Abstract
The rapid development of multiple vaccines providing strong protection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been a major achievement. There is now compelling evidence for the role of neutralizing antibodies in protective immunity. T cells may play a role in resolution of primary SARS-CoV-2 infection, and there is a widely expressed view that T cell-mediated immunity also plays an important role in vaccine-mediated protection. Here we discuss the role of vaccine-induced T cells in two distinct stages of infection: firstly, in protection from acquisition of symptomatic SARS-CoV-2 infection following exposure; secondly, if infection does occur, the potential for T cells to reduce the risk of developing severe COVID-19. We describe several lines of evidence that argue against a direct impact of vaccine-induced memory T cells in preventing symptomatic SARS-CoV-2 infection. However, the contribution of T cell immunity in reducing the severity of infection, particularly in infection with SARS-CoV-2 variants, remains to be determined. A detailed understanding of the role of T cells in COVID-19 is critical for next-generation vaccine design and development. Here we discuss the challenges in determining a causal relationship between vaccine-induced T cell immunity and protection from COVID-19 and propose an approach to gather the necessary evidence to clarify any role for vaccine-induced T cell memory in protection from severe COVID-19.
Subject terms: Clinical trials, Viral infection
Understanding of the role of T cells in SARS-CoV-2 infection is of great importance for the design of next-generation vaccines. In this Perspective, Davenport and colleagues discuss the challenges in determining a causal relationship between vaccine-induced T cell immunity and protection from COVID-19.
Introduction
The orchestrated roles of innate and adaptive immunity are crucial for preventing and controlling infectious threats. Immune memory established following recovery from infection, or via vaccination, provides at least partial immune protection from subsequent reinfection or disease. Immunological memory has multiple components, with putative roles for circulating antibodies, memory B cells, and memory CD4+ T cells and CD8+ T cells. Understanding the relative contribution of these different immune mechanisms in protection from SARS-CoV-2 infection and identifying immune correlates of protection are critical for long-term control of COVID-19. A simplified mechanistic correlate identifying the responses that mediate protection facilitates rational design of novel vaccines and allows approval of new vaccines through ‘immunobridging’ (acceptance on the basis of immune responses rather than efficacy trials). In addition, immune correlates support predictions of the durability of immunity in the face of waning immune responses and against emerging new variants of concern. Studies of vaccines for other acute viral infections, such as influenza, measles and polio, show that neutralizing antibodies (nAbs) are commonly an important correlate of protection1 (Box 1). By contrast, there is a clear and dominant role for T cell immunity in maintaining immune control of chronic infections such as tuberculosis and many herpes virus infections. In reality, however, protective immunity to most pathogens does not refer to a binary outcome (susceptible versus protected) but instead reflects a spectrum, from complete protection (prevention of the acquisition of viral infection) through to amelioration of symptomatic disease severity. Importantly, elements of immune memory responses are likely to be multifaceted, particularly in protection against severe COVID-19, and the mechanisms of protection from establishment of infection and from severe infection may only partially overlap. Although it may be overly simplistic to attempt to assess an independent role for antibodies and T cells, understanding which immune mechanisms contribute to protection at different stages of infection could assist in rationally focusing improvements for future vaccines and immunotherapeutics for SARS-CoV-2.
In this Perspective, we consider how immune memory generated by vaccination contributes to protection from subsequent COVID-19. In clinical trials this protection is typically measured as vaccine efficacy against ‘symptomatic infection’ or ‘severe infection’ (including hospitalization and ventilation), although protection against asymptomatic infection has also been measured in some studies where participants undergo regular PCR screening for infection2. Here we adopt a functional definition of ‘protection from infection’, meaning the ability of an immune response to protect against the establishment of detectable infection (with use of whatever screening method might be used in a particular study). This might in some cases mean complete protection against viral entry and replication, but in other cases it may include the possibility of low-level asymptomatic viral replication. Similarly, we adopt a functional definition of ‘severe SARS-CoV-2 infection’, meaning typically infection leading to hospitalization3.
Importantly, we aim to distinguish between immune responses and immune protection. That is, although different aspects of immune responses to SARS-CoV-2 vaccination can be measured, only a subset of these may meaningfully contribute to immune protection from infection or progression to severe disease. Although we briefly discuss the role of immune responses in the control of primary SARS-CoV-2 infection in naive individuals, our main focus is on understanding how immune responses after vaccination predict or control the outcome of a subsequent infectious challenge. Understanding this relationship between different immune parameters and clinical outcome is key to predicting vaccine efficacy, the rational design of next-generation vaccines and forecasting the impact of waning immunity and SARS-CoV-2 variants on long-term population-level protection. Importantly, immune memory against SARS-CoV-2 may act at different stages to affect infection outcomes. For example, nAb responses or mucosal responses may act to block viral entry and establishment of infection, while recall of humoral or cellular immunity may act later to reduce the severity of infection4. It is clear that vaccination reduces the risk of SARS-CoV-2 infection. However, this protection is not perfect, and ‘breakthrough infection’ still occurs in a proportion of vaccinated individuals, particularly with novel viral variants5,6. Importantly, even when breakthrough infection occurs, vaccine-induced immunity may still provide protection from progression to severe infection7,8. Thus, after discussing the role of immune responses in the resolution of primary infection, we focus on the roles of vaccine-induced adaptive immune responses in both protection from SARS-CoV-2 infection and protection from severe disease, with a focus on the role of SARS-CoV-2-specific T cells.
Box 1 Diverse infections require divergent mechanisms of immune control.
A major question in understanding immunity to SARS-CoV-2 infection is how to differentiate between immune responses and the dominant mechanism of immune protection. That is, both infection and vaccination tend to elicit a combination of T cell and antibody responses. However, there are many infections where only a subset of these responses are thought to be the most critical mediators of protection (in tuberculosis, for example, where T cells are thought to play the major role). As the levels of antibody and cellular responses are often correlated, it can be difficult to dissect their independent roles. However, conditions of immunosuppression or immunodeficiency can give some insights.
An absolute lack of T cells, such as occurs in T cell-deficient forms of severe combined immunodeficiency, is incompatible with life, and therefore studying vaccine responses in the setting of absolute T cell deficiency is not feasible. However, other forms of T cell deficiency exist, including the severe acquired CD4+ T cell depletion associated with HIV/AIDS, which is associated with exacerbation of a number of latent or chronic infections, such as tuberculosis and herpes virus infections, as well as susceptibility to a variety of fungal, protozoan and other opportunistic infections. Because HIV/AIDS (and other T cell deficiency disorders) are associated with B cell dysfunction and/or deficiency, it is not possible to attribute all these infections solely to CD4+ T cell dysfunction. However, the fact that common acute viral infections such as influenza (and seasonal coronavirus infections) are not dramatically more severe in people with HIV/AIDS may suggest that, in the context of established immunity, these viruses can be controlled at least temporarily with poorly functioning and/or reduced levels of CD4+ T cells. Here it is again important to differentiate between the requirement for coordinated collaboration between T cells and B cells in the establishment of immunity in primary infection versus the roles of these cells in mediating protection once immunity is established.
HIV/AIDS-associated CD4+ T cell lymphopenia has been associated with poorer outcomes in active SARS-CoV-2 infection83–85, including prolonged primary SARS-CoV-2 infection and extensive viral evolution in the context of a slowly developing antibody response16. Emerging data on SARS-CoV-2 vaccination in the setting of HIV infection suggest largely preserved levels of neutralizing antibodies86,87. However, other studies suggest an increased risk of breakthrough infections after vaccination88. Understanding the impact of T cell deficiency occurring after the establishment of immunity to SARS-CoV-2 infection may directly address the role of memory T cells in protection from COVID-19.
T cells in primary SARS-CoV-2 infection
Both antibody and T cell responses are induced by primary SARS-CoV-2 infection in unvaccinated individuals, and there is an abundance of evidence that a robust multicomponent immune response correlates with resolution of infection in the absence of prior vaccination9. Clinical studies have correlated more rapid generation of both antibody and T cell responses to SARS-CoV-2 with improved infection outcomes10–12. Coordinated T cell and antibody responses reduce the severity of primary SARS-CoV-2 infection13. Moreover, studies in individuals with immunodeficiencies have suggested that T cell responses have an important role in controlling primary SARS-CoV-2 infection when antibody responses are impaired14. Importantly, a robust CD4+ T follicular helper cell response is essential for the development of nAb responses to primary SARS-CoV-2 infection15. Low CD4+ T cell counts in HIV infection have been associated with prolonged viral persistence owing to delayed antibody development and viral escape mutation16. Higher convalescent HLA-B*07:02-restricted CD8+ T cell responses are present in individuals with milder infection17. A role for both antibody and T cell responses in resolving primary SARS-CoV-2 infection is also supported by several studies in mouse and macaque infection models18,19.
A number of studies have also investigated the potential role of pre-existing T cell responses to seasonal coronaviruses and their potential cross-reactivity with SARS-CoV-2. Higher levels of cross-reactive CD8+ T cells were reported in convalescent individuals who had experienced mild disease compared with those who had experienced severe disease20. In a study of health-care workers, both infected–recovered and exposed–uninfected (seronegative) health-care workers were observed to have similar levels of T cells that cross-react between SARS-CoV-2 and other coronaviruses21. In addition, a study of T cell responses in household contacts suggested that higher levels of nucleocapsid-specific, IL-2-secreting T cells were found in individuals who did not become infected compared with those who became infected22.
There is little controversy that a robust, multicomponent adaptive immune response is critical for mitigating primary SARS-CoV-2 infection13. However, the mechanistic role of different elements of this response can be difficult to dissect in primary infections. Moreover, the rapid spread of infection and uptake of vaccination will mean a reducing frequency of primary infections over time. The analysis of vaccine-primed immune responses and their association with infection outcome is directly relevant to increasing the efficacy of vaccines and is the focus of this Perspective going forward.
Vaccine-induced protection from infection
nAbs protect from SARS-CoV-2 infection
It is well established that nAbs are critical for both preventing SARS-CoV-2 infection and reducing the risk of progression to severe disease (Fig. 1), and the mean level of plasma nAbs elicited by different vaccines is predictive of their efficacy against symptomatic SARS-CoV-2 infection3 (Fig. 1a). In addition, studies of antibody levels soon after vaccination show that vaccinated individuals with low levels of nAbs were more likely to subsequently experience breakthrough infection6,23. In vitro neutralization activity is also predictive of vaccine efficacy against symptomatic infection with SARS-CoV-2 variants24 (Fig. 1b). Monoclonal nAbs to the SARS-CoV-2 spike protein have shown a high level of protection if administered prophylactically or as postexposure prophylaxis25,26, demonstrating a mechanistic role for antibodies in preventing infection (Box 2). Work across several animal models of SARS-CoV-2 infection also defines a role for nAbs in protection from SARS-CoV-2 infection27.
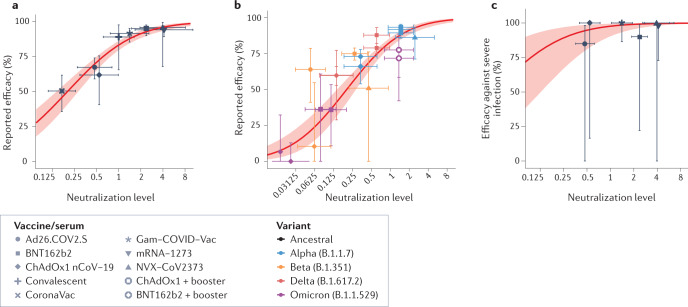
Fig. 1. Neutralizing antibodies predict protection from acquisition of SARS-CoV-2 infection.
a | Relationship between the mean in vitro neutralizing antibody level from phase I/II trials of different vaccines and the reported phase III efficacy against symptomatic infection with the ancestral SARS-CoV-2 strain (adapted from ref.3). The x axis is scaled as a proportion of the mean neutralization titre of convalescent participants analysed in the same study. b | Relationship between the predicted neutralization against SARS-CoV-2 variants and the reported efficacy of different vaccines against the variants (adapted from ref.24, with additional data overlaid from ref.63). c | Relationship between the neutralizing antibody level and protection from severe SARS-CoV-2 infection (with the ancestral virus; adapted from ref.3).
Box 2 Lessons from passive antibody therapy.
Although neutralizing antibody responses are clearly correlated with protection from acquisition of SARS-CoV-2 infection and severe disease, a major question is whether they act directly and independently to mediate this protection. A number of SARS-CoV-2-neutralizing monoclonal antibodies have been developed that have been used both prophylactically and therapeutically89,90. The results of these studies allow us to compare vaccine-mediated protection (involving a combination of antibody and cellular responses) with the protection from antibodies alone. For example, O’Brien and colleagues studied the effects of administration of monoclonal antibodies to household contacts within 96 h of diagnosis of the index case25. They observed overall 81% protection from acquisition of symptomatic SARS-CoV-2 infection. However, as some of the individuals may have been exposed before treatment, this may include postexposure prophylaxis of many participants. To overcome this, they also studied protection against the late acquisition of symptomatic SARS-CoV-2 infection (arising more than 2 weeks after treatment), assumed to have been acquired after treatment. Prophylactic administration of monoclonal antibodies provided 92.6% protection from late acquisition of SARS-CoV-2 infection, which closely matches the efficacy seen after the use of vaccines that induce the highest levels of neutralizing antibodies3. Therapeutic administration of monoclonal antibodies early after confirmed infection (before day 5 after symptom onset) has also been shown to provide up to 85% protection from progression to severe disease26,90.
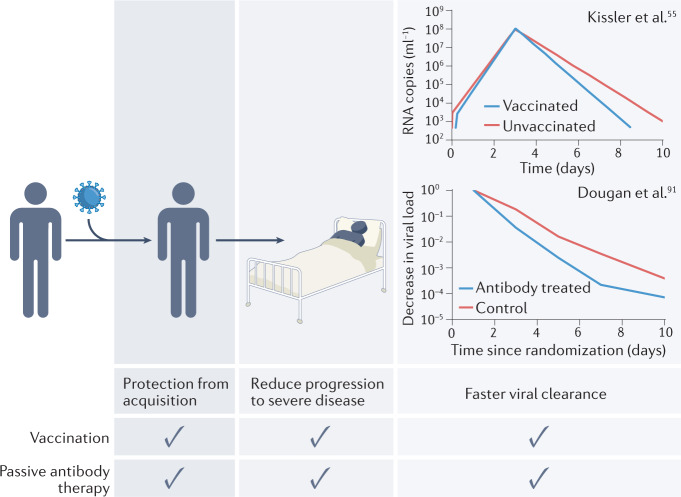
Another feature of vaccine-mediated immune control of SARS-CoV-2 infection is the more rapid clearance of the virus after the peak of infection55 (see the figure), which has sometimes been attributed to T cell-mediated lysis of infected cells. Interestingly, passive antibody administration leads to a similar increase in the rate of viral clearance91. The mechanisms for this are unclear, and may include antibody-dependent cellular cytotoxicity lysis of infected cells (as supported by animal model studies92), or simply a reduction in the level of ongoing cycles of infection through neutralization and clearance of free virus. Animal models also clearly support the utility of prophylactic and early therapeutic administration of monoclonal neutralizing antibodies27,93–95.
Taken together, this analysis suggests that passive antibody administration and vaccination can protect from both acquisition of and progression to severe SARS-CoV-2 infection, and that they have similar effects on viral kinetics. The levels of the antibody delivered in therapeutic SARS-CoV-2 monoclonal antibody treatment are much higher than those observed after vaccination90. However, this analysis strongly supports the idea that neutralizing antibodies play a mechanistic role in protection from SARS-CoV-2 infection, and that it may not be necessary to invoke other mechanisms to explain the protection seen after vaccination.
Role of T cell responses in protection from SARS-CoV-2 infection
Memory T cell responses have been posited to play a significant role in immune protection from acquisition of SARS-CoV-2 infection9,28,29, although direct evidence for this is limited. At least two major roles for T cells in the adaptive immune response are proposed. First, CD4+ T cells are central facilitators of effective humoral immunity via the provision of ‘helper’ signals that modulate the differentiation and selection of B cells. In addition, both CD4+ T cells and CD8+ T cells have the potential to mediate direct antiviral function through either the secretion of antiviral cytokines or the directed killing of infected host cells. A critical role for CD4+ T cell help (T follicular helper cells) in the generation of effective nAb responses to SARS-CoV-2 infection is clear15,30,31. However, a role for vaccine-elicited memory CD4+ T cell and CD8+ T cell responses in directly mediating protection from SARS-CoV-2 infection (independent of initial T cell help for generating nAb responses) is unclear. In contrast to nAb analyses, where robust correlations between vaccine-induced nAbs and prevention of infection are available3,6,23, definitive data correlating the levels of memory T cells in the blood with protection from SARS-CoV-2 infection in vaccinated humans are lacking.
A significant limitation in studying a link between memory T cell immunity and protection in humans is the lack of a standardized assay for measuring T cell responses and for comparison across studies. Measurement of a pathogen-specific antibody is a useful and convenient marker of B cell-mediated immunity that is routinely used in research and diagnostic assays for acute and chronic viral infections (for example, hepatitis B, measles, mumps, rubella and varicella zoster). By contrast, the most established memory T cells research assays, such as the enzyme-linked immunosorbent spot assay, are time-consuming and labour-intensive, largely precluding their advancement into the diagnostic setting, with the exception of interferon-γ release assays in the diagnosis of Mycobacterium tuberculosis infection. As a result, our ability to correlate functional T cell assays to clinical outcomes is less established as compared with antibody assays. The recent development of an interferon-γ release assay for SARS-CoV-2 is welcome in this regard32. In addition, studying T cells in blood may not reflect the levels of localized immunity in the lung, although there is as yet no strong evidence to support a major role for vaccine-induced localized or non-spike-specific T cell responses33 (Box 3).
The lack of data supporting a clear role for T cells in preventing SARS-CoV-2 infection may be due, in part, to challenges in deconvoluting the concomitant generation of both humoral and cellular immunity. That is, the prominent protective effect of nAb responses in highly efficacious vaccines may ‘mask’ any secondary role for T cell responses. Additionally, even a weak correlation between T cell and antibody responses may further confound analyses of correlates of protection15,33.
Box 3 Mucosal responses and responses to non-spike proteins.
Previous SARS-CoV-2 infection is thought to elicit a range of localized (lung and mucosal) immune responses that are not expected to be present after intramuscular immunization. Similarly, infection elicits responses to multiple viral antigens. By contrast, most current vaccines incorporate only the spike immunogen, and all are delivered intramuscularly, and thus vaccine-induced antibody and T cell responses are focused on the spike protein. One whole inactivated vaccine (CoronaVac) has been widely used and elicits responses to multiple SARS-CoV-2 antigens. However, given the intramuscular route of administration, vaccination with CoronaVac is not expected to prime a tissue or mucosal immune responses to the extent natural infection might. Comparison across natural infection, whole virus vaccination and spike-only vaccination provides the opportunity to identify the contribution of localized and non-spike responses. However, as infection and vaccination can elicit very different levels of neutralizing antibody (nAb) responses, we need to first account for these differences. Thus, for example, if tissue responses are important for immune protection, then previous infection should provide significantly greater levels of protection than predicted by the levels of circulating antibodies. Similarly, if responses to non-spike antigens play a major role in protection, then we might predict that both infection and whole virus vaccination should show higher protection than predicted from the nAb response alone. However, analysis of the data shows that the protection from acquisition of symptomatic SARS-CoV-2 infection generated by natural infection and CoronaVac vaccination is predicted well by the level of plasma nAbs3. This suggests a limited contribution of mucosal, tissue and non-spike responses to the level of protection already predicted by nAb levels measured in plasma.
Disentangling the contribution of nAbs and T cells in protection from SARS-CoV-2 infection
A current challenge in this field is the difficulty of studying T cell responses in isolation, given the significant reliance on T cells for proficient nAb generation. One potential way to address the role of T cells in protection from SARS-CoV-2 infection is to identify contexts where T cell and nAb responses may be at least partially ‘decoupled’, and then analyse which response predicts protection.
Infection with SARS-CoV-2 variants of concern is one situation where nAb responses and T cell responses may diverge (Fig. 2a). nAb titres for variants of concern (including Omicron) can be much lower (more than tenfold) than responses to the ancestral virus and the vaccine immunogen24,34–36. However, there is relatively little loss of T cell cross-reactivity to variants of concern, including Omicron37–39. Thus, exposure of vaccinated individuals to SARS-CoV-2 variants of concern presents a situation in which T cell immunity should remain largely intact, whereas effective concentrations of nAbs are greatly reduced (Fig. 2a). Clinical trials consistently show that vaccine efficacy in preventing infection is reduced for these variants40,41 at a level predicted by nAb levels24 (Fig. 1b), despite the largely intact T cell recognition42. Similar results have been observed in macaques, where decreased neutralization titres for the Beta variant coincided with reduced control of SARS-CoV-2 infection, despite preserved T cell recognition43.
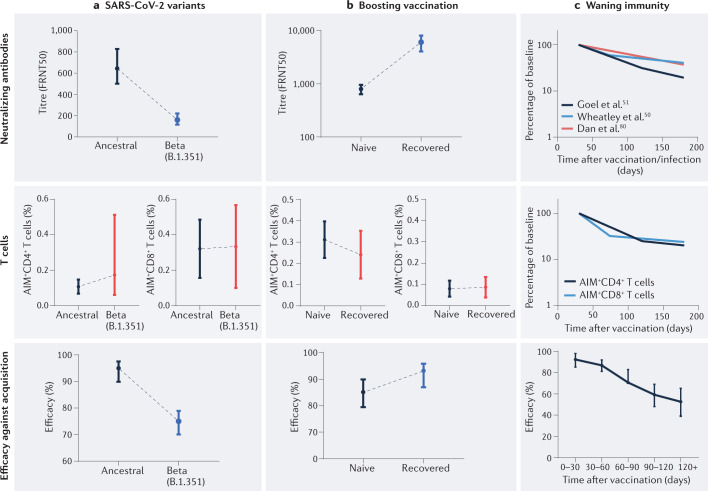
Fig. 2. Protection from acquisition of SARS-CoV-2 infection tracks the neutralizing antibody responses.
From comparison of situations where neutralizing antibody (nAb) and memory T cell responses are decoupled, protection appears to be predicted by the level of nAbs. a | The nAb levels, circulating spike-specific (AIM+) CD4+ T cell levels and AIM+CD8+ T cell levels, and protection against symptomatic infection for the Beta variant versus the ancestral SARS-CoV-2 strain. nAb titres (measured as the 50% focus-reduction neutralizing antibody titre (FRNT50)) are reduced 3.9-fold, 8.8-fold and at least 10-fold for the Delta (B.1.167.2), Beta (B.1.351) and Omicron (B.1.1.529) variants, respectively24,63. By contrast, T cell responses to the variants are largely preserved, including to the Beta, Delta and Omicron variants37–39,65. Protection decreases as expected from the reduced nAb level24,41. b | Vaccination of previously infected individuals leads to higher peak nAb levels than seen in vaccination of naive individuals44. However, peak CD4+ T cell and CD8+ T cell responses are similar following vaccination of previously infected individuals and naive individuals45. The effectiveness after BNT162b2 vaccination in recovered individuals (recovered from infection with the Delta variant) is significantly higher than the effectiveness after vaccination in naive individuals (93% versus 85%, P = 0.006), tracking the increased nAb responses49. c | The observed waning of nAb responses (data obtained from refs50,51,80) has led to the suggestion that the relative contribution of T cell-mediated immunity to protection may increase over time. However, the decay rate of antigen-specific T cells in the circulation is similar to that observed for nAb titres45. Although protection wanes with time7, it is not possible to differentiate the contribution of humoral or T cell responses, as they decay at similar rates.
Boosting of immune responses in previously infected individuals through vaccination also provides an example of dissociation between nAb levels and T cell levels44,45 (Fig. 2b). Vaccination of previously infected individuals results in peak nAb levels severalfold higher than the levels observed after vaccination of naive individuals44,46,47. However, spike-specific T cell immunity does not appear to be similarly boosted, with spike-specific T cell frequencies comparable to those observed after vaccination of naive individuals45,48 (Fig. 2b). Thus, if protection from infection tracked with T cells, then we might expect little difference in protection after vaccination of naive or recovered individuals. However, vaccination has been observed to have a significantly greater effectiveness in preventing infection in recovered individuals versus naive individuals49, which argues for a critical role for nAbs.
It is now clear that nAb responses wane after SARS-CoV-2 infection or vaccination with a half-life of 3–4 months50,51, and that a decline in vaccine efficacy against symptomatic SARS-CoV-2 infection tracks closely with this decay7,8. The observed waning of nAb responses could theoretically lead to an increasingly important role for CD4+ T cells and CD8+ T cells over time51. Comparisons of the stability or waning of particular immune responses can be challenging owing to differences in assay sensitivity and comparing antibody titres with cell numbers. However, a direct comparison of the decay of nAb responses and of SARS-CoV-2-specific CD4+ T cell and CD8+ T cell numbers show similar half-lives50,51 (Fig. 2c), although other studies have even suggested a rise in T cell numbers52. Epidemiological data clearly show a waning in the level of protection from symptomatic SARS-CoV-2 infection over the first 6 months after vaccination7,8. However, as nAb responses and T cell numbers appear to decay with similar rates, this does not provide a definitive argument for the importance of T cells in late protection from infection. Indeed, decoupling the relative contribution of T cell and humoral responses to immune protection over time is likely to be extremely challenging.
A final argument that has been raised to argue for T cell protection from acquisition of SARS-CoV-2 infection relates to the onset of protection after vaccination. Studies have argued that clinical protection is observed within the first 2 weeks after the first vaccine dose, when T cell and binding antibody responses can be detected, while nAbs remain below the limit of detection (usually a titre of 1:10 or 1:20)53. However, a major limitation here is the sensitivity of in vitro neutralization assays. The highest concentration of serum used is typically 1:10 to 1:20 (as lower serum dilutions affect the assay itself). However, the inability to detect neutralization at this level does not imply the absence of an in vivo effect (there is obviously no dilution in vivo). Indeed, the estimated neutralization titre for 50% protection from symptomatic SARS-CoV-2 infection (54 IU ml−1) is below the sensitivity of most in vitro assays, despite the clear evidence of protection in vivo3. However, further investigation and validation of this early-onset protection may provide interesting insights into protection from SARS-CoV-2 infection.
T cells as a ‘backup’ for antibody-mediated protection from SARS-CoV-2 infection?
In a healthy vaccinated individual, protection from acquisition of SARS-CoV-2 infection clearly appears to correlate with nAb levels (Figs 1,2). Can T cells act as a ‘backstop’ to prevent SARS-CoV-2 infection when nAb responses are low or absent? Identifying subgroups with compromised or low nAb responses provides an opportunity to study this. For example, individuals receiving low-potency vaccines, studied late after vaccination, or treated with immunosuppressive agents may reveal the potential of T cells for protection. A major clinical concern is for immunocompromised individuals who may fail to make appropriate nAb responses. This is a difficult population to study given the clinical heterogeneity of immunosuppression and also the interaction between different immune responses. For example, patients treated with anti-CD20 (a B cell-depleting antibody) and then vaccinated show reduced antibody responses to vaccination. However, they also show reduced CD4+ T follicular helper cell responses, other relatively normal CD4+ T cell responses (for example, T helper 1 cells) but augmented CD8+ T cell responses54. This highlights the potential for a compensatory increase in the number (and role) of some T cell responses in the absence of nAb responses, although the mechanisms of this are unclear. Importantly, this occurs in the context of B cell deficiency in the ‘priming’ stage of vaccination. Anti-CD20 treatment after vaccination may have quite different effects on established immunity. Thus, immunosuppressive therapy may often produce a complex picture of both B cell and T cell immune perturbation, and the mechanisms of protection may not always mirror those in immunocompetent individuals. However, understanding immune protection in this vulnerable population may provide insights into an area where boosting T cell responses has the potential to greatly improve infection outcomes.
Vaccine-induced protection from severe disease
The role of nAbs in protection from severe disease
The previous analysis investigated the correlates of protection from SARS-CoV-2 infection, which likely involves nAb-mediated blocking of the early establishment of infection (although the site and mechanisms of action are not definitively established)4. Analyses of protection from severe disease are more difficult, however, as there are relatively few prospective data on severe disease from phase II vaccine trials, with fewer than 100 severe cases observed overall3. Despite this important limitation, we still find that nAb responses remain predictive of protection from severe disease and that the nAb titre associated with 50% protection from severe SARS-CoV-2 infection is around six times lower than the level required to protect against acquisition of infection (around 8 IU ml−1 versus 54 IU ml−1)3. Because of the lower levels of nAbs associated with protection from severe disease, this protection is expected to be sustained for much longer than protection from acquisition of infection24 (Fig. 1c). Similarly, protection against severe disease from SARS-CoV-2 variants is also thought to be maintained because lower levels of nAbs are associated with protection from severe infection. A major challenge here is that the level of nAbs associated with protection from severe disease is below the detection limit of many neutralization assays. In addition, it is unclear whether a nAb level of 8 IU ml−1 is itself active in protection (a ‘mechanistic correlate’ of protection) or whether this is simply a (surrogate) biomarker of another mechanism such as plasmablast or memory B cell recall potential (which may act to rapidly boost nAb levels in early infection)1.
Whereas vaccination and prior infection can mediate strong protection from acquisition of SARS-CoV-2 infection (ranging from around 50% to around 95% protection), ‘breakthrough infections’ in vaccinated individuals are commonly observed, particularly with the Beta and Omicron variants5,6. However, the risk of progression to severe disease in breakthrough infections is greatly reduced compared with that in primary infections3,41. Studies of viral dynamics in breakthrough infections with the Delta variant suggest that viral loads in nasopharyngeal swabs at presentation are similar between vaccinated individuals and naive individuals55. However, viral clearance rates are significantly greater in vaccinated individuals55,56, with divergence between groups occurring around day 6 after symptom onset (Fig. 3a). This suggests that a change in immune control occurring after clinical presentation may increase the subsequent viral clearance rate and contribute to protection from severe disease.
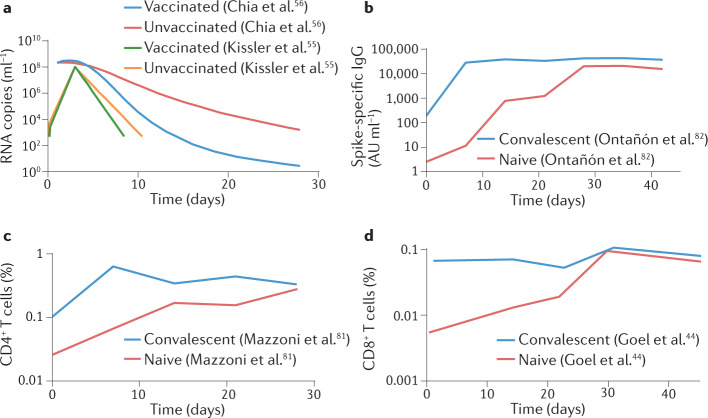
Fig. 3. Understanding protection from severe SARS-CoV-2 infection.
Vaccination provides greater protection from severe infection with SARS-CoV-2 than from acquisition of mild or asymptomatic infection. a | Studies show that breakthrough infection in vaccinees (with the Alpha variant or the Delta variant) leads to similar viral RNA levels at presentation or the peak to the levels seen in unvaccinated individuals. However, more rapid viral clearance is observed after the peak in vaccinated individuals. Data extracted from refs55,56. Recall of neutralizing antibody responses (part b) (data from refs81,82), CD4+ T cell responses (part c) (data from ref.81) and CD8+ T cell responses (part d) (data from ref.44) occurs rapidly after vaccination of previously infected individuals. AU, arbitrary units.
Studies of passive antibody administration (primarily in unvaccinated individuals) suggest that high (monoclonal) antibody levels delivered in the first week after symptom onset can play a significant role in reducing the progression to severe infection and can accelerate viral clearance (Box 2). A rapid rise in nAb responses after breakthrough infection in vaccinated individuals has been shown in this time window, suggesting that B cell recall and nAb production may play a similar role in reducing the severity of infection57,58. An analysis of the dynamics of immune recall early after breakthrough SARS-CoV-2 infection in the Delta-dominant era shows rapid recall of antibody responses occurring around day 5 after symptom onset (around day 8 after infection), but weak or no recall of CD4+ T cell and CD8+ T cell responses in blood around the same time58 (Fig. 3b–d). A cross-sectional study on samples taken an average of 10–11 days after Delta or Omicron breakthrough infection showed some activation of spike-specific T cells, as well as spike-specific B cells59. However, the time point sampled is typically after control of viral levels has been achieved, making it difficult to dissect the role of particular immune responses in the control of the virus. Also, the observations of T cell numbers in blood of course do not preclude a different potential role for the activation or activity of these cells in tissues.
Thus, recall of nAb responses occurs in a time window where they are at least temporally associated with the rapid decline in viral levels seen in vaccinated individuals, and passive administration of antibodies has been shown to be effective in ameliorating disease outcome. Understanding the relationship between pre-infection nAb levels, the timing and magnitude of nAb recall, the clearance rate of the virus, and improved clinical outcomes of SARS-CoV-2 infection is an important area for future investigation.
T cells and protection from severe disease
The lack of an association between protection from acquisition of SARS-CoV-2 infection and either T cell responses measured in blood or T cell capacity to recognize SARS-CoV-2 variants is perhaps unsurprising, given their mechanisms of action. That is, whereas pre-existing antibodies may act directly to neutralize an incoming virus, T cells require antigen presentation for activation and subsequent antiviral activity. Animal studies of simian immunodeficiency virus infection and M. tuberculosis infection have shown that T cells do ‘too little too late’ to prevent the establishment of infection, but can play a role in later control60,61. Similarly, one study suggests that although T cells do not protect from influenza virus infection, in the absence of nAbs, CD8+ T cell responses may reduce the severity of infection62.
Studies of vaccine protection from infection with SARS-CoV-2 variants provide one opportunity to investigate the relationship between protection from severe disease and immune recognition by antibodies and T cells. For example, geometric mean vaccine-induced nAb responses to the Omicron variant compared with the ancestral strain are decreased more than tenfold (compared with a reduction of around 3.9-fold for the Delta strain)24,36,63. However, T cell responses to the Omicron variant compared with the ancestral strain are reported to be decreased by a mean of only 10–30%39,64,65, and are not significantly different between the Delta strain and the Omicron strain64,65. Two studies have shown reduced vaccine effectiveness in preventing both symptomatic infection and hospitalization in the Omicron-dominant period compared with the Delta-dominant period, which appears consistent with the greater drop in nAb recognition of the Omicron variant versus the Delta variant66,67. However, if immunity acts in a ‘two-step’ fashion, where antibodies block viral entry but T cells moderate disease severity4, then reduced protection from hospitalization with particular SARS-CoV-2 variants may, in part, be due to a higher number of symptomatic infections in vaccinated individuals, while retaining the same level of protection from progression from symptomatic to severe disease. A more recent study managed to directly address vaccine protection against progression from symptomatic to severe SARS-CoV-2 infection by studying vaccinated SARS-CoV-2-positive individuals and following their risk of subsequent hospitalization68. This showed that vaccinated individuals infected with the Omicron variant had reduced vaccine protection from progression to hospitalization compared with vaccinated individuals with Delta variant infection (after the intrinsic reduction in severity for Omicron variant infection had been accounted for), again consistent with the reduction in nAb recognition of the Omicron variant68. Although these epidemiological studies are supportive of a major role for nAbs in determining disease severity, a direct assessment of whether the ‘risk of progression’ from symptomatic to severe infection correlates better with antibody neutralization or with T cell recognition of SARS-CoV-2 variants is a critical question, and further studies are required to directly analyse this relationship.
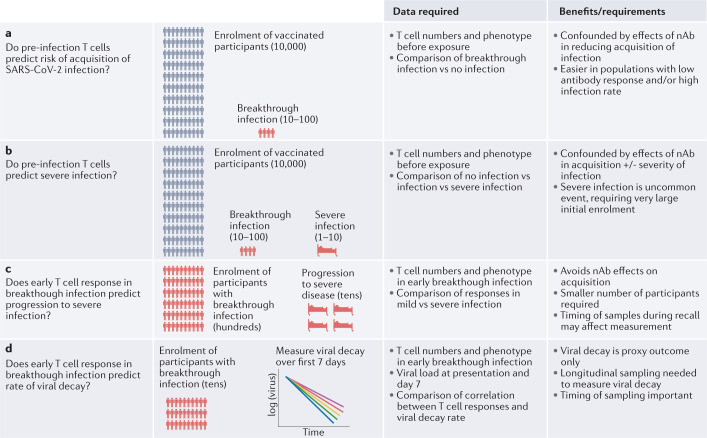
Clinical studies to analyse T cells as a correlate of protection from severe COVID-19 are likely to be challenging (Fig. 4). Firstly, T cells are typically measured in blood, whereas T cell function in the mucosal or tissue site of infection may be more important. Secondly, the lack of a standardized T cell assay limits comparison of results across different studies. One approach might be to study pre-infection T cell responses as a correlate of protection from acquisition of SARS-CoV-2 infection and subsequent progression to severe disease in a cohort of vaccinated individuals (as has been done for antibody responses in acquisition of infection2,6,23 (Fig. 4a)). However, two factors make studying correlates of severe disease difficult. First, the low frequency of severe disease (particularly in vaccinated individuals) means that large numbers of vaccinated or previously infected individuals would need to be studied to identify a sufficient sample size of individuals with severe disease (Fig. 4b). Second, because nAbs confer such strong protection both against the establishment of infection and from severe disease (and may be correlated with T cell responses), it will be difficult to disentangle nAb protection from any additional contribution of T cells to protection from severe disease.
Fig. 4. Investigating T cell protection from severe SARS-CoV-2 infection.
Vaccination provides a high level of protection from acquisition of SARS-CoV-2 infection, and an even higher level of protection from severe disease. Thus, immunity might be thought of as having complementary actions at two stages of infection: first blocking establishment of infection and, second, even if breakthrough infection occurs, acting to reduce the severity of infection. a | The levels of neutralizing antibodies (nAbs) have been shown to be lower in vaccinated individuals with breakthrough infection. Similar studies investigating whether T cells contribute to protection from acquisition of SARS-CoV-2 infection are needed. b | Prospective studies of pre-infection T cell numbers as predictors of disease severity are challenging, due to the low frequency of severe disease. c | Studying whether the levels of CD4+ T cell and CD8+ T cell response predict disease severity in individuals with breakthrough infection may reduce the number of individuals needed to be studied. However, rapid changes in T cell numbers during the recall response may complicate analysis. d | Studying the relationship between T cell responses and viral clearance rate can be performed on smaller cohorts and has the potential to reveal a role for T cells in control of viral replication (although this is only a proxy measure for disease severity).
An alternative approach to looking for a role for T cells in infection severity is to study the magnitude and timing of recall responses in individuals with breakthrough infections and how the level of CD4+ T cells or CD8+ T cells predicts either viral clearance or the severity of the clinical outcome (Fig. 4c). That is, by focusing only on individuals with breakthrough infection, this approach can both reduce the number of individuals needed for such studies and eliminate the confounding effect of nAbs protecting against acquisition of infection. The major outcome would be to establish whether early T cell responses (measured as soon as possible after infection) or peak T cell responses predict the risk of progression to severe COVID-19. However, the timing of sampling is a major issue, and this would likely require a large number of individuals to definitively establish it. An alternative approach would be to study the association between T cell responses and SARS-CoV-2 viral decay dynamics. Studies suggest that there is faster viral clearance in vaccinated individuals55,56, and thus an analysis of the relationship between T cell responses and the slope of viral RNA decay from the peak could provide a proxy measure of immune control (Fig. 4d).
A major issue with analysing the effect of T cells in breakthrough SARS-CoV-2 infection is that nAbs are likely to remain an important confounder. Studies of ‘T cell-only’ vaccines have the potential to isolate the effects of T cells, and have been conducted in mice and hamsters19,69–71. In these animal models of high-dose infection, high levels of vaccine-induced CD8+ T cells (~3–10% of total CD8+ T cells in the spleen of mice when measured with tetramers70) can be present at the time of challenge, and this led to reductions in viral levels (~2–30-fold) and protection from disease70,71. High levels of T cells were also induced in the lungs after intranasal or intravenous administration of vaccines in mice72, which may have contributed to the protection observed. A study of intranasal vaccines in macaques showed reductions in SARS-CoV-2 levels after challenge73. Current SARS-CoV-2 vaccines result in low levels of circulating CD8+ T cell and CD4+ T cell responses in humans (around 0.1% of the total CD8+ and CD4+ populations)74. Early studies of T cell-focused vaccines have now been reported in humans, with induction of T cell responses of around 0.2–0.5% early after vaccination75. Although it is difficult to directly compare responses in specific-pathogen-free mice and humans, this does suggest that the current levels of response seen in human trials may be significantly lower than the levels achieved in mice. Larger human efficacy trials have the potential to demonstrate a protective effect of inducing T cell responses independent of antibody responses, although generating the appropriate magnitude, localization or durability of the T cell response could prove challenging.
These factors highlight the difficulty in establishing a role for T cells in protective immunity to SARS-CoV-2 infection beyond a general assumption that they are likely to be helpful. We propose that investigation of the role of T cells in severe COVID-19 needs to focus on individuals with breakthrough infection and progress in a two-stage approach: firstly, identifying the relationship between nAbs (measured at presentation and peak) as predictors of viral decay and/or progression to severe COVID-19; then, using this observed relationship with antibodies, to ask whether consideration of the levels of CD4+ T cells and CD8+ T cells can improve upon this prediction.
Immune protection in immunocompromised individuals
Understanding the role of T cells in protection from severe COVID-19 in patients with a primary immunodeficiency and those receiving secondary immunosuppression is critical to optimizing the management of these vulnerable patient groups. Immunodeficiency may contribute to a poor outcome after vaccination in several ways. Firstly, poor vaccine immunogenicity is a hallmark of immunodeficiency, which is likely to predispose to susceptibility to acquisition of infection. Immunocompromised people have been over-represented in breakthrough infections, corresponding to 44% of postvaccination infections despite accounting for only 2.7% of the population (in the pre-Delta era)76. In addition, failure to mount a sufficient recall response may lead to increased severity and duration of shedding and may facilitate viral evolution that may lead to new variants of concern77. The lower vaccine-mediated protection from acquisition of infection may mean that prospective studies of correlates of protection from acquisition of infection and progression to severe COVID-19 may be possible with smaller patient numbers (notwithstanding the limited numbers and heterogeneities of these patient groups).
Studies of breakthrough infection in immunocompromised and immunosuppressed individuals are of utmost importance in the coming months, and should be aimed at identifying responses that are associated with reduced progression to severe COVID-19. Importantly, in immunocompromised individuals with a defective or absent B cell compartment but a functional T cell compartment, T cell responses are still likely to be perturbed and/or may play a different role in the absence of antibodies62. Identifying responses that can be augmented to provide increased protection from severe SARS-CoV-2 infection in highly vulnerable populations is an urgent research priority.
Conclusion
Dissecting the role of T cell immunity in vaccine-mediated protection from SARS-CoV-2 is an important mechanistic issue for the design of future vaccines and for understanding vulnerability to severe COVID-19 (Box 4 for a summary of outstanding questions). It is also important to know whether measuring T cell responses adds to the predictive ability of our existing immune correlates. Current evidence does not support a significant role for T cell responses in protection from acquisition of SARS-CoV-2 infection. However, a role for T cells in protection from progression to severe SARS-CoV-2 infection has yet to be fully explored (Fig. 4). This reflects similar findings in influenza virus infection, where T cells are thought to contribute to reduced disease severity without affecting the risk of acquisition of infection62,78. However, as mentioned in the Introduction, it is easy to make the discussion a binary one between ‘all antibodies’ and ‘all T cells’ contributing to protection, whereas the immune system typically defies such simplified dichotomies. T cells clearly contribute to the development of antibody responses, and similarly B cells can play a role in regulation of T cell responses79. In addition, studies of both immunocompetent and B cell-immunodeficient individuals suggest that T cell and B cell responses can compensate for each other in some circumstances54. It also seems likely that breakthrough infection and severe COVID-19 might reflect differences in viral factors, such as inoculum size. For example, it may be that nAbs can prevent acquisition of infection with smaller inocula but are unable to fully neutralize a larger inoculum (potentially leading to the need for subsequent T cell control).
Studies of SARS-CoV-2 susceptibility and severity in immunocompromised individuals may not always reflect the same role of these responses in typical infection. However, determining which responses can mediate protection in the context of immunodeficiency is clearly a major priority in protecting this highly vulnerable population. Identifying whether and in what context boosting T cell responses can increase protection from severe SARS-CoV-2 infection could provide a novel avenue for protection of many at-risk groups. Further studies are urgently needed to provide evidence to support the ongoing study and analysis of T cell immunity in SARS-CoV-2 infection.
Box 4 Outstanding questions on the role of vaccine-induced T cells in immunity to SARS-CoV-2 infection.
Acquisition of SARS-CoV-2 infection
Do T cells play a more important role in protection from acquisition of SARS-CoV-2 infection in the presence of B cell immunodeficiency?
Can boosting T cell responses to higher levels augment the protection from neutralizing antibodies (nAbs)?
Protection from severe COVID-19
Do T cell responses reduce the severity of illness in breakthrough infections when nAb responses are taken into account?
What is the role of T cell responses in controlling infection in different immunodeficiency states?
Can boosting T cell responses provide increased protection from severe COVID-19 in vaccinated and/or immunosuppressed populations?
Key data required
Fine kinetics of T cell and nAb responses during breakthrough infections to correlate with control of viraemia
Well-established vaccinated cohorts of defined immunodeficiency states and followed up for breakthrough infection severity
Statistical modelling of nAb and T cell contributions to protection, where T cell contributions can be analysed after nAb effects have been accounted for
Acknowledgements
This work was supported by Australian NHMRC programme grant 1149990 to S.J.K. and M.P.D., Australian MRFF award 2005544 to S.J.K., J.A.J., A.K.W. and M.P.D., MRFF award 2007221 to J.T. and US NIH grants AI105343, AI082630, AI108545, AI155577 and AI149680 to E.J.W. S.J.K., D.S.K., J.A.J., A.K.W., D.C. and M.P.D. are supported by NHMRC fellowships.
Glossary
- Breakthrough infection
SARS-CoV-2 infection occurring in partially immune individuals (either previously vaccinated or previously infected).
- Immune correlates of protection
Immune responses that correlate with and predict a particular infection outcome. These may be a mechanistic correlate, which actually mediates the immune protection. Alternatively, they may simply be a surrogate marker of the underlying mechanistic response. Different correlates may exist for protection from acquisition of infection and protection from progression to severe COVID-19.
- Neutralizing antibodies
(nAbs). Antibodies, usually measured in plasma or serum, that inhibit live SARS-CoV-2 replication in vitro. nAbs bind to the spike protein of SARS-CoV-2. Surrogate assays such as pseudovirus neutralization assays and ACE2-binding inhibition assays generally correlate well with live virus neutralization assays.
- Protection from SARS-CoV-2 infection
In clinical studies, protection is typically defined as the absence of detectable SARS-CoV-2 by PCR, with or without the presence of symptoms. With use of this approach, an unknown proportion of asymptomatic or very mild infections may be missed (depending on the sensitivity of the assay and the frequency of screening). However, we use this term to discriminate between protection from initial establishment of infection and progression to severe COVID-19.
- Protection from severe COVID-19
Prevention of hospitalization, intensive care admission, invasive ventilation or death.
- SARS-CoV-2-specific T cells
CD4+ T cells or CD8+ T cells, usually measured in blood, that respond to SARS-CoV-2-specific proteins or peptides. T cell responses are typically enumerated by HLA tetramers, by their ability to produce cytokines or other molecules, or by more complex assays of cell killing or helper functions. SARS-CoV-2-specific T cells can respond to both the spike protein and multiple other SARS-CoV-2 proteins following infection or if the antigens are included in a vaccine.
Author contributions
All authors contributed intellectual input to the manuscript. The first draft was written by M.P.D. and S.J.K., and all authors reviewed the manuscript.
Peer review
Peer review information
Nature Reviews Immunology thanks Eui-Cheol Shin, Antonio Bertoletti, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
E.J.W. reports consulting or is an advisor for Merck, Marengo, Janssen, Related Sciences and Synthekine and was a founder of Surface Oncology, Danger Bio and Arsenal Biosciences. All other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stephen J. Kent, Email: skent@unimelb.edu.au
Miles P. Davenport, Email: m.davenport@unsw.edu.au
References
- 1.Plotkin SA. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 4.Cromer D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacisuleyman E, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N. Engl. J. Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M, et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartof SY, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitelly H, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 9.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas C, et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021;27:1178–1186. doi: 10.1038/s41591-021-01355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbe A, et al. T cell assays differentiate clinical and subclinical SARS-CoV-2 infections from cross-reactive antiviral responses. Nat. Commun. 2021;12:2055. doi: 10.1038/s41467-021-21856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan AT, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rydyznski Moderbacher C, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012 e1019. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bange EM, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juno JA, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 16.Cele S, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe. 2022;30:154–162 e155. doi: 10.1016/j.chom.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y, et al. An immunodominant NP105-113-B*07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease. Nat. Immunol. 2022;23:50–61. doi: 10.1038/s41590-021-01084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahan K, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang Z, et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J. Exp. Med. 2021;218:e20202187. doi: 10.1084/jem.20202187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallajosyula V, et al. CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci. Immunol. 2021;6:eabg5669. doi: 10.1126/sciimmunol.abg5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swadling L, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2021;601:110–117. doi: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kundu R, et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022;13:80. doi: 10.1038/s41467-021-27674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert PB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cromer D, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien MP, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N. Engl. J. Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta A, et al. Early Treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 27.Baum A, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipsitch M, Grad YH, Sette A, Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020;20:709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertoletti A, Le Bert N, Qui M, Tan AT. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol. Immunol. 2021;18:2307–2312. doi: 10.1038/s41423-021-00743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaan Lakshmanappa Y, et al. SARS-CoV-2 induces robust germinal center CD4 T follicular helper cell responses in rhesus macaques. Nat. Commun. 2021;12:541. doi: 10.1038/s41467-020-20642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lederer K, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295 e1285. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan AT, et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J. Clin. Invest. 2021;131:e152379. doi: 10.1172/JCI152379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon MML, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021;6:eabl9105. doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhou D, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361 e2346. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cele S, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riou C, et al. Escape from recognition of SARS-CoV-2 Beta variant spike epitopes but overall preservation of T cell immunity. Sci. Transl. Med. 2021;14:eabj6824. doi: 10.1126/scitranslmed.abj6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarke A, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021;2:100355. doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kustin T, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 2021;27:1379–1384. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang P, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 42.Alter G, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596:268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandrashekar A, et al. Prior infection with SARS-CoV-2 WA1/2020 partially protects rhesus macaques against reinfection with B.1.1.7 and B.1.351 variants. Sci. Transl. Med. 2021;13:eabj2641. doi: 10.1126/scitranslmed.abj2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel RR, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Painter MM, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142 e2133. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatatos L, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021 doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anichini G, et al. SARS-CoV-2 antibody response in persons with past natural infection. N. Engl. J. Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angyal A, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2021;3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pouwels KB, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wheatley AK, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goel RR, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:eabm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilich T, et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021;13:eabf7517. doi: 10.1126/scitranslmed.abf7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalimuddin S, et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med. 2021;2:682–688 e684. doi: 10.1016/j.medj.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apostolidis SA, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kissler SM, et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N. Engl. J. Med. 2021;385:2489–2491. doi: 10.1056/NEJMc2102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chia PY, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirotsu Y, et al. Active immunization by COVID-19 mRNA vaccine results in rapid antibody response and virus reduction in breakthrough infection by Delta (B.1.617.2) Res. Sq. 2021 doi: 10.21203/rs.3.rs-957198/v1. [DOI] [Google Scholar]
- 58.Koutsakos M, et al. Dynamics of immune recall following SARS-CoV-2 vaccination or breakthrough infection. medRxiv. 2021 doi: 10.1101/2021.12.23.21268285. [DOI] [Google Scholar]
- 59.Kared H, et al. Immunity in Omicron SARS-CoV-2 breakthrough COVID-19 in vaccinated adults. medRxiv. 2022 doi: 10.1101/2022.01.13.22269213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf AJ, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davenport MP, Ribeiro RM, Perelson AS. Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J. Virol. 2004;78:10096–10103. doi: 10.1128/JVI.78.18.10096-10103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sridhar S, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 63.Khoury DS, et al. A meta-analysis of early results to predict vaccine efficacy against Omicron. medRxiv. 2021 doi: 10.1101/2021.12.13.21267748. [DOI] [Google Scholar]
- 64.Keeton R, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarke A, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859 e811. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferdinands JM, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.UK Health Security Agency. COVID-19 vaccine weekly surveillance reports (weeks 39 to 12, 2021 to 2022) (UK Health Security Agency, 2021).
- 68.Nyberg T, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022 doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noh JY, Jeong HW, Kim JH, Shin EC. T cell-oriented strategies for controlling the COVID-19 pandemic. Nat. Rev. Immunol. 2021;21:687–688. doi: 10.1038/s41577-021-00625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pardieck IN, et al. A third vaccination with a single T cell epitope protects against SARS-CoV-2 infection in the absence of neutralizing antibodies. bioRxiv. 2021 doi: 10.1101/2021.12.15.472838. [DOI] [Google Scholar]
- 71.Matchett WE, et al. Cutting edge: nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J. Immunol. 2021;207:376–379. doi: 10.4049/jimmunol.2100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joag V, et al. Cutting edge: mouse SARS-CoV-2 epitope reveals infection and vaccine-elicited CD8 T cell responses. J. Immunol. 2021;206:931–935. doi: 10.4049/jimmunol.2001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishii H, et al. Neutralizing antibody-independent SARS-CoV-2 control correlated with intranasal vaccine-induced CD8+ T-cell responses. Cell Rep. Med. 2022 doi: 10.1016/j.xcrm.2022.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt T, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heitmann JS, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature. 2021;601:617–622. doi: 10.1038/s41586-021-04232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tenforde MW, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corey L, et al. SARS-CoV-2 variants in patients with immunosuppression. N. Engl. J. Med. 2021;385:562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayward AC, et al. Natural T cell-mediated protection against seasonal and pandemic influenza. results of the flu watch cohort study. Am. J. Respir. Crit. Care Med. 2015;191:1422–1431. doi: 10.1164/rccm.201411-1988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohib K, et al. Antigen-dependent interactions between regulatory B cells and T cells at the T:B border inhibit subsequent T cell interactions with DCs. Am. J. Transpl. 2020;20:52–63. doi: 10.1111/ajt.15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dan JM, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzoni A, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J. Clin. Invest. 2021;131:e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ontañón J, et al. Influence of past infection with SARS-CoV-2 on the response to the BNT162b2 mRNA vaccine in health care workers: kinetics and durability of the humoral immune response. EBioMedicine. 2021;73:103656. doi: 10.1016/j.ebiom.2021.103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geretti AM, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin. Infect. Dis. 2021;73:e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhaskaran K, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vizcarra P, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lombardi A, et al. Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. Lancet Reg. Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brumme ZL, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines. 2022;7:28. doi: 10.1038/s41541-022-00452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coburn SB, et al. COVID-19 infections post-vaccination by HIV status in the United States. medRxiv. 2021 doi: 10.1101/2021.12.02.21267182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor PC, et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stadler E, et al. Determinants of passive antibody effectiveness in SARS-CoV-2 infection. medRxiv. 2022 doi: 10.1101/2022.03.21.22272672. [DOI] [Google Scholar]
- 91.Dougan M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N. Engl. J. Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamin R, et al. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature. 2021;599:465–470. doi: 10.1038/s41586-021-04017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wheatley AK, et al. Landscape of human antibody recognition of the SARS-CoV-2 spike. Cell Reports. 2021;37:109882. doi: 10.1016/j.celrep.2021.109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du S, et al. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell. 2020;183:1013–1023 e1013. doi: 10.1016/j.cell.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez DR, et al. Prevention and therapy of SARS-CoV-2 and the B.1.351 variant in mice. Cell Rep. 2021;36:109450. doi: 10.1016/j.celrep.2021.109450. [DOI] [PMC free article] [PubMed] [Google Scholar]