex of household head | Female | 836 | 6883 | 0.90(0.83, 0.98) | 0.88(0.81, 0.96)* |
| Male | 3106 | 23,273 | 1 | 1 | |
| Place of delivery | Home | 531 | 7554 | 1 | 1 |
| Health facility | 3410 | 22,602 | 2.09(1.90, 2.31) | 1.43(1.29. 1.59)* | |
| Community-level education | Low | 1926 | 15,256 | 1 | 1 |
| High | 2016 | 14,900 | 1.22(1.11, 1.35) | 1.05(1.01, 1.17)* | |
| Community-level poverty | Low | 2077 | 15,294 | 1 | 1 |
| High | 1864 | 14,863 | 0.87(0.79, 0.96) | 1.17(1.00, 1.29) | |
| Community-level ANC utilization | Low | 1966 | 15,793 | 1 | 1 |
| High | 1976 | 14,363 | 1.14(1.03, 1.26) | 1.04(0.94, 1.16) | |
ANC Antenatal care, CI Confidence interval
*p-value < 0.05, **other = lpg, natural gas, biogas, kerosene, coal, lignite, agricultural crop, straw/shrubs/grass, other,
Discussion
This study aimed to determine the minimum acceptable diet feeding practice and associated factors among children in east Africa. Accordingly, the prevalence of minimum acceptable diet child feeding practice in the region was 11.56% [95%CI = 11.22%, 11.90%]. The prevalence of minimum acceptable diet feeding practices in this study was higher than the findings of other studies [5, 26, 27]. The prevalence was lower than reports in Africa and Asia [20, 28].
In the multilevel multivariable analysis factors such as the age of the child, respondent age, source of cooking fuel, exposure to media, household wealth status, mother educational level, working status, sex of household head, place of delivery and community-level education were significantly associated with feeding minimum acceptable diet. Children with age of 12–17 months had higher odds to feed a minimum acceptable diet than a child with ages 6–8 months. This finding is in agreement with the study done in Ethiopia [5], Ghana [20], Uganda [21] and Indonesia [22]. This may be attributed to the late introduction of complementary feeding and the start of complementary feeding with only limited items (only milk or cereal). Mothers might also be able to perceive that the younger the children, the weaker the intestine’s capacity to digest such foods as banana, eggs, pumpkin, carrots, green vegetables and meat [29]. This may be further justified by traditional beliefs and practices, when introducing complementary food to infants, they may develop diarrhea due to poor hygienic conditions, but mothers may equate this problem with taking new food items and ultimately they would not encourage the child to eat foreign foods.
Similarly, employed mothers had higher odds to provide a minimum acceptable diet for their children. This finding is supported by studies conducted in Ethiopia [5, 30] and Serilanka [31]. This may be related to the earning capacity of the mother, which is an important factor in feeding the child with an appropriate diet. Increased access to resources, broader social networks and increasing awareness of their social environment could also improve the chances of feeding the child with the minimum appropriate diet [5]. Similarly, older aged women had a higher chance of providing MAD to their children compared with young women. This finding was supported by another study done among Indian population [32]. Such significant effects of maternal age on complementary feeding practice suggest that the mother’s experience may play a significant role inappropriate infant and young child feeding practices [32]. In this study exposure to public media was a significant predictor of feeding a minimum acceptable diet, which is in line with another study [9]. This might be associated with the influence of media exposure on behavioral change to improve the complementary feeding practice through enhancing mothers’ knowledge on feeding a minimum acceptable diet to their children [5].
The mother’s education level was significantly associated with feeding the child a minimum acceptable diet. A similar finding was reported from studies in Ethiopia [9], Tanzania [30], Ghana [20] and east Africa [23]. This may be related to higher maternal education improving the job opportunity of mothers and the decision-making process of households, which in turn is correlated with an improvement in the use of health services [33]. Similarly, higher household wealth statuses were significantly associated with minimum acceptable diet feeding practice. This finding was in agreement with another study done elsewhere [15, 23, 30]. The present study found that birthing in a health facility was significantly associated with higher odds of minimum acceptable diet feeding practice and this finding is supported by a study done in west Africa [24]. This might be as institutional delivery increases exposure to health information and improves mothers’ knowledge about infant and young child feeding [34]. In this study, children from a household who used traditional biomass as a source of fuel (wood, animal dug and others) had a lower chance to feed a minimum acceptable diet. This might be associated with improving access to affordable and reliable modern forms of energy services is essential, especially for developing countries in reducing poverty and promoting economic development [35]. Children from communities of higher educational level had more odds to be fed a minimum acceptable diet than children from the community of lower educational level and this finding is supported by the studies conducted in Tanzania [30] and east Africa [23]. This may also be because trained mothers were more likely to have sufficient knowledge, easy to understand the practice of child feeding, received lessons in school on child feeding that would improve their comprehension of the value of child feeding [5].
Strength and limitations of the study
This study was based on nationally representative data with large sample size. Besides, it was based on an appropriate statistical approach (multilevel analysis) to accommodate the community or cluster level variability of minimum acceptable diet feeding. Moreover, since it is based on the national survey data the study has the potential to give insight to policymakers and program planners to design appropriate intervention strategies at the national level. However, this study had limitations in that the DHS is mostly based on respondents’ self-report and might have the possibility of recall bias.
Conclusion
In this study, the prevalence of minimum acceptable diet feeding practices in eastern Africa was found to be below. Both individual and community-level factors were associated with minimum acceptable diet feeding practice. Child age, mother’s educational level, source of cooking fuel, media exposure, sex of household head, household wealth status, mother working status, age of the mother, place of delivery and community level of education were the significant determinants of minimum acceptable diet feeding practices. Therefore, giving special attention to children aged 6–23 months and practicing appropriate feeding practices should be implemented to decrease devastating health problems of the child associated with inappropriate minimum acceptable diet feeding practice. Also, designing public health interventions targeting higher-risk children such as those from the poorest household and strengthening mothers' education on acceptable child feed practices are recommended.
Acknowledgements
We greatly acknowledge MEASURE DHS for granting access to the Demographic and Health Surveys data.
Abbreviations
- CI
Confidence Interval
- CSA
Central Statistical Agency
- DHS
Demographic Health Survey
- EA
Enumeration Area
- ICC
Intraclass Correlation Coefficient
- LLR
Likelihood Ratio
- LMIC
Low and middle-income country
- PCV
Proportional change in Variance
- WHO
World Health Organization
Authors’ contributions
MGW, TSA, GAT, AZA, ZTT, AML, YY and ABT conceived the study. MGW, TSA, GAT, AZA, ZTT, AML, YY and ABT analyzed the data. MGW, TSA, GAT, AZA, ZTT, AML, YY and ABT drafted the manuscript and reviewed the article. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
All result-based data are within the manuscript and the data set is available online and any one can access it from www.measuredhs.com.
Declarations
Ethics approval and consent to participate
As the study was a secondary data analysis of publicly accessible survey data, ethical approval and participant consent was not required. However, we asked the DHS Program and permission was granted to download and use the data for this study from http://www.dhsprogram.com. The procedures approved by the Institution Review Board for DHS public-use datasets do not allow the identification of respondents, families, or sample populations in any way. In the data sets, there are no names of individuals or household addresses.
Consent for publication
Not applicable.
Competing interests
No conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Misganaw Gebrie Worku, Email: misgeb2008@gmail.com.
Tesfa Sewunet Alamneh, Email: tesfasewunet23@gmail.com.
Getayeneh Antehunegn Tesema, Email: getayenehantehunegn@gmail.com.
Adugnaw Zeleke Alem, Email: aduzeleke2201@gmail.com.
Zemenu Tadesse Tessema, Email: zemenut1979@gmail.com.
Alemneh Mekuriaw Liyew, Email: Alemnehmekuriawliyew@gmail.com.
Yigizie Yeshaw, Email: yigizieyeshaw29@gmail.com.
Achamyeleh Birhanu Teshale, Email: achambir08@gmail.com.
References
- 1.Aggarwal A, Verma S, Faridi M. Complementary feeding—reasons for inappropriateness in timing, quantity and consistency. Indian J Pediatr. 2008;75(1):49. doi: 10.1007/s12098-008-0006-9. [DOI] [PubMed] [Google Scholar]
- 2.Disha A, Rawat R, Subandoro A, Menon P. Infant and young child feeding (IYCF) practices in Ethiopia and Zambia and their association with child nutrition: analysis of demographic and health survey data. Afr J Food Agric Nutr Dev. 2012;12(2):5895–5914. [Google Scholar]
- 3.World Health Organization. Global nutrition targets 2025: Stunting policy brief. No. WHO/NMH/NHD/14.3. World Health Organization; 2014.
- 4.Quinn V, Guyon A, Ramiandrazafy M. Successfully Scaling Up Exclusive Breastfeeding—Lessons from Madagascar. Child Health and Nutrition Research Initiative (CHNRI). 2006.
- 5.Tassew AA, Tekle DY, Belachew AB, Adhena BM. Factors affecting feeding 6–23 months age children according to minimum acceptable diet in Ethiopia: a multilevel analysis of the Ethiopian demographic health survey. PLoS ONE. 2019;14(2):e0203098. doi: 10.1371/journal.pone.0203098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malawi D. Malawi demographic and health survey 2010 (final report). National Statistical Office (NSO) and ICF Macro, Zomba, Malawi, and Calverton, Maryland, USA. 2011.
- 7.santé Omdl, Staff WHO, Organization WH, UNICEF., UNAIDS. Global strategy for infant and young child feeding. World Health Organization; 2003.
- 8.Organization WH. Guideline: counselling of women to improve breastfeeding practices. World Health Organization; 2018. [PubMed]
- 9.Molla M, Ejigu T, Nega G. Complementary feeding practice and associated factors among mothers having children 6–23 months of age, Lasta District, Amhara region, Northeast Ethiopia. Advances in Public Health. 2017;2017.
- 10.Who U, Usaid A, Aed U. Indicators for assessing infant and young child feeding practices. Geneva: World Health Organization; 2008. [Google Scholar]
- 11.Who U, Usaid A, Ucdavis I. Indicators for assessing infant and young child feeding practices: conclusions of a consensus meeting held 6–8 November 2007 in Washington DC, USA. Geneva: World Health Organization; 2008. [Google Scholar]
- 12.Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. The lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 13.Ogbo FA, Agho K, Ogeleka P, Woolfenden S, Page A, Eastwood J, et al. Infant feeding practices and diarrhoea in sub-Saharan African countries with high diarrhoea mortality. PLoS ONE. 2017;12(2):e0171792. doi: 10.1371/journal.pone.0171792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogbo FA, Nguyen H, Naz S, Agho KE, Page A. The association between infant and young child feeding practices and diarrhoea in Tanzanian children. Trop Med Health. 2018;46(1):2. doi: 10.1186/s41182-018-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel A, Pusdekar Y, Badhoniya N, Borkar J, Agho KE, Dibley MJ. Determinants of inappropriate complementary feeding practices in young children in India: secondary analysis of national family health survey 2005–2006. Matern Child Nutr. 2012;8:28–44. doi: 10.1111/j.1740-8709.2011.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogbo FA, Page A, Idoko J, Claudio F, Agho KE. Trends in complementary feeding indicators in Nigeria, 2003–2013. BMJ Open. 2015;5(10):e008467. doi: 10.1136/bmjopen-2015-008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garemo M, Elamin A, Gardner A. Weight status and food habits of preschool children in Abu Dhabi, United Arab Emirates: NOPLAS project. Asia Pac J Clin Nutr. 2018;27(6):1302. doi: 10.6133/apjcn.201811_27(6).0018. [DOI] [PubMed] [Google Scholar]
- 18.Loney T, Aw T-C, Handysides DG, Ali R, Blair I, Grivna M, et al. An analysis of the health status of the United Arab Emirates: the ‘big 4’public health issues. Glob Health Action. 2013;6(1):20100. doi: 10.3402/gha.v6i0.20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchodigni IM, Hounkpatin WA, Ntandou-Bouzitou G, Avohou H, Termote C, Kennedy G, et al. Complementary feeding practices: determinants of dietary diversity and meal frequency among children aged 6–23 months in Southern Benin. Food Security. 2017;9(5):1117–1130. doi: 10.1007/s12571-017-0722-y. [DOI] [Google Scholar]
- 20.Issaka AI, Agho KE, Burns P, Page A, Dibley MJ. Determinants of inadequate complementary feeding practices among children aged 6–23 months in Ghana. Public Health Nutr. 2015;18(4):669–678. doi: 10.1017/S1368980014000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokori A, Schonfeldt H, Hendriks SL. Child factors associated with complementary feeding practices in Uganda. South Afr J Clin Nutr. 2017;30(1):7–14. doi: 10.1080/16070658.2016.1225887. [DOI] [Google Scholar]
- 22.Ng CS, Dibley MJ, Agho KE. Complementary feeding indicators and determinants of poor feeding practices in Indonesia: a secondary analysis of 2007 demographic and health survey data. Public Health Nutr. 2012;15(5):827–839. doi: 10.1017/S1368980011002485. [DOI] [PubMed] [Google Scholar]
- 23.Gewa CA, Leslie TF. Distribution and determinants of young child feeding practices in the East African region: demographic health survey data analysis from 2008–2011. J Health Popul Nutr. 2015;34(1):6. doi: 10.1186/s41043-015-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Issaka AI, Agho KE, Page AN, Burns PL, Stevens GJ, Dibley MJ. Determinants of suboptimal complementary feeding practices among children aged 6–23 months in seven francophone W est A frican countries. Matern Child Nutr. 2015;11:31–52. doi: 10.1111/mcn.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewey K. Guiding principles for complementary feeding of the breastfed (PAHO and WHO). Pan Am Heal Organ World Heal Organ. 2001;18–25.
- 26.Ahmed KY, Page A, Arora A, Ogbo FA. Trends and factors associated with complementary feeding practices in Ethiopia from 2005 to 2016. Matern Child Nutr. 2020;16(2):e12926. doi: 10.1111/mcn.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdurahman AA, Chaka EE, Bule MH, Niaz K. Magnitude and determinants of complementary feeding practices in Ethiopia: a systematic review and meta-analysis. Heliyon. 2019;5(7):e01865. doi: 10.1016/j.heliyon.2019.e01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutter CK, Daelmans BM, de Onis M, Kothari MT, Ruel MT, Arimond M, et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics. 2011;128(6):e1418–e1427. doi: 10.1542/peds.2011-1392. [DOI] [PubMed] [Google Scholar]
- 29.Tekaly G, Kassa M, Belete T, Tasew H, Mariye T, Teshale T. Pre-lacteal feeding practice and associated factors among mothers having children less than two years of age in Aksum town, Tigray, Ethiopia, 2017: a cross-sectional study. BMC Pediatr. 2018;18(1):310. doi: 10.1186/s12887-018-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogbo FA, Ogeleka P, Awosemo AO. Trends and determinants of complementary feeding practices in Tanzania, 2004–2016. Trop Med Health. 2018;46(1):40. doi: 10.1186/s41182-018-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senarath U, Godakandage SS, Jayawickrama H, Siriwardena I, Dibley MJ. Determinants of inappropriate complementary feeding practices in young children in Sri Lanka: secondary data analysis of demographic and health survey 2006–2007. Matern Child Nutr. 2012;8:60–77. doi: 10.1111/j.1740-8709.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhami MV, Ogbo FA, Osuagwu UL, Agho KE. Prevalence and factors associated with complementary feeding practices among children aged 6–23 months in India: a regional analysis. BMC Public Health. 2019;19(1):1034. doi: 10.1186/s12889-019-7360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S, Creanga AA, Gillespie DG, Tsui AO. Economic status, education and empowerment: implications for maternal health service utilization in developing countries. PLoS ONE. 2010;5(6):e11190. doi: 10.1371/journal.pone.0011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yisak H, Ambaw B, Walle Z, Alebachew B, Ewunetei A. Minimum acceptable diet and associated factors among HIV-exposed children aged 6–24 months in Debre Tabor Town, Ethiopia. HIV/AIDS (Auckland, NZ) 2020;12:639. doi: 10.2147/HIV.S274764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malla S, Timilsina GR. Household cooking fuel choice and adoption of improved cookstoves in developing countries: a review. The World Bank; 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All result-based data are within the manuscript and the data set is available online and any one can access it from www.measuredhs.com.