Abstract
Immunotherapy has reached clinical success in the last decade, with the emergence of new and effective treatments such as checkpoint blockade therapy and CAR T-cell therapy that have drastically improved patient outcomes. Still, these therapies can be improved to limit off-target effects, mitigate systemic toxicities, and increase overall efficacies. Nanoscale engineering offers strategies that enable researchers to attain these goals through the manipulation of immune cell functions, such as enhancing immunity against cancers and pathogens, controlling the site of immune response, and promoting tolerance via the delivery of small molecule drugs or biologics. By tuning the properties of the nanomaterials, such as size, shape, charge, and surface chemistry, different types of immune cells can be targeted and engineered, such as dendritic cells for immunization, or T cells for promoting adaptive immunity. Researchers have come to better understand the critical role the immune system plays in the progression of pathologies besides cancer, and developing nanoengineering approaches that seek to harness the potential of immune cell activities can lead to favorable outcomes for the treatment of injuries and diseases.
Keywords: Nano-immunoengineering, Immunotherapy, Nanoparticles, Cancer, Vaccines, CAR T-cell therapy, Tolerance, Tissue regeneration, Gene delivery
Introduction
The immune system is an inevitable part of the human body, interfacing with every organ system. Primary immune functions are to maintain homeostasis; insufficient or excess immune response can lead to pathologies such as cancer, infectious disease, chronic inflammation, and more. Recent advances in biomedical and pharmaceutical engineering have allowed researchers to engineer immune cells to further our understanding of the complex processes and develop better treatments. Moreover, since many biomedical processes (drug release, cellular uptake, signal transduction) occur on the nanoscale, controlling the immune system by nanoscale engineering, or nano-immunoengineering, can lead to more favorable outcomes.
Herein, we define the concept of nano-immunoengineering as engineering approaches that seek to enhance, control, or regulate immune cell functions by incorporating nanoengineering concepts and designs. The knowledge accumulated from these results will be used to design better treatments for various biomedical applications. These include, but are not limited to, designing better synthetic and biomaterials for delivering immunomodulatory drugs or biologics [1], increasing drug and gene delivery efficiency in target immune cells [2], and enhancing immune cell behavior with nanomaterials [3]. Being that this is a new field that is rapidly expanding, with growing interest in public institutions, private sectors and funding bodies, the translational impact of nano-immunoengineering is expected to increase even more in the next decade [4].
In this Review, we discuss the different aspects of nano-immunoengineering, emphasizing zero-dimension nanomaterial platforms that could modulate the immune system to induce an immune response against cancers and pathogens, or promote tolerance and tissue regeneration (Fig. 1). We will place a heavy emphasis on nanoparticle platforms because the site of action predominantly occurs in the cardiovascular and lymphatic system or deep tissues where nanoparticles could penetrate and accumulate more efficiently. This is in contrast to other two-dimension or three-dimension nanomaterials such as nanofibers, nanowires, nanosheets and nanoscaffolds, where their applications are better suited for topical or surgical procedures. We discuss the implication of these platforms and highlight the cutting-edge technologies that have the potential to revolutionize the field of immunotherapy.
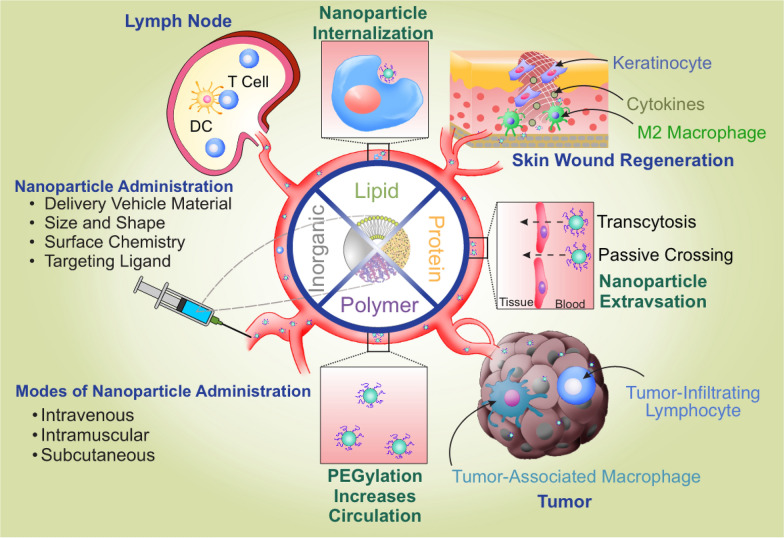
Fig. 1.
Overview of nano-immunoengineering designs and applications. Four major types of materials suited for biomedical applications include lipids, polymers, proteins, and inorganic materials. Therapeutic cargoes encapsulated in lipid coatings or grafted with poly (ethylene glycol) (PEG) can increase stability and circulation half-life. Nanoparticles can extravasate into tumors or tissues depending on the size, shape, charge, and hydrophobicity. In the tumors, the nanoparticles could enhance T cell functions or reprogram tumor-associated immune cells to improve anti-tumor efficacy; in the tissues, nanoparticles engulfed by dendritic cells (DCs) could either promote host immunity or tolerance when they migrate to the lymph nodes; in injured tissues, nanoparticles could reprogram resident immune cells such as macrophages to a healing phenotype to accelerate wound healing
Enhancing anti-tumor immunity for cancer immunotherapy
One of the most prominent applications of nano-immunoengineering is in combating cancer. The advent of immunotherapy, combined with next-generation sequencing and systems biology, allows scientists and clinicians to develop therapies for precision oncology. Still, there is a gap between translating such information into therapies; this includes developing carriers that can maximize bioactivity and bioavailability and enhance target immune cell functions through such properties. In this section, we introduce applications of nano-immunoengineering in the delivery of immunomodulatory agents and cancer vaccines, as well as enhancing and modulating adoptive cell therapy (ACT) (Fig. 2).
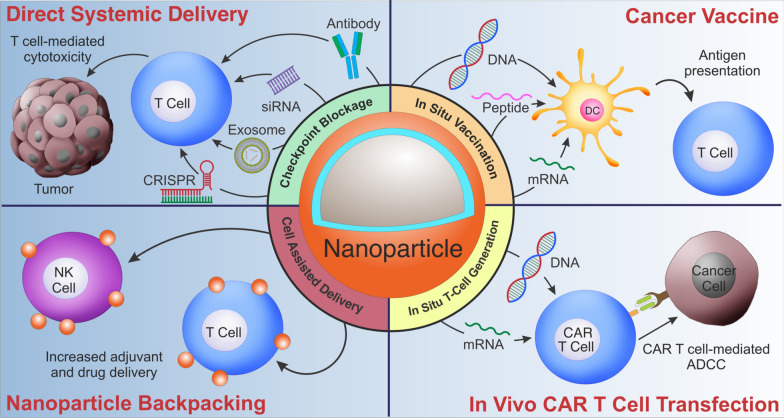
Fig. 2.
Nano-immunoengineering for cancer therapy. Nanoparticles can be utilized for various applications, including (i) delivering immunomodulatory molecules, including checkpoint inhibitors in the form of proteins or nucleic acids to enhance T cell immunity or reprogram the tumor microenvironment; (ii) generating effective cancer vaccines via transfecting DCs; (iii) attaching nanoparticles to T cells and NK cells to maximize localized delivery of adjuvant or drugs and limit systemic toxicities; iv) producing CAR T-cells in situ by transfecting endogenous T cells
Nanotechnology for effective delivery of immunomodulatory agents
Nanotechnology offers several advantages in the delivery of therapeutic cargoes for modulating the activity of immune cells. This includes more selective targeting, controlled release of payload, greater biodistribution, and prolonged and enhanced effects of the immunomodulator via the design of the nanoparticle (surface charge, size, hydrophobicity, stiffness, pore size, biocompatibility, and biodegradability). In addition to directly enhancing the anti-tumor immunity of immune cells, such as T cells or NK cells, “re-educating” tumor-associated immune cells such as tumor-associated macrophages (TAMs) represents a promising strategy for immunotherapy and immunoengineering. As macrophages are highly plastic cells, they can polarize to the pro-inflammatory, tumoricidal M1 phenotype, or the anti-inflammatory, pro-tumorigenic M2 phenotype. In particular, TAMs, classified as M2d, represent a critical therapeutic target since they constitute the major immune cell populations in tumors and mediate immunosuppression via the secretion of IL-10 and TGF-β and coordinate with other cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Treg) [5]. Strategies that seek to deplete [6] or re-polarize [7] TAMs represent have been heavily investigated, and this tumor microenvironment (TME) reprogramming approach represents a promising therapeutic strategy for cancer immunotherapy by virtue of enhancing anti-tumor immunity. Herein, we provide a brief overview of how nanotechnology can be combined with immunomodulatory agents, focusing specially on checkpoint blockade and microenvironment reprogramming; a more extensive review of checkpoint inhibitors and their application in nanomedicine can be found elsewhere [8].
Delivery of protein-based immunomodulators
A particular field that has significantly advanced in the last decade is the development of checkpoint inhibitors, mostly in the form of monoclonal antibodies (mAbs). Especially, programmed cell death protein 1 (PD-1) is a major immune checkpoint expressed by activated T cells, B cells, and monocytes. The engagement of PD-1 and its ligand, PD-L1, elicits an inhibitory signal in activated T cells; many cancers hijack this pathway to evade immune surveillance. Blocking this interaction using PD-1 antibodies can overcome this immunosuppression and enhance anti-tumor immunity. While mAb-based checkpoint blockade therapy has tremendous clinical success, systemic administration can still suffer lower efficacy due to premature degradation of the antibody and off-target toxicities. Several groups have worked on the direct delivery of PD-1 antibodies using biodegradable nanocarriers like PLGA [9, 10] or large pore mesoporous silica-upconversion nanoparticles (UCNP) [11]. PD-1 antibodies can be co-delivered with other adjuvants or small molecule inhibitors to improve efficacy. For translational use, efforts focusing on developing biodegradable platforms will allow more excellent safety and release of the encapsulated PD-1 antibodies. Recent advances have focused on triggered-release systems based on tumor microenvironment cues, such as pH, reactive oxygen species (ROS), or matrix metalloproteinase (MMP) expression. The expression level of MMP has been correlated with tumor metastasis, making such biological cues an excellent target. A MMP-mediated, biodegradable DNA nano-cocoon has been developed in which both CpG-oligodeoxynucleotides (CpG-ODN) and PD-1 antibodies could be co-loaded into the same nanocomplex for preventing postsurgical tumor relapse [12]. The release of the cargoes is mediated by the cleavage of the triglycerol monostearate capsules encasing the restriction enzyme Hhal by MMPs. When freed, the Hhal can degrade the nano-cocoon via restriction digest, thereby releasing CpG-ODN and the encapsulated PD-1 antibodies. The CpG DNA herein not only acted as a delivery vehicle, but also as a therapeutic agent that could enhance the anti-tumor response in a B16F10 metastasis mouse model. In a separate study utilizing MMP2-mediated degradation, Liu et al. co-encapsulated IMD-0354-containing lipid nanoparticles (~ 32 nm) with PD-1 antibodies in a nanogel (final size ~ 120 nm) to achieve PD-1 blockade and TAM repolarization. IMD-0354 is a NF-Kβ pathway inhibitor that can downregulate PD-1 expression on the surface of activated T cells. The combination approach allowed targeting both T cells and M2 TAMs, leading to significant tumor growth inhibition and extended survival of mice bearing B16 tumors. Besides PD-1 antibodies, other checkpoint inhibitors have been directly delivered or combined with other nanomaterials to achieve greater therapeutic efficacy, including CTLA-4 [13, 14] and CD47 [15].
Delivery of nucleic acid-based immunomodulators
Another promising approach to modulate the immune system is by delivering nucleic acids such as plasmid DNA (pDNA), messenger RNA (mRNA), short interfering RNA (siRNA), and microRNA. In contrast to mAbs, whose microheterogeneity patterns can influence their characteristics and encapsulation efficiency [16], the negative charge associated with nucleic acids allows them to be readily encapsulated using cationic materials with high efficiency. In particular, siRNA-mediated knockdown has been formulated into various types of nanoparticles composed of lipids [17], polymers [18], and inorganic matrices [19] for modulating the target immune cell. A significant advantage of RNA therapeutics over DNA therapeutics for immunotherapy is that RNA can function readily in the cytosol, whereas DNA must localize to the nucleus for proper expression. This generates a major barrier for nonviral plasmid DNA delivery into immune cells, as many of them, including primary T cells, NK cells, DCs, and macrophages, are refractory to transfection with pDNA using chemical-based methods. Therefore, major work in nanoparticle-based nucleic acid delivery has focused on RNA over DNA as the therapeutic cargo for immunomodulation. Recent efforts have focused on developing biodegradable platforms for clinical translation, like protein therapeutics. These biodegradable platforms could consist of either polymeric, lipid, or inorganic materials. A hybrid lipid calcium phosphate nanoparticle (LCP NPs) was developed that encapsulated PD-1 siRNA [20]. This material was originally developed by the Leaf Huang group for enhanced delivery of nucleic acids due to the synergistic effect of lipid-mediated membrane fusion and the proton sponge effect from the degradation of calcium phosphate in eliciting endosome escape [21]. Furthermore, calcium phosphate has the advantage of being biocompatible and completely biodegradable under acidic pH. These small (~ 30 nm) nanoparticles could readily encapsulate nucleic acids via the electrostatic interaction between Ca2+ and the PO43− backbone of DNA or RNA. The delivered siRNA could readily knock-down PD1 expression in tumor-infiltrating lymphocytes, resulting in greater killing efficacy and cytokine production [20]. Compared to tumor-infiltrating lymphocytes, tumor-infiltrating monocytes and macrophages contribute significantly to tumor progression, invasion, and metastases. Hanafy et al. has developed lipid nanoparticles containing acid-labile PEG linkers for the encapsulation of PD-1 siRNA for the downregulation of PD-1 on TAMs as opposed to lymphocytes. They observed increased uptake of the acid-sensitive PEG lipid nanoparticles in J774A.1 macrophages. Notably, both the PD-1 expression in the CD68 + TAMs and tumor size were greatly reduced in a B16-F10 tumor mouse model. The authors attributed the results to the re-polarization of M2 to M1 macrophages upon checkpoint blockade. In addition to PD-1 blockade, knockdown strategies that target key genes involved in the pro-tumorigenic functions of TAM also generated positive outcomes. In a recent study, lipid nanoparticles based on an ionizable lipid CL4H6 were developed for the silencing of activator of transcription 3 (STAT3) and hypoxia-inducible factor 1 α (HIF-1α) in TAMs [17]. STAT3 and HIF-1α are known to interfere with tumor suppression and increase tumor angiogenesis [22, 23]. The nanoparticles were produced using an ethanol dilution method, giving rise to homogenous (PDI 0.0–0.2) and small (~ 90 nm) particles, with a relatively neutral zeta potential with a high encapsulation efficiency (> 90%) of the siRNA. TAMs readily took up the lipid nanoparticles compared to other cell populations (i.e. tumor cells, endothelial cells, and other leukocytes) even in the absence of targeting ligands. Screening using both RAW 264.7 cells (murine macrophages) and bone marrow-derived macrophages (BMDM) showed, as well as TAMs in vivo, showed that a ratio of 60:40 or 70/30 mol% of CL4H6:chol enabled the greatest silencing activity. The inhibition of STAT3 and HIF-1α (by 37% and 48%, respectively) led to the infiltration of CD11 + macrophages as well as an increase in the presence of CD169+ (M1) macrophages. In addition, quantitative PCR revealed a decrease in CD31 and TGF-β levels, as well as an increase in IFN-γ and TNF-α levels, accompanied by a significant reduction of tumor size. To enhance the efficacy and targeting of TAMs, researchers have focused on key receptors, such as CD163 and CD206. Of the many receptors, CD206, or the macrophage mannose receptor 1 (MRC1), has been commonly utilized as the target for several nanoparticle delivery systems [24–26]. Zhang et al. synthesized a poly(β-amino ester) (PBAE) nanocarrier coated with poly(glutamic acid)- mannose for the targeted co-delivery of in vitro-transcribed (IVT) IRF5 and IKK mRNA (3:1) in an ovarian cancer mouse model [26]. The nanocarriers successfully reprogrammed the M2 TAMs into an M1 phenotype, slowed the tumor growth, and doubled the survival time in the mice. This platform was further applied to mice with pulmonary melanoma metastases and glioma. However, the authors did not observe complete eradication of the tumors in the various mouse models tested, suggesting that this approach is best used in combination with other therapies for efficacy.
Gene editing approach for immunomodulation
The CRISPR-Cas system has emerged as a powerful tool in modulating the immune system. Due to the large loading capacity required for the CRISPR-Cas system, nonviral nanocarriers could enable such delivery in vivo. For instance, Li et al. reported the synthesis of nanoparticles with different PEG densities containing CRISPR-Cas for the in vivo targeting of B cells [27]. However, for therapeutic applications, the delivery of the ribonucleoproteins (RNPs) is preferred to limit off-targeting effects and unwanted gene editing, genome toxicity, and immunogenicity. By using a truncated Cas9 targeting sequence and poly(glutamic acid) (PGA) as an RNP stabilizer, Nguyen et al. showed fourfold improved HDR efficiency in CD4+ T cells using this nanoparticle platform combined with electroporation [28]. This enhanced efficiency was also observed in other types of immune cells such as CD8+ T cells, B cells, NK cells, and hematopoietic stem cells (HSCs). To bypass the use of electroporation for RNP delivery, the Rotello group developed a nanocomposite platform using engineered RNP with gold nanoparticles. By incorporating the RNP with an oligo(glutamic acid) tag, the protein could readily associate with gold nanoparticles that contained arginine head groups via carboxylate-guanidinium interaction to form nanocomposites that were about 285 nm in size [29]. The relatively large size of these nanocomposites provided a passive targeting strategy for macrophages in vivo. Notably, compared to other nanocarrier systems that were less efficient at endosome escape (hence lower editing efficiency), this approach led to direct cytosolic delivery of the RNPs, and knockout of the PTEN gene in macrophages in vivo was successful. While many current oncology studies have focused more on the delivery of the RNP into tumor cells rather than directly into immune cells, this strategy can still lead to enhanced intratumor immune response via the suppression of the immune checkpoints. In addition, the co-delivery of small molecule drugs can lead to immunogenic cell death, further contributing to anti-tumor immunity. Liu et al. reported a virus-like nanoparticle (VLN) that co-delivered the CRISPR/Cas system along with small molecule drugs for combination therapy [30]. The particle core comprised of thiolated mesoporous silica nanoparticles (MSN) in which the pores were loaded with axitinib, a tyrosine kinase inhibitor that suppresses tumor growth via the MAPK-ERK and P13K-AKT pathway; the pores were “sealed off” by RNP with sgRNA targeting PD-L1 that were conjugated to the surface via disulfide bonds. The VLN core was further coated with a layer of lipids to enhance the stability and particle uptake in tumor cells. This triggered release system could be initiated by intracellular glutathione, upon which the RNP could dissociate, along with the release of axitinib in the target cancer cells. The VLN was able to achieve a knockdown efficiency up to 58.2% and reduced the expression of PD-L1 by up to 41.3% in B16F10 cells. These in vitro results reflected a significant reduction of Treg population and tumor size in vivo.
Exosomes for immunomodulation
Besides synthetic nanomaterials, naturally-derived nanoparticles such as exosomes have also been applied for immunomodulation. Exosomes are small (30–150 nm), spherical extracellular vesicles (EVs) generated by cells that contain a variety of biomolecules such as proteins, mRNAs, and microRNAs for cellular communication. Exosomes include tetraspanins such as CD9, CD37, CD63, and CD81, which can be employed as biomarkers to isolate them for biosensing and disease detection. Exosomes have lately acquired popularity in immunotherapy, owing in part to their ease of preparation, storage, and manipulation when compared to ACT. Exosomes harvested from various types of immune cells, such as DCs, NK cells, CD8+T cells and M1-polarized macrophages, have all shown to exert anti-tumor effects or potentiate such responses [31–34]. Notably, the cytotoxic activity of the exosomes is mainly dependent on the cytokines used to activate the immune cells, such as IL-12 for CD8+ T cells [35] and IL-15/IL-21 for NK cells [36]. Besides cancer cells within tumors, exosomes derived from CD8+ T cells and NK cells have been shown to mediate cytotoxic activity against tumor stromal cells such as mesenchymal stromal cells (MSCs) and tumor-associated fibroblasts (TAFs) [37] and circulating tumor cells (CTCs) [38], respectively. Exosomes secreted by CAR T-cells have been shown to elicit strong anti-tumor effects against breast cancer cells expressing EGFR and HER2 as well as mesothelin (MSLN) in vitro and in vivo [39, 40]. In vitro analysis demonstrated the presence of CAR and CD63 and MHC I proteins and CD3, CXCR4 and CD57, with undetectable amounts of CD45 RA and PD-1. More notably, the CAR-containing exosomes carried cytolytic enzymes like perforin and granzyme B and displayed substantial cytotoxic action against cancer cells unaffected by immunological checkpoints like PD-1 [41]. Another promising aspect is that intraperitoneal injection of the CAR-exosomes did not lead to cytokine release syndrome (CRS) in mice. Since exosomes can be isolated and stored as off-the-shelf products, CAR exosomes represent a promising alternative to CAR T-cells for cancer immunotherapy.
Nanotechnology for cancer vaccines
Cancer vaccines refer to vaccines that either i) prevent the viral infections that lead to the development of certain cancer (e.g., cervical cancer by human papillomavirus (HPV)) or ii) prevent or treat the cancers in high-risked individuals, known as a prophylactic and therapeutic vaccine, respectively. Currently, the two major challenges in cancer vaccine development are the high variability of the tumor-associated antigens (TAA) in different tumors, and the immunosuppressive TME [42]. Consequently, the careful selection of TAA as a cancer vaccine will dictate such vaccine's efficacy and safety. Tumor lysates encompass the full array of TAA and can elicit potent anti-tumor immunity [43]. Adjuvant and combination immunotherapies with peptide or nucleic acid-based vaccines are being investigated as potential ways to bolster stronger and longer-lasting immune responses against cancer cells [44, 45].
Nanotechnology in peptide-based vaccines
Successful eradication of tumors requires the generation of MHC I-restricted cytotoxic T lymphocytes (CTLs). This is achieved via delivering TAA as a peptide or gene in combination with the robust stimulation of DCs, which can further activate TAA-specific T cells. Tyrosinase-related protein 2 (Trp2) has been identified as a TAA of melanoma, and the delivery of the epitope peptide (SVYDFFVWL) has been adapted in different nanoplatforms [46, 47]. Tsai et al. developed a simple polyplex formulation by mixing arginine-modified Trp2 with CpG at various ratios. This approach allowed for the interrogation of the role of individual vaccine components in the immune system in a “carrier-free” manner. While a ratio of 5:1 Trp2R9 to CpG led to the highest antigen loading and greatest uptake in DCs, the expression of activation markers including CD40, CD80, and CD86 were less than the DCs treated with free CpG. This could be explained by the R9's stronger binding to the CpG, resulting in less release and stimulation of Toll-like receotir (TLR) 9. Nonetheless, complexing with Trp2Rx led to greater T cell proliferation and IFN-γ release as well as a reduced tumor burden. Alternatively, both the Trp2 peptide and CpG could be co-encapsulated into LCP NPs for efficient cytosolic delivery into DCs (Fig. 3). Interestingly, one of the lipids used in the study, dioleoyl-3-trimethylammonium propane (DOTAP), also possesses immuno-stimulating properties such as upregulating the production of cytokines and enhancing the cross-presentation of antigen by DCs in addition to serving as a carrier material. These capabilities are mediated through the induction of reactive oxygen species and activation of TLR4 intracellularly. Cationic nanoparticles are also efficiently taken up by DCs when compared with other cell types. Because DCs are critical antigen-presenting cells (APCs) primarily responsible for starting T cell immune responses, cationic nanoparticles' rapid absorption, together with their immunostimulatory properties, could potentially increase the immunogenicity of cancer vaccines. The composition of cationic nanoparticles carrying cancer vaccines can be greatly modified and varied with different types of antigens, excipients, adjuvants, and material components [48]. This design flexibility makes cationic nanoparticles a promising platform for vaccine delivery and offers endless possibilities for further optimization.
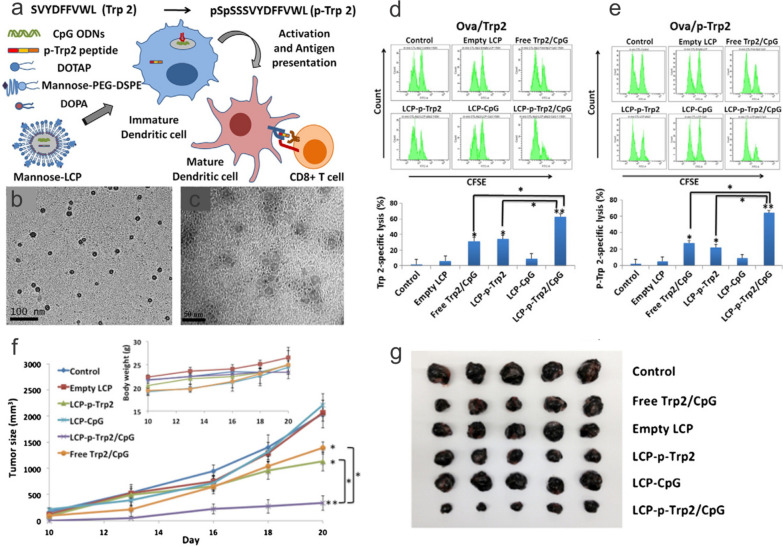
Fig. 3.
Lipid calcium phosphate nanoparticle (LCP NP)-mediated co-delivery of Trp2 peptide and CpG in B16F10 subcutaneous tumor. a Synthesis LCP NPs that encapsulate phosphorylated Trp2 (p-Trp2) and CpG for the delivery into DCs via the mannose receptor. TEM image of b hydrophobic LCP cores and c mannose-functionalized, aqueous LCP NPs. In vivo CTL response assay examining mice immunized with control peptide/CpG or mannose-LCP NPs containing both Trp2/CpG against splenocytes pulsed with d Trp2 or e p-Trp2. f, g Reduction of B16F10 tumor sizes in mice immunized with LCP NPs containing both Trp2 and CpG.
Reproduced with permission from ref [47]
Polymeric nanoparticles composed of poly(D,L-lactide-co-glyoclide) (PLGA) are also promising TAA delivery platforms. PLGA is a biocompatible polymer and has a well-established safety profile. Nanoparticles fabricated with PLGA are small enough to be administered via conventional vaccine routes (subcutaneous, intramuscular). This quality is important when discussing the delivery of TLR7/8 agonists, such as peptide/protein-based TAAs, which are typically limited by their poor retention at the injection site. TLR7/8 agonists are cytokine inducers that can be used as cancer adjuvants to activate DCs and incite a robust T cell response. A platform, such as PLGA nanoparticles, that could improve their availability and exposure to DCs may provide great immunogenic improvements. Studies suggest that using nanoparticles to encapsulate these peptide-based TAAs and their adjuvants provides protection from degradation and enhanced/targeted delivery to DCs. This can ultimately strengthen T cell response reactions. Because TLR7 and 8 are located on the luminal side of endo/lysosomes, peptide TAAs must be effectively delivered through the cellular membrane and internalized into these endo/lysosomes to incite an immune response. PLGA nanoparticles are a favorable delivery platform for these TLR agonists since they efficiently enter these endosome/lysosomes after being endocytosed into the cell. Another reason why PLGA nanoparticles are efficient as in vivo vaccine delivery vehicles is because they prevent the rapid clearance of antigens from the injection site. This is accomplished by protecting their payloads from biodegradation and efficiently directing themselves to lymph nodes rather than to systemic circulation where they are rapidly cleared [49]. These properties demonstrate how PLGA nanoparticles encapsulating TLR7/8 agonists can be used to improve cancer vaccines.
Nanotechnology in nucleic acid-based vaccines
Although the use of these peptide-based antigens as cancer vaccines in clinical trials has demonstrated fewer side effects than conventional therapies, they have shown to provide moderate therapeutic benefits in only a small portion of patients. The risk of tumorigenesis, the threshold concentration of TAA needed for stimulation, and the presence of immunosuppressive cytokines such as IL-10 and TGF-β that can offset proper anti-tumor response preclude the wide use of tumor lysates for vaccination [50]. Genomic sequencing allows the identification of neoantigens that can overcome the aforementioned variability challenge; for example, autologous DCs can be loaded, or “pulsed” with the neo-antigen identified using high-throughput sequencing and readministered back to the patient, where they can migrate to the lymph nodes to present antigens and activate T lymphocytes [51]. Exosomes harvested from DCs have been successfully applied as cancer vaccines [52]. However, these personalized vaccines tend to be costly and time-consuming. By combining the strength of genomic sequencing technology with nanoengineering, in situ vaccination can be achieved to develop personalized DNA or mRNA cancer vaccine against specific TAA to increase both safety and efficacy. One of the most common methods of in situ vaccination is using an oncolytic virus, but systemic activation leading to CRS is a major safety concern. In situ vaccination with nonviral pDNA or mRNA vaccines delivered via nanocarriers is a safer and more cost-effective method than traditional vaccination methods. This concept has recently been demonstrated using lipidoid nanoparticles [42]. A key advantage of pDNA/mRNA vaccines is that they elicit both CTLs and helper T cells simultaneously via both MHC class I and II pathways [53, 54]. Another advantage is that multiple antigens can be encoded, allowing greater immunization [55].
The ability to rapidly design the gene construct, the relatively low cost for large-scale manufacturing, high stability and hence ease of storage, and the capacity to induce expression of target antigen for a more-extended period make DNA vaccines ideal compared to mRNA vaccines. It has been shown that plasmid DNA can persist in muscle cells for up to six months [56]. Moreover, expression of the gene endogenously will allow post-translational modifications of native protein conformation suitable for antigen presentation. While early reports demonstrated the potential of DNA as vaccines for generating tumor-specific immunity in vivo, the efficacy of DNA vaccine did not translate in human clinical trials. One key limitation is the low immunogenicity of DNA vaccines [57]. Specifically, the low gene transfer efficiency of DNA in APCs such as DCs is a major underlying challenge. Successful gene transfer is vital for efficiently generating MHC Class I-restricted CTLs efficiently since somatic cells like myocytes lack the MHCII or co-stimulatory molecules for T cell priming following intramuscular injection [58, 59]. The CTLs are critical in eliminating TAA-expressing cells. In addition, the DCs can further activate CD4+ T lymphocytes, which can assist activation of CTLs and promote the generation of CD8+ memory cells. To enable expression, various types of polymer and lipid platforms have been used to complex with pDNA for therapeutic vaccine applications. This includes chitosan [60], PLL [58], PEI [61, 62], PBAE [63].
Earlier works focusing on chitosan with DNA have shown the potential of inducing an immune response [64]. Furthermore, the mucoadhesive property of chitosan makes it well suited for delivery to mucosal sites such as the airway [65]. PLL is another cationic material that has been widely utilized for plasmid DNA vaccine development. Interestingly, it has been shown that PLL-coated, 40–50 nm polystyrene nanoparticles could readily transfect DCs compared to 1 µm sized particles in vitro. Moreover, both 20 nm and 1 µm particles could not generate a proper immune response, while the 50 nm particles elicited the strongest immunogenicity. The authors attributed this result to the differential uptake pathway, and noted that different materials of similar sizes, such as gold and silica, could produce similar effects [58]. Many works have incorporated mannose for targeted delivery to increase the uptake and hence the total efficacy of the DNA vaccine since DCs also express MRC1 like macrophages [66]. In a separate study, PEI was used to condense OVA-encoding pDNA for delivery into DCs [62]. Specifically, the role of PEI as a cancer vaccine adjuvant was investigated. It was found that the DCs were successfully transfected and migrated to the draining lymph nodes in vivo. The animals treated with PEI/DNA had an increased CTL activity and a reduced tumor growth. The vaccinated animals also had increased inflammation and cell death in the injection site, which could be partially attributed to PEI-mediated cytotoxicity [67]. Unlike mRNA, however, DNA must traffic to the nucleus and cross the nuclear membrane to be transcribed. Furthermore, as endosomes escape, free DNA becomes dissociated from the carrier material and vulnerable to nuclease or cytosolic DNA sensor-mediated destruction, especially in professional APCs like DCs and macrophages [68–70]. Hence, strategies that aim at protecting the DNA cargoes after endosome escape and increasing nuclear transport of the pDNA should considerably improve transfection efficiency [71, 72].
Compared to DNA vaccines, mRNA vaccines require higher maintenance for storage and transport due to their instability from the presence of the 2’ hydroxyl. The relative ease of delivery and expression (since DNA vaccine has the additional nuclear membrane to overcome) and the transient nature have attracted attention in the last decade as a vaccine candidate over pDNA. Regardless of whether pDNA or mRNA, the delivery of these nucleic acids is often accompanied by a nanoparticle to carry this genetic payload. Although other delivery vehicles exist for mRNA vaccines, as will be discussed in a later section, many of the current nanocarriers of clinical importance are composed of lipids. In this regard, emphasis will be placed on the design of the lipid nanoparticles as well as the selection of their compositions. It is important to note that lipid nanoparticles differ from liposomes in that liposomes have at least one lipid bilayer with an aqueous core, whereas lipid nanoparticles have a presence within the core as well. When observing the composition of clinically relevant lipid nanoparticles, there are often 4 main components that include (i) neutral phospholipids, (ii) ionizable cationic lipids, (iii) cholesterol, and iv) polyethylene-glycol (PEG)-lipids. The neutral phospholipids and cholesterol aid in the overall structure and stability of the lipid nanoparticle, while the ionizable cationic lipids and PEG-lipids provide more functionality and improve storage conditions. Cationic ionizable lipids are composed of three parts, (i) an ionizable head group, (ii) a linker region, and (iii) lipid tails. The ionizable head group and linker region both facilitate endosomal escape and contribute to the overall pKa, which has been shown to elicit an optimal adaptive immune response when tailored to 6.6–6.9 for mRNA vaccines [73]. The lipid tails influence the geometry, and thus endosomal escape capabilities, in addition to the toxicity and storage conditions of the lipid nanoparticle [74]. For example, by incorporating ester linkages into the hydrocarbon tail, the lipid nanoparticle can degrade quicker because of the cleavage caused by the metabolic activity of esterases present in the cell, but placing these esters too close to the head group could influence the system’s overall pKa [75]. Moreover, because ionizable lipids are cationic, they can interact with anionic mRNA during particle formation to ensure that they are encapsulated in the lipid nanoparticle. PEG-lipids are often used in small molar percentages in the overall formulation but provide a steric hindrance to prevent lipid nanoparticle aggregation and contribute to the nanoparticle's overall size [76].
Nanotechnology in adoptive cell transfer for cancer immunotherapy
Nanoparticles in immune cell-assisted delivery
While therapeutic nanoparticles functionalized with PEG can enhance circulation half-life and hence overall bioavailability, the current solid tumor delivery paradigm is ineffective, with less than 1% of the dosage reaching the target [77]. One alternative approach is to functionalize drugs or adjuvants encapsulated in nanocarriers onto immune cells, such as T cells and NK cells delivery in vivo. These adjuvant nanoparticles as “cellular backpacks” have been studied extensively in T cells to enhance T cell functions [78]. Key advantages of backpacking adjuvants include (i) limiting systemic toxicity from high dosing of adjuvants and (ii) allowing small molecule drugs to be administered (that cannot be genetically expressed). In an earlier study, multilamellar liposomes (~ 300 nm) with lipids containing maleimide headgroups were developed [79]. The maleimide functional groups can readily conjugate with the free thiols on the surface of T cells and HSCs and remain for days even after stimulation in vitro; on the other hand, the maleimide-liposomes were readily internalized by immature DCs. These surface anchoring nanoparticles were nontoxic and could achieve week-long sustained release of the therapeutic payloads. Importantly, attaching these nanoparticles to the cells at ~ 100 particles per cell did not interfere with vital cellular functions in cytotoxic T cells, including proliferation, cytotoxicity, diapedesis, and tumor homing ability in vivo. To investigate the effects of adjuvant-loaded nanoparticles on CD8+ T cells, IL-15Sa and IL-21 were co-encapsulated into multilamellar lipid nanoparticles and anchored to the T cells. These backpacked T cells showed long persistence in mice with melanoma lung and bone marrow tumors. Impressively, the surface anchored nanoparticles provided 11-fold enhancement in the immunostimulatory effects to the CD8+ T cells compared to that by co-administered, non-attached nanoparticles. Using a similar strategy, Stephan et al. encapsulated NSC-87877, a small molecule inhibitor of SHP1 and SHP2, into stable liposomes containing hydrogenated soybean phosphatidylcholine and cholesterol and studied the spatial distribution of the nanoparticles on the effector T cells [80]. Interestingly, the nanoparticles were discovered to be localized in the uropod during migration but concentrated to the immunological synapse upon cell target recognition. This phenomenon did not interfere with the killing of target tumor cells. The encapsulated drug was found to be released slowly over 6 days, during which the tumor-specific T-cells could expand. Infusion of the nanoparticle-modified T-cells showed significant tumor infiltration and extended survival in a prostate cancer mouse model. The authors used mass spectrometry to identify CD45 as the principal membrane anchor for the nanoparticles linked to the T cell surface, as well as adhesion proteins (LFA-1, CD2, CD97), CD98, the transferrin receptor, and the MHC-1 complex, to mention a few. Zheng et al. prepared liposomes containing the hydrophobic TGF-β inhibitor (TGF-βI) SB525334 using the ethanol injection method and anchored the particles to the pmel-1 CD8+ T cells via CD45 (non-internalizing receptor) or CD90 (Thy1.1, internalizing receptor) (Fig. 4) [81]. It was found that ACT T cells pre-loaded with CD45-targeting TGF-βI liposomes ex vivo led to superior tumor infiltration of ACT T cells than those backpacked with CD90-targeting liposomes and reduction of tumor size in a B16F10 melanoma murine model. This CD45-targeted loading strategy was later adopted for the loading of stimulus-responsive nanogels containing interleukin IL-15 super-agonist (IL-15Sa) [78] and IL-2 [82] onto T cells for better drug encapsulation and regulation of release. Table 1 summarizes the surface functionalization of nanoscale dimension cargoes onto various types of immune cells.
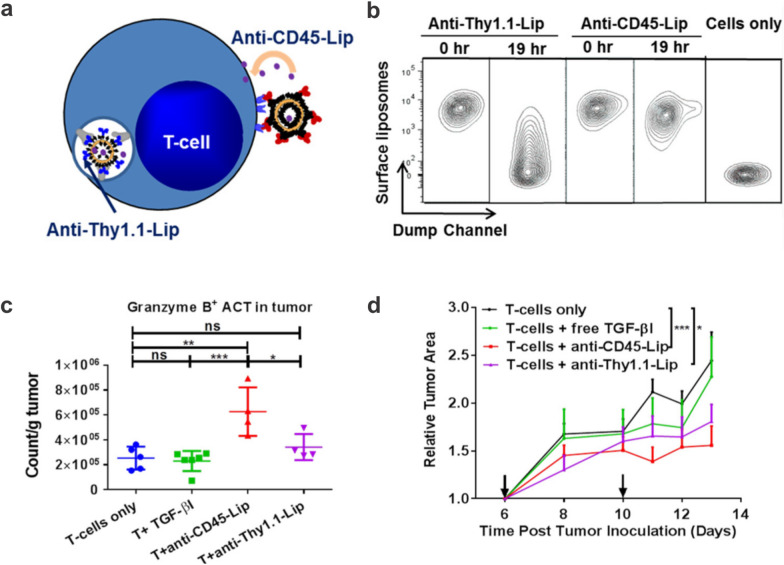
Fig. 4.
Surface engineering of T cells with nanoparticles. a Schematic diagram depicting the liposomes anchoring to either CD45 or Thy1.1 receptor on T cells. b Flow cytometry showed that remaining liposomes on cell surface-bound to CD45, a non-internalizing receptor, were significantly greater than those bound to Thy1.1, an internalizing receptor. c T cells loaded with anti-CD45 liposomes containing TGF-β inhibitors led to greater infiltration in tumors. d CD45 bound liposomes decreased the tumor size of B16F10 tumors compared to either free or Thy1.1 bound liposomes.
Reproduced with permission from ref [81]
Table 1.
Surface engineering of immune cells with nanomaterials for delivery
| Cell Source | Cargo | Size | Functionalization Strategy |
Application | References |
|---|---|---|---|---|---|
| T cell | Multilamellar liposomes | ~ 300 nm | Maleimide-cell surface thiol | B16F10 melanoma lung and bone marrow tumors | [79] |
| Lipid-coated PLGA | ~ 230 nm | Maleimide-cell surface thiol | B16F10 melanoma lung and bone marrow tumors | [79] | |
| Liposomes | ~ 200 nm | Maleimide-cell surface thiol | Prostate tumor | [80] | |
| Lipid nanocapsules | ~ 340 nm | Maleimide-cell surface thiol | Lymphoma cells | [83] | |
| Liposomes | ~ 83 nm | CD45 antibody conjugation | B16F10 melanoma | [81] | |
| Cytokine nanogels | ~ 100 nm | Covalent conjugation via crosslinker/ electrostatic interaction | B16F10 melanoma | [82] | |
| Lipid nanocapsules | ~ 240 nm* | Maleimide-cell surface thiol | Functional modification of CTLs | [84] | |
| CAR T-cell | Cytokine nanogels | ~ 80–130 nm | CD45 antibody conjugation/ electrostatic interaction | B16F10 melanoma | [78] |
| Multilamellar liposomes | ~ 160 nm* | Maleimid-cell surface thiol | SKOV 3 ovarian cancer and leukemia | [85] | |
| NK cell | Liposomes | ~ 138 nm | NK1.1 antibody conjugation | SW620 colon cancer cells | [86] |
| Graphene oxide-PEG nanoclusters | ~ 50–300 nm | CD16 antibody conjugation | Activation of NK cells | [87] | |
| CAR NK- cell | Liposomes | ~ 220 nm* | Maleimide-cell surface thiol | SKOV 3 Ovarian cancer | [88] |
| Leukocyte | Liposomes | ~ 118 nm | Binding between E-selectin receptor and apoptosis inducing ligand TRAIL | Circulating colon cancer cells (COLO 205) | [89] |
| B cell | Multilamellar Liposomes | ~ 300 nm | Maleimide-cell surface thiol | B16F10 melanoma lung and bone marrow tumors | [79] |
| HSC | Multilamellar Liposomes | ~ 300 nm | Maleimide-cell surface thiol | B16F10 melanoma lung and bone marrow tumors | [79] |
Nanoparticles in car immune cell manufacturing
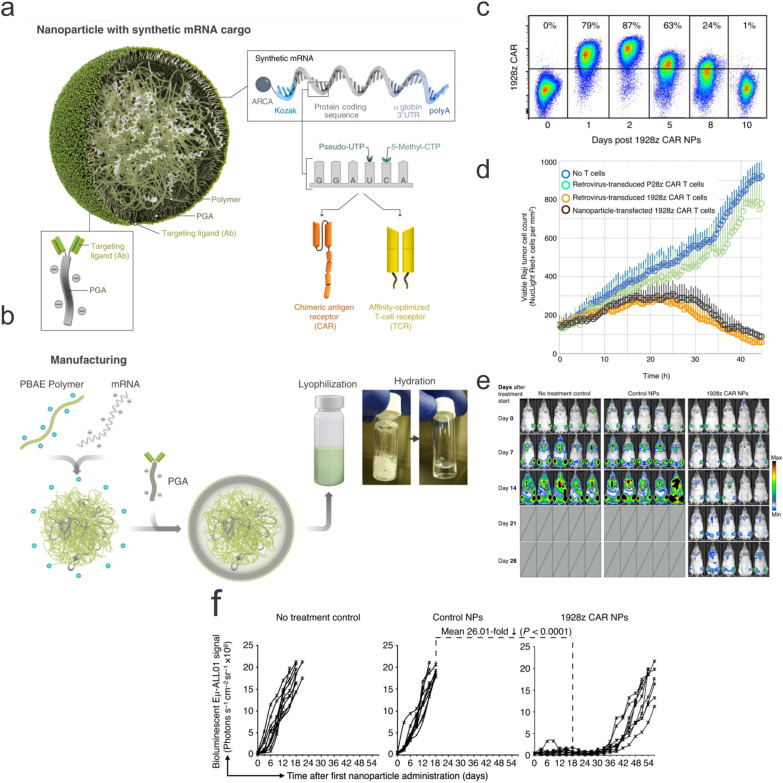
One important application of nanotechnology for the engineering of immune cells (T cells, NK cells, and macrophages) with CAR is the nonviral delivery of the CAR constructs, either in the form of DNA or RNA, into these cells. While viral vectors demonstrate superior gene transfer efficiency, with approximately 70% of clinical trials using viral vectors such as lentiviruses and adenoviruses [92], limitations associated with viral vectors such as immunogenicity, tumorigenicity, limited packaging capacity, and difficulty to scale continue to plague viral vector application for successful commercialization [93–95]. On the other hand, nonviral vectors offer advantages such as better safety profiles, reduced immunogenicity, greater loading capacity, and ease of scaling [96, 97]. In particular, nonviral vectors allow for T cells' in-situ programming, which significantly reduces the time and cost of preparation for ACT T cells. This is demonstrated by the Stephan group, who designed an anti-CD19 194-1BBz CAR encoded in the piggyBac transposon that was encapsulated in a PBAE nanocarrier modified with a peptide containing the microtubule-binding and nuclear localization sequence [98]. The cationic particle core was further coated with PGA-functionalized with anti-CD3e for targeting T cells in vivo. The size of the polymeric nanoparticles was approximately 150 nm in size and -8 mV in zeta potential. While the in vitro and in vivo transfection efficiencies were low (~ 3% and ~ 1.5%, respectively), the engineered T cells showed enhanced tumor lysis and cytokine production compared to those transduced with lentiviral vectors in vivo. The same group later demonstrated the delivery of in IVT mRNA encoding anti-CD19 1928z CAR using the same PBAE nanocarrier formulation [99] (Fig. 5). The anti-CD8 antibody functionalized nanocarriers were readily bound to T cells and demonstrated superior transfection efficiency (~ 75%) in vitro compared to the plasmid-encapsulating nanocarriers. The reprogrammed CAR T-cells could readily recognize the CD19 + Raji cells, completely eradicate the tumors, or lead to significant tumor regression. The potential of this technology was further demonstrated in mice bearing solid tumors, in which nanoparticle-reprogrammed CART-cells expressing antiROR1 could efficiently eliminate LNCaP C4-2 prostate tumor cells and extend the survival of mice by 42 days. However, compared to stably transfected or transduced T cells, IVT mRNA transfected CAR T-cells showed transient expression of 1928z CAR for about 8 days. Nonetheless, repeated dosing of the mRNA nanoparticles could reach the same levels of gene transfer (~ 10%) into the host T cells, demonstrating that this approach could still lead to long-term expression of CARs on T cells. Regardless of whether the cargo is DNA or RNA, one major advantage of these polymeric nanocarriers is that they can be lyophilized and reconstituted, offering the potential as an off-the-shelf product for storage and transport [100].
Fig. 5.
Polymeric nanocarrier for the delivery of CAR IVT mRNA into T cells. a Schematic illustration of the PBAE nanocarrier encapsulating the IRF5 and IKK IVT mRNA. The polymeric nanoparticles created from self-assembly could be readily lyophilized and redispersed, increasing flexibility for storage and transport. c Transfection efficiencies of the nanocarriers into T cells. d The nanoparticle-reprogrammed T cells showed comparable lysis activity to lentivirus-transduced T cells against Raji lymphoma cells. e Only the transfected T cells were able to alleviate tumor burden and f extend average survival time in C57BL/6 mice.
Reproduced with permission from ref [99]
Like in situ vaccination, the critical factor that limits the use of this approach is the gene transfer efficiency, especially for DNA, in host T cells. Primary T cells are refractory to transfection. Tailoring the structural properties of polymeric or lipid-based materials could lead to better gene transfer into T cells and therapeutic efficacy overall. For example, Olden et al. investigated a library of sunflower and comb-shaped poly(2-dimethylaminoethyl methacrylate) (pDMAEMA) for T cell transfection. They found that one of the candidates, l-pHEMA25-g-(pDMAEMA16)25 (CP-25–16), could achieve a reporter gene transfection efficiency of ~ 25% with mRNA and ~ 18% with DNA in human primary CD4+ and CD8+ T cells while maintaining high (> 90%) viability in vitro [101]. In another study, Richter et al. developed a block copolymer micelle incorporating lipoic acid into poly{[2-(dimethylamino)ethyl methacrylate]101-b-[n-(butyl methacrylate)124-co-(lipoic acid methacrylate)22]} (p(DMAEMA101-b-[nBMA124-co-LAMA22])) and showed ~ 29% transfection efficiency in K-562 cells while cells transfected with pDMAEMA showed negligible expression of the reporter [102]. Ayyadevara et al. had found that the mere addition of Ca2+ could increase the transfection of polyplex into Jurkat cells compared to polyplex-only control, while other cations such as Na+ or Mg2+ did not lead to such effect [103]. The increase in transfection was partially attributed to the increased association of polyplex with the cell membrane.
In addition to polymeric-based nanocarriers, lipid nanocarriers have also been investigated to deliver CAR genes into T cells. Lipids represent one of the most widely used materials as vectors for nonviral gene transfer. This is because they are generally considered safe, biocompatible with low immunogenicity, and easy to use. In contrast to polymeric nanoparticles, lipid nanoparticles are more clinically developed for the delivery of RNA [104]. Many of the cationic lipids developed for transfection have positively charged head groups and hydrophobic tails connected by a linker, which is either an ester, ether or amide functional group that determines the overall flexibility and biodegradability of the cationic lipid [105]. Some examples of the widely used cationic lipids for transfection include (N-[1-(2,3-dioleyloxy)propyl]- N,N,N-trimethylammonium chloride) (DOTMA), (2,3- dioleyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl1-propanaminium trifluoroacetate) (DOSPA), and (N-[1-(2,3- dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate) (DOTAP) [97]. They can form complexes spontaneously in the aqueous environment with the negatively charged nucleic acids through the positively charged head groups. However, since cationic lipids tend to be cytotoxic, researchers have adopted ionizable lipids (lipids that are neutral at physiological pH but become positively charged at lower pH) for clinically relevant lipid nanoparticle formulations [106–108]. Billingsley et al. synthesized and evaluated a library of ionizable lipids, and found that the top candidate, C14-4, when formulated with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), cholesterol, and C14-PEG, was able to induce the greatest luciferase expression in Jurkat cells and primary CD4+ and CD8+ T cells without increased cytotoxicity compared to Lipofectamine [109]. The authors then assessed the delivery of IVT CD19 CAR mRNA into primary T cells using the same formulation and compared it with electroporation, the most commonly used method for the nonviral delivery of CAR in vitro and ex vivo. The C14-4 formulation exhibited comparable CAR expression to electroporation when measured by flow cytometry, but much less cytotoxicity. Importantly, the CAR T-cells generated by the ionizable lipid nanocarriers demonstrated cancer-killing activity on par with those transfected by electroporation and transduced by lentiviral vectors in a co-culture assay with Nalm-6 acute lymphoblastic leukemia cells. The same group further screened and identified additional candidates A16 and B10 as promising ionizable lipid formulation for functional delivery of mRNA into primary T cells with low cytotoxicity [110].
Besides T cells, NK cells also have received increased attention as the source for CAR engineering. One key advantage is that transplanted NK cells do not give rise to Graft Versus Host Disease (GvHD) due to the lack of T cell receptors (TCRs). Therefore, attempts to generate off-the-shelf, allogenic CAR NK cells have received a lot of research focus. In one instance, Kim et al. has developed a core–shell iron oxide nanoparticle platform for the transfection of CAR NK cells [111]. The core is made of zinc-doped iron oxide layered by caffeic acid and polydopamine, followed by an outer layer of PEI. The nanoparticle was able to deliver both EGFP and CAR encoding EGFR into NK-92MI cells. Importantly, the transfected NK-92MI cells could elicit cytotoxicity against MDA-MB-231 cells in vitro and in vivo. In addition to acting as a nanocarrier for gene delivery, the iron oxide nanoparticle also enabled MR imaging in vivo as an excellent T2 contrast agent. This allowed tracking of the CAR NK cells once administered. In contrast to conventional CAR design, which contains the scFv against the target antigen, Wang et al. engineered CAR NK cells with a piggyBac construct encoding NKG2D as the extracellular domain, and DAP10 and CD3ζ as the co-signaling domain via the biodegradable PBAE nanocarrier [112]. Compared to the control NK-92 cells, the transfected NK-92 cells exhibited greater degranulation activity and IFN-γ production, as well as cytolysis against solid tumor cell lines GBM43, GBM10, A549 and PC3. The cytolysis activity of the NKG2D.CAR-NK-92 cells were further enhanced by CD73 blockade both in vitro and in vivo.
While T cells and NK cells can exert powerful anti-tumor activities, they are susceptible to the immunosuppressive TME, rendering them relatively ineffective for solid tumors. On the other hand, Macrophages are resilient and abundantly present in many solid tumors due to the active recruitment of bone marrow-derived monocytes to the TME and subsequent differentiation and polarization to the pro-tumoral (M2) phenotype. Taking advantage of this phenomenon, Klichinsky et al. developed CAR macrophages (CAR-Ms) using an adenoviral vector (Ad5f35) that could elicit a high degree of gene expression, since macrophages are very difficult to transfect with high efficiency [113]. In multiple cancer models, CAR-Ms displayed direct anti-tumor effects in vitro and in vivo; additionally, these altered macrophages were engaged in antigen cross-presentation and activating T cells in vivo, which is crucial for fostering active anti-tumor immunity. The switch towards a pro-inflammatory (M1) state upon antigen recognition and involvement in antigen presentation were similarly found in CAR-Ms generated from iPSCs via GSEA analysis [114, 115]. The TAMs could be redirected to a CAR-expressing M1 state using a nonviral, piggyBac vector encoding an anti-ALK CAR and the IFNγ gene. The CAR-Ms reprogrammed in situ mirrored the ACT CAR-Ms reported by Klinchinsky et al. They exerted anti-tumor activity via CAR-mediated phagocytosis, presented antigens, and activated CD8+ T cells. However, as with the other nanoparticle-based in situ programming approaches, the gene transfer efficiency was significantly lower than that of viral vectors, indicating that achieving high gene transfer efficiency in these immune cells remains a critical bottleneck that, if overcome, could lead to significantly improved therapeutic outcomes. Nevertheless, CAR-Ms represent a promising avenue for tackling solid tumors, and that compared to CAR T-cell therapy, which can lead to CRS, the pro-inflammatory cytokines generated by CAR-Ms are more confined to the TME, further reducing the risk of CAR-based therapies.
Nanotechnology for modulating car T-cell activity
Nanotechnology also offers ways to manipulate CAR T-cell activity besides acting as delivery vehicles for gene transfer. One of the challenges associated with CAR T-cell therapy is the adverse effect associated with CRS because of the lack of control of activated T cells activity. A conventional approach to modulate such activity is by engineering ON and OFF switches in CAR T-cells [116–118]. Alternatively, nanoparticles with intrinsic material properties can be tailored to manipulate the activation or deactivation of the CAR constructs. By harnessing these properties, including the material’s propensity to generate or respond to light, heat, or magnetic field, the state of the CAR T-cells can be tuned to reduce off-target effects and CRS. Nguyen et al. developed a light-switchable CAR (LiCAR) that could only be activated by a blue light when the CAR T-cells engaged the target ligand. This is accomplished by including photoresponsive domains into the split CAR constructs; upon illumination and binding to a substrate, the split CAR constructs dimerize and activate intracellular signaling pathways [119]. The core–shell upconversion nanoplates (UCNPs) acted as light transducers for LiCAR, in which the Yb3+ served as the sensitizer and Tm3+ as the emitter for the NIR light. The dimensions of the UCNPs (~ 200 nm in diameter and ~ 85 nm in height) allowed for enhanced upconversion luminescence, which is crucial for in vivo application. To increase the efficiency of the LiCAR/UCNP system, the authors coupled streptavidin-functionalized UCNP to the LiCAR T-cells, and observed superior cancer-killing compared to tumors protected from light or treated with LiCAR T-cells without UCNP. Importantly, the LiCAR/UCNP system reduced the two major adverse effects commonly associated with conventional CAR T-cell therapy: the “on-target, off-tumor” cytotoxicity and CRS. Mice treated with LiCAR T-cells exhibited reduced B cell aplasia, a common side effect associated with anti-CD19 CAR T-cell therapy, compared to mice that received WT CAR T-cells. Furthermore, SCID-beige mice (to model CRS) treated with LiCAR T-cells showed no weight loss and reduced serum IL-6 levels when compared to mice treated with WT CAR T-cells. These results indicated that the LiCAR/UCNP system could offer a greater safety profile using the optical properties of nanomaterials for controlling cellular behavior. To further validate the utility of optical nanomaterials for CAR T-cell engineering, Miller et al. designed thermal-responsive gene switches governed by the HSPB1 core promoter that allows for CAR expression at elevated temperatures (40–42 °C) [120]. Combined with plasmonic gold nanorods injected intravenously into the tumor-bearing mice and exhibiting photothermal effects upon NIR light (650–900 nm) exposure, the thermal-activated TS-Fluc αCD19 CAR T cells could achieve targeted elimination of CD19 + Raji cells in a spatially confined manner. However, while the light is an attractive source for stimulus-responsive control, tissue penetration of light and water absorption are still major issues [121, 122], especially in larger hosts. This issue is further exacerbated in solid tumors, in which CAR T-cells are already inefficient against. Future efforts into nanomaterial-CAR T-cell platforms focusing on using stimuli that do not readily interact with body tissues, such as magnetic fields, could provide greater therapeutic efficacy. For example, anchoring magnetic nanoclusters to T cells and using MRI guidance could increase the accumulation of ACT T cells [123].
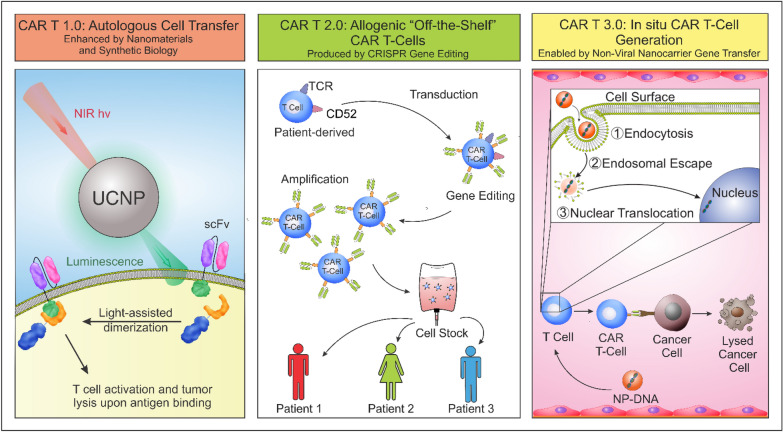
CAR-based therapies show great promise for oncology-immunotherapy. Current CAR T-cell therapy is based on autologous T cell engineering, which can be costly, and patients can experience CRS or neurotoxicity. The off-targeting effects can be minimized by including more complex designs and incorporating logic gates and nanomaterials (CAR 1.0) [119]. To further improve the therapy for wider patient access and eliminate GVHD, the CAR T-cells can be modified via CRISPR gene editing to remove the TCR alpha constant (TRAC) locus and the CD52 (CAR 2.0) [30, 124]. However, since this is a “universal product”, each patient might respond differently, hence affecting final patient outcome. To decrease the time and costs associated with ACT, a potential direction is to adapt an in situ CAR T-cell generation approach to reprogram endogenous T cells into CAR T-cells (CAR 3.0) [98]. This can be a personalized approach with increased efficacy and decreased overall costs combined with genomic sequencing technology (Fig. 6).
Fig. 6.
Next-generation approaches for improving CAR T-cell therapy. Advances that combine synthetic biology and optogenetics allow the design of switchable CAR T-cells with greater specificity and spatiotemporal control of activity to improve current autologous ACT (CAR 1.0). A step forward would be to engineer CAR T-cells with CRISPR multiplex gene editing to inactivate or knockout genes such as TRAC and CD52 (CAR 2.0). The edited cells can then be expanded and stored as an “off-the-shelf” product. To decrease the time and costs associated with ACT, a potential direction is to adapt an in situ CAR T-cell manufacturing approach to reprogram endogenous T cells into CAR T-cells (CAR 3.0). Combined with genomic sequencing technology, this can be a personalized approach with increased efficacy and decreased overall costs
Inducing acute immunity towards infectious diseases
Infectious diseases pose a foremost threat to global health and are the leading causes of death for individuals living in poverty. Despite this fact, there are limited treatment options for many of these diseases, therefore safe and efficacious vaccines only exist for a small portion of all diseases. A vaccine's ultimate purpose is to generate a high affinity and antigen specific antibody response against the immunization agent. This is usually observed by detecting an increase in IgG or IgA, which are the dominant types of antibodies produced by memory B cells following vaccination. Typically, vaccine platforms entail either live attenuated or whole inactive vaccines. Live attenuated vaccines have been available since the 1950’s and were derived from the disease-causing pathogen that has been weakened under laboratory conditions to cause either no or mild disease effects while offering the individual immunity to the pathogen. These attenuated pathogens can replicate within the host, and the stimulation of these pathogens provides enough time for memory cells to be produced if the individual is ever exposed to the pathogen. Live attenuated vaccines tend to be long lasting due to the formation of memory cells; common diseases that use this approach include tuberculosis, polio, measles, and influenza [125–127]. Conversely, inactivated whole-cell vaccines utilize pathogens that have been killed through either physical or chemical means such that they cannot cause disease. Even though this technique is regarded as safer since there are no live components, inert whole-cell vaccines may not always provoke an immune response, and the immune response that is elicited may be short-lived and require multiple doses to be successful. Typical applications include vaccines for hepatitis A, typhoid, and influenza [128–130]. As was alluded to earlier, these conventional approaches to creating vaccines have several limitations, including a complicated manufacturing process, potentially severe side effects, and severe infections. To this end, subunit vaccines, specifically those that employ nanoparticles as vaccine delivery vehicles, have been of particular interest. Much like inactivated whole-cell vaccines, subunit vaccines do not use live components of a pathogen but only the antigenic components to elicit an immune response. Several nanoparticle platforms have been developed applying the concept of subunit vaccines and have been demonstrated to induce both humoral and cellular immune responses against the pathogen they are modified with, as described in the upcoming sections.
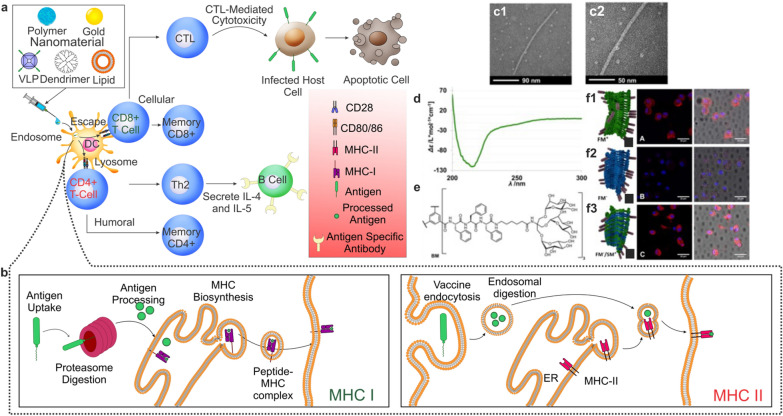
Despite the development of a plethora of antibiotics over the years, the treatment of bacterial infections is still plagued by several challenges, specifically owing to an increase in antibiotic-resistant bacteria strains. To this end, a wide range of nanoparticle platforms, encompassing dendrimers, liposomes, polymeric, protein, and inorganic nanoparticles, have been implemented to enhance the therapeutic effectiveness of antibiotics and their function as vaccine adjuvants. For the latter, nanoparticles are targeted towards APCs, including DCs, macrophages, and B cells to uptake extracellular proteins, process them, and present these peptides to CD4+ T cells to elicit long-term humoral immune responses against the antigen in the form of antigen-specific antibodies (Fig. 7a, b). Of particular interest in the field of nanoparticle vaccines are liposomes. Liposomes are a promising delivery vehicle as their compositions can make them nontoxic, nonimmunogenic, and biodegradable. Moreover, liposomes are very modular as their size, lipid composition, charge, and loaded antigen cargo can be easily modified [131]. As such, antigens can easily be encapsulated in the hydrophilic core and protected from enzymatic degradation by a lipid bilayer, which functions to increase the bioavailability of the antigen by allowing for facile transport through the cell membrane [132].
Fig. 7.
Bacterial vaccine and antigen-presenting pathways. a Various nanoparticles that are used to deliver antigens or antigen fragments for bacterial vaccines. Following the delivery of these antigen-bound/encapsulated platforms, the cell uptakes the vaccine via endosomal pathways. From here, the antigen can undergo processing in either the endosome, via the MHC-II pathway, or be processed by proteases in the cytoplasm before the presentation by the MHC-I complex. Antigen presentation via MHC-II results in humoral immunity where Th2 cells activate B cells to produce antibodies against the bacteria. Conversely, MHC-I presentation results in the differentiation of T cells into CTLs that can initiate cell death by cellular immunity. b Pathways for MHC-I and MHC-II antigen processing. In MHC-I presentation, the antigen can escape the endosome and is processed by proteases. The processed antigen is transported to the endoplasmic reticulum (ER), where MHC biosynthesis occurs, complexes with the MHC-I ligand, and is transported to the cell membrane for presentation to T cells. During MHC-II pathways, the antigen is digested in the endosome and fuses with MHC-II intracellularly to form the peptide MHC-II complex prior to being shuttled to the cell surface for presentation. c TEM characterization of negatively stained self-assembly polymer to demonstrate monodispersity and size range of mannose-decorated multicomponent supramolecular polymers targeted towards APCs. d Circular dichroism spectra of dendritic glycopeptide peptide. e Chemical structure of amphiphilic glycopeptide used as a monomer for self-assembly. f Cellular uptake of self-assembled polymers with and without mannosylated monomers (f1 and f2) and mannosylated monomers without a fluorescent label (f3).
Reproduced with permission from ref [135]
Bacterial infections and subunit vaccines
Several studies have highlighted the usage of liposomes as a delivery vehicle for subunit vaccination. For example, a recent study encapsulated utilized a liposome-based approach to encapsulate two tuberculosis (TB) antigens (Ag85B and ESAT-6) as a novel subunit vaccine and delivered this system into C57BL/6 mice [133]. The liposome comprised phosphatidylserine encapsulating the two TB antigens and was delivered first subcutaneously and then intranasally. It was observed that mice exposed to the liposome containing the Ag85B antigen produced large amounts of IFN-γ, which is essential for TB resistance. Following stimulation, splenocytes were discovered to overexpress IL-17A, which has been hypothesized to produce CD4+ cells that occupy the lung post-infection and produce chemokines that recruit IFN-γ secreting CD4+ T cells to help control the infection [134].
Aside from liposomes, another promising approach to synthesizing nanoparticle vaccines is through the usage of polymers and dendrimers. Much like liposomes, polymer platforms are highly modular and versatile. By carefully tuning the monomeric units, it is possible to create biodegradable, biocompatible, and nontoxic polymeric particles as possible vaccine vehicle candidates. This idea was highlighted in a study that utilized mannose-decorated supramolecular polymers to facilitate the uptake of the platform by APCs [135]. This study used a combination of benzene-tricarboxamides and triazine-branched nonaphenylalanines to guide supramolecular polymerization, which was carried out in conjunction with mannose functional monomers (Fig. 7c–f). Although this system was not used to encapsulate an antigen, the platform could be internalized by macrophages and demonstrated the potential to be further developed in future applications. A similar system was designed in 2016 using a combination of PLGA and PEI to encapsulate a model antigen and impart a positive charge to facilitate cellular uptake and endosomal escape [136]. This platform encapsulated OVA and could induce cross-presentation of the model antigen on MHC-I molecules for a strong and antigen-specific response in CD8+ cells.
While less commonly used in bacterial infections, virus-like particle (VLP) vaccine platforms have also been reported in developing vaccines against anthrax infections. The VLP that will be discussed is derived from the bacteriophage Escherichia virus T4 (T4) and was genetically fused in their Hoc or Soc region to the antigen of interest. Anthrax infections are caused by Bacillus anthracis, which is a gram-positive bacterium, that can usually be treated by antibiotics but is fatal if inhaled. Recently, a B. anthracis vaccine was created by fusing the anthrax protective antigen (PA) to the T4 Soc capsid proteins [137]. Several animal models, including mice, rats, and rabbits, were subjected to the anthrax lethal toxin at 100% lethal dosage, and all the treated animals could survive. This was attributed to the vaccine's ability to elicit both humoral and cellular immunity in the treated animals that were notably absent in the nontreated controls, which died two days following injection.
Inorganic nanoparticles, specifically gold nanoparticles (AuNPs), have been utilized as another approach to create vaccines against bacterial infections owing to their ability to be easily functionalized and to act as an adjuvant. This is especially true with gold, which has piqued interest due to its inherent stability, low toxicity, capacity to activate macrophages, and ability to cause immune responses in lymphocytes that create antibodies against certain antigens [138–140]. In recent years, a number of studies have been conducted to develop subunit vaccines against bacterial infections using gold nanoparticles as the primary building block. One example utilizes a subunit of the flagellin of Pseudomonas aeruginosa as a vaccine candidate due to the presence of thiol-containing cysteine residues near the N-terminus that can form Au–S bonds [141]. This flagellum is known to interact with TLR5 to elicit an immune response. Additionally, P. aeruginosa is a gram-negative bacterium that causes infections in immunocompromised patients and is responsible for 10% of hospital-acquired infections. Moreover, this bacterium has exhibited multidrug resistance, making antibiotic treatment difficult or unfeasible. By conjugating the flagellum to the AuNP, the authors could create a highly immunogenic system, which would be favorable to be internalized by DCs and macrophages due to the presence of the AuNP, which resulted in the formation of antibodies that would recognize the whole bacteria. Similar studies were performed for other bacterial targets, including tetanus toxin and Listeria monocytogenes, where a botanical adjuvant was utilized in conjunction with the AuNP to increase the systemic response of IgG and IgA in one study. In a separate study an Au glyconanoparticle with a listeriolysin O peptide was used to target DCs and induce a robust T cell protective response [142, 143].
Viral infections and mRNA vaccines
A great amount of work has been dedicated to designing vaccines for viral infections following the novel coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), specifically in the form of IVT mRNA vaccines. While the previously described subunit vaccines utilized a fragment of the target antigen in order to elicit an immune response, mRNA vaccines function differently to produce a similar humoral response. Specifically, the mRNA for a protein of interest, the spike protein in the case of SARS-CoV-2, is generated and injected into the patient using a nanocarrier. The principle behind IVT mRNA technology is as follows. First, mRNA containing a 5’ cap, 5’ and 3’ untranslated regions, the open reading frame, and a 3’ poly(A) tail is incorporated into a nanocarrier that will facilitate cell membrane interactions and endosomal escape for in vitro translation, these will be discussed in an upcoming section. After entering the cell, a fraction of the exogenous mRNA will escape the endosome and enter the cytoplasm. The mRNA will then undergo translation using the cell’s native machinery. Eventually, exonucleases will degrade the mRNA and terminate the translation process for the protein of interest. The newly expressed proteins undergo post-translational modifications and are degraded into antigen peptides loaded onto MHC molecules for presentation to immune effector cells. From here, cellular or humoral immunity will arise and provide the patient immunity to the antigen that was processed and presented. In this way, if the patient is exposed to the SARS-CoV-2 virus in the future, existing antibodies are capable of binding to the virus and preventing it from replicating. With this general idea in mind, several strategies have been employed to design optimal mRNA lipid nanoparticles to be utilized as a vaccine regarding their efficacy, toxicity, and stability.
The design of lipid nanoparticles for mRNA vaccines should take into consideration several elements including: i) mRNA target and possible nucleotide modifications to ensure immunogenicity and recognition by RNA sensors, ii) an optimized lipid nanoparticle formulation that can encapsulate and deliver the target mRNA, and iii) long-term storage of the nanoparticle. Other types of nanoparticles, both organic and inorganic, are summarized in Table 2. Nucleotide modifications are crucial when developing mRNA vaccines, as they can otherwise be recognized by RNA sensors, including TLR7 and TLR8, and elicit an unfavorable innate immune response [144]. While the end goal of these vaccines is to activate the immune system, doing so preemptively may drastically reduce mRNA translation and render the treatment ineffective [145]. Standard mRNA modifications that reduce immunogenicity include modifying nucleotides with pseudouridine, 2-thiouridine, N6-methyladenosine, N1-methylpseudouridine, and 5-methylcytidine as they aid in preventing mRNA from activating TLRs, or making the mRNA undetectable by RIG-I and PRK pathways in the cases of pseudouridine and 2-thiouridine [146]. By employing nucleotide modifications, researchers can enhance the stability of mRNA used in these nanoparticle vaccines and, ultimately, increase protein translation.
Table 2.
Nanoparticle vaccines targeted towards viral antigens
| Nanoparticle | Size (diameter) | Antigen | Viral target | References |
|---|---|---|---|---|
| Gold | 20–40 nm | West Nile Virus envelope protein | West Nile Virus | [147] |
| Gold | 12 nm | M2e | Influenza A | [148] |
| Iron oxide | 25 nm | HIV-1 Envelope glycoprotein SOSIP trimers | HIV-1 | [149] |
| Mesoporous silica | 120 nm | E2 | Bovine viral diahorrea virus | [150] |
| Chitosan | 571.7 nm | Killed swine influenza A virus H1N2 | Swine influenza virus (H1N2) | [151] |
| PLGA | 200–300 nm | Inactivated virus H1N2 | Swine influenza virus (H1N2) | [152] |
| PLA/PLGA | 474–940 nm | Hepatitis B surface antigen | Hepatitis B virus | [153] |
| PLGA/lecithin | 300 nm, 1 μm, and 3 μm | HPV-L1 Pentamer | Human papillomavirus | [154] |
| PLGA | 972.5 nm | Influenza split vaccine antigen | Influenza H5N1 | [155] |
| MD39-6xHis:Ni–NTA (1:40) | 150 nm | BG505 MD39 trimer | HIV | [156] |
| DOPC:DOPG (4:1) and DMPC:DOPC:DOPG (2:2:1) | 64.5 nm, 150 nm, and 203 nm | membrane-proximal external region (MPER) from envelope subunit gp41 | HIV | [157] |
| Virus-like particles | 10 nm | HPV16 L1 capsomeres | HPV | [158] |
| Protein nanoparticle (ferritin) | 20–50 nm | HIV-1 envelope and H1 influenza hemagglutinin | HIV and Influenza (H1N1) | [159] |
Other approaches to optimizing the mRNA are to i) employ a cap at the 5’ end to eliminate free phosphate groups in the mRNA, which can both increase the stability and translation efficiency by recruiting additional transcriptional machinery, ii) incorporate untranslated regions (UTRs) that regulate transcription, and iii) reduce RNA exonuclease activity with a poly(A) tail on the 3- end. There have been several capping agents that have been employed over the years, including eukaryotic translation initiation factor 4E (eIF4E), anti-reverse cap analogs (ARCA), and CleanCap [146, 160, 161]. In essence, the development of new capping agents was to promote the orientation of the cap on the mRNA, as 20% of eIF4E capped mRNAs were either not capped or capped incorrectly, resulting in poor efficacy. UTRs influence both the translation efficiency and mRNA half-life. 5’ UTRs influence protein expression and should be designed to have a minimal amount of GC content as this can cause complex geometries that may hinder ribosomal recruitment and, therefore, start codon recognition [162]. Similarly, 3’ UTRs should be kept to an optimal length as longer sequences decrease the half-life of the mRNA [163]. Finally, the poly(A) tail promotes mRNA transcription by binding poly(A)-binding proteins (PABP) that recruit eIF4E and eIF4G on the 5’ cap to promote a circular mRNA structure that diminishes immunogenicity [164].
Two of the most prominent mRNA vaccines currently available are for the recent novel coronavirus disease caused by SARS-CoV-2, specifically Pfizer/BioNTech’s BNT162b2 and Moderna’s mRNA-1273 vaccines. Recently, the immunopathogenesis of COVID-19 has been extensively reviewed [165]. The spike protein is a class I fusion glycoprotein that has been identified as the major surface receptor on the coronavirus and is the primary target for generating neutralizing antibodies. However, producing viable cell lines with clinical-grade spike proteins can be an arduous and time-consuming task, which is detrimental to developing a vaccine quickly and efficiently. To this end, researchers at both of these pharmaceutical companies decided to leverage an mRNA vaccine that elicits both cellular and humoral immunity with a notably decreased manufacturing time [166, 167]. Furthermore, it was previously demonstrated that modifying uridine in mRNA, via a N1-methylpseudouridine modification, allows for mRNA vaccines to avoid endosomal TLR-mediated microbial-associated molecular pattern (MAMP) activation and prevents inciting an innate immune response [168]. Thus, the mRNA-1273 Moderna vaccine utilizes a N1-methylpseudouridine modification on their optimized spike protein mRNA [SARS-CoV-2S(2P)] in addition to 5’ and 3’ UTRs and a 3’ poly(A) tail (Fig. 8c) [169]. When designing the lipid nanoparticle to deliver mRNA-1273, Moderna chose a ratio of SM-102: 1,2-distearoyl-sn-glycero-3 phosphocholine (DSPC): cholesterol (ionizable cationic lipid: neutral lipid: cholesterol: (heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate} PEG2000-DMG (monomethoxypolyethyleneglycol-2,3-dimyristylglycerol) 50:10:38.5:1.5) with a known pKa of 6.75 [170, 171]. The pKa of these ionizable lipids falls within the optimal 6.6–6.9 range described previously for eliciting an adaptive immune response following mRNA vaccination. Furthermore, the small amount of PEG-ylated lipids used is consistent with previous reports used to control the lipid nanoparticles size, prevent mRNA leakage, and increase stability. Pfizer-BioNTech utilized the same mRNA modifications with a different lipid composition and molar ratios for their BNT162b2 mRNA vaccine containing the same 4 major components, (4-hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) (an ionizable lipid), 2-[(polyethylene glycol)-2000]-N,Nditetradecylacetamide, DSPC, cholesterol. Unlike Moderna’s lipid nanoparticle, Pfizer’s has a pKa of 6.09 that is below the cited optimal range for eliciting an mRNA vaccine adaptive immune response [171, 172].
Fig. 8.
mRNA modifications and delivery for viral vaccines. a General lipid nanoparticle formulations for mRNA delivery, peg-ylated lipids improve circulation while cholesterol and cationic lipids provide structural stability and facilitate endosomal escape and cellular uptake, respectively. b Cryo-EM image of a lipid nanoparticle. c Commonly utilized mRNA modifications that increase the efficacy of the delivered mRNA by increasing protein expression and mRNA stability while mitigating immune activation via pattern recognition receptors. d Mechanism of action for viral vaccines. Following delivery and cellular uptake of the mRNA encapsulated lipid nanoparticles , the transcript is able to escape the endosome and undergo translation, in this schematic the mRNA codes for the spike protein. T he spike protein is then processed by proteases and presented to T cells in lymph nodes via the MHC-II and TCR complex. Next, T cells begin to proliferate and differentiate into effector T cells and T follicle helper cells that can stimulate B cells to produce antibodies specific o the spike protein antigen. Following future exposure to SARS-CoV2, the patient’s immune system will be primed and capable of combating the virus. e Serum samples collected 23 days (“preboost”) and 46 days (“postboost”) from hACE2 transgenic mice treated following initial priming. Lipid nanoparticle RBD-hFc mRNA treated mice display elevated SARS-CoV-2 spike-specific IgG antibodies (e1) and neutralizing antibodies (e2) following the booster compared to mRNA delivered without a lipid nanoparticle and lipid nanoparticles without the target mRNA. f Survival of mice groups following treatment shows that boosted mice had a higher survival rate. g Weight of treated mice returned normal two weeks post booster for mice treated with lipid nanoparticle RBD-hFc mRNA. Untreated mice did not survive past day 7. Reproduced with permission from ref [173, 174].
Promoting tolerance for autoimmunity and suppressing chromic inflammation
Autoimmune diseases are marked by abnormally low activity or overactivity of the patient’s immune system, which limits the body’s ability to combat foreign invaders or causes the body to lose tolerance towards native antigens, and develop autoantibodies that recognize their own tissue as foreign, respectively [175]. According to the American Autoimmune Related Disease Association (AARDA) approximately 50 million Americans suffer from autoimmune disorders (https://aarda.org/). However, the factors that contribute to autoimmune diseases are immense and complex as they can combine factors from both innate and adaptive immunity. In pathological autoimmune disorders innate immune components, such as pattern-recognition receptors (PRRs), such as TLRs, are stimulated and activate myeloid cells, innate-lymphoid cells, and various populations of T cells that ultimately contribute to the disease pathology. However, when the adaptive immune system is responsible for the disease, B and T cells increase the range of antigens they can recognize but, in doing so, lose the ability to distinguish between exogenous and self-antigens. Affecting the lining of synovial tissue in diarthrodial joints, the lining of the intestines, nervous tissue, and systemically throughout the body are some of the manifestations of autoimmune diseases. These include rheumatoid arthritis (RA), Inflammatory Bowel Disease (IBD), multiple sclerosis (MS), and systemic lupus erythematosus (SLE), to name a few [176–179].
Traditionally, treatment of autoimmune diseases has centered around suppressing the autoimmune response of the disorder by delivering immunosuppressants or other therapeutic agents that interfere with cell activation or migration via systemic or locally targeted methods [180, 181]. However, while some disease-modifying anti-rheumatological drugs (DMARDs) are very potent and efficient in providing symptomatic relief and slowing the progression of the disease, they do so at the risk of moderate to severe off-target effects that may result in toxicity. For instance, calcineurin inhibitors, such as cyclosporine, are used to treat RA by blocking T cell activation and IL-2 production, which is associated with T cell proliferation, but may also confer nephrotoxicity concerns to the treated patient [182]. A rise in the number of biologics for the treatment of autoimmune diseases has occurred in response to the associated health issues created by the off-target effects of DMARDs. These biologics are intended to ameliorate these health concerns. Compared to traditional drugs, biological drugs tend to be antibodies, interferons, fusion proteins, or synthetic proteins that either block the effects of pro-inflammatory cytokine or act on immune-competent cells, including T cell and B cell targeted biologics. One example is the biologic Tocilizumab, a monoclonal antibody, that is an IL-6 receptor antagonist responsible for inhibiting the pro-inflammatory cytokine signaling of IL-6 in RA patients and is intravenously injected [183]. While biologics are generally safer than DMARDs and can be used in conjunction to aid in the treatment of patients suffering from autoimmune diseases, there exist several limitations that still remain to be addressed including: i) delivery to the target cells, ii) degradation and clearance in vivo, and iii) transport across biological barriers. To this end, there have been various reports of utilizing nanotechnology to address the aforementioned limitations.
One promising application of nanotechnology in treating autoimmune diseases has been observed in patients suffering from MS. Multiple sclerosis (MS) is an autoimmune disease that affects the central nervous system (CNS), causing patients to have deficits in neurocognitive ability, sensation, motor, and autonomic functions as a result of the activation of CD4+ autoreactive T cells and a cascade of cellular events that eventually leads to chronic inflammation and destruction of the myelin sheath by myelin-reactive auto-T cells [184]. In short, immune cells, such as microglia, that reside in the CNS begin to upregulate MHC-I and -II, cell surface costimulatory molecules, and cytokines that allow CD4+ and CD8+ T cells, monocytes, macrophages, and DCs to enter into the CNS, via the blood–brain barrier (BBB), following their activation [185]. Of these newly migrated cells, myelin-reactive auto-T cells initiate an inflammatory cascade and cause the demyelination of neurons, resulting in axonal damage and loss of neuronal function. The demyelination processes provide a positive feedback loop to further promote the inflammatory process while simultaneously causing damage to the BBB, stimulating oxidative and nitrosative stress pathways, and activating macrophages [186]. While there is no available treatment to regenerate myelin following its destruction, nanotechnology has been utilized in MS to deliver therapeutic agents across the BBB in a targeted manner to alleviate symptoms of inflammation.
Immunotherapy via drug delivery
Using lipid nanocarriers, one approach has been to deliver therapeutic molecules through the BBB by entering the post-capillary venules. Lipid nanocarriers can encapsulate both hydrophilic and hydrophobic molecules, pass through the BBB by entering post-capillary venules, and have their surfaces engineered to interact with the BBB or specific cells of interest [187]. In 2019, an intranasal lipid nanocarrier was developed to deliver the selective dihydroorotate dehydrogenase inhibitor Teriflunomide (TFM) [188]. TFM has been demonstrated to have a cytostatic effect on proliferating B cells and T cells as well as activated astrocytes and microglia by preventing the synthesis of various cytokines and interfering with the interaction between T cells and DCs [189]. Normally, therapeutic molecules have difficulty penetrating the BBB due to this barrier’s innate hydrophilicity and can also cause hepatotoxicity if delivered systemically. However, the authors could circumvent these limitations by loading TFM into a lipid nanocarrier. Moreover, by adopting an intranasal delivery method, the lipid nanocarriers can avoid hepatic clearance and toxicity and also have a large surface area of vascular tissue to permeate through to increase the number of particles reaching their target location. Due to their small size (~ 100 nm) and composition (i.e., combining HPMC K4M and poloxamer 407) these lipid nanocarriers allow for biodegradable, biocompatible, and mucoadhesive systems.
Another noteworthy study used solid lipid nanoparticles (SLNs) to administer the polyamine methylthioadenosine orally (MTA). In this study, SLNs were synthesized by a microencapsulation technique where steric acid, MTA, Tween 80, and phospholipid 90 G (PL) were combined and mixed isothermally to generate approximately 100 nm SLNs with a negative zeta potential (~ -8 mV). It has previously been described that oral delivery of this SLN system can promote the delivery of several drugs to the CNS [190, 191]. In order to mimic MS, a mouse model was utilized where the animal was subjected to the copper chelating agent cuprizone in order to induce demyelination. Pharmacokinetically, it was found that by incorporating MTA into a SLN the researchers were able to enhance the concentration of the compound that was being delivered at various time points across 4 h. Moreover, the circulation time was nearly tripled using the SLN, where the elimination half-life of the compound was 28 min when administered alone versus 1.25 h when delivered using the lipid platform. Following demyelination, the mice experienced decreased locomotor activity; however, measurements obtained from actophotometer and Rotarod tests demonstrated that the mice treated with MTA loaded in the SLN were able to improve their locomotor activity to 71% relative to the cuprizone-only group, whereas the MTA-only group improved locomotor active by 49%. Similarly, the rotarod test demonstrated an increase in overall coordination, grip strength, and balance of the animals by 95% in the MTA-SLN condition relative to controls and a 68% increase in the MTA-only condition. A histopathological study revealed that the cuprizone-only group had average myelination of 57%, whereas the MTA and MTA-SNL conditions had 65% and 80% myelination, respectively, after 30 days of treatment. Overall, this study shows the advantages of utilizing nanotechnology in the effective delivery and treatment of MS.
Rheumatoid arthritis (RA) has been another area of interest for delivering therapeutic drugs utilizing nanotechnology. RA is a chronic and systemic autoimmune disease that predominantly affects the synovial joints, where it is characterized by inflammation of the synovium that can lead to cartilage and bone erosion, and can lead to progressive disability and premature death as there are risks associated with extra-articular symptoms such as keratitis, pericarditis, and pulmonary granulomas [192]. One key contributor to the pathogenesis of RA and other autoimmune diseases is Th17 cells. Th17 cells are known to express pro-inflammatory cytokines (i.e., IL-17[A-E], IL-6, IL-21, IL-22, and TNF-α), and their differentiation is promoted by exposure to a series of cytokines (TGF-β, IL-6, and IL-21) that activate STAT3 and induce the expression of lineage-specific transcription factors RORγt and RORα [193, 194]. Of the aforementioned cytokines produced by Th17 cells, IL-17A contributes to RA pathology by stimulating fibroblast-like synoviocytes (FLS), causing osteoclasts to mature, and recruiting other immune cells, such as neutrophils, macrophages, and B cells [195]. While usually responsible for lubricating the joints by producing hyaluronic acid and lubricin, once activated, FLS matures and begins heavily expressing TLR1-6 due to an increase in TNF-α and IL-1β [196, 197]. The overexpression of toll-like receptors (i.e., TLR3) and secreting factors like IL-6 in conjunction with cell–cell interactions and type I interferons ultimately leads to enhanced B cell differentiation, which leads to a variety of autoantibodies, T cell, and macrophage activation, which can promote inflammation [176, 198]. Moreover, activated FLS can begin destroying the extracellular matrix and surrounding cartilage by overproducing matrix metalloproteinases (MMPs) to promote a pro-inflammatory environment further.
To this end, a liposome drug delivery system was designed using a novel peptide (ART-1) to preferentially target inflamed cells in joints. This platform also encapsulated IL-27 as a therapeutic agent as it has been demonstrated to inhibit Th17 differentiation while also modulating angiogenesis, osteoclastogenesis, and matrix degradation that are associated with tissue damage and inflammation [199, 200]. The liposome was formulated with DOPC (1, 2-Dioleoyl-sn-glycero-3-phosphocholine), DOPE (1, 2-Dioleoyl-sn-glycero-3-phos-phoethanolamine), cholesterol, and DSPE-PEG (2000) amine (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino (polyethyleneglycol)-2000] and the ART-1 lipopeptide was custom synthesized and incorporated in the formulations, with a size range from 53 nm-165 nm. When this platform was delivered to the animal model, it was determined that IL-27 containing liposomes without the targeting peptide failed to inhibit disease progression to the same extent the targeted liposomes were able to. Furthermore, by directing the liposome containing the therapeutic molecules to inflamed joints, systemic exposure to the cytokine was minimized, as were off-target responses (Fig. 9).
Fig. 9.
Peptide targeted delivery of liposomes to modulate cytokine release in arthritis. a Schematic highlighting liposome components and in vivo delivery to arthritic rats. b Material characterization of ART-1-IL-27-liposomes. b1 and b2 illustrate the uniformity and monodispersity of the ART-1 targeted liposome by TEM and DLS respectively. c Zeta potential measurement to highlight positive induced charge following ART-1 incorporation. d Flow cytometry data corresponding to cellular uptake of ART-1-FITC-liposomes and Control-FITC-liposomes at 250 nM concentrations at various time points. Liposomes modified with ART-1 are capable of targeting and have better uptake efficiency than the control liposomes in vitro. e Real-time fluorescent imaging of naïve and arthritic rats 4 h post I.V. injection. There is increased accumulation in the arthritic joints of the rats with the ART-1 targeting ligand relative to the control. Naïve rats do not demonstrate particle accumulation. f Arthritic score of groups of rats treated with combinations of the ART-1-IL-27 liposome. The group that received with ART-1-IL-27-lipsosme demonstrate better recovery with a lower arthritic score than the control and groups treated with either ART-1 liposome, Control-IL-27 liposome, or Plain IL-27.
Reproduced with permission from ref [200]
Another critical component that contributes to the pathology of RA is activated macrophages. Once activated by the cascade of events described above, macrophages can also release pro-inflammatory cytokines (i.e., IL-1, IL-6, and TNF-α), digestive enzymes, and proteases such as collagenases and MMPs, and produce ROS that can further damage healthy tissues. To this end, nanotechnology has been implemented to deliver therapeutic agents to combat several of these deleterious pathways observed in RA. When macrophages are subjected to prolonged inflammation, they begin to express different markers than would be observed in a healthy state. It has been known that of these receptors, folate receptor-β (FRβ) is selectively elevated in RA synovial macrophages [201]. Thus, folate-mediated targeting, specific for FRβ, has been one approach utilized to specifically target activated synovial macrophages. For instance, a study performed in 2015 was able to develop a liposome functionalized with FRβ that can encapsulate the DMARD methotrexate (MTX) to decrease the harmful side effects and improve the drug's delivery efficiency [202]. In this study, the liposome was composed of DOPE, cholesterol, and N-(carbonyl methoxypolyethylene glycol-2000)- 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-MPEG) and functionalized with a folic acid peptide specific for FRβ in order to encapsulate MTX and precisely deliver the drug to synovial macrophages. A collagen-induced mouse arthritis model was utilized, and it was found that the formulation used was able to reduce expression of both CD39 and CD73, the expression of which is correlated with FRβ expression levels, in joint-infiltrating macrophages. By specifically targeting this population of macrophages, the authors could reduce off-target effects and observe a more favorable clinical score for the targeted system than the bare liposome or MTX treated conditions. This group also utilized the same platform to deliver siRNA for myeloid cell leukaemia-1 (MCL1) to induce apoptosis in synovial macrophages [203].
While inflammatory processes are necessary to initiate healing processes, chronic inflammation, as is observed in chronic wounds, can prevent the injury site from returning to a point of homeostasis. Wound healing is a four-phase immune system response following skin damage that aims to neutralize pathogens and regenerate tissue to close the wound. The first part, homeostasis, starts at the time of injury and ends with the arrival and coagulation of platelets. Patelets then enhance the transition to the inflammation phase by excreting TGF-β1 and Platelet-Derived Growth Factors (PDGFs), which attract neutrophils to the injury site. Neutrophils also release antimicrobial substances to counter bacterial pathogens, and cytokines that recruit macrophages to the injury site. M1 macrophages are responsible for removing cellular debris and apoptotic cells. In the third phase, the proliferation of keratinocytes, fibroblasts, and epithelial cells regenerates skin tissue. The wound healing process turns to an anti-inflammatory state by releasing TGF-β1 and cytokines, and repolarizing macrophages to an M2 state. The release of IFN-γ by macrophages favors fibrogenesis. The final phase of the process sees little cell proliferation and ECM remodeling but rather a decrease of immune cell activity. Macrophages and fibroblasts undergo apoptosis or exit the injury site [204]. For patients suffering from chronic wounds, the wound healing process is unable to progress from the inflammatory stage and tissue remodeling does not occur.
Nanoparticles can enhance tissue regeneration by repolarizing macrophages, before the injury site shifts into the third anti-inflammatory phase. Macrophages then secrete anti-inflammatory biological substances, which help drive the injury site to an anti-inflammation state. One-way nanoparticles can do this is by delivering small molecules. Xia et al. loaded Rebamipide onto chitosan nanoparticles that inhibit the NF-kB signaling pathway, preventing polarization into the M1 phenotype [205]. The small molecule can also be in the form of a sugar unit. Dong et al. delivered galactose-α-1,3-galactose (α-gal) in liposomes to mice suffering from splinted wounds. α-gal showed to accelerate wound healing and closure by enhancing macrophage invasion and lowering the M1 to M2 ratio [206]. In addition, the small molecule does not necessarily have to dissociate from the nanoparticle. Silica nanoparticles covered in mannose cluster the mannose receptor on the macrophage and polarize the cell into M2. The authors found higher levels of fibroblast proliferation [207].
Immunotherapy via biologics
Alternatively, new research has been conducted on developing immunological tolerance with DCs that have immune-suppressive capabilities against a specific antigen. Rather than trying to modulate cellular signaling pathways to reduce inflammatory signals or prevent cells from overproducing antibodies and/or migrating to the disease site, this approach takes advantage of existing cellular functions of the immune system to alleviate autoreactive lymphocytes that are responsible for the disease. Specifically, DCs are highly phagocytic immune surveillance cells that recognize a plethora of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) via PRRs and TLRs organized on their outer membrane. By internalizing potential foreign material, DCs can initiate and regulate functions observed with adaptive immunity by presenting antigens via the MHC-II complex to T cells to stimulate their differentiation. In addition to stimulating T cell differentiation, DCs also tightly regulate T cell development by both central and peripheral tolerance. During central tolerance, DCs present self-antigens to T cells located in the thymus and induce apoptosis when they respond strongly. A similar phenomenon is observed in lymph nodes for peripheral tolerance checks to maintain homeostasis and prevent autoimmune responses [208, 209].
Traditionally, a combination of pharmacological agents, including rapamycin or dexamethasone, cytokines (e.g., TGF-β, TNF-α, IL-1β and/or IL-10), and a variety of the autoantibody desired to generate tolerance towards are introduced to ex vivo purified DCs to generate tolerogenic DCs (tolDCs) [210]. By tuning the chemical cocktail used to induce tolDCs, they can exhibit various phenotypes, including differences in migratory behavior, cytokine release, and type of tolerogenic outcome induced in T cells. After generating the tolDCs with the desired phenotype, the cells can then be transplanted back into the patient to diminish the cellular autoimmune responses via peripheral tolerance mechanisms to cause T cell anergy, deletion, or conversion to a Treg phenotype. While this approach has proven promising in clinical trials, there are several limitations that need to be overcome including: i) the isolation, purification, and culturing conditions of primary DCs requires careful technical experience and ii) generating tolDCs has to be performed on a per-patient basis as there is a risk of histocompatibility complications [211]. However, by implementing nanotechnology and functionalizing nanoparticles with antibodies present on DCs, including CD205, CD206, CD40, or CD11c, various groups have been able to formulate nanoparticle vaccines using tolDCs to modulate cellular responses in a targeted manner [212–215].
One example of generating tolDCs can be observed in the treatment of MS. During this study, several potential autoantigens were identified that play an active role in the demyelination, BBB penetrating, and inflammatory processes, including oligodendrocyte-associated proteins such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG). After MBP was chosen as their target to promote tolerance, small unilamellar vesicles (SUVs) were synthesized via a solid-phase technique and were fabricated from egg phosphatidylcholine (PC) and monomannosyl dioleyl glycerol (ManDOG), which served as a targeting agent towards mannose receptors. These SUVs were then mixed with the MBP peptides to encapsulate them. Controls without the peptide and without the mannose targeting agent were also synthesized similarly but without the specified component. DCs were isolated from the rat’s blood that was utilized in the study for ex vivo data, and the liposome formulation was also delivered systemically to elucidate the in vivo targetability and efficacy. Ultimately, it was discovered that exposure to mannose residues on the surface of liposome carriers is necessary to facilitate delivery to DCs as the nontargeted liposome with the same peptides loaded were significantly less efficient [216]. This was observed by noting the mannosylated liposome’s ability to promote anti-inflammatory, neuroprotective, and regenerative effects. Following treatment, there was a twofold decrease in the concentration of circulating antibodies for MBP, implying systemic suppression of autoreactive B cells, a significant downregulation of IL-2 and IFN-γ, and enhanced expression of BDNF, which is known to induce remyelination [217]. A similar study from 2015 utilized biodegradable polymeric nanoparticles composed of poly(lactic-coglycolic acid) (PLGA) that were 400-500 nm to deliver a MBP peptide [218]. Similar to the previous study, the authors were able to induce potent T cell tolerance to MBPs, reduce immune cell infiltration, and limit cytokine production by protecting their MPN that was conjugated to the surface of the polymeric particle and delivered via IV infusion.
Another promising technology for the delivery of biologics is cell-derived exosomes. Exosomes derived from MSCs have been demonstrated to exhibit immune suppressive properties that can aid in regulating the immune system [219]. In the context of RA, MSC-derived exosomes can ameliorate symptoms such as cartilage degradation and have even been found to induce endogenous cartilage repair [220]. Recently, work done by Tavasolian et al. demonstrated that by isolating exosomes from healthy MSCs that overexpress anti-inflammatory miRNA, specifically miR-146a and miR-155, they could significantly alter Treg populations in collagen-induced arthritis mice. The control over Tregs was observed in conjunction with an increase in anti-inflammatory cytokines, such as IL-10 and TGF-β, and demonstrated that these microRNA transduced exosomes could elicit a therapeutic effect on the immune system CIA-mice [221]. Similar studies were performed by Meng et al. and Zheng et al. The former utilized MSC-derived exosomes carrying miR-320a to regulate RA-FLSs in patient derived samples and was able to attenuate arthritis and bone damage in vivo in mice with CIA by suppressing RA-FLS’s ability to activate, migrate, and invade [222]. Meanwhile, the latter utilized miR-192-5p to downregulate expression of RAC2, which is commonly upregulated in RA synovium and macrophages and can lead to an overproduction of nitric oxide and an inflamed synovium. Using CIA rats, the authors were able to show a decrease in the amount of proinflammatory cytokines (i.e., IL-1β and TNF-α) produced and a reduction in nitric oxide accumulating in the serum [223].
Nanoparticles have also been utilized to deliver biologics to induce macrophage polarization for accelerated woundhealing. The delivery of biological substances also shifts the macrophage into the wound healing state. Xiao et al. loaded a Fibroblast Growth Factor (bFGF) onto an iron oxide nanoparticle. Sustained release of bFGF over 10 days can polarize the macrophage to the M2 state. In vivo experiments showed accelerated wound healing through increased muscle thickness over the control [224]. In addition to growth factor delivery, nucleic acid delivery can also repolarize macrophages. Whitehead et al. delivered TNF-α siRNA in a lipidoid nanoparticle to macrophages. and downregulates MCP-1 while reducing the number of macrophages entering the wound in the M1 state. Lower levels of M1 macrophages lead to rapid wound healing [225].
Nanoparticles can also deliver biologics for heart tissue regeneration. The heart tissue regeneration process is similar to that of wound tissue regeneration, and the stimuli for repolarization are the same [226]. Conjugating IL-4 to the AuNP leads to the secretion of anti-inflammatory cytokines that shift the polarization of the macrophage into the M2 state. The result was functional heart muscle regeneration [227]. Delivery of CD146 and IGF1 shows the higher phagocytic activity of M2 macrophages in systems undergoing myogenesis [228]. Gold-silver nanoparticles promote MHC protein expression, upregulate the expression of myogenic transcription factors, while also activating the p38 MAPK signaling pathway. The Au-AgNPs was able to thicken the skeletal muscle in an in vivo model [229]. On the other side of the spectrum, Lee et al. silenced the transcription factor IRF5 via delivery of RNAi in a lipid nanoparticle. Suppression of the IRF5 gene leads to higher M2 macrophage cell count, faster wound regeneration, and enhanced infarct healing [230].
Summary and future perspectives
Nano-immunoengineering provides a powerful and versatile way for scientists and engineers to control and interrogate immune cell functions. One of the biggest appeals of these nanoparticles is that they can serve as delivery vehicles for in situ cell programming, allowing for cell immunization (e.g., delivering nucleic acids to DCs) or cell engineering (CAR T-cells) in vivo. Such a feat is difficult using current viral vector technology due to the smaller packaging capacity of viruses. This is especially important considering the future generations of CAR that incorporate additional co-stimulatory domains or dimerization domains for enhanced anti-tumor immunity or safety, where the constructs can exceed the cloning capacity of viral vectors. Synthetic nanomaterials can overcome such limitation and enable the delivery of these intricate constructs. The pre-existing immunity in the population for adeno-associated virus (AAV)-based therapies or immunogenicity and tumorigenicity for lentivirus-based therapies further preclude the wide use of viral vector technology. On the other hand, nano-immunoengineering of nonviral vectors can be easily tuned to have gain of function modalities, such as PEG engineering to increase colloidal and serum stability, antibody conjugation to improve targeting, and nuclear localization signal to improve gene transfer efficiency. The latter is especially critical, as one major obstacle concerning the delivery of nucleic acid cargoes for nano-immunoengineering, particularly DNA, is the low gene transfer efficiency. We envision that increasing the delivery of pDNA will drastically increase the overall effectiveness of the in-situ engineering applications and hence a better therapeutic outcome. This allows for scaled-up manufacturing of the therapeutic products and decreased overall costs and preparation time.
Through proper selection of the materials, researchers can better control the safety profile of the systems or tune the property of the nanomaterials for controlled and sustained release. For instance, inorganic nanoparticles such as iron oxide can be used as MR contrast agents for tracking immune cells, or UCNP as light transducers for controlling synthetic protein activities to limit off-target toxicities. In some cases, such as cancer vaccines, the delivery material themselves (e.g., cationic lipids and polymers) could serve as adjuvants to enhance the immune response. Conversely, the degradation of PLGA (and the release of lactic acid) can dampen the local immune microenvironment and provide immunomodulatory effects. Therefore, selecting the optimal nanomaterials for the specific application requires careful consideration for maximized efficacy. A deeper understanding of the physiochemical properties of these nanomaterials and how they interface with the immune system will allow researchers to address underlying challenges in current therapies, as well as explore new frontiers in treatment. In essence, nano-immunoengineering has the potential to effectively counteract disease progression in a cost-effective manner, while still being able to maintain safety and therapeutic efficacy, hence enabling a wider population to be benefited from the next-generation immunotherapies.
Acknowledgements
Not applicable.
Author contributions
Initially, STC and K‑BL discussed the structures and contents of review article. STC conceptualized the review article structure and narrative in addition to providing expertise in the review of nanoimmunoengineering for cancer application. BC provided scientific expertise in the review of nanomaterials for infectious disease and autoimmunity application. JS provided assistance in the review of immune tolerance and additional figure generation. GP provided assistance in the review of cancer vaccines. K‑BL had the role of giving detailed comments on the manuscript and improving critical revision of the review article. All authors read and approved the final manuscript.
Funding
K-BL acknowledges the partial financial support from the NSF (CBET-1803517), the New Jersey Commission on Spinal Cord (CSCR17IRG010; CSCR16ERG019), NIH R21 (R21AR071101), NIH R01 (1R01DC016612, 3R01DC016612-01S1, and 5R01DC016612-02S1), New Jersey Health Foundation (PC16-21CV), and the New Jersey Alliance for Clinical and Translational Science (NJACTS). STC acknowledges the Rutgers Biotechnology Training Program NIH T32 GM135141.
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xie Y-Q, Wei L, Tang L. Immunoengineering with biomaterials for enhanced cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2018;10(4):e1506. doi: 10.1002/wnan.1506. [DOI] [PubMed] [Google Scholar]
- 2.Neshat SY, Tzeng SY, Green JJ. Gene delivery for immunoengineering. Curr. Opin. Biotechnol. 2020;66:1–10. doi: 10.1016/j.copbio.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg MS. Immunoengineering: how nanotechnology can enhance cancer immunotherapy. Cell. 2015;161(2):201–204. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Green JJ. Immunoengineering has arrived. J. Biomed. Mater. Res., Part A. 2021;109(4):397–403. doi: 10.1002/jbm.a.37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao N-B, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin. Cancer Res. 2010;16(13):3420. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4(5):341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Zhang Z. The application of nanotechnology in immune checkpoint blockade for cancer treatment. J. Control. Release. 2018;290:28–45. doi: 10.1016/j.jconrel.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Ordikhani F, et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI insight. 2018;3(20):e122700. doi: 10.1172/jci.insight.122700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J. Control. Release. 2019;306:15–28. doi: 10.1016/j.jconrel.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal. J. Nanobiotechnol. 2021;19(1):243. doi: 10.1186/s12951-021-00975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, et al. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Adv. Mater. 2016;28(40):8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014;26(48):8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11(5):4463–4474. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, et al. Sendai virus acts as a nano-booster to excite dendritic cells for enhancing the efficacy of CD47-directed immune checkpoint inhibitors against breast carcinoma. Mater. Chem. Front. 2021;5(1):223–237. doi: 10.1039/D0QM00393J. [DOI] [Google Scholar]
- 16.Hintersteiner B, et al. Microheterogeneity of therapeutic monoclonal antibodies is governed by changes in the surface charge of the protein. Biotechnol. J. 2016;11(12):1617–1627. doi: 10.1002/biot.201600504. [DOI] [PubMed] [Google Scholar]
- 17.Shobaki N, et al. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J. Control. Release. 2020;325:235–248. doi: 10.1016/j.jconrel.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Kwak SY, et al. PLGA nanoparticles codelivering siRNAs against programmed cell death protein-1 and its ligand gene for suppression of colon tumor growth. Mol. Pharm. 2019;16(12):4940–4953. doi: 10.1021/acs.molpharmaceut.9b00826. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, et al. Enha ncing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms. Nanomaterials. 2019 doi: 10.3390/nano9020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, et al. Silencing PD-1 and PD-L1 with nanoparticle-delivered small interfering RNA increases cytotoxicity of tumor-infiltrating lymphocytes. Nanomedicine (Lond) 2019;14(8):955–967. doi: 10.2217/nnm-2018-0237. [DOI] [PubMed] [Google Scholar]
- 21.Li J, et al. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release. 2010;142(3):416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J. Biomed. Sci. 2019;26(1):78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werno C, et al. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis. 2010;31(10):1863–1872. doi: 10.1093/carcin/bgq088. [DOI] [PubMed] [Google Scholar]
- 24.Zhu S, et al. Targeting of tumor-associated macrophages made possible by PEG-sheddable mannose-modified nanoparticles. Mol. Pharm. 2013;10(9):3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, et al. Anti-CD206 antibody-conjugated Fe3O4-based PLGA nanoparticles selectively promote tumor-associated macrophages to polarize to the pro-inflammatory subtype. Oncol. Lett. 2020;20(6):298. doi: 10.3892/ol.2020.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019;10(1):3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, et al. Optimized nanoparticle-mediated delivery of CRISPR-Cas9 system for B cell intervention. Nano Res. 2018;11(12):6270–6282. doi: 10.1007/s12274-018-2150-5. [DOI] [Google Scholar]
- 28.Nguyen DN, et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat. Biotechnol. 2020;38(1):44–49. doi: 10.1038/s41587-019-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y-W, et al. In vivo editing of macrophages through systemic delivery of CRISPR-Cas9-ribonucleoprotein-nanoparticle nanoassemblies. Advanced therapeutics. 2019;2(10):1900041. doi: 10.1002/adtp.201900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27(1):154–157. doi: 10.1038/cr.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitt JM, et al. Dendritic cell–derived exosomes for cancer therapy. J. Clin. Investig. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HY, et al. CD8+ T cells stimulated by exosomes derived from RenCa cells mediate specific immune responses through the FasL/Fas signaling pathway and combined with GM-CSF and IL-12, enhance the anti-renal cortical adenocarcinoma effect. Oncol. Rep. 2019;42(2):866–879. doi: 10.3892/or.2019.7208. [DOI] [PubMed] [Google Scholar]
- 34.Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. Ther. 2017;25(7):1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, et al. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8+ T cells. Sci. Rep. 2017;7(1):13365. doi: 10.1038/s41598-017-14000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enomoto Y, et al. Cytokine-enhanced cytolytic activity of exosomes from NK Cells. Cancer Gene Ther. 2021 doi: 10.1038/s41417-021-00352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo N, et al. Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat. Commun. 2018;9(1):435. doi: 10.1038/s41467-018-02865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y-T, et al. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv. Sci. 2021;8(6):2003747. doi: 10.1002/advs.202003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu W, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019;10(1):4355. doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang P, et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell. Immunol. 2021;360:104262. doi: 10.1016/j.cellimm.2020.104262. [DOI] [PubMed] [Google Scholar]
- 41.Willis GR, Kourembanas S, Mitsialis SA. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 2017 doi: 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, et al. In situ cancer vaccination using lipidoid nanoparticles. Sci. Adv. 2021 doi: 10.1126/sciadv.abf1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, et al. Nanoparticle cancer vaccines: design considerations and recent advances. Asian J. Pharm. Sci. 2020;15(5):576–590. doi: 10.1016/j.ajps.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 2016;28(7):319–328. doi: 10.1093/intimm/dxw027. [DOI] [PubMed] [Google Scholar]
- 45.Crews DW, Dombroski JA, King MR. Prophylactic cancer vaccines engineered to elicit specific adaptive immune response. Front. Oncol. 2021;11:626463–626463. doi: 10.3389/fonc.2021.626463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai SJ, Amerman A, Jewell CM. Altering antigen charge to control self-assembly and processing of immune signals during cancer vaccination. Front. Immunol. 2021 doi: 10.3389/fimmu.2020.613830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z, et al. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release. 2013;172(1):259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Heuts J, et al. Cationic nanoparticle-based cancer vaccines. Pharmaceutics. 2021;13(5):596. doi: 10.3390/pharmaceutics13050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H, Griffith TS, Panyam J. Poly(d, l-lactide-co-glycolide) nanoparticles as delivery platforms for TLR7/8 agonist-based cancer vaccine. J. Pharmacol. Exp. Ther. 2019;370(3):715–724. doi: 10.1124/jpet.118.254953. [DOI] [PubMed] [Google Scholar]
- 50.González FE, et al. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum. Vaccin. Immunother. 2014;10(11):3261–3269. doi: 10.4161/21645515.2014.982996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Y-Z, Zhao X, Song X-R. Ex vivo pulsed dendritic cell vaccination against cancer. Acta Pharmacol. Sin. 2020;41(7):959–969. doi: 10.1038/s41401-020-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahlund CJE, et al. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017;7(1):17095. doi: 10.1038/s41598-017-16609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens AJ, Burgess-Brown NA, Jiang S. Beyond just peptide antigens: the complex world of peptide-based cancer vaccines. Front. Immunol. 2021 doi: 10.3389/fimmu.2021.696791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol. Rev. 2011;239(1):62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 55.Stegantseva MV, et al. Multi-antigen DNA vaccine delivered by polyethylenimine and Salmonella enterica in neuroblastoma mouse model. Cancer Immunol. Immunother. 2020;69(12):2613–2622. doi: 10.1007/s00262-020-02652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ledwith BJ, et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology. 2000;43(4–6):258–272. doi: 10.1159/000053993. [DOI] [PubMed] [Google Scholar]
- 57.Yang B, et al. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014;10(11):3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minigo G, et al. Poly-l-lysine-coated nanoparticles: A potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25(7):1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 59.Liu MA. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines. 2019;7(2):37. doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z, et al. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS ONE. 2013;8(4):e60190. doi: 10.1371/journal.pone.0060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meleshko AN, et al. Phase I clinical trial of idiotypic DNA vaccine administered as a complex with polyethylenimine to patients with B-cell lymphoma. Hum. Vaccin. Immunother. 2017;13(6):1–6. doi: 10.1080/21645515.2017.1285477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y-F, Yang Y-W. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur. J. Pharm. Sci. 2010;40(2):75–83. doi: 10.1016/j.ejps.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 63.DeMuth PC, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat. Mater. 2013;12(4):367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy K, et al. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 65.Vila A, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57(1):123–131. doi: 10.1016/j.ejpb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Sun B, et al. Mannose-functionalized biodegradable nanoparticles efficiently deliver DNA vaccine and promote anti-tumor immunity. ACS Appl. Mater. Interfaces. 2021;13(12):14015–14027. doi: 10.1021/acsami.1c01401. [DOI] [PubMed] [Google Scholar]
- 67.Deng R, et al. Revisit the complexation of PEI and DNA—how to make low cytotoxic and highly efficient PEI gene transfection non-viral vectors with a controllable chain length and structure? J. Control. Release. 2009;140(1):40–46. doi: 10.1016/j.jconrel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Rehman ZU, Hoekstra D, Zuhorn IS. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano. 2013;7(5):3767–3777. doi: 10.1021/nn3049494. [DOI] [PubMed] [Google Scholar]
- 69.Liu D, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26(9):1735–1749. doi: 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu Y, et al. Inhibition of cGAS-mediated interferon response facilitates transgene expression. IScience. 2020;23(4):101026. doi: 10.1016/j.isci.2020.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni R, Feng R, Chau Y. Synthetic approaches for nucleic acid delivery: choosing the right carriers. Life (Basel) 2019;9(3):59. doi: 10.3390/life9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Y, et al. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano. 2013;7(6):5376–5384. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassett KJ, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nogueira SS, et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 2020;3(11):10634–10645. doi: 10.1021/acsanm.0c01834. [DOI] [Google Scholar]
- 75.Sabnis S, et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26(6):1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanez Arteta M, et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. 2018;115(15):E3351. doi: 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1(5):16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 78.Tang L, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018;36(8):707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephan MT, et al. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stephan MT, et al. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, et al. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS Nano. 2017;11(3):3089–3100. doi: 10.1021/acsnano.7b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y-Q, et al. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomaterials Science. 2019;7(4):1345–1357. doi: 10.1039/C8BM01556B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang B, et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Transl. Med. 2015 doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones RB, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53. doi: 10.1016/j.biomaterials.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siriwon N, et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol. Res. 2018;6(7):812. doi: 10.1158/2326-6066.CIR-17-0502. [DOI] [PubMed] [Google Scholar]
- 86.Chandrasekaran S, et al. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials. 2016;77:66–76. doi: 10.1016/j.biomaterials.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loftus C, et al. Activation of Human Natural Killer Cells by Graphene Oxide-Templated Antibody Nanoclusters. Nano Lett. 2018;18(5):3282–3289. doi: 10.1021/acs.nanolett.8b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siegler EL, et al. Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol. Ther. 2017;25(12):2607–2619. doi: 10.1016/j.ymthe.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitchell MJ, et al. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc Natl Acad Sci U S A. 2014;111(3):930–935. doi: 10.1073/pnas.1316312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joo K-I, et al. Crosslinked multilamellar liposomes for controlled delivery of anticancer drugs. Biomaterials. 2013;34(12):3098–3109. doi: 10.1016/j.biomaterials.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin H, et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 93.Baum C, et al. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 2006;17(3):253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 94.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 95.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 96.Pack DW, et al. Design and development of polymers for gene delivery. Nat. Rev. Drug Discovery. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 97.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem. Rev. 2009;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 98.Smith TT, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017;12(8):813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parayath NN, et al. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020;11(1):6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fonte P, Reis S, Sarmento B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release. 2016;225:75–86. doi: 10.1016/j.jconrel.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 101.Olden BR, et al. Cationic polymers for non-viral gene delivery to human T cells. J. Control. Release. 2018;282:140–147. doi: 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richter F, et al. Improved gene delivery to K-562 leukemia cells by lipoic acid modified block copolymer micelles. J. Nanobiotechnol. 2021;19(1):70. doi: 10.1186/s12951-021-00801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ayyadevara VSSA, Roh K-H. Calcium enhances polyplex-mediated transfection efficiency of plasmid DNA in Jurkat cells. Drug Delivery. 2020;27(1):805–815. doi: 10.1080/10717544.2020.1770371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suhr OB, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet. J. Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sum HC, Wettig S, Slavcev AR. Impact of DNA vector topology on non-viral gene therapeutic safety and efficacy. Curr. Gene Ther. 2014;14(4):309–329. doi: 10.2174/1566523214666140612154929. [DOI] [PubMed] [Google Scholar]
- 106.Ramishetti S, et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv. Mater. 2020;32(12):1906128. doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 107.Guimaraes PPG, et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim M, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021 doi: 10.1126/sciadv.abf4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Billingsley MM, et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20(3):1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Billingsley MM, et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 2022;22(1):533–542. doi: 10.1021/acs.nanolett.1c02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim K-S, et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials. 2019;221:119418. doi: 10.1016/j.biomaterials.2019.119418. [DOI] [PubMed] [Google Scholar]
- 112.Wang J, et al. Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J. Immunother. Cancer. 2018;6(1):136. doi: 10.1186/s40425-018-0441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klichinsky M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020;38(8):947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020;13(1):153. doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang M, et al. Nanocomplex-Mediated In Vivo Programming to Chimeric Antigen Receptor-M1 Macrophages for Cancer Therapy. Adv. Mater. 2021;33(43):2103258. doi: 10.1002/adma.202103258. [DOI] [PubMed] [Google Scholar]
- 116.Wu C-Y, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350(6258):aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jan M, et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci. Transl. Med. 2021;13(575):eabb6295. doi: 10.1126/scitranslmed.abb6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nguyen NT, et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 2021;16(12):1424–1434. doi: 10.1038/s41565-021-00982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller IC, et al. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat. Biomed. Eng. 2021;5(11):1348–1359. doi: 10.1038/s41551-021-00781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahrar K, et al. Preclinical assessment of a 980-nm diode laser ablation system in a large animal tumor model. JVIR. 2010;21(4):555–561. doi: 10.1016/j.jvir.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bozkulak O, et al. The 980-nm diode laser for brain surgery: histopathology and recovery period. Lasers Med. Sci. 2004;19(1):41–47. doi: 10.1007/s10103-004-0302-1. [DOI] [PubMed] [Google Scholar]
- 123.Nie W, et al. Magnetic nanoclusters armed with responsive PD-1 antibody synergistically improved adoptive T-cell therapy for solid tumors. ACS Nano. 2019;13(2):1469–1478. doi: 10.1021/acsnano.8b07141. [DOI] [PubMed] [Google Scholar]
- 124.Depil S, et al. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 2020;19(3):185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 125.Belshe RB, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 126.Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479–480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 127.Chandra RK. Reduced secretory antibody response to live attenuated measles and poliovirus vaccines in malnourished children. BMJ. 1975;2(5971):583–585. doi: 10.1136/bmj.2.5971.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levine M, et al. Large-scale field trial of TY21A live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987;329(8541):1049–1052. doi: 10.1016/S0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 129.Greco D, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N. Engl. J. Med. 1996;334(6):341–349. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 130.Plotkin S. History of vaccination. Proc. Natl. Acad. Sci. 2014;111(34):12283. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006;1(3):297–315. doi: 10.2217/17435889.1.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giddam AK, et al. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine (Lond.) 2012;7:1877–1893. doi: 10.2217/nnm.12.157. [DOI] [PubMed] [Google Scholar]
- 133.Diogo GR, et al. Immunization with mycobacterium tuberculosis antigens encapsulated in phosphatidylserine liposomes improves protection afforded by BCG. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 135.Straßburger D, et al. Mannose-decorated multicomponent supramolecular polymers trigger effective uptake into antigen-presenting cells. ChemBioChem. 2018;19(9):912–916. doi: 10.1002/cbic.201800114. [DOI] [PubMed] [Google Scholar]
- 136.Song C, Noh Y-W, Lim YT. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int. J. Nanomed. 2016;11:3753. doi: 10.2147/IJN.S122726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao P, et al. A bacteriophage T4 nanoparticle-based dual vaccine against anthrax and plague. MBio. 2018 doi: 10.1128/mBio.01926-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Staroverov SA, et al. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull. Exp. Biol. Med. 2011;151(4):436–439. doi: 10.1007/s10517-011-1350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gregory AE, et al. Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine. 2012;30(48):6777–6782. doi: 10.1016/j.vaccine.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y-T, et al. The use of a gold nanoparticle-based adjuvant to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials. 2011;32(31):7988–7998. doi: 10.1016/j.biomaterials.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Dakterzada F, et al. Induction of humoral immune response against Pseudomonas aeruginosa flagellin(1–161) using gold nanoparticles as an adjuvant. Vaccine. 2016;34(12):1472–1479. doi: 10.1016/j.vaccine.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 142.Barhate G, et al. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J. Pharm. Sci. 2014;103(11):3448–3456. doi: 10.1002/jps.24161. [DOI] [PubMed] [Google Scholar]
- 143.Rodriguez-Del Rio E, et al. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33(12):1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 144.Lind NA, et al. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Beuckelaer A, et al. Type I interferons interfere with the capacity of mRNA lipoplex vaccines to elicit cytolytic T cell responses. Mol. Ther. 2016;24(11):2012–2020. doi: 10.1038/mt.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 147.Niikura K, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7(5):3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 148.Tao W, et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antiviral Res. 2017;141:62–72. doi: 10.1016/j.antiviral.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ringe RP, et al. Neutralizing antibody induction by HIV-1 envelope glycoprotein SOSIP trimers on iron oxide nanoparticles may be impaired by mannose binding lectin. J. Virol. 2020;94(6):e01883–e1919. doi: 10.1128/JVI.01883-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mahony D, et al. In vivo delivery of bovine viral diahorrea virus, E2 protein using hollow mesoporous silica nanoparticles. Nanoscale. 2014;6(12):6617–6626. doi: 10.1039/C4NR01202J. [DOI] [PubMed] [Google Scholar]
- 151.Dhakal S, et al. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dhakal S, et al. Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J. Control. Release. 2017;247:194–205. doi: 10.1016/j.jconrel.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 153.Thomas C, et al. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011;8(2):405–415. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- 154.Jia C, et al. A novel human papillomavirus 16 L1 pentamer-loaded hybrid particles vaccine system: influence of size on immune responses. ACS Appl. Mater. Interfaces. 2018;10(42):35745–35759. doi: 10.1021/acsami.8b11556. [DOI] [PubMed] [Google Scholar]
- 155.Jia J, et al. Adjuvanticity regulation by biodegradable polymeric nano/microparticle size. Mol. Pharm. 2017;14(1):14–22. doi: 10.1021/acs.molpharmaceut.6b00434. [DOI] [PubMed] [Google Scholar]
- 156.Tokatlian T, et al. Enhancing humoral responses against HIV envelope trimers via nanoparticle delivery with stabilized synthetic liposomes. Sci. Rep. 2018;8(1):16527. doi: 10.1038/s41598-018-34853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hanson MC, et al. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33(7):861–868. doi: 10.1016/j.vaccine.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hassett KJ, et al. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur. J. Pharm. Biopharm. 2015;94:220–228. doi: 10.1016/j.ejpb.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Georgiev IS, et al. Two-component ferritin nanoparticles for multimerization of diverse trimeric antigens. ACS Infect. Dis. 2018;4(5):788–796. doi: 10.1021/acsinfecdis.7b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu S, et al. mRNA vaccine era—mechanisms drug platform and clinical prospection. IJMS. 2020 doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype. Viruses. 2021;13(2):270. doi: 10.3390/v13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leppek K, Das R, Barna M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018;19(3):158–174. doi: 10.1038/nrm.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.von OrlandiniNiessen AG, et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol. Ther. 2019;27(4):824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wesselhoeft RA, et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell. 2019;74(3):508–520.e4. doi: 10.1016/j.molcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang L, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dowd KA, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354(6309):237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pardi N, et al. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discovery. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nelson J, et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 2020 doi: 10.1126/sciadv.aaz6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Corbett KS, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schoenmaker L, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Buschmann MD, et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021;9(1):65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vogel AB, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 173.Elia U, et al. Lipid nanoparticle RBD-hFc mRNA vaccine protects hACE2 transgenic mice against a lethal SARS-CoV-2 infection. Nano Lett. 2021;21(11):4774–4779. doi: 10.1021/acs.nanolett.1c01284. [DOI] [PubMed] [Google Scholar]
- 174.Oberli MA, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gharagozloo M, Majewski S, Foldvari M. Therapeutic applications of nanomedicine in autoimmune diseases: from immunosuppression to tolerance induction. Nanomedicine. 2015;11(4):1003–1018. doi: 10.1016/j.nano.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 176.Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020;16(6):316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin. Dev. Immunol. 2004;11:839572. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chu F, et al. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018;318:1–7. doi: 10.1016/j.jneuroim.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 179.Kato R, et al. CD4+CD25+LAG3+ T cells with a feature of Th17 cells associated with systemic lupus erythematosus disease activity. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elli EM, et al. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front. Oncol. 2019 doi: 10.3389/fonc.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 2020;16(3):167–178. doi: 10.1038/s41584-020-0374-8. [DOI] [PubMed] [Google Scholar]
- 182.Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009;4(2):481. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 183.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2004;23(1):683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 185.Hemmer B, et al. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Clin. Pract. Neurol. 2006;2:201–211. doi: 10.1038/ncpneuro0154. [DOI] [PubMed] [Google Scholar]
- 186.Kallaur AP, et al. Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple sclerosis: increased peripheral inflammation but less acute neuroinflammation. Mol. Neurobiol. 2016;53(8):5191–5202. doi: 10.1007/s12035-015-9443-4. [DOI] [PubMed] [Google Scholar]
- 187.Kucharz K, et al. Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles. Nat. Commun. 2021;12(1):4121. doi: 10.1038/s41467-021-24323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gadhave DG, Kokare CR. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: optimization and in vivo studies. Drug Dev. Ind. Pharm. 2019;45(5):839–851. doi: 10.1080/03639045.2019.1576724. [DOI] [PubMed] [Google Scholar]
- 189.Warnke C, Stuve O, Kieseier BC. Teriflunomide for the treatment of multiple sclerosis. Clin Neurol Neurosurg. 2013;115(Suppl 1):S90–S94. doi: 10.1016/j.clineuro.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 190.Kumar P, et al. Oral delivery of methylthioadenosine to the brain employing solid lipid nanoparticles: pharmacokinetic, behavioral, and histopathological evidences. AAPS PharmSciTech. 2019 doi: 10.1208/s12249-019-1296-0. [DOI] [PubMed] [Google Scholar]
- 191.Kumar P, et al. Preclinical explorative assessment of dimethyl fumarate-based biocompatible nanolipoidal carriers for the management of multiple sclerosis. ACS Chem. Neurosci. 2018;9(5):1152–1158. doi: 10.1021/acschemneuro.7b00519. [DOI] [PubMed] [Google Scholar]
- 192.van der Linden MPM, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62(12):3537–3546. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 193.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 194.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lubberts E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015;11(7):415–429. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- 196.Ospelt C, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58(12):3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 197.Seibl R, et al. Expression and regulation of toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 2003;162(4):1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fox DA, et al. Cell-cell interactions in rheumatoid arthritis synovium. Rheum. Dis. Clin. North Am. 2010;36(2):311–323. doi: 10.1016/j.rdc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Meka RR, et al. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun. Rev. 2015;14(12):1131–1141. doi: 10.1016/j.autrev.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Meka RR, Venkatesha SH, Moudgil KD. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J. Control. Release. 2018;286:279–288. doi: 10.1016/j.jconrel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nakashima-Matsushita N, et al. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(8):1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 202.Nogueira E, et al. Enhancing methotrexate tolerance with folate tagged liposomes in arthritic mice. J. Biomed. Nanotechnol. 2015 doi: 10.1166/jbn.2015.2170. [DOI] [PubMed] [Google Scholar]
- 203.Nogueira E, et al. Neutral PEGylated liposomal formulation for efficient folate-mediated delivery of MCL1 siRNA to activated macrophages. Colloids Surf. B. 2017;155:459–465. doi: 10.1016/j.colsurfb.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 204.Larouche J, et al. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv. Wound Care. 2018;7(7):209–231. doi: 10.1089/wound.2017.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sun M, et al. Rebamipide-loaded chitosan nanoparticles accelerate prostatic wound healing by inhibiting M1 macrophage-mediated inflammation via the NF-κB signaling pathway. Biomater. Sci. 2020;8(3):912–925. doi: 10.1039/C9BM01512D. [DOI] [PubMed] [Google Scholar]
- 206.Kaymakcalan OE, et al. Antigen-mediated, macrophage-stimulated, accelerated wound healing using α-gal nanoparticles. Ann Plast Surg. 2018;80(4 Suppl 4):S196–s203. doi: 10.1097/SAP.0000000000001360. [DOI] [PubMed] [Google Scholar]
- 207.Gan J, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219:119340. doi: 10.1016/j.biomaterials.2019.119340. [DOI] [PubMed] [Google Scholar]
- 208.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009;206(3):549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Iberg CA, Jones A, Hawiger D. Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol. 2017;38(11):793–804. doi: 10.1016/j.it.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boks MA, et al. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction—a comparative study of human clinical-applicable DC. Clin. Immunol. 2012;142(3):332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 211.Flórez-Grau G, et al. Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fan Y, Moon JJ. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines. 2015 doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dhodapkar Madhav V, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. . 2014 doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dominguez AL, Lustgarten J. Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine. 2010;28(5):1383–1390. doi: 10.1016/j.vaccine.2009.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cruz LJ, et al. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: a comparative study. J. Control. Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 216.Belogurov AA, Jr, et al. Liposome-encapsulated peptides protect against experimental allergic encephalitis. FASEB J. . 2013;27(1):222–231. doi: 10.1096/fj.12-213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.VonDran MW, et al. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J. Neurosci. 2011;31(40):14182. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hunter Z, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8(3):2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen W, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016;64(4):831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 220.Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95(12):2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 221.Tavasolian F, et al. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr. Gene Ther. 2020;20(4):297–312. doi: 10.2174/1566523220666200916120708. [DOI] [PubMed] [Google Scholar]
- 222.Meng Q, Qiu B. Exosomal MicroRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression. Front. Physiol. 2020 doi: 10.3389/fphys.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zheng J, et al. Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192–5p delays inflammatory response in rheumatoid arthritis. Int. Immunopharmacol. 2020;78:105985. doi: 10.1016/j.intimp.2019.105985. [DOI] [PubMed] [Google Scholar]
- 224.Wu J, et al. Mussel-inspired surface immobilization of heparin on magnetic nanoparticles for enhanced wound repair via sustained release of a growth factor and M2 macrophage polarization. ACS Appl. Mater. Interfaces. 2021;13(2):2230–2244. doi: 10.1021/acsami.0c18388. [DOI] [PubMed] [Google Scholar]
- 225.Kasiewicz LN, Whitehead KA. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater. 2016;32:120–128. doi: 10.1016/j.actbio.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 226.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Raimondo TM, Mooney DJ. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc. Natl. Acad. Sci. 2018;115(42):10648. doi: 10.1073/pnas.1806908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee W, et al. Thermosensitive hydrogel harboring CD146/IGF-1 nanoparticles for skeletal-muscle regeneration. ACS Appl. Bio Mater. 2021;4(9):7070–7080. doi: 10.1021/acsabm.1c00688. [DOI] [PubMed] [Google Scholar]
- 229.Ge J, et al. Gold and gold-silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through p38 MAPK signaling pathway and promote in vivo skeletal muscle regeneration. Biomaterials. 2018;175:19–29. doi: 10.1016/j.biomaterials.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 230.Courties G, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 2014;63(15):1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.