Abstract
Objectives
The aim of this study was to explore risk factors for the prognosis of lung cancer with simple brain metastasis (LCSBM) patients and to establish a prognostic predictive nomogram for LCSBM patients.
Materials and methods
Three thousand eight hundred and six cases of LCSBM were extracted from the Surveillance, Epidemiology, and End Results (SEER) database from 2010 to 2015 using SEER Stat 8.3.5. Lung cancer patients only had brain metastasis with no other organ metastasis were defined as LCSBM patients. Prognostic factors of LCSBM were analyzed with log-rank method and Cox proportional hazards model. Independent risk and protective prognostic factors were used to construct nomogram with accelerated failure time model. C-index was used to evaluate the prediction effect of nomogram.
Results and conclusion
The younger patients (18–65 years old) accounted for 54.41%, while patients aged over 65 accounted for 45.59%.The ratio of male: female was 1:1. Lung cancer in the main bronchus, upper lobe, middle lobe and lower lobe were accounted for 4.91%, 62.80%, 4.47% and 27.82% respectively; and adenocarcinoma accounted for 57.83% of all lung cancer types. The overall median survival time was 12.2 months. Survival rates for 1-, 3- and 5-years were 28.2%, 8.7% and 4.7% respectively. We found female (HR = 0.81, 95% CI 0.75–0.87), the married (HR = 0.80; 95% CI 0.75–0.86), the White (HR = 0.90, 95% CI 0.84–0.95) and primary site (HR = 0.45, 95% CI 0.39–0.52) were independent protective factors while higher age (HR = 1.51, 95% CI 1.40–1.62), advanced grade (HR = 1.19, 95% CI 1.12–1.25) and advanced T stage (HR = 1.09, 95% CI 1.05–1.13) were independent risk prognostic factors affecting the survival of LCSBM patients. We constructed the nomogram with above independent factors, and the C-index value was 0.634 (95% CI 0.622–0.646). We developed a nomogram with seven significant LCSBM independent prognostic factors to provide prognosis prediction.
Keywords: Lung cancer with simple brain metastasis, Prognostic analysis, SEER database, Nomogram
Introduction
Brain metastasis is one of the common metastatic mode of lung cancer [1]. It has the characteristics of advanced degree of malignancy, high mortality and difficulty in treatment. The main source of brain metastasis is also lung cancer [2]. And the brain is a specific metastatic organ of non-small cell lung cancer [3].
We studied the prognosis of lung cancer with simple brain metastasis (LCSBM), that was, lung cancer only had brain metastasis with no other organ metastasis. Some study showed the 5-year disease-free survival (DFS) and overall survival (OS) of non-small cell lung cancer were 82.4% and 85.4%, respectively [4]. It’s obvious that patients with LCSBM have a poorer prognosis [5]. However, the factors affecting prognosis have not been fully studied. At present, the most commonly used method is still the TNM staging system, with an unsatisfactory predictive effect [6]. Judging the prognosis is necessary in aspect of clinicians analyzing and evaluating the patients' conditions in order to better adjust the treatment strategies. Individualized prognostic diagnosis can lead to individualized treatment, which also has a good promotion effect on the development of precision medicine.
In view of this, an accurate and practical prognostic model is necessary. We selected data from the SEER database and established a nomogram to quantify the contribution of each risk factor to the prognosis. The SEER database is the abbreviation for the Surveillance, Epidemiology, and End Results (SEER) database. It is a program expanding over time to now include 18 registries, with information over enough to cover about 30% of the United States population, collecting data of their morbidity, mortality and survival [7]. A nomogram allows the model to be presented in an intuitive and simple form that can quickly achieve good results without any mathematical foundation or complex calculations.
The purpose of our study was to analyze the prognostic risk factors for LCSBM patients, and to establish a prediction model of patient prognosis in order to help clinical practice. This allows physicians to access the states of patients and lay the foundation for individualized treatment. What’s more, it will provide a credible explanation of the condition for patients and their families to avoid the lack of confidence and over-expectation, and facilitate the communication between the doctor and the patient.
Material and methods
Source
Data of patients with lung cancer brain metastasis from Surveillance, Epidemiology and End Results (SEER) database was searched and collected through queries using the latest version of the SEER 18 Registries Research Data (2010–2015), which was released in April 2017 with the SEER*Stat 8.3.5 software.
Patient screening
Inclusion criteria:
Patients aged 18 years or older diagnosed with LCSBM (and younger than 80 at diagnosis).
Patients diagnosed between 2010 and 2015.
Patients diagnosed only primary neoplasms without multiple primary neoplasms elsewhere.
Patient diagnosed with pathological results.
Patients with complete follow-ups.
Patients died of LCSBM rather than other causes.
Exclusion criteria:
Unknown demographic information including diagnostic age, gender, marital status and race;
Unknown clinical information including primary site, TNM stage and grade;
Unknown treatment information including surgery and others;
Patients received chemotherapy or radiotherapy at diagnosis;
Patients with multiple primary tumors.
Variable selection
There were 2 main types based on biology and treatment: small cell lung cancer and non-small cell lung cancer [8], and non-small cell lung cancer could be divided into squamous cell carcinoma, adenocarcinoma and large cell carcinoma. Since these sub-categories had an important impact on prognosis, our study discussed these types separately. Pathological grade was an important manifestation of the malignant degree of lung cancer. The advanced grade of pathology was closely related to the poor prognosis. According to the degree of differentiation of lung cancer, this study classified lung cancer into high differentiation (grade I), moderate differentiation (grade II), poorly differentiated (grade III) and undifferentiated (grade IV).The primary site was divided into: (1) Main bronchus; (2) Upper lobe, lung; (3) Middle lobe, lung and (4) Lower lobe, lung according to the anatomical structure. Location would be a meaningful taxonomy due to the different cellular structures. For the TNM staging system, the SEER database used the seventh edition of the TNM staging [9]. In order to maintain consistency in data measurement standards, this study also used the same staging criteria. Laterality was divided into left, right and others.
In terms of treatment, patients were divided into surgery group and non-surgical group. The SEER database did not contain information of radiotherapy and chemotherapy, so there was no relevant classification.
In terms of general conditions and epidemiological indicators, the study used X-tile software to obtain the best segmental age for diagnosis. And the race was divided into white, black and other (American Indian/AK Native, Asian/Pacific Islander). Marital status was classified as unmarried and married.
In addition, the patient's study endpoint was death or the deadline was March 2018.
Statistical analyses
Univariate analysis and Cox proportional hazards model were performed by SPSS (v25.0). Prognostic overall survival was analyzed using Kaplan–Meier curves. Each of individual prognostic factors of LCSBM patients were analyzed by log-rank method. Introducing meaningful variables of single factor analysis into Cox proportional hazards model for multivariate analysis, the independent risk factors were obtained, P < 0.05 was statistically significant. By R Studio (v3.6.2), Independent risk factors were included in the accelerated failure time model to construct nomogram. C-index was used to access the predictive capacity of nomogram.
Result
Patient characteristics from SEER database
In our study, younger patients (18–65 years old) accounted for 54.41%, while patients aged over 65 accounted for 45.59%. Married people were slightly more than half. The major race was white, accounting for 78.24% of all selected patients, while blacks and others accounted for 13.40% and 8.36%, respectively. The ratio of male to female patients was about 1:1. The most common type of histology was adenocarcinoma, accounting for 57.83%. For laterality, left and right accounted for 41.93% and 58.07%, respectively.
Univariate analysis showed that the factors affecting the prognosis of LCSBM included the following factors (Table 1): age (χ2 = 163.16, p = < 0.001), marital Status (χ2 = 43.985, p = < 0.001), primary Site (χ2 = 10.727, p = 0.013), race (χ2 = 16.999, p = < 0.001), surgery (χ2 = 184.795, p = < 0.001), gender (χ2 = 31.99, p = < 0.001), grade (χ2 = 71.301, p = < 0.001), histologic type (χ2 = 98.416, p = < 0.001), T Stage (χ2 = 58.295, p = < 0.001) and N stage (χ2 = 25.029, p = < 0.001) (Table 1).
Table 1.
Clinicopathologic characteristics of patients
| Clinicopathologic parameters | Number of cases | Average survival (month) | 95% CI | χ2 | p | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Age | 163.160 | < 0.001 | ||||
| 18–65 | 2071 | 54.41 | 15.061 | 14.107–16.014 | ||
| ≥ 66 | 1735 | 45.59 | 8.806 | 8.070–9.542 | ||
| Marital status | 43.985 | < 0.001 | ||||
| Non-married | 1786 | 46.93 | 10.377 | 9.550–11.205 | ||
| Married | 2020 | 53.07 | 13.808 | 12.890–14.727 | ||
| Primary site | 10.727 | 0.013 | ||||
| Main bronchus | 187 | 4.91 | 9.0410 | 6.979–11.102 | ||
| Upper lobe, lung | 2390 | 62.80 | 12.503 | 11.708–13.299 | ||
| Middle lobe, lung | 170 | 4.47 | 13.994 | 10.790–17.198 | ||
| Lower lobe, lung | 1059 | 27.82 | 11.686 | 10.520–12.852 | ||
| Race | 16.999 | < 0.001 | ||||
| White | 2978 | 78.24 | 11.861 | 11.172–12.549 | ||
| Black | 510 | 13.40 | 11.708 | 10.061–13.355 | ||
| Other | 318 | 8.36 | 15.783 | 13.418–18.148 | ||
| Surgery | 184.795 | < 0.001 | ||||
| No surgery | 3463 | 90.99 | 10.550 | 9.978–11.121 | ||
| Surgery | 343 | 9.01 | 28.233 | 25.065–31.401 | ||
| Gender | 31.990 | < 0.001 | ||||
| Male | 1970 | 51.76 | 10.804 | 10.008–11.600 | ||
| Female | 1836 | 48.24 | 13.706 | 12.746–14.667 | ||
| Grade | 71.301 | < 0.001 | ||||
| I | 140 | 3.68 | 19.020 | 14.870–23.170 | ||
| II | 880 | 23.12 | 15.229 | 13.807–16.651 | ||
| III | 2446 | 64.27 | 10.996 | 10.270–11.722 | ||
| IV | 340 | 8.93 | 9.712 | 8.026–11.398 | ||
| Laterality | 0.475 | 0.491 | ||||
| Left | 1596 | 41.93 | 12.216 | 11.293–13.138 | ||
| Right | 2210 | 58.07 | 12.153 | 11.318–12.988 | ||
| Histologic type | 98.416 | < 0.001 | ||||
| Squamous cell carcinoma | 555 | 14.58 | 8.131 | 7.085–9.176 | ||
| Adenocarcinoma | 2201 | 57.83 | 14.318 | 13.411–15.225 | ||
| Small cell lung cancer | 335 | 8.80 | 9.832 | 8.156–11.507 | ||
| Large cell carcinoma | 101 | 2.65 | 8.456 | 6.289–10.623 | ||
| Others | 614 | 16.13 | 10.049 | 8.656–11.442 | ||
| T stage | 58.295 | < 0.001 | ||||
| T1 | 430 | 11.30 | 16.512 | 14.280–18.744 | ||
| T2 | 1283 | 33.71 | 13.424 | 12.311–14.537 | ||
| T3 | 1014 | 26.64 | 10.74 | 9.625–11.855 | ||
| T4 | 1079 | 28.35 | 10.234 | 9.219–11.248 | ||
| N stage | 25.029 | < 0.001 | ||||
| N0 | 1078 | 28.32 | 14.532 | 13.183–15.882 | ||
| N1 | 400 | 10.51 | 12.973 | 11.025–14.921 | ||
| N2 | 1778 | 46.72 | 11.123 | 10.302–11.943 | ||
| N3 | 550 | 14.45 | 10.118 | 8.739–11.496 | ||
| Overall | 3806 | 100.00 | 12.234 | 11.604–12.864 | ||
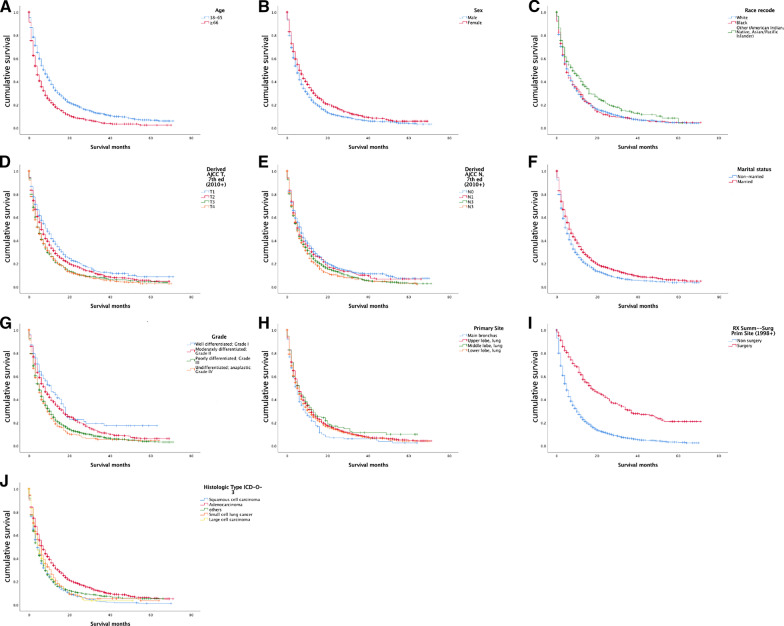
The prognostic survival time of patients aged 18–65 (average survival time: 15.1 months, 95% CI 14.107–16.014) was better than that of patients aged 66 and over (average survival time: 8.8 months, 95% CI 8.070–9.542). The prognosis of unmarried patients (10.4 months) was worse than that of married (13.8 months) and the prognosis of males was worse than that of females (10.8 months vs 13.7 months). For the primary site, middle lobe of lung had the best prognosis, better than main bronchus; upper lobe and lower lobe (14.0 months vs 9.0 months vs 12.5 months vs 11.7 months) (Fig. 1).
Fig. 1.
Charts from A–J are Kaplan-Meier Curve of prognostic factors. Note: figures refer to age, gender, race, T stage, N stage, marital status, grade, primary site, surgery, histologic type, respectively
Multivariate analysis of prognostic factors
Introducing the significant factors of single factor analysis into Cox proportional risk model for multi-factor analysis and then we got independent prognostic factors as follows: age (HR = 1.506, 95% CI 1.402–1.617), marital status (HR = 0.804, 95% CI 0.749–0.864), gender (HR = 0.806, 95% CI 0.751–0.866), race (HR = 0.896, 95% CI 0.844–0.951), grade (HR = 1.185, 95% CI 1.123–1.251), T stage (HR = 1.092, 95% CI 1.054–1.132) and primary site (HR = 0.451, 95% CI 0.390–0.521) (Table 2).
Table 2.
Independent factors for the prognosis of lung cancer brain metastasis
| Independent risk factors | Regression coefficient | SE | P | HR | 95% CI |
|---|---|---|---|---|---|
| Age | 0.409 | 0.036 | < 0.001 | 1.506 | 1.402–1.617 |
| Marital status | − 0.218 | 0.036 | < 0.001 | 0.804 | 0.749–0.864 |
| Gender | − 0.215 | 0.037 | < 0.001 | 0.806 | 0.751–0.866 |
| Race | − 0.110 | 0.030 | < 0.001 | 0.896 | 0.844–0.951 |
| Grade | 0.170 | 0.028 | < 0.001 | 1.185 | 1.123–1.251 |
| T stage | 0.088 | 0.018 | < 0.001 | 1.092 | 1.054–1.132 |
| Primary site | − 0.797 | 0.074 | < 0.001 | 0.451 | 0.390–0.521 |
As shown in Table 2, the protective factors included married, women, white people and primary site, while risk factors for poor prognosis include: higher age, advanced grade and advanced T stage.
Development and verification of prediction model nomogram
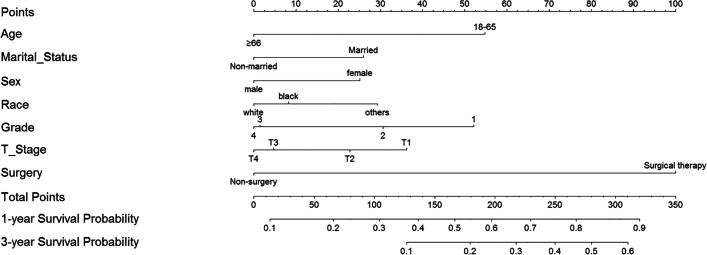
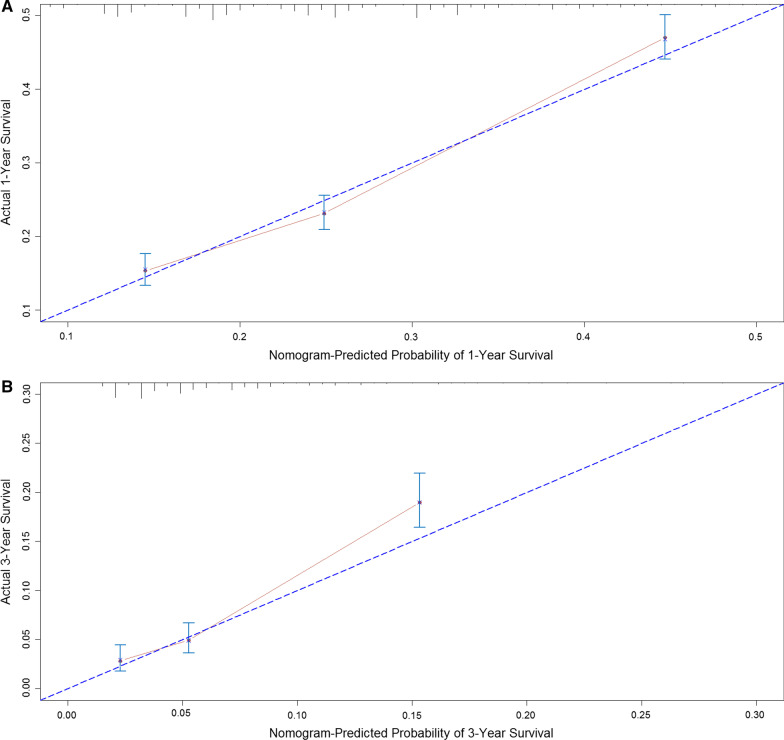
With the results of multivariate analysis, we constructed a nomogram (Fig. 2). The risk factors introduced in the model were given different weights according to the degree of influence, and different scores were obtained according to the individual information of the patients. Adding the scores together to get the final score, and the prognostic prediction results could be found in the nomogram. Internally validation was done by discrimination and calibration method. The calibration plots showed correlation between observed OS and nomogram predicted OS. C-index of the predictive model in this study was 0.634 (95% CI 0.622–0.646), showing a good prediction effect (Fig. 3).
Fig. 2.
Nomogram for predicting 1- and 3-year cancer-specific survival of patients with lung cancer brain metastasis. Note: Grade: I = 1; II = 2; III = 3; IV = 4
Fig. 3.
Nomogram model calibration curves. A 1-years calibration curves; B 3-years calibration curves. Note: The x-axis shows the nomogram predicted probability, and the y-axis gives the actual survival as estimated by the Kaplan–Meier method
Discussion
Analysis of demographic results
We used the SEER database to obtain information of 3806 patients diagnosed with LCSBM, and extracted general status indicators, pathological indicators and treatment status indicators to comprehensively analyze the risk factors of LCSBM prognosis. This was the first nomogram predicting the prognosis of LCSBM patients, which could better optimize the diagnosis and treatment plan and help improve the prognosis of patients. At the same time, there were some deviations in this study, which would be discussed in the limitations of the study later.
In our study, the average survival time for patients with LCSBM was 12.2 months. Survival rates for 1-, 3- and 5-years were 28.2%, 8.7% and 4.7%, respectively. In general, lung cancer is more likely to occur in the old, but our study showed that the proportions of young people and that of old people diagnosed with LCSBM were similar, suggesting that young people might be more prone to brain metastasis, which deserved further study. In terms of race, white patients occupied a major part. Brain metastasis might be related to genes in combination with age and race.
The most common histological type was adenocarcinoma in this study, while main type of lung cancer was squamous cell carcinoma, indicating that brain tissue had more affinity for lung adenocarcinoma cells. It was showed that adenocarcinoma-associated genes were closely related to brain metastasis, and the specific small RNA associated with metastasis in lung adenocarcinoma had been identified [10]. Most patients with brain metastases were pathological grade III, because advanced malignant tumors were prone to brain metastasis, just like breast cancer [11].
For the primary site, the upper lobe tumors accounted for more than half, and lung cancer often occurred in the upper lobe, which was consistent.
Risk factors for small intestinal neoplasms
Patients with LCSBM had some similarities with other types of lung cancer patients. First, the mean prognosis of the old group was worse than that of the young group, which was consistent with most studies [12, 13]. Second, Deng et al. reported that marital status was a prognostic factor for distant metastasis of non-small cell lung cancer [14]. Marriage as an important external environment might be psychologically reflected in the prognosis [15]. Third, the higher the histological grade, the stronger the malignancy and the worse the prognosis. In addition to our research, there was no literature reporting whether the prognosis of LCSBM was related to grade, but it had been reported that the prognosis of patients with breast cancer brain metastases was related to histologic grade [16]. Therefore, we believed that this conclusion was reasonable. Fourth, in aspect of histological classification, Miller did a research showing that prognosis of adenocarcinoma was better than that of squamous cell carcinoma in non-small cell lung cancer with brain metastasis [17]. Our study reconfirmed this result. As the T or N stage increased, the tumor progressed gradually, and the survival time gradually decreased, which was in line with clinical experience [6, 16, 18]. Our study found that from the initial stage to the end stage, the difference in prognosis between different TNM stages was only about half a year. It was obvious that LCSBM had high level of malignancy. Finally, surgical resection could effectively prolong the prognosis of patients [19, 20]. Although the data showed that surgery could prolong the prognosis of patients well, many patients with brain metastases did not meet the surgical indications so that patients who could be operated were few. As a result, our study included a large number of inoperable people to improve the adaptability of the model.
More importantly, our study suggested that LCSBM was a special type of lung cancer. First, race was an important factor influencing the prognosis of LCSBM. Thus, the prognosis of LCSBM patients might be related to genes. It had been shown that EGFR mutation was an independent predictor of probabilistic and prognostic factors for brain metastases (BM) and it was also an overall survival (OS)-positive predictor of BM patients [21]. Lee believed that the presence of EGFR activating mutations should be used as an indicator of prognosis in patients with lung adenocarcinoma and brain metastases [22]. And according to Fu P, race was an independent predictor of EGFR mutation [23]. This confirmed our point of view to some extent. Another study showed that Robo1 was also a cancer-promoting gene that might promote the development and progression of lung cancer and lung cancer brain metastasis [24]. Nowadays, the novel immunotherapy about programmed death ligand-1 (PD-L1) and programmed death ligand-1 (PD-1) receptor is getting wide attention, and many clinical trials showed that the objective response rate in patients PD-L1–positive tumors obviously higher than in those with PD-L1–negative tumors, PD-L1 protein expression may become a novel biomarker in the future to guide the clinical use of immunotherapy [25, 26]. ALK and ROS1 rearrangements have been proved as oncogenic drivers. Tejas Patil et al., reviewed 579 patients with stage IV NSCLC, and the results showed that the incidence of brain metastases for treatment-naïve, stage IV ROS1+ and ALK+ NSCLC were both up to about 34% and with no difference across ROS1, ALK, EGFR, KRAS, BRAF or other mutations groups [27, 28].
In summary, genetic differences were likely to be the underlying cause of different prognosis; Second, gender was an independent factor affecting prognosis, and the average survival time of women is 26.9%, which was higher than that of males. This was consistent with the findings of non-small cell lung cancer, showing that male is the independent risk factors for shortening the prognosis [29]. Under long-term chemotherapy, female also had a better prognosis than male [30]. This suggested that genes and hormones were involved; Third, Li et al. collected tumor tissue samples from 118 patients with non-small cell lung cancer, and found that EGFR gene mutations and high copy number of genes were more common in female patients [31]. The above-mentioned EGFR gene was associated with brain metastasis of lung adenocarcinoma, so we believed that the gender difference in prognosis may be derived from the gene.
In general, lung cancer patients with simple brain metastases have a poor prognosis, but its prognostic risk factors are unclear. From the results, we have identified these: patients with simple brain metastasis may be related to genetic differences, and endocrine status may affect the prognosis of patients.
Nomogram for small intestinal neoplasms
In some studies, TNM staging system did not yield a suitable prognosis [32]. TNM staging system is a general-purpose model which has distinct deviations for specific diseases, especially those with low morbidity. And nomogram is a good alternative [33], it can quickly and intuitively get the patients’ prognosis. Nowadays, nomogram is currently used in a variety of fields.
In our subject-related areas, current researches included prognostic analysis of tumor brain metastasis [34], survival analysis of non-small cell lung cancer (NSCLC) after surgical resection [33], and prognosis of NSCLC brain metastasis after surgery [35, 36], but there had not been a LCSBM prognostic prediction model. Therefore, it was necessary to examine prognostic risk factors for such patients and establish a reliable prediction model. Our model was proven to achieve reliable accuracy and to meet the needs of doctors and patients. Doctors could adjust the treatment according to the specific information of the patients and carry out targeted individualized treatment. At the same time, for patients, brain metastasis indicates a small amount of time. Accurate prognosis is responsible for patients, and it will become an important reference to treatment choice and psychological preparation. Therefore, in order to maximize the effectiveness of treatment and improve the prognosis and quality of life of patients, our model is meaningful.
Insufficient study
Although the evidence in this test was sufficient, the argument was reasonable, and an innovative viewpoint was put forward, there were certain deficiencies. First of all, the data source of this trial is the SEER database. It collected the data of residents from different regions in the United States. To draw conclusions that can apply to another area, the data from the local should be used to validate the model in advance. Second, for the SEER database, it only included some common information such as age, race, gender, histology level, TNM staging, etc. However, there are many other risk factors influencing the prognosis, and it is impossible to exhaust the enumeration. Genomic status, protein expression, family history, etc. were all excluded, which would cause bias. Third, although the information on radiotherapy and chemotherapy has recorded in the database, it was not recommended for the construction of nomogram due to the incompleteness of the data and the bias was impacted by the patient's willing to treat as well. Fourth, the test was a retrospective analysis based on the database, with the limitations of the trial itself, requiring further validation of the prospective cohort study to obtain sufficient evidence.
Acknowledgements
Not applicable.
Abbreviations
- LCSBM
Lung cancer with simple brain metastasis
- DFS
Disease-free survival
- OS
Overall survival
- SEER
Surveillance, Epidemiology, and End Results
- BM
Brain metastases
- NSCLC
Non-small cell lung cancer
Author contributions
JY, ZC and JF contributed to the conception of the study and wrote the whole manuscript. JY, ZC and JF contributed equally to this article. CX and YW helped to revise the manuscript. ZZ, QL, SG and LJ helped to analyze data associated with the manuscript. JW is the last corresponding author, who is responsible for the submission and other details involved with this article. YS and GJ helps in manuscript preparation and revise.
Funding
This work was supported by the National Natural Science Foundation of China (82170033, 81372236, 31521003), Natural Science Foundation of Shanghai (21ZR1479200), Shanghai Changhai Hospital Scientific Research Fund (2019SLZ002, 2019YXK018), Shanghai Postdoctoral Science Foundation (12R21411-500).
Availability of data and materials
All data in this paper are from SEER database, and we guarantee that all clinical data are anonymous. Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.com.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaying Yuan, Zhiyuan Cheng and Jian Feng have contributed equally to this work and shared first authorship
Contributor Information
Gengxi Jiang, Email: jiang9909@hotmail.com.
Yan Shang, Email: shangyan751200@163.com.
Junjie Wu, Email: wjjxcc@126.com.
References
- 1.Gibson AJW, Li H, D'Silva A, Tudor RA, Elegbede AA, Otsuka SM, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35(9):117. doi: 10.1007/s12032-018-1182-8. [DOI] [PubMed] [Google Scholar]
- 2.Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31(4):481–488. doi: 10.1016/j.nec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Ortuzar W, Hanna N, Pennella E, Peng G, Langer C, Monberg M, Scagliotti G. Brain metastases as the primary site of relapse in two randomized phase III pemetrexed trials in advanced non-small-cell lung cancer. Clin Lung Cancer. 2012;13(1):24–30. doi: 10.1016/j.cllc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Dai L, Yan W, Kang X, Fu H, Yang Y, Zhou H, et al. Exploration of postoperative follow-up strategies for early staged NSCLC patients on the basis of follow-up result of 416 stage I NSCLC patients after lobectomy. Zhongguo Fei Ai Za Zhi. 2018;21(3):199–203. doi: 10.3779/j.issn.1009-3419.2018.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanibuchi M, Kim SJ, Fidler IJ, Nishioka Y. The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Invest. 2014;61(3–4):241–253. doi: 10.2152/jmi.61.241. [DOI] [PubMed] [Google Scholar]
- 6.Oberije C, De Ruysscher D, Houben R, van de Heuvel M, Uyterlinde W, Deasy JO, et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: toward survival prediction for individual patients. Int J Radiat Oncol Biol Phys. 2015;92(4):935–944. doi: 10.1016/j.ijrobp.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 9.Wittekind C. TNM-System 2010 : Zur 7. Auflage der TNM-Klassifikation maligner Tumoren [2010 TNM system: on the 7th edition of TNM classification of malignant tumors] Pathologe. 2010;31(5):331–2. doi: 10.1007/s00292-010-1349-3. [DOI] [PubMed] [Google Scholar]
- 10.Daugaard I, Venø MT, Yan Y, Kjeldsen TE, Lamy P, Hager H, et al. Small RNA sequencing reveals metastasis-related microRNAs in lung adenocarcinoma. Oncotarget. 2017;8(16):27047–27061. doi: 10.18632/oncotarget.15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow L, Suen D, Ma KK, Kwong A. Identifying risk factors for brain metastasis in breast cancer patients: implication for a vigorous surveillance program. Asian J Surg. 2015;38(4):220–223. doi: 10.1016/j.asjsur.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, Schild SE, et al. Brain metastasis from non-small cell lung cancer (NSCLC): prognostic importance of the number of involved extracranial organs. Strahlenther Onkol. 2014;190(1):64–67. doi: 10.1007/s00066-013-0439-6. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, et al. Brain metastasis: prognostic value of the number of involved extracranial organs. Strahlenther Onkol. 2013;189(12):996–1000. doi: 10.1007/s00066-013-0442-y. [DOI] [PubMed] [Google Scholar]
- 14.Deng J, Ren Z, Wen J, Wang B, Hou X, Xue Z, et al. Construction of a nomogram predicting the overall survival of patients with distantly metastatic non-small-cell lung cancer. Cancer Manag Res. 2018;10:6143–6156. doi: 10.2147/CMAR.S183878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvikstad A, Vatten LJ. Cancer risk and prognosis in Norway: comparing women in their first marriage with women who have never married. J Epidemiol Community Health. 1996;50(1):51–55. doi: 10.1136/jech.50.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Zhang K, Siegal GP, Wei S. Clinicopathological factors associated with survival in patients with breast cancer brain metastasis. Hum Pathol. 2017;64:53–60. doi: 10.1016/j.humpath.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Suh JH, Barnett GH, et al. The impact of tumor biology on survival and response to radiation therapy among patients with non-small cell lung cancer brain metastases. Pract Radiat Oncol. 2017;7(4):e263–e273. doi: 10.1016/j.prro.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 18.She Y, Zhao L, Dai C, Ren Y, Zha J, Xie H, et al. Preoperative nomogram for identifying invasive pulmonary adenocarcinoma in patients with pure ground-glass nodule: a multi-institutional study. Oncotarget. 2017;8(10):17229–17238. doi: 10.18632/oncotarget.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Rahman O. Outcomes of surgery as part of the management of metastatic non-small-cell lung cancer: a surveillance, epidemiology and end results database analysis. Cancer Invest. 2018;36(4):238–245. doi: 10.1080/07357907.2018.1466895. [DOI] [PubMed] [Google Scholar]
- 20.Karagkiouzis G, Spartalis E, Moris D, Patsouras D, Athanasiou A, Karathanasis I, et al. Surgical management of non-small cell lung cancer with solitary hematogenous metastases. In Vivo. 2017;31(3):451–454. doi: 10.21873/invivo.11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han G, Bi J, Tan W, Wei X, Wang X, Ying X, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget. 2016;7(35):56998–57010. doi: 10.18632/oncotarget.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Shin DY, Kim JW, Keam B, Kim TM, Kim HJ, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86(3):363–368. doi: 10.1016/j.lungcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Fu P, Panneerselvam A, Clifford B, Dowlati A, Ma PC, Zeng G, et al. Simpson's paradox - aggregating and partitioning populations in health disparities of lung cancer patients. Stat Methods Med Res. 2015;24(6):937–948. doi: 10.1177/0962280211434179. [DOI] [PubMed] [Google Scholar]
- 24.Li XX, Jin L, Sun ZF, Gu F, Li WL, Ma YJ. Robo1 expression in non-small cell lung cancer and its brain metastasis. Zhonghua Zhong Liu Za Zhi. 2013;35(3):198–201. doi: 10.3760/cma.j.issn.0253-3766.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11(7):964–975. doi: 10.1016/j.jtho.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Yu Y, Lu S. Effectiveness of PD-1/PD-L1 inhibitors in the treatment of lung cancer: Brightness and challenge. Sci China Life Sci. 2020;63(10):1499–1514. doi: 10.1007/s11427-019-1622-5. [DOI] [PubMed] [Google Scholar]
- 27.Patil T, Smith DE, Bunn PA, Aisner DL, Le AT, Hancock M, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol. 2018;13(11):1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remon J, Pignataro D, Novello S, Passiglia F. Current treatment and future challenges in ROS1- and ALK-rearranged advanced non-small cell lung cancer. Cancer Treat Rev. 2021;95:102178. doi: 10.1016/j.ctrv.2021.102178. [DOI] [PubMed] [Google Scholar]
- 29.Bauml J, Mick R, Zhang Y, Watt CD, Vachani A, Aggarwal C, et al. Determinants of survival in advanced non–small-cell lung cancer in the era of targeted therapies. Clin Lung Cancer. 2013;14(5):581–591. doi: 10.1016/j.cllc.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu XY, Huang XE. Screening for patients with non-small cell lung cancer who could survive long term chemotherapy. Asian Pac J Cancer Prev. 2015;16(2):647–652. doi: 10.7314/apjcp.2015.16.2.647. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Zhang LJ, Wang WP, Guo K, Shao JY, Rong TH. Correlation between EGFR gene mutation and high copy number and their association with the clinicopathological features in Chinese patients with non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2011;33(9):666–670. [PubMed] [Google Scholar]
- 32.Wu J, Zhou L, Huang L, Gu J, Li S, Liu B, et al. Nomogram integrating gene expression signatures with clinicopathological features to predict survival in operable NSCLC: a pooled analysis of 2164 patients. J Exp Clin Cancer Res. 2017;36(1):4. doi: 10.1186/s13046-016-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 34.Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol. 2015;4(2):18. doi: 10.3978/j.issn.2304-3865.2015.06.01. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Zheng W, Ying L, Wu J, Wu S, Ma S, et al. A nomogram to predict brain metastases of resected non-small cell lung cancer patients. Ann Surg Oncol. 2016;23(9):3033–3039. doi: 10.1245/s10434-016-5206-3. [DOI] [PubMed] [Google Scholar]
- 36.Ji X, Zhuang Y, Yin X, Zhan Q, Zhou X, Liang X. Survival time following resection of intracranial metastases from NSCLC-development and validation of a novel nomogram. BMC Cancer. 2017;17(1):774. doi: 10.1186/s12885-017-3763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this paper are from SEER database, and we guarantee that all clinical data are anonymous. Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.com.