Introduction
Congenital muscular dystrophy (CMD) is a rare, inherited neuromuscular disease comprised of many heterogeneous subgroups. CMD has a prevalence of about one in 100,000 children and is characterized by early onset progressive muscle weakness that presents from birth to up to two years of age.1 Patients with CMD secondary to collagen VI deficiency (COLVI-RD) experience a rapid decline in pulmonary function with more than 50% of vital capacity lost by 10 years of age.2 By the second decade of life, 70% of patients develop respiratory failure requiring assisted ventilation and experience early mortality.
Progressive muscle weakness is recognized to cause declining lung function in patients with muscular dystrophy, but the etiology of respiratory failure in this population is multifactorial. Changes in the mechanical properties of the chest wall and in lung compliance play a role in respiratory decline.3 More specifically, progressive contractures of the costovertebral joints distort the chest wall decreasing compliance and limiting normal chest motion.4,5 As these structural chest wall deformities develop and respiratory muscle weakness worsens, lung compliance also decreases.6 Respiratory muscle weakness, chest wall deformity and decreased lung compliance impair respiratory function more profoundly than might be expected with muscle weakness alone. Therefore, patients with muscular dystrophy experience respiratory decline that is out of proportion to what is expected from muscle weakness alone.7
Passive stretch has been proposed to prevent chest wall contractures as stretching slows the development of contractures and maximizes muscle function in patients with CMD.8–10 Therefore, we hypothesize that stretching of the chest wall by hyperinflation could potentially attenuate the rate of respiratory compromise.
The clinical courses for COLVI-RD and LAMA2-related muscular dystrophy patients are severe and progressive with clearly defined trajectories.11 The primary aim of this study was to test the feasibility and efficacy of individualized hyperinflation therapy on slowing the decline of pulmonary function in children with both these forms of CMD.
Methods
Recruitment
We recruited participants from two tertiary children’s hospitals and externally using an international registry of patients from the Cure Congenital Muscular Dystrophy Foundation (CureCMD-IR; https://www.curecmd.org/). Inclusion criteria were: 1) age 5–21 years old, 2) forced vital capacity (FVC) of ≥ 30 and ≤ 80% predicted based on modified ATS criteria 12, 3) confirmed collagen type VI-related CMD or LAMA2-related muscular dystrophy by clinical history and muscle/skin biopsy or gene mutation, 4) absence of other chronic medical conditions, and 5) absence of tracheostomy and ventilator dependence. Our exclusion criteria included: 1) non-compliance with study protocols and/or 2) difficulty in tolerating hyperinflation.
We used minimization, an adpative method for randomization that has been used previously to minimize imbalance between groups in randomized controlled trials.13 Criteria of randomization were limited to age, gender, and range of PFT restriction (mild, moderate and severe). Upon enrollment of each participant, the study statistician generated an allocation sequence code using the aforementioned minimization algorithm and provided the allocation sequence directly to the study coordinator for intervention assignment. No other personnel received the allocation assignment. Institutional Review Boards at both sites approved the study. The study was registered at ClinicalTrials.gov (NCT01836627).
Hyperinflation protocol
We utilized the CoughAssist® T70 machine (Phillips Respironics, Murrysville, PA, USA) (CAM) for pressure-volume assessments and also to provide hyperinflation therapy to the treatment group.
The pneumotachometer was connected in-line with the CAM via a full-face mask, and data were recorded on a laptop computer. The hyperinflation protocol started with an insufflation pressure of 10 cm water pressure (cwp) for 3 seconds followed by passive exhalation and recording of the exhaled volume. Testing was done at each pressure five times to ensure reproducibility. We incrementally increased pressure by 5 cwp and repeated until 1) the delivered volume increased by <10% of that delivered at the preceding pressure, and 2) the participant did not tolerate the pressure. We identified the preceding pressure as the ideal insufflation pressure (Figure 1). In the event the increasing pressures became uncomfortable, we prescribed the highest tolerated pressure. The hyperinflation regimen was then set at the insufflation pressure administered for 3 seconds, then released with a 9 second pause before the next breath at 5 breaths/minute for 15 minutes.
Figure 1:
Titration plot of a pressure-volume curve (PVC) of a subject with inspiratory capacity (IC) noted. The black arrow indicates the inflection point of the curve, and represents the identified hyperinsufflating pressure subsequently prescribed.
Only patients in the treatment group received a CAM with individualized settings for hyperfinaltion therapy. Control group patients continued with routine care prescribed by their local pulmonologist.
Testing Procedures and Assessments
Pulmonary Function
We performed all lung volume measurements using a pneumotachometer (Series 4700, Hans Rudolph Inc., Shawnee, KS, USA) with amplifier attached to a tightly applied facemask. We measured forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak flow (PEF), slow vital capacity (SVC), and inspiratory capacity (IC) in the seated and then supine positions. Acknowledging the limitations of a patient with respiratory muscle weakness, the modied ATS criteria for acceptability were used to maximize the quality of the data.12 Measurements were made in the morning and afternoon of the same day.
Study Procedures
Baseline visit
We obtained consent/assent at the baseline visit. We also obtained a medical history and performed a physical exam. We determined height from ulna length as contractures preclude accurate measurements of standing height or arm span.14 We performed baseline spirometry and determined individualized parameters for the hyperinflation regimen. Participants and caregivers completed questionnaires and were informed about the Daily Phone Diary (DPD). Control participants were discharged with a follow-up assessment date without additional equipment. Participants in the treatment group were given a CAM with pressures set to the prescribed pressures and specific instructions on how to perform the hyperinflation therapy.
Follow up contact
We allowed a period of up to 4 weeks for participants in the treatment arm to accommodate to the therapy until they were using the treatment at the goal of 15 minutes, twice daily. Participants then had research visits at 4, 8, and 12 months, during which the titration protocol and spirometry were repeated for all participants. If a treatment subject had a higher insuflation pressure, the CAM was adjusted to administer the new higher pressure.
Outcomes
Our primary outcome of interest was change in forced vital capacity. When monitoring for change in FEV1 over time, FEV1 would track the change in FVC if restriction in total lung capacity was the main reason for a lower FEV1. Secondary outcomes included quality of life (QOL) which was measured using the PedsQL15, and adherence to the hyperinflation protocol which was quantified using the Daily Phone Diary (DPD) and the downloadable memory cards from CAMs.(14–17)
Sample Size
Thirty five participants were required to investigate changes in pulmonary function and demonstrate an effect of >0.5 in the rate of change in FVC in the hyperinflation group compared to controls. These calculations were based on the difference in slopes estimated using a linear mixed effects model. Seventeen participants were required in the hyperinflation group to demonstrate adherence over a 12-month period. Using a mean across time of 0.10 (standard deviation 0.15) for optimal adherence there was 85% power to demonstrate a successful rate of adherence (at least 80%) to the hyperinflation treatment. This sample size was determined using a repeated measures model including a correlation for longitudinal DPD measurements (rho=0.8), 10% attrition and a two-sided alpha of 0.05.
Statistical Analysis
Descriptive statistics for all demographic, medical, and adherence variables were calculated. Wilcoxon rank sum test or Fisher exact test were used to examine differences between the control and treatment groups for race, age, gender, and baseline PFT data. Mixed-effects regression models adjusting for potential confounders (age, gender, BMI, and adherence rate between visits) were used to assess for changes in lung function. Subject level random effects were assigned to control for correlation between longitudinal measurements where the baseline FVC values were used as a covariate. The magnitude of the change in lung function between visits was calculated by substracting the change in each of the variables in the treatment group from the change observed in controls. Similar statistical models were used for assessing the change in maximum pressure and maximum volume data. Separate linear regression models were fitted to QOL scores at visits 2, 3, and 4, adjusting for covariates: baseline QOL and baseline FVC (model for QOL at visit 2) or the first principal component derived from the FVC values in earlier visits (models for QOL at visits 3, and 4). Bonferroni adjustment was made for multiple testing. The reported results are the least square mean (LSM) with standard error (SE) estimates. We excluded electronic monitoring adherence data from analyses if it was missing for more than 50% of the study interval. We imputed DPD data given it is highly correlated with CAM data 16 when available. We also calculated the frequencies of barriers to insufflation therapy. SAS version 9.4 (NC, USA) was used for analyses with statistical significance considered as p<0.05.
Results
Participants
There were 45 participants recruited for the study and 34 (75%) agreed to participate. Eighteen participants were randomized to the treatment group and 16 to the control group. Of those randomized, 1 participant dropped out from the treatment group due to inability to tolerate hyperinflation therapy. Two participants spontaneously dropped out of the control group. There were no differences in demographics or pulmonary function between the 2 groups at baseline (Table 1).
Table 1:
Subject demographics and pulmonary function measurements by group at recruitment into the study. Subsequently, 2 Control and 1 treatment subject withdrew from the study.
| Treatment (n=18) | Control (n=16) | Overall (N=34) | P-Value (Trt vs Cont) | |
|---|---|---|---|---|
| Age in Years (MED ± IQR) | 10.1 ± 3.9 | 10.5 ± 5.3 | 10.2 ± 4.5 | 0.96 |
| Male | 50% | 50% | 50% | 1.00 |
| Race | ||||
| • White | 83.3% | 81.3% | 82.4% | 1.00 |
| • Black-African American | 0 | 12.5% | 5.9% | 0.21 |
| • Asian | 11.1% | 0 | 5.9% | 0.49 |
| • Biracial | 5.6% | 6.3% | 5.9% | 1.00 |
| Forced Vital Capacity (% reference)(MED ± IQR) | 55.5±29.3 | 55.5±31.3 | 55.5±30.8 | 0.77 |
| Lung Disease Severity | ||||
| • Mild (FVC: 70–79%) | 27.8 | 31.3 | 29.4 | 1.0 |
| • Moderate (FVC: 60–69%) | 16.7 | 12.5 | 14.7 | 1.0 |
| • Moderate-Severe (FVC: 50–59%) | 16.7 | 12.5 | 14.7 | 1.0 |
| • Severe (FVC: 35–49%) | 38.9 | 37.5 | 38.2 | 1.0 |
| • Very Severe (FVC: < 35%) | 0 | 6.3 | 2.9 | 0.47 |
MED: median, IQR: interquartile range, Trt: treatment, Cont: control
Adherence
The overall adherence rate was 44±20.4% for the total study period and adherence declined over the course of the trial. At Visit 2, adherence was 51.8±18.4% compared to 36.1±27.5% and 38.1±22.6% in the period between Visits 2 to 3 and Visits 3 to 4, respectively.
Change in Pulmonary Function
A. Change in Seated Pulmonary Function Measurements
Absolute FVC
From baseline to visit 4, absolute FVC in the seated position was 1.2±0.06, 1.13±0.06, 1±0.06 and 1.1±0.06 liters respectively in the control group. In the treatment group, FVC was 1.09±0.06, 1.21±0.07, 1.1±0.06, and 1.1±0.06 liters from baseline to visit 4 respectively (Figure 2). At visits 2 and 3 the magnitude of the change in FVC from visit 1 for the treatment group was higher compared to control by 0.21±0.05 liters (P=0.0006), 0.17±0.04 liters (P=0.0004) respectively. There was no difference in the change in FVC between visits 1 and 4 between the study groups.
Figure 2:
Mean (± SD) absolute FVC values at individual visits for the control and treatment groups. The asterix (*) indicates significant difference between the treatment and control groups after adjusting for baseline FVC and other covariates (age, gender, BMI, and adherence rate between visits). The magnitude of the change in absolute FVC from visit 1 was significant at visit 2 and 3 for the treatment group.
Absolute FEV1
There was no significant gain in absolute FEV1 in the treatment group compared to controls between visits 1 and 3, and between visits 1 and 4.
B. Change in Seated Pulmonary Function As Percent Predicted
FVC % Predicted
There was a similar trend for the FVC% predicted as for the absolute FVC. From baseline to visit 4 for the control group, FVC% predicted in the seated position was 51.6±1.8%, 45.7±1.8%, 42.4±1.8%, 44.7±1.8% respectively. In the treatment group FVC% predicted was 41.9±1.8%, 45.1±2.2%, 39.4±1.9%, 38.5±1 8% from baseline to visit 4 respectively (Figure 3). At visits 2 and 3 the magnitude of the change in FVC% predicted from visit 1 for the treatment group was higher compared to control by 9±2% (P=<0.0001), 7±1.6% (P=0.0001) respectively. There was no difference in the change in FVC% predicted between visits 1 and 4 between the two groups.
Figure 3:
Mean (± SD) FVC% predicted values at individual visits for the control and treatment groups. The asterix (*) indicates significant difference between the treatment and control groups after adjusting for baseline FVC and other covariates (age, gender, BMI, and adherence rate between visits). The magnitude of the change in FVC% predicted values from visit 1 was significant at visit 2 and 3 for the treatment group.
FEV1 % Predicted
The magnitude of the change in FEV1% predicted was higher by 7±2.2% (P=0.01) in the treatment group between visits 1 and 2.
C. Reproduciblity of Pulmonary Function
The results were reproducible when the measurements were repeated in the afternoon session (Table 2). The treatment group experienced a gain in FVC between visits 1 and 2 by 0.216±0.058 litres (P=0.0014) and in FVC% predicted by 6.35±2.58% (P=0.0863). There was also a similar gain in FEV1 by 0.1726±0.06 L/sec (P=0.04) and in FEV1% predicted by 6.93±2.21% (P=0.0109).
Table 2:
Pulmonary function measurements in the mornings and afternoons at baseline of control and treatment groups. Values represented are the mean with SD for continuous data and percentage for categorical data.
| Variable | Control N =14 | Treatment N= 17 | P |
|---|---|---|---|
| Age | 11.3 ± 4.3 | 11 ± 3.7 | 0.8651 |
| Gender (% male) | 57 | 53 | 1.000 |
| Race (% white) | 79 | 82 | 1.000 |
| AM measurements | |||
| FVC seated (L) | 1.25 ± 0.72 | 1.07 ± 0.64 | 0.26 |
| FVC supine (L) | 1.10 ± 0.74 | 1.06 ± 0.63 | 0.84 |
| FEV1 seated (L) | 1.02 ± 0.54 | 0.93 ± 0.61 | 0.23 |
| FEV1 supine (L) | 0.88 ± 0.52 | 0.89 ± 0.59 | 0.40 |
| IC seated (L) | 0.84 ± 0.37 | 0.84 ± 0.47 | 0.57 |
| IC supine (L) | 0.83 ± 0.46 | 0.79 ± 0.43 | 0.67 |
| Maximum insufflation pressure (cwp) | 13.3 ± 2.3 | 14.5 ±5.3 | 0.09 |
| Maximum volume (L) | 1.16 ± 0.63 | 1.05 ± 0.58 | 0.09 |
| PM measurements | |||
| FVC seated (L) | 1.26 ± 0.75 | 1.14 ± 0.65 | 0.48 |
| FVC supine (L) | 1.12 ± 0.74 | 1.00 ± 0.58 | 0.51 |
| FEV1 seated (L) | 1.01 ± 0.57 | 0.96 ± 0.58 | 0.60 |
| FEV1 supine (L) | 0.89 ± 0.52 | 0.76 ± 0.49 | 0.33 |
| IC seated (L) | 0.82 ± 0.36 | 0.83 ± 0.42 | 0.95 |
| IC supine (L) | 0.80 ± 0.39 | 0.78 ± 0.41 | 0.80 |
| Maximum insufflation pressure (cwp) | 11.7 ± 3.0 | 16.1 ± 5.3 | < 0.001 |
| Maximum volume (L) | 0.93 ± 0.33 | 1.08 ± 0.46 | 0.18 |
D. Pulmonary Function in the Supine Position
The only measurement that demonstrated a gain in the supine position was absolute FVC between the first and the second visit for the treatment group (0.17±0.05L for FVC (P=0.007) and 4.8±2% for FVC% predicted (P=0.1)).
E. Inspiratory capacity
There was no change in inspiratory capacity between groups, neither in the seated nor in the supine positions.
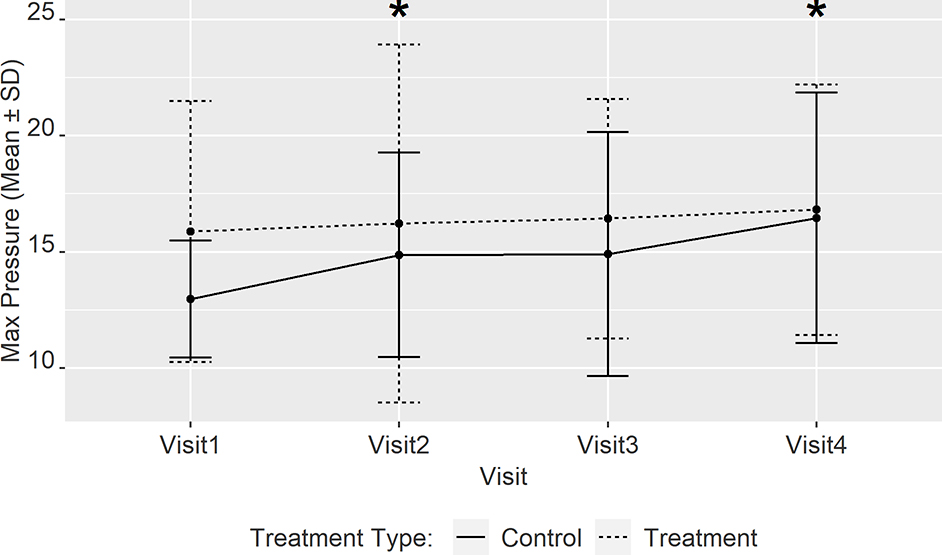
Changes in the maximum titration pressure and corresponding volume
From baseline to visit 4, the maximum insufflation pressure expressed as LSM was 12.9±1.3, 14.7±1.2, 15.2±1.2, 16.5±1.2 cwp respectively in the control group. In the treatment group the pressures were 16.6±1.2, 15.6±1.2, 17.0±1.2, 16.5±1.2 cwp from baseline to visit 4 respectively. At visit 2, 3 and 4, the magnitude of the change in maximum pressure for the treatment group was lower compared to control by −2.8±0.8 (P= 0.003), −2±0.8 (P= 0.07) and −3.7±0.8 (P < 0.001) cwp respectively.
There was no significant difference in the change in maximum volume achieved at maximal pressure between the 2 groups from visit 1 to any of the follow-up visits. Therefore, the control group required successive increases in maximum insufflation pressure to reach the same maximum volume while the treatment group did not require any change in pressure and reached the same maximum volume at all visits (Figure 4).
Figure 4:
Mean (± SD) maximal insufflation pressures at individual visits for the control and treatment groups. The asterix (*) indicates significant difference between the treatment and control groups after adjusting for baseline FVC and other covariates (age, gender, BMI, and adherence rate between visits) and results are reported expressed as LS means (± SE).
Impact on QOL
There were no statistically significant changes in children-reported QOL between the 2 groups. We found a statistically significant difference between the two groups on the caregiver-reported PedsQL score at visit 2 (increased by 6.7 points (P= 0.02), after controlling for baseline FVC and baseline caregiver-reported QOL scores (Figure 5).
Figure 5:
Least square mean (LSM) and standard error (SE) estimates of mother PedsQL total score by visit and treatment type. Models are adjusted for the baseline score and the FVC values (* indicates statistically significant difference at 5% level between treatment and control groups)
Barriers to Adherence
With regard to barriers to adherence collected from the DPD over the course of the study, 80% (n=8) of families who completed treatment reported experiencing at least one barrier. Caregivers indicated that the primary difficulties to using insufflation therapy related forgetting, competing activities (e.g., family obligations, social events), the child being embarrassed doing it in front of others, difficulty finding time, and child oppositional behaviors.
Exit questionnaire data
Following completion of the trial, nine of 13 caregivers and children had a positive experience with CAM. The benefits of CAM were having more energy, shorter illness duration when sick, stronger cough/exhalation and the child finding it easier to breathe. Optimal timing for treatment was characterized as before school or immediately after school and following dinner/bedtime.
Discussion
Our prospective randomized trial of hyperinflation therapy in children showed improved vital capacity four months after initiation of hyperinflation therapy. Additionally, there was a slower decline of absolute and percent predicted vital capacity values for the remainder of the study in the treatment group compared to controls. The initial gain in vital capacity in participants randomized to hyperinflation may have been achieved through an increase in the inspiratory capacity, a decrease in the functional residual capacity (which comprise the expiratory reserve volume and the residual volume), or a combination of both factors. In our study, we did not appreciate a significant increase in the inspiratory capacity. Therefore, the gain and or the slower decline in vital capacity in the treatment group is likely due to a reduction in residual volume. Residual volume can increase with disease progression in patients with neuromuscular disease.17,18 Preserving or improving chest wall compliance may facilitate more complete exhalation, thereby increasing ERV and reducing RV. Our findings support the hypothesis that hyperinflation therapy for children with CMD achieves a beneficial effect on lung function through a change in chest wall compliance and recoil.
The maximum insufflation pressure required to reach the largest lung volume increased over time in the control group, though remained stable in the treatment group. In both groups, the volume reached at maximum inflation did not change over time. Therefore, the gradual increase in maximum insuflation pressure in the control group, without a corresponding increase in maximal insufflating volume, suggests worsening compliance in the controls compared to the treatment group. This is in line with the natural history of the pulmonary manifestations of neuromuscular disease in causing reduced chest wall compliance over time.4,5 The lack of an increase in the insufflation pressure in the treatment group shows a positive effect of hyperinflation therapy preventing that progression.
These observations perhaps explain the greater decrease in FVC% predicted at the end of the study for the control over the treatment groups. The application of high-span pressure ventilation and higher in-exsufflation pressures have been attributed to improved chest wall mechanics and reversal of early-onset pectus exavatum in spinal muscular atrophy.19 In addition, the use of mechanical insufflation/exsufflation at modest pressures has been shown to improve peak cough flow in patients with severe respiratory muscle weakness.20 These studies illustrate benefits emerging from improvements in length-tension relationships of respiratory muscles, and preservation of respiratory compliance.
Several studies have attempted to adopt hyperinflation protocols in patients with neuromuscular disorders.21,22 Overall, such regimens appear beneficial, though methods of quantifying benefits, and degrees of benefit, vary.23–25 Direct comparisons between our findings and previous studies are complex for a variety of reasons. There are studies limited to patients with Duchenne Muscular Dystrophy21,23 and these patients vary from our population in the rate of decline of lung function and the chest wall deformites that develop over time. Heterogeneous samples and cross-sectional study design22 also limit direct comparison to our longitudinal results. However, at least two previous studies21,23 address the potentially important role of improved lung compliance that may occur with hyperinflation regimens. This is an important point to consider in the context of other documented benefits: enhanced peak cough flow in children with NMD, improved lung volumes and delayed decline in respiratory function.26
In our sample, adherence to our insufflation therapy regimen was suboptimal and difficult to maintain. Adherence is an essential factor to consider, therefore, since longer term tolerability of hyperinflation will likely be key to realizing the full benefits of this therapy. After adjusting for adherence, we identified improvements in lung volume at study visits. The treatment group experienced increases in both absolute FVC and FVC% predicted at Visit 2 when adherence was at its best. Reduced adherence to hyperinflation therapy noted at successive visits likely attenuated the benefits of therapy for vital capacity. More importantly, FVC stabilized over 8 months when comparing patients in the treatment with control group. The adherence data provide important insight into the duration and frequency of treatment needed to impact lung function. A clinically significant gain of lung volume between visits 1 and 2 was achieved with an average adherence of 51%; however, as adherence decreased between visits 2 and 3 (36%) and 3 and 4 (38%), lung volume also decreased. Therefore, a simpler protocol may actually decrease the burden of the intervention and enhance adherence, yielding an improved and sustainable impact on lung volume. Additionally, as many patients had advanced disease at baseline, our findings may underestimate the potential benefits of insufflation therapy. Initiating therapy earlier in the course of disease, coupled with measures to improve adherence, may demonstrate more pronounced benefits in lung function.
QOL is an important patient-reported outcome and may have implications for adherence. While patient self-reported QOL did not change, caregivers in the treatment group noted changes over time in their child’s QOL. It is possible that caregivers were able to make external observations regarding their child’s functioning that were unnoticed by the child him/herself, which has been found in the larger literature.27 It is not surprising that the participants’ QOL did not improve or worsen over time given the relatively short duration of the study. We did not capture serious events requiring hospitalizations, or periods of care escalation, in either group that could negatively impact QOL. Families in our sample reported a variety of benefits of hypersinsufflation therapy: improved cough quality, easier breathing, and reduced duration of respiratory illnesses, among others.
Limitations of the study include not measuring dynamic compliance or static lung volumes (for TLC, ERV, and RV) that may better explain the changes in chest wall mechanics and the impact on lung volumes. In addition, lung function was measured after randomization. As a result, significant differences in FVC and maximum insufflation pressures between the two groups were measured at baseline with controls having higher values. The inclusion of baseline pulmonary function in the randomization criteria would have seriously hampered the feasibility of the study given the rarity of CMD in cmparison to other types of muscular dystrophies. These differences may explain the greater decline in lung function and the lower maximum insufflation pressure in controls compared to the treatment group. However, adjustment for baseline FVC was made when the change in lung function between visits was calculated.
Conclusion
While the progressive dystrophic course of CMD cannot be modified yet with definitive therapy, the secondary anatomical and functional effects on the respiratory system are potentially modifiable. This prospective randomized trial is the first to demonstrate the ability of passive hyperinflation to preserve vital capacity. Future studies should include children earlier in the disease progression and over longer durations of time.
Acknowledgements
We would like to thank the patients that participated in this study as well as Rachel Alvarez, Secretary, Board of Directors and Executive Director, Outreach Committee for CureCMD.
FUNDING: Supported by The National Institutes of Health (R34NH113390–01 [RA]); The CureCMD Foundation.
Footnotes
SUMMARY CONFLICT OF INTEREST STATEMENTS
No conflicts: HS, ACM, JEP, KMC, JMM, AMR, MMH, RS, DGT, CLS, RA
OHM reports grants from Cure CMD Foundation, during the conduct of the study.
PRIOR ABSTRACT PUBLICATION
Thematic poster presentation at the International Conference of the American Thoracic Society, May 23, 2017, Washington DC. Am J Respir Crit Care Med 2017;195:A7685.
References
- 1.Kang PB, Morrison L, Iannaccone ST, et al. Evidence-based guideline summary: evaluation, diagnosis, and management of congenital muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2015;84(13):1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadeau A, Kinali M, Main M, et al. Natural history of Ullrich congenital muscular dystrophy. Neurology. 2009;73(1):25–31. [DOI] [PubMed] [Google Scholar]
- 3.Ward ME, Ward JW, Macklem PT. Analysis of human chest wall motion using a two-compartment rib cage model. J Appl Physiol. 1992;72(4):1338–1347. [DOI] [PubMed] [Google Scholar]
- 4.Mortola JP, Saetta M, Fox G, Smith B, Weeks S. Mechanical aspects of chest wall distortion. J Appl Physiol. 1985;59(2):295–304. [DOI] [PubMed] [Google Scholar]
- 5.Heldt GP, McIlroy MB. Distortion of chest wall and work of diaphragm in preterm infants. J Appl Physiol. 1987;62(1):164–169. [DOI] [PubMed] [Google Scholar]
- 6.Misuri G, Lanini B, Gigliotti F, et al. Mechanism of CO(2) retention in patients with neuromuscular disease. Chest. 2000;117(2):447–453. [DOI] [PubMed] [Google Scholar]
- 7.De Troyer A, Borenstein S, Cordier R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax. 1980;35(8):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott OM, Hyde SA, Goddard C, Dubowitz V. Prevention of deformity in Duchenne muscular dystrophy. A prospective study of passive stretching and splintage. Physiotherapy. 1981;67(6):177–180. [PubMed] [Google Scholar]
- 9.Vignos PJ, Wagner MB, Karlinchak B, Katirji B. Evaluation of a program for long-term treatment of Duchenne muscular dystrophy. Experience at the University Hospitals of Cleveland. J Bone Joint Surg Am. 1996;78(12):1844–1852. [DOI] [PubMed] [Google Scholar]
- 10.Harris SE, Cherry DB. Childhood progressive muscular dystrophy and the role of physical therapy. Phys Ther. 1974;54(1):4–12. [DOI] [PubMed] [Google Scholar]
- 11.Jain MS, Meilleur K, Kim E, et al. Longitudinal changes in clinical outcome measures in COL6-related dystrophies and LAMA2-related dystrophies. Neurology. 2019;93(21):e1932–e1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer OH, Finkel RS, Rummey C, et al. Characterization of pulmonary function in Duchenne Muscular Dystrophy. Pediatr Pulmonol. 2015;50(5):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 14.Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Dev Med Child Neurol. 2004;46(7):475–480. [DOI] [PubMed] [Google Scholar]
- 15.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 16.Pascoe JE, Sawnani H, Mayer OH, et al. Adherence and barriers to hyperinsufflation in children with congenital muscular dystrophy. Pediatr Pulmonol. 2017;52(7):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demedts M, Beckers J, Rochette F, Bulcke J. Pulmonary function in moderate neuromuscular disease without respiratory complaints. Eur J Respir Dis. 1982;63(1):62–67. [PubMed] [Google Scholar]
- 18.Katz SL, Gaboury I, Keilty K, et al. Nocturnal hypoventilation: predictors and outcomes in childhood progressive neuromuscular disease. Arch Dis Child. 2010;95(12):998–1003. [DOI] [PubMed] [Google Scholar]
- 19.Bach JR, Bianchi C. Prevention of pectus excavatum for children with spinal muscular atrophy type 1. Am J Phys Med Rehabil. 2003;82(10):815–819. [DOI] [PubMed] [Google Scholar]
- 20.Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J. 2003;21(3):502–508. [DOI] [PubMed] [Google Scholar]
- 21.Katz SL, Barrowman N, Monsour A, Su S, Hoey L, McKim D. Long-Term Effects of Lung Volume Recruitment on Maximal Inspiratory Capacity and Vital Capacity in Duchenne Muscular Dystrophy. Ann Am Thorac Soc. 2016;13(2):217–222. [DOI] [PubMed] [Google Scholar]
- 22.Stehling F, Bouikidis A, Schara U, Mellies U. Mechanical insufflation/exsufflation improves vital capacity in neuromuscular disorders. Chron Respir Dis. 2015;12(1):31–35. [DOI] [PubMed] [Google Scholar]
- 23.McKim DA, Katz SL, Barrowman N, Ni A, LeBlanc C. Lung volume recruitment slows pulmonary function decline in Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2012;93(7):1117–1122. [DOI] [PubMed] [Google Scholar]
- 24.Fauroux B, Guillemot N, Aubertin G, et al. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest. 2008;133(1):161–168. [DOI] [PubMed] [Google Scholar]
- 25.Dohna-Schwake C, Ragette R, Teschler H, Voit T, Mellies U. IPPB-assisted coughing in neuromuscular disorders. Pediatr Pulmonol. 2006;41(6):551–557. [DOI] [PubMed] [Google Scholar]
- 26.Chiou M, Bach JR, Jethani L, Gallagher MF. Active lung volume recruitment to preserve vital capacity in Duchenne muscular dystrophy. J Rehabil Med. 2017;49(1):49–53. [DOI] [PubMed] [Google Scholar]
- 27.Eiser C, Varni JW. Health-related quality of life and symptom reporting: similarities and differences between children and their parents. Eur J Pediatr. 2013;172(10):1299–1304. [DOI] [PubMed] [Google Scholar]