Abstract
Introduction
Over the course of 2021, numerous key clinical trials with valuable contributions to clinical cardiology were published or presented at major international conferences. This review seeks to summarise these trials and reflect on their clinical context.
Methods
The authors reviewed clinical trials presented at major cardiology conferences during 2021 including the American College of Cardiology (ACC), European Association for Percutaneous Cardiovascular Interventions (EuroPCR), European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), American Heart Association (AHA), European Heart Rhythm Association (EHRA), Society for Cardiovascular Angiography and Interventions (SCAI), TVT-The Heart Summit (TVT) and Cardiovascular Research Technologies (CRT). Trials with a broad relevance to the cardiology community and those with potential to change current practice were included.
Results
A total of 150 key cardiology clinical trials were identified for inclusion. Interventional cardiology data included trials evaluating the use of new generation novel stent technology and new intravascular physiology strategies such as quantitative flow ratio (QFR) to guide revascularisation in stable and unstable coronary artery disease. New trials in acute coronary syndromes focused on shock, out of hospital cardiac arrest (OOHCA), the impact of COVID-19 on ST-elevation myocardial infarction (STEMI) networks and optimal duration/type of antiplatelet treatment. Structural intervention trials included latest data on transcatheter aortic valve replacement (TAVR) and mitral, tricuspid and pulmonary valve interventions. Heart failure data included trials with sodium-glucose cotransporter 2 (SGLT2) inhibitors, sacubitril/valsartan and novel drugs such as mavacamten for hypertrophic cardiomyopathy (HCM). Prevention trials included new data on proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. In electrophysiology, new data regarding atrial fibrillation (AF) screening and new evidence for rhythm vs. rate control strategies were evaluated.
Conclusion
This article presents a summary of key clinical cardiology trials published and presented during the past year and should be of interest to both practising clinicians and researchers.
Keywords: Acute coronary syndrome, Antiplatelets, Atrial fibrillation, Cardiology, Coronary revascularisation, Shock, Mechanical support, Heart failure, Lipids, Mitral clip, Transcatheter aortic valve implantation, Left atrial appendage closure, Transcatheter tricuspid valve interventions
Key Summary Points
| A concise summary of 150 key cardiology trial presented at major international conferences during 2021. |
| Clinically relevant trials with potential to impact and change current practice. |
| Updates across the spectrum of cardiology including interventional and structural, acute coronary syndromes, antiplatelet therapies, electrophysiology, atrial fibrillation, preventative therapies, and heart failure. |
Introduction
In 2021, multiple clinical trials with the potential to influence current practice and future guidelines were presented and major international meetings including the American College of Cardiology (ACC), European Association for Percutaneous Cardiovascular Interventions (EuroPCR), European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), American Heart Association (AHA), European Heart Rhythm Association (EHRA), Society for Cardiovascular Angiography and Interventions (SCAI), TVT-The Heart Summit (TVT) and Cardiovascular Research Technologies (CRT). In this article we review key studies across the spectrum of cardiovascular subspecialties including acute coronary syndromes (ACS), interventional and structural, electrophysiology and atrial fibrillation, heart failure and preventative cardiology.
Methods
The results of clinical trials presented at major international cardiology meetings in 2021 were reviewed. In addition to this, a literature search of PubMed, Medline, Cochrane library and Embase was completed including the terms “acute coronary syndrome”, “atrial fibrillation”, “coronary prevention”, “electrophysiology”, “heart failure” and “interventional cardiology”. Trials were selected on the basis of their relevance to the cardiology community and the potential to change future clinical guidelines or guide further phase 3 research. This article is based on previously completed work and does not involve any new studies of human or animal subjects performed by any of the authors.
Advances in Interventional Cardiology
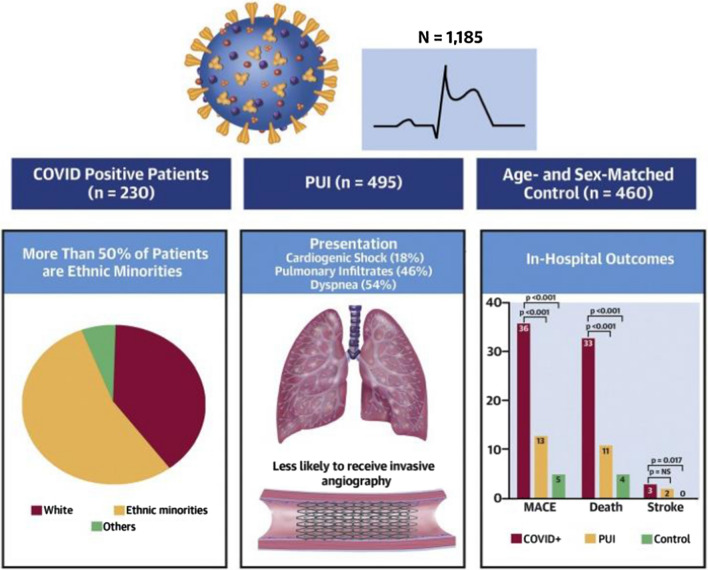
COVID-19 remains an ongoing strain on global healthcare systems. Previous observational analysis of the US multicentre NACMI (North American COVID-19 and STEMI) registry highlighted that patients with ST-elevation myocardial infarction (STEMI) and concurrent COVID-19 infection had more complex presentation, higher mortality and were less likely to undergo primary percutaneous coronary intervention (PPCI) compared with historical matched controls [1]. This year’s analysis of 1185 patients with STEMI (230 COVID-19 positive, 495 suspected COVID-19 positive and 460 controls) demonstrated that patients with COVID-19 were more likely to present with cardiogenic shock (29% vs. 5%; p < 0.01) and cardiac arrest (12% vs. 11%; p < 0.01) [2]. Furthermore, 78% of patients with COVID-19 did not receive angiography, with a notably higher mortality vs. patients with COVID-19 who did (48% vs. 28%; p = 0.006) (Fig. 1). This data reinforces that this is a high-risk group who are underinvestigated but benefit (when appropriate) from urgent revascularisation.
Fig. 1.
A summary of key findings of the NACMI Registry of acute myocardial infarction in patients with coronavirus disease 2019 [1]. COVID coronavirus disease, MACE major adverse cardiac events, NACMI North American COVID-19 and STEMI. Reproduced with kind permission of the Journal of the American College of Cardiology (Garcia et al. [2])
Substantial evidence supports reducing door to balloon times to improve outcomes in STEMI, but multiple factors exist which can cause delay (notably transfers, activation of the cardiac catheterisation lab and diagnostic clarification in equivocal cases). In a single-centre retrospective observational study, Abrahim et al. found that training emergency medical service (EMS) providers to use a mobile phone app with GPS tracking reduced mean door to balloon time from 67.8 to 56.3 min (p < 0.0001) [3]. Early prospective randomised multicentre evaluation of this straightforward strategy appears warranted.
The effectiveness of a second-generation robotic PCI system was investigated in the PRECISION GRX (Multicenter Post-Market Registry for the Evaluation of the CorPath® GRX System Effectiveness in Percutaneous Coronary Interventions) trial [4]. The two co-primary endpoints were clinical success, defined by procedure completion with less than 30% residual stenosis in the absence of a major adverse cardiac event (MACE), and technical success, defined by clinical success without the need for manual assistance or conversion. Clinical success was seen in 98.2% of all lesions. Technical success was achieved in 89.8% of lesions. In 14.7%, manual conversion was required, and the vast majority were unplanned. The results of this trial were hugely encouraging and will likely prompt further research to help reduce radiation exposure and orthopaedic complaints amongst interventional cardiologists.
Patient knowledge about indication for their PCI, when it was performed, and the type and size of stent used may influence the quality of their subsequent medical care particularly from a new clinician. However, Saferstein et al. reported that in an observational study of 310 patients who had undergone previous PCI [5], only 16.9% were able to provide the correct information about their previous procedures, indicating poor information retention. Of note 74.5% of the respondents would be happy to store personal medical information on their mobile phones, suggesting that an app or file containing relevant data would be useful to incorporate in discharge information.
The aetiology of a subset of classical STEMIs (particularly in younger patients) may be due to plaque erosion rather than plaque rupture and theoretically following thrombectomy such patients might not require culprit vessel stenting. The EROSION III trial (OCT- vs Angio-based Reperfusion Strategy for STEMI) randomised 246 patients with STEMI, after initial angiography and thrombectomy if required, to optical coherence tomography (OCT) guidance with subsequent mechanism-based management (n = 112) vs. standard care based on angiography alone (n = 114) [6]. OCT guidance was associated with a reduction in the primary efficacy endpoint of need for stent implantation (43.8% vs. 58.8%; p = 0.024). For those deemed as having plaque erosion (29%), OCT guidance was associated with a marked increase in those being managed conservatively (86% vs. 14%). This interesting study has conceptual merit but requires a larger study powered for MACE endpoints before such an approach can be definitively recommended.
Physiological Assessment of Coronary Artery Lesions
Complete revascularisation has been found superior to culprit lesion only PCI in STEMI, with increasing evidence for multivessel PCI (either acutely, during initial hospitalisation or within 45 days [7], but it is unclear if guiding the complete revascularisation by fractional flow reserve (FFR) is superior to angiography-only guidance. In the multicentre FLOWER-MI (FLOW Evaluation to Guide Revascularization in Multi-Vessel ST-Elevation Myocardial Infarction) study, 1171 patients with STEMI, with successful PCI to the culprit artery, were randomised to FFR (n = 590) vs. angiography only (n = 581) guided complete revascularisation [8]. The primary outcome of death, myocardial infarction (MI) and unplanned hospitalization leading to urgent revascularisations at 1 year was not found to be significantly different for FFR vs. angiography-only guidance (5.5 vs. 4.2%; hazard ratio HR 1.32 [95% confidence interval (CI) 0.78–2.23]; p = 0.31), although given the wide confidence intervals, the findings are not conclusive. In a sub-analysis, those with at least one PCI had lower event rates at 1 year, compared with patients with deferred PCI, suggesting that deferring lesions judged relevant by visual estimation but with FFR > 0.80 might not be optimal in this context, but future randomised studies are needed to confirm this [9]. Curzen et al. showed in the RIPCORD study that when FFR data was added systematically to angiography in patients with chest pain, the management plan changed in 26% of cases [10]. In RIPCORD2, 1100 patients were randomised to systematic FFR after angiography vs. angiography alone [11]. The co-primary outcomes assessed at 1 year were total hospital costs and quality of life/angina status. Systematic FFR was not associated with any difference in median total hospital costs (£4510 vs. £4136; p = 0.137), inpatient costs, outpatient costs, nights in hospital, outpatient visits, quality of life or angina status at 1 year. This suggests that while targeted FFR clearly remains valuable, systematic FFR in all vessels is unnecessary. Similarly, the FUTURE (FUnctional Testing Underlying coronary REvascularization) trial, which randomised 927 patients with multivessel disease to systematic FFR guidance versus a conventional approach, was stopped early because of futility with no significant difference in ischaemic cardiovascular (CV) events or death at 1-year follow-up [12].
The previous FAME trials have shown the benefits of FFR vs. angiography alone or vs. medical therapy in patients with coronary artery disease (CAD). The goal of the FAME 3 (Comparison of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention and Coronary Artery Bypass Graft Surgery in Patients With Multivessel Coronary Artery Disease) randomised trial (n = 1500) was to demonstrate non-inferiority of FFR-guided PCI vs. coronary artery bypass grafting (CABG) for patients with three-vessel disease (excluding those with left main disease and those not suitable for one or other treatment arm) [13]. However, in the overall cohort, FFR-guided PCI failed to achieve non-inferiority for the primary composite endpoint at 1 year (death, MI, stroke, or repeat revascularisation) (10.6% vs. 6.9%; HR 1.5, 95% CI 1.1–2.2; p = 0.35 for non-inferiority). Of note, the subgroup of patients with less-complex disease (SYNTAX score < 23) fared better with PCI than with CABG surgery (5.5% vs. 8.6%) but this subgroup analysis is hypothesis-generating only. FAME 3 was thus a somewhat disappointing trial from an interventionist perspective, especially as the benefits of CABG may increase over the medium term and supports current revascularisation guideline positions.
Previous data from the iFR-SWEDEHEART trial showed PCI outcomes guided by instantaneous wave-free ratio (iFR) were non-inferior vs. FFR for the primary endpoint at 1 year of MACE (death, MI, unplanned revascularisation). New 5-year follow-up data presented at TCT [14] were reassuring showing MACE rates remained similar for iFR vs. FFR (21.5% vs. 19.9%; HR 1.09, 95% CI 0.90–1.33), as were rates of death, MI and unplanned revascularisation as individual endpoints, supporting both iFR and FFR as suitable physiological tools, with iFR having the advantage of being quicker to undertake.
Another physiological measurement, quantitative flow ratio (QFR), was evaluated in the FAVOR III trial (Comparison of Quantitative Flow Ratio Guided and Angiography Guided Percutaneous InterVention in Patients With cORonary Artery Disease), which randomised 3847 patients with stable or unstable CAD, enrolled at 26 hospitals in China, to a QFR vs. angiography-guided PCI strategy [15]. The primary composite endpoint was of all-cause death, MI or ischaemia-driven revascularisation. At 1 year, the QFR-guided strategy was associated with a reduction in the primary endpoint of death, MI or ischaemia-driven revascularisation (5.8% vs. 8.8%; HR 0.65 [95% CI 0.51–0.83]; p = 0.0004), driven by fewer MIs and ischaemia-driven revascularisations. Interestingly, similar to findings of the RIPCORD study with FFR [10], use of QFR physiology changed management in 25% patients, demonstrating a possible role for implementation in daily practice.
Revascularisation in Multivessel Coronary Artery Disease
Given the paucity of contemporary trial data for patients with complex CAD deemed ineligible for CABG who undergo PCI, the OPTIMUM (Outcomes of Surgically Ineligible Patients with Multivessel CAD) registry [16] prospectively enrolled 6726 such patients with three-vessel CAD or left main stem (LMS) disease following their heart team discussion. At 30 days, the observed rate of death was 5.6%, which was in line with the predicted risk of death using the EuroSCORE II and Society of Thoracic Surgeons (STS) risk calculators (5.7% and 5.3%, respectively). In those who survived to 6 months (87.7%) over 82% had no angina vs. 40.5% at baseline. While the mortality rate at 6 months reflects the high-risk nature of the population, for survivors, the OPTIMUM registry confirms quality-of-life benefits from PCI.
The relative merits of left main revascularisation of LMS disease by PCI vs. CABG remain controversial. In a meta-analysis of four large randomised controlled trials (RCTs) (SYNTAX, NOBLE, EXCEL and PRECOMBAT) [17], studying 4394 patients with a median SYNTAX score of 25, PCI was associated with a similar rate of 5-year death (11.2% vs. 10.2%; HR 1.10, 95% CI 0.91–1.32; p = 0.33), but also a higher rate of spontaneous MI and repeat revascularisation. The authors reiterated the importance of a heart team approach to optimise individual patient outcomes.
With an ageing population, complex intravascular calcification (with associated procedural complexity) is increasingly encountered. The DISRUPT CAD III (Global IDE Study of the Shockwave Coronary Intravascular Lithotripsy (IVL) System) international multicentre registry evaluated use of intravascular lithotripsy in 431 patients [18]. At 1 year, the primary safety endpoint of MACE (cardiac death, all-cause MI or target vessel revascularisation) was 13.8%, with a target lesion failure (TLF) rate of 11.9% (driven by periprocedural MI of 9.2%). There were no Q-wave MIs beyond 30 days. While encouraging, prospective trial data vs. conventional lesion preparation is still required to confirm safety, relative efficacy and cost-effectiveness.
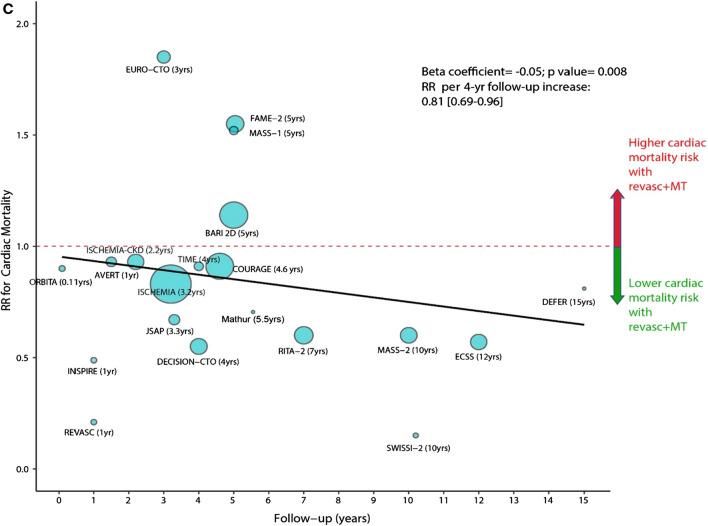
The value of PCI plus medical therapy vs. medical therapy alone for stable CAD continues to be debated. In an extensive meta-analysis of 25 trials involving 19,806 patients, a PCI strategy was associated with a lower risk of cardiac death (relative risk (RR) 0.79; p < 0.01) and spontaneous MI (RR 0.74; p < 0.01) but no significant difference in all-cause mortality (RR 0.94; p = 0.11) (Fig. 2) [19]. While the findings were encouraging, conclusions remain guarded since the meta-analysis included studies dating back to 1979 (when medical therapy was less than optimal).
Fig. 2.
Figure demonstrating meta-regression of rate ratios for cardiac mortality with revascularisation plus medical therapy vs. medical therapy alone in relation to follow-up duration. The size of the data markers is proportional to the size of trial. Rate ratios lower than 1 indicate cardiac death reduction with revascularisation. The solid line represents the meta-regression slope of the change in cardiac death rate ratio for revascularisation plus medical therapy vs. medical therapy alone with increasing length of follow-up. Reproduced with permission from the European Heart Journal (Naverese et al. [19])
Emergency CABG in acute MI carries higher risk but contemporary data are limited. In the United States National Inpatient Sample, of 11,622,528 admissions with acute MI between 2000 and 2017, 9.2% were treated by emergency CABG [20]. Use of emergency CABG fell significantly from 2000 to 2017 for all MI (10.5% to 8.7%), whether STEMI (10.2% to 5.2%) or non-STEMI (10.8% to 10.0%) (p < 0.001 for all). Surgery was more likely in patients who were aged less than 75 years, white, male and treated at large or urban teaching hospitals. Those who underwent CABG in more recent years (2012–2017 vs. 2000–2011) were more likely to have NSTEMI (80.5% vs. 56.1%), non-cardiac multi-organ failure (26.1% vs. 8.4%), cardiogenic shock (11.5% vs. 6.4%) or mechanical circulatory support (19.8% vs. 18.7%) (p < 0.001 for all). Despite the patients being sicker, in-hospital mortality for CABG-treated acute MI has decreased from 5.3% in 2000 to 3.6% in 2017 (adjusted odds ratio (OR) 0.89, 95% CI 0.88–0.89), suggesting good case selection.
It is generally accepted that around 50% of vein grafts fail within 10 years of CABG. To address this, Puskas investigated whether a venous external support (VEST) device made with a braided weave of cobalt-chromium applied over the vein grafts to provide permanent reinforcement could reduce intimal hyperplasia, which may be one of the mechanisms for early graft failure [21]. A total of 224 patients undergoing CABG with two vein grafts were enrolled, each patient having one vein graft randomised to VEST device support and one vein graft serving as a control. It was anticipated early graft failure at 1 year would occur in 13%, but in fact 42% of vein grafts had occluded. Only 113 patients were able to undergo intravascular ultrasound (IVUS) of both grafts and, among these, VEST support was not associated with any reduction in the primary endpoint of intimal hyperplasia area (mean 5.11 vs. 5.79 mm2; p = 0.072), although conclusions were confounded by the high early graft failure rate.
Advances in Stent Technology
The use of ultrathin strut biodegradable-polymer sirolimus-eluting stent (BP-SES) in STEMI was investigated in the BIOSTEMI trial [22] (Biodegradable Polymer Sirolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents in Patients With STEMI) which randomised 1300 patients to BP-SES vs. a durable polymer everolimus-eluting stent (DP-EES). The primary endpoint was target lesion failure (TLF), a composite of cardiac death, target vessel MI and clinically indicated target lesion revascularisation (TLR). Use of BP-SES was associated with significant reduction in TLF at 2 years (5.1% vs. 8.1%; 95% Bayesian credible interval 0.4–0.84; posterior probability of superiority = 0.998), although there were no significant differences in single endpoints of cardiac death, target vessel MI or definite stent thrombosis.
The mechanism of TLF reduction remains unclear. While thinner struts are associated with improved clinical outcomes in bare metal stents (BMS), reducing strut thickness may affect drug delivery from drug-eluting stents (DES). In the multicentre, single-blinded, non-inferiority CASTLE trial which randomised 1440 patients to imaging-guided PCI with BP-SES vs. DP-SES [23] did not find a significant difference in TLF between BP-SES vs. DP-EES at 12-month interim analysis (HR 0.59 [95% CI 0.26–1.36]), although full results are awaited. However, in a systematic review by Madhavan et al. [24] of 16 trials, including 20,701 patients, ultrathin-strut DES vs. conventional second-generation thin-strut DES were associated with a 15% reduction in long-term TLF at a weighted mean of 2.5 years follow-up (RR 0.85, 95% CI 0.76–0.96; p = 0.008).
Nevertheless, to optimally evaluate the potential benefit of thinner struts in DES, it is desirable to compare otherwise similar DES with respect to stent design, polymer and drug eluted. Menown et al. undertook a pre-specified comparison of 400 patients receiving at least one thin strut (84–88 µm) cobalt chromium, biodegradable polymer, Biolimus A9-eluting stents (CoCr-BP-BES) in the prospective Biomatrix Alpha registry vs. 857 patients who received at least one Biomatrix Flex stainless steel biodegradable polymer Biolimus A9-eluting stents (SS-BP-BES) in the LEADERS study (historical control) [25]. The primary endpoint was MACE (cardiac death, MI or clinically driven target vessel revascularisation (cd-TVR)). At 2 years, the thinner strut CoCr-BP-BES was associated with a reduction in MACE (6.65% vs. 13.23%; unadjusted HR 0.48 [0.31–0.73]; p = 0.0005) which remained significant after propensity analysis (7.4% vs. 13.3%; HR 0.53 [0.35–0.79]; p = 0.004) and a reduction in definite or probable stent thrombosis (1.12% vs. 3.22%; adjusted HR 0.32 [0.11–0.9]; p = 0.034) (Fig. 3). After landmark analysis at day 3 to account for differences in periprocedural MI definitions, the reduction in 2-year MACE no longer reached significance but there was still a significant reduction in the patient-orientated composite endpoint (11.7% vs. 18.4%; HR 0.6 [0.43–0.83]; p = 0.006) and a trend to lower target vessel failure (5.8% vs. 9.1%; HR 0.63 [0.4–1.00]; p = 0.078). The study shows use of thinner struts is of overall clinical benefit in DES.
Fig. 3.
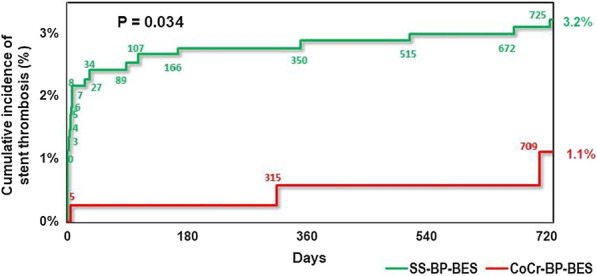
Figure demonstrating definite or probable stent thrombosis at 2 years for Biomatrix Alpha vs. LEA[1]DERS with propensity-adjustment. Stent thrombosis was adjudicated using identical criteria in both studies. CoCr-BP-BES cobalt chromium biodegradable polymer Biolimus A9-eluting stent, MACE major cardiac adverse events, SS-BP-BES stainless steel biodegradable polymer Biolimus A9-eluting stent (Menown et al. [25])
Direct stenting may be advantageous in certain scenarios. In the OPTIMIZE trial (OPtical Coherence Tomography (OCT) Compared to Intravascular Ultrasound (IVUS) and Angiography to Guide Coronary Stent Implantation), Rao randomised 1639 patients to the Svelte DES and Slender integrated delivery system (Svelte Medical Systems) vs. conventional EE-DES [26]. At 1 year, the Svelte system showed an excess of periprocedural troponin elevation, but at 2 years, using a stricter, more clinically relevant definition of periprocedural MI, showed non-inferiority vs. conventional EE-DES (2-year target vessel MI 10.23% vs. 8.81%; p = 0.48). This highlights the emerging uncertainty as to the clinical relevance of minor elevations in high-sensitive troponin alone in clinical trials.
Findings of the ReCr8 trial previously reported similar rates of TLF at 12 months in an all-comers population (n = 1491) randomised to the polymer-free Cre8 stent (Alvimedica) vs. Resolute Integrity ZES (Medtronic). The 1–3-year analysis of this trial [27] continued to report similar rates of TLF (4.9% vs. 5.1%; p for noninferiority = 0.0031), confirming an acceptable safety profile although not defining whether the Cre8 stent should be used preferentially in certain clinical settings.
Amphilimus-eluting stents, which were found to be non-inferior to zotarolimus-eluting stents in the ASTUTE and INSPIRE-1 trials may be of particular benefit in patients with diabetes owing to their ability to enable higher drug diffusion across the vessel wall. In the SUGAR trial, 1175 patients with diabetes and CAD were randomised to an amphilimus-eluting stent (Cre8 EVO) vs. conventional Resolute Onyx stent [28]. The Cre8 EVO stent was associated with a 35% reduction in the primary endpoint of TLF at 1 year (7.2% vs. 10.9%; HR 0.65 [95% CI 0.44–0.96]; p noninferiority < 0.001; p superiority = 0.030). The 2-year results are eagerly awaited.
Bioresorbable scaffolds have had a troubled past, most notably the Absorb device, which was discontinued because of safety concerns, including in COMPARE-ABSORB (ABSORB Bioresorbable Scaffold vs. Xience Metallic Stent for Prevention of Restenosis in Patients at High Risk of Restenosis), a twofold increased risk of device-oriented adverse events, such as TV-MI and late scaffold thrombosis [29]. However, of interest, at 3 years TLF rates were similar between Absorb and Xience (8.9% vs. 7.4%; HR 1.21, 95% CI 0.86–1.70); thus further follow-up to 7 years is planned to see if there are any late benefits with scaffolds.
Thinner strut second-generation scaffolds may also hold promise. In the small prospective multicentre FUTURE-II trial (Firesorb Sirolimus Target Eluting Bioresorbable Vascular Scaffold in Patients With Coronary Artery Disease), 430 patients undergoing PCI to low-risk lesions were randomised to Firesorb or Xience with no significant difference in patient-level in-segment late loss at 1 year [30]. Given these encouraging findings, the FUTURE-III trial (n = 1200 single-arm study to define TLF) has commenced.
The Fantom second-generation sirolimus-eluting bioresorbable scaffold with relatively thin struts (125 µm) was studied in the FANTOM II registry of 240 patients with stable CAD and a single lesion of length 20 mm or less and diameter between 2.5 and 3.5 mm [31]. At 5 years the composite endpoint of CV death, MI and clinically driven TLR occurred in 5.8% of patients which again is an encouraging finding. Four small, randomised trials were presented at TCT 2021 evaluating use of limus drugs (which may be less inflammatory) vs. paclitaxel for drug-coated balloons (DCBs). Three trials compared the sirolimus-coated SeQuent SCT (B. Braun) vs. the paclitaxel-coated SeQuent Please Neo (B. Braun). The first trial randomised 70 patients with coronary de novo lesions, treated at one of six centres in Malaysia [32]. The primary outcome of late lumen loss at 6 months for sirolimus vs. paclitaxel groups met non-inferiority (0.10 ± 0.32 mm vs. 0.01 ± 0.33 mm). Notably, late lumen enlargement was less frequent with the sirolimus device (32% vs. 58%; p = 0.019). Two further trials with identical protocols (FIM Malaysian and German-Swiss) [33] randomised 101 patients with in-stent restenosis. The primary outcome of in-lesion late lumen loss at 6 months was identical (0.3 mm vs 0.3 mm, 95% CI −0.24 to 0.24, p = ns). Whilst these three studies were informative, they were not powered sufficiently to detect clinical differences and longer-term data would be of interest. In the BIO-RISE CHINA trial, a Biolimus A9 DCB (10 times the lipophilicity of sirolimus) was compared vs. plain old balloon angioplasty (POBA) in patients with small-vessel CAD. The Biolimus A9 DCB was associated with reduction in the primary endpoint of in-segment late lumen loss at 9 months [34] (0.17 ± 0.32 mm vs. 0.29 ± 0.35 mm; p = 0.0034). Given this encouraging result, the ongoing REFORM trial is comparing the Biolimus A9 DCB vs. paclitaxel-coated SeQuent Please for in-stent restenosis.
Vascular Access
The STAT2 multicentre trial randomised 443 patients undergoing radial compression post PCI to a trans-radial (TR) band plus haemostatic patch (Statseal) vs. a TR band alone [35]. In both arms, the TR band deflation was attempted after 60 min. Use of Statseal along with the TR band was associated with a marked reduction in the primary endpoint of time until successful haemostasis (66 vs. 113 min; p < 0.001). Complications (including bleeding requiring intervention, haematoma, or radial artery occlusion) were numerically fewer in the StatSeal arm than with the TR band alone, but this did not meet significance (4.5% vs. 8.6%; p = 0.08). Use of the Statseal may thus help to shorten length of stay and allow earlier discharge.
The COLOR trial (Complex Large-Bore Radial Percutaneous Coronary Intervention) randomised 388 patients undergoing PCI in complex coronary lesions with large-bore guiding catheters to transradial (Tr) vs. transfemoral (TF) [36]. Tr access was associated with a significantly reduction in the primary endpoint of access site-related clinically significant bleeding or vascular complications requiring intervention at discharge (3.6% vs. 19.1%; p < 0.001) without any loss in rates of procedural success (86% vs. 89.2%; p = 0.285).
Advances in Structural Cardiology
Transcatheter Aortic Valve Intervention
Data from UK TAVI and PARTNER 2A (Placement of aortic transcatheter valve trial) have consolidated the role of transcatheter aortic valve implantation (TAVI) in intermediate to high-risk aortic stenosis (AS) with early evidence from trials such as PARTNER 3 and EVOLUT Low risk (Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients) also suggesting a role in low-risk patients (STS PROM score ≤ 3%) [1]. Two-year data from the EVOLUT Low risk trial [37] reported no difference in the primary outcome of death or disabling stroke at 24 months (1.9% in TAVI vs. 2.1% in surgical aortic valve replacement (SAVR); p = 0.742). Follow-up to 10 years is planned for each trial.
Five-year data from the SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trial which compared self-expanding CoreValve TAVI vs. SAVR in intermediate-risk patients (n = 1745, mean age 80 years; median STS-PROM 4.5%) [38] reported non-inferiority for the endpoint of death or stroke (31.3% vs. 30.8%; p = 0.85) and no difference in valve thrombosis, although higher re-intervention (3.5% vs. 1.9%; p = 0.02), more paravalvular leak (3.0% vs. 0.7%; p < 0.001), smaller effective orifice areas (EOA) (1.8 vs. 2.2 cm2; p < 0.001) and higher gradient gradients (11.2 vs. 8.6 mmHg; p < 0.001). Notably, only a small portion (16%) received the second-generation CoreValve Evolut R valve which may have underestimated TAVI performance.
Current guidance for asymptomatic severe AS with preserved left ventricular (LV) function mandates watchful waiting prior to intervening; however, trials such as RECOVERY have suggested that earlier intervention may translate to lower rates of CV death. AVATAR (Aortic Valve ReplAcemenT vs Conservative Treatment in Asymptomatic SeveRe Aortic Stenosis) was a multicentre trial randomising 157 patients (mean age 67 years, 43% female) to early surgery (n = 78) vs. conservative therapy (n = 79). Patients with a positive exercise test were excluded in addition to those with very high gradients (> 5.5 m/s), impaired left ventricular ejection fraction (LVEF), previous CABG or prior valve surgery. At 32 months, the trial met its composite primary outcome of reduction in all-cause death, heart failure, acute MI or stroke vs. conservative management (15.2% vs. 34.7%; p = 0.02) [39]. This signals that early intervention in asymptomatic severe AS may confer a mortality benefit. Further data from EARLY TAVR will help shed light on whether this mortality benefit applies to the transcatheter group.
Of increasing relevance in modern healthcare is the economic cost vs. benefit of new procedures. In a sub-analysis of the PARTNER 3 low-risk data set, Cohen performed a cost analysis of 1000 patients undergoing TAVI with the SAPIEN 3 device [40]. Although TAVI resulted in shorter procedure duration (mean 59 vs. 208 min), hospitalisation (mean 1.9 vs. 6.5 days) and intensive care unit (ICU) time (mean 0.8 vs. 2.7 days), the overall expense of the initial hospitalization costs for both TAVI and surgery were comparable ($47,196 vs. $46,606; p = 0.59), largely driven by the TAVR device costs. However, at 2 years there was a notably lower cost in the TAVR group, driven by reduction in follow-up costs vs. SAVR ($19,638 vs. $22,258; p = 0.13). As more devices enter the market and costs fall as a result of competition, it is possible this cost divergence will grow.
Continued efforts to streamline TAVI to reduce costs have resulted in dedicated next-day discharge pathways such as the Vancouver 3M (Multidisciplinary, Multimodality, but Minimalist) pathway [41]. In 3M TAVR (Multidisciplinary, Multimodality but Minimalist Transfemoral Transcatheter Aortic Valve Replacement), the safety and efficacy of this pathway was assessed in a multicentre propensity-matched study of 351 patients who underwent transfemoral TAVI as part of the S3i registry embedded in the PARTNER 2A trial. After matching, mean patient age was 82 years and the mean STS score (5.2–5.3). The 3M group spent less time in hospital (1.6 vs. 3.9 days; p < 0.001) with significantly lower overall hospitalization costs ($45,595 vs. $56,438; p < 0.001). Furthermore, there was no difference in clinical outcomes including mortality, stroke, MI or need for repeat procedures (all p = ns).
Indeed, with the increasing robustness in data for TAVI, determining patient selection vs. surgery has become increasingly contentious. Notably, the updated 2021 European Society Cardiology (ESC) guidelines released this year have recommended that patients over the age of 75 years are offered TAVI in preference to SAVR. Furthermore, a new class IIb recommendation for intervention in patients with asymptomatic severe AS with LV dysfunction (without another cause) was added to the guidelines [42]. Rewording of several recommendations from ‘SAVR should be considered’ to ‘Intervention should be considered’ has placed more emphasis on shared decision-making and reflects greater equipoise between SAVR and TAVI.
A significant number of patients undergoing TAVI have concomitant atrial fibrillation (AF). Following the results of the PoPular TAVI trial, ESC guidelines have changed to recommend oral anticoagulation (OAC) without the addition of an antiplatelet in patients with an underlying indication for anticoagulation [42]. Consequently, ENVISAGE-TAVI AF (Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation) sought to establish the non-inferiority of edoxaban compared to vitamin K antagonism (VKA) in patients with AF undergoing TAVI [43]. Edoxaban met non-inferiority for the primary efficacy outcome (HR 1.05, 95% CI 0.85–1.31; p = 0.01 for non-inferiority); however, edoxaban did not meet non-inferiority for the primary safety outcome of bleeding (HR 1.40, 95% CI 1.03–1.91; p = 0.93 for non-inferiority). The higher bleeding rates were driven by gastrointestinal (GI) bleeding which may be reflective of the higher mean patient age of 82.1 years. Similarly, the ALANTIS trial (Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis) sought to evaluate apixaban vs. standard of care in TAVI (VKA if indication for OAC; antiplatelet therapy if no indication), enrolling 1500 patients with a mean age of 82 years [44]. Apixaban failed to demonstrate a reduction in the composite primary endpoint (time to death, stroke, MI, systemic emboli, intracardiac or valve thrombosis, DVT, PE or major bleeding) vs. standard care (18.4 vs. 20.1%; HR 0.92, 95% CI 0.73–1.16). There were lower rates of valve thrombosis noted in the apixaban group (8.9% vs. 13.0%, p = 0.038); however, this did not translate into improved clinical outcomes. Interestingly, unlike in ENVISAGE-TAVI AF, this provides a signal that apixaban may be a safe, suitable (and more practical) alternative to VKAs in this patient group.
The risk of ongoing valve degeneration and durability was further assessed in the FAABULOUS 2 trial (18F-Fluoride Assessment of Aortic Bioprosthesis Durability and Outcome) [45]. This multicentre cross-sectional observational cohort study analysed 47 patients undergoing TAVI using echocardiography, computed tomography (CT) angiography, and 18F-NaF Positron emission-tomography (PET) scanning. All patients had repeat echocardiography at 1 month, 2 and 5 years with PET/CT scanning repeated at either 1 month (n = 9), 2 years (n = 22), or 5 years (n = 16). Matched comparisons were made to patients undergoing bioprosthetic SAVR (n = 57) using the same protocol. Rates of bioprosthetic valve degeneration were similar between groups across modalities; echocardiography (6% vs. 8% respectively; p = 0.78), CT (15% vs. 14% respectively; p = 0.87) and PET (15% vs. 29% respectively; p = 0.09). This interesting data suggests that mid-range durability is similar between TAVI and bioprosthetic SAVR; however, one must note that numbers were small and non-randomised.
Bicuspid valve AS has often been excluded from many of the main TAVI trials with long-term safety and efficacy being unclear. The Low-Risk Bicuspid Study has previously demonstrated low rate of all-cause mortality or disabling stroke (1.3%) at 30 days after TAVI with an Evolut R or PRO prosthesis [1]. Forrest presented 1-year outcomes of TAVI in bicuspid vs. tricuspid patients obtained using a propensity-matched analysis, pairing 145 patients to the TAVI arm of the Evolut Low-Risk Trial [46]. There was no difference in the combined endpoint of death, disabling stroke or major bleeding at 1 year (1.4% vs. 2.8%; p = ns). Furthermore, rates of pacemaker implantation (16.6% vs. 17.9%; p = ns) and rehospitalisation (3.5% vs. 4.9%; p = ns) were similar between groups. Similarly, Williams presented a sub-analysis of the PARTNER 3 trial, assessing the 1-year safety and efficacy of the SAPIEN 3™ valve in low-risk bicuspid patients [47]. Patients pooled from bicuspid registries were propensity matched to 148 patients from the tricuspid arm of the PARTNER 3 trial. At 1 year, the rates of combined endpoint of death, stroke or hospitalisation were similar between the two groups (bicuspid 10.9% vs. tricuspid 10.2%, log-rank p = 0.8). Although the 1-year data are favourable in these trials, longer-term outcomes are needed given the relatively younger mean age of this patient group.
Valve in valve (Viv) TAVI has become increasingly utilised since device approval in 2015. However, uncertainty remains as to whether bioprosthetic valve fracture (BVF) is necessary in these patients. Brinkmann et al. conducted a randomised multicentre trial of 160 patients undergoing ViV TAVI with (n = 81) and without BVF (n = 79). Devices used included Mosaic (Medtronic), Mitroflow (Sorin Group USA), Perimount, and Magna (Edwards Lifesciences). ViV TAVI with BVF had higher success rates (93% vs. 68%; p < 0.001) and a higher reduction in mean transvalvular gradient (10.8 vs. 15.8 mmHg; p < 0.001). The rates of in-hospital events were similar between groups (3.7% vs. 7.6%; p = 0.325) [48]. Although this data suggests BVF VIV TAVI has superior outcomes, the optimal timing of BVF is unclear and indeed is still a point of contention amongst operators.
Coronary artery occlusion is a rare (0.7%) but serious complication of TAVI. Transcatheter electrosurgical aortic leaflet laceration as a means of mitigating the risk of this complication was originally assessed in the novel BASILICA investigational device exemption (IDE) trial. Real-world registry data obtained form 25 international centres reported an 86.9% rate of procedure success (defined as successful BASILICA traversal and laceration without mortality, coronary obstruction or emergency intervention) [49]. Thirty-day mortality was 2.8% and stroke was 2.8%, with 0.5% disabling stroke with 1-year survival 83.9%. Although this data suggests more procedural expertise is needed, the increasing rate of valve-in-valve procedures and the need to implant higher to avoid pacemaker implantation highlights the possible future utility of this procedure.
Safe access site closure is a central component to reducing complications in TAVI. Various devices exist to facilitate femoral site closure including suture-based technologies (ProGlide; Abbott Vascular) and plug-based vascular closure device (Manta; Teleflex). CHOICE-CLOSURE (Comparison of Catheter-based Strategies for Interventional Access Site Closure During Transfemoral Transcatheter Aortic Valve Implantation) randomised 516 patients (mean age 80.5 years; 55.4% men) to undergo TAVI with vascular site closure with MANTA VCD (n = 258) or ProGlide (n = 258) [50]. Baseline characteristics, including surgical risk, were similar between groups. Major and minor access site complications were higher in the MANTA group (19.4% vs. 12.0%; RR 1.61, 95% CI 1.07–2.44; p = 0.029) vs. ProGlide. Furthermore, the MANTA group required longer time to haemostasis (240 s vs. 80 s; p < 0.001) and required greater use of an additional VCD to achieve complete haemostasis (58.5% vs. 0.0%; p < 0.001), suggesting that suture-based closure with the Proglide device was safer and superior at achieving haemostasis.
Transcatheter Mitral Valve Interventions
The landmark COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) previously demonstrated that transcatheter mitral valve (TCMV) repair with the Abbot MitraClip in moderate-to-severe or severe secondary mitral regurgitation (MR) refractory to medical therapy was superior to medical therapy alone [1]. The single-arm CLASP (Edwards PASCAL TrAnScatheter Mitral Valve RePair System Study) feasibility study sought to assess the safety and efficacy of the Edwards PASCAL™ transcatheter valve repair system in severe symptomatic MR (functional and degenerative). Building on the positive 1-year data (92% survival and 88% free from heart failure hospitalisation), the 2-year outcomes from CLASP2 reported an 80% survival rate with 84% free from heart failure hospitalisations [51]. Optimal device strategy remains unclear and the results of the head-to-head trial between the Abbot and Edwards devices are awaited in the CLASP IID/IIF trial.
There are now several feasibility studies endorsing the role of mitral valve transcatheter edge-to-edge repair (TCEER); however, real-world data on procedural and device failure requiring surgical intervention is limited. The CUTTING-EDGE (Mitral Valve Surgery After Transcatheter Edge-to-Edge Repair) registry was a multicentre, international registry analysis of 332 patients (mean age 73.8 years) which looked at outcomes of timing of surgery post TCEER failure [52]. Across three groups, aborted TCEER with subsequent surgery (21.2%), completed TCEER with acute surgery (17.6%) or completed TCEER with delayed surgery (61.2%), respective 30-day mortality rates were 26.6%, 15.8% and 13.8%. The majority (91%) required mitral valve replacement. Interestingly, 51.3% of patients were low or intermediate surgical risk (median STS PROM score was 4.0%). This data supports other studies which demonstrate that mortality post failed TCEER is extremely high. This suggests that TCEER should only be offered to patients at high surgical risk with informed decision-making between the patient and multidisciplinary teams.
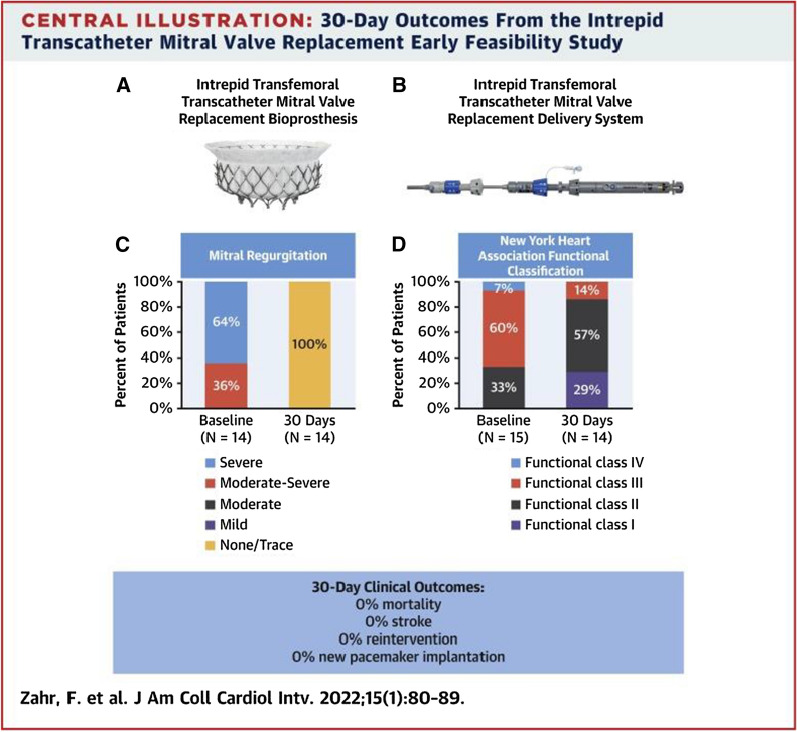
Recent studies have examined the feasibility of TCMV replacement. The Intrepid transfemoral transeptal transcatheter mitral valve replacement (TTMVR) trial was a prospective multicentre feasibility study (n = 15; median age 80 years) of the Intrepid™ TTMVR system (Medtronic) in patients with moderate-severe/severe, symptomatic MR at high surgical risk (median STS PROM 4.7%). Fourteen implants were successful with no/trace MR post procedure. One required conversion to sternotomy [53]. Overall, there were no deaths, strokes or re-interventions at 30 days (Fig. 4). Although favourable, more data are needed with longer follow-up to validate this new technology.
Fig. 4.
Central illustration from Zahr et al.’s paper demonstrating 30-day outcomes from the intrepid transcatheter mitral valve replacement early feasability study. A Image of the Intrepid transfemoral transcatheter replacement bioprothesis. B Delivery system. C Summary of 30-day results demonstrating improvement in mitral regurgitation. D Improvement in NYHA score. Reproduced with permission from the Journal of the American College Cardiology (Zahr et al. [53])
Patients undergoing mitral valve (MV) surgery often have concomitant severe tricuspid regurgitation (TR) which warrants double valve intervention. However, in patients with a dilated tricuspid valve (TV) annulus but only moderate (or less) regurgitation, the rationale for intervention is less clear. Gammie et al. conducted an international multicentre randomised study of patients with degenerative MV disease (n = 401; average age 67 years) undergoing surgery to receive either tricuspid annuloplasty (TA) or no additional procedure [54]. All patients had tricuspid annular dilatation of at least 40 mm but with moderate regurgitation or better. At 2 years the composite primary endpoint of reoperation, progression of TR by two grades or death, was significantly lower in the surgery plus TA group (3.9% vs. 10.2%; RR 0.37, 95% CI 0.16–0.86; p = 0.02). Mortality was significantly lower in the TA group (3.2% vs. 4.5%; RR 0.69, 95% CI 0.25–1.88) but pacemaker implantation rates were significantly higher (14.1% vs. 2.5%; 95% CI 2.27–14.60). Longer-term follow-up data are required as it remains unclear whether reduction in TR translates into tangible clinical benefit.
Transcatheter Tricuspid and Pulmonary Interventions
Several trials addressed novel approaches to transcatheter tricuspid valve (TCTV) intervention including valve replacement, tricuspid edge-to-edge repair (TEER) and annuloplasty ring repair. The Triband study (Transcatheter Repair of Tricuspid Regurgitation With Edwards Cardioband TR System Post Market Study) evaluated the Cardioband TV reconstruction system (Edwards Lifesciences, Irvine, CA, USA) which aims to reduce functional annular dilatation in severe functional TR and thereby facilitate better leaflet coaptation. This single-arm, multicentre prospective study enrolled 61 patients with severe functional TR despite best medical treatment. All-cause mortality and major adverse events (MAE) rates at 30 days were 1.6% and 19.7%, respectively, with 85% of patients achieving one grade reduction in TR (p < 0.001) and 69% achieving a TR grade of moderate or below at 30 days (p < 0.001). This was reflected in a 17-point reduction in Kansas City Cardiomyopathy Questionnaire (KCCQ) score (p < 0.001) [55].
Similarly, the TriClip transcatheter tricuspid valve repair (Abbott) was evaluated in TRILUMMINATE (Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater TR). This was a prospective single-arm international study of 85 patients (mean age 78 ± 7.9 years; average EuroSCORE I of 8.7 ± 10.7%) with severe TR and no other indication for valve intervention [56]. Of the 85 patients enrolled, at 1 year, TR improved to moderate or less in 71% vs. 8% at baseline (p < 0.0001) which was associated with significant functional benefits in 6-min walk test (6MWT) results (272.3 ± 15.6 to 303.2 ± 15.6 m, p = 0.0023). Notably this population were of high surgical risk (average EuroSCORE II of 8.7 ± 10.7%) and many had undergone previous valve interventions (33%). This data demonstrates that TEER produced sustained improvements in TR and clinical outcomes in a high-risk population.
The strategy of TCEER was addressed in CLASP TR, a single-arm, multicentre US early feasibility study evaluating the PASCAL transcatheter valve repair system in 63 patients with symptomatic severe TR despite optimal medical therapy [57]. At 6 months, 89% of patients improved by at least one TR grade and 70% saw at least a two-grade reduction in TR. All-cause death occurred in 3.2% with 2.3% from CV causes. Severe bleeding occurred in five patients (7.9%) with one patient requiring intervention (1.6%). Building on these feasibility studies, the CLASP II TR trial is currently enrolling patients and will seek to compare the PASCAL device vs. standard medical therapy.
Transfemoral tricuspid valve replacement (TTVR) was evaluated in TRISCEND (Investigation of Safety and Clinical Efficacy After Replacement of Tricuspid Valve With Transcatheter Device), a single-arm, multicentre and prospective trial evaluating the safety of feasibility of the EVOQUE TTVR system (Edwards Lifesciences, Irvine, CA, USA) in 56 patients with severe TR (Fig. 5). Notably, the aetiology of TR was mixed with 43% of patients having undergone a prior valve intervention [58]. At 6 months, TR reduced to mild in 49% or trivial levels in the remaining 51% (p < 0.001 vs. baseline). There was a significant improvement in New York Heart Association (NYHA) score from baseline with 89% improving to class I or II at 6 months (p < 0.001). Similarly, KCCQ score improved on average by 27 points (p < 0.001 for all). Survival was also favourable at 96% with 94% remaining free from HF hospitalisation at 6 months [59]. Future data are eagerly awaited for this exciting new mode of transcatheter intervention.
Fig. 5.
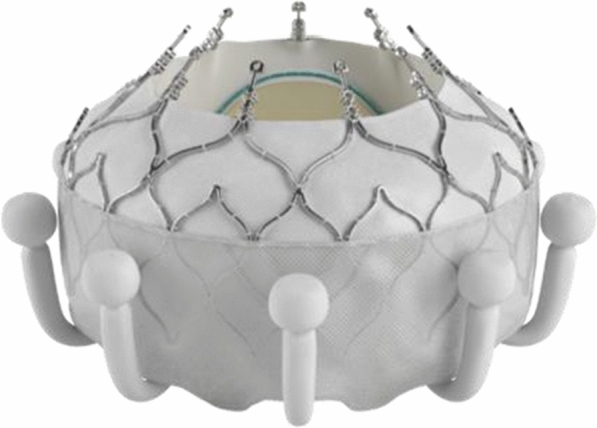
Image of the EVOQUE™ transfemoral tricuspid valve replacement (Edwards Lifesciences LLC, Irvine, CA). Image supplied with permission from Edwards Lifesciences LLC
Patients with significant pulmonary regurgitation (PR) associated with right ventricular (RV) dysfunction often require surgical pulmonary valve replacement (PVR) or repair. Often these are young patients with congenital abnormalities who have undergone prior open-heart surgery. Novel transcatheter options are thus being explored. HARMONY TPV study (The Medtronic Harmony™ Transcatheter Pulmonary Valve Clinical Study) was a prospective safety and feasibility international study of the Harmony valve, a 22-mm valve (TPV22) and a modified version of the original 25 mm mitral valve (mTPV25) in patients (n = 67) with significant PR (Fig. 6). At 1 year, PR was trace or none in all patients with no death, endocarditis, stent fracture or thrombosis noted. Two patients required a further transcatheter procedure. This technology could potentially improve survival in congenital patients for whom further open-heart surgery is not a viable option [60], but longer-term follow data are required.
Fig. 6.
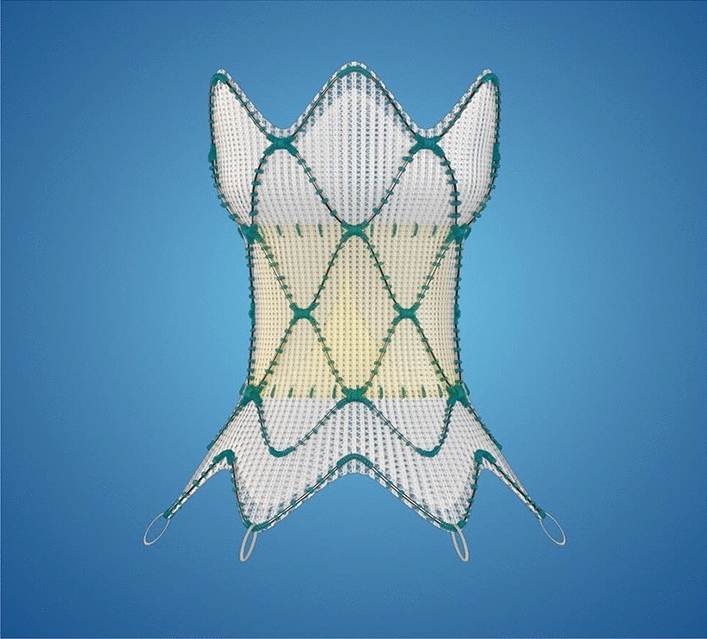
The Medtronic Harmony™ Transcatheter Pulmonary Valve replacement system.
Reproduced with permission from Medtronic, Inc
Catheter-Based Left Atrial Appendage Closure
Following PROTECT AF and PREVAIL, the Watchman left atrial appendage closure (LAA) closure device (Boston Scientific) was approved by the US Food and Drug Administration (FDA) in 2015 as a non-pharmacological alternative for the reduction of stroke risk in non-valvular AF [61]. Several years of registry data has since been collected and the first 3-year (2016–18) outcomes data involving 38,158 patients from the National Cardiovascular Data Registry (NCDR) LAA closure Registry, presented at ACC last year, demonstrated favourable procedural success and complication rates. Price presented an analysis of thromboembolic and bleeding events in this registry data at ACC21 involving 36,681 patients (mean age 76 years; 59% men). Mean CHA2DS2-VASc score was 4.8 (SD 1.5), mean HAS-BLED score was 3.0 (SD 1.1) with 69.5% having experienced prior clinically relevant bleeding. The estimated stroke rate at 1 year was low at 1.53%, demonstrating a much lower rate (77% less) than would be expected in this high-risk population [62]. Furthermore, bleeding rates were 6.2% with an all-cause mortality of 8.52%, reflective of the morbidity of this population group.
Similarly, Prague-17 (Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-Risk Atrial Fibrillation Patients trial) evaluated 402 patients with high-risk non-valvular AF (previous bleeding requiring hospitalisation or treatment; previous cardioembolic event on anticoagulation; and/or CHADSVASC ≥ 3 or HASBLED ≥ 2) by randomising to left atrial appendage closure (LAAC) device or non-warfarin oral anticoagulant (NOAC) therapy [63]. At a median follow-up of 19.9 months, the composite primary outcome (stroke, TIA, systemic embolism, CV death, major or non-major clinically relevant bleeding and procedure/device-related complications) occurred in 10.99% of the LAAC group vs. 13.42% in the NOAC group (HR 0.84, 95% CI 0.53–1.31; p = 0.44; p = 0.004 for noninferiority). In conclusion in high-risk patients with AF, LAAC was non-inferior to NOAC therapy in reducing major outcomes.
LAA closure is indicated for stroke prevention in select patients with AF who cannot tolerate OAC; however, the presence of thrombus in the LAA contraindicates the procedure. Paradoxically the intensification of antithrombotic therapy in this group poses an increased risk of bleeding. The LAPTOP registry (Management and outcomes of patients with left atrial appendage prior to percutaneous closure) was a multicentre retrospective analysis of 121 patients who were noted to have LAA thrombus prior to LAA closure [64]. Of these, 53 patients underwent closure with 68 undergoing antithrombotic intensification. At 18 months, there was no significant difference in the primary endpoint of major adverse events (bleeding, death or stroke) between groups (26.1 vs. 31.5%; HR 1.4 (0.7–2.8); p = 0.365). Notably, device-related thrombus (DRT) occurred in 13% of patients, much higher than in previous trials and conversely rates of bleeding in the medically managed groups were high at 9.6%. This data suggests that direct LAA closure may be a possibility in the presence of thrombus; however, more data are likely needed before this is reflected in current guidance.
Several trials seeking to clarify superiority of varying LAA closure devices have been presented this year. In SWISS-APERO (Comparison of Amplatzer Amulet™ vs Watchman™ Device in Patients Undergoing Left Atrial Appendage Closure), the Amulet IDE device was further compared with new-generation Watchman FLX device [65]. Residual LAA patency was compared between groups using CTCA at 45 days in 221 patients (mean age 77 years, 71% men). Of note in the Watchman arm, 22.7% of patients received the first-generation device and the rest received the Watchman FLX. No difference in LAA patency between groups was noted at 45 days (67.6% vs. 70.0%; risk ratio 0.97, 95% CI 0.80–1.16). Procedure-related complications were higher with the Amulet device (9.0% vs. 2.7%; p = 0.047).
The Amulet IDE (AMPLATZER Amulet™ Left Atrial Appendage Occluder Randomized Controlled Trial) (n = 1878, mean age 75 years) compared the Amplatzer Amulet device (Abbott) with the first-generation Watchman device (Boston Scientific) in patients who had AF with a high stroke risk, could tolerate short-term warfarin therapy but not chronic anticoagulation, and had imaging suggesting either device could be implanted. Implant success rate was higher in the Amulet group (98.4% vs. 96.4%) with smaller residual device regurgitation at 45 days in the Amulet group (98.9% vs. 96.8%; p < 0.0001 for noninferiority). The primary safety endpoint (composite of all-cause mortality, major bleeding or procedure related complications) and effectiveness endpoint (composite of ischaemic stroke or systemic embolism) were similar between groups (p < 0.001 for non-inferiority) [66].
The results of the PINNACLE FLX trial, published in 2020, led to FDA approve Boston Scientific’s second-generation LAA closure device, the Watchman FLX [1]. The follow-up 2-year outcomes data of the device exemption trial (n = 400; mean age 73.8 ± 8.6 years, mean CHA2DS2-VASc score 4.2 ± 1.5) were presented at TVT21 by Kar. Rates of stroke or embolism were low at 3.4% (performance goal 8.7%). Device-related thrombus occurred in seven patients (1.8%) within the first year with no further episodes in the second year; however, it must be noted that imaging rates after 1 year were low which may skew these findings [67]. Although such data support the role of LAA closure in patients unable to tolerate OAC, they do not prove non-inferiority, but the ongoing CHAMPION-AF may help answer this question.
Interestingly data from the LAAOS III (Left Atrial Appendage Occlusion Study III) provide support to the utility of LAAO in reduction of stroke risk. This trial randomised 4770 patients (mean age 71 years) with AF and elevated risk of stroke (CHADsVasc > 2) undergoing open-heart surgery for another indication to surgical LAAC vs. no LAAC [68]. At 3.8 years, use of surgical LAAC was associated with a reduction in the primary endpoint of ischaemic stroke or embolism (4.8% vs. 7.0%; p = 0.001). OAC usage rates at 3 years were similar between groups (75% vs. 78%) with no significant difference in bleeding between groups noted. Although not suggestive that LAAO is a replacement to OAC, it does highlight a segmented benefit in stroke risk reduction when added to OAC.
Other surgical methods to reduce post-operative AF were evaluated in the PALACS trial (Posterior Left pericardiotomy for the prevention of postoperative Atrial fibrillation after Cardiac Surgery). In this single-centre trial, 420 patients undergoing CABG, aortic valve surgery or surgery on aorta were randomised to pericardiotomy or standard care and the primary endpoint was rate of post-operative AF during hospital stay [69]. In the pericardiotomy group the rate of AF was significantly lower than the control group: 17% vs. 32% (RR 0.55, 95% CI 0.39–0.78). There was no difference in complication rate or length of stay in hospital. Given the short follow-up time in this trial it is impossible to extrapolate to whether there is any benefit in reduction of AF in the long term.
Acute Coronary Syndromes, Cardiogenic Shock and Antiplatelet Therapy
Acute Coronary Syndromes
Initial reports suggested an association between messenger RNA (mRNA) COVID-19 vaccines and the development of myocarditis. A recent review of a large Israeli healthcare database of 2.5 million patients demonstrated an estimated incidence of post-vaccine myocarditis of 2.13 cases per 100,000. The highest incidence was reported in male patients between the ages of 16 and 29 years (10.69 cases per 100,000 persons); however, most cases were classified as mild (76%) and only one case was associated with cardiogenic shock [70]. These data are reassuring that myocarditis remains rare and mild for the majority of patients affected.
The association of COVID with myocardial injury and troponin release is well documented; however, its relative impact on survival is not well understood. Kini et al. performed an observational, retrospective analysis of troponin levels of 4695 patients, 72 h before and 48 h after testing positive for COVID-19. The risk of death was found to be higher in patients with both acutely elevated troponin (47.3%; HR 4.72, 95% CI 4.15–5.36) and chronic myocardial injury (43.0%; HR 4.17, 95% CI 3.44–5.06) with the caveat of worse prognostic impact in acute injury if patients were aged less than 65 years (p interaction = 0.043) or did not have CAD (p interaction = 0.041) [71]. Although relationships between troponin elevation and mortality have been previously well established, this data suggests a role for troponin analysis in COVID-19 with regards to risk stratification and prognostication.
The IAMI (Influenza Vaccination After Myocardial Infarction) trial was a double-blind placebo-controlled trial randomising post-MI patients to receive an influenza vaccine on discharge (n = 1272) or placebo (n = 1260). The primary endpoint was a composite of all-cause death, MI or stent thrombosis at 12 months and occurred in 67 (5.3%) patients who received a vaccine vs. 91 (7.2%) who received placebo (HR 0.72 [95% CI 0.52–0.99]; p = 0.040) [72]. While the trial was stopped early because of the COVID pandemic and thus may be underpowered, it still supports a benefit of adding influenza vaccination to standard care in this patient group.
MI is not common in young patients and may not necessarily share the same pathophysiological seen in older demographics. Nevertheless, its diagnosis carries significant morbidity and mortality implications. Following on from the YOUNG-MI Registry which reported higher rates of autoimmune systemic inflammatory diseases, the Myocardial Infarction and Mental Stress 2 (MIMS2) study evaluated the impact of mental stress among 283 MI survivors (mean age 51 years) [73]. At 5 years, high stress vs. low stress as assessed by questionnaire was associated with higher re-infarction rate (37% vs. 17%; HR 2.7, 95% CI 1.5–4.9); however, of note, this was no longer significant when adjusting for underlying autoimmune disease, suggesting a possible relationship between the two variables.
Mitigation of inflammatory cascade activation in stable CAD and acute coronary syndromes (ACS) has been explored using colchicine in trials such as LODOCO and COLCOT [1]. COVERT-MI (Colchicine for Left Ventricular Remodelling Treatment in Acute Myocardial Infarction) was a multicentre RCT of 195 patients with first presentation STEMI randomised to colchicine vs. placebo [74]. Colchicine failed to show a reduction in infarct size (in LV mass) at 5 days as assessed by cardiac MRI (26.0 g IQR 16.0–44 vs. 28.4 g IQR 14.0–40.0; p = 0.87) or 3 months (mean 17 g vs. 18 g of LV mass; p = 0.92) and additionally, there was no difference in LV reverse remodelling (+ 2.4% vs. −1.1%; p = 0.49). Interestingly colchicine was associated with increased LV thrombus at 5 days (22.2% vs. 7.4%; p = 0.01) but not at 3 months (5.3% vs. 2.6%; p = 0.68). Although the trial was small, it highlights the complexity of the immunological response and need for ongoing research.
Rapid 0/1 h protocols for ruling out MI are being increasingly adopted in emergency departments, but it is unclear if they confer superior outcomes vs. conventional 0/3 h pathways. The multicentre RAPID-TnT trial (Rapid Assessment of Possible ACS in the Emergency Department With High-Sensitivity Troponin T) randomised 3378 patients (median age, 58 years; 53% male) to undergo rapid 0/1 h testing (reported to < 5 ng/L) or standard masked 0/3 h testing (< 29 ng//L). At 1 year, there was no difference in the primary endpoint of death or MI between both groups (0/1 h, 5.0% vs. 0/3 h, 3.8%; HR 1.32 [95% CI 0.95–1.83]; p = 0.10) [75]. Furthermore, there was no difference in the rate of angiography (0/1 h unmasked, 14.2%; 0/3 h masked, 12.4%; p = 0.13), suggesting that both pathways are safe for emergency department utilisation.
There has been concern that implementation of high-sensitivity troponin testing may be leading to increased testing and resource utilisation. Ganguli et al. performed a retrospective registry analysis of 7564 patients at the Mass General Brigham system, demonstrating that patients undergoing fifth-generation troponin evaluation received fewer CT scans (− 1.5 per 100 patient-visits; 95% CI − 1.8 to − 1.1), stress tests (− 5.9 per 100 patient-visits; 95% CI − 6.5 to − 5.3) and PCI (− 0.65 per 100 patient-visits; 95% CI − 1.01 to − 0.30) [76]. Similarly, the ACTION study (Clinical Impact of High-Sensitivity Cardiac Troponin T Implementation in the Community) evaluated fifth-generation troponin testing in 3536 patients and found, vs. previous assays, that hospital stays were shorter (4.3 to 4.2 h; p = 0.01) with less stress testing (6.5% to 4.9%; p = 0.02) [77]. These interesting data suggest that high-sensitivity troponin may lead to fewer unnecessary tests and shorter hospital stays, ultimately leading to reduced healthcare costs.
Cardiogenic Shock
The optimal time for angiography in patients with out of hospital cardiac arrest (OOHCA) remains unclear. The previous COACT trial (Coronary Angiography after Cardiac Arrest) suggested no difference in mortality with immediate vs. delayed angiography in patients achieving return of spontaneous circulation (ROSC) but without evidence of STEMI [78] and only 5% had evidence of a true thrombotic lesion on angiography. The TOMAHAWK (Immediate Unselected Coronary Angiography Versus Delayed Triage in Survivors of Out-of-hospital Cardiac Arrest Without ST-segment Elevation) trial thus randomised 530 resuscitated OOHCA patients to early vs. delayed angiography excluding those with left bundle branch block (LBBB) or STEMI [79]. At 30 days, mortality in the immediate angiography group was 54% vs. 48% in the ICU group (p = 0.06) with a signal to higher mortality and severe neurological deficit in the early angiography group (64.3% vs. 55.6%; RR 1.16, 95% CI 1.00–1.34) and, as with COACT, only a small proportion (40%) of the patient group had an angiographically significant lesion which likely explains the absence of clinical benefit with an early approach.
Previous data have suggested a link between air pollution and CV disease, but limited data exists between pollution and OOHCA. Gentile et al. conducted a retrospective analysis of the mean daily concentration of pollutants in the Lombardy region of Italy throughout 2019 and its association with OOHCA (1922 patients, median age 80 years, 57% male) [80]. After adjustment for temperature, a Probit regression analysis demonstrated all pollutants examined (fine particulate matter (PM10, PM2.5), benzene, carbon monoxide, nitrogen dioxide, sulfur dioxide and ozone) were associated with a higher incidence of OOHCA (p < 0.001). This association provides further impetus for improving air quality in our cities.
Mortality in refractory cardiac arrest remains exceedingly high with a lack of unified pathways in many centres. The PRAGUE OOHCA trial randomised 264 patients with OOHCA of presumed cardiac cause undergoing on-scene chest compressions to a hyperinvasive treatment approach with extracorporeal membrane oxygenation (ECMO) and mechanical cardiopulmonary resuscitation (CPR) vs. standard care [81]. The hyperinvasive strategy was associated with a longer median resuscitation time (58 vs. 46 min; p = 0.037) and improved neurological recovery at 30 days (18.2% vs. 30.6%; p = 0.02) but no difference in the primary endpoint of survival at 180 days (22.0% vs. 31.5%; p = 0.09). Notably, in patients with prolonged (> 45 min) CPR (n = 26) the hyperinvasive strategy resulted in a significantly improved Cerebral Performance Category at 180 days (p = 0.018). On the basis of the improved secondary endpoints of neurological recovery, the trial was stopped early. Invasive management may therefore be beneficial for patients with prolonged downtime; however, the logistics of implementing such a pathway remain unclear.
Therapeutic hypothermia is recommended by international resuscitation guidelines for the management of unconscious post cardiac arrest survivors to mitigate the risk of death and poor neurological outcome. Current guidelines recommend at least 24 h of targeted therapeutic hypothermia in the range of 32–36 °C; however, contention remains as to the optimal therapeutic target. CAPITAL-CHILL (Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest) was an investigator-driven, single-blind trial which randomised 389 patients (mean age 60 years; 80% male) who remained comatose following successful resuscitation from OOHCA to moderate (31 °C) vs. mild (34 °C) hypothermia for 24 h (after which all were warmed to normal body temperature at a rate of 0.25 °C/h). Moderate hypothermia was associated with similar rates of all-cause death and poor neurological outcome (48.4% vs. 45.4%) but numerically higher rates of stoke (4.4% vs. 1.6%; p = 0.22), seizure (12.5% vs. 7.1%; p = 0.08) and bleeding (23.4% vs. 19.7%; p = 0.39) [82], suggesting that aggressive cooling does not improve and indeed may worsen outcomes in OOHCA.
TTM2 (Targeted Hypothermia Versus Targeted Normothermia After Out-of-hospital Cardiac Arrest) was a multicentre trial which randomised 1900 comatose patients after OOHCA of presumed cardiac or unknown cause to targeted temperature management at 33 °C or 36 °C [83]. The primary outcome was all-cause mortality at 180 days and the composite secondary outcome was poor neurologic function or death at 180 days. There was no difference in mortality between groups at 180 days (50% vs. 48%; hazard ratio with a temperature of 33 °C, 1.06; 95% CI 0.89–1.28; p = 0.51). Additionally, there was no difference in neurological recovery between groups (54% vs. 52%; risk ratio, 1.02; 95% CI 0.88–1.16; p = 0.78). This trial adds further evidence that aggressive cooling in the post arrest patient does not improve survival or neurological outcome. Data are awaited from the ongoing ICECAP study which is evaluating the duration of cooling.
Previous preclinical models have suggested intracoronary cooling may reduce infarct size in STEMI. COOL-AMI (Cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction) randomised patients with STEMI to the ZOLL® Proteus™ intravascular cooling system vs. standard care [84]. Of note the trial originally intended to enrol 500 patients but was terminated prematurely because of excessive delay from randomisation to balloon time in the cooling arm (61 vs. 32 min; p < 0.001) and consequently longer total ischaemic time (232 vs. 188 min; p < 0.001). Within the 111 patients randomised, the cooling strategy was associated with numerically higher 30-day MACE (8.6% vs. 1.9%; p = 0.117) likely related to the longer ischaemic time in this group. Given the marked operational delay implementing cooling with this device, alternative cooling strategies are required to adequately study the concept. One such system for rapid selective coronary cooling is currently being tested in the EURO-ICE trial.
Antiplatelets
In 2021, several major trials studied whether shortened dual antiplatelet therapy (DAPT) reduced bleeding risk without increasing risk of further ischaemic events. The MASTER DAPT study (Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk) randomly assigned 4434 patients at high bleeding risk (HBR) to 1 month DAPT (abbreviated therapy) vs. ongoing DAPT for at least 3 months (standard therapy). PCI was undertaken with a biodegradable-polymer sirolimus-eluting stent in all patients. Abbreviated vs. standard therapy met non-inferiority for the primary outcomes of net adverse clinical events (NACE; death, MI, stroke or major bleeding) (p < 0.001) and MACE (death, MI or stroke) (p = 0.001) at 335 days. Having met non-inferiority, further analysis showed the abbreviated strategy was associated with significant reduction in major or clinically relevant non-major bleeding (6.5% vs. 9.4; p superiority < 0.001). In a pre-specified sub-analysis of those with acute or recent MI (n = 1780) outcomes mirrored the overall trial with similar NACE and major adverse cardiovascular and cerebrovascular events (MACCE) and decrease in major or clinically relevant non-major bleeding [85]. Whilst the results are very reassuring when a biodegradable-polymer stent is used, it is not clear whether the findings can be applied to all stents.
Previous data from the STOP DAPT-2 trial (Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2) reported that after PCI, 1 month of DAPT followed by 11 months of clopidogrel monotherapy was superior to 12 months of DAPT at preventing net adverse ischaemic events [86]. The STOP DAPT-2 ACS trial (Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 Acute Coronary Syndrome) tested the same abbreviated vs. conventional DAPT protocol in patients with ACS (n = 4156). Interestingly the abbreviated DAPT arm failed to meet non-inferiority for the combined primary outcome of CV death, MI, stroke, stent thrombosis, TIMI major or minor bleeding (3.2% vs. 2.8% respectively; HR 1.14, 95% CI 0.80–1.62; p = 0.06). Of particular note was the significant increased numbers of MI in the 1-month DAPT group (1.59% vs. 0.85%; HR 1.91, 95% CI 1.06–3.44) [87]. While the investigators presented pooled data from STOP DAPT-2 plus STOP DAPT-2 ACS (n = 5997), suggesting that 1-month DAPT duration was non-inferior to 12 months DAPT [88], further research is clearly required to define the optimal duration post ACS.
The TWILIGHT trial (Ticagrelor with or without Aspirin in High-Risk Patients after PCI) in patients post PCI deemed at high risk of bleeding or ischaemic events previously reported reduction in bleeding, without increase in ischaemic events, associated with ticagrelor monotherapy after 3 months DAPT [1]. In a pre-specified sub-analysis, the investigators found a similar risk of bleeding events and ischaemic events between sexes when a multivariate adjustment was applied, to account for differences in baseline characteristics (adjusted HR 1.20, 95% CI 0.95–1.52; p = 0.12) [89]. A patient-level meta-analysis (n = 24,096) of data from STOP DAPT-2, TWILIGHT and four other trials reported that monotherapy following abbreviated DAPT vs. conventional DAPT was associated with a similar risk of death, MI or stroke but lower bleeding risk [90]. In an observational study, Wang et al. evaluated 4875 patients who were event-free at 12 months post PCI from the prospective Fuwai PCI registry. Those who continued DAPT for more than 12 months (mean 663 days) vs. those who stopped earlier (mean 350 days) had a lower incidence death, MI or stroke at 30 months (1.5% vs. 3.8%; adjusted HR 0.37, 95% CI 0.26–0.55), without a significant difference in the secondary outcome of bleeding [91]. Although the findings are at odds with TWILIGHT, the non-randomised design may have been confounded by differences in baseline factors and possibly the predominant use of clopidogrel.
Potent P2Y12 inhibitors lead to earlier platelet inhibition in patients presenting STEMI. The COMPARE CRUSH trial which randomised 727 patients with STEMI to pre-hospital crushed vs. integral prasugrel tablets previously failed to show that crushed prasugrel improved TIMI 3 flow or ST-segment resolution. New follow-up at 1 year still showed no benefit with the crushed strategy regarding death, MI and urgent revascularisation (7.7%vs. 6.6%; OR 1.25, 95% CI 0.67–2.33; p = 0.49) [92]. Thus, while it may be convenient in the setting of nausea, there does not appear to be a clinical advantage for crushing P2Y12 inhibitors in STEMI.
De-escalating from more potent P2Y12 inhibitors to clopidogrel at 1 month post PCI in the TOPIC (Timing of platelet inhibition after acute coronary syndrome) trial previously showed a significant reduction in bleeding events with this strategy [93]. The TALOS-AMI (Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute MI undergoing percutaneous coronary intervention) study randomised 2697 patients with ACS who had received 1 month of DAPT with ticagrelor and aspirin and experienced no major ischaemic or bleeding events to de-escalation with aspirin and clopidogrel vs. continued ticagrelor and aspirin. De-escalation was associated a reduction in the primary endpoint at 1 year of CV death, MI, stroke and bleeding (4.6% vs. 8.2%; HR 0.55, 95% CI 0.40–0.76; p for superiority < 0.001). The benefit was mainly due to reduced bleeding but there was no excess in ischaemic events (2.1% vs. 3.1%; HR 0.69, 95% CI 0.42–1.14) [94].
Following PCI, the preferred antiplatelet for long-term monotherapy remains unclear. The HOST-EXAM trial (Aspirin vs. clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention) randomised 5530 patients after 6–12 months of DAPT post PCI to clopidogrel 75 mg vs. aspirin 100 mg for 24 months. Clopidogrel was associated with a reduction in the primary endpoint of death, MI, stroke, readmission due to ACS, or BARC bleeding type 3 or greater (5.7% vs. 7.7%; HR 0.73, 95% CI 0.59–0.90; p = 0.0035) and a reduction in bleeding events (2.3% vs. 3.3%; HR 0.70; p = 0.036), suggesting it may be preferable to aspirin for long-term monotherapy [95].
Loss of function (LOF) mutations in the CYP2C19 gene responsible for metabolising clopidogrel are common. Despite this, a role for genotype-guided antiplatelet therapy following PCI has not been established. The TAILOR PCI trial (Tailored Antiplatelet Initiation to Lessen Outcomes due to Decreased Clopidogrel Response After Percutaneous Coronary Intervention) randomised 5302 patients undergoing PCI to genotype-guided therapy (LOF carriers ticagrelor; non-carriers clopidogrel) vs. non genotype-guided therapy (all clopidogrel). Previously, 12 months analysis reported no difference in the primary endpoint of CV death, MI, stroke, stent thrombosis or severe recurrent ischaemia [1]. This year, extended follow-up of 4747 patients for a median of 39 months also reported no significant difference in the primary endpoint (HR 0.95, 95% CI 0.70–1.29; p = 0.74), suggesting no clear role for routine genotype-guided therapy post PCI [96].
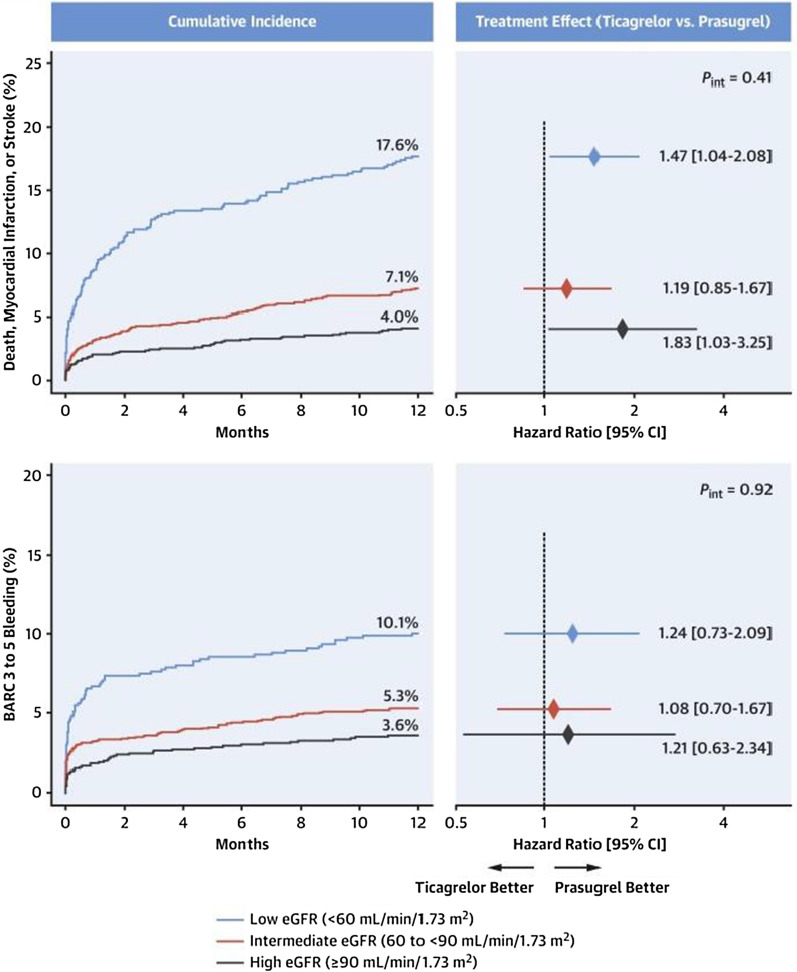
Chronic kidney disease is a recognised risk factor for ischaemic and bleeding events. The ISAR-REACT 5 trial (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5 trial) previously reported prasugrel vs. ticagrelor in patients with ACS had superior efficacy at 1 year with no difference in bleeding. This year, new subgroup data were presented with patients categorised into three groups: low estimated glomerular filtration rate (eGFR) (< 60 mL/min/1.73 m2), intermediate eGFR (≥ 60 and < 90 mL/min/1.73 m2), and high eGFR (≥ 90 mL/min/1.73 m2) (Fig. 7). Prasugrel was associated with a significant or numerical reduction in the efficacy endpoint (death, MI or stroke) in each eGFR subgroup, including a 28% reduction in those with low eGFR (14.7% vs. 20.5%; p = 0.029) but yet no significant difference in bleeding [97].
Fig. 7.
Graphs showing the cumulative incidence of the primary (top left) and secondary safety (bottom left) endpoints according to eGFR, over 12 months. Figures to the right show the hazard ratio treatment effect (ticagrelor vs. prasugrel) according to eGFR (reproduced with the permission of Elsevier) [97]
The optimal dose of aspirin for secondary prevention in established atherosclerotic cardiovascular disease (ASCVD) has not been clearly determined (ESC guidelines recommend low dose aspirin, but ACC-AHA guidelines do not define a recommended dose) [98–100]. The ADAPTABLE (Comparative Effectiveness of Aspirin Dosing in Cardiovascular Disease) study randomised 15,076 patients with established ASCVD to 81 mg vs. 325 mg aspirin. At a median of 26.2 months follow-up, despite a 40% participant crossover from high to lower dose, no significant difference was noted for the primary efficacy outcome (death, hospitalization for MI or stroke; HR 1.02, 95% CI 0.91–1.14). No significant difference in the primary safety outcome of hospitalization for major bleeding was noted (which may have been confounded by the 40% crossover). Thus, low dose aspirin appears equally efficacious and is likely safer than high dose aspirin [101].
DAPT has been shown to approximately double the risk of GI bleeding vs. monotherapy [102]. The OPT PEACE (Optimal Antiplatelet Therapy for Prevention of Gastrointestinal Injury) trial randomised patients with no evidence of GI ulceration on capsule endoscopy (CE) despite 6 months of DAPT post-PCI to a further 6 months of aspirin monotherapy (n = 168) vs. clopidogrel monotherapy (n = 169) vs. DAPT (aspirin plus clopidogrel; n = 168). Surprisingly, the primary outcome, gastric or small intestinal injury on follow-up CE, was 92.4% vs. 96.2% vs. 99.2% in the respective arms (p = 0.18 for aspirin vs. clopidogrel; p = 0.02 for any monotherapy vs. DAPT). Secondary outcomes of GI bleeding were significantly lower with monotherapy than with DAPT (0.6% vs. 5.4%; p = 0.001), and there were no target lesion failures in any group [103]. This study highlights how common subclinical GI injury is with any antiplatelet therapy (even monotherapy), although supports consideration of abbreviated DAPT therapy.
In patients with ACS, the optimal time to perform CABG after stopping P2Y12 inhibitors has not been previously studied in randomised fashion and thus clinical practice varies. The RAPID CABG trial randomised patients with ACS (mean age 64 years, 93% with multivessel disease) to early CABG 2–3 days (n = 71) vs. late CABG 5–7 days (n = 69) after stopping ticagrelor. No significant difference was seen in the primary outcome of severe/massive bleeding as defined by the Universal Definition of Perioperative Bleeding class 3 or 4 (early CABG 4.6% vs. late CABG 5.2%; p for non-inferiority = 0.0253). No ischaemic events were seen in the early CABG group, but six events occurred in the late CABG arm (one MI, four recurrent angina, one ventricular tachycardia) [104]. Thus stopping ticagrelor for a shorter period prior to CABG appears safe and may be preferable.
The use of pre-hospital glycoprotein IIb/IIIa (GP2b3a) inhibitors in patients with STEMI has been shown to improve ST-segment resolution but clinical outcome data are limited. The phase 2A, CEL-02 trial (Pharmacokinetics, pharmacodynamics, and tolerability of subcutaneous administration of a novel glycoprotein IIb/IIIa inhibitor, RUC-4, in patients with STEMI), assessed the novel GP2b3a inhibitor, zalunfiban (RUC-4), administered by subcutaneous injection in the pre-hospital setting. Twenty-seven patients with STEMI received RUC-4 in escalating doses (0.075 mg/kg [n = 8], 0.090 mg/kg [n = 9] or 0.110 mg/kg [n = 10]) with the primary endpoint of high-grade platelet inhibition (> 77%) at 15 min being met in 3/8, 7/8 and 7/8 patients in the three cohorts with a dose–response relationship [105]. In light of these promising results, a randomised phase 2B trial is currently underway to assess clinical outcomes.
The potent antiplatelet effects of ticagrelor can be problematic with regarding to the risk of bleeding, particularly if urgent surgery is required. The phase III REVERSE-IT trial (Rapid and Sustained Reversal of Ticagrelor–Intervention) evaluated the recombinant monoclonal antibody fragment bentracimab as an intravenous ticagrelor reversal agent. This single-arm study included 150 participants who required urgent ticagrelor reversal (142 planned urgent surgery, 8 with major bleeding). The primary reversal endpoint was minimum platelet inhibition on the platelet function assay within 4 h following bentracimab administration. Platelet reactivity was shown to be significantly reduced at 4 h and sustained for 24 h (p = 0.001 at all time points). Importantly, platelet inhibition results were consistent among patients requiring urgent surgery and those with major bleeding [106]. These results suggest that this agent could be utilised clinically, particularly in cases where surgery cannot be deferred until the antiplatelet effects of ticagrelor wear off.
Advances in Cardiac Devices and Electrophysiology
Screening in Atrial Fibrillation
The novel Apple Heart study, published in 2019, demonstrated the feasibility of AF screening in the general population using an Apple Watch device [107]. Building on this, the FITBIT (Fitbit Atrial Fibrillation From PPG Data Validation) study evaluated the rates of AF detection using the wearable smart watch in 455,699 participants using photoplethysmography signals to detect irregular heart rhythms. A total number of 4728 participants (1%) had notification of an irregular heart rhythm with 1162 returned for analysis. AF was confirmed in 340 (32.2%) but not confirmed in 822 (70.7%), showing a very high false positive rate which detracted from the reasonably high rate of AF detection [108]. It is likely with improved population selection and appropriate pre-test probability, assessment will lead to improved sensitivity and specificity.
Early detection and treatment of AF may lead to a reduction in stroke risk but the value of screening asymptomatic patients remains unclear. The LOOP (Atrial Fibrillation Detected by Continuous ECG Monitoring) study evaluated whether screening patients for AF with an implantable loop recorder (ILR) would lead to a higher diagnosis of AF and subsequent lower risk of stroke [109]. This multicentre Danish trial randomised 6003 patients (mean age 74.7 years; 47.3% women) with one or more risk factors for AF to ILR implantation or standard care. At 65 months, the use of ILR was associated with higher rates of AF detection (31.8% vs 12.2%, HR 3.17, 95% CI 2.81–3.59, p < 0.0001) without a reduction in stroke rates (HR 0.80, 95% CI 0.61–1.05, p = 0.11).
In STROKE-STOP 2 (Systematic ECG Screening for Atrial Fibrillation Among 75 Year Old Subjects in the Region of Stockholm and Halland, Sweden), 28,786 patients were randomised in a multicentre RCT to AF screening vs. standard care. In those whom AF was detected, anticoagulation was offered with a primary composite outcome of stroke, systemic embolism, bleeding leading to hospital admission and all-cause mortality. After a median follow-up of 6.9 years, AF screening was associated with a small but statistically significant reduction in primary outcome (31.9% vs. 33%; HR 0.96 [95% CI 0.92–1.00]; p = 0.045) [110].
A more personalised approach to AF may be the solution to dealing with the heterogeneity of the AF population. The I-STOP-aFib (Individualized Studies of Triggers of Paroxysmal Atrial Fibrillation) trial set out to determine whether avoidance of specific triggers would lead to an improvement in quality of life (QOL) via the Atrial Fibrillation Effect on QualiTy-of-life (AFEQT) score and reduction in frequency of AF. In this novel study, 499 patients with symptomatic AF were randomised in a 1:1 ratio to either an n-of-1 trigger group where patients identified a personal trigger of their AF and avoided it or a control group who just tracked their symptoms. No improvement in AFEQT score was noted vs. the control (1.7% vs. 0.5%; p = 0.17); however, a reduction in AF events was noted (RR 0.6, 95% CI 0.43–0.83; p < 0.0001) [111].
In conclusion while new trial data show AF screening is likely to detect AF at an earlier stage, the benefits in terms of reduction in stroke risk remain unclear, perhaps reflective of the relative heterogeneity of the AF population.
Intervention in Atrial Fibrillation
The long-term debate over rate vs. rhythm control in patients with AF and heart failure has leaned in favour of rhythm control thanks to recent trials such as the CASTLE AF and EAST-AFNET 4, suggesting early rhythm control in the form of anti-arrhythmic drugs (AADs) or catheter ablation techniques led to a reduction in hard outcomes [112].
The RAFT-AF trial (Randomized Ablation-based Atrial Fibrillation Rhythm Control Versus Rate Control Trial in Patients With Heart Failure and High Burden Atrial Fibrillation) set out to directly compare catheter-based rhythm control strategies with standard rate control options. In total 411 patients with a high burden of AF, heart failure with NYHA class 2 or 3 and already on at least 6 weeks of HF medications were randomised in a 1:1 fashion to catheter ablation or rate control options [113]. The rhythm control group showed a non-significant trend to reduction in death and heart failure events (23.4% vs. 32.5%; 0.71 (95% CI 0.49–1.03); p = 0.066) and an improvement in secondary outcomes including symptomatic benefit, improved exercise tolerance and improvements in NT-pro-BNP and LVEF. Unfortunately, the trial was stopped early because of futility concerns and did not enrol the target number of patients, making further interpretation of results difficult.
The RACE 3 trial previously reported that a targeted risk factor management approach with mineralocorticoid receptor antagonists (MRAs), angiotensin-converting enzyme inhibitor (ACE-i)/angiotension receptor blockers (ARBs), statins and cardiac rehabilitation vs. standard therapy improved maintenance of sinus rhythm at 1 year. It was hoped this might translate into clinical benefit with longer follow-up. However, new 5-year data failed to show ongoing benefit for the targeted approach either for maintaining sinus rhythm (46% vs. 39%; OR 1.297; CI 0.756–2.225; p = 0.346) or CV morbidity/mortality [114].
The CRYOFIRST trial (Catheter Cryoablation Versus Antiarrhythmic Drug as First-Line Therapy of Paroxysmal Atrial Fibrillation) assessed upfront pulmonary vein isolation (PVI) via cryoablation vs. AADs for prevention of atrial arrhythmia recurrence in patients with rhythm control-naïve paroxysmal AF (PAF). This was a multicentre RCT comprising 118 patients followed up for 1 year with a primary endpoint after a 90-day blanking period of freedom from recurrence of atrial arrhythmia. In the cryoablation arm 82.2% were arrhythmia free vs. 67.6% in the AAD arm (HR 0.48, 95% CI 0.26–0.86; p = 0.013) (Fig. 8). There was no difference in serious adverse events between groups (RR 0.79; p = 0.28). This study, although small, suggests an initial benefit for upfront cryoablation for patients with treatment-naïve PAF but did have the caveat of a 90-day blanking period where further ablations or adjustments to AADs could occur [115].
Fig. 8.
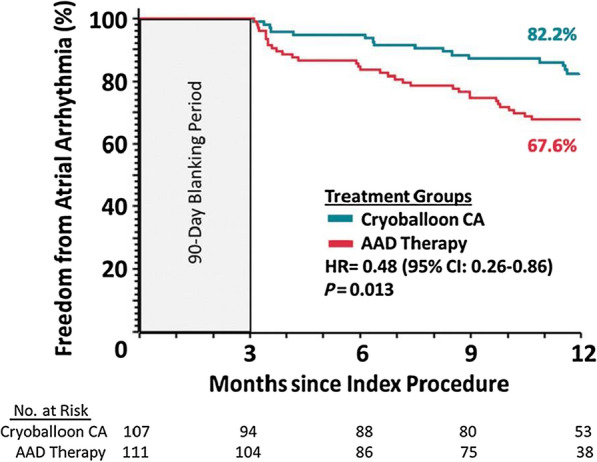
Illustration reproduced from Kaplan–Meier curve demonstrating time to first atrial arrhythmia recurrence in the cryoablation cohort and the antiarrhythmic drug cohort. At 12 months from index procedure there were significantly more patients who were free from arrhythmia in the cryoablation group compared with the control group: 82.2% vs. 67.6% (HR 0.48, 95% CI 0.26–0.86; p = 0.013). Reproduced with permission from Oxford University Press (OUP) (Kuniss et al. [115])
PVI has been the standard ablation method for AF for many years; however, the freedom from AF recurrence after first ablation remains high at approximately 20–40% [116]. The DECAAF study in 2014 reported that atrial tissue fibrosis is an independent risk factor for likelihood of recurrent arrhythmia in patients undergoing catheter ablation for AF [117]. The DECAAF 2 trial (Efficacy of Delayed Enhancement MRI-Guided Ablation vs Conventional Catheter Ablation of Atrial Fibrillation) randomised 843 patients, comparing a primary endpoint of atrial arrhythmia recurrence in patients undergoing PVI and fibrosis-guided ablation to PVI alone. At 12–18 months, no significant difference in recurrence of atrial arrhythmia vs. control (43% vs. 46.1%; HR 0.95, 95% CI 0.77–1.17; p = 0.63) was noted, suggesting no benefit in a fibrosis-guided ablation over standard PVI. In a subgroup analysis, a trend was noted to a lower rate of recurrence in patients with lower levels of fibrosis at baseline which may help guide which patients are likely to benefit from ablation [118].
Imaging-guided ablation can also be used to reduce ablation time with a targeted approach based on atrial wall thickness. The Ablate-by-LAW trial (Feasibility and Efficacy of Tailoring Ablation Index to Left Atrial Wall Thickness During Atrial Fibrillation Ablation) was undertaken to determine if adapting ablation index (AI) to left atrial wall thickness (LAWT) is safe and effective in patients undergoing PVI. To determine LAWT, a CT-produced 3D map was integrated with the CARTO navigation system and AI. A total of 90 consecutive patients with AF undergoing their first ablation procedure were recruited with primary endpoints of efficacy, safety and freedom from AF recurrence. At a mean follow-up of 16 ± 4 months, 93.3% of patients were free of AF recurrence with no major complications occurring. First-pass isolation of the right pulmonary veins (PVs) was 93% with 97% in left PVs. To conclude, a personalised approach to ablation based on LAWT is safe, effective and with low risk of recurrence [119].
Cardiac Devices and Arrhythmia
Screening and primary prevention for arrhythmias in patients post MI has previously been reserved for patients with an ejection fraction (EF) less than 35%. The SMART-MI-ICM trial (Implantable Cardiac Monitors in High-risk Post-infarction Patients With Cardiac Autonomic Dysfunction and Moderately Reduced Left Ventricular Ejection Fraction) sought to evaluate if there was a role for arrhythmia screening in post-MI patients with LVEF between 35% and 50%. At 21 months, a significant difference in the rate of arrhythmia detection was noted in the ICM group vs. control (29.9% vs 6%, HR 6.3, 95% CI 3.4–11.8, p < 0.0001) [120]. Although this highlights a high proportion of serious arrhythmias in this patient group, it is unclear if there is any morbidity or mortality benefit from earlier detection.
A more aggressive ablative approach to managing patients with permanent AF is atrioventricular node (AVN) ablation with biventricular pacing. The APAF-CRT trial previously showed a reduction in HF admissions with this approach [121]. The APAF-CRT Mortality trial was a sub-study assessing the effect of AVN ablation and biventricular pacing on mortality in patients with severe symptomatic AF and heart failure. A study population of 133 patients with severe symptomatic AF, narrow QRS and at least one HF admission in last 12 months were randomised in 1:1 fashion to ablation and cardiac resynchronisation therapy (CRT) vs. pharmacological therapy. The study was stopped early owing to efficacy at a median follow-up of 29 months with the intervention group reaching an endpoint in 11% of patients vs. 29% of patients in the control group (HR 0.26, 95% CI 0.10–0.65; p = 0.004). There were similar benefits in patients with EF > 35% and EF < 35%, indicating a survival benefit of an ablate and biventricular pace strategy over medications in patients irrespective of EF [122].
The updated 2021 ESC guidelines on cardiac pacing and CRT reflect the data from the APAF-CRT mortality trial and recommend CRT over RV pacing in patients undergoing AVN ablation for AF as a class 2a recommendation with level of evidence C [123]. Another relevant update to the 2021 guidelines was the use of His bundle pacing in patients undergoing CRT in which implantation of coronary sinus lead is unsuccessful with a class 2a recommendation and level of evidence B.
This update is supported by the HIS-Alternative trial presented at EHRA 2021. This trial compared His bundle CRT pacing vs. conventional LV CRT pacing, in a population of 50 patients followed up for 6 months with an outcome of improvement in LVEF. There was some crossover between the groups but an intention-to-treat analysis was undertaken. In the His bundle pacing group LVEF increased by 16 ± 7% vs. 13 ± 6% in the conventional group which was statistically non-significant. There were, however, higher pacing thresholds in the His bundle group vs. conventional group 2.3 ± 1.4 V vs. 1.4 ± 0.5 V (p < 0.01). In conclusion, His bundle CRT pacing had a similar physiological response as conventional CRT albeit with a slightly higher pacing threshold and would be a reasonable alternative [124].
Subcutaneous implantable cardioverter defibrillators (S-ICDs) are an alternative to transvenous cardioverter defibrillators (ICDs), used especially in younger patients and those at risk of device infection. The EFFORTLESS trial (Evaluation of factors impacting clinical outcome and cost effectiveness of the S-ICD) is a multicentre 5-year follow-up study which assessed complication rates and rates of inappropriate shocks in a diverse population of patients who received a S-ICD. Overall complication rates were low with 9.4% of patients having a system or procedure-related complication and 16.9% receiving an inappropriate shock. To conclude, at 5 years complication rates remained reasonably low for S-ICD with the majority of inappropriate shocks being seen in the older generation devices [125].
Advances in Heart Failure
Heart Failure with Reduced Ejection Fraction (HFrEF)
SGLT2 Inhibitors
The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors is well established in chronic heart failure but safety in acute heart failure has required further study. The EMPULSE trial (Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure With Reduced Ejection Fraction) randomised 530 patients with acute heart failure to empagliflozin vs. placebo [126]. Use of empagliflozin was associated with greater incidence of clinical benefit (as defined by rates death, HF events, time to first HF event, or change in Kansas City Cardiomyopathy Questionnaire-Total Symptom Score (KCCQ-TSS)) (53.9% vs. 39.7%; p = 0.0054). No safety concerns were noted, suggesting it appeared beneficial to commence SGLT2 inhibitors during acute HF admissions.
The DAPA-HF trial previously reported that the SGLT2 inhibitor dapagliflozin reduced the incidence of CV death and worsening HF in patients with HFrEF [127]. While benefits through natriuresis are established, a new analysis reported a significant reduction in the composite of serious ventricular arrhythmia, resuscitated cardiac arrest or sudden death with dapagliflozin vs. placebo [128] (5.9% vs. 7.4%; HR 0.79, 95% CI 0.63–0.99). The mechanism for this effect is unclear at present but favourable effects on cardiac metabolism, haemodynamic and reverse remodelling may contribute.
Sacubitril/Valsartan
Sacubitril/valsartan has been an important addition to the treatment of patients with HFrEF in recent years. PARADISE-MI (Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure Events after Myocardial Infarction) examined the use of sacubitril/valsartan in 5661 patients with an acute MI complicated by reduced ejection fraction (< 40%) and/or pulmonary congestion with 2830 patients randomised to receive sacubitril/valsartan while the remaining 2831 received ramipril [129]. The composite endpoint of CV death or HF event occurred in 338 (11.9%) of the sacubitril/valsartan group and 373 (13.2%) of the ramipril group (HR 0.90, 95% CI 0.78–1.04; p = 0.17). This suggests that further work is required to identify whether there is a subgroup of patients that may benefit from this drug in the acute period following an MI.
The LIFE study examined the use of sacubitril/valsartan in another group of interest, those with HFrEF and NYHA class IV symptoms, in which 335 patients were randomised to receive sacubitril/valsartan (n = 167) or valsartan alone (n = 168) and were followed up for 24 weeks [130]. Drug efficacy was measured by looking at days alive out of hospital and free of HF events and there was no significant difference between the groups, 111 days in the valsartan group vs. 103 days in the sacubitril/valsartan group (p = 0.45). There was also no significant difference in the NT-proBNP in either group vs. baseline. The patients studied in this trial were sicker than previous published work and the results do suggest that the main benefit of this drug may be for patients at an earlier stage in their disease.
Novel Therapies
Omecamtiv mecarbil is a novel, selective cardiac myosin activator that has previously been shown to improve cardiac performance [1]. A new analysis of the GALACTIC-HF trial (Registrational Study With Omecamtiv-Mecarbil to Treat Chronic Heart Failure With Reduced Ejection Fraction) suggests this drug may have greater benefit for those patients with the lowest ejection fractions. All participants had HFrEF with an LVEF of less than 35% to be included in the trial and in a further analysis they were subdivided into quartiles by LVEF. In the lowest quartile (LVEF < 22%) omecamtiv mecarbil vs. placebo was associated with a 17% reduction in risk of the primary endpoint of CV death or HF event (HR 0.83, 95% CI 0.73–0.95) [131]. In the highest quartile (defined by LVEF > 35%), the benefit fell short of significance (HR 0.99, 95% CI 0.84–1.16). This data suggests that this drug should be considered for those patients in the most advanced stages of heart failure.
The GUIDE-HF (Hemodynamic-GUIDEd Management of Heart Failure) study reported that the CardioMEMS implantable pulmonary artery pressure monitor did not appear to improve outcomes in patients with heart failure and NYHA II–IV symptoms. A total of 1022 patients were enrolled in the study and there were 253 primary endpoint events (a composite of all-cause mortality and HF events) among 497 patients in the haemodynamics-guided management group and 289 events among 503 patients in the control group (HR 0.88, 95% CI 0.74–1.05; p = 0.16) [132]. Interestingly there was some suggestion of benefit of the monitor when a pre-COVID-19 impact analysis was completed and although the reason for this is unclear it has been hypothesised that the loss of benefit may have been as a result of increased patient compliance due to the pandemic.
In an intervention without additional medications, the REHAB-HF trial showed benefit of a tailored rehabilitation programme for patients with a recent acute decompensation of heart failure. A total of 349 patients were randomised to receive the rehabilitation intervention (n = 175) or usual care (n = 174) [133]. Although there was no significant difference in rehospitalisation rate or mortality between the two groups, there was a significant difference in the Short Physical Performance Battery scores post intervention with the rehabilitation group at 8.3 ± 0.2 and the control group at 6.9 ± 0.2 (mean between-group difference, 1.5; 95% CI 0.9–2.0; p < 0.001). This suggests the programme results in improved physical function for these patients and in turn this is likely to improve quality of life.
Heart Failure with Preserved Ejection Fraction (HFpEF)
There are currently few options for effective, evidence-based therapy in heart failure with preserved EF (HFpEF). Clear benefits of SGLT2 inhibitors have previously been demonstrated for patients with HFrEF but less is known about their utility in HFpEF. Several trials presented this year have examined the use of SGLT2 inhibitors in this group. The EMPEROR-preserved trial (Empagliflozin Outcome trial in Patients With chronic heart Failure With Preserved Ejection Fraction) assigned 5988 patients with NYHA class II–IV heart failure and an EF greater than 40% to receive either empagliflozin vs. placebo [134]. Over a follow-up period of just over 2 years the primary composite endpoint of CV death or hospitalisation for heart failure occurred in 13.8% in the empagliflozin group and 17.1% in the placebo group (HR 0.79, 95% CI 0.69–0.90; p < 0.001) (Table 1). This effect was largely driven by the reduction in HF hospitalisations (Fig. 9). The reductions in the primary endpoint were seen when comparing subgroups with (HR 0.79, 95% CI 0.67–0.94) and without diabetes (HR 0.78, 95% CI 0.64–0.95). Interestingly EMPEROR-preserved included patients with moderate to severe renal impairment and over half of all participants had some level of renal dysfunction. Importantly there was no impact on the efficacy or safety of empagliflozin in the participants with renal impairment. A sub-analysis of the trial reported that the benefit seen with empagliflozin compared to placebo was significant and consistent across the whole range of participants’ kidney function which included down to an eGFR of 20 ml/min/1.73 m2 [135].
Table 1.
Primary and secondary outcomes of the EMPEROR-preserved trial (Anker et al. [134])
| Empagliflozin (n = 2997) | Placebo (n = 2991) | Hazard ratio or difference (95% confidence interval) | p value | |
|---|---|---|---|---|
| Overall primary composite endpoint—no. (%) | 415 (13.8) | 511 (17.1) | 0.79 (0.69–0.90) | < 0.001 |
| Hospitalisation for heart failure—no. (%) | 259 (8.6) | 352 (11.8) | 0.71 (0.60–0.83) | |
| Cardiovascular death—no. (%) | 219 (7.3) | 244 (8.2) | 0.91 (0.76–1.09) | |
| eGFR mean slope change per year (ml/min/1.73 m2) | − 1.25 ± 0.11 | − 2.62 ± 0.11 | 1.36 (1.06–1.66) | < 0.001 |
| Change in KCCQ score at 52 weeks | 4.51 ± 0.31 | 3.18 ± 0.31 | 1.32 (0.45–2.19) | |
| All-cause mortality—no. (%) | 422 (14.1) | 427 (14.3) | 1.00 (0.87–1.15) |
Fig. 9.
Clinical outcomes in participants randomised to a fixed-dose combination primary prevention pill versus a control group (Joseph et al. [146])
Sotagliflozin, a novel agent which acts to inhibit both SGLT2 and SGLT1, was also shown to improve outcomes in HFpEF in a meta-analysis presented this year. The SOLOIST-WHF and SCORED trials both examined the use of sotagliflozin in patients with heart failure and a meta-analysis of data from both trials was presented at the ACC meeting this year. In patients with T2DM and HFpEF with LVEF greater than 50% there was a significantly lower rate of the composite primary endpoint of CV death, HF hospitalisation or urgent HF visit in the sotagliflozin group vs. the placebo group (37.5% vs. 59.0%; p = 0.009) [136]. Patients without T2DM were not included in SCORED and SOLOIST-WHF. It would be useful for future studies to examine their use in patients with HFpEF but not T2DM given the treatment options are so limited for this group at present.
The PIROUETTE trial (The Efficacy and Safety of Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction) examined the use of the antifibrotic drug pirfenidone, traditionally used for idiopathic pulmonary fibrosis, in patients with HFpEF. Ninety-four patients with HFpEF (LVEF > 45%) were randomised to pirfenidone (n = 47) vs. placebo (n = 47) [137]. The pirfenidone group saw a significant reduction in cardiac extracellular volume measured by cardiac MRI at 1 year vs. the placebo group (− 0.7 vs. 0.5; p = 0.009). There was no increase in adverse events in the treatment group of this trial. Despite the changes seen on MRI imaging there were no significant improvements in diastolic function, 6-min walk test or quality of life scoring. Further studies will be required to confirm which patients with HFpEF this therapy offers benefit to.
Hypertrophic Cardiomyopathy
The EXPLORER-HCM trial (Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy) previously reported that mavacamten, a cardiac myosin inhibitor, led to significant improvements in peak oxygen consumption (pVO2) and NYHA class of symptoms in patients with hypertrophic cardiomyopathy with significant left ventricular outflow tract (LVOT) obstruction [138]. A further analysis of EXPLORER-HCM has now also studied quality of life. A total of 251 patients with symptomatic obstructive hypertrophic cardiomyopathy (gradient ≥ 50 mmHg) and NYHA class II–III symptoms were assigned to mavacamten (n = 123) or placebo (n = 128) for 30 weeks. At 30 weeks, the change in KCCQ-OS (overall summary) score was greater with mavacamten (14.9 [SD 15.8]) than placebo (5.4 [SD 13.7]) (difference + 9.1, 95% CI 5.5–12.8; p < 0.0001) and interestingly the beneficial effect was lost when the drug was stopped. The previous efficacy data and these new quality of life data support use of this drug for this group of patients.
Chemotherapy-Related Heart Failure
It is known that breast cancer therapy containing anthracyclines and anti-human epidermal growth factor receptor 2 antibodies and radiotherapy can cause cancer treatment-related cardiac dysfunction. In the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), 120 patients were randomised to receive candesartan and metoprolol or placebo along with their cancer treatment [139]. There was no significant difference in change in LVEF between groups after a median of 23 months follow-up (candesartan, 1.7% [95% CI 0.5–2.8]; no candesartan, 1.8% [95% CI 0.6–3.0]; metoprolol, 1.6% [95% CI 0.4–2.7]; no metoprolol, 1.9% [95% CI 0.7–3.0]). This suggests that administering cardioprotective medication along with breast cancer therapy as standard is unlikely to be beneficial and this should instead be tailored to individual risk.
Advances in Prevention
Lipids
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors markedly reduce low-density lipoprotein (LDL) cholesterol levels and reduce CV event rates but until now have required subcutaneous administration [1]. Two parallel phase 1 trials of a daily oral PCSK9 inhibitor, SPIRE-1 and SPIRE-2, have shown promising results. SPIRE-1 enrolled 16,817 patients with an LDL-C of at least 70 mg/dl and SPIRE-2 enrolled 10,621 patients with an LDL of at least 100 mg/dl [140]. The rate of familial hypercholesterolemia was higher in SPIRE-2 than SPIRE-1. The agent, currently named MK-0616, was shown to reduce plasma PCSK9 levels by more than 90% and LDL-C levels reduced by more than 50% when given daily for 2 weeks to 100 patients already on statin therapy. The drug was also shown to be well tolerated in the study with no significant increase in adverse events or deaths in the treatment groups. These results indicate an oral PCSK9 inhibitor may be effective, which would certainly be more convenient for patients requiring this treatment. Unfortunately, these two trials were stopped prematurely as the sponsor has discontinued further development of the drug because of immunogenicity issues.
The utility of high dose eicosapentoic acid (EPA) for improving CV risk has been debated over the last few years. The REDUCE-IT trial previously reported reduced CV events with the use of this agent in contrast to other omega-3 studies and suggested this might be due to higher serum EPA levels achieved in REDUCE-IT [1]. However, a new analysis of the STRENGTH trial (Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) failed to show any association between higher plasma levels of EPA and reduced cardiac events. In this study, 13,078 patients were randomised to receive either ω-3 carboxylic acid (CA) or an inert comparator, corn oil [141]. With a composite endpoint of CV death, MI, stroke, coronary revascularisation or unstable angina with hospital admission the adjusted hazard ratio for the highest tertile of plasma EPA levels vs. corn oil was 0.98 (95% CI 0.83–1.16; p = 0.81). This result raises questions about the mechanism of benefit seen in REDUCE-IT and the role of fish oils remains unclear.
Hypertension
With the therapeutic effect of renal artery denervation (RDN) now proven in the management of resistant hypertension, trials are ongoing to establish its optimum role. The RADIANCE-HTN TRIO study randomised 136 patients, already on three antihypertensive agents with a blood pressure (BP) of greater than 140/90 mmHg, to either ultrasound RDN (n = 69) or a sham procedure (n = 67) [142]. The RDN group had a greater decrease in their ambulatory systolic BP then the sham group (− 8.0 mmHg vs. − 3.0 mmHg) with a median between group difference of − 4.5 mmHg (95% CI − 8.5 to − 0.3; p = 0.022). This adds further evidence to suggest that in some patients with truly resistant hypertension, RDN may be a viable option and that completing this procedure using ultrasound rather than radiofrequency ablation is both safe and effective.
The QUARTET study (Quadruple UltrA-low-dose Treatment for hypertension) examined the use of a combination pill containing four BP-lowering agents (irbesartan, amlodipine, indapamide and bisoprolol) in 591 patients with hypertension who were either on no agent or monotherapy at enrolment with 300 patients assigned to receive the quadpill treatment and 291 to receive standard monotherapy [143]. At 12 weeks BP control rates (standard office BP < 140/90 mmHg) were higher in the quadpill group (76%) vs. the control group (58%) (RR 1.30, 95% CI 1.15–1.47; p < 0.0001) and systolic BP was on average 6.9 mmHg lower in the in the quadpill group (95% CI 4.9–8.9; p < 0.0001). This treatment approach may offer better BP control and simplify treatment for patients.
Aggressive treatment of hypertension always needs to be weighed with tolerability of the medications used and it remains unclear what the appropriate target BP for older adults should be. The STEP trial (Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension) assigned 8511 adults aged 60–80 years to either intensive treatment with a target BP of 110–130 mmHg (n = 4232) or standard treatment with a target of 130–150 mmHg (n = 4268) [144]. The mean systolic BP was lower in the intensive treatment group than the standard treatment group at 1 year (127.5 mmHg vs. 135.3 mmHg) and over a median follow-up of 3.34 years the primary composite endpoint of stroke, ACS, acute HF, coronary revascularisation, AF or CV death occurred in significantly fewer of the intensive treatment group vs. the standard treatment group (HR 0.74, 95% CI 0.60–0.92; p = 0.007). The incidence of hypotension was significantly greater in the intensive treatment group (3.4% vs. 2.6%; p = 0.03); however, there was no significant difference between groups in the occurrence of syncope, dizziness or fracture, indicating the intensive control strategy was safe.
Interestingly an analysis of the NHANES cohort showed that 18.5% of patients with known hypertension were co-prescribed drugs known to increase BP [145]. These medications included antidepressants, nonsteroidal anti-inflammatory drugs (NSAIDs), steroids and oestrogens which are all commonly prescribed but also have alternatives. This study highlights the need to review other medications alongside antihypertensives in patients with hypertension.
Primary Prevention
A meta-analysis of the TIPS-3, HOPE-3 and Polyran trials examined the use of a polypill for primary prevention and CV risk reduction. Each trial had assessed the use of a fixed-dose combination pill containing at least two antihypertensive agents, a statin and in some cases aspirin and compared this to a placebo or usual care arm. There were 18,162 participants included in the analysis and the participants randomised to receive the polypill had significantly less incidence of a composite endpoint of CV death, MI, stroke or arterial revascularisation than the standard care participants (3.0% vs. 4.9%; HR 0.62, 95% CI 0.53–0.73; p < 0.0001) (Fig. 9) [146]. While the largest risk reduction was seen in those participants taking preparations containing aspirin there was a trend towards more gastrointestinal bleeding in this group.
Basic interventions remain important in the management of CV risk. The Salt Substitute and Stroke Study (SSaSS) completed in rural China demonstrated reduced major CV events in a group of adults using a low sodium salt substitute. Over 20,000 participants with a previous stroke or aged 60 years or older with hypertension were randomised to receive a salt substitute or regular salt [147]. After a mean follow-up period of 4.74 years the rate of major CV events, stroke and death was lower in the salt substitute group vs. the regular group (49.09 CV events vs. 56.29 CV events per 1000 person-years; rate ratio 0.87, 95% CI 0.80–0.94; p < 0.001), (29.14 stroke events vs. 33.65 stroke events per 1000 person-years; rate ratio 0.86, 95% CI 0.77–0.96; p = 0.006) and (39.28 deaths vs. 44.61 deaths per 1000 person-years; rate ratio 0.88, 95% CI 0.82–0.95; p < 0.001).
The FIDELITY analysis (Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease) combined data from the FIDELIO-DKD and FIGARO-DKD trials to examine the utility the nonsteroidal MRA finerenone in treating patients with type 2 diabetes and chronic kidney disease [148]. Over 13,000 adults with T2DM and chronic kidney disease (CKD) (40% of whom had eGFR > 60 mL/min/1.73 m2 but raised urinary albumin–creatinine ratio) were randomised to finerenone vs. placebo. Finerenone was associated with a reduction in the primary endpoint of CV death, MI, stroke or HF hospitalisation (12.7% vs. 14.4%; HR 0.86, 95% CI 0.78–0.95; p = 0.018) and in the renal endpoint of kidney failure, sustained decrease in eGFR or renal death (5.5% vs. 7.1%; HR 0.77, 95% CI 0.67–0.88; p = 0.0002). This trial highlights the importance of measuring urine albumin–creatinine ratio in patients with type 2 diabetes as it may identify those with early renal impairment who may benefit from finerenone.
Undertreatment of hypertension and hyperlipidaemia increase baseline CV risk and novel ways to manage this using technology require consideration. One study this year reported on a remote digital care programme that was run by “navigators” and pharmacists to deliver care for patients with high CV risk based on an algorithm [149]. Patients (n = 10,803) were provided with BP monitors and frequent contacts were done by telephone call or text and email if patients were comfortable with this and no in-person contact was necessary. In those who completed the programme, 92% reached their BP goal and 94% achieved their LDL goal. This approach improved CV risk profiles, limited face-to-face contacts and reduced required physician time.
Limitations
While all summarised trials have been presented at major cardiology conferences in 2021 not all trials have been published as yet in peer-reviewed journals.
Conclusion
This paper has highlighted and summarised the key cardiology trials that were published and presented during 2021. Many will guide clinical practice and influence guideline development. Others have shown encouraging early data which will guide future study.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author contributions
PS—Lead author, collated clinical trials for review and divided sections across authors. Wrote structural, shock and ACS sections as well as abstract, introduction and summary table. Compiled all sections into one document and collated references. Implemented final edits from supervising consultant and reviewers. BC—Wrote intervention section. KL—Wrote Heart failure and prevention section; JC—Wrote antiplatelet section; MS—Wrote EP/Devices section; IM—Supervising consultant. Guided style and format of review paper. Reviewed and edited final document with changes fed back to and incorporated into final paper by lead author.
Disclosures
Ian Menown has received grants to institution, honoraria and/or conference sponsorship from Biosensors, Meril Life, Bayer, Boehringer Ingelheim, and Daichii Sankyo. Other authors have nothing to disclose.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
References
- 1.Kearney A, Linden K, Savage P, Menown IBA. Advances in clinical cardiology 2020: a summary of key clinical trials. Adv Ther. 2021;38(5):2170–2200. doi: 10.1007/s12325-021-01711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia S, Dehghani P, Grines C, et al. Initial findings from the North American COVID-19 myocardial infarction registry. J Am Coll Cardiol. 2021;77(16):1994–2003. doi: 10.1016/j.jacc.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahim C, Capitana A, Stanaway N, et al. Reducing EMS-to-balloon time—there's an app for that. J Am Coll Cardiol. 2021;77(18_suppl_2):16. doi: 10.1016/S0735-1097(21)01359-0. [DOI] [Google Scholar]
- 4.Mahmud E. Safety and efficacy of the second generation robotic-assisted system for percutaneous coronary intervention: final results of the multicenter PRECISION GRX study. Presented at SCAI 2021. April 29, 2021.
- 5.Safirstein JG, Kowalski M, Chiu S, et al. Knowledge retention after percutaneous coronary intervention. J Am Coll Cardiol. 2021;77(18_suppl_1):968. doi: 10.1016/S0735-1097(21)02327-5. [DOI] [Google Scholar]
- 6.Jia H, Dai J, He L, Xu Y, Shi Y, Zhao L, Sun Z, Liu Y, Weng Z, Feng X, Zhang D, Chen T, Zhang X, Li L, Xu Y, Wu Y, Yang Y, Wang C, Li L, Li J, Hou J, Liu B, Mintz GS, Yu B. EROSION III: A multicenter RCT of OCT-guided reperfusion in STEMI with early infarct artery patency. JACC Cardiovasc Interv. 2022 Mar 23:S1936-8798(22)00420-4. 10.1016/j.jcin.2022.01.298. [DOI] [PubMed]
- 7.Secemsky EA, Butala N, Raja A, et al. Temporal changes and institutional variation in use of percutaneous coronary intervention for ST-elevation myocardial infarction with multivessel coronary artery disease in the United States: an NCDR research to practice project. JAMA Cardiol. 2021;6(5):574–580. doi: 10.1001/jamacardio.2020.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puymirat E, Cayla G, Simon T, et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N Engl J Med. 2021;385:297–308. doi: 10.1056/NEJMoa2104650. [DOI] [PubMed] [Google Scholar]
- 9.Denormandie P, Simon T, Cayla G, et al. Compared outcomes of ST-segment-elevation myocardial infarction patients with multivessel disease treated with primary percutaneous coronary intervention and preserved fractional flow reserve of nonculprit lesions treated conservatively and of those with low fractional flow reserve managed invasively: insights from the FLOWER-MI trial. Circ Cardiovasc Interv. 2021;14(11):e011314. doi: 10.1161/CIRCINTERVENTIONS.121.011314. [DOI] [PubMed] [Google Scholar]
- 10.Curzen N, Rana O, Nicholas Z, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ Cardiovasc Interv. 2014;7(2):248–55. 10.1161/CIRCINTERVENTIONS.113.000978. [DOI] [PubMed]
- 11.Curzen N. RIPCORD 2: does routine pressure wire assessment influence management strategy of coronary angiography for diagnosis of chest pain. Presented at ESC 2021. August 29, 2021. [DOI] [PubMed]
- 12.Rioufol G, Dérimay F, Roubille F, et al. FUTURE Trial Investigators. Fractional flow reserve to guide treatment of patients with multivessel coronary artery disease. J Am Coll Cardiol. 2021;78(19):1875–1885. doi: 10.1016/j.jacc.2021.08.061. [DOI] [PubMed] [Google Scholar]
- 13.Fearon WF, Zimmermann FM, De Bruyne B, et al., On behalf of the FAME 3 Investigators. Fractional flow reserve-guided PCI as compared with coronary bypass surgery. N Engl J Med. 2022. 10.1056/NEJMoa2112299. [DOI] [PubMed]
- 14.Götberg M. iFR-SWEDEHEART: five-year outcomes of a randomised trial of iFR-guided vs. FFR-guided PCI. Presented at TCT 2021, Orlando. 2021.
- 15.Xu B, Tu S, Song L, Jin Z, Yu B, Fu G, et al. FAVOR III China study group. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398(10317):2149–2159. doi: 10.1016/S0140-6736(21)02248-0. [DOI] [PubMed] [Google Scholar]
- 16.Kandzari DE, Salisbury AC, Grantham JA, et al. Outcomes of percutaneous revascularization for the management of surgically ineligible patients with multivessel or left main coronary artery disease: primary results from the OPTIMUM registry. Presented at TCT 2021, Orlando. 2021. [DOI] [PubMed]
- 17.Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents vs coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet. 2021. 10.1016/S0140-6736(21)02334-5. [DOI] [PubMed]
- 18.Price M. Intravascular lithotripsy for treatment of severely calcified coronary lesions: 1-year results from the disrupt CAD III study. Presented at TCT 2021, Orlando. 2021. [DOI] [PMC free article] [PubMed]
- 19.Naverese EP, Lansky AJ, Kereiakes DJ, et al. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: a systematic review and meta-analysis. Eur Heart J. 2021;42(45):4638–4651. doi: 10.1093/eurheartj/ehab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patlolla SH, Kanwar A, Cheungpasitporn W, et al. Temporal trends, clinical characteristics, and outcomes of emergent coronary artery bypass grafting for acute myocardial infarction in the United States. J Am Heart Assoc. 2021;10(15):e020517. doi: 10.1161/JAHA.120.020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puskas J. Efficacy and safety of an external support device for saphenous vein coronary bypass grafts: the VEST trial. Presented at AHA 2021, 13 Nov 2021.
- 22.Pilgrim T, Muller O, Heg D, et al. Biodegradable- vs durable-polymer drug-eluting stents for STEMI: final 2-year outcomes of the BIOSTEMI trial. J Am Coll Cardiol Intv. 2021;14:639–648. doi: 10.1016/j.jcin.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M. A randomised study comparing imaging guided implantation of Orsiro and Xience - CASTLE study. Presented at EuroPCR 2021, 20 May 2021.
- 24.Madhavan MV, Howard JP, Naqvi A, et al. Long-term follow-up after ultrathin vs conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomised controlled trials. Eur Heart J. 2021;42(27):2643–2654. doi: 10.1093/eurheartj/ehab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menown IBA, Mamas MA, Cotton JM, et al. Thin strut CoCr biodegradable polymer Biolimus A9-eluting stents versus thicker strut stainless steel biodegradable polymer Biolimus A9-eluting stents: two-year clinical outcomes. J Interv Cardiol. 2021;1(2021):6654515. doi: 10.1155/2021/6654515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SV. Two-year results of the OPTIMIZE IDE trial: A randomised evaluation of sirolimus-eluting coronary stents with fixed-wire and rapid-exchange delivery systems and a novel bioresorbable drug carrier. Presented at: TCT 2021, Orlando. October 20, 2021.
- 27.Van Hemert ND, Voskuil M, Rozemeijer R, et al. 3-Year clinical outcomes after implantation of permanent-polymer vs polymer-free stent: ReCre8 landmark analysis. JACC Cardiovasc Interv. 2021;14(22):2477–2486. doi: 10.1016/j.jcin.2021.08.078. [DOI] [PubMed] [Google Scholar]
- 28.Romaguera R, Salinas P, Gomez-Lara J, et al. Amphilimus- vs. zotarolimus-eluting stents in patients with diabetes mellitus and coronary artery disease: the SUGAR trial. Eur Heart J. 2022;43(13):1320–1330. doi: 10.1093/eurheartj/ehab790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits PC, On behalf of the COMPARE-ABSORB investigators. 3-year results from the COMPARE-ABSORB trial. Presented at virtual CRT 2021. 13 Mar 2021.
- 30.Song L, Xu B, Chen Y, Zhou Y, Jia S, Zhong Z, Su X, Ma Y, Zhang Q, Liu J, Wang Y, Guan C, Zheng M, Qiao S, Gao R. FUTURE-II trial investigators. Thinner strut sirolimus-eluting BRS versus EES in patients with coronary artery disease: FUTURE-II trial. JACC Cardiovasc Interv. 2021;14(13):1450–1462. doi: 10.1016/j.jcin.2021.04.048. [DOI] [PubMed] [Google Scholar]
- 31.Lutz M, on behalf of the FANTOM II investigators. Safety and performance of the FANTOM sirolimus-eluting bioresorbable coronary scaffold: first report 5-year clinical outcomes. Presented at: TCT 2021, Orlando. October 20, 2021.
- 32.Ahmad WA. A randomised trial of sirolimus-coated vs paclitaxel-coated balloons in de novo coronary lesions. Presented at TCT 2021, Orlando. 6 Nov 2021.
- 33.Scheller B. One-year outcomes of two parallel randomised trials of sirolimus-coated and paclitaxel-coated balloons in coronary in-stent restenosis lesions. Presented at TCT 2021, Orlando. 6 Nov 2021.
- 34.Han Y. BIO-RISE China: a randomised trial of a biolimus-coated balloon vs POBA in small vessel coronary artery disease. Presented at TCT 2021, Orlando. 6 Nov 2021.
- 35.Seto A, Safirstein J, Tehrani D, Schussler J. Radial hemostasis is facilitated with a potassium ferrate hemostatic patch (StatSeal): the randomised controlled Statseal with TR Band assessment trial (STAT2). Presented at: Virtual SCAI 2021. April 28, 2021.
- 36.Meijers TA, Aminian A, van Wely M, et al. Randomized comparison between radial and femoral large-bore access for complex percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(12):1293–1303. doi: 10.1016/j.jcin.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Forrest J. Complete 2-year follow-up from the Evolut low risk trial. Presented at: EuroPCR 2021, Paris. May 18, 2021.
- 38.Van Mieghem NM. 5-year clinician and echocardiographic outcomes from the randomised SURTAVI trial. Presented at TCT 2021, Orlando, 5 Nov 2021.
- 39.Banovic M, Putnik S, Penicka M, et al. Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis: The avatar trial [published correction appears in Circulation. 2022 Mar;145(9):e761]. Circulation. 2022;145(9):648–658. 10.1161/CIRCULATIONAHA.121.057639. [DOI] [PubMed]
- 40.Cohen DJ. Economic outcomes of TAVR vs. SAVR for low-risk patients: results from the PARTNER 3 trial. Presented at TCT 2021, Orlando, 5 Nov 2021.
- 41.Butala N. Economics of minimalist TAVR: the 3M TAVR economic study. Presented at TVT 2021, 22 July 2021, Miami.
- 42.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease [published correction appears in Eur Heart J. 2022 Feb 18;:]. Eur Heart J. 2022;43(7):561–632. 10.1093/eurheartj/ehab395. [DOI] [PubMed]
- 43.Van Mieghem NM, Unverdorben M, Hengstenberg C, et al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med. 2021;385(23):2150–2160. doi: 10.1056/NEJMoa2111016. [DOI] [PubMed] [Google Scholar]
- 44.Collet J-P. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: the randomised ATLANTIS trial. Presented at: Virtual ACC 2021. May 15, 2021. [DOI] [PubMed]
- 45.Kwiecinski J, Tzolos E, Cartlidge TRG, et al. Native aortic valve disease progression and bioprosthetic valve degeneration in patients with transcatheter aortic valve implantation. Circulation. 2021;144(17):1396–1408. doi: 10.1161/CIRCULATIONAHA.121.056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrest JK, Ramlawi B, Deeb GM, et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6(1):50–57. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams M. The PARTNER 3 bicuspid registry for Sapien 3 TAVR in low-risk patients. Presented at: TVT 2021, Miami. July 20, 2021.
- 48.Brinkmann C, Abdel-Wahab M, Bedogni F, et al. Outcomes of valve-in-valve transcatheter aortic valve implantation with and without bioprosthetic valve fracture. EuroIntervention. 2021;17(10):848–855. doi: 10.4244/EIJ-D-21-00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan JM, Babaliaros V, Greenbaum AB, et al. Preventing coronary obstruction during transcatheter aortic valve replacement: results from the multicenter international BASILICA Registry. JACC Cardiovasc Interv. 2021;14(9):941–948. doi: 10.1016/j.jcin.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdel-Wahab M, Hartung P, Dumpies O, et al. Comparison of a pure plug-based vs a primary suture-based vascular closure device strategy for transfemoral transcatheter aortic valve replacement: the CHOICE-CLOSURE randomised clinical trial. Circulation. 2022;145(3):170–183. doi: 10.1161/CIRCULATIONAHA.121.057856. [DOI] [PubMed] [Google Scholar]
- 51.Szerlip M, Spargias KS, Makkar R, et al. 2-year outcomes for transcatheter repair in patients with mitral regurgitation from the CLASP study. JACC Cardiovasc Interv. 2021;14(14):1538–1548. doi: 10.1016/j.jcin.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko T, Hirji S, Zaid S, et al. Mitral valve surgery after transcatheter edge-to-edge repair: mid-term outcomes from the CUTTING-EDGE International Registry. JACC Cardiovasc Interv. 2021;14(18):2010–2021. 10.1016/j.jcin.2021.07.029. [DOI] [PubMed]
- 53.Zahr F, Song HK, Chadderdon SM, et al. Thirty-day outcomes following transfemoral transseptal transcatheter mitral valve replacement: intrepid TMVR early feasibility study results. JACC Cardiovasc Interv. 2021 doi: 10.1016/j.jcin.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Gammie JS, Chu MWA, Falk V, et al. Concomitant tricuspid repair in patients with degenerative mitral regurgitation. N Engl J Med. 2022;386(4):327–339. doi: 10.1056/NEJMoa2115961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickenig G, Friedrichs K, Baldus S, et al. Thirty-day outcomes of the cardioband tricuspid system for patients with symptomatic functional tricuspid regurgitation: the TriBAND study. EuroIntervention. 2021;17(10):809–817. doi: 10.4244/EIJ-D-21-00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lurz P, von Bardeleben RS, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77:229–39. [DOI] [PubMed]
- 57.Eleid M. Transcatheter valve repair in patients with severe tricuspid regurgitation: six-month results of the CLASP TR early feasibility study. Presented at EuroPCR, Paris. May 21, 2021.
- 58.Kodali S. Transfemoral tricuspid valve replacement: TRISCEND study 30-day results. Presented at: EuroPCR, Paris. May 21 2021. [DOI] [PubMed]
- 59.Kodali S. Tricuspid valve replacement in patients with tricuspid regurgitation: 6-month outcomes from the TRISCEND study. Presented at TCT 2021, 6 Nov 2021, Orlando.
- 60.Jones TK. The Harmony transcatheter pulmonary valve: 1-year outcomes from the pivotal trial and 30-day outcomes from the continued access study. Presented at SCAI 2021, 30 Apr 2021.
- 61.Reddy VK, Doshi SK, Kar S, et al.. Left atrial appendage closure: 5-year outcomes of the PREVAIL and PROTECT-AF trials. J Am Coll Cardiol. 2017;70:2964–75. [DOI] [PubMed]
- 62.Price MJ. 1-year clinical outcomes following Watchman transcatheter LAAO for stroke prevention in patients with atrial fibrillation: a report from the NCDR LAAO Registry. Presented at: ACC Virtual 2021. May 15, 2021.
- 63.Smancik P, Herman D, Neuzil P, et al. Left atrial appendage closure vs direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(25):3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
- 64.Tirado-Conte G, on behalf of the LATOP registry investigators. Management and outcomes of patients with left atrial appendage prior to percutaneous closure. Presented at: TCT Orlando 2021. October 6, 2021.
- 65.Galea R, De Marco F, Meneveau N, et al. Amulet or watchman device for percutaneous left atrial appendage closure: primary results of the SWISS-APERO randomised clinical trial. Circulation. 2022;145(10):724–738. doi: 10.1161/CIRCULATIONAHA.121.057859. [DOI] [PubMed] [Google Scholar]
- 66.Lakkireddy D. Primary outcomes of the Amplatzer Amulet IDE randomised controlled trial. Presented at: ESC Virtual 2021. August 30, 2021.
- 67.Kar S. First report of 2-year outcomes with a next-generation left atrial appendage closure device: final results from the PINNACLE FLX IDE trial. Presented at TVT 2021, 21 July 2021, Miami.
- 68.Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081–2091. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- 69.Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. 2021;398(10316):2075–2083. doi: 10.1016/S0140-6736(21)02490-9. [DOI] [PubMed] [Google Scholar]
- 70.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kini A, Cao D, Nardin M, et al. Types of myocardial injury and mid-term outcomes in patients with COVID-19. Eur Heart J Qual Care Clin Outcomes. 2021;7(5):438–446. doi: 10.1093/ehjqcco/qcab053. [DOI] [PubMed] [Google Scholar]
- 72.Fröbert O, Götberg M, Erlinge D, et al. Influenza vaccination after myocardial infarction: a randomised, double-blind, placebo-controlled, multicenter trial. Circulation. 2021. [DOI] [PubMed]
- 73.Garcia M. Psychological distress and the risk of adverse cardiovascular outcomes in young and middle-aged survivors of myocardial infarction. Presented at the American College of Cardiology Annual Scientific Sessions. Virtual Meeting. May 16, 2021.
- 74.Mewton N, Roubille R, Bresson D, et al. Effect of colchicine on myocardial injury in acute myocardial infarction. Circulation. 2021;144(11):859–869. doi: 10.1161/CIRCULATIONAHA.121.056177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lambrakis K, Papendick C, French JK, et al. Late outcomes of the RAPID-TnT RCT: a 0/1-hour high-sensitivity troponin T protocol in suspected ACS. Circulation. 2021;144(2):113–125. doi: 10.1161/CIRCULATIONAHA.121.055009. [DOI] [PubMed] [Google Scholar]
- 76.Ganguli I, Cui J, Thakore N, et al. Downstream cascades of care following high-sensitivity troponin test implementation. J Am Coll Cardiol. 2021;77:3171–3179. doi: 10.1016/j.jacc.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ola O, Akula A, De Michieli L, et al. Clinical impact of high-sensitivity cardiac troponin-T assays in the community. J Am Coll Cardiol. 2021;77:3160–3170. doi: 10.1016/j.jacc.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary angiography after cardiac arrest without ST segment elevation: one-year outcomes of the COACT randomised clinical trial. JAMA Cardiol. 2020;5:1358–1365. doi: 10.1001/jamacardio.2020.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desch S, Freund A, Akin M, et al. Angiography after out-of-hospital cardiac arrest without ST-segment elevation. N Engl J Med. 2021;385(27):2544–2553. doi: 10.1056/NEJMoa2101909. [DOI] [PubMed] [Google Scholar]
- 80.Gentile FR, Primi R, Baldi E, et al. Out-of-hospital cardiac arrest and ambient air pollution: a dose-effect relationship and an association with OHCA incidence. PLoS ONE. 2021;16(8):e0256526. doi: 10.1371/journal.pone.0256526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bělohlávek J. Hyperinvasive approach in refractory out-of-hospital cardiac arrest: an open-label randomised controlled trial. Prague OHCA study. Presented at the American College of Cardiology Annual Scientific Sessions. Virtual Meeting. May 17, 2021.
- 82.Le May MR. Therapeutic hypothermia following out-of-hospital cardiac arrest: a randomised trial comparing mild and moderate therapeutic hypothermia. Presented at the American College of Cardiology Annual Scientific Sessions. Virtual Meeting. May 17, 2021.
- 83.Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia vs normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384:2283–2294. doi: 10.1056/NEJMoa2100591. [DOI] [PubMed] [Google Scholar]
- 84.Noc M, Laanmets P, Neskovic AN, et al. A multicentre, prospective, randomised controlled trial to assess the safety and effectiveness of cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction: the COOL AMI EU Pivotal Trial. EuroIntervention. 2021 doi: 10.4244/EIJ-D-21-00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valgimigli M, Frigoli E, Heg D, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021;385(18):1643–1655. 10.1056/NEJMoa2108749. [DOI] [PubMed]
- 86.Watanabe H, Domei T, Morimoto T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomised clinical trial. JAMA. 2019;321(24). [DOI] [PMC free article] [PubMed]
- 87.Watanabe H. Short and optimal duration of dual antiplatelet therapy after everolimus-eluting cobalt-chromium stent-2 acute coronary syndrome—STOPDAPT-2 ACS. Presented at the European Society of Cardiology Virtual Congress, 30 Aug 2021.
- 88.Obayashi Y, Yamamoto K. STOPDAPT-2 total cohort: pooled results from two randomised controlled trials of clopidogrel monotherapy after 1-month DAPT following PCI, and subgroup analyses by ACS presentation, HBR, and complex PCI. Presented at TCT 2021, Orlando, 5 Nov 2021.
- 89.Vogel B, Baber U, Cohen DJ, et al. Sex differences among patients with high risk receiving ticagrelor with or without aspirin after percutaneous coronary intervention: a subgroup analysis of the TWILIGHT randomised clinical trial. JAMA Cardiol. 2021;6(9):1032–1041. doi: 10.1001/jamacardio.2021.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. 2021;373:1332. doi: 10.1136/bmj.n1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang HY, Mo R, Guan CD, et al. Establishing the optimal duration of DAPT following PCI in high-risk TWILIGHT-like patients with acute coronary syndrome. Catheter Cardiovasc Interv. 2021. 10.1002/ccd.29741. [DOI] [PubMed]
- 92.Vogel RF. Long-term efficacy of pre-hospital treatment with crushed vs integral tablets of prasugrel in patients presenting with STEMI: 1-year results from the randomised COMPARE CRUSH trial. Presented at TCT 2021, 13 Oct 2021.
- 93.Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomised study. Eur Heart J. 2017;38(41):3070–3078. doi: 10.1093/eurheartj/ehx175. [DOI] [PubMed] [Google Scholar]
- 94.Kim CJ, Park MW, Kim MC, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398(10308):1305–1316. [DOI] [PubMed]
- 95.Koo BK, Kang J, Park KW, et al. Aspirin vs clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021;397(10293):2487–2496. [DOI] [PubMed]
- 96.Pereira N. Effect of genotype-guided oral P2Y12 inhibitors vs conventional clopidogrel on long-term ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomised clinical trial follow-up study. Presented at ACC 2021, 17 May 2021. [DOI] [PMC free article] [PubMed]
- 97.Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–34. [DOI] [PubMed]
- 98.Wöhrle J, Seeger J, Lahu S, et al. Ticagrelor or prasugrel in patients with acute coronary syndrome in relation to estimated glomerular filtration rate. JACC Cardiovasc Interv. 2021;14(17):1857–1866. doi: 10.1016/j.jcin.2021.06.028. [DOI] [PubMed] [Google Scholar]
- 99.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 100.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht310.P4876. [DOI] [PubMed] [Google Scholar]
- 101.Jones WS, Mulder H, Wruck LM, et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N Engl J Med. 2021;384(21):1981–1990. [DOI] [PMC free article] [PubMed]
- 102.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han Y, Liao Z, Li Y, et al. Magnetically-controlled capsule endoscopy for assessment of antiplatelet therapy-induced gastrointestinal injury. J Am Coll Cardiol. 2022;79(2):116–128. doi: 10.1016/j.jacc.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 104.So DYF. A randomised study of early vs. delayed coronary artery bypass surgery among patients with acute coronary syndromes treated with ticagrelor: the RAPID CABG study. Presented at AHA 2021, 13 Nov 2021.
- 105.Bor WL, Zheng KL, Tavenier AH, et al. Pharmacokinetics, pharmacodynamics, and tolerability of subcutaneous administration of a novel glycoprotein IIb/IIIa inhibitor, RUC-4, in patients with ST-segment elevation myocardial infarction. EuroIntervention. 2021;17:5. doi: 10.4244/EIJ-D-21-00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhatt DL. REVERSE-IT: effect of bentracimab on platelet inhibition and hemostasis in patients on ticagrelor with major bleeding or requiring urgent procedures. Presented at the American Heart Association Annual Scientific Sessions. Virtual Meeting. November 15, 2021.
- 107.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lubitz SA. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Presented at the American Heart Association Annual Scientific Sessions. Virtual Meeting. November 14, 2021.
- 109.Svendsen JH, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial [published correction appears in Lancet. 2021 Oct 23;398(10310):1486] Lancet. 2021;398(10310):1507–1516. doi: 10.1016/S0140-6736(21)01698-6. [DOI] [PubMed] [Google Scholar]
- 110.Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. 2021;398(10310):1498–1506. doi: 10.1016/S0140-6736(21)01637-8. [DOI] [PubMed] [Google Scholar]
- 111.Marcus GM, Modrow MF, Schmid CH, et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomised clinical trial. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 113.Tang A. A randomised ablation-based atrial fibrillation rhythm control vs rate control trial in patients with heart failure and high burden of atrial fibrillation: RAFT-AF. Presented at ACC 2021, 17 May 2021. [DOI] [PubMed]
- 114.Rienstra M. Targeted therapy of underlying conditions in patients with persistent atrial fibrillation and mild to moderate stable heart failure: long term outcome of the RACE 3 trial. Presented at: EHRA Virtual 2021. April 23, 2021.
- 115.Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. EP Europace. 2019;23(7):1033–1041. doi: 10.1093/europace/euab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Darby AE. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9(1):1427. doi: 10.4022/jafib.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311(5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 118.Marrouche NF, Greene T, Dean JM, et al. DECAAF II: efficacy of DE-MRI-guided fibrosis ablation vs. conventional catheter ablation of persistent atrial fibrillation. Presented at ESC 2021. 28 Aug 2021.
- 119.Teres C, Soto-Iglesias D, Penela D, et al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the 'ablate by-LAW' single-centre study-a pilot study. Europace. 2021 doi: 10.1093/europace/euab216. [DOI] [PubMed] [Google Scholar]
- 120.Bauer A, Sappler N, Stulpnagel L von, Klemm M, Schreinlechner M, Wenner F, Schier J et al. Implantable cardiac monitors in high-risk post-infarction patients with cardiac autonomic dysfunction and moderately reduced left ventricular ejection fraction. Presented at: ESC 2021. August 27, 2021.
- 121.Brignole M, Pokushalov E, Pentimalli F, et al. A randomised controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39(45):3999–4008. 10.1093/eurheartj/ehy555. [DOI] [PubMed]
- 122.Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021;42(46):4731–4739. 10.1093/eurheartj/ehab569. (Errata in Eur Heart J. 2021 Oct 16 and Dec 08). [DOI] [PubMed]
- 123.Glikson M, Nielsen JC, Kronborg MB, et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA) Eur Heart J. 2021;42(35):3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 124.Vinther M, Risum N, Svendsen JH, Møgelvang R, Philbert BT. A randomised trial of His pacing vs biventricular pacing in symptomatic HF patients with left bundle branch block (His-alternative) JACC Clin Electrophysiol. 2021 doi: 10.1016/j.jacep.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Lambiase P, Theuns DAMJ, Murgatroyd FD, et al. Five year outcomes of the subcutaneous implantable cardioverter-defibrillator EFFORTLESS (evaluation of factors impacting clinical outcome and cost effectiveness of the S-ICD) registry. EP Europace. 2021;23(3):116417. doi: 10.1093/europace/euab116.417. [DOI] [Google Scholar]
- 126.Voors A. Empagliflozin in patients hospitalised for acute heart failure—EMPULSE. Presented at the American Heart Association Annual Scientific Sessions. Virtual meeting. 14 Nov 2021.
- 127.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 128.Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727–3738. doi: 10.1093/eurheartj/ehab560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pfeffer MA, Claggett B, Lewis EF, et al. Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. N Engl J Med. 2021;385(20):1845–1855. [DOI] [PubMed]
- 130.Mann DL, Givertz MM, Vader JM, et al. Effect of treatment with Sacubitril/Valsartan in patients with advanced heart failure and reduced ejection fraction: a randomised clinical trial. JAMA Cardiol. 2021;e214567. [DOI] [PMC free article] [PubMed]
- 131.Teerlink JR, Diaz R, Felker GM, et al. Effect of ejection fraction on clinical outcomes in patients treated with Omecamtiv Mecarbil in GALACTIC-HF. J Am Coll Cardiol. 2021;78(2):97–108. [DOI] [PubMed]
- 132.Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398(10304):991–1001. doi: 10.1016/S0140-6736(21)01754-2. [DOI] [PubMed] [Google Scholar]
- 133.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385(3):203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 135.Zannad F. EMPEROR-preserved: empagliflozin and outcomes in heart failure with a preserved ejection fraction and CKD. Presented at kidney week 2021. 5 Nov 2021.
- 136.Bhatt D. Benefits of sodium glucose co-transporter-1/2 inhibition with sotagliflozin across the full spectrum of ejection fraction, including heart failure with preserved ejection fraction. Presented at the American College of Cardiology scientific sessions. Virtual meeting. 17 May 2021.
- 137.Miller C. Perfenidone in heart failure with preserved ejection fraction – Pirouette. Presented at the American College of Cardiology scientific sessions. Virtual meeting. 17 May 2021.
- 138.Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10293):2467–2475. doi: 10.1016/S0140-6736(21)00763-7. [DOI] [PubMed] [Google Scholar]
- 139.Heck SL, Mecinaj A, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2×2 factorial, randomised, placebo-controlled, double-blind clinical trial of Candesartan and Metoprolol. Circulation. 2021;143(25):2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johns DG. The clinical safety, pharmacokinetics, and LDL-cholesterol lowering efficacy of MK-0616, an oral PCSK9 inhibitor. Presented at the American Heart Association annual scientific sessions. Virtual meeting. 15 Nov 2021.
- 141.Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH trial. JAMA Cardiol. 2021;6(8):1–8. doi: 10.1001/jamacardio.2021.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–2486. [DOI] [PubMed]
- 143.Chow CK, Atkins ER, Hillis GS, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines vs standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398(10305):1043–1052. doi: 10.1016/S0140-6736(21)01922-X. [DOI] [PubMed] [Google Scholar]
- 144.Steffen HM, Bönner G, Middeke M. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(27):2589–2590. doi: 10.1056/NEJMc2117463. [DOI] [PubMed] [Google Scholar]
- 145.Vitarello J. Use and estimated impact of medications that raise blood pressure among US adults with hypertension: national cross sectional study. Presented at the American College of Cardiology annual scientific sessions. Virtual meeting. 16 May 2021.
- 146.Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet. 2021;398(10306):1133–1146. doi: 10.1016/S0140-6736(21)01827-4. [DOI] [PubMed] [Google Scholar]
- 147.Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385(12):1067–1077. doi: 10.1056/NEJMoa2105675. [DOI] [PubMed] [Google Scholar]
- 148.Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2021;ehab777. [DOI] [PMC free article] [PubMed]
- 149.Blood A. A remotely delivered hypertension and lipid program in 10,000 patients across and diverse healthcare network. Presented at the American Heart Association annual scientific sessions. Virtual meeting. 13 Nov 2021.