Abstract
Introduction
The use of oral fosfomycin for urinary tract infections (UTIs) caused by non-Escherichia coli uropathogens is uncertain, including Klebsiella pneumoniae, the second most common uropathogen.
Methods
A multicompartment bladder infection in vitro model was used with standard media and synthetic human urine (SHU) to simulate urinary fosfomycin exposure after a single 3 g oral dose (fAUC0–72 16884 mg·h/L, t½ 5.5 h) against 15 K. pneumoniae isolates including ATCC 13883 (MIC 2 to >1024 mg/L) with a constant media inflow (20 mL/h) and 4-hourly voiding of each bladder. The impact of the media (CAMHB + G6P versus SHU) on fosfomycin MIC measurements, drug-free growth kinetics and regrowth after fosfomycin administration was assessed. A low and high starting inoculum (5.5 versus 7.5 log10 cfu/mL) was assessed in the bladder infection model.
Results
Compared with CAMHB, isolates in SHU had a slower growth rate doubling time (37.7 versus 24.1 min) and reduced growth capacity (9.0 ± 0.3 versus 9.4 ± 0.3 log10 cfu/mL), which was further restricted with increased inflow rate (40 mL/h) and more frequent voids (2-hourly). Regrowth was commonly observed in both media with emergence of fosfomycin resistance promoted by a high starting inoculum in CAMHB (MIC rise to ≥1024 mg/L in 13/14 isolates). Resistance was rarely detected in SHU, even with a high starting inoculum (MIC rise to ≥1024 mg/L in 2/14 isolates).
Conclusions
Simulated in an in vitro UTI model, the regrowth of K. pneumoniae urinary isolates was inadequately suppressed following oral fosfomycin therapy. Efficacy was further reduced by a high starting inoculum.
Introduction
Urinary tract infections (UTIs) are among the most commonly encountered infections and the second most common cause of sepsis.1 There has been an 8-fold increase in antimicrobial resistance (AMR) among urinary pathogens over the past two decades.2 Oral fosfomycin is commonly recommended as a first-line agent in treatment guidelines. In adult patients attending emergency departments between 2010 and 2016, fosfomycin susceptibility among all uropathogens was 87.8%, and higher for Escherichia coli (97.5%).3 High susceptibility rates are maintained among MDR strains.4,5
Klebsiella pneumoniae is the second most common uropathogen implicated in uncomplicated UTIs.6 MDR strains of K. pneumoniae are challenging to treat and associated with increased mortality.7,8 Oral fosfomycin can represent the only oral antibiotic option for some strains.9,10K. pneumoniae have a WT population for fosfomycin with MIC ≤64 mg/L. Yet, in vitro activity is limited by heteroresistance and multiple mechanisms of resistance, including a chromosomally encoded fosA gene, defects in transporter genes, their regulators and target modifications.11,12 CLSI restrict oral fosfomycin susceptibility for E. coli uropathogens (S ≤64 mg/L, R >128 mg/L).13 EUCAST had considered oral fosfomycin susceptibility for all Enterobacterales; however, in 2021 they revised this to apply only to E. coli, and have lowered the breakpoint (S ≤8 mg/L, R >8 mg/L).14 Problems with fosfomycin susceptibility testing, however, have been widely described.15–19
Accurate simulation of the urinary environment is critical when translating in vitro to in vivo pharmacodynamics (PD). The availability of nutritional factors is related to MIC measurements, with reduced nutrients resulting in both a slower growth rate and could promote higher kill rate.20 Urine is nutritionally depleted and naturally antimicrobial, with hypertonicity, low pH, low oxygen content and high nitrites and urea. The measurement of fosfomycin activity, however, is promoted by the supplementation of laboratory media with glucose-6-phosphate (G6P), which is found in negligible concentrations in human urine.21 Urodynamics is an additional important factor that can promote UTI clinical cure, with increased urine production and frequent bladder voiding. These are all important factors when performing pharmacokinetics (PK)/PD studies using a dynamic bladder infection in vitro model.
To assess fosfomycin activity against K. pneumoniae within the urinary environment, we have performed in vitro PD profiling against clinical urinary isolates. The experimental parameters investigated were selected to assess the impact of the media on MIC measurements, growth kinetics and fosfomycin activity in a urodynamic simulation of UTI treatment with a single 3 g dose of oral fosfomycin. To assess the impact of baseline fosfomycin heteroresistance on the emergence of resistance, low and high starting inocula were assessed, and genomic changes in the post-exposure regrowth explored.
Methods
Media and antibiotic
Cation-adjusted Mueller–Hinton II agar (MHA, Becton Dickinson, USA), CAMHB (Becton Dickinson) and synthetic human urine (SHU) were used. SHU components are presented in Table S1 (Table S1 is available as Supplementary data at JAC Online), originally described by Ipe et al.22 Intravenous fosfomycin (Meiji Seika Pharma, Tokyo, Japan) was used. Where indicated, G6P (Sigma–Aldrich, USA) was added to CAMHB and SHU at a concentration of 25 mg/L, and to SHU at 0.2 mg/L to reflect the physiological urinary concentration.21
K. pneumoniae isolates and susceptibility testing
Clinical, non-duplicate K. pneumoniae urinary isolates were obtained from a surveillance collection at a tertiary acute care hospital in metropolitan Melbourne, Australia (Table S2; Ethics approval 533/16). Isolates underwent fosfomycin susceptibility testing by agar dilution (AD) following standard methodology.23 MIC ≤64 mg/L was used to distinguish WT from the non-WT strains. The differential effect of fosfomycin on 14 representative clinical isolates and ATCC 13883 was assessed (Table 1). These isolates underwent additional fosfomycin susceptibility testing by disc diffusion (FOT200 discs, Oxoid Ltd/Thermo Fisher Scientific, UK) and broth microdilution (BMD). Disc diffusion diameter >15 mm (ignoring inner colonies within inhibition zone) was used to distinguish WT from the non-WT strains.24 The impact of the media on MIC measurements was quantified, with the mean difference and SD calculated (see Supplementary Methods). High-level fosfomycin-resistant (HLR) subpopulations were assessed (see Supplementary Methods).
Table 1.
Baseline fosfomycin susceptibility and heteroresistance of the representative K. pneumoniae isolates
| Strain # | Standard susceptibility testing | Heteroresistance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AD MIC (mg/L)a | DD (inhibition zone, mm)b | High inoculum AD (mg/L)c | High inoculum DD (mm)d | Disc elution (time, h)e | RSP proportion in CAMHBf | RSP proportion in SHUf | |||
| Clinical isolates | |||||||||
| INF014 | 2 | WT | 25 | WT | 64 | 22 | POS (72) | 1E-07 | – |
| INF321 | 2 | WT | 24 | WT | 64 | 22 | POS (48) | 1E-06 | – |
| INF215 | 4 | WT | 24 | WT | 64 | 17 | POS (48) | 1E-07 | – |
| INF174 | 4 | WT | 23 | WT | 128 | 19 | POS (48) | 4E-07 | – |
| INF044 | 8 | WT | 23 | WT | 256 | 19 | POS (48) | 2E-06 | – |
| INF344 | 8 | WT | 21 | WT | 256 | 17 | POS (48) | 4E-06 | 9E-07 |
| INF079 | 8 | WT | 20 | WT | 256 | 18 | POS (48) | 8E-07 | – |
| INF018 | 8 | WT | 21 | WT | 256 | 15 | POS (48) | 1E-06 | 4E-06 |
| INF171 | 16 | WT | 24 | WT | 256 | 13 | POS (48) | 3E-06 | 1E-06 |
| INF142 | 32 | WT | 22 | WT | 256 | 16 | POS (72) | 3E-06 | 1E-05 |
| INF223 | 32 | WT | 22 | WT | 128 | 14 | POS (24) | 5E-07 | – |
| INF161 | 32 | WT | 20 | WT | 256 | 16 | POS (48) | 1E-05 | 4E-06 |
| INF348g | 128 | non-WT | 11 | non-WT | 512 | no zone | POS (24) | 3E-05 | 8E-06 |
| INF249g | >1024 | non-WT | no zone | non-WT | >1024 | no zone | POS (24) | 1E+00 | 7E-01 |
| ATCC | |||||||||
| 13883 | 64 | WT | 21 | WT | 1024 | 20 | POS (48) | 1E-05 | 1E-05 |
AD, agar dilution; DD, disc diffusion; RSP, resistant subpopulation; –, indicates resistant growth not detected after 24 h incubation.
AD was performed in triplicate (median value reported). An MIC ≤64 mg/L was used to distinguish WT from the non-WT strains.
DD was performed in triplicate (mean inhibition diameter reported). An inhibition diameter of >15 mm was used to distinguish WT from the non-WT strains. DD results applied the EUCAST reading guide that ignores any inner colonies within the inhibition zone.
AD performed with 1 × 106 cfu/spot.
DD performed with a lawn culture prepared directly using turbid overnight growth in CAMHB. Inhibition diameter ignores inner colonies within the inhibition zone.
Disc elution screening test with six FOT200 discs (Oxoid Ltd/Thermo Fisher Scientific, UK) added to 2 mL CAMHB, inoculated with 100 μL of a 109 cfu/mL bacterial suspension from an overnight culture in CAMHB, and assessed for turbidity (POS, positive) over 72 h incubation.
RSP was determined after 24 h incubation in the bladder infection model in CAMHB with G6P, or SHU, with an inflow rate of 20 mL/h and 4-hourly voiding. The proportion was calculated from growth quantified on MHA with 512 mg/L fosfomycin (and 25 mg/L G6P) compared with total growth on drug-free MHA. All RSPs were confirmed to have a fosfomycin MIC of ≥1024 mg/L by standard AD methodology, except for INF174 that measured MIC 512 mg/L.
Non-WT isolates (INF249 and INF348) both had a premature STOP codon in uhpB.
Bladder infection in vitro model
A modified multicompartment in vitro model that applies a continuous dilution system was used (see Supplementary Methods and Figure S1). Urinary excretion of fosfomycin after a single 3 g oral dose was simulated. Sixteen bladder compartments were run in parallel. Each bladder was inoculated with a K. pneumoniae isolate. Bladder filling and intermittent voiding mimicked normal human urodynamics. The main modification from the previous in vitro design25 was an increase in media inflow to 20 mL/h into each bladder compartment (previously 3.75 mL/h). Bladder compartments underwent 4-hourly voiding that reduced the volume to 5 mL. The impact of the media (CAMHB with G6P versus SHU) and the starting inoculum (5.5 versus 7.5 log10 cfu/mL) on regrowth and emergence of resistance was assessed following fosfomycin administration.
Fosfomycin PK
Observed urinary fosfomycin concentrations in healthy volunteers informed the in vitro simulation, with a peak concentration of 1000 mg/L after 6 h, urinary half-life 5.5 h and fAUC0–72 16884 mg·h/L.26–28 Measurement of fosfomycin concentrations was performed by ultra-high-performance liquid chromatography–tandem MS (UHPLC–MS/MS) (see Supplementary Methods).29 All 16 bladder compartments were sampled at peak concentration (6 h). Representative samples were collected from three bladder compartments at 4, 8, 24, 48 and 72 h. Measured concentrations were compared with target values using Bland–Altman plots and linear regression. Fosfomycin stability was confirmed, with <5% reduction in concentration after incubation at 37°C for 72 h.
Quantification of bacterial growth
Drug-free bacterial growth under static incubation (37°C with vigorous shaking) and dynamic incubation (in the bladder infection model) were compared in both CAMHB (with G6P) and SHU. Under drug-free conditions, the bladder infection model was run at two speeds (20 mL/h, 4-hourly voids; 40 mL/h, 2-hourly voids) to assess the impact of simulated increased urodynamics. Drug-free growth rate (presented as generation time, tgen) was obtained from linear regression of the growth curve over 2–4 h incubation, selected for when the growth was expected to be maximal. Growth capacity was the bacterial density after 24 h incubation. Comparison by one-way ANOVA.
Following fosfomycin administration in the bladder infection model, samples for bacterial density were collected at 4, 8, 24, 48 and 72 h. K. pneumoniae ATCC 13883 was run in duplicate as a biological replicate. Samples were collected directly from each bladder compartment. Bacterial cells were washed twice with saline and then underwent serial 10-fold dilutions in saline, of which 20 μL was plated from each dilution onto drug-free MHA (for total growth quantification) and on MHA with 512 mg/L fosfomycin and 25 mg/L G6P (for HLR quantification, performed every 24 h). Plates were incubated aerobically at 37°C for 16–20 h. Plates with fosfomycin were re-incubated for an additional 24 h. Limit of detection was 50 cfu/mL.
Molecular assessment of fosfomycin resistance
Baseline (parent) isolates underwent WGS as reported previously.30,31 A targeted exploration of key fosfomycin resistance genes and promoter regions was performed (murA, fosA, uhpT, glpT, uhpA, uhpB, uhpC, cyaA, ptsI and glpR) to identify sequence variations that distinguished non-WT isolates (fosfomycin MIC >64 mg/L) from those with lower MICs (see Supplementary Methods). Baseline HLR subpopulation and post-exposure regrowth strains were sequenced and compared with their respective parent strains. Sequence variations in fosfomycin resistance genes and promoter regions were assessed, as well as a broad exploration of single nucleotide variations (SNVs) across the chromosome (see Supplementary Methods). All sequence reads were deposited in the NCBI Sequence Read Archives (project accession PRJNA701073).
Results
Baseline fosfomycin susceptibility
Fifty K. pneumoniae clinical urinary isolates were selected for fosfomycin AD MIC testing. The fosfomycin MIC distribution was similar to EUCAST32 and a collection published from the Netherlands24 (Figure 1 and Table S2). Fourteen representative clinical isolates, and K. pneumoniae ATCC 13883, were selected for further testing based on their fosfomycin MIC (range 2 to >1024 mg/L). All isolates had disc diffusion diameters of ≤25 mm (Table 1). Isolates had a wide variety of STs, virulence and AMR genes (Table S3).
Figure 1.
Fosfomycin MIC distribution among clinical K. pneumoniae urinary isolates (n = 50). MIC performed by agar dilution. Solid bars represent isolates with MICs within the WT range.
Baseline fosfomycin-resistant subpopulations
All representative isolates had a detectable HLR subpopulation after overnight incubation in CAMHB with G6P. The HLR subpopulation was present in the WT strains at a proportion of 10–6–10–7 (Table 1). When incubated in SHU, only 8 of 15 isolates had a detectable HLR subpopulation. Given that the total growth capacity in SHU was 1 log10 cfu/mL lower compared with incubation in CAMHB, the HLR subpopulation was likely below the limit of detection in some strains. Using a high-density inoculum with modified AD and disc diffusion testing demonstrated universal elevations in MIC measurements and reductions in inhibition zone diameters (Table 1). All isolates were positive by a modified disc elution fosfomycin heteroresistance screening test (see Supplementary Methods).
Impact of the media on fosfomycin MIC measurements
Fosfomycin MIC measurements in different media are presented in Table S4. Compared with AD (MHA with 25 mg/L G6P), testing by BMD (CAMHB with 25 mg/L G6P) had a mean (±SD) difference of 1.1 ± 1.0 MIC dilutions. Testing without G6P supplementation increases the mean (±SD) difference in MIC measurements by 4.9 ± 2.0 dilutions (by AD in MHA) and by 4.0 ± 1.5 dilutions (by BMD in CAMHB [pH 7.3]), except isolate INF348 that maintained MIC within ≤1 2-fold dilution. All MIC values were ≥512 mg/L when tested in SHU (pH 5.6), with an insignificant change with the addition of a physiological amount of G6P (0.2 mg/L21). In contrast, when 25 mg/L G6P was added to SHU, all MIC measurements reduced, except for isolate INF348, which maintained an MIC of 1024 mg/L. In CAMHB, an acidic environment (pH 5.6) promoted a small increase in fosfomycin activity, reflected by a single MIC dilution reduction compared with pH 7.3 (mean difference: –0.7 ± 0.8 dilutions in CAMHB with G6P; –1.4 ± 0.6 dilutions in CAMHB without G6P). The impact of reducing the amount of nutrients in CAMHB was dependent on dilution with saline or water. Compared with full-strength CAMHB, MICs were maintained within ≤1 2-fold dilution with a 1:10 dilution with PBS (mean difference: –0.5 ± 1.0 dilutions in CAMHB with G6P; 0.5 ± 0.7 dilutions in CAMHB without G6P). In contrast, CAMHB (with and without G6P) diluted 1:10 with distilled water, MICs were greatly reduced (mean difference: –4.1 ± 1.7 dilutions in CAMHB with G6P; –4.0 ± 1.0 dilutions in CAMHB without G6P).
Impact of the media and simulated urodynamics on bacterial growth
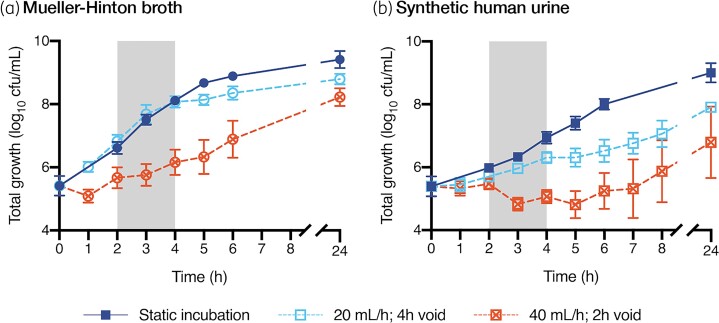
Bacterial growth under drug-free static conditions was slower in SHU compared with CAMHB (tgen 37.7 min in SHU versus 24.1 min in CAMHB [with G6P]) with a reduced growth capacity (9.0 ± 0.3 log10 cfu/mL in SHU versus 9.4 ± 0.3 log10 cfu/mL in CAMHB [with G6P], mean difference: –0.41 log10 cfu/mL, P = 0.0077) (Figure 2 and Table 2). When incubated in CAMHB under dynamic conditions in the bladder infection model, the change in bacterial density was most affected when the media inflow rate was increased to 40 mL/h with 2-hourly voids (tgen: 73.4 min). Compared with static incubation, growth capacity in CAMHB was reduced during dynamic incubation (mean difference at 20 mL/h: –0.62 log10 cfu/mL, P = 0.0002; mean difference at 40 mL/h: –1.2 log10 cfu/mL, P < 0.0001). Dynamic incubation in SHU resulted in greater impacts in growth kinetics (tgen at 20 mL/h: 60.1 min; tgen at 40 mL/h: bacterial density failed to increase over the initial 6 h of incubation) (Figure 2 and Table 2). Compared with static incubation, growth capacity in SHU was further reduced during dynamic incubation (mean difference at 20 mL/h: –1.1 log10 cfu/mL, P≤0.0001; mean difference at 40 mL/h: –2.2 log10 cfu/mL, P = 0.0001).
Figure 2.
Drug-free growth under static and dynamic incubation conditions. Bacterial growth is presented as the average (±SD) of the 14 clinical isolates and the ATCC strain. Static incubation performed in 20 mL media, incubated at 36°C with vigorous shaking (200 rpm), without media inflow or outflow. Dynamic incubation performed in the bladder infection in vitro model under two different conditions: media inflow at 20 mL/h with 4-hourly voids compared with 40 mL/h with 2-hourly voids. The grey shaded area highlights the time over which the growth rate was assessed (2–4 h). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 2.
Drug-free growth under static and dynamic incubation settings
| Media | Incubation conditionsa,b | Time period | ||
|---|---|---|---|---|
| Initial growth (2–4 h) | Maximum growth (24 h) | |||
| Δ in bacterial density (log10 cfu/mL) | Average tgen (min) | Total bacterial density (log10 cfu/mL) | ||
| CAMHB + G6P | Static | 1.5 (±0.2) | 24.1 | 9.4 (±0.3) |
| CAMHB + G6P | 20 mL/h; 4 h void | 1.3 (±0.2) | 28.9 | 8.8 (±0.2) |
| CAMHB + G6P | 40 mL/h; 2 h void | 0.5 (±0.3) | 73.4 | 8.2 (±0.3) |
| SHU | Static | 1.0 (±0.1) | 37.7 | 9.0 (±0.3) |
| SHU | 20 mL/h; 4 h void | 0.6 (±0.2) | 60.1 | 7.9 (±0.1) |
| SHU | 40 mL/h; 2 h void | –0.4 (±0.2) | NA | 6.8 (±1.1) |
NA, not applicable (due to a reduction in bacterial density that was observed over the initial 2–4 h time period).
Bacterial growth is presented as the average (±SD) of the 14 clinical isolates and the ATCC strain. The tgen (min) is calculated from the average change in bacterial density over a 2 h time period (2–4 h).
Static incubation in 20 mL media, incubated at 36°C with vigorous shaking (200 rpm), without any media inflow or outflow.
Dynamic incubation in the bladder infection in vitro model with constant media inflow and intermittent voiding.
Response to fosfomycin exposure in the bladder infection model
In vitro fosfomycin concentrations from the bladder compartments confirmed the targeted simulated fosfomycin exposure after a single 3 g oral dose (Figure S2). Despite humanized fosfomycin exposure, post-exposure regrowth was commonly observed across all representative K. pneumoniae isolates, regardless of baseline fosfomycin MIC (Table 3). HLR clinical isolate, INF249 (baseline MIC ≥1024 mg/L), regrew maximally in all dynamic experiments, with the quantification of the resistant subpopulation equal to the total growth at all sampling timepoints.
Table 3.
Post-exposure outcome (change in bacterial density at 72 h from starting inoculum) following single dose fosfomycin, comparison of high and low starting inoculum and incubation in different media
| Strain # | Media: CAMHB + G6P | Media: SHU | ||||||
|---|---|---|---|---|---|---|---|---|
| Low inoculuma | High inoculumb | Low inoculumc | High inoculumd | |||||
| TG (MIC) | RSP (Proportion) | TG (MIC) | RSP (Proportion) | TG (MIC) | RSP (Proportion) | TG (MIC) | RSP (Proportion) | |
| Clinical isolates | ||||||||
| INF014 | –3.2 (2) | – | 1.6 (>1024) | 7.1 (5E-01) | 2.8 (2) | – | 0.5 (512) | 3.2 (7E-04) |
| INF321 | – | – | – | – | 3.0 (8) | – | 0.2 (8) | – |
| INF215 | 1.8 (4) | – | 1.4 (>1024) | 7.1 (8E-01) | 3.1 (>1024) | 4.2 (3E-03) | 0.8 (>1024) | 5.2 (7E-02) |
| INF174 | – | – | 2.1 (>1024) | 6.9 (6E-01) | 2.6 (8) | 0.3 (1E-06) | 0.3 (16) | 1.9 (9E-05) |
| INF044 | –0.4 (8) | – | 1.4 (>1024) | 7.0 (2E-01) | 2.9 (16) | 1.3 (5E-05) | 0.8 (16) | 1.2 (5E-06) |
| INF344 | –1.5 (8) | – | 1.8 (>1024) | 6.8 (8E-01) | 3.1 (8) | 2.2 (3E-05) | 1.2 (16) | 2.3 (3E-05) |
| INF079 | 1.7 (16) | – | 1.5 (>1024) | 6.7 (5E-03) | 2.7 (32) | 0.5 (1E-06) | 0.6 (16) | 4.0 (5E-03) |
| INF018 | 0.8 (16) | – | 1.6 (>1024) | 6.8 (5E-02) | 3.0 (32) | 1.0 (5E-06) | 0.5 (16) | 2.5 (1E-03) |
| INF171 | 3.1 (16) | 2.4 (8E-05) | 1.4 (1024) | 4.6 (5E-03) | 3.1 (8) | 1.9 (2E-05) | 0.8 (32) | 0.9 (2E-05) |
| INF142 | 3.1 (>1024) | 6.2 (2E-01) | 1.3 (>1024) | 5.5 (5E-02) | 2.4 (32) | 1.7 (5E-05) | –0.3 (32) | – |
| INF223 | 3.3 (32) | 0.3 (3E-07) | 1.3 (>1024) | 5.4 (3E-01) | 2.8 (32) | – | –0.1 (32) | 0.2 (2E-05) |
| INF161 | 3.2 (32) | 1.3 (3E-06) | 1.3 (>1024) | 6.2 (5E-01) | 2.7 (32) | 1.3 (9E-06) | 0.9 (256) | –0.1 (2E-05) |
| INF348 | 3.5 (256) | 4.2 (1E-03) | 1.5 (>1024) | 6.0 (1) | 2.8 (256) | 2.9 (3E-04) | 0.5 (>1024) | 2.9 (1) |
| INF249 | 3.0 (>1024) | 3.1 (8E-01) | 1.1 (>1024) | 1.2 (1) | 3.1 (>1024) | 2.9 (8E-01) | 1.4 (>1024) | 1.5 (1) |
| ATCCe | ||||||||
| 13883 | 3.7 (>1024) | 6.6 (3E-01) | 1.5 (>1024) | 6.2 (1) | 2.5 (128) | 2.7 (4E-04) | 1.0 (64) | 2.6 (1E-02) |
| – | – | 1.7 (>1024) | 5.6 (3E-01) | 2.5 (64) | 4.1 (1E-02) | 0.8 (128) | 1.8 (4E-03) | |
TG, total growth; RSP, resistant subpopulation; –, indicates no growth detected at 72 h, either TG or RSP growth.
TG, change in log10 cfu/mL at 72 h of the TG quantified on drug-free MHA, and the MIC determined by AD; RSP, change in log10 cfu/mL at 72 h of the RSP growth quantified on MHA with 512 mg/L fosfomycin (and 25 mg/L G6P), and the proportion that the RSP growth makes up of the total population. The RSP growth was confirmed by AD to have an MIC ≥1024 mg/L.
The bladder infection model was run with an inflow rate of 20 mL/h with 4-hourly intermittent voiding.
All fosfomycin MICs were performed by AD.
Average starting inoculum 5.4 (±0.2) log10 cfu/mL.
Average starting inoculum 7.5 (±0.1) log10 cfu/mL.
Average starting inoculum 5.3 (±0.1) log10 cfu/mL.
Average starting inoculum 7.5 (±0.1) log10 cfu/mL.
ATCC strain tested in duplicate.
CAMHB with G6P
For the remaining 14 isolates (excluding the HLR strain INF249), following a high starting inoculum (7.5 ± 0.1 log10 cfu/mL), 13 isolates had maximal regrowth at 72 h with a rise in fosfomycin MIC to ≥1024 mg/L. Only isolate INF321 (MIC 2 mg/L) had undetected regrowth (Figure 3a). A resistant subpopulation was detected in the starting inoculum in half of the isolates (INF018, INF171, INF142, INF223, INF161, INF348, ATCC 13883 [both replicates]) at a density between 2.0 and 3.4 log10 cfu/mL. In contrast, following a low starting inoculum (5.4 ± 0.2 log10 cfu/mL), three isolates had undetected regrowth (INF321, INF215 and ATCC 13883 [one of two replicates]) (Figure 3b), only two isolates had a rise in MIC to ≥1024 mg/L (INF142 and ATCC 13883 [single replicate]) and the remaining isolates regrew without a significant change in fosfomycin MIC. Resistant subpopulations were below the limit of detection in the low starting inoculum (except INF249). There was a trend in isolates with baseline MICs ≤16 mg/L to have sub-maximal regrowth at 72 h (≤7.0 log10 cfu/mL) without a detectable resistant subpopulation (Table 3).
Figure 3.
Growth response after a simulated single 3 g dose of oral fosfomycin. Each experiment is represented by two graphs illustrating the total growth (quantitative growth on drug-free MHA) and the fosfomycin-resistant growth (quantitative growth on MHA supplemented with 512 mg/L fosfomycin and 25 mg/L G6P). (a) Testing in CAMHB with 25 mg/L G6P with a low starting inoculum. (b) Testing in CAMHB with 25 mg/L G6P with a high starting inoculum. (c) Testing in SHU with a low starting inoculum. (d) Testing in SHU with a high starting inoculum. Solid lines represent the total growth. Dashed lines represent fosfomycin resistant growth. The 15 isolates are grouped by their baseline fosfomycin MIC measurement (2–4, 8, 16–32 and >32 mg/L). ATCC 13883 was run in duplicate. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
SHU
Regardless of the starting inoculum, following fosfomycin exposure, all isolates regrew to a maximal growth capacity, but with limited post-exposure amplification of the resistant subpopulation (Figure 3c and d). Resistant subpopulations were detected in the high and low starting inoculums in the same isolates as the CAMHB experiments. Following a high starting inoculum (7.5 ± 0.1 log10 cfu/mL), only two isolates had a rise in MIC to ≥1024 mg/L (INF215 and INF348), and two isolates had smaller rises in MIC (INF014 [MIC rise from 2 to 512 mg/L] and INF161 [MIC rise from 32 to 256 mg/L]). With a low starting inoculum (5.3 ± 0.1 log10 cfu/mL), only one isolate had a post-exposure rise in MIC ≥1024 mg/L (INF215) (Table 3).
Molecular mechanisms of fosfomycin resistance in baseline clinical isolates
Comparative WGS on the 50 K. pneumoniae clinical isolates demonstrated 5 of 7 non-WT strains had a premature STOP codon in uhpB (n = 4 ST29, n = 1 ST34). The other 2 isolates had unique mutations: INF011 (ST1393) L14S in glpT and T63A in uhpC; INF319 (ST461) L100M in uhpC (Table S2). Of the 15 isolates tested in the bladder infection model, two genomes failed quality control (INF018-postexp-SHU and INF321-HLR-MHBG6P). When compared with their respective parent strains, the HLR subpopulation and post-exposure regrowth derivates had a median seven chromosomal SNVs (range 0–26; Table S5). Non-synonymous mutations were identified in 36 distinct genes, the majority of which (n = 28, 78%) were mutated in only a single genome. Putative deletions were commonly detected in a wide variety of genes, the most common identified in a sodium/solute symporter family protein and was observed in 8/15 isolates (INF018, INF044, INF079, INF161, INF215, INF223, INF321, INF344) (Table S6).
Fosfomycin gene mutations in the baseline HLR subpopulation
Isolates INF249 (MIC >1024 mg/L) and INF348 (MIC 128 mg/L) had the same truncation in uhpB in all derivative strains that was detected in the parent strain (Table S5). In CAMHB with G6P, only three HLR subpopulation strains (INF142, INF171, INF174) had new genomic changes compared with their parent strain. These included mutations in uhpC, uhpT and ptsI, respectively (Table S5). When incubated in SHU, four HLR subpopulation strains (INF018, INF161, INF171, INF348) had new genomic changes compared with their parent strain. Only one strain (INF171) had a mutation in the same gene (uhpT) identified after incubation in CAMHB with G6P. The other identified strains (INF018, INF161, INF348) had mutations in uhpT, ptsI and glpT, respectively (Table S5).
Fosfomycin gene mutations in the regrowth population after fosfomycin exposure
Isolates INF249 (MIC >1024 mg/L) and INF348 (MIC 128 mg/L) maintained the same truncation in uhpB detected in the parent strains. In CAMHB with G6P, only four strains (INF079, INF142, INF171, INF348) had new mutations compared with their parent strain, despite 13 strains demonstrating a rise in fosfomycin MIC to ≥1024 mg/L. These include mutations in glpT in three strains, and INF142 had an insertion sequence upstream of fosA. These mutations were not identified in the baseline HLR subpopulation of these strains. When exposed to fosfomycin in SHU, only two strains (INF079, INF171) had new mutations in the fosfomycin resistance genes, including mutations in glpT and uhpT, respectively. Neither of these strains demonstrated a post-exposure rise in fosfomycin MIC. INF079 did not have mutations detected in a baseline HLR subpopulation. INF171 had a mutation detected in the same gene (uhpT) in the baseline HLR subpopulation; however, the mutation in the post-exposure regrowth was a different deletion and present in 50% of the population (Table S5).
Discussion
The regrowth of K. pneumoniae urinary isolates appears to be inadequately suppressed following urinary fosfomycin exposure after a simulated single 3 g oral dose in an in vitro bladder infection model. Efficacy was reduced with a high starting inoculum and in nutrient-depleted media simulating the biomatrix of urine. A low starting inoculum enabled the most fosfomycin activity. Although WGS testing identified mutations in derivate strains across the chromosome and in known fosfomycin resistance genes, many strains had elevated fosfomycin MIC measurements without an identified causative genomic change.
The evidence supporting fosfomycin efficacy for UTIs caused by non-E. coli urinary isolates is limited, including complicated UTIs and the use of multiple doses of fosfomycin. Clinical studies have reported higher persistence and recurrence rates in K. pneumoniae isolates treated with oral fosfomycin, and a divergence between in vitro susceptibility (92%) and microbiological cure (46%).10,33 Fosfomycin heteroresistance and treatment selection of a pre-existing resistant subpopulation have been implicated in limited efficacy.16,34,35 Our study, however, demonstrated different genomic changes in the baseline HLR subpopulation compared with the post-exposure derivative strains, when aligned with the parent strain.
The mechanisms of fosfomycin resistance are multiple in both gene and gene function. It is difficult to link the fosfomycin phenotype to a specific genotype. FosA, an enzyme that catalyses the Mn2+-and K+-dependent glutathione-mediated degradation of fosfomycin, is an intrinsic gene conserved among all K. pneumoniae strains.12 This contrasts with E. coli strains with an acquired plasmid-borne fosA3 gene, which affords an increase in the fosfomycin MIC to ≥1024 mg/L.36,37 Among the 50 baseline isolates, we identified 5 of 7 of the non-WT strain with a premature STOP codon in uhpB. UhpB encodes the kinase of the two-component response regulator (UhpA) required for uhpT expression. UhpT is a fosfomycin–G6P symporter. Down-regulation, or complete lack of expression, reduces the import of fosfomycin into the bacterial cell. This is supported by the fosfomycin susceptibility results observed in our isolate INF348, where G6P supplementation failed to enhance fosfomycin activity in the setting of having a premature STOP codon in uhpB. While we did not identify any experimentally derived isolates with acquired mutations in uhpB, we did identify five with disruptions in uhpT (four with elevated MICs) and one with a disruption in uhpC (with elevated MIC). Additionally, there were five derived isolates with acquired mutations in glpT (four with elevated MICs), which encodes for a second fosfomycin symporter. One additional isolate carried an insertion sequence insertion upstream of fosA, which may result in increased expression of this gene. In agreement with Ortiz-Padilla et al.,38 we speculate that an elevation in fosfomycin MIC >64 mg/L is due to the combination of impaired fosfomycin symporters (UhpT and GlpT) with the chromosomally mediated FosA production. However, most of our experimentally derived isolates, including 30 with elevated fosfomycin MICs, did not acquire mutations in uhp, glpT or fosA or promoter regions, indicating that additional, yet unknown, mechanisms play a role in fosfomycin resistance. Our analyses also identified many distinct acquired SNVs in these isolates; however, these were distributed across different regions of the chromosome (Table S5), and we were unable to identify specific candidate resistance-causing mutations.
The site of infection is a vital aspect when translating in vitro simulations to human infections. To simulate the bladder environment, a synthetic urine alternative, SHU, was tested in parallel with standard laboratory media. We found that although regrowth was common in both media, there was reduced emergence of resistance in SHU. This was also supported by the observation that all isolates had fosfomycin MIC measurements ≥512 mg/L when performed by BMD in SHU (Table S4). The reduced nutrients and pH 5.6 of SHU, compared with CAMHB, had a much less of an impact on MIC measurements compared with the presence or absence of G6P. This exemplifies how the environment impacts upon antibiotic activity and PD outcome, particularly important when considering the urinary environment.39 Similarly, Hughes and Andersson40 describe different bacterial evolutionary trajectories to antibiotic resistance, reporting that strong antibiotic selection pressure results in higher resistance and reduced fitness, whereas weak antibiotic selection selects for less resistance but mutants with greater fitness.
A major modification to the in vitro model compared with previous testing was a >5-fold increase in the media flow rate into each bladder compartment, thereby providing an improved dynamic simulation of human urodynamics. In this study, applying a 20 mL/h inflow rate resulted in faster and larger volume increases in the bladder compartments, before each timed, high-speed void. The simulation of urodynamics and large volume shifts is vital for informing UTI PD. This dynamic incubation also serves as an inherent test of uropathogen fitness, where successful strains must maintain an adequate growth rate to sustain a viable regrowth population.
The limitations of our in vitro model include the lack of an immune system and bladder tissue architecture. Furthermore, we demonstrated an occasion where the ATCC strain had discrepant results with its biological replicate, likely due to random differences between the two replicates in relation to the resistant subpopulation proportion included in the starting inocula. This is further reflective of the multiple experimental variables at play. However, testing multiple different strains with a broad spectrum of baseline susceptibility ensures no single result greatly impacts upon the final conclusions.
In this study, fosfomycin failed to eradicate K. pneumoniae uropathogens, regardless of baseline susceptibility. This suggests caution when considering the use of fosfomycin as monotherapy for the treatment of UTIs due to K. pneumoniae. These data support the recent change in the EUCAST oral fosfomycin breakpoints to restrict susceptibility testing to E. coli. The increased activity of fosfomycin observed with a low starting bacterial inoculum may support the use of fosfomycin if administered early in infection, along with increased fluid intake, frequent voiding and a functioning immune system. The role of combination antibiotic therapy, and the development of a FosA inhibitor, may facilitate a more certain role for fosfomycin against infections caused by K. pneumoniae.
Supplementary Material
Acknowledgements
Thank you to Dr Adam Jenney and Dr Kathryn Holt for providing the baseline collection of K. pneumoniae clinical isolates.
Funding
This study was supported by internal funding. A.Y.P. and J.A.R. are part funded through an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellowships (APP1117940 and APP1117065, respectively). J.A.R. would like to acknowledge funding for a NHMRC Centre of Research Excellence (APP1099452).
Transparency declarations
I.J.A. has consultancies/advisory boards for MSD. J.M. has received research grants from Pfizer, Gilead, Astellas, MSD, F2G and VenatoRx. J.A.R. has consultancies/advisory boards for MSD, QPEX, Discuva Ltd, Accelerate Diagnostics, Bayer and Biomerieux; speaking fees for MSD, Biomerieux and Pfizer; and industry grants from MSD, The Medicines Company, Cardeas Pharma, Biomerieux, QPEX and Pfizer. A.Y.P. has received investigator-initiated research funding from MSD. All other authors: none to declare.
Supplementary data
Methods, Tables S1 to S6 and Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Keijzers G, Macdonald SP, Udy AAet al. . The Australasian Resuscitation In Sepsis Evaluation: Fluids or vasopressors in emergency department sepsis (ARISE FLUIDS), a multi-centre observational study describing current practice in Australia and New Zealand. Emerg Med Australas 2020; 32: 586–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bezabih YM, Sabiiti W, Alamneh Eet al. . The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J Antimicrob Chemother 2021; 76: 22–9. [DOI] [PubMed] [Google Scholar]
- 3. Quaegebeur A, Brunard L, Javaudin Fet al. . Trends and prediction of antimicrobial susceptibility in urinary bacteria isolated in European emergency departments: the EuroUTI 2010–16 Study. J Antimicrob Chemother 2019; 74: 3069–76. [DOI] [PubMed] [Google Scholar]
- 4. Vardakas KZ, Legakis NJ, Triarides Net al. . Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 2016; 47: 269–85. [DOI] [PubMed] [Google Scholar]
- 5. Zarakolu P, Eser OK, Otlu Bet al. . In-vitro activity of fosfomycin against Escherichia coli and Klebsiella pneumoniae bloodstream isolates and frequency of OXA-48, NDM, KPC, VIM, IMP types of carbapenemases in the carbapenem-resistant groups. J Chemother 2021; 1–6. 10.1080/1120009X.2021.1963618. [DOI] [PubMed] [Google Scholar]
- 6. Flores-Mireles AL, Walker JN, Caparon Met al. . Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13: 269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ernst CM, Braxton JR, Rodriguez-Osorio CAet al. . Adaptive evolution of virulence and persistence in carbapenem-resistant Klebsiella pneumoniae. Nat Med 2020; 26: 705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peirano G, Chen L, Kreiswirth BNet al. . Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147. Antimicrob Agents Chemother 2020; 64: e01148-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhanel GG, Zhanel MA, Karlowsky JA. Oral Fosfomycin for the Treatment of Acute and Chronic Bacterial Prostatitis Caused by Multidrug-Resistant Escherichia coli. Can J Infect Dis Med Microbiol 2018; 2018: 1404813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seroy JT, Grim SA, Reid GEet al. . Treatment of MDR urinary tract infections with oral fosfomycin: a retrospective analysis. J Antimicrob Chemother 2016; 71: 2563–8. [DOI] [PubMed] [Google Scholar]
- 11. Abbott IJ, Dekker J, van Gorp Eet al. . Impact of bacterial species and baseline resistance on fosfomycin efficacy in urinary tract infections. J Antimicrob Chemother 2020; 75: 988–96. [DOI] [PubMed] [Google Scholar]
- 12. Klontz EH, Tomich AD, Gunther Set al. . Structure and dynamics of FosA-mediated fosfomycin resistance in Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 2017; 61: e01572-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Edition: M100-S31. 2021.
- 14. EUCAST . Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0, 2021. http://www.eucast.org.
- 15. Diez-Aguilar M, Martinez-Garcia L, Canton Ret al. . Is a new standard needed for diffusion methods for in vitro susceptibility testing of fosfomycin against Pseudomonas aeruginosa? Antimicrob Agents Chemother 2016; 60: 1158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ballestero-Tellez M, Docobo-Perez F, Rodriguez-Martinez JMet al. . Role of inoculum and mutant frequency on fosfomycin MIC discrepancies by agar dilution and broth microdilution methods in Enterobacteriaceae. Clin Microbiol Infect 2017; 23: 325–31. [DOI] [PubMed] [Google Scholar]
- 17. Elliott ZS, Barry KE, Cox HLet al. . The role of fosA in challenges with fosfomycin susceptibility testing of multispecies Klebsiella pneumoniae carbapenemase-producing clinical isolates. J Clin Microbiol 2019; 57: e00634-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cottell JL, Webber MA. Experiences in fosfomycin susceptibility testing and resistance mechanism determination in Escherichia coli from urinary tract infections in the UK. J Med Microbiol 2019; 68: 161–8. [DOI] [PubMed] [Google Scholar]
- 19. Mojica MF, De La Cadena E, Hernandez-Gomez Cet al. . Performance of disk diffusion and broth microdilution for fosfomycin susceptibility testing of multidrug-resistant clinical isolates of Enterobacterales and Pseudomonas aeruginosa. J Glob Antimicrob Resist 2020; 21: 391–5. [DOI] [PubMed] [Google Scholar]
- 20. Mouton JW. Soup with or without meatballs: Impact of nutritional factors on the MIC, kill-rates and growth-rates. Eur J Pharm Sci 2018; 125: 23–7. [DOI] [PubMed] [Google Scholar]
- 21. Abbott IJ, van Gorp E, Wijma RAet al. . Evaluation of pooled human urine and synthetic alternatives in a dynamic bladder infection in vitro model simulating oral fosfomycin therapy. J Microbiol Methods 2020; 171:105861. [DOI] [PubMed] [Google Scholar]
- 22. Ipe DS, Ulett GC. Evaluation of the in vitro growth of urinary tract infection-causing Gram-negative and Gram-positive bacteria in a proposed synthetic human urine (SHU) medium. J Microbiol Methods 2016; 127: 164–71. [DOI] [PubMed] [Google Scholar]
- 23. International Organization for Standardization (ISO) . ISO 20776-2. Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Testing Devices—Part 2. ISO, 2008.
- 24. van den Bijllaardt W, Schijffelen MJ, Bosboom RWet al. . Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J Antimicrob Chemother 2018; 73: 2380–7. [DOI] [PubMed] [Google Scholar]
- 25. Abbott IJ, Meletiadis J, Belghanch Iet al. . Fosfomycin efficacy and emergence of resistance among Enterobacteriaceae in an in vitro dynamic bladder infection model. J Antimicrob Chemother 2018; 73: 709–19. [DOI] [PubMed] [Google Scholar]
- 26. Wijma RA, Koch BCP, van Gelder Tet al. . High interindividual variability in urinary fosfomycin concentrations in healthy female volunteers. Clin Microbiol Infect 2018; 24: 528–32. [DOI] [PubMed] [Google Scholar]
- 27. Wenzler E, Bleasdale SC, Sikka Met al. . Phase I study to evaluate the pharmacokinetics, safety, and tolerability of two dosing regimens of oral fosfomycin tromethamine in healthy adult participants. Antimicrob Agents Chemother 2018; 62: e00464-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wenzler E, Ellis-Grosse EJ, Rodvold KA. Pharmacokinetics, safety, and tolerability of single-dose intravenous (ZTI-01) and oral fosfomycin in healthy volunteers. Antimicrob Agents Chemother 2017; 61: e00775-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker SL, Lipman J, Roberts JAet al. . A simple LC-MS/MS method using HILIC chromatography for the determination of fosfomycin in plasma and urine: application to a pilot pharmacokinetic study in humans. J Pharm Biomed Anal 2015; 105: 39–45. [DOI] [PubMed] [Google Scholar]
- 30. Gorrie CL, Mirceta M, Wick RRet al. . Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 2017; 65: 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorrie CL, Mirceta M, Wick RRet al. . Antimicrobial-resistant Klebsiella pneumoniae carriage and infection in specialized geriatric care wards linked to acquisition in the referring hospital. Clin Infect Dis 2018; 67: 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. EUCAST . Data from the EUCAST MIC distribution website. https://mic.eucast.org.
- 33. Neuner EA, Sekeres J, Hall GSet al. . Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56: 5744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao M, Bulman ZP, Lenhard JRet al. . Pharmacodynamics of colistin and fosfomycin: a ‘treasure trove’ combination combats KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 2017; 72: 1985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fransen F, Hermans K, Melchers MJBet al. . Pharmacodynamics of fosfomycin against ESBL- and/or carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2017; 72: 3374–81. [DOI] [PubMed] [Google Scholar]
- 36. Ito R, Mustapha MM, Tomich ADet al. . Widespread fosfomycin resistance in gram-negative bacteria attributable to the chromosomal fosA gene. mBio 2017; 8: e00749-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang Y, Shen P, Wei Zet al. . Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents 2015; 45: 66–70. [DOI] [PubMed] [Google Scholar]
- 38. Ortiz-Padilla M, Portillo-Calderon I, de Gregorio-Iaria Bet al. . Interplay among Different Fosfomycin Resistance Mechanisms in Klebsiella pneumoniae. Antimicrob Agents Chemother 2021; 65: e01911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin-Gutierrez G, Docobo-Perez F, Rodriguez-Beltran Jet al. . Urinary tract conditions affect fosfomycin activity against Escherichia coli strains harboring chromosomal mutations involved in fosfomycin uptake. Antimicrob Agents Chemother 2018; 62: e01899-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hughes D, Andersson DI. Evolutionary trajectories to antibiotic resistance. Annu Rev Microbiol 2017; 71: 579–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.