Abstract
Through the analysis of a series of 25 peptides composed of various portions of the histatin 5 sequence, we have identified P-113, a 12-amino-acid fragment of histatin 5, as the smallest fragment that retains anticandidal activity comparable to that of the parent compound. Amidation of the P-113 C terminus increased the anticandidal activity of P-113 approximately twofold. The three histidine residues could be exchanged for three hydrophobic residues, with the fragment retaining anticandidal activity. However, the change of two or more of the five basic (lysine and arginine) residues to uncharged residues resulted in a substantial loss of anticandidal activity. A synthetic d-amino-acid analogue, P-113D, was as active against Candida albicans as the l-amino-acid form. In vitro MIC tests in low-ionic-strength medium showed that P-113 has potent activity against Candida albicans, Candida glabrata, Candida parapsilosis, and Candida tropicalis. These results identify P-113 as a potential antimicrobial agent in the treatment of oral candidiasis.
Fungal infections have become increasingly significant as a consequence of the growing population of immunocompromised patients. For example up to 46% of AIDS patients experience symptoms of oral candidiasis (46). The search for novel antifungal agents continues because of the emergence of fungal pathogens resistant to drugs that have a favorable therapeutic index, such as fluconazole, and because of toxicity issues associated with drugs such as amphotericin B (6, 11, 46). Among the candidates for new classes of antifungal agents are antimicrobial peptides (6, 46). We are investigating the potential of one particular family, the histatin peptides, as anticandidal agents in the treatment of oral candidiasis.
Histatins are a family of small, cationic, histidine-rich peptides secreted into saliva by human parotid and submandibular-sublingual glands (33, 34, 43). Histatin 1 and histatin 3, 38 and 32 amino acids in length, respectively, are encoded by distinct genes (43). The other 10 histatin peptides isolated from saliva are believed to arise from histatins 1 and 3 by proteolytic processing.
Activities ascribed to histatin peptides include a role in formation of the enamel pellicle of teeth (21), inhibition of hemagglutination (31), coaggregation (30), protease activity (32), and neutralization of lipopolysaccharide (41). However, a prominent and perhaps principal function may be antimicrobial. Streptococcus mutans, a bacterium responsible for dental carries, is susceptible to killing by histatin peptides (27). In addition, the three principal histatin peptides, histatins 1, 3, and 5, have been shown to kill C. albicans at low, micromolar concentrations, with histatin 5 having the most potent activity (33, 35, 37, 47, 48, 49). Thus, histatin peptides, and in particular, histatin 5, may perform a function in the oral cavity analogous to that of cationic antimicrobial peptides expressed in other tissues.
There are examples of fragments of peptides or proteins that retain the antimicrobial activity of the parent molecule or whose activities even exceed that of the parent molecule (15, 24). Previous reports suggest that fragments of histatin 5 in fact retain some anticandidal activity (19, 37, 43, 49). Therefore, an analysis of peptide fragments of histatin 5, the most potent member of the naturally occurring histatins, was undertaken. The goal of the study was to assess the most promising of these peptides against a battery of Candida albicans strains and against other Candida pathogens also known to cause oral candidiasis.
In order to test the antimicrobial activities of optimized histatin peptides more efficiently, a broth dilution susceptibility test that measures the MICs of peptides was developed. Using this test, we show that an optimized derivative of histatin 5, called P-113, has potent in vitro activity against the major Candida pathogens that cause oral candidiasis. The spectrum of activity of P-113 includes strains resistant to fluconazole, generally the first-choice drug in antifungal therapy, raising the possibility that P-113 could be a valuable drug that could combat this disease.
MATERIALS AND METHODS
Materials.
P-113, P-113D, histatin 5, other histatin derivatives, and the magainin derivative MSI-78 were synthesized by Multiple Peptide Systems, San Diego, Calif. Magainin 2 was purchased from Bachem Bioscience, King of Prussia, Pa. The unamidated form of P-113 was synthesized by UCB-Bioproducts, Braine-l' Alleud, Belgium. Histatin 5 peptide fragments were prepared by Quality Control Biochemicals, Hopkinton, Mass. The purities (>95%) and authenticities of all peptides were determined by analytical reverse-phase high-pressure liquid chromatography and mass spectroscopy (2).
Strains.
C. albicans ATCC 10231 and C. albicans ATCC 44505 were used as standard susceptible strains. Clinical isolates of C. albicans were obtained from William Powderly (Washington University, St. Louis, Mo.). Clinical isolates of other Candida species were obtained from Mike Rinaldi (University of Texas, San Antonio).
Cell killing assays.
Killing assays were performed by a previously described method (48), with modifications. C. albicans ATCC 44505 was grown on Sabouraud dextrose agar (Fisher Scientific) overnight at 35°C, and several colonies were suspended in 10 mM potassium phosphate (pH 7.4). Fifty microliters of cells (104 CFU/ml) was placed in each well of one-half-area titer plates (no. 3696 96-well plates; Costar), and the contents of each well were mixed with an equal volume of the same buffer containing P-113 or other test compounds. After 1 h of incubation at 37°C, liquid was removed by inversion of the microtiter plates and a drop of Sabouraud dextrose agar was added to allow growth of the cells remaining on the surface of the well. After incubation at 30°C for 4 to 5 h to allow growth of viable cells, the numbers of live and dead cells in each well were counted by direct observation with an inverted microscope.
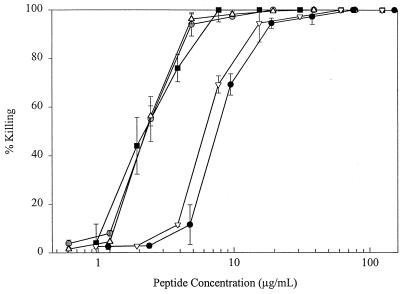
Killing assays were performed with strain C. albicans ATCC 44505. The killing assay determinations in Fig. 1 were averaged from two independent experiments, each run in duplicate, and the difference for each datum point (the variance) is depicted by error bars. The 50 and 90% lethal doses (LD50s and LD90s, respectively) in Tables 2 and 3 were determined by using the function y = y0 + A(log x)+B[(log x)2]+C[(log x)3] (where y is the percent killing for peptide concentration x, y0 is the constant of regression, and A, B, and C are regression variables) using Sigma Plot 5.0 to produce dose-response curves. LD50s and LD90s were averaged from two independent experiments, each run in duplicate. The difference in LD50s and LD90s obtained for each independent trial (the variance) is shown as the range of values.
FIG. 1.
Killing activity of histatin derivatives and MSI 78 peptide against C. albicans ATCC 44505. Cells were incubated with peptide for 1 h at 37°C, incubated for 4 to 5 h at 30°C, and inspected for viability with an inverted microscope as described in Materials and Methods. Values are averages of two experiments done in duplicate, and error bars are the variance of the two experiments. Peptides are MSI 78 (■), P-113 ( ), P-113D (▵), histatin 5 (●), and magainin 2 (▿).
), P-113D (▵), histatin 5 (●), and magainin 2 (▿).
TABLE 2.
Killing activities of histatin derivitives against C. albicans
| Peptide | Sequenceb | LD50c (μg/ml) | LD90c (μg/ml) |
|---|---|---|---|
| Histatin 5 | DSHAKRHHGYKRKFHEKHHSHRGY | 7.3 ± 0.52 | 8.3 ± 0.3 |
| P-123 | DSHAKRHHGYKRKF | >80 | >80 |
| P-103 | KRHHGYKRKFHEKHHSHR | 10.7 ± 5.49 | 24.8 ± 8.1 |
| Histatin 9 | RKFHEKHHSHRGYR | 39 ± 15.8 | >80 |
| P-112 | DSHAKRHHGYKR | >80 | >80 |
| P-113unama | AKRHHGYKRKFH | 3.9 ± 0.63 | 7.8 ± 1.33 |
| P-113 | AKRHHGYKRKFH–NH2 | 2.3 ± 0.65 | 4.7 ± 0.16 |
| P-113D | AKRHHGYKRKFH–NH2 | 2.2 ± 0.3 | 4.8 ± 0.13 |
| P-114 | HHGYKRKFHEKH | >80 | >80 |
| P-115 | YKRKFHEKHHSH | >80 | >80 |
| P-116 | KFHEKHHSHRGY | >80 | >80 |
| P-117 | KRHHGYKRKFH | 13.7 ± 6.6 | >80 |
| P-118 | AKRHHGYKRKF | 10.0 ± 6.1 | >80 |
| P-119 | AKRHHGYKRK | >80 | >80 |
| P-120 | AKRHHGYKR | >80 | >80 |
P-113unam, P-113 in the unamidated form.
The designation–NH2 signifies carboxyl-terminal amidation; all peptides except P-113 and P-113D are not amidated.
LD50s, LD90s, and ranges (variances) were calculated as averages of two independent experiments, each of which was done in duplicate, as described in Materials and Methods.
TABLE 3.
Killing activities of P-113 substitution derivatives against C. albicans
| Peptide no. | Peptide | Sequencea | Molecular momentb | LD50c (μg/ml) | LD90c (μg/ml) |
|---|---|---|---|---|---|
| P-113 | AKRHHGYKRKFH–NH2 | 0.343 | 2.3 ± 0.63 | 4.7 ± 0.16 | |
| 1 | 113-F4.5.12 | AKRFFGYKRKFF–NH2 | 0.527 | 2.2 ± 0.5 | 4.3 ± 0.22 |
| 2 | 113-Y4.5.12 | AKRYYGYKRKFY–NH2 | 0.404 | 2.5 ± 0.4 | 4.3 ± 0.28 |
| 3 | 113-L4.5.12 | AKRLLGYKRKFL–NH2 | 0.508 | 3.7 ± 0.43 | 7.3 ± 0.21 |
| 4 | 113-Q2.10 | AQRHHGYKRQFH–NH2 | 0.270 | >80 | >80 |
| 5 | 113-Q3.9 | AKQHHGYKQKFH–NH2 | 0.224 | 31.7 ± 24 | >80 |
| 6 | 113-Q2.3.9.10 | AQQHHGYKQQFH–NH2 | 0.267 | >80 | >80 |
| 7 | 113-K6 | AKRHHKYKRKFH–NH2 | 0.497 | 5.6 ± 0.23 | 10.0 ± 0.20 |
| 8 | 113-H8 | AKRHHGYHRKFH–NH2 | 0.526 | 3.0 ± 0.31 | 5.0 ± 0.24 |
| 9 | 113-K6H8 | AKRHHKYHRKFH–NH2 | 0.573 | 2.6 ± 0.28 | 5.3 ± 1.0 |
The designation–NH2 signifies amidation of the carboxyl terminus. Residues in boldface type indicate changes from the P-113 sequence.
The molecular moment was calculated by the method of Eisenberg and colleagues (8, 9), as described in Materials and Methods.
LD50s, and LD90s, and ranges (variances) were calculated as averages of two independent experiments, each of which was done in duplicate, as described in Materials and Methods.
Broth dilution assays.
Cells were diluted to a final concentration of 104 CFU/ml in LYM broth containing test and control compounds, as indicated. The volume of the cell suspension was 100 μl per well in a 96-well plate. The composition of LYM broth (final concentration in the assay) is as follows: 5.4 mM KCl, 5.6 mM Na2HPO4, 0.5 mM magnesium sulfate, and 1.0 mM sodium citrate, all to final concentrations. In addition, 0.4 mg of ZnCl2, 2.0 mg of FeCl3 · 6H2O, 0.1 mg of CuSO4 · 5H2O, 0.1 mg of MnSO4 · H2O, and 0.1 mg of Na2B4O7 · 10H2O, all per liter of medium, were added. Also, glucose, an amino acid mixture, and a vitamin mixture, all from Life Technologies (RPMI-1640 Select-Amine Kit), were supplemented as instructed by the vendor (Accumed International, Inc., Westlake, Ohio).
After incubation at 30°C for 17 to 24 h, the MIC of P-113 was determined as the lowest concentration of compound that showed no visible growth (visual determination), which corresponds to less than 0.01 optical density unit (at 600 nm) above the background (Molecular Devices Thermomax plate reader).
The MICs for every clinical isolate were based on at least two independent experimental determinations. The same value was usually observed for the two independent tests. When a difference in test results was observed for a particular isolate, a third test was required, and the value from the majority of tests was reported. MICs for an isolate were generally within a twofold range.
To determine resistance to fluconazole, cells were tested in LYM broth and the MIC was determined as described above. Resistance was defined as an optical density greater than 30% of that for the growth control in the presence of 50 μg of fluconazole/ml.
Circular dichroism.
Circular dichroism spectra of P-113 were recorded at 25°C with a 62DS spectropolarimeter equipped with a rectangular quartz cell with a path length of 0.1 cm. Spectra were recorded between 190 and 260 nm every 0.5 nm, with a time constant of 1 and a 1-nm bandwidth. Data were collected from 5 to 10 separate scans and averaged. P-113 sample concentrations of 95 and 185 μM were prepared in aqueous buffer containing 20 mM NaCl, 20 mM KCl, 1 mM CaCl2, 0.1 mM MgCl2, and 2.5 mM KH2PO4 (adjusted to pH 7.45) and in 100% trifluoroethanol (Sigma).
Molecular moment calculations.
The mean hydrophobic moment (〈μH〉) values for the histatin peptide fragments at different angles (δ) were calculated by the method of Eisenberg and colleagues (8, 9) by the equation
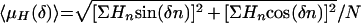 |
where N is the number of residues and n is the specific residue within the peptide sequence; Hn is the hydrophobic value, according to the normalized consensus hydropathy scale (8) assigned to residue n; and δ is the angle (in radians) between successive residues (e.g., δ is equal to 100° for an α helix).
RESULTS
Comparison of P-113 histatin derivative and other antimicrobial peptides.
The optimal histatin fragment for development as a therapeutic agent, when economic considerations are taken into account, is the smallest peptide that retains full anticandidal activity. P-113, a peptide composed of 12 amino acids from histatin 5 and amidated on its C terminus, has shown promising activity in preventing gingivitis in humans (29). In order to determine if P-113 also retained the antifungal properties of the parent compound, histatin 5, the peptides were compared by the C. albicans cell killing assay. P-113, which had an LD50 of 2.3 μg/ml and an LD90 of 4.7 μg/ml, was at least as active as histatin 5 on a molar basis and more active than histatin 5 on a weight basis (Fig. 1). It is interesting that the activity of the mirror-image peptide of P-113, called P-113D, which contains all the amino acid residues in the d conformation, was the same as that of P-113 (Fig. 1). The activity of P-113 compared favorably with that of the natural antimicrobial peptide, magainin 2, and MSI 78, a magainin derivative that was optimized for antimicrobial activity (10). MSI 78 was twice as active as P-113 on a molar basis and had activity comparable to that of P-113 on a weight basis, whereas magainin 2 was less active than P-113 (Fig. 1).
Development of broth dilution assay for MIC determination.
In contrast to the potent activity observed in killing assays, histatins and their derivatives showed little activity in broth dilution tests with a standard medium such as RPMI 1640 medium (data not shown). In order to determine the in vitro efficacy of P-113 against a panel of clinical isolates, a broth dilution susceptibility test was developed. We assumed that there might be components in RPMI 1640 medium that interfered with the antimicrobial activity of P-113 on the basis of the fact that the peptides were effective in inhibiting growth if the RPMI 1640 medium was diluted 10-fold. Our experiments revealed that the antifungal killing activities of histatin 5 and P-113 in buffer were antagonized by NaCl and by the presence of millimolar concentrations of calcium or magnesium divalent cations (data not shown). On the basis of these results, we removed calcium nitrate and sodium chloride, which are not essential for growth, from RPMI 1640 medium. In addition, sodium citrate was added to chelate magnesium, allowing the availability of this essential element throughout the incubation, but at reduced free concentrations. Finally, the addition of trace elements contributed to improved fungal growth and to slightly better potencies of the histatin peptides in our modified medium, LYM broth (defined in Materials and Methods). In the absence of P-113, growth was comparable in LYM broth and RPMI 1640 medium (data not shown).
C. albicans cells of either strain ATCC 10231 or strain ATCC 44505 were inhibited by 3.1 μg of P-113 per ml in LYM broth (Table 1); there was no visible growth after overnight incubation at 30°C in the presence of peptide. To confirm the effects of the growth medium components, the antagonistic effects of salt were tested. If calcium nitrate was added to the LYM broth, little or no antifungal activity was detected. If sodium citrate was removed from the LYM broth, presumably increasing the free concentration of magnesium ions, then the MIC rose to 12.5 μg/ml, a fourfold decrease in activity. The addition of sodium chloride to the LYM broth resulted in an increase in the MIC to 25 μg/ml. Thus, the exclusion of monovalent and divalent salts from the growth medium proved to be important in detecting the maximal growth-inhibitory activity of P-113. Importantly, the susceptibilities of C. albicans strains to either amphotericin B or fluconazole was not altered in LYM broth compared to those in RPMI 1640 medium (data not shown).
TABLE 1.
Antifungal activity of P-113
| Organism | Origina | Identification code | P-113 MIC (μg/ml) |
|---|---|---|---|
| Candida albicans | ATCC | 10231 | 3.1 |
| Candida albicans | ATCC | 44505 | 3.1 |
| Candida albicans | HIV pt. isolate | 443 | 3.1 |
| Candida albicans | HIV pt. isolate | 84 | 3.1 |
| Candida albicans | HIV pt. isolate | 111 | 3.1 |
| Candida albicans | HIV pt. isolate | 206 | 3.1 |
| Candida albicansb | HIV pt. isolate | 207 | 3.1 |
| Candida albicansb | HIV pt. isolate | 78 | 1.6 |
| Candida albicansb | HIV pt. isolate | 20 | 3.1 |
| Candida albicans | HIV pt. isolate | 118 | 3.1 |
| Candida albicans | HIV pt. isolate | 269 | 3.1 |
| Candida albicans | HIV pt. isolate | 162 | 3.1 |
| Candida albicans | HIV pt. isolate | 359 | 3.1 |
| Candida albicansb | HIV pt. isolate | 575 | 3.1 |
| Candida albicans | HIV pt. isolate | 52 | 3.1 |
| Candida albicans | HIV pt. isolate | 125 | 3.1 |
| Candida albicans | HIV pt. isolate | 209 | 3.1 |
| Candida albicans | HIV pt. isolate | 123 | 3.1 |
| Candida albicans | HIV pt. isolate | 508 | 3.1 |
| Candida albicans | HIV pt. isolate | 283 | 3.1 |
| Candida albicansb | HIV pt. isolate | 5 | 3.1 |
| Candida glabrata | ATCC | 98-2229 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2237 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2267 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2272 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2255 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2279 | 0.8 |
| Candida glabrata | Clinical isolate | 98-2282 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2314 | 3.1 |
| Candida glabrata | Clinical isolate | 98-2320 | 1.6 |
| Candida glabrata | Clinical isolate | 98-2332 | 3.1 |
| Candida krusei | ATCC | 14243 | 1.6 |
| Candida kefyr | ATCC | 66028 | 6.3 |
| Candida parapsilosis | Clinical isolate | 98-2318 | 3.1 |
| Candida parapsilosis | Clinical isolate | 98-2305 | 3.1 |
| Candida parapsilosis | Clinical isolate | 98-2297 | 3.1 |
| Candida tropicalis | Clinical isolate | 98-2327 | 1.6 |
| Candida tropicalis | Clinical isolate | 98-2323 | 3.1 |
| Candida tropicalis | Clinical isolate | 98-2091 | 1.6 |
ATCC, American Type Culture Collection (Manassas, Va.); HIV pt. isolate, isolate from a human immunodeficiency virus-infected patient.
C. albicans strains resistant to fluconazole (MICs, >50 μg/ml).
Susceptibility testing of clinical isolates.
The modified broth dilution method was used to determine the susceptibilities of a number of clinical isolates of C. albicans to P-113. Following an overnight incubation, the various clinical isolates were all found to be sensitive to 3.1 μg of P-113 per ml (Table 1). It is noteworthy that several of the C. albicans strains were resistant to fluconazole but not to the antimicrobial activity of P-113. Other pathogens that cause oral candidiasis were also susceptible to growth inhibition in the presence of low concentrations of P-113 (Table 1). Some of these species, such as Candida glabrata, are intrinsically resistant to fluconazole.
Identification of P-113 as the core histatin fragment retaining full antimicrobial activity.
An evaluation of histatin 5 fragments began with the testing of the synthetic peptides P-123, P-103, and histatin 9, which represent the N-terminal, middle, and C-terminal segments of histatin 5, respectively. Significant killing activity against C. albicans was retained only by P-103 (Table 2). Next, two series of nested fragments of 8 and 12 residues in length, respectively, were tested, focusing on this middle segment of histatin 5. The anticandidal activities of the eight-amino-acid peptides was either greatly reduced or absent, an indication that larger peptides are required for activity. However, one peptide containing 12 amino acids of the P-113 sequence demonstrated anticandidal activity equivalent to that of histatin 5. Thus, the core anticandidal activity appeared to reside within these 12 residues. Variants missing either a single residue from the N terminus or up to three residues from the C terminus of the P-113 sequence were synthesized and tested (Table 2, P-117 through P-120). In all cases removal of residues from the P-113 sequence decreased the anticandidal activity. Overall, the data summarized in Table 2 identify the 12-amino-acid sequence found in P-113 as the smallest derivative of histatin 5 that retains full anticandidal activity.
Because some antimicrobial peptides are amidated on their C termini, it was of interest to determine the effect of amidation on activity. The peptide containing a C-terminal amide group, P-113, was almost twofold more potent than the unamidated peptide of the same amino acid sequence (Table 2).
Structural and compositional studies of P-113.
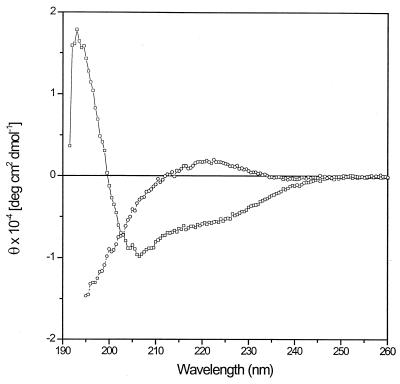
The fact that P-113 is reduced in size compared to histatin 5 might alter its structural properties. Histatins form an α helix in trifluoroethanol solution, suggesting that they are helical in hydrophobic environments (37, 38). This capacity to take on an amphipathic α-helical structure (containing hydrophobic residues on one face of the helix and hydrophilic residues on the opposite face) is thought to be important for the antimicrobial activity of histatins. To determine if P-113 retained the ability to form an amphipathic α helix in hydrophilic and hydrophobic environments, circular dichroism spectra of histatin fragment P-113 were obtained in aqueous buffer and in 100% trifluoroethanol (Fig. 2). In aqueous buffer, P-113 exhibited a positive band at 220 nm and a strong minimum below 200 nm, parameters characteristic of polypeptide random coils. In contrast, in trifluoroethanol P-113 produced negative bands at 206 to 208 nm and at about 220 nm and a strong positive band at 192 nm, spectral features characteristic of an α-helical conformation. The α-helical content estimated from the absolute molar ellipticity values obtained at 222 nm was 19% (5) or 27% (4), depending on the method of calculation. The mean residue ellipticity was independent of the P-113 concentration (data not shown), indicating that the peptide was in a monomeric form.
FIG. 2.
Circular dichroism spectra of peptide P-113. Circular dichroism spectra were measured in aqueous buffer (○) or 100% trifluoroethanol (□), as described in Materials and Methods. The P-113 concentration was 185 μM. θ, molar ellipticity per residue.
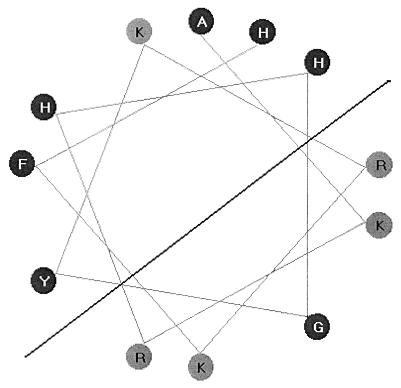
To test the importance of structural features of P-113, several modified peptides were synthesized and tested for their anticandidal activities. The retention of some propensity to form an amphipathic α helix, illustrated in the helical-wheel projection (Fig. 3), suggests that the histidine side chains would be hydrophobic and uncharged. In the first group of modifications, to test the importance of hydrophobic residues at positions 4, 5, and 12 of P-113, the histidine residues were replaced by either phenylalanine, tyrosine, or leucine residues, all of which are unequivocally hydrophobic in nature. Each of the three modified peptides retained potent antimicrobial activity against C. albicans (Table 3, peptides 1 to 3). These results are consistent with the idea that the histidine side chains are part of the hydrophobic face of an amphipathic helix. It is interesting that histidine residues proved not to be essential for in vitro antimicrobial activity, despite their preponderance in P-113 and histatins.
FIG. 3.
Helical-wheel projection of P-113. A view from the top to the bottom of the helical axis shows residues at locations which are separated by 100° (360° and 3.6 residues per rotation). Basic amino acid residues are denoted by black letters.
The second group of modifications was designed to test for the importance of cationic charges. The substitution of glutamine residues for either lysines or arginines resulted in reduced activity for peptides with substitutions at positions 3 and 9 or a loss of activity for those with substitutions at positions 2 and 10 against both C. albicans strains tested (Table 3, peptides 4 to 6). The replacement of all four cationic residues by glutamines resulted in a complete loss of antimicrobial activity (Table 3).
The third group of substitutions was designed to increase the amphipathicity of the peptide, as a guide to optimizing antimicrobial activity. The helical-wheel projection (Fig. 3) shows that the glycine residue at position 6 interrupts a group of positive charges on one face of the helix, and similarly, a lysine residue at position 8 is located within the nonpolar face of the putative helix. These residues were changed singly by substituting a lysine residue for a glycine at position 6 (Table 3, peptide 7) or by substituting a histidine for a lysine at position 8 (Table 3, peptide 8). Finally, peptide 9 (Table 3) contains both a lysine at position 6 and a histidine at position 8 to maximize the amphipathicities of the two faces of the helix. Indeed, the molecular moments are significantly increased for peptides 7 and 8 and especially for peptide 9 (Table 3), indicating that the amphipathicities of these modified peptides are significantly increased, assuming that the dodecamers maintain the α-helical structure. However, the killing activities of these peptides were not improved compared to that of P-113. None of 25 peptides with substitutions that were tested in the killing assay exhibited improved antimicrobial activity against C. albicans compared to that of P-113 (Table 3 and data not shown). As a result of these analyses, P-113 was selected for further development.
DISCUSSION
The emergence of resistant fungal pathogens has been a motivating force in the search for new antifungal agents. Antimicrobial peptides, including histatins, have been considered prime candidates because they probably have a mode of action distinct from those of other antifungal agents (6, 43, 46). Although the mode of action of histatins in particular has not been fully elucidated, the suggestions that they might be surface-active agents (35), induce the release of ATP (22), or act on the mitochondria (13, 17) would imply that histatins have a target different from those of the approved antifungal drugs used clinically. Several investigators have used histatins as a starting point to make peptides with increased antimicrobial activity (7, 18, 20, 39, 44, 50), whereas our aim was to optimize activity by using the natural sequence.
In order to evaluate the activity of P-113 on a large scale, a liquid-dilution MIC test was developed in which the growth medium was LYM broth, which excluded excess divalent cations and monovalent salt and which included a chelating agent and trace metals. The minimal fungicidal concentration, that is, the concentration that resulted in at least 3 log units of killing after 20 h of incubation, was twofold higher than the MIC for C. albicans strain ATCC 10231 (data not shown), indicating a strong killing effect of P-113 in LYM broth. The MIC and minimal fungicidal concentration tests show unambiguously that P-113 kills not only cells resuspended in buffer but also cells capable of growing as well. To reinforce this point, C. albicans cells grown in LYM broth in the exponential phase were then exposed to P-113. Although they were fully capable of growth, such cells were killed during the 1-h incubation at 37°C to the same extent as cells resuspended in buffer (data not shown). Thus, P-113 is a potent cidal agent against C. albicans.
The disadvantage of using LYM broth is that the special conditions required for activity (low salt and reduced divalent cation concentrations and the presence of citrate as a chelator) may not reflect conditions encountered in vivo. Whereas a standard medium such as RPMI 1640 medium may be more predictive of in vivo activity, it is possible that properties detected in media such as LYM broth uncover potentially valuable antimicrobial activity. For example, defensins are cationic peptides produced by host cells and are thought to have significant in vivo antimicrobial activity against both bacteria and fungi. They are considered an integral part of the innate host defense system and are essential for nonoxidative killing of microbes (12, 25). Whereas defensins have not been shown to have significant activity in standard MIC tests (42), they do show potent antimicrobial killing activity against C. albicans or bacteria resuspended in buffer (12, 25) and against bacteria in airway fluid (12, 16). It is also interesting that P-113D, the protease-resistant, mirror-image isomer of P-113, was effective in killing bacteria in sputum (D. M. Rothstein et al., Abstr. 14th Annual Norh American Cystic Fibrosis Conference, Pediatr. Pulmonol. Suppl. 20, p. 250, 2000), which contains divalent cations at concentrations in excess of 4 mM (26). However, divalent cations in a standard medium such as cation-adjusted Mueller-Hinton broth antagonize the antimicrobial activity of P-113D in vitro. In considering these examples, it is possible that standardized conditions are not always the “gold standard” in terms of predicting activity, even in the presence of divalent cations, in the more complex in vivo environment. It is also possible that peptides such as defensins or P-113 act in concert with other host defense factors in vivo, augmenting their effect compared to the effects determined from the results of standard susceptibility tests. The results of the in vitro experiments suggest that efficacy may depend on the formulation of P-113 in a buffer with a low salt concentration, perhaps with a chelating agent, and on restrictions of dietary intake of monovalent and divalent salts at the time of application.
Histatins may play a biological role in helping the innate immune system to maintain a balance of microorganisms in the healthy individual. Adding back histatin derivatives may restore this balance in patients suffering from oral candidiasis. It is interesting that several reports suggest that histatin levels are low in AIDS patients (23, 28, 36), although one report suggests that histatin levels are actually increased in response to AIDS (1). It is possible that a histatin derivative applied locally to the oral cavity could compensate for deficiencies in the host immune system.
P-113 was the optimal histatin derivative in our study and on a molar basis was as active as histatin 5, the most potent of the naturally occurring histatins (48, 49). Because the potency and spectrum of activity of P-113 were similar to those of histatin 5 (Fig. 1), the mode of action of P-113 may be similar or identical to that of its parent molecule. It is interesting that P-113 retains its strong antimicrobial activities, against both fungi and bacteria, despite its reduced size. The ability to form an α helix in a hydrophobic environment, such as in an encounter with the cell membranes of microorganisms, might be crucial to the activity of amphipathic α-helical peptides such as histatins (37, 38). We have shown that P-113 has at least some propensity to form a helix (Fig. 2). The measurements of the helical content, estimated to be 19 to 27%, suggest that the structure of P-113 would have a considerably less α-helical character than that of histatin 5 (37, 38). This reduction in helical content of P-113 could be due to the fact that the potential number of hydrogen bonds along the backbone for a dodecamer is reduced to 9. However, it is also possible that the measurements used to deduce the helical content may be biased against detecting α-helical structure when the peptide size is reduced (37). It is clear, in any case, that P-113 is too small to traverse a bacterial or a fungal membrane in an α-helical structure, which requires at least 20 residues (45), precluding some models of antimicrobial activity in which a pore is formed following the spanning of helical peptides across the membrane (14). A recent study questions the importance of helix formation of histatin 5 on the basis of the retention of activity in peptides containing proline substitutions, which would prevent helix formation. However, the fact that these proline substitutions were outside residues 4 to 15, which encompass the P-113 sequence, leaves open the intriguing possibility that a helical structure could form within the P-113 segment of histatin 5, resulting in the retention of activity in the substituted peptide (40).
Studies with modified peptides revealed several interesting features of P-113. As expected, peptides in which the cationic residues were removed were inactive or had reduced activity compared to that of P-113 (Table 3, peptides 4 to 6). In contrast, the histidine residues were all dispensable when they were replaced by hydrophobic residues (Table 3, peptides 1 to 3). It is possible, however, that the histidine residues of P-113 play a role in vivo if, for example, aggregation of microorganisms, tissue binding and the stability of the peptide, or other factors are important. When modifications were made to enhance the amphipathic nature of the peptide so that hydrophilic residues would reside exclusively on one face of the putative helix and hydrophobic residues would reside on the opposite face (Fig. 3), the modified peptides remained as active as P-113 (Table 3). It was somewhat surprising that there was no improvement of killing activity as a function of the molecular moment, suggesting that the amphipathicity of the putative P-113 α helix is not the overriding factor that defines the antimicrobial activity of P-113. Because P-113 was as active as the derivative molecules and is the natural sequence, P-113 was chosen for more extensive testing.
P-113 is more active than histatin 5 on a weight basis, and its compact size reduces the cost of chemical synthesis. The activity of P-113 (by weight) is comparable to those of other antimicrobial peptides such as the magainin derivative MSI 78 (10) in both killing assays (Fig. 1) and MIC tests (data not shown). P-113 has a very favorable spectrum of activity against the pathogens responsible for oral candidiasis, including strains very refractory to treatment with fluconazole (Table 1). Despite its potent and broad spectrum of activity, P-113 appears to have a particularly strong safety profile. No adverse effects related to P-113 have been reported in clinical trials with over 400 patients, in which the efficacy of P-113 was tested in a mouth-rinse formulation for the prevention of gingivitis (29). The data reported from the present study suggest that P-113 may be an excellent candidate in the prevention and treatment of Candida-based fungal infections. Future investigations should reveal the antimicrobial activity of P-113 in the environment of the oral cavity.
ACKNOWLEDGMENTS
We thank William Powderly (Washington University, St. Louis, Mo.) for providing clinical isolates of C. albicans and Mike Rinaldi (University of Texas, San Antonio) for providing the other Candida isolates. We also thank Richard Darveau (University of Washington, Seattle), Eva Helmerhorst (Boston University School of Dental Medicine), Marcia Osburne (Aventis Genomics Center, Cambridge, Mass.), and Hagan Bayley (Texas A&M University) for helpful discussions.
This study was supported in part by NIH grant DEO 7652 (to F.G.O.).
REFERENCES
- 1.Bercier J G, Al-Hashimi I, Haghighat N, Rees T D, Oppenheim F G. Salivary histatins in patients with recurrent oral candidiasis. J Oral Pathol Med. 1999;28:26–29. doi: 10.1111/j.1600-0714.1999.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 2.Bieman K. Mass spectrometry of peptides and proteins. Annu Rev Biochem. 1992;61:977–1010. doi: 10.1146/annurev.bi.61.070192.004553. [DOI] [PubMed] [Google Scholar]
- 3.Blondelle S E, Houghten R A. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry. 1992;31:12688–12694. doi: 10.1021/bi00165a020. [DOI] [PubMed] [Google Scholar]
- 4.Bolotina I A, Chekhov V O, Lugauskas V I, Finkel'shtein A V, Ptitsyn O B. Determination of the secondary structure of proteins from their circular dichroism spectra. I. Protein reference spectra for alpha-, beta- and irregular structures. Mol Biol (Moscow) 1980;14:891–902. [PubMed] [Google Scholar]
- 5.Chen Y H, Yang J T, Chau K H. Determinations of the helix and beta forms of proteins in aqueous solutions by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 6.De Lucca A J, Walsh T J. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother. 1999;43:1–11. doi: 10.1128/aac.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driscoll J, Duan C, Zuo Y, Xu T, Troxler R, Oppenheim F G. Candidacidal activity of human salivary histatin recombinant variants produced by site-directed mutagenesis. Gene. 1996;177:29–34. doi: 10.1016/0378-1119(96)00265-x. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg D, Weiss R M, Terwilliger T C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA. 1984;81:140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs P C, Barry A L, Brown S D. In vitro antimicrobial activity of MSI-78, a magainin analog. Antimicrob Agents Chemother. 1998;42:1213–1216. doi: 10.1128/aac.42.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgopapadakou N H. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998;1:547–557. doi: 10.1016/s1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Gyurko C, Lendenmann U, Troxler R F, Oppenheim F G. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother. 2000;44:348–354. doi: 10.1128/aac.44.2.348-354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock R E. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 15.Hancock R E, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 16.Hancock R E, Scott M G. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmerhorst E J, Breeuwer P, van't Hof W, Walgreen-Weterings E, Oomen L C, Veerman E C. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst E J, Hodgson R, van't Hof W, Veerman E C, Allison C. The effects of histatin-derived basic antimicrobial peptides on oral biofilms. J Dent Res. 1999;78:1245–1250. doi: 10.1177/00220345990780060801. [DOI] [PubMed] [Google Scholar]
- 19.Helmerhorst E J, Reijnders I M, van't Hof W, Simoons-Smit I, Veerman E C. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother. 1999;43:702–704. doi: 10.1128/aac.43.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmerhorst E J, Van't Hof W, Veerman E C, Simoons-Smit I. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J. 1997;326:39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen J L, Lamkin M S, Oppenheim F G. Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J Dent Res. 1992;71:1569–1576. doi: 10.1177/00220345920710090501. [DOI] [PubMed] [Google Scholar]
- 22.Koshlukova S E, Lloyd T L, Araujo M W, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 23.Lal K, Pollock J J, Santarpia III R P, Heller H M, Kaufman H W, Fuhrer J, Steigbigel R T. Pilot study comparing the salivary cationic protein concentrations in healthy adults and AIDS patients: correlation with antifungal activity. J Acquir Immune Defic Syndr. 1992;5:904–914. [PubMed] [Google Scholar]
- 24.Lee K H, Hong S Y, Oh J E, Kwon M, Yoon J H, Lee J, Lee B L, Moon H M. Identification and characterization of the antimicrobial peptide corresponding to C-terminal beta-sheet domain of tenecin 1, an antibacterial protein of larvae of Tenebrio molitor. Biochem J. 1998;334:99–105. doi: 10.1042/bj3340099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 26.Levy J, Smith A L, Kenny M A, Ramsey B, Schoenknecht F D. Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: comparison with activity in serum. J Infect Dis. 1983;148:1069–1076. doi: 10.1093/infdis/148.6.1069. [DOI] [PubMed] [Google Scholar]
- 27.MacKay B J, Denepitiya L, Iacono V J, Krost S B, Pollock J J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984;44:695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandel I D, Barr C E, Turgeon L. Longitudinal study of parotid saliva in HIV-1 infection. J Oral Pathol Med. 1992;21:209–213. doi: 10.1111/j.1600-0714.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 29.Mickels, N., C. McManus, J. Massaro, P. Friden, V. Braman, R. D'Agostino, F. Oppenheim, M. Warbington, S. Dibart, and T. Van Dyke. Clinical and microbial evaluation of a histatin containing mouthrinse in humans with experimental gingivitis. J. Clin. Periodontol., in press. [DOI] [PubMed]
- 30.Murakami Y, Nagata H, Amano A, Takagaki M, Shizukuishi S, Tsunemitsu A, Aimoto S. Inhibitory effects of human salivary histatins and lysozyme on coaggregation between Porphyromonas gingivalis and Streptococcus mitis. Infect Immun. 1991;59:3284–3286. doi: 10.1128/iai.59.9.3284-3286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami Y, Takeshita T, Shizukuishi S, Tsunemitsu A, Aimoto S. Inhibitory effects of synthetic histidine-rich peptides on haemagglutination by Bacteroides gingivalis 381. Arch Oral Biol. 1990;35:775–777. doi: 10.1016/0003-9969(90)90103-h. [DOI] [PubMed] [Google Scholar]
- 32.Nishikata M, Kanehira T, Oh H, Tani H, Tazaki M, Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem Biophys Res Commun. 1991;174:625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- 33.Oppenheim F G, Xu T, McMillian F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 34.Oppenheim F G, Yang Y C, Diamond R D, Hyslop D, Offner G D, Troxler R F. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem. 1986;261:1177–1182. [PubMed] [Google Scholar]
- 35.Pollock J J, Denepitiya L, MacKay B J, Iacono V J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984;44:702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock J J, Santarpia III R P, Heller H M, Xu L, Lal K, Fuhrer J, Kaufman H W, Steigbigel R T. Determination of salivary anticandidal activities in healthy adults and patients with AIDS: a pilot study. J Acquir Immune Defic Syndr. 1992;5:610–618. [PubMed] [Google Scholar]
- 37.Raj P A, Edgerton M, Levine M J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 38.Raj P A, Soni S D, Levine M J. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J Biol Chem. 1994;269:9610–9619. [PubMed] [Google Scholar]
- 39.Ramalingam K, Gururaja T L, Ramasubbu N, Levine M J. Stabilization of helix by side-chain interactions in histatin-derived peptides: role in candidacidal activity. Biochem Biophys Res Commun. 1996;225:47–53. doi: 10.1006/bbrc.1996.1129. [DOI] [PubMed] [Google Scholar]
- 40.Situa H, Balasubramanianb S V, Bobek L A. Role of alpha-helical conformation of histatin-5 in candidacidal activity examined by proline variants. Biochim Biophys Acta. 2000;1475:377–382. doi: 10.1016/s0304-4165(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama K. Anti-lipopolysaccharide activity of histatins, peptides from human saliva. Experientia. 1993;49:1095–1097. doi: 10.1007/BF01929920. [DOI] [PubMed] [Google Scholar]
- 42.Takemura H, Kaku M, Kohno S, Hirakata Y, Tanaka H, Yoshida R, Tomono K, Koga H, Wada A, Hirayama T, Kamihira S. Evaluation of susceptibility of gram-positive and -negative bacteria to human defensins by using radial diffusion assay. Antimicrob Agents Chemother. 1996;40:2280–2284. doi: 10.1128/aac.40.10.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai H, Bobek L A. Human salivary histatins: promising anti-fungal therapeutic agents. Crit Rev Oral Biol Med. 1998;9:480–497. doi: 10.1177/10454411980090040601. [DOI] [PubMed] [Google Scholar]
- 44.Tsai H, Raj P A, Bobek L A. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect Immun. 1996;64:5000–5007. doi: 10.1128/iai.64.12.5000-5007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Heijne G, Manoil C. Membrane proteins: from sequence to structure. Protein Eng. 1990;4:109–112. doi: 10.1093/protein/4.2.109. [DOI] [PubMed] [Google Scholar]
- 46.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, Lal K, Santarpia III R P, Pollock J J. Salivary proteolysis of histidine-rich polypeptides and the antifungal activity of peptide degradation products. Arch Oral Biol. 1993;38:277–283. doi: 10.1016/0003-9969(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 48.Xu T, Levitz S M, Diamond R D, Oppenheim F G. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu T, Oppenheim F G. Salivary antimicrobials: where are we? Ann N Y Acad Sci. 1993;694:117–131. [Google Scholar]
- 50.Zuo Y, Xu T, Troxler R F, Li J, Driscoll J, Oppenheim F G. Recombinant histatins: functional domain duplication enhances candidacidal activity. Gene. 1995;161:87–91. doi: 10.1016/0378-1119(95)00237-z. [DOI] [PubMed] [Google Scholar]