Abstract.
Neurophotonics was launched in 2014 coinciding with the launch of the BRAIN Initiative focused on development of technologies for advancement of neuroscience. For the last seven years, Neurophotonics’ agenda has been well aligned with this focus on neurotechnologies featuring new optical methods and tools applicable to brain studies. While the BRAIN Initiative 2.0 is pivoting towards applications of these novel tools in the quest to understand the brain, this status report reviews an extensive and diverse toolkit of novel methods to explore brain function that have emerged from the BRAIN Initiative and related large-scale efforts for measurement and manipulation of brain structure and function. Here, we focus on neurophotonic tools mostly applicable to animal studies. A companion report, scheduled to appear later this year, will cover diffuse optical imaging methods applicable to noninvasive human studies. For each domain, we outline the current state-of-the-art of the respective technologies, identify the areas where innovation is needed, and provide an outlook for the future directions.
Keywords: optical imaging, molecular sensors, optogenetics, fluorescence, label free, blood flow, multimodal
1. Introduction
The Neuron Doctrine formulated by Ramón y Cajal at the turn of the 19th century is an embodiment of neuroscience discovery empowered by optics. Cajal used Camillo Golgi’s “black reaction” to sparsely impregnate neurons with light-absorbing silver nitrate in histological brain sections. Inspecting these sections with a light microscope, Cajal concluded that information was flowing in one specific direction—from dendrites to axons—within a network of discrete nerve cells connected by synapses. This conceptual advance, enabled through utilization of a light-absorbing probe in combination with widefield microscopy, laid the foundation for modern neuroscience. Fast-forward to the present day, and optical tools for measurement and manipulation of neuronal activity are so ubiquitous and versatile that “neurophotonics” has become a common word in the scientific vocabulary: we talk about neurophotonic technologies and research centers, we have the Neurophotonics journal, and recognize neurophotonics as its own field of research.
While optical methods have occupied center stage in neuroscience from the times of Cajal and Golgi, the last decade has seen a rapid advance of neurophotonic technologies, in large part thanks to the BRAIN Initiative1,2 as well as other large-scale neuroscience projects in the US and around the world.3 At the dawn of the BRAIN Initiative, the research community was deeply engaged in discussion about what kinds of tools were needed to accelerate neuroscience discovery. Questions were asked about the technological goals to maximize the progress in understanding the brain: Should we measure each spike in every neuron? Should we prioritize one model species over another? Should we pick one specific neuronal circuit and summon all forces to understand it as completely as we can? After intense debates in the media, townhall meetings and deliberations at the National Institutes of Health and beyond, the final answer was: We need it all! We need to understand the brain across scales, from its molecular composition to large-scale architecture, from single-cell gene expression to whole-brain activity patterns, and for each aspect of the brain’s organization, we need to use those model species that make the most sense. Thus, we also need a variety of tools with which to tackle these different biological parameters and phenomena. Halfway through the BRAIN Initiative, we can safely say that this inclusive scope is paying off. We now have a large array of diverse experimental and computational tools to study the brain across species, scales, levels of description, in animals and humans. Notably, the lion’s share of these technologies falls under the general umbrella of neurophotonics.
Here, we review the current state-of-the-art tools that are, in general, applicable to animal models and usually considered under the umbrella of microscopy. We start with an overview of high-resolution structural imaging tools that have emerged and/or matured within the last decade. Then, we highlight several newly developed optical sensors and modulators of brain activity followed by discussion of a suite of one-photon (1P) and multiphoton fluorescence microscopy methods aimed at large-scale imaging of brain activity with high temporal resolution. We include a number of technologies for imaging cerebral blood flow and metabolism and feature several imaging approaches that integrate optical tools with other measurement modalities providing complementary information and/or helping to overcome fundamental limitations of purely optical methods. Finally, we highlight the importance of computational tools that complement optical technologies extending their capabilities.
Given the vast and rapidly expanding landscape of neurophotonic tools, the current scope is not exhaustive but rather biased on the collective scientific interest of this group of authors. Most often, these tools entail some level of invasiveness and in general are not applicable to live brain imaging in humans. A companion paper is focused on noninvasive (diffuse) optical imaging technologies targeted for humans.
2. Imaging of Microscopic Brain Structure
A singular advance that underlies the modern high-resolution, high-throughput imaging of labeled brain structures in animal species is the development of a diverse palette of bright, photostable fluorescent proteins.4 These proteins are genetically encoded, which allows targeting of fluorescent labeling to specific cells, subcellular compartments, and biochemical events (e.g., gene transcription). In parallel, advancement of new biochemical methods of tissue index matching, building upon the original CLARITY protocol,5 have enabled volumetric acquisition of samples as thick as the whole mouse brain. On the imaging side, light-sheet microscopy6 has emerged as a method of choice for high-throughput acquisition of cleared brain samples.7 Beyond the diffraction-limited resolution of light microscopy, a suite of super-resolution technologies has leveraged the principle of manipulating the ‘on’ and ‘off’ state of the fluorescent labels.8,9 Expansion microscopy10 provides an alternative solution for overcoming the diffraction limit by physically expanding the sample. Expansion microscopy can be used to view small structures, such as synaptic vesicles, with no need for complex super-resolution instrumentation, but, in contrast to the “classical” super-resolution methods, is not compatible with live imaging.
Complementary to fluorescent labeling, label-free imaging relies on intrinsic properties of brain tissue.11–16 High-resolution label-free structural imaging is of particular relevance for human brain tissue, where expression of fluorescent proteins does not apply for neuroethics reasons. Label-free imaging techniques leverage optical phenomena of interaction of light with live and fixed brain tissue including autofluorescence, reflectance, birefringence (polarization and direction dependence of refractive index) that yields retardance and changes in light polarization and scattering.
2.1. Fluorescent Proteins
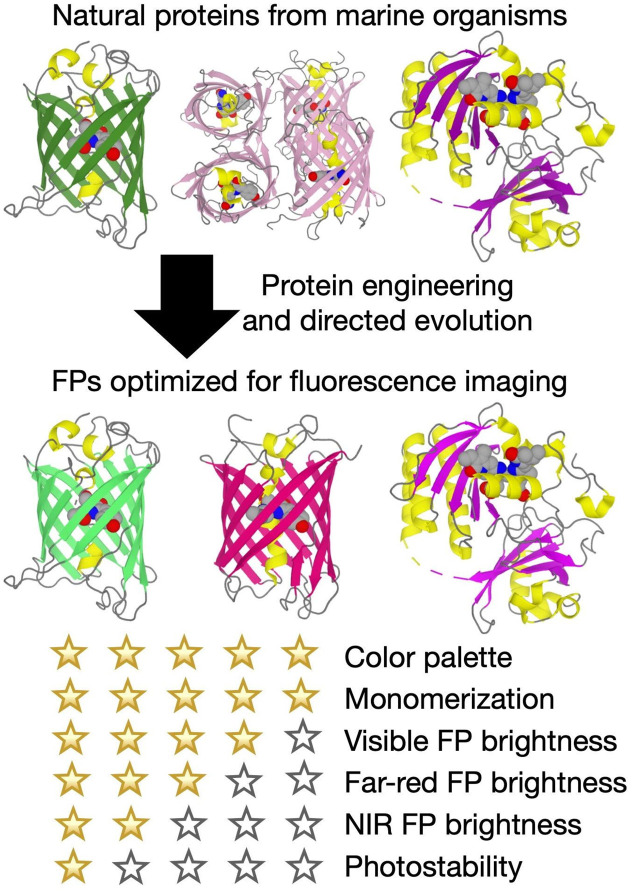
Naturally occurring fluorescent proteins (FPs) from various marine organisms, including the archetypical Aequorea victoria green FP (avGFP),17 provide the foundation for all genetically encoded molecular probes for fluorescence imaging of brain structure and function used today. The gene for an FP is introduced into the tissues of a model organism and then transcribed and translated by the normal cellular machinery. Expression in specific neuronal cell types or brain regions can be achieved with appropriate combinations of gene enhancers and promoters, adeno-associated virus (AAV) serotypes and injection site, and recombinase-dependent expression strategies. Having inspired countless genetic manipulation strategies, numerous advances in microscopy, and the development of powerful functional imaging probes, FPs are the substrate upon which a vast ecosystem of neurophotonic technologies now flourish.
Essentially all FPs in use today have been engineered using their wild-type progenitors as a starting point (Fig. 1). Nature and natural evolution have played the biggest role in providing researchers with a diversity of FP-based tools, and undoubtedly there are many secrets yet to be revealed.18 However, for adapting natural FPs to the unnatural demands of neurophotonic applications, laboratory engineering and directed evolution play essential roles. Such approaches have been overwhelmingly successful at providing a color palette of bright FPs for multi-color, multi-parameter imaging,19 and monomeric FPs for use as minimally-perturbing fusion protein labels.20 However, these efforts have been only partially successful at providing high-performance far-red FPs,21 brightly fluorescent near-infrared FPs,22 or substantial improvements in photostability.
Fig. 1.
Fluorescent protein engineering.
Looking to the future, new protein engineering methods, or more intensive application of established methods, will be critical for overcoming these and other limitations. It is impossible to predict exactly which approaches are most likely to provide the next generation of FPs but, based on precedent, progress is certain to be achieved in a piecemeal fashion. For example, complementary approaches of particular promise include innovative library screening schemes23 and rational computational design.24 For some applications, alternative approaches such as semi-synthetic fluorogenic proteins,25,26 and bright luciferase plus luciferin pairs,27,28 may supplant FPs. In one form or another, FPs, or their functional equivalents, will remain at the forefront of neurophotonic research for the foreseeable future.
2.2. Tissue Clearing for High-Throughput, High-Resolution Imaging of Macrobiomolecules
Classic histological protocols for labeling of fixed tissue sections often include a step of dehydration followed by “clearance.” In the process of dehydration, the water is removed and replaced by the alcohol (ethanol). In the process of clearance, a clearing agent (often methyl salicylate) is used to displace the ethanol while also removing fat molecules rendering the tissue optically transparent. In neuroscience, these protocols have been widely used in combination with bright field and epi-fluorescence microscopy for morphological analyses, i.e., for reconstruction of dendritic and axonal arbors across different neuronal cell types.29 While useful, these methods were limited to relatively thin brain sections to allow the chemicals to penetrate throughout. In addition, uneven shrinkage of serial sections prevented stitching sections into volumes. More recently, transition to organic solvents has been proposed as a way to increase sample thickness enabling volumetric reconstruction.30 However, the problem of uneven shrinkage remained. In addition, these solvents produced bleaching of endogenous fluorescence, and their toxic and corrosive nature required specialized equipment and handling. As an alternative, aqueous solutions with high refractive index were employed preventing bleaching but not achieving sufficient transparency for thick samples (reviewed in Ref. 31).
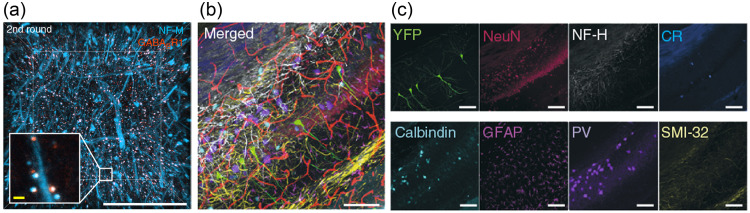
In 2013, development of CLARITY,5 a method of making large volumes of tissue optically transparent and macromolecule-permeable using a hydrogel built from within, revolutionized brain histology. This method has broken ground for the development of a growing family of tissue clearing tools with varying hydrogel composition, including those that can repeatedly expand and shrink brain tissue32 while preserving target macrobiomolecules.33 In combination with light-sheet microscopy (Sec. 4.3), these methods allow high-throughput analysis of large-scale FP expression and also support multiplexed immunostaining with many rounds of fluorescent antibody labeling and stripping34 (Fig. 2).
Fig. 2.
Visualization of biomolecules in cleared tissue. (a) Imaging of neurofilament medium unit (NF-M) and GABAB receptor subunit-1 antibodies in cleared, expanded tissue. Scale bars, . (b)–(c) Overlay of multi-round immunostained images. Scale bar, . Panel (a) is adapted from Ref. 32; panels (b) and (c) are adapted from Ref. 33.
2.3. Super-Resolution Microscopy
Super-resolution microscopy refers to a new class of fluorescence imaging techniques that have shattered the diffraction barrier of light microscopy, offering spatial resolutions down to the single-digit nanometer range close to the ultimate limit, i.e. the size of the fluorescent molecules. Since their invention some 20 years ago (recognized by the Nobel Prize in 2014), a diversifying family of super-resolution methods has been developed and deployed in neuroscience research, including STED, RESOLFT, PALM, STORM, u-PAINT, and SIM, encompassing laser-scanning and widefield imaging modalities35 (Fig. 3). While these techniques are quite distinct in design and use (concerning hardware, labeling, image analysis), they are all based on the principle of manipulating the ‘on’ and ‘off’ state of the fluorescent labels, whose spatial distribution can be read out in a diffraction-unlimited way.
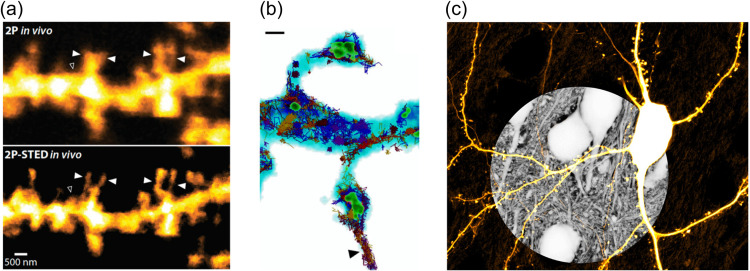
Fig. 3.
Super-resolution imaging of fine neuronal structures. (a) Comparison of 2P versus 2P-STED image of dendritic spines imaged in the mouse hippocampus in vivo (adapted from Ref. 36). (b) Correlative STED and SMLM image of dendritic spine morphology and dynamic spatial arrangement of synaptic proteins (blue, neuronal morphology; green, PSD-95 scaffold protein; colored tracks, GluA1 receptor subunit), scale bar 500 nm (adapted from Ref. 37). (c) Super-resolution shadow imaging (SUSHI) of brain tissue, revealing anatomical context of a YFP-labeled neuron (adapted from Ref. 38).
In STED microscopy,39 dye molecules are switched off by stimulated emission in a donut-shaped region around the excitation spot that scans the sample, shrinking its size. In PALM, STORM and u-PAINT, collectively referred to as SMLM (single-molecule localization microscopy), the fluorophores are imaged onto a camera, but not all at the same time. They are turned on so sparsely that each of them can be precisely localized. By acquiring thousands of images of different sets of activated fluorophores, a super-resolved image is constructed.40
These ground-breaking methods have enabled unprecedented optical access to subcellular organelles and protein arrangements in living brain cells and tissue preparations, unlocking a wealth of new and exciting biological information. For instance, they have revealed the ring-like organization of the cytoskeleton in axons,41 the columnar arrangement of pre- and postsynaptic signaling proteins,42 the structural plasticity of dendritic spines in the hippocampus,36 as well as basic physical properties of the extracellular space of brain tissue.43
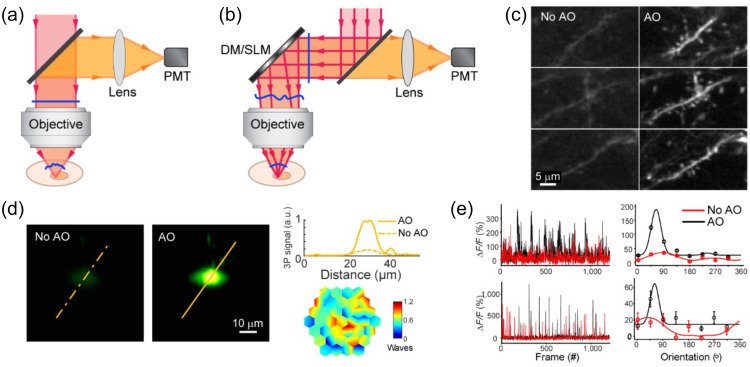
In the future, this technology will still develop rapidly in many key respects, allowing for more sophisticated experiments on more complex and biologically relevant samples, while also becoming less cumbersome and expensive. An important task is to make in vivo super-resolution imaging more of a reality, which faces special challenges in terms of depth penetration and brain motion. We can expect that recent technological advances, including those in adaptive optics and computational tools, will substantially potentiate the power of super-resolution microscopy and the biological insights that it will unearth.
2.4. Expansion Microscopy
The fundamental optical diffraction limit precludes the use of conventional light microscopy for imaging of nanoscopic structures such as the post-synaptic density. Expansion Microscopy (ExM)44 overcomes this limitation by physically magnifying tissues while reserving or highlighting biomolecules such as proteins, nucleic acids, lipids, etc.45,46
The general workflow of ExM involves the following steps: (1) infusion and synthesis of a water-swellable hydrogel throughout the specimen; (2) chemically linking biomolecules of interest to the hydrogel; (3) homogenization of the specimen enzymatically or via heat denaturation, and (4) physical expansion of the specimen in pure water.47 The workflow is designed to facilitate isotropic expansion.10,44,48–51 Most ExM methods expand the specimen by up to in the linear dimension. Using a conventional light microscope, such expansion provides image resolution for an objective lens with 270 nm diffraction limit (). If protocols for expansion52 or more53 are used, the effective imaging resolution can be further improved to .
This technology has been used to (1) study synapses in a number of model organisms including mouse brains44,51 (Fig. 4), larval and adult Drosophila brains,54,55 C. elegans,56 planarian glia,57 and larval zebrafish, revealing heterogeneity in synapse structure58 and age-associated changes in active zones;54 (2) trace neurons in large volume;32,59 and (3) interrogate neurological diseases, such as Parkinson’s disease,60 schizophrenia,61 and dementia,62 discovering disease-related subtle structural changes61,63 and protein mislocalization.62
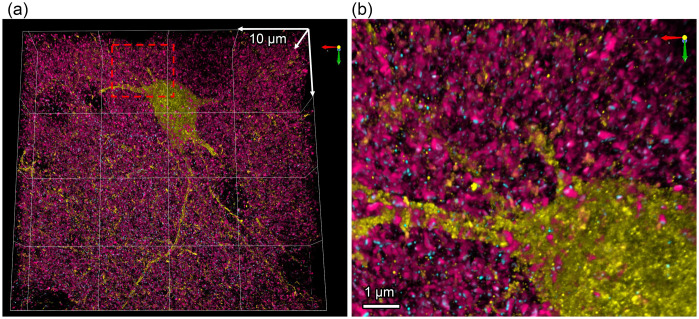
Fig. 4.
Expansion microscopy imaging of synaptic proteins. (a) Volumetric image of an inhibitory neuron genetically labelled with EYFP in a expanded mouse brain section stained with antibodies against EYFP (yellow), synaptophysin (magenta) and PSD95 (cyan). (b) Zoom-in view from (a), as indicated by the dashed red box.
In the future, we expect ExM will further advance (1) labeling strategies to overcome the bottleneck in the signal strength that is diluted by expansion itself; (2) new gel chemistry that lead to larger expansion and better resolution; (3) multiplexing strategies to address the limitation of the 4–5 colors due to spectral overlap of fluorophores; (4) development of optical objectives and microscopes and the associated imaging software specifically for imaging expanded brains or other tissues. In the next decade, we envision researchers will be able to routinely acquire large volume and highly multiplexed nanoscale images of expanded neuronal tissue to quantify changes in synaptic components and other nanoscale structure in the brain, potentially providing new insights into the molecular mechanisms of neuronal function and disease.
2.5. Label-Free Structural Imaging Based on Interaction of Light with Tissue
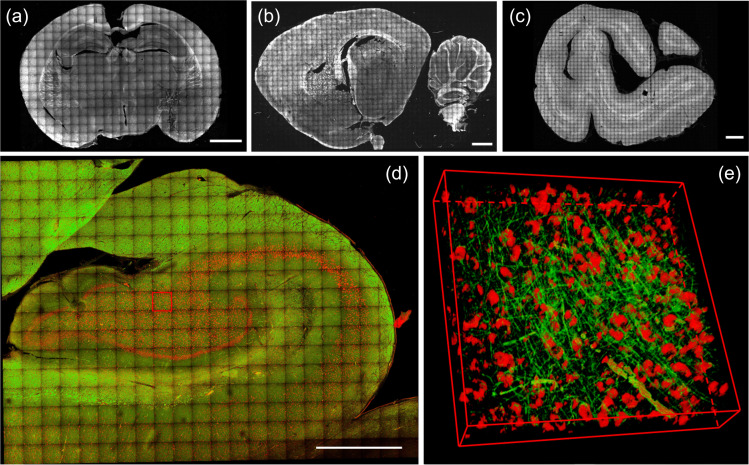
While fluorescent proteins offer selective genetic targeting and multiplexing, these approaches are limited to animal studies where genetic manipulations and editing can be performed. Label-free imaging relies on intrinsic properties of brain tissue and applies to human cerebral tissue, freshly excised or fixed. Indeed, the biological structure autofluorescence produced by red blood cells retained inside the vessels, and the lipofuscin pigments present inside the neuronal bodies, can be detected by fluorescence microscopy techniques.64 In addition, it is possible to modify the sample with specific preparations in order to increase the autofluorescence signals of particular structures. Among them, a recent protocol called MAGIC (Myelin Autofluorescence imaging by Glycerol Induced Contrast enhancement)11 enables to perform label-free fluorescence imaging of myelinated fibers in various mammalian brains, including humans, with a glycerol-based tissue treatment (Fig. 5) allowing three-dimensional (3D) connectomics analysis of the brain.
Fig. 5.
Reconstruction of brain sections treated with MAGIC: (a) mouse, (b) rat, (c) vervet monkey, (d) and human. Scale bar = 1 mm. (e) 3D rendering () of the stack indicated by the red box in d; green and red channels show the myelinated fibers enhanced by MAGIC and the autofluorescence of the cell bodies produced by lipofuscin pigments. Adapted from Costantini et al.11
Furthermore, optical methods based on different physical phenomena are extensively used to investigate both in vivo and ex vivo tissues. Raman scattering and its derivation as Coherent anti-Stokes Raman scattering (CARS)65 permits to determine the molecular composition of every tissue. Second-harmonic generation (SHG) allows to detect well organized structures like collagen, myosin, or polysaccharides66,67 while third-harmonic generation (THG) distinguishes the interface between different compartments like skin, neuron’s myelin, and adipocyte.68 Another label free technique that enables the high-resolution analysis of myelinated fibers is 3D-PLI (Polarized Light Imaging),69 based on the detection of the birefringence of the myelin sheaths surrounding axons. In particular, this technique allows the reconstruction of the 3D organization of the whole brain fiber’s architecture in thin slices.70 Finally, reflectance microscopy12,14 can be used to measure myelin contents and capillary blood flow in live preparations.
All these methodologies are usually linked together to obtain a multimodal mapping of the different tissue components.65,71 We believe that in future some of the label-free techniques will be combined with specific tissue preparation and labeling, such as clearing methods,31 as already done with CARS on mouse brain,72 coupling the morpho-chemical label free analysis with labeled 3D volumetric imaging on large samples.73,74 The combination of label-free methods with specific labeling will allow a more comprehensive analysis of tissue composition offering the possibility of molecular phenotyping at the cellular and subcellular level.
2.6. Optical Coherence Tomography
Optical coherence tomography (OCT)75 is another label-free imaging modality that uses intrinsic contrast to generate cross-sectional or volumetric images of tissues at micrometer resolution. OCT differs from the above-mentioned label-free tools in that it employs low-coherence interferometry to provide depth-resolved images, for which light backscattered and reflected from a sample is mixed with a reference light. Not only the intensity, but also the phase and polarization of the detected light carry physical information. Various OCT-based techniques have been developed for different fields, such as ophthalmology, most notably, but also in cardiology, and dermatology, to visualize tissue structure and function.
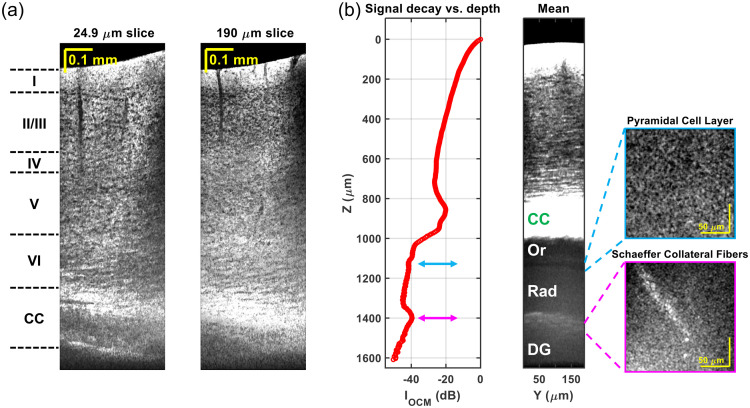
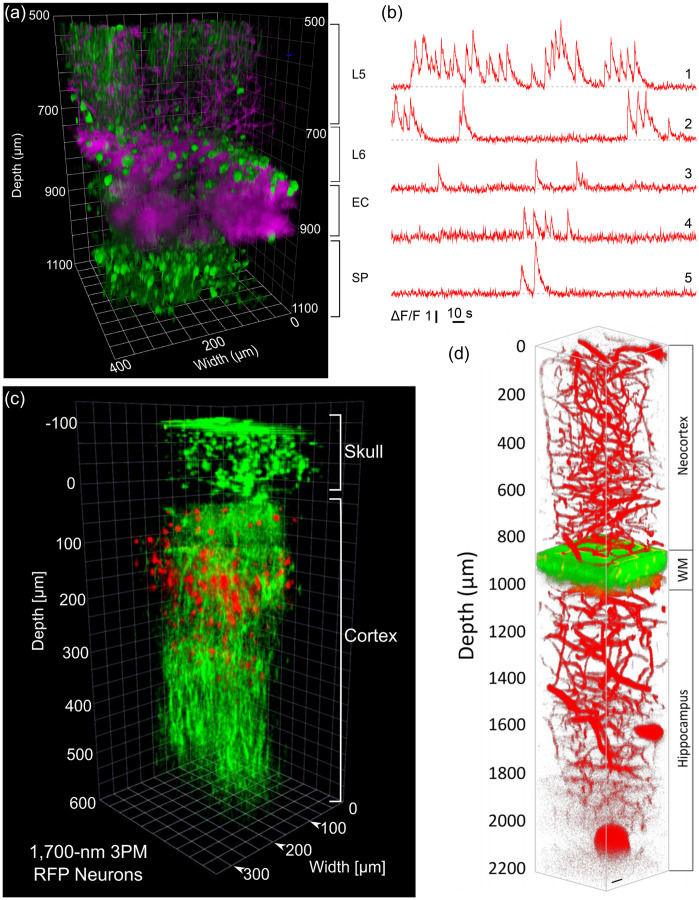
OCT applications in the brain have been emerging in recent years.76 Like the retina, many regions of the brain possess an internal organization which gives rise to natural differences in optical properties. The simplest example of this principle is the fact that white matter has a higher scattering coefficient than gray matter. On a finer scale, cellular composition, myelination, and potentially, axonal/dendritic content can also modulate scattering coefficients of individual layers in the cortical column.77 Within gray matter itself, high resolution Optical Coherence Microscopy (OCM) imaging has revealed individual myelinated axons78 and neuronal cell bodies,77 and potentially cell nuclei.79 Morphological changes can accompany cell death80,81 and therefore inform about neuronal viability, while Aβ plaques are distinguished as being highly backscattering.82 Finally, OCT is unique in enabling optical microscopic imaging more than 1 mm deep in vivo in scattering tissue, through minimally invasive preparations (Fig. 6), and can capture deep pathology that is missed by more superficial imaging techniques.83 Functional and hemodynamic extensions of OCT are discussed in Sec. 6.1.
Fig. 6.
In vivo 1700-nm optical coherence microscopy (OCM) of the mouse brain through a thinned skull preparation. (a) Minimum intensity projection sagittal images, or “slices,” with different projection thicknesses in the coronal direction, show cortical cytoarchitecture and the corpus callosum (CC), without physical tissue slicing. (b) OCM signal decay (left panel) and averaged coronal image (middle panel) show sub-cortical layers. CC, corpus callosum; Or, stratum oriens; Rad, stratum radiatum; DG, dentate gyrus. Adapted from Zhu et al.83
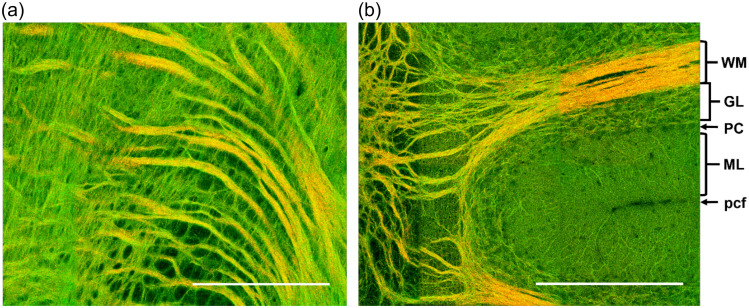
Interestingly, much of the intrinsic contrast of in vivo neuronal tissue is also apparent ex vivo in fresh84 and to some extent, even fixed85,86 tissues. This has enabled large-scale reconstructions of animal and human brains by serial optical coherence scanning (SOCS) that combines an OCT and a tissue slicer.15 The SOCS is a new tool for fundamental research into the anatomical and connectional architecture of the brain without using exogenous contrast agents or expression of foreign genes. It relies on conventional or polarization-sensitive OCT of a volume within the field of view, translating the sample laterally to image neighboring volumes, removing a layer of tissue using the slicer to expose deeper regions for imaging, and repeating these steps until the entire block is imaged. Consequently, the SOCS provides large datasets for brain imaging and mapping, and the data size is proportional to the sample volume and inversely proportional to the volumetric spatial resolution. The latter can be enhanced by utilizing OCM. The technology has been used to distinguish the gray matter and white matter,87 compared with Nissl staining86 and diffusion MRI,88,89 and applied on the rat brain,15 mouse brain,13,90–92 and human medulla oblongata.88 Figure 7 shows the power and promise of the ex vivo SOCS method, as it shows axon fibers of mouse brainstem and cerebellum and delineates the cerebellar layers without histological processing.
Fig. 7.
Ex vivo serial optical coherence scanning (SOCS) of the mouse brain. (a)–(b) Composite images with reflectivity (green) and retardance (red) contrasts for selected regions in mouse brainstem (a) and cerebellum (b). WM, white matter; GL, granular layer; ML, molecular layer; pcf, preculminate fissure. PC indicates a layer of Purkinje cell bodies whose dendrites reside in ML. Scale bar: (length of a square tile).
While ex vivo OCT provides intrinsic contrast, it is most powerful when complemented by other, more specific histological or labelled imaging techniques. In the future, we can expect the use of SOCS to complement other serial imaging modalities in creating unique and undistorted datasets from rodent, nonhuman primate, and human brains. Development of image processing and analysis tools will allow creation of new brain atlases and better understanding of the connectome. These could facilitate studying atrophies in brain diseases93 and finding new targets for neuromodulation treatments. The necessity of serial imaging and analysis of big data would require implementation of automated protocols to realize these highly significant goals.
3. Molecular Sensors and Actuators for Imaging and Manipulation of Brain Activity
Recent advances in molecular engineering have led to several families of high-quality optical sensors. We use the terms “sensor,” “indicator,” and “probe,” interchangeably, to broadly describe molecules that provide a measurable signal in response to changes in brain activity. Following the traditional definition of the term in the analytical sciences, we use “biosensor” to refer to only those sensors in which a protein component is detecting the biological change of interest. Combined with advanced microscopy, calcium ion () imaging using GCaMPs94 is now routinely employed to enable large-scale, single- or multiphoton imaging for readout of neuronal circuit activity in awake, behaving animals.95,96 In parallel, voltage sensors have been significantly improved in recent years in terms of intrinsic properties such as membrane localization, brightness, sensitivity and kinetics. These voltage sensors can be divided into three general categories: synthetic sensors,97 hybrid biosensors that use a combination of synthetic dyes and genetically encoded proteins,98 and fully genetically encoded biosensors.99–101 Synthetic voltage sensors have a long history in neuroscience including the latest red-shifted photoinduced electron transfer (PeT) highlighted below.102 Hybrid voltage biosensors combine molecular specificity of genetically encoded proteins with unsurpassed photophysical properties of synthetic fluorophores. Genetically encoded voltage biosensors are based on either opsins or voltage sensing domains; the most recent of them approach the performance of their synthetic counterparts.103 The choice of voltage sensor is driven by applications, and no single solution fits all.104
Beyond and voltage, more recent protein engineering efforts have now extended the concept of single-FP based biosensors to the design of genetically encoded biosensors for neurotransmitters and neuromodulators, including glutamate, GABA, dopamine, norepinephrine, serotonin and acetylcholine, based on either G-protein coupled receptors (GPCR) or bacterial periplasmic binding proteins (PBP).105 These biosensors enable measurements of neurotransmitter release with high spatiotemporal resolution and molecular specificity across the full course of behavioral paradigms.106–117
With growing appreciation for the concepts of the neurovascular unit118 and tripartite synapse,119 there has been a concerted effort for development of fluorescent biosensors for cellular metabolism,120,121 and targeting these and other biosensors to not only neurons but also non-neuronal cells including astrocytes (as well as immune and vascular mural cells).122
The sensors mentioned above, whether genetically encoded, hybrid or synthetic, employ fluorescence to report activity. Another notable form of luminescence is phosphorescence that has a longer lifetime and thus occurs on a slower time scale. Phosphorescence has been traditionally employed in sensing , a physiological parameter of key importance in the brain and elsewhere.123 Development of new 2P-excitable, red-shifted probes has recently enabled deep intravascular and tissue imaging of the partial pressure of ().124,125
Fluorescence and phosphorescence require that photons are delivered to the chromophore/phosphor molecule to induce emission. In bioluminescence, in contrast, emission of light occurs in the process of a chemical reaction where an enzyme oxidizes a substrate.126 Bioluminescence has been recently harnessed to generate biosensors though functional reconstitution in the presence of target molecules.127 It also has been explored for actuation by using emitted light to stimulate light-sensing proteins.128 Although the concept of bioluminescent actuators is very new, this work already has yielded promising results.129 Bioluminescent actuation joins optogenetics, a widely used tool for stimulation and inhibition of neuronal activity that has revolutionized neuroscience since its invention in 2005.130,131
3.1. Calcium Sensors
measurements are central in neuroscience due to the importance of in cellular excitability and intracellular signaling pathways.132 A correlation of signals with electrophysiological measurements of membrane potentials across neuronal compartments (soma, proximal dendrites, distal dendrites) has been demonstrated using simultaneous intracellular electrophysiological recordings.133,134 This is due to abundant expression of numerous types of voltage-gated channels.135–137
Imaging of neuronal activity using sensors was originally enabled by Roger Y. Tsien and colleagues who developed the fura-2 synthetic indicator dye and the means to deliver this dye to tissues in the form of an acetoxymethyl (AM) ester.138,139 Following these initial breakthroughs, examples of imaging to record neuronal activity soon started to appear.140,141 Ever since, the development of improved sensors has been a steadily evolving area in neuroscience due to arrival of new indicators with improved signal to noise ratio (SNR) properties, low toxicity, and optimized delivery strategies. Of particular note was the development of an exceptionally good indicator Oregon Green BAPTA-1 (OGB-1), and a clever scheme of delivering its AM ester form into thick tissues.142 Thanks to large 2P absorption cross-section of OGB-1 and other synthetic sensors, in vivo 2P imaging has been widely adopted within the neuroscience community and used for studying of neuronal circuits in healthy cerebral143–147 and cerebellar cortex,148 spinal cord,149 olfactory bulb,150,151 in glial cells,152–154 and in diseased brain.155,156
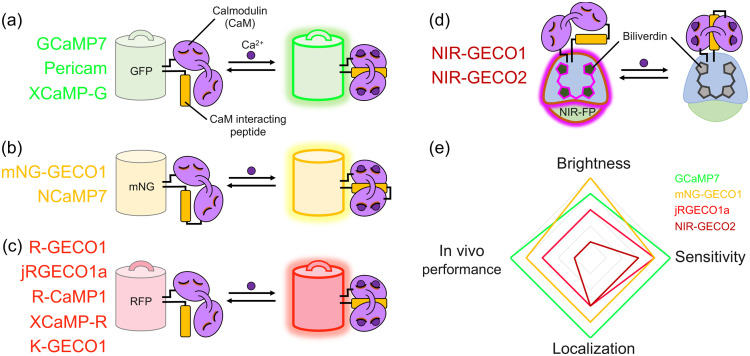
Relative to synthetic indicator dyes, genetically encoded indicators (GECIs; a.k.a. biosensors) offer a number of advantages including genetic targeting to specific cell types, virus-mediated delivery, and the possibility of creating transgenic organisms that stably express the GECI. Just as with the field of synthetic indicator dyes, the field of GECIs was enabled by the work of Roger Y. Tsien and colleagues. Specifically, they reported the Förster resonance energy transfer (FRET)-based cameleon biosensor in 1997157 and the single GFP-based camgaroo biosensor in 1999.158 These were soon followed by the improved single FP-based pericam159 and GCaMP160 biosensors which were composed of a circularly permuted GFP fused to calmodulin (CaM) and a CaM-interacting peptide.161 The first GECI that was widely used to image neuronal activity in defined cell populations was GCaMP3,162 and seventh generation GCaMP variants have been reported.163 As the toolbox of genetically encoded biosensors continues to grow (Fig. 8), it is desirable to engineer biosensors with non-overlapping spectra to simultaneously monitor multiple circuit elements. Towards this goal, there are now a number of red FP-based167–169 and near-infrared FP-based170 biosensors available, though they do not offer the same outstanding levels of performance achievable with the latest generation of the highly optimized GCaMPs. However, these red-shifted probes offer the advantage of decreased scattering and absorption effects in tissue, thus allowing for deeper and more efficient imaging in vivo.
Fig. 8.
Overview of available intensiometric single FP-based GECIs. (a) Representative GFP-based GECIs.159,163,164 (b) Representative mNeonGreen-based GECIs.165,166 (c) Representative RFP-based GECIs.164,167–169 (d) Representative near infrared FP-based GECIs.170,171 (e) Qualitative assessment of various GECIs in terms of brightness, sensitivity, localization and in vivo performance.
GECIs remain the workhorse of circuits-to-behavior neuroscience studies. The development and application of brighter and colorful biosensors along with other molecular tools will push the boundaries of exploration in neuroscience beyond the current limits.
3.2. Synthetic Voltage Sensors
Traditionally, neuronal electrical properties have been evaluated using intracellular electrophysiological recordings that are highly accurate but also terminal, invasive, low-throughput and labor intensive, and particularly difficult to perform in the brains of behaving mammals.
Synthetic sensors (dyes) provided an early impetus for probing transmembrane neuronal potential with optical imaging as an alternative to electrophysiological recordings. Nearly 50 years ago, experiments in squid axons revealed changes to the optical properties of dyes in response to membrane potential changes.172 Since that time, efforts to design fluorescent dyes that respond to biological membrane potential broadly fall into two different camps: slow-response dyes that display voltage-dependent partitioning within the membrane, and fast-response dyes whose optical properties are modulated by changing electric fields.173 Voltage-sensitive dyes are especially useful in model systems that lack ready tools for genetic manipulation and have been used extensively for mapping brain function in mammals and non-human primates.174
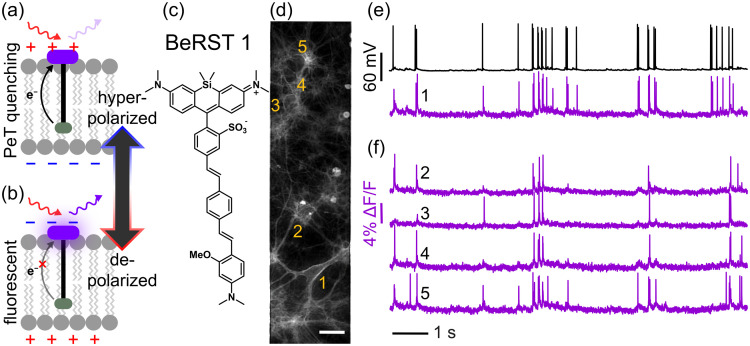
More recently, voltage-sensitive fluorophores based on voltage-sensitive photoinduced electron transfer (PeT)175,176 have emerged as another avenue for synthetic voltage sensors177 (Fig. 9). Changes in membrane potential alter the rate of PeT within the donor-acceptor framework of the dyes. At hyperpolarized potentials, PeT from an electron-rich aniline donor is accelerated by the hyperpolarized potential, preventing emission of a photon from the fluorophore excited state, and quenching fluorescence [Fig. 9(a)]. At depolarized potentials, the inverted membrane potential inhibits PeT and the dye brightens [Fig. 9(b)].179 The newest PeT-based indicators compare favorably to genetically encoded indicators, in terms of speed, sensitivity, and brightness.180 Additionally, the positive-going, turn-on response to action potentials, coupled with compatibility with multiphoton excitation,102,181,182 make PeT-based synthetic voltage indicators an attractive method for imaging rapid membrane potential changes in intact brains. However, an outstanding problem is delivery of fluorescent dyes to specific cells.
Fig. 9.
Voltage imaging with PeT sensors. (a)–(b) Mechanism of voltage sensing in PeT-based voltage-sensitive fluorophores. (c) Structure of a prototypical voltage-sensitive fluorophore, Berkeley Red Sensor of Transmembrane potential, BeRST 1. (d) Fluorescence image of cultured hippocampal neurons loaded with BeRST 1. Scale bar is . (e)–(f) Example traces from simultaneous electrophysiological (black) and optical recording (magenta) of spontaneous voltage fluctuations from neuron 1 (e) and spontaneous activity from neurons 2 to 5 (f); the corresponding neurons are labeled in (d). Adapted from Walker et al.178 and Liu and Miller,179 © 2020 American Chemical Society.
In the future, a number of innovations could improve the utility of chemically-synthesized voltage indicators: improved or universal methods of delivery into intact brains; enhanced photostability for long-term imaging; larger multiphoton excitation cross-sections for use with 2P and 3P imaging; chemical-only cell targeting, without the requirement for genetic encoding, for example, by targeting native receptors;183,184 longer wavelength indicators for in vivo imaging;185,186 and, possibly, indicators that harness modalities other than light for noninvasive imaging.
3.3. Hybrid Voltage Sensors
Hybrid voltage sensors address the challenge of targeting fluorescent dyes to specific cell types. Multiple hybrid strategies have been explored. One attempt was using a “pro-drug” strategy in which a genetically encoded enzyme on the cell surface uncages a modified fluorescent voltage indicator.187,188 Fluorescein-based voltage-sensitive fluorophores can be adapted to this strategy,189,190 but the method has yet to be applied beyond cultured neurons. Alternatively, voltage-sensitive fluorophores can be tethered to neuronal surfaces with genetically-encoded, self-labeling enzymes to enable fast voltage imaging from defined cells and sub-cellular structures as was demonstrated in cultured hippocampal neurons191–193 or brain slice.193 Voltage indicators based on rhodamines can also be targeted in a similar fashion, allowing single-trial detection of action potentials in mouse brain slices.193
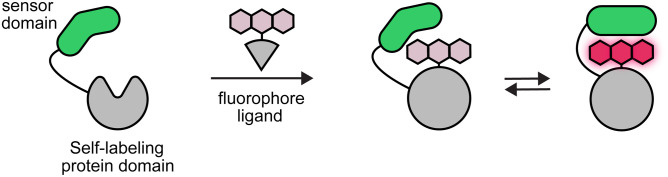
Recent efforts have introduced another solution to the problem of cell-type-specific targeting through engineering “chemigenetic” probes that couple simple synthetic fluorophores acting as the reporter molecule with a sensor protein domain partner. In chemigenetic probes, a sensor protein domain is appended to a self-labeling protein domain98,194,195 such as HaloTag196,197 that irreversibly binds a synthetic fluorophore ligand (Fig. 10). This allows genetic encoding of the protein portion and uses simple and cell-permeable small-molecules, such as Janelia Fluor (JF) dyes,25,198 as reporter fluorophores.
Fig. 10.
Schematic of chemigenetic probes. A biosensor domain is attached to a self-labeling domain that irreversibly binds a fluorophore ligand. A change in the biosensor domain alters the bound fluorophore properties allowing functional recording of biochemical signals.
Chemigenetic probes are engineered so that a change in the sensor protein domain alters the local environment around the dye and changes its fluorescence properties (Fig. 10). This approach yielded bright and photostable voltage and probes with highly tunable photophysical and chemical properties.98,194,195 Chemigenetic voltage probe Voltron98 increased photon yield from voltage imaging experiments to enable recording from many cells simultaneously. HaloCaMP and HASAP195 are bright far-red and voltage probes that push the useful imaging spectrum to longer wavelengths. These recent developments provide a template for the design of new fluorescent probes for different analytes and biochemical processes.
Further advances in synthesizing tunable far-red dyes, alongside innovative protein engineering for building protein scaffolds able to modulate dye fluorescence, promise to enable functional imaging deeper in tissue. Moreover, improved bioavailability of dyes will allow routine imaging in live animals. Another equally exciting avenue is to develop orthogonal self-labeling proteins like HaloTag that work well in vivo with corresponding bioavailable ligands for multicolor imaging.
3.4. Genetically Encoded Voltage Biosensors
Recent advances in the development of genetically encoded voltage indicators (GEVIs, a.k.a. voltage biosensors) have led to significant improvement in their optical properties offering recording of transmembrane voltage via fluorescence imaging without the need to deliver extrinsic (synthetic) sensors or fluorophores. In particular, a few recent GEVIs, including SomArchon, QuasAr3, ASAP3 and Ace2N have achieved sufficient sensitivity to capture individual action potentials in behaving mice.99,100,199–201 Figure 11 shows an example of voltage imaging of multiple cells simultaneously using SomArchon, which allows for the detection of individual action potentials and subthreshold membrane voltage fluctuations in behaving mice.99 Additionally, since SomArchon operates at the near-infrared wavelength, it can be used in conjunction with blue-light-activated optogenetic actuators for simultaneous voltage imaging and optogenetic control.
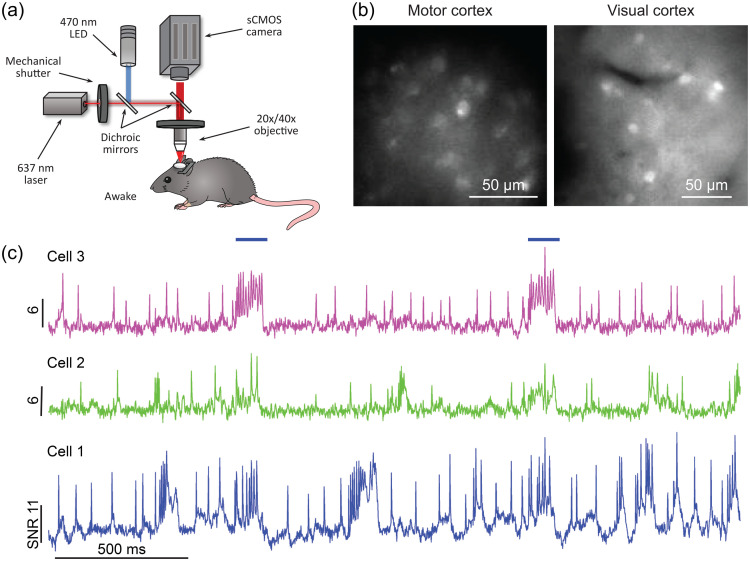
Fig. 11.
Single cell voltage imaging in awake mice using a wide-field imaging setup. (a) Experimental setup: awake mice were head-fixed under a wide-field microscope. (b) Representative SomArchon-expressing neurons visualized via EGFP fluorescence in cerebral cortex. (c) Voltage imaging time-courses from three representative neurons in hippocampus; horizontal bars denote optogenetic stimulation. Reproduced with permission from Piatkevich et al.99
Several different types of GEVIs exist (for a recent comprehensive review, see Ref. 202). SomArchon and QuasAr3 are opsin-type GEVIs that use the voltage-dependent protonation of opsins as an optical readout. Indicators like Ace2N use an opsin to sense transmembrane potential, and changes in the protonation state of the opsin change the efficiency of energy transfer to a bright fluorescent protein (FP). ASAP3 is an FP fusion with a voltage sensitive protein domain which undergo conformational transitions upon transmembrane voltage fluctuations. These conformational changes are translated to the FP and change the fluorescence intensity of the FP.203–209 GEVIs have been used to tackle intriguing scientific questions, such as how spiking activity of specific neuron types is modulated during different behavioral processes and relate to subthreshold membrane voltage fluctuations and extracellularly measured local field potential dynamics.
With continued improvement in both voltage sensors’ molecular designs and optical instrumentation in the coming years, we expect that voltage imaging will be increasingly accessible to neuroscientists for high-speed kilohertz analysis of many individual neurons during behavior. Such capability will enable time-resolved analysis of transmembrane voltage, both action potentials and subthreshold membrane fluctuations, of distinct cell types and cell compartments in behavior and pathology. Finally, combining voltage imaging with other precision analysis technologies, such as cellular imaging and cell-specific proteomic and transcriptomic analysis, neuroscientists will be able to link the voltage dynamics of specific neurons and brain circuits to their cellular and biochemical states and tissue environment.
3.5. Genetically Encoded Biosensors for Neurotransmitters
The development and refinement of genetically encoded and voltage indicators have paved the way for the development of genetically encoded biosensors for neurotransmitters, neuromodulators, and neuropeptides. Such biosensors are categorized by two scaffolds: microbial periplasmic binding protein (PBP) and G-protein coupled receptors (GPCR) [Figs. 12(a)–12(b)].
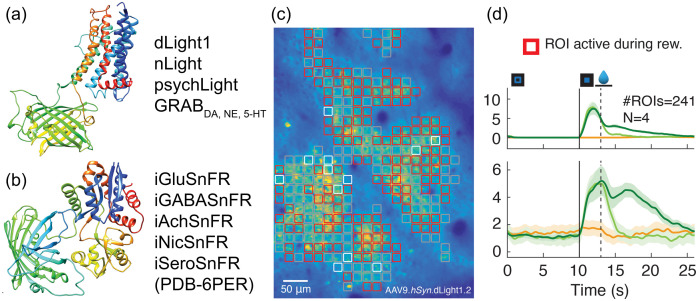
Fig. 12.
Genetically encoded sensors for neurotransmitters. (a)–(b) Design scaffolds for sensors based on cognate GPCR (a) or E. coli PBP (b). (c) 2P imaging of dopamine dynamics in the mouse cortex using dLight1.2 when mouse performs a visual-motor learning task. Heat map of dLight1.2 expression pattern in layer 2/3 of M1 cortex is overlaid with computationally defined regions of interest colored by the type of responses observed during the task. (d) Average task-induced dLight1.2 transients (bottom) and mouse running velocity (top) aligned to trial onset (0 s). For details see Ref. 210.
Microbial PBPs form a large protein superfamily that bind numerous classes of small molecules and peptides. Ligand binding in PBPs induces a large Venus flytrap-like conformational change, which is highly conserved [Fig. 12(a)]. These unique features have been used to develop a toolkit of highly sensitive sensors for other neurochemicals, including GABA (iGABASnFr), ATP (iATPSnFR), acetylcholine (iAchSnFR) and nicotine (iNicSnFr).211–214 However, there are several analytes for which bacterial PBPs do not exist. Recently, a PBP-based biosensor iSeroSnFr for serotonin has been engineered using machine-learning-guided evolution of an existing PBP-based biosensor, iAchSnFr,211 to redesign its binding pocket to report serotonin release at physiological concentrations.215
As an alternative to PBP-based biosensors, biosensors for neuromodulators have been developed by fusing GPCRs with fluorescent proteins, leveraging on ligand specificity, affinity, and binding kinetics that has evolved in the GPCRs. The first generation of GPCR-sensors were FRET-based.216–219 However, use of these biosensors in vivo has been limited due to low dynamic range and sensitivity. GPCRs have seven transmembrane (TM) alpha helices, where the largest conformational change upon activation is thought to occur for TM domains 5 to 7.220 Thus far, circularly permuted FPs (cpFPs) have been inserted in the intracellular loop 3 (IL3) domain of GPCRs, which bridges TM5 and TM6, to detect this conformational change upon ligand binding [Fig. 12(b)]. Using this versatile strategy, the Light and GRAB sensor family, consisting of dopamine, norepinephrine, and serotonin biosensors, has been developed and applied for in vivo recording of neuromodulator dynamics210,221–226 [Fig. 12(c)]. In addition, red-shifted color variants of dLight1 was engineered for multiplexed neurochemical detection (Patriarchi et al., 2020). GRAB biosensors have also been expanded to acetylcholine, adenosine and more recently endocannabinoid.224–226
The intrinsic properties of these neurotransmitter biosensors demand further iterative optimization to be broadly applied to study spatial and temporal patterns of activity in synaptically connected neuronal circuits. Computational modeling would provide theoretic guidance for future optimization. In addition, a high-throughput screening system using fluorescence-activating cell sorting (FACS) combined with image-based screening or the well-known CRISPR/Cas9 system is a pressing need to speed up the process of optimization. Extending the sensitivity, specificity, and color palette of these biosensors will continue to create rich opportunities for studies aimed at understanding the role of specific neurotransmitters in calculations performed by neuronal networks.
3.6. Molecular Probes for Cell Metabolism
Brain metabolism is highly dynamic, fluctuating with the local level of neuronal activity and with consequent energy demands. Substantial amounts of ATP are required to support the ion gradients that serve as the batteries for electrical activity, and energy is also needed to support neurotransmitter release and recycling. This energy is provided mainly by the metabolism of glucose, although other fuel molecules can also contribute. Individual brain cells also can differ substantially in their metabolic demands and responses: not only may there be differences between different classes of neurons, but various glial cells—astrocytes (the focus of Sec. 3.7) and oligodendrocytes—may play specific roles in energy metabolism.
Because the metabolic responses are rapid (a few seconds to a few minutes) and can be localized to individual cells and subcellular compartments, imaging of fluorescent biosensors can be the ideal approach to investigating how cellular brain metabolism works. Genetically encoded biosensors have been developed for glucose227–230 and ATP;231–233 for the key electron-carrying cofactors (Fig. 13) and NADPH/NADP234–237 and for the metabolic intermediates pyruvate238 and lactate.239 These have been imaged both in vitro and in vivo to elucidate metabolic responses in neurons and astrocytes,240,241 though some features remain quite controversial.
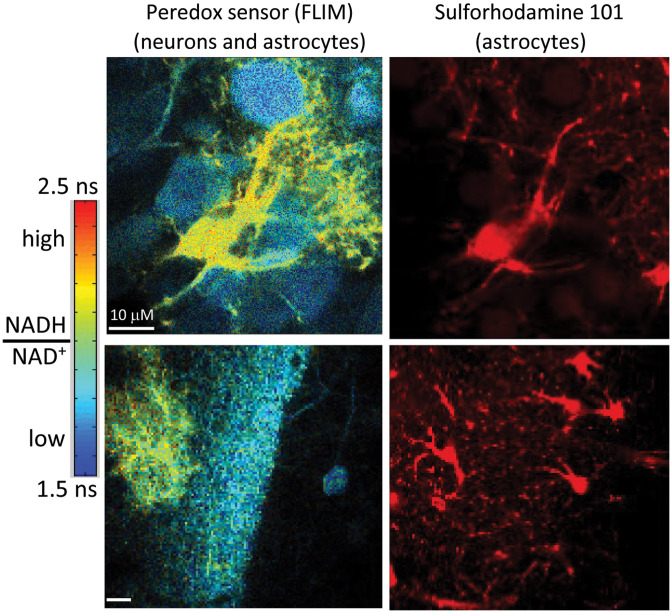
Fig. 13.
Metabolic imaging of the Peredox biosensor of redox, expressed in neurons and astrocytes of acute hippocampal slice. Left: pseudocolor images of the biosensor, which reports high ratio as increased fluorescence lifetime. Right: counterstain with sulforhodamine 101 marks astrocytes specifically and shows that they are the cells with elevated levels in the left-hand images, while neurons have lower levels. Not all cells in the slice express the genetically encoded biosensor, which was introduced with a viral vector. See further discussion of astrocytes in Sec. 3.7.
A particular challenge for using metabolic biosensors is that quantitative readouts are essential for interpretation; unlike the ubiquitous biosensors used to monitor neuronal activity, for which the temporal pattern itself is informative, metabolite levels must be compared between different cells and different conditions, even when biosensor expression levels vary. Ratiometric imaging can be used to normalize for expression levels, but ratio imaging requires more spectral bandwidth and reduces the opportunity for multiplexing several biosensors; excitation-ratiometric measurements can be challenging for scanning microscopes; and single-FP ratiometric biosensors also tend to be quite pH sensitive.120 A valuable alternative is to use fluorescence lifetime imaging: the lifetime readout is independent of biosensor concentration, and rapid lifetime imaging is increasingly practical.120,241,242 Lifetime-based biosensors are slightly harder to develop, but the ability to multiplex and to get instrument-independent quantitation makes them a valuable target for biosensor development in the coming years.
3.7. Sensors for Astrocytes
Astrocytes are one of the major non-neuronal cell types in the mammalian central nervous system (CNS), comprising up to 20% of the human brain.243 Astrocytes are found throughout the CNS in non-overlapping domains.244 These cells possess a characteristic morphology, with a central cell body radiating numerous membranous processes, which extend out into the neuropil interacting with neurons, other glia, and blood vessels.243 Astrocytes are electrically silent, which meant that for many years they were dismissed as being merely parenchymal support cells – or “brain glue.” The development of new optical imaging tools and their application to astrocytes has forced a dramatic rethink of this preconception.122 Indeed, in situ imaging experiments, performed largely in acute tissue slices from rodents, have provided compelling evidence that astrocytes are key components and regulators of neuronal circuits.
Use of small organic ion-sensitive dyes and genetically encoded biosensors has demonstrated that astrocytes contribute to local synaptic homeostasis, through active buffering of 245 and .246 Meanwhile, use of metabolic biosensors suggests that astrocytes also act as “power plants,” supplying lactate (a key energy substrate) to neurons.240 Astrocyte-specific expression of genetically encoded neurotransmitter biosensors has shown that these cells are well placed to sense local neuronal activity and neurotransmitter release;247 crucially, structural imaging of membrane-associated fluorescent proteins,248 and tagged glutamate transporters,249 suggests this activity can also trigger dynamic changes in astrocyte processes, which reciprocally modulate synaptic transmission. Such modifications may well be linked to transient increases in cytosolic , which are also evoked by local neuronal activity, and which, under certain circumstances, are thought to trigger the release of neuroactive substances (gliotransmitters) from astrocytes that act to modify neuronal activity.250
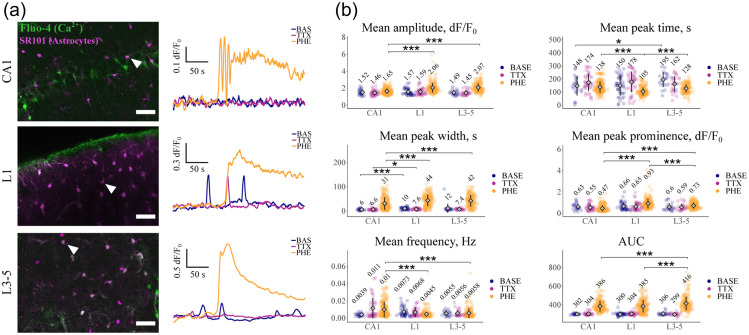
This multifaceted modulation of neurons and synapses by astrocytes suggests these cells provide an additional layer of information processing to that occurring in neurons, one which appears crucial to the modulation of animal behavior.251 However, the role of astrocytes is complicated by the recent discovery of astrocyte subtypes, which exist both between and within brain regions.252 These subtypes show differences at the molecular, anatomical and physiological levels, and are likely specialized to match and regulate local neuronal circuits253 (and see Fig. 14).
Fig. 14.
Astrocyte subpopulations in the mouse CNS show significant differences in signaling. transients in sulforhodamine101 (SR101)-labeled astrocytes were detected using Fluo-4. Measurements were made in mouse acute tissue slices containing cortical layer 1 (L1), cortical layers 3-5 (L3-5) and the hippocampal CA1 region (CA1). transients (expressed as fluorescence changes relative to baseline: ) were initially recorded under conditions of baseline activity (BASE) and after sequential application of tetrodotoxin (TTX) (to isolate astrocytes from neuronal activity) and tetrodotoxin plus the -adrenergic receptor agonist phenylephrine (PHE). (a) Representative astrocytes (arrowheads) from three brain areas and the transients recorded from these cells under the various experimental conditions. Scale bar, . (b) Analysis of various transient parameters, recorded under identical experimental conditions. Numerical values are the calculated means for each condition. AUC: area under curve. Modified from Batiuk et al.254 *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Future work will benefit greatly from genetic tools (e.g. new Cre- mouse lines or viral vector systems) that allow precise targeting of these astrocyte subtypes,255 including in gene knockout models. These will allow the contribution of astrocyte subtypes to local circuit function to be studied in great depth. Such work will also benefit from more specific methods for the acute and chronic manipulation of astrocyte activity,256–258 as well as an expanded/comprehensive palette of sensors, allowing the mechanistic basis of astrocyte function to be elucidated. Such a toolbox will, of necessity, include sensors for ions, receptor activation (particularly G protein-coupled receptors), metabolic functions and key intracellular signaling pathways (including aspects of structural plasticity and mRNA/protein trafficking).259,260
Ideally, such probes will allow high levels of multiplexing (single wavelength probes) and be optimized for in vivo use (good signal-to-noise ratio, sensitivity, dynamic range, stability in the cellular environment, resistance to photobleaching, lack of toxicity, etc.), as recent work has demonstrated that astrocyte function is strongly influenced by behavioral state.261 Probe development will go hand-in-hand with improvements in head-mounted camera technology for use in awake behaving animals (e.g., UCLA miniscope project) and silicon probe design (for the simultaneous measurement of neuronal activity).262
In summary, genetic manipulation of regional and subregional astrocyte genes, in combination with (in vivo) functional studies of astrocytes and their interactions with local neurons, will bring new insights into diversified astrocyte function and the consequences for control of local neuronal circuits and subsequent animal behavior. Such information may well provide novel insights into CNS diseases. It is likely studies of other CNS cell types, such as oligodendrocytes, microglia and pericytes will benefit from adopting similar strategies.
3.8. Probes for Oxygen Measurements by Phosphorescence Quenching
Tissue oxygen levels can be measured by the phosphorescence quenching method263,264 using molecular probes with controllable quenching parameters and defined bio-distributions. This approach relies on the ability of oxygen () to quench emission originating from the triplet excited states of probe molecules. Oxygen levels are derived from phosphorescence decay times, which are independent of the optical properties of the medium and local probe concentrations. “Protected” oxygen probes for truly quantitative imaging of oxygen in vivo consist of platinum (Pt) or palladium (Pd) porphyrins encapsulated inside hydrophobic dendrimers, coated at the periphery with hydrophilic polyethylene glycol (PEG) residues.265–267
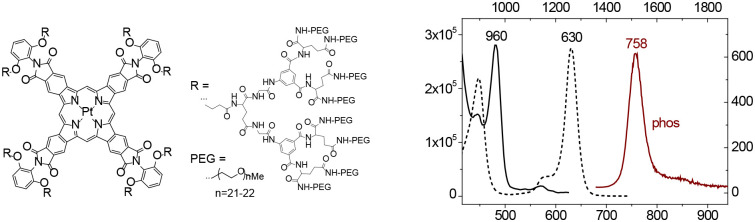
Over the past decade, a special subset of phosphorescent probes has been developed specifically for 2P phosphorescence lifetime microscopy (2P PLIM or simply 2PLM) of oxygen.124 The 2PLM technique has become particularly popular in neuroimaging, enabling imaging of oxygen distributions and concentration gradients in live brain with micron-scale resolution.268–274 While being excellent linear absorbers, Pt and Pd porphyrins have vanishingly small 2P absorption cross-sections. In the original 2P oxygen probes (e.g., PtP-C343) the ability to absorb light by a 2P mechanism was boosted by supplementing the porphyrins with multiple 2P chromophores comprising 2P antennae.124,275,276 However, later a more potent probe was developed, known as Oxyphor 2P125 (Fig. 15), which relies on a single porphyrin whose 2P-active excited electronic states have been tuned by way of synthetic manipulations, rendering a chromophore with strong 2PA and exceptionally bright phosphorescence.277,278 Presently, Oxyphor 2P is the probe of choice for 2PLM as well as for other applications of phosphorescence-based oximetry (e.g. Ref. 279).
Fig. 15.
Structure, optical absorption spectra and basic photophysical properties of probe Oxyphor 2P. In the spectra (right): dotted black line, 1P absorption; solid black lin, 2P absorption; solid dark red line, phosphorescence.
The next challenge in the phosphorescent probes development area is to combine oxygen imaging with sensing of other environmental analytes (e.g. temperature, proton gradients, metal ions, etc.), explore higher orders of non-linearity (e.g. 3PA), and create sensors that can be tagged selectively to particular types of tissues and cells.
3.9. Bioluminescent Tools for Imaging, Modulating, and Integrating
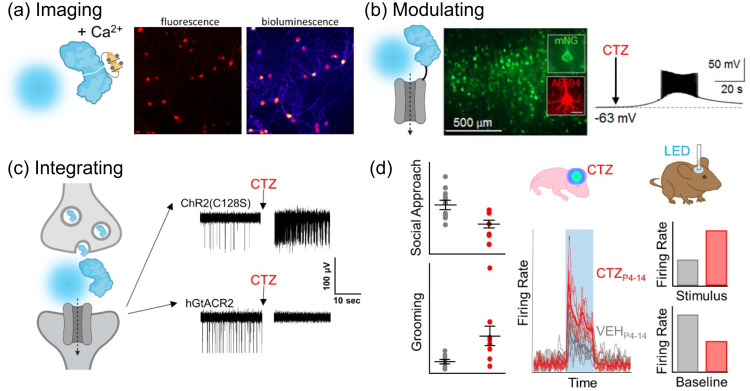
Photons are typically generated from a physical light source, but they can also be produced by a genetically encoded luciferase, an enzyme that oxidizes a substrate, a luciferin, thereby emitting bioluminescence. Bioluminescent imaging enables minimal background and no photobleaching as there is no need for an excitation light source. Bioluminescence can be repurposed beyond imaging and be utilized as a versatile genetically encoded light source for controlling optogenetic actuators.
Core principles behind this platform technology are fusions of a light-emitting luciferase and a light-sensing photoreceptor such as opsins (channelrhodopsin, pumps),280,281 enzymes (adenylate cyclase, Cre recombinase),128,282 or transcription factors (CRY2/CIB, GAVPO).128,283 Expressing light-emitting luciferases and light-sensing proteins in pre- and postsynaptic neurons permit causal interrogation of synaptically connected populations through an “optical synapse” [Fig. 16(a)]. Engineered split luciferases allow for functional reconstitution, allowing light emission to be harnessed to activate light sensing proteins when , voltage, neurotransmitters, and neuropeptides are detected or altered [Fig. 16(b)]. By making light itself activity dependent, optogenetic proteins can be modulated by luciferases serving as an integrator of biological processes [Fig. 16(c)].
Fig. 16.
Bioluminescent tools for imaging, modulating, and integrating. (a)–(c) Luciferases are genetically encoded light emitters that can be employed for imaging neuronal activity when used as a dependent split molecule (a), for modulating neuronal activity when tethered to an optogenetic element (b), and for integrating neuronal activity by expressing the light emitter pre- and the light biosensor post-synaptically (c). (d) Example of experiments taking advantage of the bimodal feature of luminopsins: genetically targeted brain circuits were activated chemogenetically (luciferin) during a defined time window of postnatal development; the same neurons were interrogated in adult animals optogenetically (LED).
Light emission from luciferases can be harnessed to control a range of biological processes by coupling bioluminescence with light-sensing proteins. Fusions of luciferase and opsin (luminopsins) have been used in vivo for improving motor deficits in a Parkinson’s disease mouse model,284 suppression of seizure activity,285 enhancing neuronal repair and functional recovery after ischemic stroke286 and spinal cord injury, modulating spatial and episodic short-term memory,287 studying the effects of developmental hyperexcitation on behavior and circuit activity in adulthood129 [Fig. 16(d)], and promoting axon regeneration after peripheral nerve injury.288
We can expect that this technology will benefit from discovery of new organisms with novel luciferase-luciferin combinations.289 Molecular engineering of luciferases towards brighter and faster indicators in conjunction with tuning light sensing proteins will enable imaging, modulation, and integration of neuronal activity in freely moving mice at increasing sensitivities. This will further be enabled by chemical engineering of synthetic luciferins extending the color palette and intensity of emitted light and advances in miniscopes.290 The trajectory of bioluminescent probes will allow imaging, controlling, and integrating neuronal activity with increasing sophistication.
3.10. Optogenetic Actuators
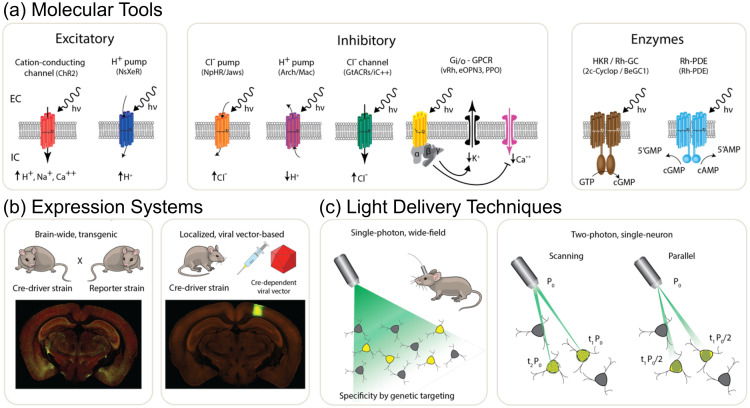
Optogenetic techniques have revolutionized neuroscience this past decade, offering an unprecedented toolkit for manipulating neuronal circuits with light.130 Driven by the goal of understanding the organization and function of the brain, optogenetics began with the discovery of the light-gated cation-conducting channelrhodopsins ChR1 and ChR2291,292 and the application of ChR2 for light-based excitation of mammalian neurons.131,293,294 This groundbreaking advance was followed by efforts of multiple groups to enhance and diversify the optogenetic toolbox.295,296 These efforts have culminated in the engineering of dozens of novel optogenetic tools for activation and silencing of neuronal activity, for modulation of G-protein signaling pathways and for direct modulation of second-messenger pathways [Fig. 17(a)]. Optogenetic tools have also been developed for light-based control over gene expression, synaptic release, and biochemical signaling pathways. While these have been recently reviewed elsewhere,297–301 we will focus here on the most commonly applied tools, which are based on microbial and vertebrate rhodopsin proteins. Common to all these novel tools is the utilization of naturally occurring light-sensitive proteins as actuators to drive cellular processes. The critical advance, and the main attraction of optogenetic tools for neuroscience research, is that these tools are all genetically encoded. This allows the use of genetic engineering techniques to direct the expression of optogenetic tools to precisely defined cell populations, through implementation of transgenic techniques and/or viral vector technology [Fig. 17(b)]. Selectivity is achieved via specific promoter or enhancer sequences that are exclusively expressed in the target cell populations. Cells expressing the light-sensitive protein respond to light in a manner defined by the properties of the specific protein used, while their non-expressing neighbors will remain unaltered. Together, the intersection of genetic specificity and the natural insensitivity of most brain tissue elements to light, render optogenetics a powerful technique for functional dissection of brain circuits in living animals. Optogenetics has been used for a wide range of experimental purposes, from functional dissection of neuronal circuit connectivity302 through investigation of the contribution of defined neuronal populations to highly specific behaviors303 to the development of light-driven interventions in animal models of neurological disorders304 and, most recently, treatment of human patients suffering from neurodegenerative disease.305
Fig. 17.
Optogenetic tools, genetic targeting and optical techniques. (a) Three major classes of rhodopsin-based optogenetic tools: excitatory pumps and channels (left); inhibitory opsins composed of chloride-conducting channelrhodopsins, proton and chloride pumps, and G-protein-coupled rhodopsins coupled to the inhibitory Gi/o pathway (center); enzyme-rhodopsins (right); shown are the histidine kinase group and the phosphodiesterase group. (b) Expression systems: two examples of gene delivery techniques used to express optogenetic tools in target neuron populations: crossing a recombinase driver mouse line with another that expresses a recombinase-dependent optogenetic actuator (left) or stereotactic injection of a viral vector encoding an optogenetic actuator. (c) Light-delivery techniques for optogenetics: (left) wide-field illumination of neurons expressing an optogenetic actuator through a chronically implanted multimode optical fiber or (right) 2P illumination of individual neurons using spiral-scanning of a diffraction limited spot or parallel illumination of extended shapes using wavefront shaping approaches. Images in panel (b) are from the Allen Institute for Brain Science (https://connectivity.brain-map.org/, experiments #741951571 and #288264301).
Optogenetic techniques require optical access to cells expressing optogenetic actuators, to allow light-based modulation of the targeted cells. While this is simple to achieve in vitro (Boyden et al., 2005), applying optogenetic manipulations in vivo requires technology for delivering light to the living brain. The first experiments using ChR2 in behaving rodents used multimode optical fibers coupled through cannulas that were chronically implanted or affixed to the skull.295,306 This arrangement allowed reversible connection of the optical fiber without the need to physically insert a light guide into brain tissue for each experiment. This simple technical approach has been the basis for numerous studies using 1P excitation [Fig. 17(c)] in a vast number of brain regions and neuronal circuits. However, one must consider that such manipulations are far from being able to accurately mimic the natural activity patterns expressed by populations of neurons in the unperturbed brain and might therefore lead to uninterpretable results due to “off-manifold” effects.307
In recent years, 2P techniques have been combined with highly refined optogenetic actuators to allow the control of single neurons308–310 and precisely defined multi-neuron ensembles [Fig. 17(c)], see Sec. 6). Using pulsed near-infrared lasers combined with spiral scanning or parallel illumination approaches,311,312 these developments have the potential to advance optogenetic techniques to the point that they can be utilized to “write in” information at the same level of spatial and temporal precision that the brain uses to encode information.313–316 These exciting developments will surely yield important insight into the natural “language” that neurons use to communicate, encode the external world, and execute behavior. These are long-standing questions in neuroscience. The “optogenetic revolution” has brought us to the exciting place where we can begin to address these questions with remarkable detail and precision.
3.10.1. Optogenetic tools: light-sensitive proteins
Optogenetic tools are derived from a wide range of organisms, from archaea to fungi, algae, and animals. Genes encoding opsin proteins are widespread in nature, being found in all three domains of life and viruses.317,318 Metagenomics led to a revolution in the field of opsin research in the early 2000s and now both metagenomic and metatranscriptomic techniques are driving the rapid expansion of our collective understanding of the taxonomic and functional diversity of this family of proteins.319 While channelrhodopsins were the first to be applied as optogenetic tools,131 the current optogenetic toolbox contains tools based on light-gated ion pumps, cation and anion-conducting channels, G-protein coupled receptors and light-activated enzymes. The diversity of optogenetic tools allows for unprecedented flexibility in experimental design, but also demands an understanding of the basic working principles of the complexities and limitations associated with utilizing light-sensitive proteins.
3.10.2. Light-activated ion pumps and channels
The most widely used opsins in optogenetics are cation-conducting and anion-conducting channelrhodopsins, termed CCRs and ACRs respectively. In the early days, the cation conducting Channelrhodopsin-2 from the alga Chlamydomonas reinhardtii (CrChR2 or just ChR2) and its first mutant (ChR2-H134R, a mutation introduced to enhance the amplitude of functional photocurrents) monopolized optogenetic investigation.292,320 ChR2 is a CCR, mostly permeable to protons but which also conducts other cations, including sodium but to a much lesser extent.321 Naturally occurring ACRs, first discovered in 2015, conduct a variety of anion species.322,323 Expressed exogenously in host cells, CCRs can act as photoactivatable depolarizing actuators while the function of ACRs is dependent on the chloride reversal potential. In many circumstances, the chloride concentration difference is such that ACR activation leads to hyperpolarization and neuronal inhibition. ACRs have been used extensively to silence adult mammalian neurons but they do have some limitations. Photoactivation of ACRs can lead to excitation in some cells (based on chloride reversal potential) and antidromic propagation when they are expressed in the axon.324–326 In contrast to channelrhodopsins, ion pumping rhodopsins have the advantages of high ion specificity, active transport of ions against the electrochemical gradient, and a wide variety of functions. Inward proton pumps (e.g. NsXeR) can be used to depolarize neurons,327 while outward proton (e.g. eArch3.0), outward sodium (e.g. eKR2), and inward chloride pumps (e.g. eNpHR3.0) hyperpolarize neurons and lead to inhibition.328–331 Unfortunately, in contrast to the robust ion flux generated by some channelrhodopsins, pumps are limited by a one-to-one photon-to-ion stoichiometry which can translate into weak photocurrents or photodamage induced by prolonged and intense illumination in efforts to overcompensate for these limitations.332 Additionally, excessive buildup of ions over time can damage cells, especially protons which can alter the intracellular or extracellular pH. These inconsistencies and limitations have motivated the community to attempt to identify new tools for neuronal inhibition. G-protein coupled receptors (GPCRs, discussed below) which engage G-protein signaling cascades or highly selective potassium channels (owing to the pronounced potassium gradient of neurons) would solve some of the issues with pumps and channels as hyperpolarizing actuators. While significant efforts to engineer a potassium channel from other channelrhodopsins enjoyed only partial success, naturally occurring potassium channels from Hyphochytrium catenoides were characterized recently and were termed Kalium Channel Rhodopsins (KCRs).333 Even though KCRs still need to be tested and validated for in vivo experiments, this new discovery has long been awaited by the optogenetics community and these actuators are a welcome addition to the toolbox.
3.10.3. Opsin engineering and enhancement
Not long after the first optogenetics experiments were performed in the early 2000s, it became clear that the capabilities and diversity of opsins available to neuroscientists would need to increase substantially to match the scientific potential of the technique and overcome known and yet unforeseen roadblocks. In addition to the ongoing microbial ecological research to discover naturally occurring opsins, the engineering of opsins with desirable properties through targeted mutation and other means (such as fusions) became a fixture of the field. Efforts to engineer opsins with improved properties such as ion selectivity, spectral diversification, kinetics, light sensitivity, and ion conductance have seen success in recent years and have remained active areas of research. Spectral tuning is of particular interest, because red shifted light (i) penetrates more deeply into tissues, (ii) is less energetic than blue shifted light and thus causes less photodamage, and (iii) can be helpful to eliminate cross talk when opsins are paired with other tools such as fluorescent labels and voltage or indicators. A limited list of engineered opsins of interest include those developed for (1) altered ion selectivity: ChloCs,334 (2) spectral properties: ReaChR335 and Phobos,336 (3) light sensitivity: ChRmine,314 and (4) kinetics: Chronos,337 f-Chrimson.338 The selection of optogenetic actuators for specific experimental applications should be guided by their ionic selectivity, kinetics, action spectrum, and photocurrent amplitude.
3.10.4. G-protein coupled rhodopsins and light-activated enzymes
Animal rhodopsins are GPCRs which catalyze GDP/GTP exchange through engagement of heterotrimeric G-proteins after light absorption.317 The utility of GPCRs in optogenetics research has advanced significantly in recent years with the implementation of bistable melanopsins and parapinopsins. Human and mouse melanopsin variants (hOpn4L, mOpn4L) from retinal ganglion cells can be activated and inactivated with blue light and yellow light, respectively, an advantage in optogenetics experiments where temporal control of activity is desired.339 While both opsins induce dependent inward rectifying (GIRK) currents and are highly light sensitive, mOpn4L exhibits superior on and off kinetics as well as less reduction in amplitude from sustained stimulation. However, these Opn4 variants both showed mixed activation of multiple G-protein pathways, leading to complex effects on neuronal physiology.339 Herlitze and colleagues later described Lamprey Parapinopsin (UVLamP or PPO) which has improved activation and deactivation kinetics compared to mOpn4L and reduced spectral overlap between the active and inactive states resulting in shorter light pulses required for full activation and deactivation.340 More recently, two new bistable opsins were characterized which couple selectively to the inhibitory signaling pathways and allow for efficient optogenetic silencing at presynaptic terminals. The targeting-enhanced mosquito homolog of encephalopsin, termed eOPN3, as well as the lamprey parapinopsin (PPO) were both shown to inhibit synaptic vesicle release through coupling.341,342 PPO allows switchable synaptic silencing, activated with blue-violet light, and deactivated with green light. eOPN3 exhibits a red-shifted action spectrum, high light sensitivity, and rapid activation. It returns to the inactive state spontaneously within minutes, making it an excellent inhibitory tool on the time scale from minutes to hours. Another notable member of the bistable GPCR-rhodopsin family, the zebrafish opsin Opn7b also couples to the pathway and is inactivated by light stimulation. Opn7b was applied as an “inverse optogenetic tool.” (Ref. 343).
The modulation of secondary messengers for the interrogation of cyclic nucleotide mediated cellular processes and signaling pathways has far reaching implications in neuroscience and biological systems in general and is an area where the spatiotemporal control afforded by optogenetics can provide a valuable advantage over pharmacological techniques.344 There are three classes of enzyme-rhodopsins that have so far been described in the literature: histidine kinase rhodopsin (HKR), rhodopsin guanylyl cyclase (Rh-GC), and rhodopsin phosphodiesterase (Rh-PDE).345–347 While the use of enzymerhodopsins for in vivo optogenetics research is not yet a staple, there are promising advances in the use of photoactive enzymes from flavoproteins. For example, the system comprising photoactive adenylyl cyclase from Beggiatoa (bPAC) and a cAMP-gated K+ channel from Spriochaeta thermophila (SthK) has been successfully applied to inhibit diverse neuron types in mice and Zebrafish embryos.348 More recently, a variant of bPAC was developed which exhibits lower dark activity (biPAC) and was then applied to investigate cAMP signaling in hypothalamic neurons and its impact on sexual behavior in mice.349
4. Imaging Brain Activity with One-Photon (1P) Excitation
Due to the rapidly expanding arsenal of fluorescent probes (discussed in Sec. 3), there is a growing need for technologies to enable fast and efficient imaging of these probes in brain tissue. The main advantage of 1P fluorescent methods is the efficiency of excitation quantified as the absorption cross-section: the probability of photon absorption by a fluorophore molecule. In comparison with multiphoton methods (Sec. 5), the probability of 1P absorption for virtually all of these probes is very high. Therefore, 1P excitation does not require pulsed lasers and can be achieved with relatively inexpensive diode-pumped solid-state laser (DPSS) lasers, LEDs, or even a filtered white light source such as a xenon or tungsten–halogen lamp.
With 1P excitation, one way to achieve fast, large-scale imaging is by using wide-field illumination of the entire brain surface and capturing the emitted fluorescent light with a camera. This “mesoscale” imaging (Sec. 4.1) is fast, because no scanning is involved, but lacks sectioning along the z-axis and has limited spatial resolution in the XY-plane due to light scattering. Therefore, mesoscale imaging is well suited for studies of cortical activity that do not require resolving single cells or cortical layers. Cell-type specificity can be achieved by genetic targeting of fluorescent probes.
1P fluorescence imaging technology has been miniaturized to enable measurements in freely behaving animals.350 These wearable “miniscopes” (Sec. 4.2) have been widely applied, in part due to a relatively simple, open-source, low-cost design.351,352
Laser scanning microscopy improves resolution at the price of imaging speed, because of the need to scan the excitation beam. Among different approaches, light-sheet microscopy6 has been successfully applied for in vivo imaging of brain activity in various organisms from semitransparent zebra fish embryos353 to mouse cortex354,355 (Sec. 4.3).
Optical imaging of deeper brain regions usually requires removal of the overlaying tissue or insertion of bulky lenses. As a compromise between the invasiveness of the measurement and the information content, fiber photometry has been used in many studies for detection of the fluorescence signal without spatial resolution. A number of recording channels can be increased using hundreds of splayed microfibers, each following a path of least resistance through tissue.356 More recently, a proof-of-principle demonstration of deep brain imaging has been achieved by scanning the excitation beam through an implanted multimodal optical fiber to form an image (Sec. 4.4). Stabilization of this minimally invasive technology will enable cellular and subcellular resolution beyond the penetration limit of multiphoton imaging (we discuss multiphoton imaging in Sec. 5).
4.1. Wide-Field Calcium Imaging
In vivo wide-field imaging in awake mice expressing GECIs (see Sec. 3.1) allows simultaneous imaging of many brain areas as mice perform complex behavioral tasks.357 Sensory integration, perception, memory and learning are just several examples of the cognitive functions that can be studied using this approach.358–362 In addition, mesoscale imaging can be used to characterize the organization of spontaneous cortical activity363–366 and functional mapping of cortical areas358,367 [Figs. 18(a)–18(b)].
Fig. 18.
Mapping of brain areas and task-induced neuronal activity with mesoscale imaging. (a) Functional mapping of cortical areas. Example maps from three mice (m1, m2, m3) under anesthesia in response to different sensory stimuli: whisker, visual, auditory, forelimb, and hindlimb. Mean evoked activity map is shown for each stimulus type for each mouse. Color denotes normalized fluorescence (ΔF/F). The maps are registered onto the brain atlas (black lines; © 2004 Allen Institute for Brain Science. Allen Mouse Brain Atlas. Available from: http://mouse.brain-map.org/). Overlays of the 5 maps are shown on the right. (b) Parcellation of the dorsal cortical surface into 25 areas. Cortices are color-coded: auditory (green), association (pink), somatosensory + V1 (blue), and motor (red). (c) Behavioral and wide-field imaging setup in mice performing a whisker-dependent memory guided task. (d) Body movement vector in two example hit trials, one active and one passive. (e) Short term memory (STM) maps (averaged during the quiet delay period) for an active and passive trial showing frontal and posterior patches of activity, respectively. Color denotes normalized fluorescence (ΔF/F).
Very much like a conventional epifluorescence microscope, a wide-field or mesoscale fluorescence imaging system consists of two major components: an excitation path delivering light to the brain surface and an emission path that projects an image of the light emitted by the fluorescent indicator onto a camera chip. The light source in most state-of-the art wide-field setups is either a DPSS laser or an LED matching the excitation peak of a fluorescent sensor. A corresponding emission filter in front of the camera blocks excitation light from reaching the camera. The camera is often the most expensive component in the imaging setup. Many imaging setups use sensitive and expensive sCMOS or EMCCD cameras, particularly when the fluorescence signal is weak due to sparse labelling or limited brightness of the sensor. Tandem lens systems, as initially described by Ratzlaff and Grinvald in 1991368 are often used to collect and project the emitted light onto the camera chip.369 Excitation light can enter in between the lens pair via a dichroic mirror. Alternatively, the brain surface can be illuminated obliquely via one or multiple lightguides positioned independently from the excitation path.370
The advantages of wide-field imaging are its large field of view (the entire dorsal cortical surface in the mouse) and the ability to image through the intact skull offering minimally invasive and stable conditions. In addition, a typical wide-field imaging setup is fairly inexpensive, appealing to many labs worldwide. The disadvantages are that 1P imaging is mainly sensitive to superficial cortical layers, and suffers from light scattering, particularly when imaging through the skull. In addition, the raw fluorescence signal can be contaminated by hemoglobin absorption. While many of commonly used fluorescent sensors for wide-field imaging are green (e.g., GCaMPs), hemoglobin strongly absorbs light in the blue-green spectrum. This leads to hemodynamic artifacts due to the hemodynamic response (an increase in the blood volume and decrease in blood oxygenation) associated with an increase in neuronal activity. Several methods have been developed to correct for this artifact. These methods require illumination with additional wavelengths, thereby adding further components along excitation and emission paths. One method uses estimated changes in concentration of oxy- and deoxyhemoglobin, derived from reflectance measurements at, e.g., 530 and 630 nm.363 Specifically for GCaMP imaging, one can use -independent GCaMP fluorescence upon excitation at .371 Due to the significant decrease in hemoglobin absorption at red wavelengths, hemodynamic artifacts are less prominent with red-shifted indicators, such as jRGECO1a, leading to relaxed requirement for hemodynamic correction of the collected fluorescence signals.117
An example of the insights gained from wide-field imaging is shown in Figs. 18(c)–18(e) adapted from Ref. 359. This study demonstrated that short-term memory was maintained in distinct cortical locations depending on the behavioral strategy of the mouse, either in a frontal area during an active strategy or a posterior area during a passive strategy.
Presently, wide-field imaging is being extended to specific neuronal populations via cell-type-specific targeting of GECIs.164 Further development of red-shifted GECIs and other types of indicators, such as voltage sensors, may enable deeper and faster imaging. Combining wide-field imaging with fiber photometry and/or electrophysiological recordings will permit simultaneously imaging/recording of neuronal activity from both cortical and subcortical areas. Finally, 1P fluorescence imaging is being implemented in freely moving mice (Sec. 4.2), opening the door to study more natural behaviors.
4.2. Wearable 1P Microscopes
Table-top microscopy systems, such as the one shown in Fig. 18 above, require head fixation of the animal under the microscope objective. This condition is incompatible with many behaviors. Wearable imaging systems have been developed to enable measurements in freely moving model organisms, from rodents to zebra finches and primates.352,372,373 These miniaturized microscopes (or “miniscopes”) exist in several implementations regarding imaging capabilities, size, and weight depending on experimental needs. Many of them are currently tethered (i.e., connect to the data acquisition system through flexible wires and, in some cases, fibers), allowing measurements during behavioral assays commonly used in research settings. Most of them rely on fluorescent indicators to provide the specificity and contrast necessary to study the cellular or molecular signaling of interest.
1P miniscopes typically employ integrated LEDs or fiber-coupled DPSS lasers for fluorescence excitation and CMOS (complementary metal oxide semiconductor) image sensors for detection [Fig. 19(a)].352,372,373 They offer high frame rates (up to at full resolution) across large fields of view (from up to 10 mm diameter). Spatial resolution varies with the field of view from few microns to tens of microns.375,376 One of the main drawbacks of 1P miniscopes is their potential out-of-focus signal contamination and limited optical depth penetration in densely labeled tissue ( depending on tissue type). Deeper regions can be imaged using tissue-implanted relay optics, such as gradient index (GRIN) lenses [Figs. 19(b)–19(c)]. The relatively low equipment cost and ease of use of 1P miniscopes have facilitated their widespread adoption.
Fig. 19.
1P wearable microscopes for cellular-resolution imaging in freely behaving animals. (a) Example 1P miniscope for high-speed, cellular-resolution imaging in freely behaving mice. This implementation is wire-free and weighs 4-5 grams, including a 1.1-gram single-cell lithium-polymer battery. It allows recordings of duration at 20 Hz, 320 x 320 pixels resolution, x2 pixel subsampling across a FOV. Data is logged onto a MicroSD card for offline analysis. (b) Example raw images of GCaMP6f indicator-expressing (top) and computationally identified cells (bottom) in the dorsal hippocampus of control and epileptic mice. Imaging was performed using a tissue-implanted GRIN lens. (c) Spatially tuned activity pattern across 20 example cells from control and epileptic mice trained to run on a 2-m linear track for water reward. Adapted from Ref. 374 with permission from Nature Publishing Group.
Wearable imaging systems have enabled biological studies difficult or unfeasible with their larger stationary counterparts (e.g., social interactions, sleep-wake transitions, seizure activity).374,376 They have provided insight into how genetically defined cell types encode external (e.g., sensory and motor) and internal (e.g., cortical state) information in various brain regions. More recently, miniscopes have also enabled real-time measurement of cellular activity in the spinal cord of behaving mice.372,377–379
In the future, we can expect these imaging systems to become even more powerful. Improved electronics and optics (e.g., new CMOS sensors, custom micro-optics, electronic focusing systems) will enhance 1P miniscopes’ ability to record at higher resolution, sensitivity, contrast, and speed, in multiple colors and across larger FOVs or tissue volumes.352,372,378–380 While initial tether-free designs exist [Fig. 19(a)], further improvements in onboard or wireless power and data transmission technology are needed to reduce overall device weight and boost recording duration at desired image resolutions and frame rates.
Complementary to these hardware improvements, new indicators (e.g., transmitter and voltage sensors) and computational tools [Fig. 19(b)] (see also Sec. 9) are likely to enable real-time analyses and interrogation of nervous system activity. These innovations will permit longitudinal investigation of various disease, treatment, or behavioral conditions best studied in freely moving animals.
4.3. Imaging in 3D with Light-Sheet Microscopy
The standard method of forming 3D images in intact, living specimens such as the brain is to scan a high numerical aperture (NA) point of light to different positions within a volume of interest. For instance, sequential raster scan of individual planes is often used in confocal microscopy to reconstruct a volume. However, this approach becomes increasingly challenging for high-speed imaging, both because scanners begin to exceed physical limits, and integration time per pixel becomes vanishingly small, nearing the fluorescence lifetime of many fluorophores.381 To overcome these limitations, it is possible to acquire data using parallel illumination of multiple regions at once. In multiphoton microscopy (Sec. 5), this approach has taken many forms, from multi-point scanning382,383 to extended axial range imaging.384–386 An embodiment of this approach using 1P excitation is light-sheet microscopy, which illuminates an entire plane within the sample, achieving optical sectioning by generating a focused image of this illuminated plane onto a camera detector. This approach greatly increases integration time per pixel compared to point scanning, while its selective illumination of only the plane being imaged makes light-sheet microscopy especially light-efficient.381
Light-sheet microscopy has traditionally been applied to imaging cleared tissues (Sec. 2.2), where generating a thin light sheet and focusing onto this light sheet with a second, orthogonal objective is relatively simple for samples up to the size of a whole mouse brain.387,388 Another application has been to image small organisms such as Drosophila embryos and zebrafish during early development, and also for functional imaging in the larval zebrafish brain and heart.389,390 However, the standard use of two orthogonal objective lenses in these light-sheet systems limits both sample size and geometry. 3D imaging speed is also limited by the need to either translate the sample through the light-sheet plane or scan the light sheet in synchrony with repositioning of the detection focal plane. An approach that overcomes these speed and geometry constraints is swept confocally aligned planar excitation (SCAPE) microscopy, a single-objective light-sheet approach, which uses a confocal de-scanning to enable an obliquely incident light sheet at the sample to be scanned from side to side, while the image of the oblique plane is de-scanned, rotated and mapped onto the face of a fast camera [Fig. 20(a)].354,355 The system’s single, stationary objective permits a wide range of intact samples to be imaged in an upright or inverted geometry and does not require restraint or physical translation of the sample. This approach permits 3D microscopy of a wide range of living organisms at speed of up to 300 volumes per second, leveraging the low phototoxicity associated with light-sheet excitation and high light efficiency to capture dynamics as well as 3D movement in behaving animals.
Fig. 20.
SCAPE microscopy: elements and application. (a) Top: SCAPE microscopy uses an oblique light sheet to illuminate the sample, and emitted light is collected by the same objective lens. SCAPE sweeps the oblique light sheet back and forth across the sample, de-scanning and rotating returning light to focus onto a stationary camera. Bottom: example images of a drosophila larva, captured as the light sheet scans. (b) Top: experimental imaging configurations to image neuronal activity in zebrafish larvae and awake, behaving mouse. Bottom: visualization of spontaneous neuronal GCaMP activity in the whole brain of a larval zebrafish. Volume rendering shows a maximum intensity projection over time with inset showing time-courses of 6 cells at 6 different depths in the brain. (c) Top and middle: spontaneous activity of apical dendrites of layer 5 neurons in whisker barrel cortex via GCaMP6f imaging in awake behaving mouse. Maximum intensity projection from the top (XY) and side (YZ) with colors denoting time of peak activity. Bottom: time-courses for the seven numbered regions of interest indicated above.
SCAPE microscopy has been applied to high-speed imaging of a range of different samples including proprioceptive neurons in crawling Drosophila larvae,391 whole-brain imaging in adult Drosophila,392 zebrafish larvae [Fig. 20(b)],381 and red blood cell dynamics in the beating hearts of zebrafish larvae,355,393 and neuronal activity in freely moving C. elegans worms.355 SCAPE is also effective for imaging the mouse nervous system, capturing activity in apical dendrites in layers 1–3 of the awake mouse cortex at 10 volumes per second [Fig. 20(c)],7,354,381,394 and profiling response properties of 10,000’s of olfactory sensory neurons in the intact mouse olfactory epithelium.395 All the prior examples utilized 1P excitation at 488 and 561 nm illumination. However, SCAPE and related light-sheet approaches are also suitable for 2P excitation which can enable deeper imaging into the living mammalian brain. In the future, 2P SCAPE will provide a competitive alternative to multiplexed 2P strategies outlined in Sec. 5. Efforts are also underway to implement SCAPE via an implanted GRIN lens enabling imaging of deep brain regions.
4.4. Deep Imaging Through Multimode Optical Fibers
GRIN lenses, prisms and fiber bundles have been successfully used to image deeper brain regions.396–401 However, insertion of these optical elements into the brain is invasive. One strategy to minimize this problem is imaging though hair-thin multimode fibers (MMFs). Light transport through MMFs is a complex, hardly predictable, yet deterministic process. It can be described by the transmission matrix (TM),402 a linear operator whose components can be experimentally measured by means of wavefront manipulation and interferometry.403 For this application, the TM can be conceptually thought of as a lookup table between the light “shape” entering and exiting the fiber.
Once available, the TM prescribes an optical field entering the MMF required to form the desired field distribution at the focal plane [Fig. 21(a)]. When applied to deep-tissue fluorescence imaging, the fiber is being advanced through the overlying tissue until it arrives at a subcortical brain region under investigation [Fig. 20(b)], which is then sequentially exposed to a series of illumination fields. These fields most commonly form diffraction-limited spots sequentially displayed across the focal plane, mimicking a laser scanning microscope. The emitted fluorescence is collected through the same fiber, spectrally separated, and recorded by a photodetector such as a photomultiplier tube (PMT) [Fig. 20(c)].
Fig. 21.
Deep brain imaging through an optical fiber. (a) Coherent light propagated through a MMF forms a speckle pattern at the output (left). Manipulation of the wavefront entering the MMF yields a desired optical field, e.g., a focal spot (right). (b)–(c) Experimental configuration for in vivo imaging. (d) Inhibitory neurons expressing tdTomato imaged in vivo (panel adapted from Ref. 403). (e) The trade-off between the fiber size and resolution. (f) Dendritic spines of a hippocampal neuron ex vivo resolved using a MMF (bottom). Comparison of the same field of view imaged with a confocal microscope (top). Panels (e) and (f) are adapted from Ref. 404. (g) In vivo neurons expressing GCaMP6f (left) and their activity visualized as standard deviation over time (right). Bottom traces show the change of signal in time after background (BG) subtraction from two regions of interest (1 and 2). Panel adapted from Ref. 405 © Optica.
In context of neuroscience, holographic endoscopy has been used for structural imaging of neurons in cultures,405 acute brain slices404,406 and in vivo403–405 [Fig. 20(d)]. Among all other endoscopes, imaging with MMFs features the smallest probe footprint inducing tissue damage while maintaining subcellular resolution [Fig. 20(e), Ref. 404]. Even dendritic spines and axonal boutons can be resolved using MMFs with diameters [Fig. 20(f), Ref. 404]. The first indication that spatially-resolved functional imaging of intracellular is possible to implement through the means of holographic endoscopy has been shown recently [Fig. 20(g), Ref. 405].
Using fibers with higher numerical apertures, imaging of custom-designed regions of interests and optimizing the animal models for indicator sparsity required in 1P imaging should ultimately open the door for minimally invasive imaging of neuronal activity anywhere in the brain. Moreover, high speed scanning of custom-designed trajectories along blood vessels containing a fluorescent indicator would allow imaging of blood flow similarly to a 2P technique pioneered by David Kleinfeld and collaborators.407 Importantly, the same probe could be used not only for imaging but also for spatially resolved optical stimulation of opsins (i.e., optogenetics).
All these imaging modalities funneled through a hair-thin optical probe would push the microscopic visual reach beyond the fundamental limits of optical far-field imaging technologies. The main challenge lies currently in transitioning this technology into awake animals and the realm of chronic experiments.
5. Imaging Brain Activity with Multiphoton Excitation
There are a few fundamental challenges when imaging brain activity. One challenge already discussed above (Sec. 4) is to be able to capture fast signaling events (1–100 ms) flowing rapidly between neurons. This challenge scales with the size of neuronal network under investigation. Another challenge is that brain tissue is often highly scattering, which limits the depth penetration of 1P microscopy methods. For example, in the mouse cortex, 1P voltage imaging can be used to measure spikes in cortical neurons with cell bodies located in layers I and II, i.e., less than below the surface.99,201 Deeper penetration requires multiphoton imaging.
Here, we introduce a suite of multiphoton technologies that have been developed and/or significantly advanced in the last decade to penetrate deep into scattering tissue and cover a large field of view while retaining high resolution and speed. We start by highlighting a multi-region 2P microscopy design that has been developed to improve the imaging field of view (Sec. 5.1).408–410 These microscopes rely on very large objectives with high NA. The problem of multiregion imaging has also been addressed by optical systems with multiple arms and/or objectives.411–413
Next, we provide a brief overview of several strategies to increase the imaging speed through temporal and spatial multiplexing and “light sculpting,” i.e., engineering of the point spread function (PSF, a.k.a. the focal spot) (Sec. 5.2). This is followed by a more detailed discussion of two fast imaging strategies: discrete sampling of specific voxels of interest with acousto-optic lenses414 (Sec. 5.3) and multipass temporal multiplexing where the excitation laser pulses sequentially excite fluorescence in axially displaced voxels (Sec. 5.4).415
In 2P microscopy, excitation of fluorophore molecules by scattered photons results in the background signal leading to deterioration of signal to background ratio (SBR) with depth. This background can be reduced using two photons of different color derived from two laser beams that overlap only in the focal plane (Sec. 5.5). In 3P microscopy416 (Sec. 5.6), the probability of simultaneous absorption of 3 photons outside of the focal volume is very low, allowing deeper penetration. In addition, 3P excitation requires less energetic, red-shifted photons that scatter less in tissue.
The ability of multiphoton technologies to image the brain tissue deeper, clearer, and faster makes these tools well compatible with the “circuits-to-behavior” neuroscience studies. Ideally, these studies would be conducted in freely behaving animals. To this end, several miniaturized, portable multiphoton designs have been explored (Sec. 5.7).417,418 The major limitation for these miniaturization efforts is the fundamental tradeoff between the objective diameter, resolution, and field of view. To overcome this limitation, alternative strategies are being explored including microlens arrays419,420 and nanofabricated metalenses.421 In the meantime, however, performance of tabletop multiphoton microscopes remains unsurpassed.
5.1. Multi-Region 2-Photon (2P) Imaging
Many conventional 2P imaging systems have fields-of-view (FOVs) that are limited to or less, and typically a single image plane is monitored per acquisition. However, neuronal circuitry can be distributed across many cubic millimeters, and capturing dynamics at this spatial scale, with a sub-second time resolution, can lead to novel insights into brain function. Thus, it is useful to pursue large-scale 2P imaging.
Measuring neuronal activity across large FOVs ( or more) with subcellular resolution requires relatively large diameter optics, such as microscope objectives that are wide.408,422,423 Objectives with moderate-to-high numerical apertures () and large FOVs (i.e., long focal lengths) provide access to an order of magnitude more pixels. However, the sampling time of the raster scan is unchanged, so scanning the full FOV results in frame rates that are too slow ( per frame) to be useful for many applications. To this end, strategically choosing specific subregions within the large FOV and fast jumping between these subregions408,410,422 can be used to sample across large FOVs while still maintaining sub-second temporal resolution. This technique can be combined with temporal multiplexing422,424 and spatial multiplexing409 to achieve fast, simultaneous acquisition of multiple volumes (Fig. 22). An alternative approach to imaging in multiple regions simultaneously is to have multiple, small ROIs under separate, independently positioned objectives.411,412
Fig. 22.
Large field-of-view, multiregion 2P imaging. (a) FOV available in conventional 2P systems are often confined to , which is smaller than many cortical areas. Larger FOVs (2-mm diameter, or the 5 x 5 mm region of the Diesel2p system410 provide optical access to multiple cortical areas simultaneously. (b) Neuronal activity can be monitored across these FOVs through rapid beam steering and/or multiplexed beams. (c)–(d) The Diesel2p is a system that provides a large FOV (c) and dual scan engines coupled into a single objective (d) for independent multiplexed imaging and/or 2P optogenetic manipulations of neuronal activity. (e) With large FOV, 2P imaging systems, subcellular resolution is available across a large imaging volume, containing on the order of a million neurons. Adapted from Ref. 410.
Multiphoton imaging has been scaled up in the axial dimension as well. For example, one can temporally multiplex 2P imaging and 3P imaging (Sec. 5.6) to simultaneously image superficial and deeper neocortical layers.425 Many innovations in conventional 2P imaging have found application in multi-region 2P systems as well, including adaptive optics410 (Sec. 5.8), axially extended Bessel beam volume scanning426 (Sec. 5.2), and quad-region scanning.427
5.2. Sampling Strategies for Fast 2P Imaging
Conventional 2P microscopy is based on raster scanning of a near-infrared laser beam focused into a “point” (diffraction-limited) PSF. However, as previously described, sequential scanning of this PSF throughout large () volumes is too slow to image neuronal activity, which occurs on the timescale of milliseconds. Figure 23 summarizes various approaches for fast 2P imaging with large FOVs that have been proposed in recent years. Among them is the multi-region microscopy discussed in Sec. 5.1. Another (not mutually exclusive) approach is “sculpting” the PSF in space and time in order to increase the imaging speed (or to image larger volumes whilst maintaining acquisition rates).
Fig. 23.
A summary of state-of-the-art approaches for fast 2P imaging. (a) Commonly used excitation modalities: (i) high NA gaussian beams,428 (ii) low NA gaussian beams,429 (iii) Bessel beams,430 (iv) stereoscopic low NA gaussian beams,386 (v) line foci for tomographic imaging182 and (vi) temporally focused, low NA Gaussian beams.311,431 Top row: simulated intensities of incident beams in the objective back focal plane (BFP) used to generate different excitation PSFs. Middle row: simulated excitation PSFs (red colormap) and the proportional 2P excited fluorescence (green colormap) generated in the focal plane (XY). Bottom row: same as middle but axial (xz) slices. The color bars and principal axes in (i) are applicable to all simulated data apart from (vi) where different colors are used to represent the different wavelengths constituting the ultrafast pulse with central wavelength used for temporal focusing. refers to the pulse duration which is shortest at the focal plane where 2P excitation is maximized. Abbreviations: |E| refers to the amplitude of the electric field; , the intensity, and , the proportional 2P fluorescence; NA, numerical aperture; TwINS, 2P imaging of neurons using stereoscopy. (b) A summary of different implementations of (i) lateral, (ii) axial, and (iii) temporal multiplexing that have been applied to fast 2P imaging. (i) Left: the incident beam is divided into two beamlets each directed to simultaneously image different regions of interest (ROI).432 Right: the excitation field is divided into multiple laterally separated spots which sample different portions of the same field of view.433 (ii) Multiple planes throughout a volume can be imaged simultaneously using axially multiplexed beams432 or by directing beamlets with different wavelengths to different sample depths.424 (iii) Since the lifetime of common fluorophores is shorter (shown, not to scale) than the separation between subsequent pulses generated by suitable mode-locked lasers, each pulse can be divided into multiple beamlets, which can be laterally (upper) or axially (lower) displaced to perform fast 2P imaging.415,434,435 (c) Sequential and parallel acquisition modes used for fast 2P imaging. (i)–(v) Methods based on single pixel detection: (i),428 (ii),430 (iii),182 (iv),436 and (v).437 (xii)–(x) Common parallel excitation methods combined with camera detection: (vii),438,439 (viii),429 and (ix).440 Abbreviations: PD, point detector; SPAD, single-photon avalanche diode array. (d) (i) Simulated widefield PSF and detection PSFs used for extended depth of field imaging: (ii),441 (iii),383 (iv),442 and (v).443 Abbreviations: PM, phase mask; WF, widefield; DH, double helix PSF; LF, light field.
Diverse photonic technologies354,444–452 have increased the rate of sequential scanning, and volumetric imaging rates have also been increased by converting lateral scanning of a patterned substrate in space into axial displacements.453,454 Some techniques aim to reduce the pixel dwell time of 2P microscopy to its fundamental limit (determined by the excited state fluorophore lifetime) by dividing high-energy ultrafast laser pulses into beamlets that sequentially excite fluorescence in different voxels.415,424,434,435 The temporal resolution of 2P microscopy has also been improved by optimizing the scan trajectory450 or by discretely sampling voxels distributed throughout the region of interest.100,436,455
An alternative approach for fast 2P imaging is to sculpt the excitation PSF and increase the instantaneous volume of excitation to reduce the total number of sequential measurements. This includes line scanning,182 and axially elongated excitation PSFs such as Bessel beams which are scanned in the directions orthogonal to their elongation.426,430,456,457 The acquisition of stereoscopic (tomographic) information is necessary in the case of elongated foci and densely labelled samples.182,386 Illumination with spatial and temporal focusing of femtosecond pulses has also been used to increase frame rates.431,438,439 Another subclass of parallel methods spatially multiplex the excitation beam, project multiple foci in 3D, and record fluorescence using 1D425,432,437,449 or 2D detectors.383,433,441,458–460
5.3. Two-Photon Imaging in 3D with an Acousto-Optic Lens
For many applications, such as imaging transients in neuronal cell bodies, specific dendrites, axons, or synapses, the ideal sampling strategy would not be a complete coverage of a volume, but rather a set of regions of interest (ROIs) strategically positioned to overlap with locations of interest. This can be achieved by using random-access 3D imaging with an acousto-optic lens (3D-AOL) to steer a 2P laser beam to arbitrary preselected locations in the imaging volume.461 Moreover, the same technology can be used to correct for brain movement in real time, which can be problematic for imaging of small structures during behavioral tasks.462 Selective imaging of ROIs with 3D-AOL microscopy enables considerably higher temporal resolution than imaging the entire volume, since the inertia-free focus and scanning is rapid and the ROIs typically occupy a small fraction of the volume.414,436,463 In addition, this type of acquisition streamlines curation, analysis, storage, and sharing, because only the required data are collected.
3D-AOL laser scanners consist of two orthogonally arranged pairs of AO deflectors, with counter-propagating acoustic waves that fill the crystals, creating dynamic diffraction patterns that can steer and focus a 2P laser beam in 436,455,464 [Figs. 24(a), 24(b)]. Linear acoustic drives enable random access focusing of a laser beam to any point in the 3D imaging volume (called 3D-RAMP)436 or random access focusing and continuous line scanning in different x–y planes,455 while nonlinear drives enable continuous line scans in any arbitrary direction (x, y, z) at tens of kilohertz.467 Unlike resonant galvanometers, 3D-AOL line scans can be varied in orientation, length, and dwell time (). A compact design version of the 3D-AOL has a 10 × 10 × 25 cm footprint and can be introduced into the optical path of conventional 2P microscopes providing an imaging volume of .414 Real-time correction for brain movement is achieved by tracking a fluorescent object in the imaging volume [typically a bead; Fig. 24(c)] with closed-loop FPGA-based image processing and compensating for the movement with a rigid translation of the imaging volume using the 3D-AOL scanner with update rates up to 1 kHz.462
Fig. 24.
Principles and applications of 3D-AOL microscopy. (a) Schematic of pair of AO deflectors (AODs) with chirped sound waves that curve the optical wavefront of a laser beam (red lines). Modified from Ref. 463. (b) Two pairs of orthogonally aligned AODs that make up a spherical AOL that can focus and scan a laser beam in 3D. Cubes below illustrates multiple linear (left) and nonlinear (right) drive-based imaging modes. Modified from Ref. 414. (c) Left: illustration of 3D movement correction with XY and Z scans of a bead interleaved with multiplane imaging. Right: sequence of operation to track a reference object in 3D and compensate imaging for brain movement. Modified from Ref. 462. (d) Selective imaging of sparsely distributed inhibitory interneurons in the cerebellum. Top left: awake mouse on a treadmill. Top right: locations of somatic ROIs in 3D-AOL imaging volume of mouse expressing GCaMP6f in Golgi cells. Middle: montage of 72 selectively imaged Golgi cell somata. Bottom: activity traces extracted from the cells during locomotion, whisking (WMI) and mild air puff to the whiskers (Puff). Modified from Ref. 465. (e) Image of pyramidal cell with dendritic branches selectively imaged with multiple line scans (Ribbon scanning). Aligned images of individual dendritic segments with spines clearly visible (middle). Modified from Ref. 466. (f) Schematic illustrating multiscale imaging functionality of 3D-AOL microscopy, which enables simultaneous imaging of neuronal populations, dendrites, spines and axons present in a local circuit.
3D-AOL microscopy has been used with GECIs to image neuronal population activity in the forebrain of tethered zebrafish while performing swim bouts,462 and populations of excitatory463,468,469 and inhibitory neurons465,470 in mice [Fig. 24(d)]. Its high spatial resolution has enabled imaging of large populations of axons in the cerebellum471 and selective imaging of dendritic trees and spines with millisecond precision with either 3D-RAMP436,463 or multiple line scans466 [Fig. 24(e)]. It is particularly suitable for high-speed multiscale imaging of neurons and neuronal structures that are sparsely distributed within local circuits [Fig. 24(f)].
Current developments include increasing the imaging volume and speeding up movement-stabilized imaging of entire dendritic trees. In the medium-term, this technology could be optimized for movement-stabilized voltage imaging100 and photo-stimulation applications. On the longer-term, ultra-high-speed adaptive optics capability of this technology467,472 could be useful for a range of applications, including higher spatiotemporal imaging of neuronal circuits, optical tweezers, and nanofabrication.
5.4. Dense Volumetric Imaging with Light Beads Microscopy
Another approach to address a trade-off between resolution, speed of acquisition and the volume size of the recorded neuronal population is Light Beads Microscopy (LBM)415 that pushes multiphoton imaging to the limits dictated only by the tolerable biological radiation exposure and the nature of fluorescence. In LBM, a set of axially separated and temporally distinct foci record the entire axial imaging range near-simultaneously, enabling volumetric recording at per second only limited by fluorescence lifetime (Fig. 25). Combined with an acquisition strategy that allows recoding of each distinct spatial voxel by only one laser pulse, this results in maximization of generated fluorescence light per unit excitation power.
Fig. 25.
Light Beads Microscopy: elements and application. (a), (b) The Many-fold Axial Multiplexing Module (MAxiMuM) enables 30-fold temporal multiplexing in the axial direction using a cavity-based design resulting in a set of 30 axially focused beams, “Light Beads” at the sample with and . A high energy pulse undergoes 30 roundtrips in a slightly imbalanced 8f reimaging cavity and during each round trip a sub-pulse at an appropriate delay is coupled out. Choosing the reflectivity of the output coupler, the exponential falloff of pulse energy can be exactly matched to the required power increase in scattering tissue for all beads. (c) Volumetric recording of 807,748 neurons within at 2.2 Hz within the mouse cortex. 3D rendering of neuron spatial coordinates for a 9-minute recording. (d) Subset of 50 traces from the total of 807,748 extracted neurons, offset: 0.5 .
LBM has been applied to achieve cellular resolution recordings within a volume of spanning both hemispheres of the mouse cortex and containing more than 1 million neurons at as well as other configuration in which volume size is traded for recording speed. These data provided evidence of covariance of neuronal activity within the population of stimulus-tuned neurons separated by several millimeters across the brain at single-trial level representing information on internal states or uncontrolled aspects of external stimuli and behavior.415
We expect that in the future, SNR of LBM can be further enhanced using shorter laser pulses and higher bandwidth amplification between the photodetectors and digitizers. Together with development of more efficient, red-shifted indicators these developments are expected to extend the depth of imaging and the size of recorded neuronal population within the limits of tolerable sample exposure to laser light. The ability to record from such large neuronal populations with cellular resolution opens up a range of opportunities for understanding the neurocomputation principles underlying multiregional encoding and processing of sensory and behavioral information across the mammalian brain.
5.5. Non-Degenerate 2P Microscopy
In the 2P imaging modalities described above (Secs. 5.1–5.4), a fluorophore was excited by the simultaneous absorption of two photons of the same energy within the near infrared spectrum, derived from the same pulsed laser beam. This is known as “degenerate” 2P (D-2P) excitation. Alternatively, the same energy needed for the transition to the excited state can be delivered via absorption of two photons of different energy (i.e., different color) [Fig. 26(a)]. This is “non-degenerate” 2P excitation (ND-2PE). ND-2PE has a long history in the chemical physics and microscopy.474–477 Using ND-2PE, we can vary the energies of the individual photons ( and ) while keeping the sum energy constant. The first laser beam can be tuned within the standard near-infrared range of excitation wavelengths used in conventional 2P microscopy, placing the second laser beam within the short wavelength infrared wavelengths range for excitation of visible emission fluorophores (green to red). Due to the requirements of two temporally synchronized pulsed beams [Fig. 26(b)], it is preferential to use a single source as a master oscillator. Commercially available turn-key options include dual output femtosecond lasers with one beam fixed at 1040-nm and a second tunable beam.478 To gain more tuning flexibility, one can also use a femtosecond Ti:Sapphire laser pumping an Optical Parametric Oscillator (OPO).479–481
Fig. 26.
ND-2PE with collinear and displaced beams. (a) Schematic Jablonski energy diagram for D-2PE, D-3PE, and ND-2PE. , fluorescence signal intensity; and , intensities of the two laser beams; and , the wavelength of the two laser beams; , , and , cross-sections for D-2PE, D-3PE, and ND-2PE; [C], fluorophore concentration. (b) Simplified schematic of ND-2PE microscope setup: GS-XY, galvanometer scanner; SL, scan lens; TL, tube lens; DM, dichroic mirror; OL, objective lens; PMT, photomultiplier tube. (c) Left: color-coded normalized non-degenerate absorption cross-section for EGFP as a function of the combination of NIR and IR wavelengths. The cross-section value at each combination of wavelengths was normalized by the peak value of this fluorophore. The isocline corresponding to the ground to excited state transition energy is indicated in dashed black line. Right: normalized cross-section values as a function of the equivalent degenerate wavelength are shown in red. Independently measured degenerate cross-section values normalized by its peak within the equivalent range of the total photon energy are shown in black. The black dashed line indicates the position of the peak degenerate absorption used for the normalization procedure (930 nm). These wavelengths correspond to the energy isocline shown in panel (b). (d) Simultaneous excitation of four different fluorescent proteins in Brainbow mouse cortical tissue using ND-2PE with , and . Panel (d) is reproduced with permission from Ref. 473. (e) Left: D-2PE excites the sample both near the surface and within the focal volume. Right: by selecting the two excitation beams for ND-2PE outside of the excitation spectrum of the fluorophores and spatially displacing the beams, the sample is excited only in the focal volume where two beams overlap. (f) Simulation results for the normalized values of signal, background, and SBR versus the beam displacement from the colinear configuration.
ND-2PE results in an increase in the probability of 2P excitation,480,482 quantified as the excitation cross-section – an important parameter that, for a given fluorophore concentration and laser power, translates into brighter fluorescence. Because laser illumination may have adverse effects on live biological tissues, large cross-section is always a desirable feature at the focus of fluorophore engineering.277 Importantly, ND-2PE does not alter the shape of 2P absorption spectrum, so that that the peak absorption remains the same [Fig. 26(c)].
ND-2PE has been used to extend the excitation wavelength range providing a third “virtual” excitation wavelength.473,483,484 Using synchronized pulses from a femtosecond laser and an OPO, Mahou et al. demonstrated simultaneous excitation of four different fluorescent proteins in Brainbow mouse cortical tissue473 [Fig. 26(d)]. ND-2PE has also been explored to increase spatial resolution (Refs. 485, 486 2004 #2024) and penetration depth in scattering media.487,488 Importantly, ND2P excitation can be used to suppress the background (out-of-focus fluorescence) by choosing their wavelength outside of the D-2PE spectrum of the fluorophores and shaping the beams to overlap only at the focal spot.478,488–491 For example, one can arrange the beams to enter the back aperture of the objective side-by-side parallel to each other and the optical axis [Fig. 26(e)]. This arrangement reduces their overlap above the focal plane minimizing the background at the price of reducing the effective numerical aperture (NA) for each beam.478,492 Simulation studies using a beam propagation model have shown that the amount of enhancement in the signal-to-background ratio (SBR) depends on the amount of displacement [Fig. 26(f)]. Both the efficiency of ND-2PE (signal) and out-of-focus fluorescence (background) decrease by increasing the spatial separation of the beams. However, the background decreases at a higher rate leading to the overall increase in SBR.478
Further studies are required to optimize the key parameters important for achieving the optimal SBR in ND-2PE: the amount of displacement, the beam size, and the quality of the beam overlap in the focal volume. These improvements would allow increasing the depth penetration of 2P microscopy, particularly in cases of dense fluorescence labeling.
5.6. Three-Photon (3P) Microscopy
As we discussed above, 2P microscopy (2PM)428 in combination with genetically expressed fluorescent probes493 has been enormously influential in neuroscience. It opened the door for studies of brain structure and function with cellular and subcellular resolution in living animals. The main limitation of this technology is depth penetration: SBR deteriorates when imaging deep into the brains.494 This problem is commonly known as out-of-focus background fluorescence (mentioned in Sec. 5.5). 3P microscopy (3PM, Fig. 27)497 overcomes this “fundamental” limitation.
Fig. 27.
Deep imaging with 3PM. (a) 3PM of GCaMP6s-labeled neurons in the mouse cortex and the hippocampus. Green, fluorescence; magenta, third-harmonic generation (THG). (b) Activity recording in the Stratum Pyramidale layer of the hippocampus at depth. Only a small portion of the 48-minute recording session is shown. Reproduced with permission from Ref. 416. Springer Nature. (c) Imaging through the intact mouse skull. Red, RFP-labeled neurons in a Brainbow mouse, male, 12 weeks. Green, THG. The zero depth is set just beneath the skull. Reproduced with permission from Ref. 495. Springer Nature. (d) In vivo 3PM of mouse brain vasculature labeled with quantum dots (Qtracker 655) to depth. Reproduced with permission from Ref. 496, © 2019 American Chemical Society.
The deep tissue penetration capability of 3PM derives from the longer excitation wavelengths to reduce tissue scattering497–499 and the higher-order nonlinear excitation to steepen the drop in the probability to excite fluorescence away from the focus.415,497,500,501 In contrast to 2PM, 3PM is not limited by out-of-focus fluorescence, so far even in densely labeled samples.
Long wavelength 3PM is being applied to deep tissue imaging beyond the depth limit of 2PM, such as 3PM of GCaMP-labeled neurons in the mouse hippocampus497 and 3PM of quantum-dot-labeled mouse brain vasculature at depth.496 3PM is capable of imaging through a highly turbid layer such as through the intact skull of adult mouse495 and adult zebrafish.502 2PM and 3PM can be combined for simultaneous imaging of both the cortical layers and the subcortical regions.424 By leveraging blue-shifted 3P excitation, simultaneous imaging of multiple fluorophores can be achieved. For example, commonly used blue, green, yellow and red fluorescent proteins can be 3P excited using a single excitation beam at 1340 nm,503 enabling four color, deep tissue imaging.
While long wavelength 3PM has significantly increased the penetration depth of high spatial resolution fluorescence imaging, there are a number of limitations that must be overcome for 3PM to achieve its full potential. 3P excitation is inherently weaker than 2P excitation.504,505 In fact, the 3P signal strength sets the practical limit of the imaging depth of 3PM today.506 Therefore, improving the 3P signal is essential to increasing the 3P imaging depth. Large 3P cross-sections,503 adaptive optics,507,508 and imaging the regions of interest only509 can improve the 3P signal. Furthermore, the laser source for 3PM is not yet optimized for deep tissue penetration, and the complexity and cost of the excitation source is a major barrier for the applications of 3PM. An excitation source with cost and user experience similar to a Ti:Sapphire laser for 2PM is necessary to make deep tissue 3PM a routine instrument for brain research. Finally, foundational knowledge of many parameters for 3PM is not yet available. For example, the spectral properties of 3P cross-sections have not been studied for nearly all dyes and fluorescent proteins at wavelengths . Although the scattering and absorption characteristics of the mouse brain between 1300 nm and 1700 nm have been measured recently,495,510 systematic studies of tissues are needed for other brains (e.g., fly, fish, non-human primate, etc.) to define the application space for 3PM and lower the barrier of adoption of the 3PM technique beyond the mouse brains.
5.7. Wearable Multiphoton Microscopes
Both 2P and 3P microscopes have been miniaturized to enable imaging in freely behaving animals. Compared to 1P miniscopes (Sec. 4.2), their 2P counterparts offer higher resolution, both laterally and axially, and larger depth penetration (up to ) [Figs. 28(a)–28(c)].372,373,418 Depending on the scanning modality, frame rates of up to (but typically ) can be achieved. 3P miniscopes offer even larger imaging depths (up to in the cortex) but require low-repetition-rate amplified laser systems and special fibers in less widespread use [Figs. 28(d)–28(f)].417 Due to their reliance on short-pulse lasers and photomultiplier tubes (PMTs) for fluorescence excitation and detection, respectively, multiphoton miniscopes are fiber-coupled. The use of hollow-core (HC) photonic crystal fibers has allowed efficient fluorescence excitation at defined wavelengths [Figs. 28(a), 28(d)]. However, multiphoton miniscopes’ attainable field of view tends to be smaller (up to but typically ) than their 1P counterparts [Figs. 19(b), 28(b), 28(e)].372,373 Additionally, their relatively low frame rate and depth-of-field can complicate stable recordings during animal behavior-induced or tissue-intrinsic movements.
Fig. 28.
2P and 3P wearable microscopes for cellular-resolution imaging in freely behaving animals. (a) Example 2P miniscope for high-resolution imaging at depth. This implementation weighs 4.2 grams and includes an electrically tunable lens (ETL) (top right) for rapid focus adjustment across a focal range (bottom right). Using a microelectromechanical system (MEMS) scanner, the miniscope allows 10 Hz recordings at 512 x 512 pixels resolution across a FOV. (b) Example images of GCaMP6s-expressing neurons acquired at the indicated focal depths (z) in the mouse prefrontal cortex. (c) transients from dendritic (top) and cell body regions of interest (ROIs) (bottom) within the focal planes shown in (b). (d) 3P miniscope for deep, high-resolution imaging. This microscope weighs 5 grams and includes a 1.2-m hollow-core photonic bandgap crystal fiber (HC-PBGF) (right) for low-dispersion delivery of the excitation light-pulses. A plastic optical fiber collects the emitted fluorescence for remote detection by photomultiplier tubes (PMTs). (e) Image of GCaMP6s-labeled rat cortical neurons at the indicated focal depth (z) in the posterior parietal cortex. (f) spiking from four example neurons recorded at cortical depth. (a)–(f) Adapted with permission from Nature Publishing Group: (a)–(c) from Ref. 418 and (d)–(f) from Ref. 417.
In the future, improved laser scanning and optical components may enhance multiphoton miniscopes’ FOV, frame rate, and axial beam control [Fig. 28(a)]. Other features may include the ability to perform all-optical or combined imaging and electrophysiological interrogations in unrestrained animals.352,372
5.8. Adaptive Optics in Multiphoton Microscopy
Adaptive optics (AO) is a method used in brain imaging to reduce the blurring effect of wavefront distortions and recover diffraction-limited performance deep within the brain tissue. AO was first developed for ground-based telescopes to measure and correct the distortion introduced by the turbulent atmosphere on wavefronts of distant astronomical objects.511,512 It was later adopted by ophthalmologists to correct the aberrations of the eye for high-resolution imaging of retinal structures.513,514 With the increasing popularity of multiphoton fluorescence microscopy for in vivo brain imaging, AO methods have been developed to measure and correct the distortion that brain tissue imparts on the wavefront of the excitation light [Fig. 29(a)] for high-resolution imaging at depth.515
Fig. 29.
Adaptive optics enables high-resolution multiphoton microscopy at depth. (a) Wavefront distortion of the excitation light can be (b) corrected with a deformable mirror (DM) or liquid crystal spatial light modulator (SLM) to form a diffraction-limited excitation focus. Blue: wavefront. Red: excitation light. Orange: fluorescence. (c) 2P fluorescence images of dendrites and dendritic spines ( depth) in the mouse cortex. (d) 3P fluorescence images of a neuron ( depth) in the mouse spinal cord. (e) AO increases transient strength and enables detection of orientation selectivity.
An AO system corrects the distortion or optical aberrations of the image-forming light (i.e., the excitation laser in multiphoton microscopy) by controlling its wavefront with a phase device such as a deformable mirror or liquid-crystal spatial light modulator, so that a diffraction-limited focus can be formed within the sample [Fig. 29(b)]. Effective correction requires accurate measurement of sample-induced aberrations. Methods similar to those applied in astronomy and ophthalmology, where the distortion is directly measured by a wavefront sensor (typically made of a lenslet array and a camera), have been demonstrated for sample preparations with minimal scattering.516,517 For samples with substantial scattering, indirect wavefront sensing methods have been developed. Some measure wavefront distortion by manipulating the excitation wavefront and detecting its effect on fluorescent signal/image,518–520 while others measure the electric PSF of the excitation focus directly.521
Both direct522,523 and indirect AO methods521,524–526 have been applied to 2P fluorescence microscopy for high-resolution structural and functional imaging of the brain down to layer 5b of the mouse cortex523 and through thinned skull [Fig. 29(c)].527 Combined with 3P fluorescence microscopy, AO has enabled non-invasive high-resolution imaging of structures such as subcortical synapses and spinal cord neurons [Fig. 29(d)].506,508 The recovery of diffraction-limited resolution enabled accurate measurement of activity from thalamic boutons in layer 4 of the mouse primary cortex [Fig. 29(e)], which led to the discovery of extensive orientation-selective thalamic inputs.528
The proof-of-principle experiments described above have firmly established the importance and effectiveness of AO for high-resolution brain imaging at depth. Now it is time for the field to go beyond purely technological demonstrations so that AO can contribute to biological discoveries in the brain. The biggest obstacle is how to integrate AO modules into existing and new microscopes, and to ensure its ease of use and robust performance even in the hands of users with no optical expertise. Further engineering and collaboration with microscope companies will be necessary to realize this goal.
6. Two-Photon Optogenetics
The advent of optogenetic actuators (Sec. 3.10), has ushered a new era in causal neuronal circuit interrogation. Yet, in most “traditional” applications of optogenetic perturbation an entire population of neurons is excited or suppressed together, and furthermore the experimenter often does not observe the neuronal response to the perturbation. However, in essentially every neuronal circuit, the activity of different neurons within a population is heterogeneous. Further, neuronal excitability varies in both space and time, strongly affecting responses to optical stimulation. Thus, providing more nuanced insights into key problems in systems neuroscience like the connection between neuronal code and behavior, requires the advancement of neurophotonic methods with increased precision and built-in optical readout. The resulting set of techniques are often termed “circuit optogenetics”529 or “all-optical” circuit interrogation.311,530,531
6.1. 2P Optogenetic Photostimulation
From a technical standpoint, stimulating distributed sets of pre-defined neurons in the brain with single-cell resolution has become possible through the advent of holographic 2P optogenetic photostimulation (e.g., Refs. 532–536), which has transformed the study of the behavioral relevance of specific circuits and activity patterns.313–315,537,538 2P optogenetic photostimulation utilizes the principle of non-linear 2P absorption to excite light-sensitive opsins within a confined axial plane deep into tissue.308,532,539 Holographic optical neuronal interfaces (HONIs, in analogy to their holographic optical trap (HOT) predecessors) use phase-modulating spatial light modulators (SLMs) to diffractively shape a distributed excitation pattern in two or three dimensions.449,540–543 Tightly focused diffraction-limited focal spots maximize 2P excitation efficiency within the focal volume but are not very efficient in modulating behavior of the targeted neuron, because only a small patch of the cell membrane is affected. Therefore, practical implementation of 2P optogenetics commonly relies on one of the two general strategies: rapidly scanning a diffraction-limited spot to cover a large fraction of the cell’s membrane310,314,315,532,536 or defocusing (i.e., spatially expanding) the focal spot to cover the entire cell body533,535,537,539,544–546 [Figs. 30(a)–30(b)]. The first approach generally trades robustness of optogenetic actuation with temporal precision, and typically lacks the high temporal resolution necessary for generation of precise spike timing to reproduce spatiotemporally patterned activity. The second approach requires more complex hardware. In addition, temporally focused 2P excitation311,438,544 is used to decouple the lateral and axial dimensions of the excitation spots by shaping the light pulse’s spatiotemporal profile.
Fig. 30.
Precision optogenetic modulation of neuronal circuits and behavior. (a) All-optical precision circuit interrogation is enabled by combining 2P imaging and cell-targeted holographic stimulation of a distributed pattern of multiple neurons, typically using a dedicated low-repetition rate (amplified) femtosecond laser. The illumination is extended across each neuron’s membrane by either rapid scanning, reducing the effective numerical aperture (at the cost of optical sectioning) or by parallel illumination of temporally focused extended shapes in two or three dimensions. (b) An array of methods are being developed to calibrate the projected light patterns, adjust as needed, and “smooth” their intensity profile (by minimizing holographic speckle547), and in some cases, validate and correct their precise targeting in situ inside the tissue.548 (c) Beyond target validation, all-optical precision neurophotonic interfaces enable direct readout of the effects of circuit perturbations with simultaneous imaging of neuronal activity (left panel from Ref. 536). This paradigm can be used to infer circuit connectivity and dynamics. One can also infer neuronal activity indirectly through animal behavioral reports of its (perturbed) perceptual experience (right panel, from Ref. 538), which informs us about the behavioral relevance of specific neurons and coding features.
All-optical HONIs technology is a highly active area of neurophotonics and multiple recent studies have advanced key performance attributes of the optogenetic probes and the spatiotemporal characteristics of the generated light patterns. Several new families of red-shifted optogenetic probes were developed and/or characterized primarily for this application including Chrimson,337 ReachR,549 ChRome535 and ChRmine,314 soma-targeting strategies were developed for increased efficacy and precision,533,535,550,551 and transgenic animals that express these probes under genetic control have been developed546,552 (see also Sec. 3.10). While these opsins have the desirable property of being effectively excited at the wavelengths of many common pulsed lasers, their extended excitation shoulder at shorter wavelengths means that they suffer from unwanted “cross-talk” excitation from the 2P imaging laser (which becomes worse for more sensitive probes). One possible alternative strategy being developed is to combine a blue-shifted opsin like CoChR with a red-shifted indicator like jRCamp.534,551 While earlier studies in this area excited neurons with moderate temporal precision (typically ), sensitive probes and effective excitation from amplified laser sources with low repetition rate now enable millisecond-precise stimulation,314,533,535,538,546 turning SLM switching times into the limiting factor. Increasing this performance parameter is possible by employing rate-optimized SLMs314 (where the liquid crystal elements are also heated), by sequentially stacking several SLMs,314 or by sequentially illuminating different sectors of the SLM553,554 —a technique first developed for rapid pulse shaping.555 While holographic patterning of diffraction-limited focal spots in 3D is quite straightforward, development of temporally focused holographic stimulation required creative optical solutions.311,531,542,556–558
6.2. Combining Precision Optogenetics with Psychophysical Measurements
These advances, together with a complementary toolbox of experimental and analysis techniques, have set the stage for neurophotonic probing of the precise relationship between subtle circuit manipulations and the observed behavior [Fig. 30(c)]. Population-wide circuit perturbation often leads to dramatic and easily detectable behavioral effects. In contrast, early attempts to apply precision optogenetics in awake behaving animals have failed to evoke measurable behavioral effects.532 To date, several (non-exclusive) strategies to address this challenge have emerged. The first one is to intensify the manipulation, e.g., by increasing its duration.313–315 The second strategy is to train the animal to detect or discriminate among purely synthetic percepts. In the process of such training, animals become increasingly sensitive to subtle perturbations, reducing their perceptual thresholds by several orders of magnitude. This approach underlies recent studies on the coding logic of olfactory,538,559,560 and visual561 perception. Efforts in this area are often inspired by classical studies that have used subtle electrical stimulation to bias sensory psychophysical curves.562
Due to the above-mentioned neurophotonic advances, complex challenges in determining the behavioral relevance of specific features of neuronal circuit activity can now uniquely be addressed with all-optical circuit interrogation solutions and with unprecedented detail. For example, this toolbox now allows experiments like “leave one out” and cross-excitation of two equivalent populations, providing unprecedented data for computational studies. Likewise, precise manipulation of stimulus timing and inter-neuron synchrony can also be precisely studied. Moreover, changing the parameters of optogenetic actuation by light, including frequency and duration, not only affects the stimulation efficacy, but also provides an important control for possible experimental confounds.538
7. Microscopic Imaging of Blood Flow and Oxygenation
Brain cells, including astrocytes and microglia, partner with neurons to maintain healthy brain activity and metabolism (see Secs. 3.6, 3.7). Another important player in this partnership is blood vessels.563 During neuronal activity, vasoactive molecules and ions released by neurons and glia control the diameter of local microvasculature producing changes in blood flow.564,565 In parallel, active brain cells consume causing an increase in extraction from blood vessels to cerebral tissue. Therefore, measurements of blood flow and oxygenation indirectly reflect neuronal activity.566 In addition to serving as a surrogate for neuronal activity, these measurements are important in their own right, because perfusion of tissue by blood and the partial pressure of ()—in blood vessels and tissue (the interstitial space)—arefundamental physiological parameters in health and disease.
Cerebral blood flow (CBF) can be measured volumetrically with microscopic resolution using OCT that utilizes red blood cell (RBC) motion-induced Doppler shifts (Sec. 7.1). Other optical CBF measurements include laser speckle contrast imaging (LSCI)567–569 and 2P imaging of RBC velocity.407 LSCI provides a rapid widefield qualitative characterization of the motion of light scattering particles.570 2P measurements of RBC velocity require fluorescent labeling of the blood plasma or RBC.407,571
Approaches to microscopic measurements of in the brain utilize different principles and differ in their degree of invasiveness and sensitivity.572,573 Depth-resolved real time measurements of intravascular and interstitial with micron-scale resolution have been enabled by 2P imaging of phosphorescent nanoprobes (Sec. 3.8). This technique termed ‘2P phosphorescence lifetime microscopy’ or 2PLM (Sec. 7.2) also enables estimation of cerebral metabolic rate of by quantifying gradients around penetrating cortical arterioles.574,575
Measurements sensitive to cerebral blood flow, volume and oxygenation are commonly referred to as “hemodynamic.” One of the most popular hemodynamic imaging modalities is functional Magnetic Resonance Imaging (fMRI), a macroscopic noninvasive technology commonly used in humans that leverages paramagnetic properties of deoxyhemoglobin to generate signal. Optical hemodynamic imaging technologies create contrast due to differences in the absorption spectrum of oxy- and deoxyhemoglobin.576 Among them is functional near-infrared imaging (fNIRS),577 an optical counterpart of fMRI, which we cover in the companion paper dedicated to human optical imaging technologies. In animals, optical intrinsic signal imaging (OISI) has been extensively used for mapping of cortical activity.578
Photoacoustics (PA) offers another solution to hemodynamic imaging (Sec. 7.3). This is a hybrid technology that relies on absorption of photon energy to produce other physical phenomena: heat (a change in temperature) and sound (a pressure wave), which is detected by ultrasonic transducers.579 Photoacoustic microscopy (PAM), which required optical focusing, offers quantification of oxygenation in individual RBCs. Photoacoustic tomography (PAT) provides deeper imaging at the price of lower resolution.580
7.1. Imaging Brain Function with Optical Coherence Tomography
Cerebral blood flow (CBF) supports brain function by delivering oxygen and other nutrients, while removing waste. Neuronal activity modulates CBF through a process known as “neurovascular coupling.” Therefore, measurements of CBF and related hemodynamic parameters can reflect brain function. Earlier (Sec. 2.6), we discussed optical coherence tomography (OCT), which uses optical interferometry to detect echoes of light backscattered from different depths in tissue,75 in the context of imaging tissue structure. Over the years, researchers have developed a rich toolbox of functional extensions based on fluctuations of light scattered from red blood cells (RBCs), providing a measurement of brain function. These methods include Doppler,581 angiography,582 decorrelation,583 and capillary transients (RBC passage).584
Compared to 2P microscopy, in general, OCT offers slightly greater penetration depths if performed at the same excitation wavelength, enables imaging through less invasive preparations, and does not require extrinsic contrast agents. Amongst optical methods, OCT has the unique advantage of acquiring with depth priority. This enables measuring simultaneously across cortical depths, revealing subtle differences between cortical laminae585,586 that might otherwise be obscured by physiological noise. This capability has led to OCT angiography studies that pinpoint layer IV as the cortical layer with the largest RBC response to functional activation585,587 (Fig. 31). Second, OCT acquires three-dimensional volumes in seconds or even fractions of a second, enabling comprehensive assessments of microvascular network dynamics. This capability has led to studies showing flow homogenization during functional activation588,589 as well as the retrograde propagation of dilation.590 Third, OCT uses time and confocal gating to reject multiply scattered light and isolate backscattered light from sub-picoliter brain volumes. Such precise control of the light path enables quantitative measurements of capillary speed,588 oxygenation,591 hemoglobin concentration,592 and metabolic rate of oxygen consumption.592 These baseline measurements are “absolute” in the sense that they can be compared between locations or subjects, and followed over time, unlike conventional laser speckle and optical intrinsic signal imaging. Such baseline measurements provide critical context to interpret neuronal activity-associated changes in hemodynamics or metabolism.
Fig. 31.
Angiography and blood flow measurements with OCT. (a) Depth-resolved OCT angiography of the rat somatosensory cortex.587 Location of OCT cross-sections shown on volumetric angiogram [maximal intensity projection (MIP); superficial vessels in orange-yellow, deep vessels in green]. (b), (c) Cross-sectional angiogram (dynamic intensity, (b)) and static intensity (c) images. (d), (e) Localized changes in dynamic red blood cell (dRBC) content extracted from boxes in (b) and (c), respectively. (f) Depth-resolved measurements show the strongest dRBC responses in middle cortical layers.
Emerging trends include 1700 nm OCT for imaging of deep cortical and subcortical regions,83 new contrast mechanisms,593 and algorithms594 that may highlight different aspects of brain function at the level of neuroglial tissue. Proper validation against other gold standards, such as multiphoton microscopy,595 will be critical to provide a sound physiological underpinning for these and other emerging OCT techniques.
7.2. Two-Photon O2 Imaging
High-resolution, real-time quantification of the partial pressure of () in vivo has long been a much-desired objective.596–599 Development of 2P-enhanced oxygen probes (Sec. 3.8)124,125,275,600–603 and 2PLM of in the brain268,269 have provided important insights into the brain oxygen metabolism. Among them are confirmation of the “initial dip” in upon neuronal activation,269,270 evidence for artero-venous diffusional shunts in the brain269 and experimental demonstration of diffusional gradients in around individual RBCs—the so-called Erythrocyte-Associated Transients (EATs).269,604
This technology also enabled estimation of the cerebral metabolic rate of (), a notoriously challenging task,605 based on gradients immediately surrounding diving arterioles (Fig. 32).574,575 This method relies on 2 assumptions. The first assumption is that periarterial tissue gets all its from the arteriole. This is justified by the absence of capillaries within the periarteriolar space,606 the presence of a gradient in tissue around diving arterioles,270,575 and a decrease within arterioles from the vessel center to the vessel wall indicating the leaves through the arteriolar wall. This organization can be approximated by Krogh’s model of diffusion from a cylinder607 where the diving arteriole serves as a single source for the periarterial tissue. With this model, one can estimate from maps of periarterial tissue . Recently, this method was used to evaluate baseline across layer in the mouse cortex.
Fig. 32.
2P imaging of tissue . (a) MIP of a 2P vascular image stack of the top of cerebral cortex. Contrast is due to intravascular fluorescent dye. (b) Zoomed-in measurement plane. (c) Color-coded measurement points overlaid onto the survey phosphorescence image. (c) as a function of distance from the center diving arteriole.
We are only starting to understand microscopic metabolism, and many questions remain. In current practice, measurements are acquired in a “point scan” mode, where the laser beam jumps from point to point. Interleaving of point scan 2PLM with traditional line scan fluorescence measurements would allow near-simultaneous probing of and other parameters, such as neurotransmitter release and vasodilation, needed to address important questions about regulation of delivery to cerebral tissue. Combined with imaging of glucose metabolites (see Sec. 3.6), measurements will expand our understanding of the relative contribution of oxidative and non-oxidative metabolic pathways in the brain across different conditions.
7.3. Photoacoustic Imaging
Photoacoustic (PA) tomography (PAT) is a rapidly developing modality that combines optical excitation with ultrasonic detection and can be used over a range of spatial scales at high speed. PAT can utilize endogenous (label-free) contrasts and exogenous probes to provide anatomical, functional, histologic, metabolic, and molecular imaging.579,608,609
In PAT, non-ionizing laser pulses () are directed to the tissue for excitation. Some of the delivered optical energy is absorbed by the tissue and converted into heat. The heat then induces a pressure rise through thermoelastic expansion. The pressure rise launches an ultrasonic wave, which is referred to as a PA wave. The PA wave is detected by ultrasonic transducers to form an image by a computer. PAT has two primary incarnations: reconstruction-based photoacoustic computed tomography (PACT) and focused-scanning-based photoacoustic microscopy (PAM).579
PAT has found broad applications in neuroscience (Fig. 33). PAM has been used to map cortical vasculature in the mouse brain and quantify oxygen saturation and total concentration of hemoglobin at the single capillary level, based on an endogenous PA contrast—hemoglobin absorption.613 Label-free PACT has imaged detailed mouse brain structures,614 provided a measurement of the whole rat brain resting-state functional connectivity,610 and visualized the epileptic wave propagation across the whole mouse brain during a seizure.611 More recently, PACT has imaged brain activity in adult humans.615 Exogenous PA probes are also being developed to monitor glucose metabolism and detect rapid voltage and responses to external stimulations in live animals.616,617
Fig. 33.
PAT of the brain. (a) In vivo PACT image of a whole rat brain in the coronal plane.610 (b) In vivo high-resolution PACT image of a mouse brain in the coronal plane.611 (c) In vivo superresolution PACT image of a mouse brain, where green shows the conventional PACT signals, and magenta shows the enhanced signals from localized droplets.612 (d) In vivo PAM image of a mouse brain.613 (e) Cross-sectional PACT image of a mouse brain at 2.8 mm depth.614
In the future, we expect that PAT will visualize action potential pulses in the deep brain in rodents with the development of novel indicators operating in the red or near-infrared spectral range. We also expect PAT to be translated to adult human brain imaging, while its current primary barrier is the skull. The adult human skull severely attenuates the excitation light and the emitted PA waves and strongly distorts the PA waveforms, deteriorating image quality. A potential solution is to combine PAT with x-ray computed tomography (CT) or MRI, which can provide accurate skull information to correct for the wavefront distortion. In addition, machine learning methods may play a revolutionary role in this problem due to their high tolerance to skull modeling errors and high computational speed.
8. Hybrid and Multimodal Approaches
In contrast to optical imaging technologies based on detection of photons, hybrid technologies rely on absorption of photon energy to produce other physical phenomena. Above, we discussed photoacoustics (Sec. 7.3) where absorption of photon and heating launches an ultrasonic wave.579 Ultrasound (US) is also being explored to steer the light in tissue618 and in US-assisted optical imaging (Sec. 8.1).619–622 Recently, the photoacoustic process has been used for spatially confined neuronal stimulation.623
Another approach is to combine those technologies that do not interact serving as parallel and complementary information channels extending the spatiotemporal scale, resolution and specificity of each modality alone. For example, implanted “neurophotonic” probes have been developed bearing LEDs and electrodes along the penetrating shanks; LEDs deliver the light required for optogenetics actuation of light-gated ion channels and ion pumps, and electrodes record neuronal activity.624–626 An alternative solution for depth-resolved optogenetic actuation is tapered optical fibers that allow the light to exit at different locations along the tapered fiber end depending on the angle of incidence of the laser beam entering the fiber. Recording electrodes printed along the fiber enable simultaneous electrophysiological recordings (Sec. 8.2). Another multimodal approach is combining optical imaging with electrophysiological recordings using optically transparent electrode arrays627–629 (Sec. 8.3). Integration of photodetectors along the shanks of penetrating neurophotonic probes is also being explored.626 Our final example is simultaneous optical imaging (and optogenetic stimulation) with fMRI630–634 (Sec. 8.4) that can help physiological underpinning of noninvasive imaging signals and human translation.
8.1. Ultrasound-Enabled Deep Fluorescence Imaging
Light scattering in brain tissue is a barrier to studying the living brain beyond superficial depths. Conventional optical focusing methods rely on ballistic photons, which exponentially decrease with depth. Loss of photons to scattering limits the signal that can be achieved at the focal spot. In addition, scattered photons contribute to out-of-focus excitation. Taken together, these two effects decrease the signal to background ratio (SBR) with depth. Ultrasound (US) scatters less than light in tissue and can be used to image beyond superficial depths, but in general has lower specificity compared to what can be achieved with targeted fluorescent probes.
US has been used to assist optical imaging and photostimulation and has also been explored as a guidestar mechanism for optical wavefront shaping (Fig. 34). Optical wavefront shaping works by tailoring the input optical wavefront to counteract scattering as it travels through the tissue. The scattering properties of tissue (ignoring the contributions by blood cells) is static on the timescale of several seconds at depths up to a few millimeters.635 Within this time window, knowledge of the relevant parts of the transmission matrix allows the incident wavefront to be pre-distorted to form a desired pattern inside the tissue. In Sec. 5.8, we discussed the use of fluorescent guidestars in multiphoton microscopy. Non-optical guidestars can greatly extend the penetration depth of optical techniques.
Fig. 34.
Wavefront shaping system design. (a) The wavefront shaping system consists of 5 major steps: Recording, camera data transfer, phase mask computation, phase map transfer, and phase map display and playback. First, the scattered light from a guidestar inside the tissue is captured and collected by the wavefront measurement system. Depending on the type of guidestar, the measurement system may use interferometry to detect the signal from the guidestar (e.g., with an ultrasonic guidestar) or simply measure the bulk signal after filtering (e.g., fluorescence from a particle or cell). The captured data from the camera in the measurement system is then transferred to a computing device such as a PC or FPGA where it is processed to find the desired phase map to send light back to the guidestar. Then, this phase mask is transferred to the SLM and displayed to form the shaped playback wave. (b) An example of a wavefront shaping system used to focus light in brain tissue using time-reversed US-encoded light focusing. The US-tagged light is recorded by the camera and then used to calculate the correct wavefront solution to playback a light focus at the previous location of the ultrasonic focus. (c) The sum of the time required to perform each step of the wavefront sensing and shaping process forms the system latency. Typically, the wavefront measurement time is the most significant portion of the process, but when large pixel number cameras and SLMs are used, the time for data transfer becomes significant as well. Parallel processing on embedded systems such as FPGAs can be used to enhance processing time. Furthermore, SLM technologies such as DMDs can be used to reduce the display time to several tens of microseconds as compared to the much slower, millisecond-order liquid-crystal based SLMs.
The fundamental property that allows the scattering behavior of tissue to be characterized and compensated for is the deterministic nature of elastic scattering.636 In practice, this property is exploited by latching onto a signal from inside the sample provided by a guidestar to find the needed part of the transmission matrix and correctly modulate the incident light. The predominant methods to find this correct input pattern rely on either feedback optimization or direct conjugation of the signal from a guidestar. In addition to ultrasonic637 and fluorescent guidestars,638 other modalities have been explored including kinetic,639,640 magnetic particle,641 and photoacoustic642 guidestars. The performance of these systems is typically benchmarked by the number of independent elements of the transmission matrix that can be controlled and the latency between wavefront recording and playback. The spatial resolution in each case is limited by the nature of the guidestar.
Using wavefront shaping, the penetration depth is no longer limited by scattering (i.e., the transport mean free path) but by the absorption limit. This limit can be several centimeters deep within tissue at wavelengths where absorption is low in the near-infrared regime, and wavefront shaping systems have been demonstrated to successfully focus at depths of up to 2.5 cm in chicken breast and 10 cm in tissue-mimicking phantoms.643
However, there are still significant challenges imposed by the temporal dynamics of light scattering in the tissue. In vivo, blood flow (i.e., moving red blood cells, RBCs) is a major contributing factor to scattering dynamics. Due to RBC flow, the measured transmission matrix elements can become outdated within several milliseconds even at a depth of 1 millimeter and, due to the nonlinear relationship between scattering path length and penetration depth, this decorrelation time decreases below one millisecond at a depth of only 2 millimeters.644 This requires low latency wavefront sensing and shaping systems to maintain the correct wavefront solution. To date, state-of-the-art systems have achieved latencies on the order of a few milliseconds with control of 105 optical modes by leveraging fast waveform modulators such as digital micromirror devices.645 While lower latencies can be achieved with faster modulators such as acousto-optic646 or microelectromechanical light valves,647 these techniques typically only offer control over several hundred optical modes.
Recently, a proof-of-principle for another US-enabled fluorescence imaging method has been demonstrated that is not limited by fast speckle decorrelation.619 Rather, the fluctuating speckle intensity at a point of interest is co-encoded in the emitted fluorescence and US-modulated light. Using the US-modulated transmissions as a decoding key, the fluorescence from the target point at the US focus can be extracted from the overall fluorescence emission. A number of technical challenges associated with this approach still need to be addressed including decreasing the measurement time and enlarging the field of view. In the meanwhile, wavefront shaping remains the most promising approach for overcoming light scattering.
In the future, we expect wavefront shaping technologies to develop along two main axes:
(1) For some applications where the penetration depth is relatively shallow, such as imaging of deep cortical layers, wavefront distortions are more strongly associated with gross tissue heterogeneity and less impacted by blood flow. These distortions can be addressed with adaptive optics solutions such as the ones described for multiphoton microscopy in Sec. 5.8.
(2) To be able to correct for dynamic light scattering induced by moving RBCs, further technological development is needed to improve system latency by leveraging fast wavefront shaping devices like digital micromirror devices (DMD) and parallelization to solve the data transfer and processing bottlenecks. Currently, wavefront shaping techniques have largely been demonstrated in carefully controlled experiments. To move toward broader adoption, it is necessary to develop strategies tackling the temporal dynamics, especially to reach the ultimate depth limits, and to address practical implementation concerns such as how to interface the wavefront shaping systems with the types of configurations commonly used in neuroscience experiments (e.g., freely moving rodents). To achieve these goals, we envision a wavefront shaping device which will integrate the wavefront sensing and shaping elements such as the one demonstrated in Ref. 648, will be critical to address the wavefront measurement bottleneck by parallelizing the measurement and guaranteeing alignment between the measured and shaped wavefronts.
8.2. Tapered Optical Fibers as Implantable Multimodal Neuronal Interfaces
Tapered optical fibers have recently emerged as a new platform for implantable devices to optically interact with deep structures of the mouse brain. These fibers can both deliver or collect light over either large brain volumes or spatially confined subregions all around the conical shape, while generating little to no damage to the brain tissue, comparable to that caused by sharp electrodes used for electrophysiological recordings.649–651
Tapered optical fibers have the unique ability of enabling depth-resolved optogenetics and fiber photometry by exploiting the modal properties of the narrowing waveguide: high and low order modes can exchange energy with the brain tissue at different depths along the implant, resulting in a fully integrated and implantable mode-division multiplexer/demultiplexer (MODEM) for brain microcircuitry [Fig. 35(a)]. For example, if the tapered fiber is implanted in cerebral cortex, one can control which cortical layer is being illuminated by changing the input angle of light entering the fiber [see different colors in Fig. 35(a)]. Due to modal mixing of propagating light in the waveguide, the resolution of this technique is in the order of a few hundreds of micrometers. For obtaining finer spatial resolution of light delivery and/or collection, micro and nanofabrication can be employed to coat the fiber with a reflective metal thin layer followed by etching small windows along the tapered end [Fig. 35(b)].652–654
Fig. 35.
Tapered optical fibers as neuronal interfaces. (a) Example of how modes of different order outcouple at different sections of a TF. Image is in false colors. (b) High resolution patterning allows structuring the non-planar edge of metal-coated tapered optical fibers.
Together with the low invasiveness, this technology has contributed to the dissection of basal ganglia neuronal circuitry in the mouse brain: motor-action-related topographical organization of the striatum was probed by depth-selective optogenetic stimulation,655 while differentiated dopamine transients in dorsal and ventral striatum in reward processing were observed by applying depth-resolved fiber photometry.651 From the perspective of advanced detection of photons generated in deep brain regions, tapered fibers are compatible with time-correlated single photon counting technologies recently employed to probe the biochemical state of neurons,656 and can help in bringing this technology to deeper (subcortical) brain structures.657
In addition to the ability of carrying excitation or fluorescence light, tapered fibers can host extracellular recording electrodes, resulting an interesting paradigm for probing small brain volume with spatially confined light delivery and electrophysiological recordings.658 The main challenge in this respect is the non-planar surface of the narrowing waveguide, which does not allow using standard micro- and nano-fabrication techniques.
The photonic properties of tapered fibers also offer the opportunity to control nanoscale light-matter interactions on the fiber surface659 providing a suitable platform to translate in vivo the many options for enhanced biomolecular interfacing using plasmonic resonances that have been proposed in vitro.660 These include Surface Enhanced Raman Scattering (SERS), refractive index sensing, metal-enhanced fluorescence, and highly localized, ultrafast heat delivery in combination with the modulation of the radiative field through sub-wavelength apertures for light delivery and collection in highly scattering tissue.
Next generation technology will therefore have to focus on the development of methods to integrate complex optical and optoelectronic elements on the edge of the fiber taper, aiming at the realization of multifunctional neuronal interfaces targeting both small and wide brain volumes.
8.3. Transparent Electrode Arrays for Multimodal Brain Mapping
Optical methods for monitoring and stimulating neuronal activity enable investigating large-scale neuronal networks up to subcellular resolution, and across multiple signaling dimensions. Combining optical tools with electrophysiological recordings allows comprehensive investigations of brain circuits in vivo, multi scale, as it leverages the finely tuned spatial resolution of optical imaging with the high temporal resolution and fidelity of electrophysiology. Multimodal studies, however, are not possible with conventional opaque metal microelectrodes, as they block optical access and suffer from photovoltaic artifacts that can contaminate the recordings.661,662
Recent progress in materials and fabrication processes for neuroelectronic interfaces has led to the development of transparent microelectrode arrays for simultaneous, artifact-free imaging and recording in the same brain location. These devices typically consist in planar arrays of transparent microscale electrodes (spatial scales ranging from to ) patterned onto thin, flexible polymeric films (e.g., parylene, polyimide, PET). The majority of the transparent devices proposed thus far are micro-electrocorticography (microECoG) arrays [Fig. 36(a)],661–663 although there are a few examples of laminar electrodes for intracortical recordings.627,666
Fig. 36.
Multimodal measurements and interrogation of brain activity using transparent electrode arrays and neurophotonic technologies. (a) (Top) Design of a flexible, transparent 16-channel microECoG array for multimodal mapping the dynamics of epileptiform discharges in mouse models.663 The array is a grid of square electrodes with spacing (total recording area: 1.55 mm × 1.55 mm, total array footprint 2.75 mm × 2.75 mm. Scale bar: 2 mm). (Bottom) Photographs illustrating the optical transparency of the array in the recording area. Scale bars, left to right: 1 mm, , and . Reproduced from Ref. 663. (b) Simultaneous optogenetics, recording and hemodynamic imaging in Thy-Cr2 mice through graphene arrays.662 (Left) Line-scan imaging of arteriolar dilation induced by optogenetic stimulation under two different electrode sites (marker: FITC-dextran, red arrows show line-scan location). Scale bars: . (Middle) Average vascular diameter change and (right) light-evoked LFPs recorded at the graphene electrode sites. Reproduced from Ref. 662. (c) Maps of cortical responses to co-localized electrical stimulation delivered through graphene electrodes in GCamp6f mice.664 (Top) fluorescence intensity in response to cortical electrical stimulation under (left) a graphene and (right) a platinum array of the same size. (Bottom) Spatiotemporal maps of cortical activation following electrical stimulation at varying intensities delivered from a transparent graphene electrode (marked with the red star). Adapted from Ref. 664. (d) Multimodal analysis of ictal state transitions in acute mouse preparations induced by 4-aminopyridine (4-AP).663 (Top left) Schematics of the relative graphene contact positions and 4-AP application site. (Top right) Propagation of the ictal wavefront at seizure onset mapped with wide-field epifluorescence imaging. (Bottom) fluorescence intensity beneath each electrode and simultaneous microECoG recordings from the graphene array during seizure onset. Adapted from Ref. 663. (e) Closed-loop graphene device for artifact-free optogenetics. (Left) Photographs of the transparent graphene array and (right) the fully assembled battery-powered, closed-loop device integrating the graphene array with an optic fiber for optogenetic stimulation. Adapted from Ref. 665.
The enabling technology behind transparent electrodes are novel nanomaterials that combine high optical transparency for imaging with the electrical conductivity required to record neuronal signals. Graphene—a 2D monoatomic layer of carbon atoms—in particular has emerged as the material of choice for many of these technologies, due to its remarkable combination of transparency, electrical conductivity, biocompatibility, low noise, and resistance to corrosion.667–669
From the initial works demonstrating co-localized imaging with electrophysiology627 and optogenetics661 through graphene microECoG arrays, transparent electrodes have been used for simultaneous LFP recordings, 2P imaging in layers II/III of the mouse cortex, optogenetics, and hemodynamic imaging [Fig. 36(b)],662 for delivering cortical stimulation and concurrently mapping the evoked cortical activation patterns [Fig. 36(c)],664 and for studying the onset and spreading dynamics of epileptiform discharges via simultaneous electrophysiology and wide-field imaging [Fig. 36(d)].663 Graphene electrodes have also been integrated with optic fibers to realize battery-powered devices for closed-loop optogenetics [Fig. 36(e)].665
Despite the exciting results, the main drawbacks of transparent graphene microelectrodes are the high interface impedance—which limits the ability to record single extracellular spiking activity—and the complexity of processing and fabrication. In the attempt to overcome these limitations, a number of alternative materials have been explored for transparent microelectrodes, including carbon nanotubes (CNTs),670 platinum nanoparticle coatings,671 conductive polymers (e.g., PEDOT-PSS628), and gold nanomeshes.629,666
In addition to addressing the impedance, temporal resolution and scalability issues, future work is needed to evaluate the longevity and biocompatibility of graphene-based and other transparent electrode technologies for chronic in vivo implants. Furthermore, innovative approaches combining surgical preparations with custom device configurations are required to conduct multimodal investigations into deeper subcortical regions, which are inaccessible to planar microECoG arrays. Finally, there is a lack of established frameworks for analyzing high-dimensional datasets operating on different spatial and temporal scales, such as those emerging from multimodal optical and electrophysiological mapping of neuronal activity.
8.4. Optical Imaging and Functional Magnetic Resonance Imaging (fMRI)
Blood-oxygen-level-dependent (BOLD) fMRI is a widely implemented, clinically and preclinically accessible, noninvasive neuroimaging tool with whole-brain coverage. Despite these strengths, BOLD-fMRI data have relatively low spatiotemporal resolution (sub-millimeter to millimeter, ), and are insensitive to the cellular origins of activity. This can limit the utility and interpretability of BOLD-fMRI measurements.672 As a complementary neuroimaging toolset, optical imaging methods have high reporter specificity and spatiotemporal resolution (micron to sub-millimeter, tens to hundreds of Hz), but are limited to optically accessible tissue and to applications in animal models due to the introduction of fluorophores which requires invasive manipulation of the nervous system. Together, optical imaging and BOLD-fMRI provide a link between reporter specific measurements of activity and the most widely used method for assessing human brain function as well as a means of crossing disparate spatiotemporal scales.
BOLD-fMRI and optical imaging can be performed concomitantly (Fig. 37), or serially, to interrogate the physiological reporter specific underpinnings of the BOLD-fMRI signal, explore brain-wide circuit- and network-level activity patterns, and neuro-glio-vascular coupling in health and disease. To collect BOLD-fMRI and optical imaging data from the same subject, significant experimental challenges must be overcome to accommodate the confined space and high magnetic field of the MR-scanner without compromising the optical signal. This can be particularly difficult if the multi-modal data are acquired simultaneously which is necessary when studying certain aspects of brain function such as spontaneous activity (without external stimuli).673,674
Fig. 37.
Concurrent optical imaging and fMRI. (a) Optical imaging implementations within the MRI scanner. Schematic of typical wide-field imaging components (i.). Excitation light is delivered (LED light source), passes through a dichroic mirror, and is focused onto the mouse cortex (1X objective). Emitted fluorescence signal is relayed to a camera and recorded. Example imaging frames from a movie recorded from a mouse expressing GCaMP in glia (Aldh1l1-Cre/Ai162) (top right). Data are collected in-scanner. Blooms of increased activity are in hot colors; epochs of decreased activity are in cool colors. Schematic of a typical fiber photometry experiment (ii.). Excitation light delivered and signal recorded through long fiber optic cables. Both (i.) and (ii.) can be performed within an MRI scanner (iii.) where simultaneous BOLD-fMRI data can be recorded. Wide-field imaging yields measures of cortical activity (2D images), while fiber photometry can target subcortical areas (1D time courses). Simultaneously recorded glia- (green) and BOLD (orange) spontaneous activity from a cortical roi are plotted (middle). In this example, these signals are moderately correlated (Pearson’s correlation , P < 0.05). (b) In-scanner wide-field imaging. Components and light path are indicated (image reproduced from Ref. 630). For wide-field imaging, optical access to the cortex is gained by resecting the scalp and covering the skull in dental cement, glue, and glass (right). The wide-field imaging data can be registered to the MRI data using the middle cerebral arteries as anatomical landmarks and an MR angiogram. Example multi-modal measurements of spontaneous fluorescence and BOLD activity. (c) With multi-modal data registered to a common space (i.), ROIs can be imposed (e.g., Allen Atlas regions) and a connectome computed. Multi-modal spontaneous activity can be used to compute a between contrast transfer function (ii.). Here, gamma-variant fitting630 was applied to compute a transfer function using simultaneously recorded spontaneous BOLD activity and excitatory neuronal activity (Slc17a7/Cam/TeTG6f, N = 6, plotted in blue) or glia activity (Aldh1l1-Cre/Ai162, N = 5, plotted in purple). Abbreviations: functional magnetic resonance imaging (fMRI, MRI, MR), light emitting diode (LED), anterior (A), posterior (P), right (R), left (L), dorsal (D), ventral (V), Pearson’s correlation (), region(s) of interest (roi, rois), signal intensity (SI), arbitrary units (au), blood-oxygen-level-dependent (BOLD), seconds (secs), echo planar imaging (EPI), repetition time (TR), field of view (FOV), radio frequency (RF).
To date, there are a handful of implementations of BOLD-fMRI with simultaneous single/double-fiber photometry to yield ‘point’ measurements, or one-dimensional time-courses of optical data and two reports of simultaneous wide-field imaging using fiber-optic bundles to obtain two-dimensional optical images of cortical activity.630,631,634,675–681 Experimentally, each in-scanner optical imaging implementation includes a means of delivering excitation light to, and relaying optical signal from within the bore, reducing MR-susceptibility artifacts caused by surgeries that yield optical access to the brain, minimizing motion, and eliminating or keeping metal components at a safe distance from the MR-scanner. The signal-processing and analyses of these data follow a variety of strategies that depend on the implementation and scientific question.
In the near future, we expect improved in-scanner optical imaging compatibility with state-of-the-art MRI technology (e.g., imaging with an MR-cryo-coil), experimental versatility (e.g., use of novel or multiple optical probes), and data quality (e.g., better signal-to-noise). Along with these advances in data acquisition, more complex applications will be attempted including implementations in awake behaving animals, disease models, and both young and old subjects. Further, the analyses of these multi-modal data will expand and progress through more wide-spread implementation, data sharing and cross-specialty collaboration. Ultimately, BOLD-fMRI and optical imaging methods have the potential to link animal and human studies and to provide unique insight into the functional organizing principles of the brain in health and disease.
9. Computational Imaging
Computational microscopy is a class of methods that synergistically combines optical hardware and computational algorithms for enabling novel capabilities that the optics alone do not support (Fig. 38). The motivation is to overcome the many challenges in neuronal imaging. These methods have been broadly applied to 1P or multiphoton imaging of functional indicators (e.g., imaging and voltage imaging), OCT, and PA imaging.
Fig. 38.
Computational microscopy synergistically combines optical hardware and computational algorithms.
The first type of challenges stem from the physical tradeoffs between several desirable imaging parameters, including SNR, imaging speed, spatial resolution, FOV, depth of field (DOF), and the size and weight of the optical device. To overcome this, new types of computational microscopes have been developed to enable high-speed volumetric imaging, enhanced resolution, and extended DOF. For example, Light field microscopy enabled single-shot in-vivo 3D recording of neuronal activity442,682–687 [Fig. 39(a)]. Similar concepts have enabled miniaturized microscopes, such as MiniLFM,688 Miniscope3D,690 CM2,419 and FlatScope,691 for 3D recording of neuronal activity in freely moving animals [Fig. 39(b)]. Structured illumination microscopy enabled super-resolution reconstruction and background suppression for in vivo brain imaging689,692,693 [Fig. 39(c)].
Fig. 39.
Computational microscopy enables novel imaging capabilities. (a) Light field microscopy enables single-shot high-speed volumetric neuronal imaging.442,683 (b) Miniaturized light field microscope allows volumetric neuronal recording in freely moving mice.688 (c) Structured illumination microscopy achieves super-resolution reconstruction of dendrites expressing ChR2-GFP on the mouse brain in vivo.689
The second type of challenges stem from the complexity of the large-scale multi-dimensional neuronal imaging data. To overcome this, many advanced computational algorithms have been developed to perform various signal extraction tasks, such as segmentation, enhancement of SNR, image deconvolution, and spike estimation. In the past few years, parametric and model-based techniques, such as principal component analysis (PCA), non-negative matrix factorization (NMF), and sparse deconvolution, have shown tremendous success. In recent years, data-driven machine/deep learning-based techniques have become popular and achieved the state-of-the-art performance in various tasks, such as vessel segmentation in deep brain694,695 [Fig. 40(a)], neuron segmentation696,698 [Fig. 40(b)], functional signal denoising697 [Fig. 40(c)], signal extraction,699,700 3D deconvolution,701,702 and super-resolution reconstruction.703
Fig. 40.
Deep learning enables state-of-the-art neuronal signal extraction. (a) Vascular segmentation from 2P imaging data.694 (b) Active neuron segmentation in 2P imaging.696 (c) Denoising 2P imaging data.697
In the future, we can expect that computational microscopy will continue to broadly impact both the system design and signal analysis in neuronal imaging. One promising area is to jointly optimize the optics, acquisition strategy, and computational algorithm in a holistic framework to further push the imaging performance limits. We also anticipate that more advanced deep learning methods beyond the widely used supervised learning methods will be developed, which will incorporate unsupervised learning, semi-supervised learning, physical model-based priors for more data efficient and reliable signal extraction.
10. Conclusions
In this Status Report, we provided a 30,000-foot view of a suite of neurophotonic methods—ranging from molecular sensors to imaging technologies that allow high-speed imaging with large fields of view—that have emerged from the BRAIN Initiative and related large-scale neuroscience efforts. For each domain, we outlined the current state-of-the-art of the respective technologies, identified the areas where innovation is needed, and provided an outlook for future directions. Our goal was not to offer a comprehensive list of all recently developed neurophotonic tools. Further, our choice was often biased toward technologies developed in our own laboratories. We hope, nevertheless, that the overall scope does justice to the breadth of the modern neurophotonics arena spanning across the spatiotemporal scales:704 from the nanometer scale of molecular sensors to the mesoscale of cortical columns and entire brain areas (hundreds of microns); from the nanosecond scale of fluorescent lifetime to slow changes in mood, executive function, and arousal unfolding over minutes and hours.
Most often, the invasive nature of the methods described in this report limits their direct translation to humans. Exceptions exist, including intraoperative brain imaging705–707 and/or other situations where the benefit overweighs the probability of the risk. For example, optogenetic methods are being tested in clinical trials aimed at (partial) restoration of vision.708 Maybe some of the methods presented here will evolve or inspire noninvasive techniques that can be employed in humans one day. But even in cases where the direct translation is prohibited, microscopic imaging in animals has led to better interpretation of noninvasive optical signals achievable in humans. In particular, physiological underpinning of fNIRS (as well as fMRI) signals requires an understanding of the relationship between neuronal network activity, blood flow, and consumption.566,709,710 Neurophotonic methods designed to noninvasively probe deep tissue and apply to humans will be the focus of our companion report.
Each of the technologies highlighted in this report has been conceived by a small group of experts, but not without input from the bigger neurophotonics community. In every case, a dialog with the community and parallel efforts elsewhere have been instrumental in driving innovation, providing a testbed for early developments, and fine-tuning the new technology to serve specific applications. Among the most significant measures of the success for these efforts are ever-expanding collaborative ties between the technology and biology experts and growing global science network across countries and continents.3,711
As impressive and significant as the recent progress has been, we are just getting started. Our next challenge as a community is to sustain the momentum beyond the lifetime of any individual program or initiative. Needless to say, this entails, among others, retaining and nurturing the best of our students. So, if you are a student reading these lines, we would like to conclude by speaking directly to you:
Dear Student, thank you for making it to the end of this report. We appreciate your curiosity and persistence. At the risk of sounding self-congratulatory, we would like to tell you that each of the tools featured here is a story of ingenuity, perseverance, and tireless work. Each one is a personal story of trial and error, triumph and failure, a bold vision baked into a dry and meticulous scientific approach. These are technologies made by people. We are using light to illuminate the future, and it’s never been so bright! Come and join us in this endeavor!
Acknowledgments
This report was edited by Anna Devor and Darcy Peterka. Cover design by Kıvılcım Kılıç. A.D. was supported by the U.S. National Institutes of Health (NIH) grants R01MH111359, R01DA050159, and U19NS123717. A.N. was supported by NIH grants R01NS108034, U19NS112959, and U19NS123719. D.A.B. was supported by NIH grant R01NS108472. M.G.H. is currently the ERANet Chair (NCBio) at i3S Porto funded by the European Commission (H2020-WIDESPREAD-2018-2020-6; NCBio; 951923). R.A.S. is a Wellcome Principal Research Fellow (203048, 224499) and his microscopy development is co-funded by the NIH Brain initiative (U01NS113273). Fi.P., and Fe.P. acknowledge funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 677683. M.D.V. and Fe.P. acknowledge funding from the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 828972. Fi.P., M.D.V., Fe.P, O.Y., V.E., and T.C. acknowledge that this project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 101016787. Fe.P., B.L.S., and M.D.V. were funded by NIH Grant No. 1UF1NS108177-01. O.Y. and V. E. were supported by H2020-RIA (DEEPER 101016787) and the ERC (PrefrontalMap 819496). L.V.W. acknowledges funding support by NIH grants R01 NS102213, U01 NS099717, and U01 EB029823. S.L.S. was supported by NIH grants R01NS091335, R01NS121919 and National Science Foundation (NSF) grant 1934288. R.E.C, and Y.N. were supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grant 19H05633. V.J.S. was supported by NIH grants NS094681, EB029747, and EY031469. S.N.S. acknowledges funding from the NIH Ruth L. Kirschstein National Research Service Award (F31 NS115421). P.R.D. acknowledges funding from the NIH Ruth L. Kirschstein National Research Service Award (F31 NS118949). T.A. and T.L. acknowledge funding from the University of Minnesota Medical School (AIRP) and the National Ataxia Foundation. F.V. was supported by NIH grants R01NS117756 and R01NS121219. U.H. was supported by NIH Brain Initiative grants R01NS120832, U01NS099709, and NSF NeuroNex Technology Hub 1707352. G.Y. was supported by NIH grants R01 GM124038 and R01 NS102586. L. T. was funded by NIH grant R21EY030016. I.C. was supported by European Union’s Horizon 2020 Research and Innovation Framework Program under Grant Agreement No. 654148 (Laserlab-Europe); European Union’s Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2) and No. 945539 (Human Brain Project SGA3); General Hospital Corporation Center of the NIH under Award No. U01 MH117023; Italian Ministry for Education in the framework of Euro-Bioimaging Italian Node (ESFRI research infrastructure); “Fondazione CR Firenze” (private foundation). T. Č., H. U., and P. O. were supported by the European Union’s H2020-RIA (DEEPER, Grant Agreement No. 101016787), European Research Council (724530), and MEYS (CZ.02.1.01/ 0.0/ 0.0/ 15_003/0000476). S.S. was supported by NIH grants U19NS107464, R01NS109885 and UF1NS107680. V.E and R.S were supported by the European Research Council (ERC-2019-AdG 885090, HOLOVIS). N.J. was supported by NIH grant U01NS118300. A.V. was supported by the National Institute of Neurological Disorders and Stroke of the NIH under Award Nos. 5U01NS103488, 1RF1NS113251, and 1RF1NS110501, and the Kavli Foundation. D. S. P. was supported by NIH grants 5U19NS104649, 5U01NS113273, 9R44MH117430. Y. Z. was supported by NIH Director’s New Innovator Award DP2 OD025926-01 and the Kaufman Foundation. A. S. A holds a Career Award at the Scientific Interface from Burroughs Wellcome Fund and acknowledges funding from the Searle Scholar Program and NIH New innovator award 1DP2MH129956. E. M. R. L. was supported by NIH grants R01MH111424 and U01NS094358. E. W. M. acknowledges support from NIH (R01NS098088) and NSF (NeuroNex 1707350).
Biography
Biographies of the authors are not available.
Disclosures
M.D.V., B.L.S., Fe.P. are founders and hold private equity in Optogenix, a company that develops, produces, and sells technologies to deliver light into the brain. L.V.W. has a financial interest in Microphotoacoustics, Inc., CalPACT, LLC, and Union Photoacoustic Technologies, Ltd., which, however, did not support this work. T.C. is the mentor of a start-up endeavor DeepEn, which develops commercial holographic endoscopes. N.J. and Howard Hughes Medical Institute have filed patent applications that relate to adaptive optics. T.F.A. and K.M.N.S.N. have a financial interest in Agile Diffraction Ltd., which aims to commercialize the Acousto-Optic Lens (AOL) technology. D.S.P and Columbia University have patents related to multiphoton photostimulation. E.M.C.H. and Columbia University hold intellectual property rights on SCAPE, some of which are licensed to Leica Microsystems.
References
- 1.Insel T. R., Landis S. C., Collins F. S., “Research priorities. The NIH BRAIN initiative,” Science 340(6133), 687–688 (2013). 10.1126/science.1239276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devor A., et al. , “The challenge of connecting the dots in the B.R.A.I.N,” Neuron 80(2), 270–274 (2013). 10.1016/j.neuron.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grillner S., et al. , “Worldwide initiatives to advance brain research,” Nat. Neurosci. 19(9), 1118–1122 (2016). 10.1038/nn.4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez E. A., et al. , “The growing and glowing toolbox of fluorescent and photoactive proteins,” Trends Biochem. Sci. 42(2), 111–129 (2017). 10.1016/j.tibs.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung K., et al. , “Structural and molecular interrogation of intact biological systems,” Nature 497(7449), 332–337 (2013). 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B. C., et al. , “Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution,” Science 346(6208), 1257998 (2014). 10.1126/science.1257998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman E. M. C., et al. , “Light-sheet microscopy in neuroscience,” Annu. Rev. Neurosci. 42, 295–313 (2019). 10.1146/annurev-neuro-070918-050357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hell S. W., “Microscopy and its focal switch,” Nat. Methods 6(1), 24–32 (2009). 10.1038/nmeth.1291 [DOI] [PubMed] [Google Scholar]
- 9.Huang B., Bates M., Zhuang X. W., “Super-resolution fluorescence microscopy,” Annu. Rev. Biochem. 78, 993–1016 (2009). 10.1146/annurev.biochem.77.061906.092014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillberg P. W., et al. , “Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies,” Nat. Biotechnol. 34(9), 987–992 (2016). 10.1038/nbt.3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costantini I., et al. , “Autofluorescence enhancement for label-free imaging of myelinated fibers in mammalian brains,” Sci. Rep. 11, 8038 (2021). 10.1038/s41598-021-86092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascaro A. L. A., et al. , “Label-free near-infrared reflectance microscopy as a complimentary tool for two-photon fluorescence brain imaging,” Biomed. Opt. Express 6(11), 4483–4492 (2015). 10.1364/BOE.6.004483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min E., et al. , “Serial optical coherence microscopy for label-free volumetric histopathology,” Sci. Rep. 10, 6711 (2020). 10.1038/s41598-020-63460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schain A. J., Hill R. A., Grutzendler J., “Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy,” Nat. Med. 20(4), 443–449 (2014). 10.1038/nm.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Zhu J. F., Akkin T., “Serial optical coherence scanner for large-scale brain imaging at microscopic resolution,” Neuroimage 84, 1007–1017 (2014). 10.1016/j.neuroimage.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leahy C., Radhakrishnan H., Srinivasan V. J., “Volumetric imaging and quantification of cytoarchitecture and myeloarchitecture with intrinsic scattering contrast,” Biomed. Opt. Express 4(10), 1978–1990 (2013). 10.1364/BOE.4.001978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsien R. Y., “The green fluorescent protein,” Annu. Rev. Biochem. 67, 509–544 (1998). 10.1146/annurev.biochem.67.1.509 [DOI] [PubMed] [Google Scholar]
- 18.Lambert G. G., et al. , “Aequorea’s secrets revealed: new fluorescent proteins with unique properties for bioimaging and biosensing,” PLoS Biol. 18(11), e3000936 (2020). 10.1371/journal.pbio.3000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaner N. C., et al. , “Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein,” Nat. Biotechnol. 22(12), 1567–1572 (2004). 10.1038/nbt1037 [DOI] [PubMed] [Google Scholar]
- 20.Hoi H., et al. , “An engineered monomeric Zoanthus sp yellow fluorescent protein,” Chem. Biol. 20(10), 1296–1304 (2013). 10.1016/j.chembiol.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 21.Shen Y., Lai T., Campbell R. E., “Red fluorescent proteins (RFPs) and RFP-based biosensors for neuronal imaging applications,” Neurophotonics 2(3), 031203 (2015). 10.1117/1.NPh.2.3.031203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shcherbakova D. M., et al. , “Near-infrared fluorescent proteins: multiplexing and optogenetics across scales,” Trends Biotechnol. 36(12), 1230–1243 (2018). 10.1016/j.tibtech.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bindels D. S., et al. , “mScarlet: a bright monomeric red fluorescent protein for cellular imaging,” Nat. Methods 14(1), 53–56 (2017). 10.1038/nmeth.4074 [DOI] [PubMed] [Google Scholar]
- 24.Eason M. G., Damry A. M., Chica R. A., “Structure-guided rational design of red fluorescent proteins: towards designer genetically-encoded fluorophores,” Curr. Opin. Struct. Biol. 45, 91–99 (2017). 10.1016/j.sbi.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 25.Grimm J. B., et al. , “A general method to improve fluorophores for live-cell and single-molecule microscopy,” Nat. Methods 12(3), 244–250 (2015). 10.1038/nmeth.3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou J., et al. , “De novo design of a fluorescence-activating beta-barrel,” Nature 561(7724), 485–491 (2018). 10.1038/s41586-018-0509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh H. W., et al. , “Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging,” Nat. Methods 14(10), 971–974 (2017). 10.1038/nmeth.4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwano S., et al. , “Single-cell bioluminescence imaging of deep tissue in freely moving animals,” Science 359(6378), 935–939 (2018). 10.1126/science.aaq1067 [DOI] [PubMed] [Google Scholar]
- 29.Helmstaedter M., et al. , “Reconstruction of an average cortical column in silico,” Brain Res. Rev. 55(2), 193–203 (2007). 10.1016/j.brainresrev.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 30.Erturk A., et al. , “Three-dimensional imaging of solvent-cleared organs using 3DISCO,” Nat. Protoc. 7(11), 1983–1995 (2012). 10.1038/nprot.2012.119 [DOI] [PubMed] [Google Scholar]
- 31.Costantini I., et al. , “In-vivo and ex-vivo optical clearing methods for biological tissues: review,” Biomed. Opt. Express 10(10), 5251–5267 (2019). 10.1364/BOE.10.005251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku T., et al. , “Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues,” Nat. Biotechnol. 34(9), 973–981 (2016). 10.1038/nbt.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park Y. G., et al. , “Protection of tissue physicochemical properties using polyfunctional crosslinkers,” Nat. Biotechnol. 37(1), 73–83 (2019). 10.1038/nbt.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray E., et al. , “Simple, scalable proteomic imaging for high-dimensional profiling of intact systems,” Cell 163(6), 1500–1514 (2015). 10.1016/j.cell.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choquet D., Sainlos M., Sibarita J. B., “Advanced imaging and labelling methods to decipher brain cell organization and function,” Nat. Rev. Neurosci. 22(4), 237–255 (2021). 10.1038/s41583-021-00441-z [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer T., et al. , “Chronic 2P-STED imaging reveals high turnover of dendritic spines in the hippocampus in vivo,” Elife 7, e34700 (2018). 10.7554/eLife.34700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inavalli V., et al. , “A super-resolution platform for correlative live single-molecule imaging and STED microscopy,” Nat. Methods 16(12), 1263–1268 (2019). 10.1038/s41592-019-0611-8 [DOI] [PubMed] [Google Scholar]
- 38.Tonnesen J., Inavalli V., Nagerl U. V., “Super-resolution imaging of the extracellular space in living brain tissue,” Cell 172(5), 1108–1121.e15 (2018). 10.1016/j.cell.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 39.Hell S. W., “Far-field optical nanoscopy,” Science 316(5828), 1153–1158 (2007). 10.1126/science.1137395 [DOI] [PubMed] [Google Scholar]
- 40.Furstenberg A., Heilemann M., “Single-molecule localization microscopy-near-molecular spatial resolution in light microscopy with photoswitchable fluorophores,” Phys. Chem. Chem. Phys. 15(36), 14919–14930 (2013). 10.1039/c3cp52289j [DOI] [PubMed] [Google Scholar]
- 41.Xu K., Zhong G., Zhuang X., “Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons,” Science 339(6118), 452–456 (2013). 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang A. H., et al. , “A trans-synaptic nanocolumn aligns neurotransmitter release to receptors,” Nature 536(7615), 210–214 (2016). 10.1038/nature19058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrabetova S., et al. , “Unveiling the extracellular space of the brain: from super-resolved microstructure to in vivo function,” J. Neurosci. 38(44), 9355–9363 (2018). 10.1523/JNEUROSCI.1664-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F., Tillberg P. W., Boyden E. S., “Expansion microscopy,” Science 347(6221), 543–548 (2015). 10.1126/science.1260088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klimas A., Zhao Y., “Expansion microscopy: toward nanoscale imaging of a diverse range of biomolecules,” ACS Nano 14(7), 7689–7695 (2020). 10.1021/acsnano.0c04374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wassie A. T., Zhao Y., Boyden E. S., “Expansion microscopy: principles and uses in biological research,” Nat. Methods 16(1), 33–41 (2019). 10.1038/s41592-018-0219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallagher B. R., Zhao Y., “Expansion microscopy: a powerful nanoscale imaging tool for neuroscientists,” Neurobiol. Dis. 154, 105362 (2021). 10.1016/j.nbd.2021.105362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y. X., et al. , “Nanoscale imaging of clinical specimens using pathology-optimized expansion microscopy,” Nat. Biotechnol. 35(8), 757–764 (2017). 10.1038/nbt.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucur O., et al. , “Nanoscale imaging of clinical specimens using conventional and rapid-expansion pathology,” Nat. Protoc. 15(5), 1649–1672 (2020). 10.1038/s41596-020-0300-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen F., et al. , “Nanoscale imaging of RNA with expansion microscopy,” Nat. Methods 13(8), 679–684 (2016). 10.1038/nmeth.3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chozinski T. J., et al. , “Expansion microscopy with conventional antibodies and fluorescent proteins,” Nat. Methods 13(6), 485–488 (2016). 10.1038/nmeth.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truckenbrodt S., et al. , “X10 expansion microscopy enables 25-nm resolution on conventional microscopes,” Embo Rep. 19(9), e45836 (2018). 10.15252/embr.201845836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang J. B., et al. , “Iterative expansion microscopy,” Nat. Methods 14(6), 593–599 (2017). 10.1038/nmeth.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang N., et al. , “Superresolution imaging of Drosophila tissues using expansion microscopy,” Mol. Biol. Cell 29(12), 1413–1421 (2018). 10.1091/mbc.E17-10-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosca T. J., et al. , “Presynaptic LRP4 promotes synapse number and function of excitatory CNS neurons,” Elife 6, e27347 (2017). 10.7554/eLife.27347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu C. C., et al. , “Expansion microscopy of C. elegans,” Elife 9, e46249 (2020). 10.7554/eLife.46249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang I. E., et al. , “Hedgehog signaling regulates gene expression in planarian glia,” Elife 5, e16996 (2016). 10.7554/eLife.16996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freifeld L., et al. , “Expansion microscopy of zebrafish for neuroscience and developmental biology studies,” Proc. Natl. Acad. Sci. U. S. A. 114(50), E10799–E10808 (2017). 10.1073/pnas.1706281114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao R. X., et al. , “Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution,” Science 363(6424), 245 (2019). 10.1126/science.aau8302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crittenden J. R., Graybiel A. M., “Disease-associated changes in the striosome and matrix compartments of the dorsal striatum,” HBK Behav. Neurosci. 24, 783–802 (2016). 10.1016/B978-0-12-802206-1.00039-8 [DOI] [Google Scholar]
- 61.Corner A. L., et al. , “Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction,” PLoS Biol. 18(1), e3000604 (2020). 10.1371/journal.pbio.3000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortega J. A., et al. , “Nucleocytoplasmic proteomic analysis uncovers eRF1 and nonsense-mediated decay as modifiers of ALS/FTD C9orf72 toxicity,” Neuron 106(1), 90–107.e13 (2020). 10.1016/j.neuron.2020.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crittenden J. R., et al. , “Striosome-dendron bouquets highlight a unique striatonigral circuit targeting dopamine-containing neurons,” Proc. Natl. Acad. Sci. U. S. A. 113(40), 11318–11323 (2016). 10.1073/pnas.1613337113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pesce L., et al. , “Exploring the human cerebral cortex using confocal microscopy,” Prog. Biophys. Mol. Biol. 168, 3–9 (2022). 10.1016/j.pbiomolbio.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchetti M., et al. , “Custom multiphoton/raman microscopy setup for imaging and characterization of biological samples,” Methods Protoc. 2(2), 51 (2019). 10.3390/mps2020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cicchi R., et al. , “From molecular structure to tissue architecture: collagen organization probed by SHG microscopy,” J. Biophotonics 6(2), 129–142 (2013). 10.1002/jbio.201200092 [DOI] [PubMed] [Google Scholar]
- 67.Cox G., “Biological applications of second harmonic imaging,” Biophys. Rev. 3(3), 131–141 (2011). 10.1007/s12551-011-0052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weigelin B., Bakker G. J., Friedl P., “Third harmonic generation microscopy of cells and tissue organization,” J.Cell Sci. 129(2), 245–255 (2016). 10.1242/jcs.152272 [DOI] [PubMed] [Google Scholar]
- 69.Axer M., et al. , “High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging,” Front. Neuroinf. 5, 34 (2011). 10.3389/fninf.2011.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stacho M., et al. , “A cortex-like canonical circuit in the avian forebrain,” Science 369(6511), eabc5534 (2020). 10.1126/science.abc5534 [DOI] [PubMed] [Google Scholar]
- 71.Shaik T. A.-O., et al. , “Monitoring changes in biochemical and biomechanical properties of collagenous tissues using label-free and nondestructive optical imaging techniques,” Anal. Chem. 93(8), 3813–3821 (2021). 10.1021/acs.analchem.0c04306 [DOI] [PubMed] [Google Scholar]
- 72.Wei M., et al. , “Volumetric chemical imaging by clearing-enhanced stimulated Raman scattering microscopy,” Proc. Natl. Acad. Sci. U. S. A. 116(14), 6608 (2019). 10.1073/pnas.1813044116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silvestri L., et al. , “Universal autofocus for quantitative volumetric microscopy of whole mouse brains,” Nat. Methods 18(8), 953–958 (2021). 10.1038/s41592-021-01208-1 [DOI] [PubMed] [Google Scholar]
- 74.Costantini I., et al. , “Large-scale, cell-resolution volumetric mapping allows layer-specific investigation of human brain cytoarchitecture,” Biomed. Opt. Express 12(6), 3684–3699 (2021). 10.1364/BOE.415555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang D., et al. , “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kut C., et al. , “Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography,” Sci. Transl. Med. 7(292), 292ra100 (2015). 10.1126/scitranslmed.3010611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srinivasan V. J., et al. , “Optical coherence microscopy for deep tissue imaging of the cerebral cortex with intrinsic contrast,” Opt. Express 20(3), 2220–2239 (2012). 10.1364/OE.20.002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ben Arous J., et al. , “Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy,” J. Biomed. Opt. 16(11), 116012 (2011). 10.1117/1.3650770 [DOI] [PubMed] [Google Scholar]
- 79.Marchand P. J., et al. , “Visible spectrum extended-focus optical coherence microscopy for label-free sub-cellular tomography,” Biomed. Opt. Express 8(7), 3343–3359 (2017). 10.1364/BOE.8.003343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srinivasan V. J., et al. , “Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke,” PLOS ONE 8(8), e71478 (2013). 10.1371/journal.pone.0071478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li F., et al. , “Nondestructive evaluation of progressive neuronal changes in organotypic rat hippocampal slice cultures using ultrahigh-resolution optical coherence microscopy,” Neurophotonics 1(2), 025002 (2014). 10.1117/1.NPh.1.2.025002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolmont T., et al. , “Label-free imaging of cerebral β-amyloidosis with extended-focus optical coherence microscopy,” J. Neurosci. 32(42), 14548–14556 (2012). 10.1523/JNEUROSCI.0925-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J., et al. , “1700 nm optical coherence microscopy enables minimally invasive, label-free, in vivo optical biopsy deep in the mouse brain,” Light Sci. Appl. 10(1), 145 (2021). 10.1038/s41377-021-00586-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Assayag O., et al. , “Imaging of non-tumorous and tumorous human brain tissues with full-field optical coherence tomography,” NeuroImage: Clin. 2, 549–557 (2013). 10.1016/j.nicl.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bizheva K., et al. , “Imaging ex vivo healthy and pathological human brain tissue with ultra-high-resolution optical coherence tomography,” J. Biomed. Opt. 10(1), 011006 (2007). 10.1117/1.1851513 [DOI] [PubMed] [Google Scholar]
- 86.Magnain C., et al. , “Blockface histology with optical coherence tomography: a comparison with Nissl staining,” Neuroimage 84, 524–533 (2014). 10.1016/j.neuroimage.2013.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H., et al. , “Reconstructing micrometer-scale fiber pathways in the brain: multi-contrast optical coherence tomography based tractography,” Neuroimage 58(4), 984–992 (2011). 10.1016/j.neuroimage.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H., et al. , “Cross-validation of serial optical coherence scanning and diffusion tensor imaging: a study on neural fiber maps in human medulla oblongata,” Neuroimage 100, 395–404 (2014). 10.1016/j.neuroimage.2014.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lefebvre J., et al. , “Whole mouse brain imaging using optical coherence tomography: reconstruction, normalization, segmentation, and comparison with diffusion MRI,” Neurophotonics 4(4), 041501 (2017). 10.1117/1.NPh.4.4.041501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castonguay A., et al. , “Comparing three-dimensional serial optical coherence tomography histology to MRI imaging in the entire mouse brain,” J. Biomed. Opt. 23(1), 016008 (2018). 10.1117/1.JBO.23.1.016008 [DOI] [PubMed] [Google Scholar]
- 91.Liu C. J., et al. , “Visualizing and mapping the cerebellum with serial optical coherence scanner,” Neurophotonics 4(1), 011006 (2017). 10.1117/1.NPh.4.1.011006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li T. Q., Liu C. J., Akkin T., “Contrast-enhanced serial optical coherence scanner with deep learning network reveals vasculature and white matter organization of mouse brain,” Neurophotonics 6(3), 035004 (2019). 10.1117/1.NPh.6.3.035004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu C. J., et al. , “Polarization-sensitive optical coherence tomography reveals gray matter and white matter atrophy in SCA1 mouse models,” Neurobiol. Dis. 116, 69–77 (2018). 10.1016/j.nbd.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 94.Inoue M., “Genetically encoded calcium indicators to probe complex brain circuit dynamics in vivo,” Neurosci. Res. 169, 2–8 (2021). 10.1016/j.neures.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 95.Barson D., et al. , “Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits,” Nat. Methods 17(1), 107–113 (2020). 10.1038/s41592-019-0625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lohr C., et al. , “Using genetically encoded calcium indicators to study astrocyte physiology: a field guide,” Front. Cell Neurosci. 15, 690147 (2021). 10.3389/fncel.2021.690147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen L. B., Salzberg B. M., Grinvald A., “Optical methods for monitoring neuron activity,” Annu. Rev. Neurosci. 1, 171–182 (1978). 10.1146/annurev.ne.01.030178.001131 [DOI] [PubMed] [Google Scholar]
- 98.Abdelfattah A. S., et al. , “Bright and photostable chemigenetic indicators for extended in vivo voltage imaging,” Science 365(6454), 699–704 (2019). 10.1126/science.aav6416 [DOI] [PubMed] [Google Scholar]
- 99.Piatkevich K. D., et al. , “Population imaging of neural activity in awake behaving mice,” Nature 574(7778), 413–417 (2019). 10.1038/s41586-019-1641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villette V., et al. , “Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice,” Cell 179(7), 1590–1608.e23 (2019). 10.1016/j.cell.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kannan M., et al. , “Fast, in vivo voltage imaging using a red fluorescent indicator,” Nat. Methods 15(12), 1108–1116 (2018). 10.1038/s41592-018-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kulkarni R. U., et al. , “In vivo two-photon voltage imaging with sulfonated rhodamine dyes,” ACS Cent. Sci. 4(10), 1371–1378 (2018). 10.1021/acscentsci.8b00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pal A., Tian L., “Imaging voltage and brain chemistry with genetically encoded sensors and modulators,” Curr. Opin. Chem. Biol. 57, 166–176 (2020). 10.1016/j.cbpa.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 104.Kulkarni R. U., Miller E. W., “Voltage imaging: pitfalls and potential,” Biochemistry 56(39), 5171–5177 (2017). 10.1021/acs.biochem.7b00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andreoni A., Davis C. M. O., Tian L., “Measuring brain chemistry using genetically encoded fluorescent sensors,” Curr. Opin. Biomed. Eng. 12, 59–67 (2019). 10.1016/j.cobme.2019.09.008 [DOI] [Google Scholar]
- 106.Lee S. J., et al. , “Cell-type-specific asynchronous modulation of PKA by dopamine in learning,” Nature 590(7846), 451–456 (2021). 10.1038/s41586-020-03050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oe Y., et al. , “Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance,” Nat. Commun. 11, 471 (2020). 10.1038/s41467-020-14378-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Augustine V., et al. , “Temporally and spatially distinct thirst satiation signals,” Neuron 103(2), 242–249.e4 (2019). 10.1016/j.neuron.2019.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohebi A., et al. , “Dissociable dopamine dynamics for learning and motivation,” Nature 570(7759), 65–70 (2019). 10.1038/s41586-019-1235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Jong J. W., et al. , “A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system,” Neuron 101(1), 133–151.e7 (2019). 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson J. E., et al. , “Optical dopamine monitoring with dLight1 reveals mesolimbic phenotypes in a mouse model of neurofibromatosis type 1,” Elife 8, e48983 (2019). 10.7554/eLife.48983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong H., et al. , “Dorsal striatum dopamine levels fluctuate across the sleep-wake cycle and respond to salient stimuli in mice,” Front. Neurosci. 13, 242 (2019). 10.3389/fnins.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dai B., et al. , “Dopamine release in nucleus accumbens core during social behaviors in mice,” 10.1101/2021.06.22.449478 (2021). [DOI] [PMC free article] [PubMed]
- 114.Gallo E. F., et al. , “Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning,” Mol. Physchiatry (2021). 10.1038/s41380-021-01364-y [DOI] [PMC free article] [PubMed]
- 115.Kjaerby C., et al. , “Dynamic fluctuations of the locus coeruleus-norepinephrine system underlie sleep state transitions,” 10.1101/2020.09.01.274977 (2020). [DOI]
- 116.Sturgill J., et al. , “Basal forebrain-derived acetylcholine encodes valence-free reinforcement prediction error,” 10.1101/2020.02.17.953141 (2020). [DOI]
- 117.Lohani S., et al. , “Dual color mesoscopic imaging reveals spatiotemporally heterogeneous coordination of cholinergic and neocortical activity,” 10.1101/2020.12.09.418632 (2020). [DOI] [PMC free article] [PubMed]
- 118.Iadecola C., “The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease,” Neuron 96(1), 17–42 (2017). 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eroglu C., Barres B. A., “Regulation of synaptic connectivity by glia,” Nature 468(7321), 223–231 (2010). 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koveal D., Diaz-Garcia C. M., Yellen G., “Fluorescent biosensors for neuronal metabolism and the challenges of quantitation,” Curr. Opin. Neurobiol. 63, 111–121 (2020). 10.1016/j.conb.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barros L. F., et al. , “Current technical approaches to brain energy metabolism,” Glia 66(6), 1138–1159 (2018). 10.1002/glia.23248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu X., Nagai J., Khakh B. S., “Improved tools to study astrocytes,” Nat. Rev. Neurosci. 21(3), 121–138 (2020). 10.1038/s41583-020-0264-8 [DOI] [PubMed] [Google Scholar]
- 123.Wilson D. F., et al. , “Measuring oxygen in living tissue: intravascular, interstitial, and “tissue” oxygen measurements,” Adv. Exp. Med. Biol. 701, 53–59 (2011). 10.1007/978-1-4419-7756-4_8 [DOI] [PubMed] [Google Scholar]
- 124.Finikova O. S., et al. , “Oxygen microscopy by two-photon-excited phosphorescence,” Chemphyschem 9(12), 1673–1679 (2008). 10.1002/cphc.200800296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Esipova T. V., et al. , “Oxyphor 2P: a high-performance probe for deep-tissue longitudinal oxygen imaging,” Cell Metab. 29(3), 736–744.e7 (2019). 10.1016/j.cmet.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tung J. K., et al. , “Bioluminescence imaging in live cells and animals,” Neurophotonics 3(2), 025001 (2016). 10.1117/1.NPh.3.2.025001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park S. Y., et al. , “Novel luciferase-opsin combinations for improved luminopsins,” J. Neurosci. Res. 98(3), 410–421 (2020). 10.1002/jnr.24152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crespo E. L., et al. , “Bioluminescent optogenetics 2.0: harnessing bioluminescence to activate photosensory proteins in vitro and in vivo,” J. Vis. Exp. 174, e62850 (2021). 10.3791/62850 [DOI] [PubMed] [Google Scholar]
- 129.Medendorp W. E., et al. , “Selective postnatal excitation of neocortical pyramidal neurons results in distinctive behavioral and circuit deficits in adulthood,” Iscience 24(3), 102157 (2021). 10.1016/j.isci.2021.102157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deisseroth K., “Optogenetics: 10 years of microbial opsins in neuroscience,” Nat. Neurosci. 18(9), 1213–1225 (2015). 10.1038/nn.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boyden E. S., et al. , “Millisecond-timescale, genetically targeted optical control of neural activity,” Nat. Neurosci. 8(9), 1263–1268 (2005). 10.1038/nn1525 [DOI] [PubMed] [Google Scholar]
- 132.Berridge M. J., Bootman M. D., Roderick H. L., “Calcium signalling: dynamics, homeostasis and remodelling,” Nat. Rev. Mol. Cell. Biol. 4(7), 517–529 (2003). 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 133.Larkum M. E., et al. , “Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons,” J. Physiol. 549(2), 471–488 (2003). 10.1113/jphysiol.2002.037614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ross W. N., et al. , “Synaptically activated ca2+ release from internal stores in CNS neurons,” Cell Mol. Neurobiol. 25(2), 283–295 (2005). 10.1007/s10571-005-3060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miller R. J., “Multiple calcium channels and neuronal function,” Science 235(4784), 46–52 (1987). 10.1126/science.2432656 [DOI] [PubMed] [Google Scholar]
- 136.Bezanilla F., “Voltage-gated ion channels,” IEEE Trans. Nanobiosci. 4(1), 34–48 (2005). 10.1109/TNB.2004.842463 [DOI] [PubMed] [Google Scholar]
- 137.Felix R., “Molecular regulation of voltage-gated Ca2+ channels,” J. Recept. Signal Transduct. Res. 25(2), 57–71 (2005). 10.1081/RRS-200068102 [DOI] [PubMed] [Google Scholar]
- 138.Grynkiewicz G., Poenie M., Tsien R. Y., “A new generation of Ca-2+ indicators with greatly improved fluorescence properties,” J. Biol. Chem. 260(6), 3440–3450 (1985). 10.1016/S0021-9258(19)83641-4 [DOI] [PubMed] [Google Scholar]
- 139.Tsien R. Y., “A non-disruptive technique for loading calcium buffers and indicators into cells,” Nature 290(5806), 527–528 (1981). 10.1038/290527a0 [DOI] [PubMed] [Google Scholar]
- 140.Levram V., Grinvald A., “Activity-dependent calcium transients in central-nervous-system myelinated axons revealed by the calcium indicator fura-2,” Biophys. J. 52(4), 571–576 (1987). 10.1016/S0006-3495(87)83246-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuste R., Katz L. C., “Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters,” Neuron 6(3), 333–344 (1991). 10.1016/0896-6273(91)90243-S [DOI] [PubMed] [Google Scholar]
- 142.Garaschuk O., Milos R. I., Konnerth A., “Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo,” Nat. Protoc. 1(1), 380–386 (2006). 10.1038/nprot.2006.58 [DOI] [PubMed] [Google Scholar]
- 143.Garaschuk O., et al. , “Optical monitoring of brain function in vivo: from neurons to networks,” Pflugers Arch. 453(3), 385–396 (2006). 10.1007/s00424-006-0150-x [DOI] [PubMed] [Google Scholar]
- 144.Kerr J. N., et al. , “Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex,” J. Neurosci. 27(48), 13316–13328 (2007). 10.1523/JNEUROSCI.2210-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kerr J. N., Greenberg D., Helmchen F., “Imaging input and output of neocortical networks in vivo,” Proc. Natl. Acad. Sci. U. S. A. 102(39), 14063–14068 (2005). 10.1073/pnas.0506029102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ohki K., et al. , “Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex,” Nature 433(7026), 597–603 (2005). 10.1038/nature03274 [DOI] [PubMed] [Google Scholar]
- 147.Sato T. R., et al. , “The functional microarchitecture of the mouse barrel cortex,” PLoS Biol. 5(7), e189 (2007). 10.1371/journal.pbio.0050189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sullivan M. R., et al. , “In vivo calcium imaging of circuit activity in cerebellar cortex,” J. Neurophysiol. 94(2), 1636–1644 (2005). 10.1152/jn.01013.2004 [DOI] [PubMed] [Google Scholar]
- 149.Wilson J. M., et al. , “Two-photon calcium imaging of network activity in XFP-expressing neurons in the mouse,” J. Neurophysiol. 97(4), 3118–3125 (2007). 10.1152/jn.01207.2006 [DOI] [PubMed] [Google Scholar]
- 150.Chaigneau E., et al. , “Two-photon imaging of capillary blood flow in olfactory bulb glomeruli,” Proc. Natl. Acad. Sci. U. S. A. 100(22), 13081–13086 (2003). 10.1073/pnas.2133652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chaigneau E., et al. , “The relationship between blood flow and neuronal activity in the rodent olfactory bulb,” J. Neurosci. 27(24), 6452–6460 (2007). 10.1523/JNEUROSCI.3141-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nimmerjahn A., et al. , “Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo,” Nat. Methods 1(1), 31–37 (2004). 10.1038/nmeth706 [DOI] [PubMed] [Google Scholar]
- 153.Wang X., et al. , “Astrocytic Ca(2+) signaling evoked by sensory stimulation in vivo,” Nat. Neurosci. 9(6), 816–823 (2006). 10.1038/nn1703 [DOI] [PubMed] [Google Scholar]
- 154.Hirase H., “A multi-photon window onto neuronal-glial-vascular communication,” Trends Neurosci. 28(5), 217–219 (2005). 10.1016/j.tins.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 155.Takano T., et al. , “Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease,” Ann. N. Y. Acad. Sci. 1097, 40–50 (2007). 10.1196/annals.1379.004 [DOI] [PubMed] [Google Scholar]
- 156.Tian G. F., et al. , “An astrocytic basis of epilepsy,” Nat. Med. 11(9), 973–981 (2005). 10.1038/nm1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miyawaki A., et al. , “Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin,” Nature 388(6645), 882–887 (1997). 10.1038/42264 [DOI] [PubMed] [Google Scholar]
- 158.Baird G. S., Zacharias D. A., Tsien R. Y., “Circular permutation and receptor insertion within green fluorescent proteins,” Proc. Natl. Acad. Sci. U. S. A. 96(20), 11241–11246 (1999). 10.1073/pnas.96.20.11241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nagai T., et al. , “Circularly permuted green fluorescent proteins engineered to sense Ca2+,” Proc. Natl. Acad. Sci. U. S. A. 98(6), 3197–3202 (2001). 10.1073/pnas.051636098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakai J., Ohkura M., Imoto K., “A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein,” Nat. Biotechnol. 19(2), 137–141 (2001). 10.1038/84397 [DOI] [PubMed] [Google Scholar]
- 161.Nasu Y., et al. , “Structure- and mechanism-guided design of single fluorescent protein-based biosensors,” Nat. Chem. Biol. 17(5), 509–518 (2021). 10.1038/s41589-020-00718-x [DOI] [PubMed] [Google Scholar]
- 162.Tian L., et al. , “Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators,” Nat. Methods 6(12), 875–881 (2009). 10.1038/nmeth.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dana H., et al. , “High-performance calcium sensors for imaging activity in neuronal populations and microcompartments,” Nat. Methods 16(7), 649–657 (2019). 10.1038/s41592-019-0435-6 [DOI] [PubMed] [Google Scholar]
- 164.Inoue M., et al. , “Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics,” Cell 177(5), 1346–1360.e24 (2019). 10.1016/j.cell.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 165.Zarowny L., et al. , “Bright and high-performance genetically encoded Ca2+ indicator based on mneongreen fluorescent protein,” ACS Sens. 5(7), 1959–1968 (2020). 10.1021/acssensors.0c00279 [DOI] [PubMed] [Google Scholar]
- 166.Subach O. M., et al. , “Novel genetically encoded bright positive calcium indicator NCaMP7 based on the mNeonGreen fluorescent protein,” Int. J. Mol. Sci. 21(5), 1644 (2020). 10.3390/ijms21051644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao Y. X., et al. , “An expanded palette of genetically encoded Ca2+ indicators,” Science 333(6051), 1888–1891 (2011). 10.1126/science.1208592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dana H., et al. , “Sensitive red protein calcium indicators for imaging neural activity,” Elife 5, e12727 (2016). 10.7554/eLife.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen Y., et al. , “A genetically encoded Ca2+ indicator based on circularly permutated sea anemone red fluorescent protein eqFP578,” BMC Biol. 16(1), 9 (2018). 10.1186/s12915-018-0480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qian Y., et al. , “Improved genetically encoded near-infrared fluorescent calcium ion indicators for in vivo imaging,” PLoS Biol. 18(11), e3000965 (2020). 10.1371/journal.pbio.3000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qian Y., et al. , “A genetically encoded near-infrared fluorescent calcium ion indicator,” Nat. Methods 16(2), 171–174 (2019). 10.1038/s41592-018-0294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davila H. V., et al. , “A large change in axon fluorescence that provides a promising method for measuring membrane potential,” Nat. New Biol. 241(109), 159–160 (1973). 10.1038/newbio241159a0 [DOI] [PubMed] [Google Scholar]
- 173.Peterka D. S., Takahashi H., Yuste R., “Imaging voltage in neurons,” Neuron 69(1), 9–21 (2011). 10.1016/j.neuron.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grinvald A., Hildesheim R., “VSDI: a new era in functional imaging of cortical dynamics,” Nat. Rev. Neurosci. 5(11), 874–885 (2004). 10.1038/nrn1536 [DOI] [PubMed] [Google Scholar]
- 175.de Silva A. P., et al. , “New fluorescent model compounds for the study of photoinduced electron transfer: the influence of a molecular electric field in the excited state,” Angew. Chem. Int. Ed. Engl. 34(16), 1728–1731 (1995). 10.1002/anie.199517281 [DOI] [Google Scholar]
- 176.Li L.-S., “Fluorescence probes for membrane potentials based on mesoscopic electron transfer,” Nano Lett. 7(10), 2981–2986 (2007). 10.1021/nl071163p [DOI] [PubMed] [Google Scholar]
- 177.Miller E. W., et al. , “Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires,” Proc. Natl. Acad. Sci. U. S. A. 109(6), 2114–2119 (2012). 10.1073/pnas.1120694109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Walker A. S., et al. , “Optical spike detection and connectivity analysis with a far-red voltage-sensitive fluorophore reveals changes to network connectivity in development and disease,” Front. Neurosci. 15, 643859 (2021). 10.3389/fnins.2021.643859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu P., Miller E. W., “Electrophysiology, unplugged: imaging membrane potential with fluorescent indicators,” Acc. Chem. Res. 53(1), 11–19 (2020). 10.1021/acs.accounts.9b00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Milosevic M. M., et al. , “In vitro testing of voltage indicators: Archon1, ArcLightD, ASAP1, ASAP2s, ASAP3b, Bongwoori-Pos6, BeRST1, FlicR1, and Chi-VSFP-butterfly,” eNeuro 7(5), ENEURO.0060-20.2020 (2020). 10.1523/ENEURO.0060-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kulkarni R. U., et al. , “Voltage-sensitive rhodol with enhanced two-photon brightness,” Proc. Natl. Acad. Sci. U. S. A. 114(11), 2813–2818 (2017). 10.1073/pnas.1610791114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kazemipour A., et al. , “Kilohertz frame-rate two-photon tomography,” Nat. Methods 16(8), 778–786 (2019). 10.1038/s41592-019-0493-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fiala T., et al. , “Chemical targeting of voltage sensitive dyes to specific cells and molecules in the brain,” J. Am. Chem. Soc. 142(20), 9285–9301 (2020). 10.1021/jacs.0c00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wakayama S., et al. , “Chemical labelling for visualizing native AMPA receptors in live neurons,” Nat. Commun. 8, 14850 (2017). 10.1038/ncomms14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cosco E. D., et al. , “Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection,” J. Am. Chem. Soc. 143(18), 6836–6846 (2021). 10.1021/jacs.0c11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matikonda S. S., et al. , “Core remodeling leads to long wavelength fluoro-coumarins,” Chem. Sci. 11(28), 7302–7307 (2020). 10.1039/D0SC02566F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hinner M. J., Hübener G., Fromherz P., “Genetic targeting of individual cells with a voltage-sensitive dye through enzymatic activation of membrane binding,” Chembiochem 7(3), 495–505 (2006). 10.1002/cbic.200500395 [DOI] [PubMed] [Google Scholar]
- 188.Ng D. N., Fromherz P., “Genetic targeting of a voltage-sensitive dye by enzymatic activation of phosphonooxymethyl-ammonium derivative,” ACS Chem. Biol. 6(5), 444–451 (2011). 10.1021/cb100312d [DOI] [PubMed] [Google Scholar]
- 189.Liu P., et al. , “Fluorogenic targeting of voltage-sensitive dyes to neurons,” J. Am. Chem. Soc. 139(48), 17334–17340 (2017). 10.1021/jacs.7b07047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ortiz G., et al. , “Synthesis of sulfonated carbofluoresceins for voltage imaging,” J. Am. Chem. Soc. 141(16), 6631–6638 (2019). 10.1021/jacs.9b01261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grenier V., et al. , “Spying on neuronal membrane potential with genetically targetable voltage indicators,” J. Am. Chem. Soc. 141(3), 1349–1358 (2019). 10.1021/jacs.8b11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sundukova M., et al. , “A chemogenetic approach for the optical monitoring of voltage in neurons,” Angew. Chem. Int. Ed. 58(8), 2341–2344 (2019). 10.1002/anie.201812967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deal P. E., et al. , “Covalently tethered rhodamine voltage reporters for high speed functional imaging in brain tissue,” J. Am. Chem. Soc. 142(1), 614–622 (2020). 10.1021/jacs.9b12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abdelfattah A. S., et al. , “A general approach to engineer positive-going eFRET voltage indicators,” Nat. Commun. 11(1), 3444 (2020). 10.1038/s41467-020-17322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deo C., et al. , “The HaloTag as a general scaffold for far-red tunable chemigenetic indicators,” Nat. Chem. Biol. 17(6), 718–723 (2021). 10.1038/s41589-021-00775-w [DOI] [PubMed] [Google Scholar]
- 196.Los G. V., et al. , “HaloTag: a novel protein labeling technology for cell imaging and protein analysis,” ACS Chem. Biol. 3(6), 373–382 (2008). 10.1021/cb800025k [DOI] [PubMed] [Google Scholar]
- 197.Encell L. P., et al. , “Development of a dehalogenase-based protein fusion tag capable of rapid, selective and covalent attachment to customizable ligands,” Curr. Chem. Genom. 6, 55–71 (2012). 10.2174/1875397301206010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Grimm J. B., et al. , “A general method to fine-tune fluorophores for live-cell and in vivo imaging,” Nat. Methods 14(10), 987–994 (2017). 10.1038/nmeth.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gong Y., et al. , “High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor,” Science 350(6266), 1361–1366 (2015). 10.1126/science.aab0810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Adam Y., et al. , “Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics,” Nature 569(7756), 413–417 (2019). 10.1038/s41586-019-1166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fan L. Z., et al. , “All-optical electrophysiology reveals the role of lateral inhibition in sensory processing in cortical layer 1,” Cell 180(3), 521–535.e18 (2020). 10.1016/j.cell.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin M. Z., Schnitzer M. J., “Genetically encoded indicators of neuronal activity,” Nat. Neurosci. 19(9), 1142–1153 (2016). 10.1038/nn.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bando Y., et al. , “Genetic voltage indicators,” BMC Biol. 17(1), 71 (2019). 10.1186/s12915-019-0682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu Y., Zou P., Cohen A. E., “Voltage imaging with genetically encoded indicators,” Curr. Opin. Chem. Biol. 39, 1–10 (2017). 10.1016/j.cbpa.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kannan M., Vasan G., Pieribone V. A., “Optimizing strategies for developing genetically encoded voltage indicators,” Front. Cell Neurosci. 13, 53 (2019). 10.3389/fncel.2019.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Beck C., Zhang D., Gong Y., “Enhanced genetically encoded voltage indicators advance their applications in neuroscience,” Curr. Opin. Biomed. Eng. 12, 111–117 (2019). 10.1016/j.cobme.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Knopfel T., Song C., “Optical voltage imaging in neurons: moving from technology development to practical tool,” Nat. Rev. Neurosci. 20(12), 719–727 (2019). 10.1038/s41583-019-0231-4 [DOI] [PubMed] [Google Scholar]
- 208.Peng L. X., Xu Y. X., Zou P., “Genetically-encoded voltage indicators,” Chin. Chem. Lett. 28(10), 1925–1928 (2017). 10.1016/j.cclet.2017.09.037 [DOI] [Google Scholar]
- 209.Ma Y., Bayguinov P. O., Jackson M. B., “Optical studies of action potential dynamics with hVOS probes,” Curr. Opin. Biomed. Eng. 12, 51–58 (2019). 10.1016/j.cobme.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Patriarchi T., et al. , “Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors,” Science 360(6396), eaat4422 (2018). 10.1126/science.aat4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Borden P. M., et al. , “A fast genetically encoded fluorescent sensor for faithful in vivo acetylcholine detection in mice, fish, worms and flies,” 10.1101/2020.02.07.939504 (2020). [DOI]
- 212.Lobas M. A., et al. , “A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP,” Nat. Commun. 10, 711 (2019). 10.1038/s41467-019-08441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Marvin J. S., et al. , “A genetically encoded fluorescent sensor for in vivo imaging of GABA,” Nat. Methods 16(8), 763–770 (2019). 10.1038/s41592-019-0471-2 [DOI] [PubMed] [Google Scholar]
- 214.Shivange A. V., et al. , “Determining the pharmacokinetics of nicotinic drugs in the endoplasmic reticulum using biosensors,” J. Gen. Physiol. 151(6), 738–757 (2019). 10.1085/jgp.201812201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Unger E. K., et al. , “Directed evolution of a selective and sensitive serotonin sensor via machine learning,” Cell 183(7), 1986–2002.e26 (2020). 10.1016/j.cell.2020.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hoffmann C., et al. , “A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells,” Nat. Methods 2(3), 171–176 (2005). 10.1038/nmeth742 [DOI] [PubMed] [Google Scholar]
- 217.Jensen J. B., et al. , “Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels,” J. Gen. Physiol. 133(4), 347–359 (2009). 10.1085/jgp.200810075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maier-Peuschel M., et al. , “A FRET-based M2 muscarinic receptor sensor to study the mechanisms of allosteric modulation,” N-S Arch. Pharmacol. 377, 15 (2008). [Google Scholar]
- 219.Vilardaga J. P., et al. , “Measurement of the millisecond activation switch of G protein-coupled receptors in living cells,” Nat. Biotechnol. 21(7), 807–812 (2003). 10.1038/nbt838 [DOI] [PubMed] [Google Scholar]
- 220.Latorraca N. R., Venkatakrishnan A. J., Dror R. O., “GPCR dynamics: structures in motion,” Chem. Rev. 117(1), 139–155 (2017). 10.1021/acs.chemrev.6b00177 [DOI] [PubMed] [Google Scholar]
- 221.Dong A., et al. , “A fluorescent sensor for spatiotemporally resolved endocannabinoid dynamics in vitro and in vivo,” Nat. Biotechnol. (2021). 10.1038/s41587-021-01074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sun F., et al. , “A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice,” Cell 174(2), 481–496.e19 (2018). 10.1016/j.cell.2018.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sun F. M., et al. , “Next-generation GRAB sensors for monitoring dopaminergic activity in vivo,” Nat. Methods 17(11), 1156–1166 (2020). 10.1038/s41592-020-00981-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Feng J., et al. , “A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine,” Neuron 102(4), 745–761.e8 (2019). 10.1016/j.neuron.2019.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wan J. X., et al. , “A genetically encoded sensor for measuring serotonin dynamics,” Nat. Neurosci. 24(5), 746–752 (2021). 10.1038/s41593-021-00823-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wu Z., et al. , “A GRAB sensor reveals activity-dependent non-vesicular somatodendritic adenosine release,” 10.1101/2020.05.04.075564 (2020). [DOI]
- 227.Deuschle K., et al. , “Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering,” Protein Sci. 14(9), 2304–2314 (2005). 10.1110/ps.051508105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Takanaga H., Chaudhuri B., Frommer W. B., “GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor,” BBA-Biomembr. 1778(4), 1091–1099 (2008). 10.1016/j.bbamem.2007.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Diaz-Garcia C. M., et al. , “Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor,” J. Neurosci. Res. 97(8), 946–960 (2019). 10.1002/jnr.24433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Keller J. P., et al. , “In vivo glucose imaging in multiple model organisms with an engineered single-wavelength sensor,” Cell Rep. 35(12), 109284 (2021). 10.1016/j.celrep.2021.109284 [DOI] [PubMed] [Google Scholar]
- 231.Berg J., Hung Y. P., Yellen G., “A genetically encoded fluorescent reporter of ATP:ADP ratio,” Nat. Methods 6(2), 161–166 (2009). 10.1038/nmeth.1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Imamura H., et al. , “Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators,” Proc. Natl. Acad. Sci. U. S. A. 106(37), 15651–15656 (2009). 10.1073/pnas.0904764106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tantama M., et al. , “Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio,” Nat. Commun. 4, 2550 (2013). 10.1038/ncomms3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hung Y. P., et al. , “Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor,” Cell Metab. 14(4), 545–554 (2011). 10.1016/j.cmet.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao Y., et al. , “SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents,” Cell Metab. 21(5), 777–789 (2015). 10.1016/j.cmet.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tao R., et al. , “Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism,” Nat. Methods 14(7), 720–728 (2017). 10.1038/nmeth.4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sallin O., et al. , “Semisynthetic biosensors for mapping cellular concentrations of nicotinamide adenine dinucleotides,” Elife 7, e32638 (2018). 10.7554/eLife.32638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.San Martin A., et al. , “Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate,” PLOS ONE 9(1), e85780 (2014). 10.1371/journal.pone.0085780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.San Martin A., et al. , “A genetically encoded FRET lactate sensor and its use to detect the warburg effect in single cancer cells,” PLOS ONE 8(2), e57712 (2013). 10.1371/journal.pone.0057712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mächler P., et al. , “In vivo evidence for a lactate gradient from astrocytes to neurons,” Cell Metab. 23(1), 94–102 (2016). 10.1016/j.cmet.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 241.Diaz-Garcia C. M., et al. , “Neuronal stimulation triggers neuronal glycolysis and not lactate uptake,” Cell Metab. 26(2), 361–374 (2017). 10.1016/j.cmet.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yellen G., Mongeon R., “Quantitative two-photon imaging of fluorescent biosensors,” Curr. Opin. Chem. Biol. 27, 24–30 (2015). 10.1016/j.cbpa.2015.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Allen N. J., Lyons D. A., “Glia as architects of central nervous system formation and function,” Science 362(6411), 181–185 (2018). 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chung W. S., Allen N. J., Eroglu C., “Astrocytes control synapse formation, function, and elimination,” Cold Spring Harb. Perspect. Biol. 7(9), a020370 (2015). 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ziemens D., et al. , “Heterogeneity of activity-induced sodium transients between astrocytes of the mouse hippocampus and neocortex: mechanisms and consequences,” J. Neurosci. 39(14), 2620–2634 (2019). 10.1523/JNEUROSCI.2029-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shen Y., et al. , “Genetically encoded fluorescent indicators for imaging intracellular potassium ion concentration,” Commun. Biol. 2, 18 (2019). 10.1038/s42003-018-0269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Octeau J. C., et al. , “An optical neuron-astrocyte proximity assay at synaptic distance scales,” Neuron 98(1), 49–66.e9 (2018). 10.1016/j.neuron.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bernardinelli Y., et al. , “Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability,” Curr. Biol. 24(15), 1679–1688 (2014). 10.1016/j.cub.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 249.Murphy-Royal C., et al. , “Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission,” Nat. Neurosci. 18(2), 219–226 (2015). 10.1038/nn.3901 [DOI] [PubMed] [Google Scholar]
- 250.Araque A., et al. , “Gliotransmitters travel in time and space,” Neuron 81(4), 728–739 (2014). 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nagai J., et al. , “Specific and behaviorally consequential astrocyte Gq GPCR signaling attenuation in vivo with ibetaARK,” Neuron 109(14), 2256–2274.e9 (2021). 10.1016/j.neuron.2021.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pestana F., et al. , “No longer underappreciated: the emerging concept of astrocyte heterogeneity in neuroscience,” Brain Sci 10(3), 168 (2020). 10.3390/brainsci10030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ben Haim L., Rowitch D. H., “Functional diversity of astrocytes in neural circuit regulation,” Nat. Rev. Neurosci. 18(1), 31–41 (2017). 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- 254.Batiuk M. Y., et al. , “Identification of region-specific astrocyte subtypes at single cell resolution,” Nat. Commun. 11, 1220 (2020). 10.1038/s41467-019-14198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Luo L., Callaway E. M., Svoboda K., “Genetic dissection of neural circuits: a decade of progress,” Neuron 98(2), 256–281 (2018). 10.1016/j.neuron.2018.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yu X., et al. , “Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior,” Neuron 99(6), 1170–1187.e9 (2018). 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mederos S., et al. , “Melanopsin for precise optogenetic activation of astrocyte-neuron networks,” Glia 67(5), 915–934 (2019). 10.1002/glia.23580 [DOI] [PubMed] [Google Scholar]
- 258.Octeau J. C., et al. , “Transient, consequential increases in extracellular potassium ions accompany Channelrhodopsin2 excitation,” Cell Rep. 27(8), 2249–2261.e7 (2019). 10.1016/j.celrep.2019.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Depaoli M. R., et al. , “Live cell imaging of signaling and metabolic activities,” Pharmacol. Ther. 202, 98–119 (2019). 10.1016/j.pharmthera.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 260.Bi X., Beck C., Gong Y., “Genetically encoded fluorescent indicators for imaging brain chemistry,” Biosensors (Basel) 11(4), 116 (2021). 10.3390/bios11040116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Slezak M., et al. , “Distinct mechanisms for visual and motor-related astrocyte responses in mouse visual cortex,” Curr. Biol. 29(18), 3120–3127.e5 (2019). 10.1016/j.cub.2019.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Steinmetz N. A., et al. , “Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings,” Science 372(6539), eabf4588 (2021). 10.1126/science.abf4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vanderkooi J. M., et al. , “An optical method for measurement of dioxygen concentration based on quenching of phosphorescence,” J. Biol. Chem. 262, 5476–5482 (1987). 10.1016/S0021-9258(18)45596-2 [DOI] [PubMed] [Google Scholar]
- 264.Rumsey W. L., Vanderkooi J. M., Wilson D. F., “Imaging of phosphorescence: a novel method for measuring the distribution of oxygen in perfused tissue,” Science 241, 1649–1651 (1988). 10.1126/science.3420417 [DOI] [PubMed] [Google Scholar]
- 265.Vinogradov S. A., Wilson D. F., “Porphyrin-dendrimers as biological oxygen sensors,” in Designing Dendrimers, Capagna S., Ceroni P., Eds., Wiley, New York: (2012). [Google Scholar]
- 266.Lebedev A. Y., et al. , “Dendritic phosphorescent probes for oxygen imaging in biological systems,” ACS Appl. Mater. Interfaces 1(6), 1292–1304 (2009). 10.1021/am9001698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Esipova T. V., et al. , “Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging,” Anal. Chem. 83(22), 8756–8765 (2011). 10.1021/ac2022234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sakadzic S., et al. , “Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue,” Nat. Methods 7(9), 755–759 (2010). 10.1038/nmeth.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lecoq J., et al. , “Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels,” Nat. Med. 17(7), 893–898 (2011). 10.1038/nm.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Devor A., et al. , “"Overshoot” of O(2) is required to maintain baseline tissue oxygenation at locations distal to blood vessels,” J. Neurosci. 31(38), 13676–13681 (2011). 10.1523/JNEUROSCI.1968-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kazmi S. M. S., et al. , “Three-dimensional mapping of oxygen tension in cortical arterioles before and after occlusion,” Biomed. Opt. Express 4(7), 1061–1073 (2013). 10.1364/BOE.4.001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sakadzic S., et al. , “Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue,” Nat. Commun. 5, 5734 (2014). 10.1038/ncomms6734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Parpaleix A., Goulam Houssen Y., Charpak S., “Imaging local neuronal activity by monitoring PO(2) transients in capillaries,” Nat. Med. 19(2), 241–246 (2013). 10.1038/nm.3059 [DOI] [PubMed] [Google Scholar]
- 274.Lyons D. G., et al. , “Mapping oxygen concentration in the awake mouse brain,” Elife 5, e12024 (2016). 10.7554/eLife.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Brinas R. P., et al. , “Phosphorescent oxygen sensor with dendritic protection and two-photon absorbing antenna,” J. Am. Chem. Soc. 127(33), 11851–11862 (2005). 10.1021/ja052947c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Roussakis E., et al. , “Two-photon antenna-core oxygen probe with enhanced performance,” Anal. Chem. 86, 5937−5945 (2014). 10.1021/ac501028m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Esipova T. V., et al. , “Two-photon absorbing phosphorescent metalloporphyrins: effects of -extension and peripheral substitution,” J. Am. Chem. Soc. 138(48), 15648–15662 (2016). 10.1021/jacs.6b09157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Esipova T. V., et al. , “Stabilizing g-states in centrosymmetric tetrapyrroles: two-photon-absorbing porphyrins with bright phosphorescence,” J. Phys. Chem. A 121(33), 6243–6255 (2017). 10.1021/acs.jpca.7b04333 [DOI] [PubMed] [Google Scholar]
- 279.Cao X., et al. , “Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo,” Int. J. Radiat. Oncol. Biol. Phys. (RED J.) 111(1), 240–248 (2021). 10.1016/j.ijrobp.2021.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Berglund K., et al. , “Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons,” PLOS ONE 8(3), e59759 (2013). 10.1371/journal.pone.0059759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Berglund K., et al. , “Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation,” Proc. Natl. Acad. Sci. U. S. A. 113(3), E358–E367 (2016). 10.1073/pnas.1510899113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Naim N., et al. , “Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis,” J. Biol. Chem. 294(4), 1095–1103 (2019). 10.1074/jbc.AC118.004905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li T., et al. , “A synthetic BRET-based optogenetic device for pulsatile transgene expression enabling glucose homeostasis in mice,” Nat. Commun. 12, 615 (2021). 10.1038/s41467-021-20913-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zenchak J. R., et al. , “Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model,” J. Neurosci. Res. 98(3), 458–468 (2020). 10.1002/jnr.24237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tung J. K., et al. , “Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity,” Neurobiol. Dis. 109, 1–10 (2018). 10.1016/j.nbd.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yu S. P., et al. , “Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke,” J. Neurosci. 39(33), 6571–6594 (2019). 10.1523/JNEUROSCI.2010-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Song D., et al. , “Manipulation of hippocampal CA3 firing via luminopsins modulates spatial and episodic short-term memory, especially working memory, but not long-term memory,” Neurobiol. Learn. Mem. 155, 435–445 (2018). 10.1016/j.nlm.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 288.Jaiswal P. B., et al. , “Motoneuron activity is required for enhancements in functional recovery after peripheral nerve injury in exercised female mice,” J. Neurosci. Res. 98(3), 448–457 (2020). 10.1002/jnr.24109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Love A. C., Prescher J. A., “Seeing (and using) the light: recent developments in bioluminescence technology,” Cell Chem. Biol. 27(8), 904–920 (2020). 10.1016/j.chembiol.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Celinskis D., et al. , “Miniaturized devices for bioluminescence imaging in freely behaving animals,” IEEE Eng. Med. Biol. Soc. 2020, 4385–4389 (2020). 10.1109/EMBC44109.2020.9175375 [DOI] [PubMed] [Google Scholar]
- 291.Nagel G., et al. , “Channelrhodopsin-1: a light-gated proton channel in green algae,” Science 296(5577), 2395–2398 (2002). 10.1126/science.1072068 [DOI] [PubMed] [Google Scholar]
- 292.Nagel G., et al. , “Channelrhodopsin-2, a directly light-gated cation-selective membrane channel,” Proc. Natl. Acad. Sci. U. S. A. 100(24), 13940–13945 (2003). 10.1073/pnas.1936192100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li X., et al. , “Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopin and green algae channelrhodopsin,” Proc. Natl. Acad. Sci. U. S. A. 102(49), 17816–17821 (2005). 10.1073/pnas.0509030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bi A. D., et al. , “Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration,” Neuron 50(1), 23–33 (2006). 10.1016/j.neuron.2006.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yizhar O., et al. , “Optogenetics in neural systems,” Neuron 71(1), 9–34 (2011). 10.1016/j.neuron.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 296.Deisseroth K., Hegemann P., “The form and function of channelrhodopsin,” Science 357(6356), eaan5544 (2017). 10.1126/science.aan5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Levitz J., et al. , “Dual optical control and mechanistic insights into photoswitchable group II and III metabotropic glutamate receptors,” Proc. Natl. Acad. Sci. U. S. A. 114(17), E3546–E3554 (2017). 10.1073/pnas.1619652114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Levitz J., et al. , “Optical control of metabotropic glutamate receptors,” Nat. Neurosci. 16(4), 507–516 (2013). 10.1038/nn.3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kramer R. H., Mourot A., Adesnik H., “Optogenetic pharmacology for control of native neuronal signaling proteins,” Nat. Neurosci. 16(7), 816–823 (2013). 10.1038/nn.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lin J. Y., et al. , “Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI),” Neuron 79(2), 241–253 (2013). 10.1016/j.neuron.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rost B. R., et al. , “Optogenetic tools for subcellular applications in neuroscience,” Neuron 96(3), 572–603 (2017). 10.1016/j.neuron.2017.09.047 [DOI] [PubMed] [Google Scholar]
- 302.Petreanu L., et al. , “Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections,” Nat. Neurosci. 10(5), 663–668 (2007). 10.1038/nn1891 [DOI] [PubMed] [Google Scholar]
- 303.Lin D., et al. , “Functional identification of an aggression locus in the mouse hypothalamus,” Nature 470(7333), 221–226 (2011). 10.1038/nature09736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Paz J. T., et al. , “Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury,” Nat. Neurosci. 16(1), 64–70 (2013). 10.1038/nn.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sahel J. A., et al. , “Partial recovery of visual function in a blind patient after optogenetic therapy,” Nat. Med. 27(7), 1223–1229 (2021). 10.1038/s41591-021-01351-4 [DOI] [PubMed] [Google Scholar]
- 306.Adamantidis A. R., et al. , “Neural substrates of awakening probed with optogenetic control of hypocretin neurons,” Nature 450(7168), 420–424 (2007). 10.1038/nature06310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jazayeri M., Afraz A., “Navigating the neural space in search of the neural code,” Neuron 93(5), 1003–1014 (2017). 10.1016/j.neuron.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 308.Rickgauer J. P., Tank D. W., “Two-photon excitation of channelrhodopsin-2 at saturation,” Proc. Natl. Acad. Sci. U. S. Am 106(35), 15025–15030 (2009). 10.1073/pnas.0907084106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Prakash R., et al. , “Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation,” Nat. Methods 9(12), 1171–1179 (2012). 10.1038/nmeth.2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Packer A. M., et al. , “Two-photon optogenetics of dendritic spines and neural circuits,” Nat. Methods 9(12), 1202–1205 (2012). 10.1038/nmeth.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Papagiakoumou E., Ronzitti E., Emiliani V., “Scanless two-photon excitation with temporal focusing,” Nat. Methods 17(6), 571–581 (2020). 10.1038/s41592-020-0795-y [DOI] [PubMed] [Google Scholar]
- 312.Yang W. J., et al. , “Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions,” Elife 7, e32671 (2018). 10.7554/eLife.32671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Robinson N. T. M., et al. , “Targeted activation of hippocampal place cells drives memory-guided spatial behavior,” Cell 183(6), 1586–1599.e10 (2020). 10.1016/j.cell.2020.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Marshel J. H., et al. , “Cortical layer-specific critical dynamics triggering perception,” Science 365(6453), eaaw5202 (2019). 10.1126/science.aaw5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Carrillo-Reid L., et al. , “Controlling visually guided behavior by holographic recalling of cortical ensembles,” Cell 178(2), 447–457.e5 (2019). 10.1016/j.cell.2019.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chettih S. N., Harvey C. D., “Single-neuron perturbations reveal feature-specific competition in V1,” Nature 567(7748), 334–340 (2019). 10.1038/s41586-019-0997-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ernst O. P., et al. , “Microbial and animal rhodopsins: structures, functions, and molecular mechanisms,” Chem. Rev. 114(1), 126–163 (2014). 10.1021/cr4003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yutin N., Koonin E. V., “Proteorhodopsin genes in giant viruses,” Biol. Direct. 7, 34 (2012). 10.1186/1745-6150-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rozenberg A., et al. , “Microbial rhodopsins: the last two decades,” Annu. Rev. Microbiol. 75, 427–447 (2021). 10.1146/annurev-micro-031721-020452 [DOI] [PubMed] [Google Scholar]
- 320.Nagel G., et al. , “Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid Behavioral responses,” Curr. Biol. 15(24), 2279–2284 (2005). 10.1016/j.cub.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 321.Vierock J., et al. , “Molecular determinants of proton selectivity and gating in the red-light activated channelrhodopsin Chrimson,” Sci. Rep. 7, 9928 (2017). 10.1038/s41598-017-09600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Govorunova E. G., et al. , “Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics,” Science 349(6248), 647–650 (2015). 10.1126/science.aaa7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Govorunova E. G., et al. , “The expanding family of natural anion channelrhodopsins reveals large variations in kinetics, conductance, and spectral sensitivity,” Sci. Rep. 7, 43358 (2017). 10.1038/srep43358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mahn M., et al. , “High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins,” Nat. Commun. 9, 4125 (2018). 10.1038/s41467-018-06511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Messier J. E., et al. , “Targeting light-gated chloride channels to neuronal somatodendritic domain reduces their excitatory effect in the axon,” Elife 7, e38506 (2018). 10.7554/eLife.38506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kopton R. A., et al. , “Cardiac electrophysiological effects of light-activated chloride channels,” Front. Physiol. 9, 1806 (2018). 10.3389/fphys.2018.01806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shevchenko V., et al. , “Inward H+ pump xenorhodopsin: mechanism and alternative optogenetic approach,” Sci. Adv. 3(9), e1603187 (2017). 10.1126/sciadv.1603187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mattis J., et al. , “Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins,” Nat. Methods 9(2), 159–172 (2012). 10.1038/nmeth.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Gradinaru V., Thompson K. R., Deisseroth K., “eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications,” Brain Cell Biol. 36(1–4), 129–139 (2008). 10.1007/s11068-008-9027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Grimm C., et al. , “Electrical properties, substrate specificity and optogenetic potential of the engineered light-driven sodium pump eKR2,” Sci. Rep. 8, 9316 (2018). 10.1038/s41598-018-27690-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Gradinaru V., et al. , “Molecular and cellular approaches for diversifying and extending optogenetics,” Cell 141(1), 154–165 (2010). 10.1016/j.cell.2010.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cardin J. A., et al. , “Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2,” Nat. Protoc. 5(2), 247–254 (2010). 10.1038/nprot.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Govorunova E. G., et al. , “Cation and anion channelrhodopsins: sequence motifs and taxonomic distribution,” mBio 12(4), e0165621 (2021). 10.1128/mBio.01656-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wietek J., et al. , “Conversion of channelrhodopsin into a light-gated chloride channel,” Science 344(6182), 409–412 (2014). 10.1126/science.1249375 [DOI] [PubMed] [Google Scholar]
- 335.Lin J. Y., et al. , “ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation,” Nat. Neurosci. 16(10), 1499–1508 (2013). 10.1038/nn.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wietek J., et al. , “Anion-conducting channelrhodopsins with tuned spectra and modified kinetics engineered for optogenetic manipulation of behavior,” Sci. Rep. 7, 14957 (2017). 10.1038/s41598-017-14330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Klapoetke N. C., et al. , “Independent optical excitation of distinct neural populations,” Nat. Methods 11(3), 338–346 (2014). 10.1038/nmeth.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mager T., et al. , “High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics,” Nat. Commun. 9, 1750 (2018). 10.1038/s41467-018-04146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Spoida K., et al. , “Melanopsin variants as intrinsic optogenetic on and off switches for transient versus sustained activation of G protein pathways,” Curr. Biol. 26(9), 1206–1212 (2016). 10.1016/j.cub.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 340.Eickelbeck D., et al. , “Lamprey parapinopsin (“UVLamP”): a bistable UV-sensitive optogenetic switch for ultrafast control of GPCR pathways,” Chembiochem 21(5), 612–617 (2020). 10.1002/cbic.201900485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mahn M., et al. , “Efficient optogenetic silencing of neurotransmitter release with a mosquito rhodopsin,” Neuron 109(10), 1621–1635.e8 (2021). 10.1016/j.neuron.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Copits B. A., et al. , “A photoswitchable GPCR-based opsin for presynaptic inhibition,” Neuron 109(11), 1791–1809.e11 (2021). 10.1016/j.neuron.2021.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Karapinar R., et al. , “Reverse optogenetics of G protein signaling by zebrafish non-visual opsin Opn7b for synchronization of neuronal networks,” Nat. Commun. 12, 4488 (2021). 10.1038/s41467-021-24718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tsunoda S. P., Sugiura M., Kandori H., “Molecular properties and optogenetic applications of enzymerhodopsins,” in Optogenetics: Light-Sensing Proteins and Their Applications in Neuroscience and Beyond, Yawo H., et al., Eds., Springer Nature, Singapore: (2021). [DOI] [PubMed] [Google Scholar]
- 345.Luck M., et al. , “A photochromic Histidine Kinase Rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light,” J. Biol. Chem. 287(47), 40083–40090 (2012). 10.1074/jbc.M112.401604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Avelar G. M., et al. , “A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus,” Curr. Biol. 24(11), 1234–1240 (2014). 10.1016/j.cub.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yoshida K., et al. , “A unique choanoflagellate enzyme rhodopsin exhibits light-dependent cyclic nucleotide phosphodiesterase activity,” J. Biol. Chem. 292(18), 7531–7541 (2017). 10.1074/jbc.M117.775569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Sierra Y. A. B., et al. , “Potassium channel-based optogenetic silencing,” Nat. Commun. 9, 4611(2018). 10.1038/s41467-018-07038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhang S. X., et al. , “Hypothalamic dopamine neurons motivate mating through persistent cAMP signalling,” Nature 597(7875), 245–249 (2021). 10.1038/s41586-021-03845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ghosh K. K., et al. , “Miniaturized integration of a fluorescence microscope,” Nat. Methods 8(10), 871–878 (2011). 10.1038/nmeth.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Aharoni D., et al. , “All the light that we can see: a new era in miniaturized microscopy,” Nat. Methods 16(1), 11–13 (2019). 10.1038/s41592-018-0266-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Aharoni D., Hoogland T. M., “Circuit investigations with open-source miniaturized microscopes: past, present and future,” Front. Cell Neurosci. 13, 141 (2019). 10.3389/fncel.2019.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Vladimirov N., et al. , “Light-sheet functional imaging in fictively behaving zebrafish,” Nat. Methods 11(9), 883–884 (2014). 10.1038/nmeth.3040 [DOI] [PubMed] [Google Scholar]
- 354.Bouchard M. B., et al. , “Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms,” Nat. Photonics 9, 113–119 (2015). 10.1038/nphoton.2014.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Voleti V., et al. , “Real-time volumetric microscopy of in vivo dynamics and large-scale samples with SCAPE 2.0,” Nat. Methods 16(10), 1054–1062 (2019). 10.1038/s41592-019-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Perkins L. N., et al. , “Extracting individual neural activity recorded through splayed optical microfibers,” Neurophotonics 5(4), 045009 (2018). 10.1117/1.NPh.5.4.045009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Helmchen F., Gilad A., Chen J. L., “Neocortical dynamics during whisker-based sensory discrimination in head-restrained mice,” Neuroscience 368, 57–69 (2018). 10.1016/j.neuroscience.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gilad A., Helmchen F., “Spatiotemporal refinement of signal flow through association cortex during learning,” Nat. Commun. 11, 1744 (2020). 10.1038/s41467-020-15534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gilad A., et al. , “Behavioral strategy determines frontal or posterior location of short-term memory in neocortex,” Neuron 99(4), 814–828.e7 (2018). 10.1016/j.neuron.2018.07.029 [DOI] [PubMed] [Google Scholar]
- 360.Gallero-Salas Y., et al. , “Sensory and behavioral components of neocortical signal flow in discrimination tasks with short-term memory,” Neuron 109(1), 135–148.e6 (2021). 10.1016/j.neuron.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 361.Pinto L., et al. , “Task-dependent changes in the large-scale dynamics and necessity of cortical regions,” Neuron 104(4), 810–824.e9 (2019). 10.1016/j.neuron.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Esmaeili V., et al. , “Rapid suppression and sustained activation of distinct cortical regions for a delayed sensory-triggered motor response,” Neuron 109(13), 2183–2201.e9 (2021). 10.1016/j.neuron.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ma Y., et al. , “Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons,” Proc. Natl. Acad. Sci. U. S. A. 113(52), E8463–E8471 (2016). 10.1073/pnas.1525369113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Mitra A., et al. , “Spontaneous infra-slow brain activity has unique spatiotemporal dynamics and laminar structure,” Neuron 98(2), 297–305.e6 (2018). 10.1016/j.neuron.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Murphy M. C., et al. , “Macroscale variation in resting-state neuronal activity and connectivity assessed by simultaneous calcium imaging, hemodynamic imaging and electrophysiology,” Neuroimage 169, 352–362 (2018). 10.1016/j.neuroimage.2017.12.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Brier L. M., et al. , “Separability of calcium slow waves and functional connectivity during wake, sleep, and anesthesia,” Neurophotonics 6(3), 035002 (2019). 10.1117/1.NPh.6.3.035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Vanni M. P., et al. , “Mesoscale mapping of mouse cortex reveals frequency-dependent cycling between distinct macroscale functional modules,” J. Neurosci. 37(31), 7513–7533 (2017). 10.1523/JNEUROSCI.3560-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ratzlaff E. H., Grinvald A., “A tandem-lens epifluorescence macroscope – hundred-fold brightness advantage for wide-field imaging,” J. Neurosci. Methods 36(2-3), 127–137 (1991). 10.1016/0165-0270(91)90038-2 [DOI] [PubMed] [Google Scholar]
- 369.Couto J., et al. , “Chronic, cortex-wide imaging of specific cell populations during behavior,” Nat. Protoc. 16(7), 3241–3263 (2021). 10.1038/s41596-021-00527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Vanni M. P., Murphy T. H., “Mesoscale transcranial spontaneous activity mapping in GCaMP3 transgenic mice reveals extensive reciprocal connections between areas of somatomotor cortex,” J. Neurosci. 34(48), 15931–15946 (2014). 10.1523/JNEUROSCI.1818-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Allen W. E., et al. , “Global representations of goal-directed behavior in distinct cell types of mouse neocortex,” Neuron 94(4), 891–907.e6 (2017). 10.1016/j.neuron.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Nelson N. A., et al. , “Imaging spinal cord activity in behaving animals,” Exp. Neurol. 320, 112974 (2019). 10.1016/j.expneurol.2019.112974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Chen S., et al. , “Miniature fluorescence microscopy for imaging brain activity in freely-behaving animals,” Neurosci. Bull. 36(10), 1182–1190 (2020). 10.1007/s12264-020-00561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shuman T., et al. , “Breakdown of spatial coding and interneuron synchronization in epileptic mice,” Nat. Neurosci. 23(2), 229–238 (2020). 10.1038/s41593-019-0559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Scott B. B., et al. , “Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope,” Neuron 100(5), 1045–1058.e5 (2018). 10.1016/j.neuron.2018.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Rynes M. L., et al. , “Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice,” Nat. Methods 18, 417–425 (2021). 10.1038/s41592-021-01104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sekiguchi K. J., et al. , “Imaging large-scale cellular activity in spinal cord of freely behaving mice,” Nat. Commun. 7, 11450 (2016). 10.1038/ncomms11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Shekhtmeyster P., et al. , “Trans-segmental imaging in the spinal cord of behaving mice,” bioRxiv, 2021.12.23.474042 (2021). [DOI] [PMC free article] [PubMed]
- 379.Shekhtmeyster P., et al. , “Multiplex, translaminar imaging in the spinal cord of behaving mice,” 10.1101/2021.12.23.474039 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Guo C., et al. , “Miniscope-LFOV: a large field of view, single cell resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals,” 10.1101/2021.11.21.469394 (2021). [DOI] [PMC free article] [PubMed]
- 381.Hillman E. M., et al. , “High-speed 3D imaging of cellular activity in the brain using axially-extended beams and light sheets,” Curr. Opin. Neurobiol. 50, 190–200 (2018). 10.1016/j.conb.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kim K. H., et al. , “Multifocal multiphoton microscopy based on multianode photomultiplier tubes,” Opt. Express 15(18), 11658–11678 (2007). 10.1364/OE.15.011658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Xue Y., et al. , “Scanless volumetric imaging by selective access multifocal multiphoton microscopy,” Optica 6(1), 76–83 (2019). 10.1364/OPTICA.6.000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lu R., et al. , “Video-rate volumetric functional imaging of the brain at synaptic resolution,” Nat. Neurosci. 20(4), 620–628 (2017). 10.1038/nn.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Dufour P., et al. , “Two-photon excitation fluorescence microscopy with a high depth of field using an axicon,” Appl. Opt. 45(36), 9246–9252 (2006). 10.1364/AO.45.009246 [DOI] [PubMed] [Google Scholar]
- 386.Song A., et al. , “Volumetric two-photon imaging of neurons using stereoscopy (vTwINS),” Nat. Methods 14(4), 420–426 (2017). 10.1038/nmeth.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Voigt F. F., et al. , “The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue,” Nat. Methods 16(11), 1105–1108 (2019). 10.1038/s41592-019-0554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Dodt H.-U., et al. , “Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain,” Nat. Methods 4(4), 331–336 (2007). 10.1038/nmeth1036 [DOI] [PubMed] [Google Scholar]
- 389.Huisken J., et al. , “Optical sectioning deep inside live embryos by selective plane illumination microscopy,” Science 305(5686), 1007–1009 (2004). 10.1126/science.1100035 [DOI] [PubMed] [Google Scholar]
- 390.Tomer R., et al. , “Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy,” Nat. Methods 9(7), 755–763 (2012). 10.1038/nmeth.2062 [DOI] [PubMed] [Google Scholar]
- 391.Vaadia R. D., et al. , “Characterization of proprioceptive system dynamics in behaving drosophila larvae using high-speed volumetric microscopy,” Curr. Biol. 29(6), 935–944.e4 (2019). 10.1016/j.cub.2019.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Schaffer E. S., et al. , “Flygenvectors: the spatial and temporal structure of neural activity across the fly brain,” 10.1101/2021.09.25.461804 (2021). [DOI] [PMC free article] [PubMed]
- 393.Wen C., et al. , “3DeeCellTracker, a deep learning-based pipeline for segmenting and tracking cells in 3D time lapse images,” Elife 10, e59187 (2021). 10.7554/eLife.59187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Benezra S. E., et al. , “Learning enhances behaviorally relevant representations in apical dendrites,” 10.1101/2021.11.10.468144 (2021). [DOI]
- 395.Xu L., et al. , “Widespread receptor-driven modulation in peripheral olfactory coding,” Science 368(6487), eaaz5390 (2020). 10.1126/science.aaz5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Barretto R. P. J., Messerschmidt B., Schnitzer M. J., “In vivo fluorescence imaging with high-resolution microlenses,” Nat. Methods 6(7), 511–512 (2009). 10.1038/nmeth.1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Levene M. J., et al. , “In vivo multiphoton microscopy of deep brain tissue,” J. Neurophysiol. 91(4), 1908–1912 (2004). 10.1152/jn.01007.2003 [DOI] [PubMed] [Google Scholar]
- 398.Andermann M. L., et al. , “Chronic cellular imaging of entire cortical columns in awake mice using microprisms,” Neuron 80(4), 900–913 (2013). 10.1016/j.neuron.2013.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Antonini A., et al. , “Extended field-of-view ultrathin microendoscopes for high-resolution two-photon imaging with minimal invasiveness,” Elife 9, e58882 (2020). 10.7554/eLife.58882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Attardo A., Fitzgerald J. E., Schnitzer M. J., “Impermanence of dendritic spines in live adult CA1 hippocampus,” Nature 523(7562), 592–596 (2015). 10.1038/nature14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Low R. J., Gu Y., Tank D. W., “Cellular resolution optical access to brain regions in fissures: imaging medial prefrontal cortex and grid cells in entorhinal cortex,” Proc. Natl. Acad. Sci. U. S. A. 111(52), 18739–18744 (2014). 10.1073/pnas.1421753111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Popoff S. M., et al. , “Measuring the transmission matrix in optics: an approach to the study and control of light propagation in disordered media,” Phys. Rev. Lett. 104(10), 100601 (2010). 10.1103/PhysRevLett.104.100601 [DOI] [PubMed] [Google Scholar]
- 403.Turtaev S., et al. , “High-fidelity multimode fibre-based endoscopy for deep brain in vivo imaging,” Light-Sci. Appl. 7, 92 (2018). 10.1038/s41377-018-0094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Vasquez-Lopez S. A., et al. , “Subcellular spatial resolution achieved for deep-brain imaging in vivo using a minimally invasive multimode fiber,” Light: Sci. Appl. 7(1), 110 (2018). 10.1038/s41377-018-0111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ohayon S., et al. , “Minimally invasive multimode optical fiber microendoscope for deep brain fluorescence imaging,” Biomed. Opt. Express 9(4), 1492–1509 (2018). 10.1364/BOE.9.001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Turcotte R., et al. , “Focusing light in biological tissue through a multimode optical fiber: refractive index matching,” Opt. Lett. 44(10), 2386–2389 (2019). 10.1364/OL.44.002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kleinfeld D., et al. , “Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex,” Proc. Natl. Acad. Sci. U. S. A. 95(26), 15741–15746 (1998). 10.1073/pnas.95.26.15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Sofroniew N. J., et al. , “A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging,” Elife 5, e14472 (2016). 10.7554/eLife.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rumyantsev O. I., et al. , “Fundamental bounds on the fidelity of sensory cortical coding,” Nature 580(7801), 100–105 (2020). 10.1038/s41586-020-2130-2 [DOI] [PubMed] [Google Scholar]
- 410.Yu C. H., et al. , “Diesel2p mesoscope with dual independent scan engines for flexible capture of dynamics in distributed neural circuitry,” Nat. Commun. 12, 6639 (2021). 10.1038/s41467-021-26736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Yang M. K., et al. , “MATRIEX imaging: multiarea two-photon real-time in vivo explorer,” Light-Sci. Appl. 8, 109 (2019). 10.1038/s41377-019-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Lecoq J., et al. , “Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging,” Nat. Neurosci. 17(12), 1825–1829 (2014). 10.1038/nn.3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Wagner M. J., et al. , “Shared cortex-cerebellum dynamics in the execution and learning of a motor task,” Cell 177(3), 669–682.e24 (2019). 10.1016/j.cell.2019.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Nadella K. M., et al. , “Random-access scanning microscopy for 3D imaging in awake behaving animals,” Nat. Methods 13(12), 1001–1004 (2016). 10.1038/nmeth.4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Demas J., et al. , “High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy,” Nat. Methods 18(9), 1103–1111 (2021). 10.1038/s41592-021-01239-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Ouzounov D. G., et al. , “In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain,” Nat. Methods 14(4), 388–390 (2017). 10.1038/nmeth.4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Klioutchnikov A., et al. , “Three-photon head-mounted microscope for imaging deep cortical layers in freely moving rats,” Nat. Methods 17(5), 509–513 (2020). 10.1038/s41592-020-0817-9 [DOI] [PubMed] [Google Scholar]
- 418.Zong W., et al. , “Miniature two-photon microscopy for enlarged field-of-view, multi-plane and long-term brain imaging,” Nat. Methods 18(1), 46–49 (2021). 10.1038/s41592-020-01024-z [DOI] [PubMed] [Google Scholar]
- 419.Xue Y. J., et al. , “Single-shot 3D wide-field fluorescence imaging with a computational miniature mesoscope,” Sci. Adv. 6(43), eabb7508 (2020). 10.1126/sciadv.abb7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Tian F., Hu J. J., Yang W. J., “GEOMScope: large field-of-view 3d lensless microscopy with low computational complexity,” Laser Photonics Rev. 15(8), 2100072 (2021). 10.1002/lpor.202100072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Qiao P. F., Yang W. J., Chang-Hasnain C. J., “Recent advances in high-contrast metastructures, metasurfaces, and photonic crystals,” Adv. Opt. Photonics 10(1), 180–245 (2018). 10.1364/AOP.10.000180 [DOI] [Google Scholar]
- 422.Stirman J. N., et al. , “Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain,” Nat. Biotechnol. 34(8), 857–862 (2016). 10.1038/nbt.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ota K., et al. , “Fast, cell-resolution, contiguous-wide two-photon imaging to reveal functional network architectures across multi-modal cortical areas,” Neuron 109(11), 1810–1824.e9 (2021). 10.1016/j.neuron.2021.03.032 [DOI] [PubMed] [Google Scholar]
- 424.Cheng A., et al. , “Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing,” Nat. Methods 8(2), 139–142 (2011). 10.1038/nmeth.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Weisenburger S., et al. , “Volumetric Ca2+ imaging in the mouse brain using hybrid multiplexed sculpted light microscopy,” Cell 177(4), 1050–1066.e14 (2019). 10.1016/j.cell.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Lu R. W., et al. , “Rapid mesoscale volumetric imaging of neural activity with synaptic resolution,” Nat. Methods 17(3), 291–294 (2020). 10.1038/s41592-020-0760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Clough M., et al. , “Flexible simultaneous mesoscale two-photon imaging of neural activity at high speeds,” Nat. Commun. 12, 6638 (2021). 10.1038/s41467-021-26737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Denk W., Strickler J. H., Webb W. W., “Two-photon laser scanning fluorescence microscopy,” Science 248(4951), 73–76 (1990). 10.1126/science.2321027 [DOI] [PubMed] [Google Scholar]
- 429.Truong T. V., et al. , “Deep and fast live imaging with two-photon scanned light-sheet microscopy,” Nat. Methods 8(9), 757–760 (2011). 10.1038/nmeth.1652 [DOI] [PubMed] [Google Scholar]
- 430.Lu R. W., et al. , “Video-rate volumetric functional imaging of the brain at synaptic resolution,” Nat. Neurosci. 20(4), 620–628 (2017). 10.1038/nn.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Prevedel R., et al. , “Fast volumetric calcium imaging across multiple cortical layers using sculpted light,” Nat. Methods 13(12), 1021–1028 (2016). 10.1038/nmeth.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yang W. J., et al. , “Simultaneous multi-plane imaging of neural circuits,” Neuron 89(2), 269–284 (2016). 10.1016/j.neuron.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Zhang T., et al. , “Kilohertz two-photon brain imaging in awake mice,” Nat. Methods 16(11), 1119–1122 (2019). 10.1038/s41592-019-0597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Beaulieu D. R., et al. , “Simultaneous multiplane imaging with reverberation two-photon microscopy,” Nat. Methods 17(3), 283 (2020). 10.1038/s41592-019-0728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wu J. L., et al. , “Kilohertz two-photon fluorescence microscopy imaging of neural activity in vivo,” Nat. Methods 17(3), 287–290 (2020). 10.1038/s41592-020-0762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Reddy G. D., et al. , “Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity,” Nat. Neuroscie. 11(6), 713–720 (2008). 10.1038/nn.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ducros M., et al. , “Encoded multisite two-photon microscopy,” Proc. Natl. Acad. Sci. U. S. A. 110(32), 13138–13143 (2013). 10.1073/pnas.1307818110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Oron D., Tal E., Silberberg Y., “Scanningless depth-resolved microscopy,” Opt. Express 13(5), 1468–1476 (2005). 10.1364/OPEX.13.001468 [DOI] [PubMed] [Google Scholar]
- 439.Zhu G. H., et al. , “Simultaneous spatial and temporal focusing of femtosecond pulses,” Opt. Express 13(6), 2153–2159 (2005). 10.1364/OPEX.13.002153 [DOI] [PubMed] [Google Scholar]
- 440.Kumar M., et al. , “Integrated one- and two-photon scanned oblique plane illumination (SOPi) microscopy for rapid volumetric imaging,” Opt. Express 26(10), 13027–13041 (2018). 10.1364/OE.26.013027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Quirin S., et al. , “Simultaneous imaging of neural activity in three dimensions,” Front. Neural Circuit 8, 29 (2014). 10.3389/fncir.2014.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Prevedel R., et al. , “Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy,” Nat. Methods 11(7), 727–730 (2014). 10.1038/nmeth.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Broxton M., et al. , “Wave optics theory and 3-D deconvolution for the light field microscope,” Opt. Express 21(21), 25418–25439 (2013). 10.1364/OE.21.025418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Fan G. Y., et al. , “Video-rate scanning two-photon excitation fluorescence microscopy and ratio imaging with cameleons,” Biophys. J. 76(5), 2412–2420 (1999). 10.1016/S0006-3495(99)77396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Salome R., et al. , “Ultrafast random-access scanning in two-photon microscopy using acousto-optic deflectors,” J. Neurosci. Methods 154(1–2), 161–174 (2006). 10.1016/j.jneumeth.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 446.Reddy G. D., Saggau P., “Fast three-dimensional laser scanning scheme using acousto-optic deflectors,” J. Biomed. Opt. 10(6), 064038 (2005). 10.1117/1.2141504 [DOI] [PubMed] [Google Scholar]
- 447.Shain W. J., et al. , “Extended depth-of-field microscopy with a high-speed deformable mirror,” Opt. Lett. 42(5), 995–998 (2017). 10.1364/OL.42.000995 [DOI] [PubMed] [Google Scholar]
- 448.Dal Maschio M., et al. , “Three-dimensional in vivo scanning microscopy with inertia-free focus control,” Opt. Lett. 36(17), 3503–3505 (2011). 10.1364/OL.36.003503 [DOI] [PubMed] [Google Scholar]
- 449.Nikolenko V., et al. , “SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators,” Front. Neural Circuit 2, 5 (2008). 10.3389/neuro.04.005.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Gobel W., Kampa B. M., Helmchen F., “Imaging cellular network dynamics in three dimensions using fast 3D laser scanning,” Nat. Methods 4(1), 73–79 (2007). 10.1038/nmeth989 [DOI] [PubMed] [Google Scholar]
- 451.Grewe B. F., et al. , “Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens,” Biomed. Opt. Express 2(7), 2035–2046 (2011). 10.1364/BOE.2.002035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Kong L. J., et al. , “Continuous volumetric imaging via an optical phase-locked ultrasound lens,” Nat. Methods 12(8), 759–762 (2015). 10.1038/nmeth.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Chakraborty T., et al. , “Converting lateral scanning into axial focusing to speed up 3D microscopy,” Light Sci. Appl. 9, 165 (2020). 10.1038/s41377-020-00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Bawart M., et al. , “Modified Alvarez lens for high-speed focusing,” Opt. Express 25(24), 29847–29855 (2017). 10.1364/OE.25.029847 [DOI] [PubMed] [Google Scholar]
- 455.Kirkby P. A., Srinivas Nadella K. M., Silver R. A., “A compact acousto-optic lens for 2D and 3D femtosecond based 2-photon microscopy,” Opt. Express 18(13), 13720–13745 (2010). 10.1364/OE.18.013720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Lu R. W., et al. , “50 Hz volumetric functional imaging with continuously adjustable depth of focus,” Biomed. Opt. Express 9(4), 1964–1976 (2018). 10.1364/BOE.9.001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Meng G. H., et al. , “High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo,” Elife 8, e40805 (2019). 10.7554/eLife.40805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Bovetti S., et al. , “Simultaneous high-speed imaging and optogenetic inhibition in the intact mouse brain,” Sci. Rep. 7, 40041 (2017). 10.1038/srep40041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Anselmi F., et al. , “Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning,” Proc. Natl. Acad. Sci. U. S. A. 108(49), 19504–19509 (2011). 10.1073/pnas.1109111108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Moretti C., et al. , “Scanless functional imaging of hippocampal networks using patterned two-photon illumination through GRIN lenses,” Biomed. Opt. Express 7(10), 3958–3967 (2016). 10.1364/BOE.7.003958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Duocastella M., et al. , “Acousto-optic systems for advanced microscopy,” J. Phys.: Photonics 3, 012004 (2021). 10.1088/2515-7647/abc23c [DOI] [Google Scholar]
- 462.Griffiths V. A., et al. , “Real-time 3D movement correction for two-photon imaging in behaving animals,” Nat. Methods 17(7), 741–748 (2020). 10.1038/s41592-020-0851-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Fernandez-Alfonso T., et al. , “Monitoring synaptic and neuronal activity in 3D with synthetic and genetic indicators using a compact acousto-optic lens two-photon microscope,” J. Neurosci. Methods 222, 69–81 (2014). 10.1016/j.jneumeth.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Kaplan A., Friedman N., Davidson N., “Acousto-optic lens with very fast focus scanning,” Opt. Lett. 26(14), 1078–1080 (2001). 10.1364/OL.26.001078 [DOI] [PubMed] [Google Scholar]
- 465.Gurnani H., Silver R. A., “Multidimensional population activity in an electrically coupled inhibitory circuit in the cerebellar cortex,” Neuron 109(10), 1739–1753.e8 (2021). 10.1016/j.neuron.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Szalay G., et al. , “Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals,” Neuron 92(4), 723–738 (2016). 10.1016/j.neuron.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Konstantinou G., et al. , “Dynamic wavefront shaping with an acousto-optic lens for laser scanning microscopy,” Opt. Express 24(6), 6283–6299 (2016). 10.1364/OE.24.006283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Froudarakis E., et al. , “Population code in mouse V1 facilitates readout of natural scenes through increased sparseness,” Nat. Neurosci. 17(6), 851–857 (2014). 10.1038/nn.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Katona G., et al. , “Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes,” Nat. Methods 9(2), 201–208 (2012). 10.1038/nmeth.1851 [DOI] [PubMed] [Google Scholar]
- 470.Geiller T., et al. , “Large-scale 3D two-photon imaging of molecularly identified CA1 interneuron dynamics in behaving mice,” Neuron 108(5), 968–983.e9 (2020). 10.1016/j.neuron.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Lanore F., et al. , “Cerebellar granule cell axons support high dimensional representations,” Nat. Neurosci. 24(8), 1142–1150 (2021). 10.1038/s41593-021-00873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Akemann W., et al. , “Fast spatial beam shaping by acousto-optic diffraction for 3D non-linear microscopy,” Opt. Express 23(22), 28191–205 (2015). 10.1364/OE.23.028191 [DOI] [PubMed] [Google Scholar]
- 473.Mahou P., et al. , “Multicolor two-photon tissue imaging by wavelength mixing,” Nat. Methods 9(8), 815–818 (2012). 10.1038/nmeth.2098 [DOI] [PubMed] [Google Scholar]
- 474.Lakowicz J. R., et al. , “Two-color two-photon excitation of fluorescence,” Photochem. Photobiol. 64(4), 632–635 (1996). 10.1111/j.1751-1097.1996.tb03116.x [DOI] [PubMed] [Google Scholar]
- 475.Dowley M. W., Eisenthal K. B., Peticolas W. L., “Two-photon laser excitation of polycyclic aromatic molecules,” J. Chem. Phys. 47(5), 1609–1619 (1967). 10.1063/1.1712141 [DOI] [Google Scholar]
- 476.Monson P. R., Mcclain W. M., “Polarization dependence of the two-photon absorption of tumbling molecules with application to liquid 1-chloronaphthalene and benzene,” J. Chem. Phys. 53(1), 29–37 (1970). 10.1063/1.1673778 [DOI] [Google Scholar]
- 477.Frohlich D., Mahr H., “Two-photon spectroscopy in anthracene,” Phys. Rev. Lett. 16(20), 895–897 (1966). 10.1103/PhysRevLett.16.895 [DOI] [Google Scholar]
- 478.Sadegh S., et al. , “Overcoming the fundamental limit of two-photon microscopywith non-degenerate excitation,” in Biophotonics Congr.: Biomed. Opt. 2020 (Transl., Microsc., OCT, OTS, BRAIN), OSA Tech. Digest, Optica Publishing Group, p. BTu1C.4 (2020). [Google Scholar]
- 479.Sadegh S., et al. , “Measurement of the relative non-degenerate two-photon absorption cross-section for fluorescence microscopy,” Opt. Express 27(6), 8335–8347 (2019). 10.1364/OE.27.008335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Sadegh S., et al. , “Efficient non-degenerate two-photon excitation for fluorescence microscopy,” Opt. Express 27(20), 28022–28035 (2019). 10.1364/OE.27.028022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yang M. H., et al. , “Non-degenerate 2-photon excitation in scattering medium for fluorescence microscopy,” Opt. Express 24(26), 30173–30187 (2016). 10.1364/OE.24.030173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Hales J. M., et al. , “Resonant enhancement of two-photon absorption in substituted fluorene molecules,” J. Chem. Phys. 121(7), 3152–3160 (2004). 10.1063/1.1770726 [DOI] [PubMed] [Google Scholar]
- 483.Quentmeier S., Denicke S., Gericke K. H., “Two-color two-photon fluorescence laser scanning microscopy,” J. Fluoresc. 19(6), 1037–1043 (2009). 10.1007/s10895-009-0503-x [DOI] [PubMed] [Google Scholar]
- 484.Perillo E. P., et al. , “Two-color multiphoton in vivo imaging with a femtosecond diamond Raman laser,” Light-Sci. Appl. 6, e17095 (2017). 10.1038/lsa.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Lindek S., Stelzer E. H., “Resolution improvement by nonconfocal theta microscopy,” Opt. Lett. 24(21), 1505–1507 (1999). 10.1364/OL.24.001505 [DOI] [PubMed] [Google Scholar]
- 486.Ibanez-Lopez C., et al. , “Optical-sectioning improvement in two-color excitation scanning microscopy,” Microsc. Res. Tech. 64(2), 96–102 (2004). 10.1002/jemt.20073 [DOI] [PubMed] [Google Scholar]
- 487.Miller D. R., et al. , “Deep tissue imaging with multiphoton fluorescence microscopy,” Curr. Opin. Biomed. Eng. 4, 32–39 (2017). 10.1016/j.cobme.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Wang C., et al. , “Reduced deep-tissue image degradation in three-dimensional multiphoton microscopy with concentric two-color two-photon fluorescence excitation,” J. Opt. Soc. Am. B 25(6), 976–982 (2008). 10.1364/JOSAB.25.000976 [DOI] [Google Scholar]
- 489.Cambaliza M. O., Saloma C., “Advantages of two-color excitation fluorescence microscopy with two confocal excitation beams,” Opt. Commun. 184(1-4), 25–35 (2000). 10.1016/S0030-4018(00)00929-9 [DOI] [Google Scholar]
- 490.Blanca C. M., Saloma C., “Two-color excitation fluorescence microscopy through highly scattering media,” Appl. Opt. 40(16), 2722–2729 (2001). 10.1364/AO.40.002722 [DOI] [PubMed] [Google Scholar]
- 491.Kobat D., Zhu G., Xu C., “Background reduction with two-color two-beam multiphoton excitation,” in Biomed. Opt., OSA Tech. Digest (CD), Optica Publishing Group, p. BMF6 (2008). [Google Scholar]
- 492.Cheng X., et al. , “Comparing the fundamental imaging depth limit of two-photon, three-photon, and non-degenerate two-photon microscopy,” Opt. Lett. 45(10), 2934–2937 (2020). 10.1364/OL.392724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Chen T.-W., et al. , “Ultrasensitive fluorescent proteins for imaging neuronal activity,” Nature 499(7458), 295–300 (2013). 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Theer P., Denk W., “On the fundamental imaging-depth limit in two-photon microscopy,” J. Opt. Soc. Am. A Opt. Image Sci. Vision 23(12), 3139–3149 (2006). 10.1364/JOSAA.23.003139 [DOI] [PubMed] [Google Scholar]
- 495.Wang T., et al. , “Three-photon imaging of mouse brain structure and function through the intact skull,” Nat. Methods 15(10), 789–792 (2018). 10.1038/s41592-018-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Liu H., et al. , “In vivo deep-brain structural and hemodynamic multiphoton microscopy enabled by quantum dots,” Nano Lett. 19(8), 5260–5265 (2019). 10.1021/acs.nanolett.9b01708 [DOI] [PubMed] [Google Scholar]
- 497.Horton N. G., et al. , “In vivo three-photon microscopy of subcortical structures within an intact mouse brain,” Nat. Photonics 7, 205–209 (2013). 10.1038/nphoton.2012.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Kobat D., et al. , “Deep tissue multiphoton microscopy using longer wavelength excitation,” Opt. Express 17(16), 13354–13364 (2009). 10.1364/OE.17.013354 [DOI] [PubMed] [Google Scholar]
- 499.Wang M., et al. , “Comparing the effective attenuation lengths for long wavelength in vivo imaging of the mouse brain,” Biomed. Opt. Express 9(8), 3534–3543 (2018). 10.1364/BOE.9.003534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Takasaki K., Abbasi-asl R., Waters J., “Superficial bound of the depth limit of two- photon imaging in mouse brain,” eNeuro 7(1), ENEURO.0255-19.2019 (2020). 10.1523/ENEURO.0255-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wang T., et al. , “Quantitative analysis of 1300-nm three-photon calcium imaging in the mouse brain,” eLife 9, e53205 (2020). 10.7554/eLife.53205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Chow D. M., et al. , “Deep three-photon imaging of the brain in intact adult zebrafish,” Nat. Methods 17, 605–608 (2020). 10.1038/s41592-020-0819-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Hontani Y., Xia F., Xu C., “Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain,” Sci. Adv. 7, eabf3531 (2021). 10.1126/sciadv.abf3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Xu C., et al. , “Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy,” Proc. Natl. Acad. Sci. U. S. A. 93(20), 10763–10768 (1996). 10.1073/pnas.93.20.10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Xu C., Webb W. W., “Multiphoton excitation of molecular fluorophores and nonlinear laser microscopy,” Top. Fluoresc. Spectrosc. 5, 471–540 (1997). [Google Scholar]
- 506.Akbari N., et al. , “Imaging deeper than the transport mean free path with multiphoton microscopy,” Biomed. Opt. Express 13(1), 452–463 (2022). 10.1364/BOE.444696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Streich L., et al. , “High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy,” Nat. Methods 18(10), 1253–1258 (2021). 10.1038/s41592-021-01257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Rodríguez C., et al. , “An adaptive optics module for deep tissue multiphoton imaging in vivo,” Nat. Methods 18(10), 1259–1264 (2021). 10.1038/s41592-021-01279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Li B., et al. , “An adaptive excitation source for high-speed multiphoton microscopy,” Nat. Methods 17, 163–166 (2020). 10.1038/s41592-019-0663-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Wang M., et al. , “Impact of the emission wavelengths on in vivo multiphoton imaging of mouse brains,” Biomed. Opt. Express 10(4), 1905–1918 (2019). 10.1364/BOE.10.001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Babcock H. W., “The Possibility of compensating astronomical seeing,” Publ. Astron. Soc. Pac. 65, 229 (1953). 10.1086/126606 [DOI] [Google Scholar]
- 512.Tyson R. K., Principles of Adaptive Optics, CRC Press; (2015). [Google Scholar]
- 513.Liang J. Z., Williams D. R., Miller D. T., “Supernormal vision and high-resolution retinal imaging through adaptive optics,” J. Opt. Soc. Am. A 14(11), 2884–2892 (1997). 10.1364/JOSAA.14.002884 [DOI] [PubMed] [Google Scholar]
- 514.Porter J., et al. , Adaptive Optics for Vision Science: Principles, Practices, Design and Applications, Wiley; (2006). [Google Scholar]
- 515.Rodriguez C., Ji N., “Adaptive optical microscopy for neurobiology,” Curr. Opin. Neurobiol. 50, 83–91 (2018). 10.1016/j.conb.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Aviles-Espinosa R., et al. , “Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy,” Biomed. Opt. Express 2(11), 3135–3149 (2011). 10.1364/BOE.2.003135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Wang K., et al. , “Rapid adaptive optical recovery of optimal resolution over large volumes,” Nat. Methods 11(6), 625–628 (2014). 10.1038/nmeth.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Debarre D., et al. , “Image-based adaptive optics for two-photon microscopy,” Opt. Lett. 34(16), 2495–2497 (2009). 10.1364/OL.34.002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ji N., Milkie D. E., Betzig E., “Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues,” Nat. Methods 7(2), 141–147 (2010). 10.1038/nmeth.1411 [DOI] [PubMed] [Google Scholar]
- 520.Wang C., et al. , “Multiplexed aberration measurement for deep tissue imaging in vivo,” Nat. Methods 11(10), 1037–1040 (2014). 10.1038/nmeth.3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Papadopoulos I. N., et al. , “Scattering compensation by focus scanning holographic aberration probing (F-SHARP),” Nat. Photonics 11(2), 116–123 (2017). 10.1038/nphoton.2016.252 [DOI] [Google Scholar]
- 522.Wang K., et al. , “Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue,” Nat. Commun. 6, 7276 (2015). 10.1038/ncomms8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Liu R., et al. , “Direct wavefront sensing enables functional imaging of infragranular axons and spines,” Nat. Methods 16, 615–618 (2019). 10.1038/s41592-019-0434-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Jia N., Sato T. R., Betzig E., “Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex,” Proc. Natl. Acad. Sci. U. S. A. 109(1), 22–27 (2012). 10.1073/pnas.1109202108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Tao X. D., et al. , “Transcutical imaging with cellular and subcellular resolution,” Biomed. Opt. Express 8(3), 1277–1289 (2017). 10.1364/BOE.8.001277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Park J. H., et al. , “Large-field-of-view imaging by multi-pupil adaptive optics,” Nat. Methods 14(6), 581–583 (2017). 10.1038/nmeth.4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Papadopoulos I. N., et al. , “Dynamic conjugate F-SHARP microscopy,” Light-Sci. Appl. 9, 110 (2020). 10.1038/s41377-020-00348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Sun W. Z., et al. , “Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs,” Nat. Neurosci. 19(2), 308–315 (2016). 10.1038/nn.4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Chen I. W., Papagiakoumou E., Emiliani V., “Towards circuit optogenetics,” Curr. Opin. Neurobiol. 50, 179–189 (2018). 10.1016/j.conb.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Emiliani V., et al. , “All-Optical interrogation of neural circuits,” J. Neurosci. 35(41), 13917–13926 (2015). 10.1523/JNEUROSCI.2916-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Adesnik H., Abdeladim L., “Probing neural codes with two-photon holographic optogenetics,” Nat. Neurosci. 24(10), 1356–1366 (2021). 10.1038/s41593-021-00902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Packer A. M., et al. , “Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo,” Nat. Methods 12(2), 140–146 (2015). 10.1038/nmeth.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Shemesh O. A., et al. , “Temporally precise single-cell-resolution optogenetics,” Nat. Neurosci. 20(12), 1796–1806 (2017). 10.1038/s41593-017-0018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Forli A., et al. , “Two-photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo,” Cell Rep. 22(11), 3087–3098 (2018). 10.1016/j.celrep.2018.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Mardinly A. R., et al. , “Precise multimodal optical control of neural ensemble activity,” Nat. Neurosci. 21(6), 881–893 (2018). 10.1038/s41593-018-0139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Yang W., et al. , “Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions,” Elife 7, e32671 (2018). 10.7554/eLife.32671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Dal Maschio M., et al. , “Linking neurons to network function and behavior by two-photon holographic optogenetics and volumetric imaging,” Neuron 94(4), 774–789.e5 (2017). 10.1016/j.neuron.2017.04.034 [DOI] [PubMed] [Google Scholar]
- 538.Gill J. V., et al. , “Precise holographic manipulation of olfactory circuits reveals coding features determining perceptual detection,” Neuron 108(2), 382–393.e5 (2020). 10.1016/j.neuron.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Rickgauer J. P., Deisseroth K., Tank D. W., “Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields,” Nat. Neurosci. 17(12), 1816–1824 (2014). 10.1038/nn.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Papagiakoumou E., et al. , “Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses,” Opt. Express 16(26), 22039–22047 (2008). 10.1364/OE.16.022039 [DOI] [PubMed] [Google Scholar]
- 541.Golan L., et al. , “Design and characteristics of holographic neural photo-stimulation systems,” J. Neural Eng. 6(6), 066004 (2009). 10.1088/1741-2560/6/6/066004 [DOI] [PubMed] [Google Scholar]
- 542.Pégard N. C., et al. , “Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT),” Nat. Commun. 8, 1228 (2017). 10.1038/s41467-017-01031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Accanto N., et al. , “Multiplexed temporally focused light shaping through a gradient index lens for precise in-depth optogenetic photostimulation,” Sci. Rep. 9, 7603 (2019). 10.1038/s41598-019-43933-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Papagiakoumou E., et al. , “Scanless two-photon excitation of channelrhodopsin-2,” Nat. Methods 7(10), 848–854 (2010). 10.1038/nmeth.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Paluch-Siegler S., et al. , “All-optical bidirectional neural interfacing using hybrid multiphoton holographic optogenetic stimulation,” Neurophotonics 2(3), 031208 (2015). 10.1117/1.NPh.2.3.031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Chen I. W., et al. , “In vivo submillisecond two-photon optogenetics with temporally focused patterned light,” J. Neurosci. 39(18), 3484–3497 (2019). 10.1523/JNEUROSCI.1785-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Aharoni T., Shoham S., “Phase-controlled, speckle-free holographic projection with applications in precision optogenetics,” Neurophotonics 5(2), 025004 (2018). 10.1117/1.NPh.5.2.025004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Lerman G. M., et al. , “Real-time in situ holographic optogenetics confocally unraveled sculpting microscopy,” Laser Photonics Rev. 13(9), 1900144 (2019). 10.1002/lpor.201900144 [DOI] [Google Scholar]
- 549.Chaigneau E., et al. , “Two-photon holographic stimulation of ReaChR,” Front. Cell Neurosci. 10, 234 (2016). 10.3389/fncel.2016.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Baker C. A., et al. , “Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin,” Elife 5, e14193 (2016). 10.7554/eLife.14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Forli A., et al. , “Optogenetic strategies for high-efficiency all-optical interrogation using blue-light-sensitive opsins,” Elife 10, e63359 (2021). 10.7554/eLife.63359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Bounds H. A., et al. , “Multifunctional Cre-dependent transgenic mice for high-precision all-optical interrogation of neural circuits,” 10.1101/2021.10.05.463223 (2021). [DOI]
- 553.Sun S., et al. , “Large-scale femtosecond holography for near simultaneous optogenetic neural modulation,” Opt. Express 27(22), 32228–32234 (2019). 10.1364/OE.27.032228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Faini G., et al. , “Ultrafast light targeting for high-throughput precise control of neuronal networks,” 10.1101/2021.06.14.448315 (2021). [DOI] [PMC free article] [PubMed]
- 555.Frumker E., Silberberg Y., “Femtosecond pulse shaping using a two-dimensional liquid-crystal spatial light modulator,” Opti. Lett. 32(11), 1384–1386 (2007). 10.1364/OL.32.001384 [DOI] [PubMed] [Google Scholar]
- 556.Mayblum T., et al. , “New insights and system designs for temporally focused multiphoton optogenetics,” Proc SPIE 9329, 932928 (2015). 10.1117/12.2078678 [DOI] [Google Scholar]
- 557.Hernandez O., et al. , “Three-dimensional spatiotemporal focusing of holographic patterns,” Nat. Commun. 7, 11928 (2016). 10.1038/ncomms11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Sun B., et al. , “Four-dimensional light shaping: manipulating ultrafast spatiotemporal foci in space and time,” Light: Sci. Appl. 7(1), 17117 (2018). 10.1038/lsa.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Lerman G. M., et al. , “Precise optical probing of perceptual detection,” bioRxiv, 10.1101/456764 (2018). [Google Scholar]
- 560.Chong E., et al. , “Manipulating synthetic optogenetic odors reveals the coding logic of olfactory perception,” Science 368(6497), eaba2357 (2020). 10.1126/science.aba2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Dalgleish H. W., et al. , “How many neurons are sufficient for perception of cortical activity?” Elife 9, e58889 (2020). 10.7554/eLife.58889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Newsome W. T., Britten K. H., Movshon J. A., “Neuronal correlates of a perceptual decision,” Nature 341(6237), 52–54 (1989). 10.1038/341052a0 [DOI] [PubMed] [Google Scholar]
- 563.Blinder P., et al. , “The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow,” Nat. Neurosci. 16(7), 889–897 (2013). 10.1038/nn.3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Cauli B., Hamel E., “Revisiting the role of neurons in neurovascular coupling,” Front. Neuroenerg. 2, 9 (2010). 10.3389/fnene.2010.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Uhlirova H., et al. , “Cell type specificity of neurovascular coupling in cerebral cortex,” Elife 5, e14315 (2016). 10.7554/eLife.14315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Uhlirova H., et al. , “The roadmap for estimation of cell-type-specific neuronal activity from non-invasive measurements,” Philos. Trans. R. Soc. Lond. B Biol. Sci. 371(1705), 20150356 (2016). 10.1098/rstb.2015.0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Boas D. A., Dunn A. K., “Laser speckle contrast imaging in biomedical optics,” J. Biomed. Opt. 15(1), 011109 (2010). 10.1117/1.3285504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Dunn A. K., et al. , “Dynamic imaging of cerebral blood flow using laser speckle,” J. Cereb. Blood Flow Metab. 21(3), 195–201 (2001). 10.1097/00004647-200103000-00002 [DOI] [PubMed] [Google Scholar]
- 569.Dunn A. K., et al. , “Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation,” Opt. Lett. 28(1), 28–30 (2003). 10.1364/OL.28.000028 [DOI] [PubMed] [Google Scholar]
- 570.Briers J. D., “Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging,” Physiol. Meas. 22(4), R35–R66 (2001). 10.1088/0967-3334/22/4/201 [DOI] [PubMed] [Google Scholar]
- 571.Devor A., et al. , “Two-photon laser scanning microscopy as a tool to study cortical vasodynamics under normal and ischemic conditions,” in Imaging the Brain with Optical Methods, Roe A. W., Ed., pp. 245–261, Springer; (2009). [Google Scholar]
- 572.Devor A., et al. , “Frontiers in optical imaging of cerebral blood flow and metabolism,” J. Cereb. Blood Flow Metab. 32(7), 1259–1276 (2012). 10.1038/jcbfm.2011.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Devor A., et al. , “Functional imaging of cerebral oxygenation with intrinsic optical contrast and phosphorescent probes,” in Optical Imaging of Cortical Circuit Dynamics, Weber B., Helmchen F., Eds., Springer, New York: (2013). [Google Scholar]
- 574.Sakadžić S., et al. , “Two-photon microscopy measurement of cerebral metabolic rate of oxygen using periarteriolar oxygen concentration gradients,” Neurophotonics 3(4), 045005 (2016). 10.1117/1.NPh.3.4.045005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Mächler P., et al. , “Microscopic quantification of oxygen consumption across cortical layers,” 10.1101/2021.10.13.464176 (2021). [DOI]
- 576.Mayhew J., et al. , “Spectroscopic analysis of changes in remitted illumination: the response to increased neural activity in brain,” Neuroimage 10(3), 304–326 (1999). 10.1006/nimg.1999.0460 [DOI] [PubMed] [Google Scholar]
- 577.Boas D. A., et al. , “Twenty years of functional near-infrared spectroscopy: introduction for the special issue,” Neuroimage 85(Pt 1), 1–5 (2014). 10.1016/j.neuroimage.2013.11.033 [DOI] [PubMed] [Google Scholar]
- 578.Grinvald A., et al. , “Imaging the neocortex functional architecture using multiple intrinsic signals: implications for hemodynamic-based functional imaging,” Cold Spring Harb. Protoc. 2016(3), pdb top089375 (2016). 10.1101/pdb.top089375 [DOI] [PubMed] [Google Scholar]
- 579.Wang L. V., Yao J., “A practical guide to photoacoustic tomography in the life sciences,” Nat. Methods 13(8), 627–638 (2016). 10.1038/nmeth.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Yao J. J., Wang L. H. V., “Photoacoustic brain imaging: from microscopic to macroscopic scales,” Neurophotonics 1(1), 011003 (2014). 10.1117/1.NPh.1.1.011003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Wang R. K., An L., “Doppler optical micro-angiography for volumetric imaging of vascular perfusion in vivo,” Opt. Express 17(11), 8926–8940 (2009). 10.1364/OE.17.008926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Baran U., Wang R. K., “Review of optical coherence tomography based angiography in neuroscience,” Neurophotonics 3(1), 010902 (2016). 10.1117/1.NPh.3.1.010902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Srinivasan V. J., et al. , “OCT methods for capillary velocimetry,” Biomed. Opt. Express 3(3), 612–29 (2012). 10.1364/BOE.3.000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ren H., Du C., Pan Y., “Cerebral blood flow imaged with ultrahigh-resolution optical coherence angiography and Doppler tomography,” Opt. Lett. 37(8), 1388–1390 (2012). 10.1364/OL.37.001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Rakymzhan A., et al. , “Optical microangiography reveals temporal and depth-resolved hemodynamic change in mouse barrel cortex during whisker stimulation,” J. Biomed. Opt. 25(9), 096005 (2020). 10.1117/1.JBO.25.9.096005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Merkle C. W., Srinivasan V. J., “Laminar microvascular transit time distribution in the mouse somatosensory cortex revealed by dynamic contrast optical coherence tomography,” Neuroimage 125, 350–362 (2016). 10.1016/j.neuroimage.2015.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Srinivasan V. J., Radhakrishnan H., “Optical coherence tomography angiography reveals laminar microvascular hemodynamics in the rat somatosensory cortex during activation,” Neuroimage 102(Pt 2), 393–406 (2014). 10.1016/j.neuroimage.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Li Y., Wei W., Wang R. K., “Capillary flow homogenization during functional activation revealed by optical coherence tomography angiography based capillary velocimetry,” Sci. Rep. 8, 4107 (2018). 10.1038/s41598-018-22513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Erdener S. E., et al. , “Spatio-temporal dynamics of cerebral capillary segments with stalling red blood cells,” J. Cereb. Blood Flow Metab. 39(5), 886–900 (2019). 10.1177/0271678X17743877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Shin P., et al. , “High-speed optical coherence tomography angiography for the measurement of stimulus-induced retrograde vasodilation of cerebral pial arteries in awake mice,” Neurophotonics 7(3), 030502 (2020). 10.1117/1.NPh.7.3.030502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Chen S., et al. , “Imaging hemodynamic response after ischemic stroke in mouse cortex using visible-light optical coherence tomography,” Biomed. Opt. Express 7(9), 3377–3389 (2016). 10.1364/BOE.7.003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Chong S. P., et al. , “Cerebral metabolic rate of oxygen (CMRO2) assessed by combined Doppler and spectroscopic OCT,” Biomed. Opt. Express 6(10), 3941–3951 (2015). 10.1364/BOE.6.003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Tang P., et al. , “Measurement and visualization of stimulus-evoked tissue dynamics in mouse barrel cortex using phase-sensitive optical coherence tomography,” Biomed. Opt. Express 11(2), 699–710 (2020). 10.1364/BOE.381332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Tang J., et al. , “Imaging localized fast optical signals of neural activation with optical coherence tomography in awake mice,” Opt. Lett. 46(7), 1744–1747 (2021). 10.1364/OL.411897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Marchand P. J., et al. , “Validation of red blood cell flux and velocity estimations based on optical coherence tomography intensity fluctuations,” Sci. Rep. 10, 19584 (2020). 10.1038/s41598-020-76774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Swartz H. M., “Measuring real levels of oxygen in vivo: opportunities and challenges,” Biochem. Soc. Trans. 30, 248–252 (2002). 10.1042/bst0300248 [DOI] [PubMed] [Google Scholar]
- 597.Arbeit J. M., et al. , “Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy,” Int. J. Radiat. Biol. 82(10), 699–757 (2006). 10.1080/09553000601002324 [DOI] [PubMed] [Google Scholar]
- 598.Vikram D. S., Zweier J. L., Kuppusamy P., “Methods for noninvasive imaging of tissue hypoxia,” Antioxid. Redox Signaling 9(10), 1745–1756 (2007). 10.1089/ars.2007.1717 [DOI] [PubMed] [Google Scholar]
- 599.Krohn K. A., Link J. M., Mason R. P., “Molecular imaging of hypoxia,” J. Nucl. Med. 49, 129S–148S (2008). 10.2967/jnumed.107.045914 [DOI] [PubMed] [Google Scholar]
- 600.Finikova O. S., et al. , “Energy and electron transfer in enhanced two-photon-absorbing systems with triplet cores,” J. Phys. Chem. A 111(30), 6977–6990 (2007). 10.1021/jp071586f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Finikova O. S., et al. , “Dynamic quenching of porphyrin triplet states by two-photon absorbing dyes: Towards two-photon-enhanced oxygen nanosensors,” J. Photochem. Photobiol., A: Chem. 198(1), 75–84 (2008). 10.1016/j.jphotochem.2008.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Lebedev A. Y., Troxler T., Vinogradov S. A., “Design of metalloporphyrin-based dendritic nanoprobes for two-photon microscopy of oxygen,” J. Porphyrins. Phthalocyanines 12(12), 1261–1269 (2008). 10.1142/S1088424608000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Sinks L. E., et al. , “Two-photon microscopy of oxygen: polymersomes as probe carrier vehicles,” J. Phys. Chem. B 114(45), 14373–14382 (2010). 10.1021/jp100353v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Li B., et al. , “More homogeneous capillary flow and oxygenation in deeper cortical layers correlate with increased oxygen extraction,” Elife 8, e42299 (2019). 10.7554/eLife.42299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Buxton R. B., “Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism,” Front. Neuroenerg. 2, 8 (2010). 10.3389/fnene.2010.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Kasischke K. A., et al. , “Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions,” J. Cereb. Blood Flow Metab. 31(1), 68–81 (2011). 10.1038/jcbfm.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Krogh A., “The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue,” J. Physiol. 52(6), 409–415 (1919). 10.1113/jphysiol.1919.sp001839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Wang L. H. V., Hu S., “Photoacoustic tomography: in vivo imaging from organelles to organs,” Science 335(6075), 1458–1462 (2012). 10.1126/science.1216210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Li L., et al. , “Snapshot photoacoustic topography through an ergodic relay of optical absorption in vivo,” Nat. Protoc. 16(5), 2381–2394 (2021). 10.1038/s41596-020-00487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Li L., et al. , “Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution,” Nat. Biomed. Eng. 1(5), 1–11 (2017). 10.1038/s41551-017-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Zhang P., et al. , “High-resolution deep functional imaging of the whole mouse brain by photoacoustic computed tomography in vivo,” J. Biophotonics 11(1), e201700024 (2018). 10.1002/jbio.201700024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Zhang P., et al. , “In vivo superresolution photoacoustic computed tomography by localization of single dyed droplets,” Light: Sci. Appl. 8(1), 36 (2019). 10.1038/s41377-019-0147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Yao J., et al. , “High-speed label-free functional photoacoustic microscopy of mouse brain in action,” Nat. Methods 12(5), 407 (2015). 10.1038/nmeth.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Li L., et al. , “Label-free photoacoustic tomography of whole mouse brain structures ex vivo,” Neurophotonics 3(3), 035001 (2016). 10.1117/1.NPh.3.3.035001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Na S., et al. , “Massively parallel functional photoacoustic computed tomography of the human brain,” Nat. Biomed. Eng. (2021). 10.1038/s41551-021-00735-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Gottschalk S., et al. , “Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain,” Nat. Biomed. Eng. 3(5), 392–401 (2019). 10.1038/s41551-019-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Rao B., et al. , “Photoacoustic imaging of voltage responses beyond the optical diffusion limit,” Sci. Rep. 7, 2560 (2017). 10.1038/s41598-017-02458-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Chamanzar M., et al. , “Ultrasonic sculpting of virtual optical waveguides in tissue,” Nat. Commun. 10, 92 (2019). 10.1038/s41467-018-07856-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Ruan H. W., et al. , “Fluorescence imaging through dynamic scattering media with speckle-encoded ultrasound-modulated light correlation,” Nat. Photonics 14(8), 511–516 (2020). 10.1038/s41566-020-0630-0 [DOI] [Google Scholar]
- 620.Xu X. A., Liu H. L., Wang L. V., “Time-reversed ultrasonically encoded optical focusing into scattering media,” Nat. Photonics 5(3), 154–157 (2011). 10.1038/nphoton.2010.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Wang Y. M., et al. , “Deep-tissue focal fluorescence imaging with digitally time-reversed ultrasound-encoded light,” Nat. Commun. 3, 928 (2012). 10.1038/ncomms1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Si K., Fiolka R., Cui M., “Fluorescence imaging beyond the ballistic regime by ultrasound-pulse-guided digital phase conjugation,” Nat. Photonics 6(10), 657–661 (2012). 10.1038/nphoton.2012.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Jiang Y., et al. , “Optoacoustic brain stimulation at submillimeter spatial precision,” Nat. Commun. 11, 881 (2020). 10.1038/s41467-020-14706-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Zhang Y., et al. , “Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics,” Proc. Natl. Acad. Sci. U. S. A. 116(43), 21427–21437 (2019). 10.1073/pnas.1909850116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Hong G., Lieber C. M., “Novel electrode technologies for neural recordings,” Nat. Rev. Neurosci. 20(6), 330–345 (2019). 10.1038/s41583-019-0140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Moreaux L. C., et al. , “Integrated neurophotonics: toward dense volumetric interrogation of brain circuit activity-at depth and in real time,” Neuron 108(1), 66–92 (2020). 10.1016/j.neuron.2020.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Kuzum D., et al. , “Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging,” Nat. Commun. 5, 5259 (2014). 10.1038/ncomms6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Donahue M. J., et al. , “Multimodal characterization of neural networks using highly transparent electrode arrays,” Eneuro 5(6), ENEURO.0187-18.2018 (2018). 10.1523/ENEURO.0187-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Qiang Y., et al. , “Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain,” Sci. Adv. 4(9), eaat0626 (2018). 10.1126/sciadv.aat0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Lake E. M. R., et al. , “Simultaneous cortex-wide fluorescence Ca(2+) imaging and whole-brain fMRI,” Nat. Methods, 17(12), 1262–1271 (2020). 10.1038/s41592-020-00984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Schulz K., et al. , “Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex,” Nat. Methods 9(6), 597–602 (2012). 10.1038/nmeth.2013 [DOI] [PubMed] [Google Scholar]
- 632.Wang M., et al. , “Brain-state dependent astrocytic Ca(2+) signals are coupled to both positive and negative BOLD-fMRI signals,” Proc. Natl. Acad. Sci. U. S. A. 115(7), E1647–E1656 (2018). 10.1073/pnas.1711692115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Lee J. H., et al. , “Global and local fMRI signals driven by neurons defined optogenetically by type and wiring,” Nature 465(7299), 788–792 (2010). 10.1038/nature09108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Kennerley A. J., et al. , “Concurrent fMRI and optical measures for the investigation of the hemodynamic response function,” Magn. Reson. Med. 54(2), 354–365 (2005). 10.1002/mrm.20511 [DOI] [PubMed] [Google Scholar]
- 635.Brake J., Jang M., Yang C. H., “Analyzing the relationship between decorrelation time and tissue thickness in acute rat brain slices using multispeckle diffusing wave spectroscopy,” J. Opt. Soc. Am. A 33(2), 270–275 (2016). 10.1364/JOSAA.33.000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Yaqoob Z., et al. , “Optical phase conjugation for turbidity suppression in biological samples,” Nat. Photonics 2(2), 110–115 (2008). 10.1038/nphoton.2007.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Liu Y., et al. , “Optical focusing deep inside dynamic scattering media with near-infrared time-reversed ultrasonically encoded (TRUE) light,” Nat. Commun. 6, 5904 (2015). 10.1038/ncomms6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Vellekoop I. M., Mosk A. P., “Focusing coherent light through opaque strongly scattering media,” Opt. Lett. 32(16), 2309–2311 (2007). 10.1364/OL.32.002309 [DOI] [PubMed] [Google Scholar]
- 639.Ma C., et al. , “Time-reversed adapted-perturbation (TRAP) optical focusing onto dynamic objects inside scattering media,” Nat. Photonics 8(12), 931–936 (2014). 10.1038/nphoton.2014.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Zhou E. H., et al. , “Focusing on moving targets through scattering samples,” Optica 1(4), 227–232 (2014). 10.1364/OPTICA.1.000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Ruan H. W., et al. , “Focusing light inside scattering media with magnetic-particle-guided wavefront shaping,” Optica 4(11), 1337–1343 (2017). 10.1364/OPTICA.4.001337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Lai P. X., et al. , “Photoacoustically guided wavefront shaping for enhanced optical focusing in scattering media,” Nat. Photonics 9(2), 126–132 (2015). 10.1038/nphoton.2014.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Shen Y. C., et al. , “Focusing light through biological tissue and tissue-mimicking phantoms up to 9.6 cm in thickness with digital optical phase conjugation,” J. Biomed. Opt. 21(8), 085001 (2016). 10.1117/1.JBO.21.8.085001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Qureshi M. M., et al. , “In vivo study of optical speckle decorrelation time across depths in the mouse brain,” Biomed. Opt. Express 8(11), 4855–4864 (2017). 10.1364/BOE.8.004855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Wang D. F., et al. , “Focusing through dynamic tissue with millisecond digital optical phase conjugation,” Optica 2(8), 728–735 (2015). 10.1364/OPTICA.2.000728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Feldkhun D., et al. , “Focusing and scanning through scattering media in microseconds,” Optica 6(1), 72–75 (2019). 10.1364/OPTICA.6.000072 [DOI] [Google Scholar]
- 647.Tzang O., et al. , “Wavefront shaping in complex media with a 350 kHz modulator via a 1D-to-2D transform,” Nat. Photonics 13(11), 788–793 (2019). 10.1038/s41566-019-0503-6 [DOI] [Google Scholar]
- 648.Laforest T., et al. , “Co-integration of a smart CMOS image sensor and a spatial light modulator for real-time optical phase modulation,” Proc SPIE 9022, 90220N (2014). 10.1117/12.2039867 [DOI] [Google Scholar]
- 649.Pisanello F., et al. , “Multipoint-emitting optical fibers for spatially addressable in vivo optogenetics,” Neuron 82(6), 1245–1254 (2014). 10.1016/j.neuron.2014.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Pisanello F., et al. , “Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber,” Nat. Neurosci. 20(8), 1180–1188 (2017). 10.1038/nn.4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Pisano F., et al. , “Depth-resolved fiber photometry with a single tapered optical fiber implant,” Nat. Methods 16(11), 1185–1192 (2019). 10.1038/s41592-019-0581-x [DOI] [PubMed] [Google Scholar]
- 652.Balena A., et al. , “Two-photon fluorescence-assisted laser ablation of non-planar metal surfaces: fabrication of optical apertures on tapered fibers for optical neural interfaces,” Opt. Express 28(15), 21368–21381 (2020). 10.1364/OE.395187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Maglie E., et al. , “Ray tracing models for estimating light collection properties of microstructured tapered optical fibers for optical neural interfaces,” Opt. Lett. 45(14), 3856–3859 (2020). 10.1364/OL.397022 [DOI] [PubMed] [Google Scholar]
- 654.Pisano F., et al. , “Focused ion beam nanomachining of tapered optical fibers for patterned light delivery,” Microelectron. Eng. 195, 41–49 (2018). 10.1016/j.mee.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Lee J., Wang W. G., Sabatini B. L., “Anatomically segregated basal ganglia pathways allow parallel behavioral modulation,” Nat. Neurosci. 23(11), 1388–1398 (2020). 10.1038/s41593-020-00712-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Lee S. J., et al. , “Monitoring behaviorally induced biochemical changes using fluorescence lifetime photometry,” Front. Neurosci. 13, 766 (2019). 10.3389/fnins.2019.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Bianco M., et al. , “Comparative study of autofluorescence in flat and tapered optical fibers towards application in depth-resolved fluorescence lifetime photometry in brain tissue,” Biomed. Opt. Express 12(2), 993–1009 (2021). 10.1364/BOE.410244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Spagnolo B., et al. , “Integrated tapered fibertrode for simultaneous control and readout of neural activity over small brain volumes with reduced light-induced artefacts,” 10.1101/2020.07.31.226795 (2021). [DOI]
- 659.Pisano F., et al. , “Plasmonics on a neural implant: engineering light-matter interactions on the nonplanar surface of tapered optical fibers,” Adv. Opt. Mater. 10(2), 2101649 (2022). 10.1002/adom.202101649 [DOI] [Google Scholar]
- 660.Pisano F., et al. , “Single-cell micro- and nano-photonic technologies,” J. Neurosci. Methods 325, 108355 (2019). 10.1016/j.jneumeth.2019.108355 [DOI] [PubMed] [Google Scholar]
- 661.Park D. W., et al. , “Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications,” Nat. Commun. 5, 5258 (2014). 10.1038/ncomms6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Thunemann M., et al. , “Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays,” Nat. Commun. 9, 2035 (2018). 10.1038/s41467-018-04457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Driscoll N., et al. , “Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale,” Commun. Biol. 4(1), 136 (2021). 10.1038/s42003-021-01670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Park D.-W., et al. , “Electrical neural stimulation and simultaneous in vivo monitoring with transparent graphene electrode arrays implanted in GCaMP6f mice,” ACS Nano 12(1), 148–157 (2018). 10.1021/acsnano.7b04321 [DOI] [PubMed] [Google Scholar]
- 665.Liu X., et al. , “A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes,” Front. Neurosci. 12, 132 (2018). 10.3389/fnins.2018.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Seo K. J., et al. , “Transparent, flexible, penetrating microelectrode arrays with capabilities of single-unit electrophysiology,” Adv. Biosyst. 3(3), 1800276 (2019). 10.1002/adbi.201800276 [DOI] [PubMed] [Google Scholar]
- 667.Geim A. K., “Graphene: status and prospects,” Science 324(5934), 1530–1534 (2009). 10.1126/science.1158877 [DOI] [PubMed] [Google Scholar]
- 668.Kostarelos K., et al. , “Graphene in the design and engineering of next-generation neural interfaces,” Adv. Mater. 29(42), 1700909 (2017). 10.1002/adma.201700909 [DOI] [PubMed] [Google Scholar]
- 669.Rastogi S. K., et al. , “Effect of graphene on nonneuronal and neuronal cell viability and stress,” Nano Lett. 17(5), 3297–3301 (2017). 10.1021/acs.nanolett.7b01215 [DOI] [PubMed] [Google Scholar]
- 670.Zhang J., et al. , “Stretchable transparent electrode array for simultaneous electrical and optical interrogation of neural circuits in vivo,” Nano Lett. 18(5), 2903–2911 (2018). 10.1021/acs.nanolett.8b00087 [DOI] [PubMed] [Google Scholar]
- 671.Lu Y. C., et al. , “Ultralow Impedance graphene microelectrodes with high optical transparency for simultaneous deep two-photon imaging in transgenic mice,” Adv. Funct. Mater. 28(31), 1800002 (2018). 10.1002/adfm.201800002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Barkhof F., Haller S., Rombouts S. A. R. B., “Resting-state functional MR imaging: a new window to the brain,” Radiology 272(1), 29–49 (2014). 10.1148/radiol.14132388 [DOI] [PubMed] [Google Scholar]
- 673.He B. J., “Spontaneous and task-evoked brain activity negatively interact,” J. Neurosci. 33(11), 4672–4682 (2013). 10.1523/JNEUROSCI.2922-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Ito T., et al. , “Task-evoked activity quenches neural correlations and variability across cortical areas,” PLoS Comput. Biol. 16(8), e1007983 (2020). 10.1371/journal.pcbi.1007983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Albers F., et al. , “Multimodal functional neuroimaging by simultaneous BOLD fMRI and fiber-optic calcium recordings and optogenetic control,” Mol. Imaging Biol. 20(2), 171–182 (2018). 10.1007/s11307-017-1130-6 [DOI] [PubMed] [Google Scholar]
- 676.Chen X. M., et al. , “Mapping optogenetically-driven single-vessel fMRI with concurrent neuronal calcium recordings in the rat hippocampus,” Nat. Commun. 10, 5239 (2019). 10.1038/s41467-019-12850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Lee J. H., “Informing brain connectivity with optogenetic functional magnetic resonance imaging,” Neuroimage 62(4), 2244–2249 (2012). 10.1016/j.neuroimage.2012.01.116 [DOI] [PubMed] [Google Scholar]
- 678.Miyamoto D., Murayama M., “The fiber-optic imaging and manipulation of neural activity during animal behavior,” Neurosci. Res. 103, 1–9 (2016). 10.1016/j.neures.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 679.Palmer H. S., “Optogenetic fMRI sheds light on the neural basis of the BOLD signal,” J. Neurophysiol. 104(4), 1838–1840 (2010). 10.1152/jn.00535.2010 [DOI] [PubMed] [Google Scholar]
- 680.Schlegel F., et al. , “Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice,” Nat. Protoc. 13(5), 840–855 (2018). 10.1038/nprot.2018.003 [DOI] [PubMed] [Google Scholar]
- 681.Schwalm M., et al. , “Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves,” Elife 6, e27602 (2017). 10.7554/eLife.27602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Pegard N. C., et al. , “Compressive light-field microscopy for 3D neural activity recording,” Optica 3(5), 517–524 (2016). 10.1364/OPTICA.3.000517 [DOI] [Google Scholar]
- 683.Nobauer T., et al. , “Video rate volumetric Ca2+ imaging across cortex using seeded iterative demixing (SID) microscopy,” Nat. Methods 14(8), 811–818 (2017). 10.1038/nmeth.4341 [DOI] [PubMed] [Google Scholar]
- 684.Truong T. V., et al. , “High-contrast, synchronous volumetric imaging with selective volume illumination microscopy,” Commun. Biol. 3, 74 (2020). 10.1038/s42003-020-0787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Zhang Z. K., et al. , “Imaging volumetric dynamics at high speed in mouse and zebrafish brain with confocal light field microscopy,” Nat. Biotechnol. 39(1), 74–83 (2021). 10.1038/s41587-020-0628-7 [DOI] [PubMed] [Google Scholar]
- 686.Quicke P., et al. , “Subcellular resolution three-dimensional light-field imaging with genetically encoded voltage indicators,” Neurophotonics 7(3), 035006 (2020). 10.1117/1.NPh.7.3.035006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Cong L., et al. , “Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio),” Elife 6, e28158 (2017). 10.7554/eLife.28158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Skocek O., et al. , “High-speed volumetric imaging of neuronal activity in freely moving rodents,” Nat. Methods 15(6), 429–432 (2018). 10.1038/s41592-018-0008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Turcottea R., et al. , “Dynamic super-resolution structured illumination imaging in the living brain,” Proc. Natl. Acad. Sci. U. S. A. 116(19), 9586–9591 (2019). 10.1073/pnas.1819965116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Yanny K., et al. , “Miniscope3D: optimized single-shot miniature 3D fluorescence microscopy,” Light-Sci. Appl. 9(1), 171 (2020). 10.1038/s41377-020-00403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Adams J. K., et al. , “Single-frame 3D fluorescence microscopy with ultraminiature lensless FlatScope,” Sci. Adv. 3(12), e1701548 (2017). 10.1126/sciadv.1701548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Hong S., et al. , “Structured illumination microscopy for the investigation of synaptic structure and function,” Synapse Dev. 1538, 155–167 (2017). 10.1007/978-1-4939-6688-2_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Winter P. W., et al. , “Two-photon instant structured illumination microscopy improves the depth penetration of super-resolution imaging in thick scattering samples,” Optica 1(3), 181–191 (2014). 10.1364/OPTICA.1.000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Tahir W., et al. , “Anatomical modeling of brain vasculature in two-photon microscopy by generalizable deep learning,” BME Front. 2021, 1 (2021). 10.34133/2021/8620932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Stefan S., Lee J., “Deep learning toolbox for automated enhancement, segmentation, and graphing of cortical optical coherence tomography microangiograms,” Biomed. Opt. Express 11(12), 7325–7342 (2020). 10.1364/BOE.405763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Soltanian-Zadeh S., et al. , “Fast and robust active neuron segmentation in two-photon calcium imaging using spatiotemporal deep learning,” Proc. Natl. Acad. Sci. U. S. A. 116(17), 8554–8563 (2019). 10.1073/pnas.1812995116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Lecoq J., et al. , “Removing independent noise in systems neuroscience data using DeepInterpolation,” Nat. Methods 18(11), 1401–1408 (2021). 10.1038/s41592-021-01285-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Bao Y. J., et al. , “Segmentation of neurons from fluorescence calcium recordings beyond real time,” Nat. Mach. Intell. 3(7), 590–600 (2021). 10.1038/s42256-021-00342-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Rupprecht P., et al. , “A database and deep learning toolbox for noise-optimized, generalized spike inference from calcium imaging,” Nat. Neurosci. 24(9), 1324–1337 (2021). 10.1038/s41593-021-00895-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Giovannucci A., et al. , “CaImAn an open source tool for scalable calcium imaging data analysis,” Elife 8, e38173 (2019). 10.7554/eLife.38173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Wang Z. Q., et al. , “Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning,” Nat. Methods 18(5), 551 (2021). 10.1038/s41592-021-01058-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Wagner N., et al. , “Deep learning-enhanced light-field imaging with continuous validation,” Nat. Methods 18(5), 557–563 (2021). 10.1038/s41592-021-01136-0 [DOI] [PubMed] [Google Scholar]
- 703.Jin L. H., et al. , “Deep learning enables structured illumination microscopy with low light levels and enhanced speed,” Nat. Commun. 11, 1934 (2020). 10.1038/s41467-020-15784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Pavone F. S., Shoham S., Handbook of Neurophotonics, CRC Press, Boca Raton: (2020). [Google Scholar]
- 705.Richards L. M., et al. , “Intraoperative laser speckle contrast imaging with retrospective motion correction for quantitative assessment of cerebral blood flow,” Neurophotonics 1(1), 015006 (2014). 10.1117/1.NPh.1.1.015006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Sato K., et al. , “Intraoperative intrinsic optical imaging of human somatosensory cortex during neurosurgical operations,” Neurophotonics 4(3), 031205 (2017). 10.1117/1.NPh.4.3.031205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Rayshubskiy A., et al. , “Direct, intraoperative observation of similar to 0.1 Hz hemodynamic oscillations in awake human cortex: Implications for fMRI,” Neuroimage 87, 323–331 (2014). 10.1016/j.neuroimage.2013.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Kleinlogel S., et al. , “Emerging approaches for restoration of hearing and vision,” Physiol. Rev. 100(4), 1467–1525 (2020). 10.1152/physrev.00035.2019 [DOI] [PubMed] [Google Scholar]
- 709.Gagnon L., et al. , “Quantifying the microvascular origin of BOLD-fMRI from first principles with two-photon microscopy and an oxygen-sensitive nanoprobe,” J. Neurosci. 35(8), 3663–3675 (2015). 10.1523/JNEUROSCI.3555-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Kozberg M. G., et al. , “Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain,” Proc. Natl. Acad. Sci. U. S. A. 110(11), 4380–4385 (2013). 10.1073/pnas.1212785110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Adams A., et al. , “International brain initiative: an innovative framework for coordinated global brain research efforts,” Neuron 105(2), 212–216 (2020). 10.1016/j.neuron.2020.01.002 [DOI] [PubMed] [Google Scholar]