Abstract
Purpose
Low tidal volume ventilation (LTVV) is associated with mortality in patients with acute respiratory distress syndrome. We investigated the association of LTVV with mortality in COVID-19 patients.
Methods
Secondary analysis of a national observational study in COVID-19 patients in the first wave of the pandemic. We compared COVID-19 patients that received LTVV, defined as controlled ventilation with a median tidal volume ≤ 6 mL/kg predicted body weight over the first 4 calendar days of ventilation, with patients that did not receive LTVV. The primary endpoint was 28-day mortality. In addition, we identified factors associated with use of LTVV.
Results
Of 903 patients, 294 (32.5%) received LTVV. Disease severity scores and ARDS classification was not different between the two patient groups. The primary endpoint, 28-day mortality, was met in 68 out of 294 patients (23.1%) that received LTVV versus in 193 out of 609 patients (31.7%) that did not receive LTVV (P < 0.001). LTVV was independently associated with 28-day mortality (HR, 0.68 (0.45 to 0.95); P = 0.025). Age, height, the initial tidal volume and continuous muscle paralysis was independently associated with use of LTVV.
Conclusions
In this cohort of invasively ventilated COVID-19 patients, approximately a third of patients received LTVV. Use of LTVV was independently associated with reduced 28-day mortality. The initial tidal volume and continuous muscle paralysis were potentially modifiable factors associated with use of LTVV. These findings are important as they could help clinicians to recognize patients who are at risk of not receiving LTVV.
Keywords: Coronavirus disease 2019, COVID-19, Tidal volume, Mortality
Abbreviations: LTVV, Low tidal volume ventilation; PBW, Predicted body weight; PEEP, Positive end-expiratory pressure
1. Introduction
Patients with coronavirus disease 2019 (COVID-19) may need hospitalization for supplemental oxygen [1]. A substantial number of these patients need admission to an intensive care unit (ICU) for escalation of respiratory care, usually invasive ventilation [2]. Morbidity and mortality are high in COVID-19 patients that need invasive ventilation [3,4], and care for these patients remains largely supportive. One approach is to minimize additional lung injury caused by invasive ventilation.
Ventilator-induced lung injury can be prevented by using so-called lung-protective ventilation strategies [5], wherein use of a low tidal volume (VT) plays a central role. There is strong and convincing evidence that low VT ventilation (LTVV) improves outcome in patients with acute respiratory distress syndrome (ARDS) [6]. Until the COVID-19 pandemic, in patients with ARDS LTVV remained underused [[7], [8], [9], [10]]. Despite the fact that large studies of LTVV have shown no worsening of oxygenation, oxygenation can improve more rapidly with higher tidal volumes [11]. This could be an argument for health care workers to use LTVV less often. This could especially play a role in COVID-19 patients, since these patients frequently have severe hypoxemia due to extensive pulmonary infiltrates, and in some patients also pulmonary embolism. Another potential reason for underuse of LTVV in COVID-19 patients could be that airway pressures in these patients can be surprisingly low, indicating a high respiratory compliance [12,13]—at least in theory this could lead to a scenario in which use of LTVV is considered less important.
We hypothesized that LTVV is independently associated with mortality in COVID-19 patients. To test this hypothesis, we performed a secondary analysis of a large national multicenter study, named the ‘PRactice of VENTilation in corona virus disease 2019’ (PRoVENT-COVID) study, to compare 28-day mortality between patients that received LTVV versus patients that did not receive LTVV in the first 4 calendar days in the ICU. We also aimed to identify potentially modifiable patient characteristics and ventilatory parameters that had an independent association with use of LTVV.
2. Methods
2.1. Design, settings and participants
The PRoVENT-COVID study is an investigator-initiated, national, multicenter, observational study performed in 22 hospitals during the first 3 months of the national outbreak in the Netherlands [14]. The study protocol was approved by the institutional review board of each participating hospital—need for individual patient informed consent was waived seen the observational design of the study. The study was registered at www.clinicaltrial.gov (study identifier NCT04346342).
Consecutive patients aged 18 years or older were eligible for participation if they were admitted to an ICU in one of the participating hospitals and had received invasive ventilation because of acute respiratory failure due to RT–PCR confirmed COVID-19. The PRoVENT-COVID study itself had no exclusion criteria. For this current analysis, we excluded patients in whom VT or patient height, necessary for normalization of VT to the predicted body weight (PBW), was not captured. We also excluded patients that were exclusively on a spontaneous breathing mode at all the time points ventilation data were collection, and we excluded patients for whom the primary endpoint was missing, which could have happened due to transfer to a non-participating hospital.
2.2. Data collected and analysis
Demographics and data regarding premorbid diseases and home medication were collected at baseline. The current Berlin definition for ARDS was used for severity classification [15]. In the first hour of invasive ventilation in the ICU and every 8 h thereafter at fixed time points, ventilator settings and ventilation parameters were collected up to calendar day 4. Outcomes were collected up to day 90, and included intubation and life status.
VT was normalized to PBW using the following equations: VT = absolute VT / PBW [kg] (Eq. 1), and PBW = 50.0 + 0.91 * (height [cm] – 152.4) (in males, Eq. 2a) and PBW = 45.5 + 0.91 * (height [cm] – 152.4) (in females, Eq. 2b). LTVV was defined as having received a median VT ≤ 6 mL/kg PBW during controlled ventilation in the first 4 calendar days. Herein, we ignored the first VT of the first day of ventilation as initial settings could have been set in the emergency room, and could have rapidly decreased thereafter. We also ignored any VT when there was evidence of spontaneous breathing activity. This meant that we ignored a VT under any mode that requires spontaneous breathing activity, and if the measured (total) respiratory rate (RR) exceeded the set RR by more than 2 breaths per min.
2.3. Endpoints
The primary outcome was 28-day mortality. Secondary outcomes included duration of ventilation, expressed in duration of ventilation in survivors, and in the number of days free from ventilation and alive at day 28 (VFD-28). For the later, we summed the calendar days without invasive ventilation for at least 24 h and considering the last date of successful extubation; patients who had died by day 28 were considered to have had zero days free from ventilation [16]. Other endpoints were length of stay in ICU and hospital; and ICU-, hospital- and 90-day mortality.
2.4. Statistical analysis
The sample size was based on the number of available patients in the database of the PRoVENT-COVID study.
Data are reported as numbers and relative proportions for categorical variables and as median (quartile 25%–quartile 75%) for continuous variables. Categorical variables were compared using Chi-squared test or Fisher exact test, and continuous variables were compared using a Mann–Whitney U test or t-test, where appropriate. For all analyses, the group of patients that had received LTVV was used as the reference.
Cumulative distribution plots are used to present distributions of age, height, BMI, and actual VT. Kaplan–Meier curves were used to plot time until 28- and also 90-day mortality in patients that received LTVV versus patients that did not receive LTVV, and groups were compared with a log rank test. A mixed-effect multivariate Cox regression model was used to assess whether LTVV had an independent association with 28-day mortality. Centers were included as random effects to account for unobserved heterogeneity. Confounders that were selected a priori on clinical relevance were: age, gender, ARDS severity, heart rate, pH, use of prone positioning, development of acute kidney injury (AKI), and development of venous thromboembolism (VTE). In addition, respiratory system compliance was selected as a confounder based on statistical differences between groups.
Next, in a mixed-effect multivariate logistic model with centers as random factors we identified factors independently associated with use of LTVV. Confounders that were selected a priori were demographic factors, including age, gender, height, weight, and ARDS severity, ventilation parameters in the first calendar day of ventilation, including the initial VT, the use of neuromuscular blockers and the use of prone positioning.
All analyses were conducted in R v.3.6.3 (R Foundation, Vienna, Austria) and significance level was set at 0.05.
2.5. Posthoc analysis
We performed two posthoc analyses. First, we assessed and compared use of LTVV between males and females. In a second posthoc analysis we performed univariate linear and logistic regressions as posthoc analyses to assess the relationship between VT as a continuous value and clinical outcomes.
3. Results
3.1. Patients
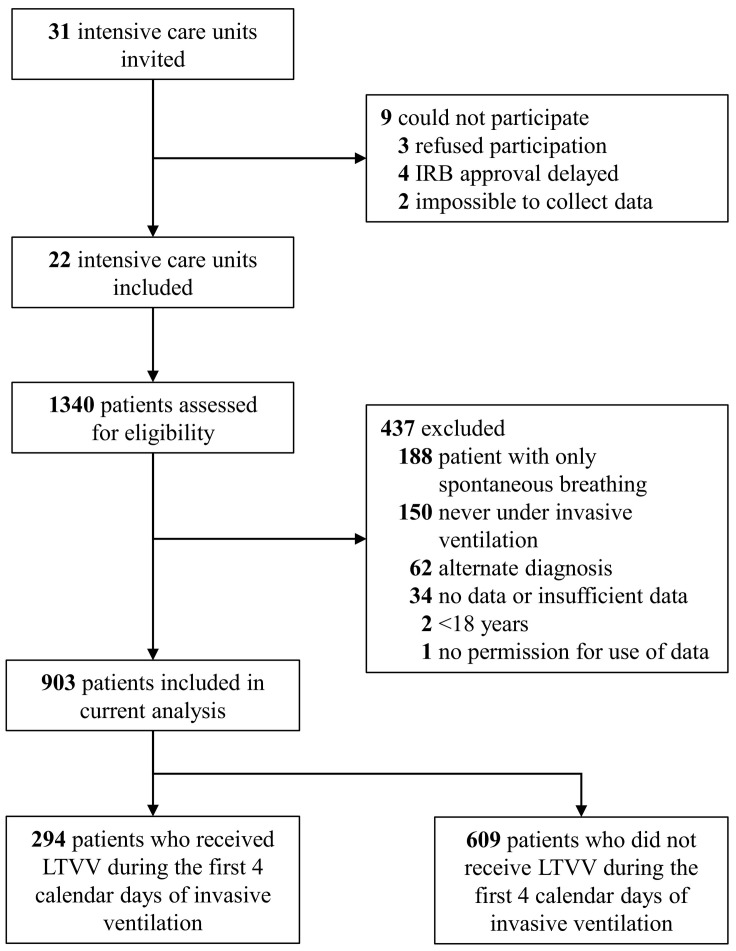
From March 1 through June 1, 2020, 1122 invasively ventilated patients with COVID-19 were included in the PROVENT-COVID study (Fig. 1 ). A total of 437 patients were excluded for the purpose of the current analysis, mainly because of spontaneous breathing activity at all time points of data collection. Patients were predominantly male, had a high BMI, and often had a history of hypertension or diabetes (Table 1 ). In most patients, ARDS severity was moderate or severe.
Fig. 1.
Consort patient flow diagram.
Table 1.
Baseline characteristics of the included patients.
| Overall cohort (n = 903) | VT ≤ 6 mL/kg PBW (n = 294) | VT > 6 mL/kg PBW (n = 609) | P valuea | |
|---|---|---|---|---|
| Male gender – no (%) | 659 (73.0) | 237 (80.6) | 422 (69.3) | <0.001 |
| Age, years | 65.00 [57.00–72.00] | 62.50 [55.25–70.00] | 66.00 [58.00–73.00] | <0.001 |
| Weight, kg | 86.00 [77.30–96.60] | 90.00 [80.00–99.00] | 85.00 [75.00–95.00] | <0.001 |
| Height, cm | 175.00 [170.00–183.00] | 180.00 [175.00–186.00] | 174.00 [168.00–180.00] | <0.001 |
| Predicted body weight, kg | 70.57 [63.29–77.85] | 75.12 [67.84–79.67] | 68.75 [61.47–75.12] | <0.001 |
| Body mass index, kg/m2 | 27.76 [25.29–30.85] | 27.64 [25.15–30.15] | 27.76 [25.39–31.14] | 0.176 |
| Use of non-invasive ventilation, no (%) | 77 (9.5) | 22 (8.2) | 55 (10.1) | 0.453 |
| Duration of noninvasive ventilation, hours | 7.50 [2.00–18.12] | 9.00 [3.75–20.50] | 7.00 [2.00–16.25] | 0.597 |
| Severity of ARDSb(Berlin definition) | 0.911 | |||
| Mild | 182 (20.4) | 60 (20.7) | 122 (20.3) | |
| Moderate | 612 (68.6) | 200 (69.0) | 412 (68.4) | |
| Severe | 98 (11.0) | 30 (10.3) | 68 (11.3) | |
| Severity of illness | ||||
| SOFA score | 7.00 [6.00–10.00] | 7.00 [6.00–10.00] | 7.00 [6.00–10.00] | 0.822 |
| Available, no of patients (%) | 412 (48.3) | 146 (53.9) | 266 (45.7) | 0.032 |
| Co-existing disorders, no (%) | ||||
| None | 225 (24.9) | 85 (28.9) | 140 (23.0) | 0.065 |
| Hypertension | 303 (33.6) | 92 (31.3) | 211 (34.6) | 0.355 |
| Heart failure | 37 (4.1) | 13 (4.4) | 24 (3.9) | 0.871 |
| Diabetes mellitus | 208 (23.0) | 70 (23.8) | 138 (22.7) | 0.764 |
| Chronic kidney disease | 37 (4.1) | 12 (4.1) | 25 (4.1) | 1.000 |
| Baseline creatinine, μmol/Lc | 77.00 [62.00–98.00] | 79.00 [64.00–98.00] | 76.00 [61.00–99.00] | 0.304 |
| Liver cirrhosis | 3 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 |
| Chronic obstructive pulmonary disease | 70 (7.8) | 22 (7.5) | 48 (7.9) | 0.938 |
| Active haematological neoplasia | 12 (1.3) | 3 (1.0) | 9 (1.5) | 0.801 |
| Active solid neoplasia | 26 (2.9) | 9 (3.1) | 17 (2.8) | 0.988 |
| Neuromuscular disease | 4 (0.4) | 2 (0.7) | 2 (0.3) | 0.833 |
| Immunosuppression | 20 (2.2) | 9 (3.1) | 11 (1.8) | 0.337 |
| Previous medication, no (%) | ||||
| Systemic steroids | 32 (3.5) | 12 (4.1) | 20 (3.3) | 0.678 |
| Inhalation steroids | 103 (11.4) | 33 (11.2) | 70 (11.5) | 0.994 |
| Angiotensin converting enzyme inhibitor | 153 (16.9) | 50 (17.0) | 103 (16.9) | 1.000 |
| Angiotensin II receptor blocker | 103 (11.4) | 24 (8.2) | 79 (13.0) | 0.044 |
| Beta-blockers | 166 (18.4) | 51 (17.3) | 115 (18.9) | 0.641 |
| Insulin | 65 (7.2) | 23 (7.8) | 42 (6.9) | 0.713 |
| Metformin | 144 (15.9) | 58 (19.7) | 86 (14.1) | 0.039 |
| Statin | 277 (30.7) | 87 (29.6) | 190 (31.2) | 0.679 |
| Calcium channel blockers | 162 (17.9) | 47 (16.0) | 115 (18.9) | 0.332 |
Data are median [quartile 25% - quartile 75%] or No (%), unless stated otherwise. Percentages may not total 100 because of rounding.
Abbreviations, APACHE: Acute Physiology and Chronic Health Evaluation; CT: computed tomography; PBW: Predicted bodyweight; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; VT: tidal volume.
The P value is computed comparing patients receiving VT ≤ 6 mL/kg PBW versus VT > 6 mL/kg PBW;
Severity of ARDS was classified according to the Berlin definition (Severe ARDS: P/F ratio < 100 mmHg; Moderate ARDS: P/F ratio between 100 and 200 mmHg; Mild ARDS: P/F ratio > 300 mmHg.
Most recent measurement in 24 h before intubation, or at ICU admission under invasive ventilation.
3.2. Incidence of LTVV
Of 903 patients, 294 (32.5%) patients received LTVV during the first 4 calendar days of invasive ventilation. These patients were younger and taller than those who did not receive LTVV (Table 1 and Supplement eFigure 1). LTVV patients received ventilation with a lower median VT (5.7 [5.3 to 5.8] (range 2.6 to 6.0) versus 6.7 [6.3 to 7.2] (range 6.0 to 10.2) mL/kg PBW; P < 0.01), a lower median peak pressure, a lower median mechanical power, but a higher median respiratory rate (Table 2 , Fig. 2 and Supplement eFigure 1). LTVV patients also had a lower median respiratory compliance. Other ventilator settings and ventilation parameters were not different. LTVV patients had a lower median PaO2 and a higher median PaCO2, but comparable median PaO2/FiO2 ratios. LTVV patients received prone position and neuromuscular blockade more often, and had a lower median plasma lactate level at baseline than those that did not receive LTVV. Pressure-controlled ventilation was used more often than volume-controlled ventilation (58 vs 26%, P < 0.01), and patients under volume-controlled ventilation received LTVV more often (Supplement eTable 1).
Table 2.
Ventilation characteristics and rescue strategies.
| Overall cohort (n = 903) | VT ≤ 6 mL/kg PBW (n = 294) | VT > 6 mL/kg PBW (n = 609) | P valued | |
|---|---|---|---|---|
| Ventilation characteristics | ||||
| VT, mL | 445.50 [402.50–493.50] | 417.50 [372.25–449.75] | 467.00 [421.00–507.50] | <0.001 |
| VT PBW, mL/kg | 6.34 [5.86–6.95] | 5.65 [5.33–5.84] | 6.70 [6.33–7.21] | <0.001 |
| VT ABW, mL/kg | 5.18 [4.50–5.85] | 4.64 [4.04–5.11] | 5.47 [4.84–6.06] | <0.001 |
| First set VT PBW, mL/kg | 6.30 [5.7–7.1] | 5.77 [5.3–6.3] | 6.59 [6.0–7.4] | <0.001 |
| PEEP, cm H2O | 13.00 [11.00–15.00] | 12.75 [10.00–15.00] | 14.00 [12.00–15.00] | 0.061 |
| Peak pressure, cm H2O | 27.00 [24.50–30.00] | 26.50 [24.00–29.25] | 27.00 [25.00–30.00] | 0.014 |
| Driving pressure, cm H2O | 14.00 [12.00–16.00] | 14.00 [12.00–16.00] | 14.00 [12.00–16.00] | 0.144 |
| Mechanical power, J/min | 19.32 [15.81–22.96] | 18.18 [15.27–21.99] | 19.84 [16.18–23.89] | <0.001 |
| Respiratory compliance, mL/cm H2O | 32.09 [26.36–38.57] | 30.36 [25.08–36.75] | 32.95 [27.34–39.17] | <0.001 |
| Total respiratory rate/min | 22.00 [20.00–25.00] | 24.00 [20.00–26.00] | 22.00 [20.00–24.00] | <0.001 |
| FiO2 | 0.45 [0.40, 0.54] | 0.45 [0.40–0.52] | 0.45 [0.40–0.55] | 0.250 |
| PaO2/FiO2 ratio, mm Hg | 170.27 [141.02–206.01] | 168.36 [140.89–204.40] | 170.65 [141.02–206.28] | 0.707 |
| SpO2/FiO2 ratio, mm Hg | 206.67 [177.48–237.89] | 210.00 [178.75–240.00] | 204.61 [176.36–237.50] | 0.388 |
| etCO2, mm Hg | 37.96 [33.75–42.76] | 39.76 [35.25–44.63] | 37.51 [33.38–42.01] | <0.001 |
| Laboratory testing | ||||
| pH | 7.36 [7.31–7.40] | 7.36 [7.31–7.40] | 7.37 [7.32–7.40] | 0.173 |
| PaO2, mm Hg | 76.14 [70.88–83.64] | 74.63 [69.20–80.26] | 76.81 [71.26–84.76] | <0.001 |
| PaCO2, mm Hg | 46.51 [42.01–52.88] | 47.63 [42.76–54.38] | 45.76 [41.26–51.98] | <0.001 |
| Lactate, mmol/L | 1.20 [1.00–1.45] | 1.10 [0.95–1.30] | 1.20 [1.00–1.53] | <0.001 |
| Kreatinine, mmol/L | 82.00 [64.50–121.00] | 85.50 [65.50–127.50] | 81.00 [64.00–119.00] | 0.141 |
| Vital signs | ||||
| Mean arterial pressure, mm Hg | 76.00 [72.00–80.00] | 76.00 [71.38–81.00] | 76.00 [72.00–80.00] | 0.335 |
| Heart rate/min | 81.00 [70.00–93.00] | 80.00 [71.00–91.00] | 81.00 [69.00–93.50] | 0.657 |
| Rescue strategies for refractionary hypoxemia | ||||
| Use of lung recruitment manoeuvres | 53 (7.1) | 15 (6.1) | 38 (7.5) | 0.601 |
| Use of prone positioning | 517 (57.6) | 182 (61.9) | 335 (55.5) | 0.078 |
| Duration of prone positioning per day, hours | 3.00 [0.00–9.50] | 4.00 [0.00–10.94] | 2.25 [0.00–9.00] | 0.026 |
| Use of neuromuscular blockade | 435 (48.2) | 168 (57.1) | 267 (43.8) | <0.001 |
| Duration of neuromuscular blockade per day, hours | 0.00 [0.00–8.00] | 4.00 [0.00–12.00] | 0.00 [0.00–8.00] | <0.001 |
| Use of extracorporeal membrane oxygenation | 11 (1.2) | 7 (2.4) | 4 (0.7) | 0.046 |
| Other | ||||
| Continuous sedationa | 897 (99.3) | 291 (99.0) | 606 (99.5) | 0.633 |
| Vasopressorb use | 848 (93.9) | 267 (90.8) | 581 (95.4) | 0.011 |
| Inotropicc use | 91 (10.1) | 30 (10.2) | 61 (10.0) | 1.000 |
| Fluid balance per day, mL | 964.25 [452.75–1461.64] | 846.50 [301.62–1413.00] | 994.00 [500.00–1483.00] | 0.014 |
| Urine output per day, mL | 1127.50 [774.38–1498.12] | 1120.00 [779.50–1447.56] | 1143.75 [773.75–1521.25] | 0.458 |
These characteristics represent aggregations over the first four days of ventilation unless stated otherwise. Data are median [quartile 25% - quartile 75%] or No (%). Percentages may not total 100 because of rounding.
Abbreviations: ABW: actual body weight; etCO2: end tidal carbon dioxide; FiO2: fraction of inspired oxygen; PaO2: arterial partial oxygen tension; PBW: predicted body weight; PEEP: positive end-expiratory pressure; SpO2: peripheral oxygen saturation; VT: tidal volume. e Assisted modus of ventilation over the first 48 h.
Sedatives consist of Midazolam, Propofol, Dexmedetomidine, Clonidine, Morphine, and Fentanyl.
Vasopressors consist of Noradrenaline, Adrenaline, and Vasopressine.
Inotropics consist of Milrinone and Dobutamine.
P values are computed comparing patients receiving VT ≤ 6 mL/kg PBW versus VT > 6 mL/kg PBW.
Fig. 2.
Cumulative distribution fractions for the ventilator characteristics. Respiratory System Compliance and Mechanical Power were lower in LTVV patients.
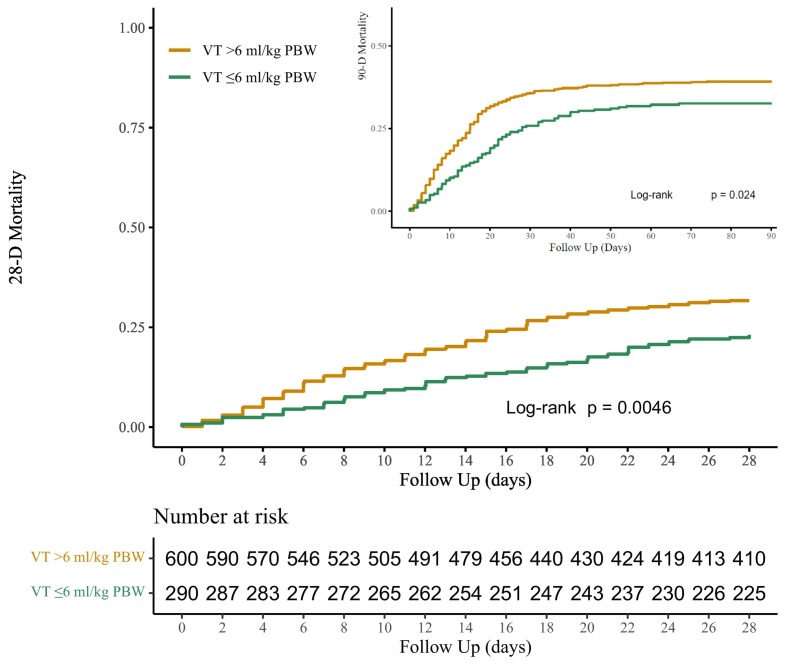
3.3. 28-day mortality
LTVV patients had a lower 28-day mortality, 23.1 versus 31.7% (P < 0.001) (Table 3 and Fig. 3 ). In multivariate analysis, LTVV had an independent association with 28-day mortality (Table 4 ). Other factors associated with 28-day mortality included age, severity of ARDS, arterial pH, heart rate, and development of acute kidney injury.
Table 3.
Clinical outcomes.
| Overall cohort (n = 903) | VT ≤ 6 mL/kg PBW (n = 294) | VT > 6 mL/kg PBW (n = 609) | P valuea | |
|---|---|---|---|---|
| Mortality | ||||
| Mortality at day 28 | 257 (28.9) | 67 (23.1) | 190 (31.7) | 0.005 |
| Mortality at day 90 | 313 (37.9) | 93 (34.1) | 220 (39.8) | 0.024 |
| In hospital mortality | 300 (36.1) | 84 (31.3) | 216 (38.3) | 0.017 |
| ICU mortality | 291 (33.0) | 80 (27.9) | 211 (35.5) | 0.013 |
| Clinical ventilator outcomes | ||||
| Ventilator free days at day 28, days | 2.48 [0.00–16.00] | 4.96 [0.00–16.99] | 0.96 [0.00–15.96] | 0.175 |
| Duration of ventilation, days | 16.00 [9.04–28.02] | 15.04 [9.52–28.04] | 16.00 [9.04–28.00] | 0.782 |
| Length of hospital stay in survivors | 29.04 [20.04–43.03] | 30.00 [19.52–42.54] | 29.04 [20.04–43.02] | 0.986 |
| Length of ICU stay in survivor | 17.04 [11.00–29.00] | 16.04 [10.00–28.01] | 18.00 [11.01–30.03] | 0.405 |
| Successful extubation | 528 (58.9) | 181 (62.2) | 347 (57.4) | 0.191 |
| Reintubation | 117 (13.1) | 37 (12.8) | 80 (13.3) | 0.917 |
| Tracheostomy | 145 (16.2) | 47 (16.1) | 98 (16.3) | 1.000 |
| Pneumothorax | 8 (0.9) | 2 (0.7) | 6 (1.0) | 0.971 |
| Extrapulmonary outcomes | ||||
| Thrombotic event | 259 (28.7) | 97 (33.0) | 162 (26.6) | 0.056 |
| Acute kidney injury | 403 (44.8) | 131 (44.7) | 272 (44.8) | 1.000 |
| Renal replacement therapy | 163 (18.1) | 59 (20.1) | 104 (17.1) | 0.316 |
Data are median [quartile 25% - quartile 75%] or No (%). Percentages may not total 100 because of rounding.
P value is computed comparing patients receiving VT ≤ 6 mL/kg PBW versus VT > 6 mL/kg PBW.
Fig. 3.
Kaplan Meier analysis for 28-day and 90-day mortality tested with log rank for significance. LTVV patients had lower mortality at both time points.
Table 4.
Mixed effect cox regression analysis on 28 day mortality.
| Univariate mixed effect cox regression | Hazard ratio (95% CI) | P value |
|---|---|---|
| LTVV | 0.67 (0.51 to 0.88) | 0.005 |
| Age, years | 1.07 (1.05 to 1.08) | <0.001 |
| Male gender | 1.30 (0.97 to 1.75) | 0.078 |
| Arterial pH | 0.00 (0.00 to 0.00) | <0.001 |
| Heart rate, bpm | 1.02 (1.01 to 1.02) | <0.001 |
| Acute kidney injury | 2.72 (2.11 to 3.52) | <0.001 |
| Respiratory compliance, mL/cm H2O | 0.99 (0.98 to 1.01) | 0.387 |
| Use of prone positioning | 1.14 (0.88 to 1.46) | 0.317 |
| Moderate vs Mild ARDSa | 1.39 (0.98 to 1.97) | 0.063 |
| Severe vs Mild ARDSa | 2.24 (1.44 to 3.49) | <0.001 |
| Thrombotic events | 1.26 (0.97 to 1.63) | 0.083 |
| Multivariate mixed effect cox regression |
Hazard ratio (95% CI) |
P value |
| LTVV | 0.68 (0.49 to 0.95) | 0.025 |
| Age, years | 1.06 (1.04 to 1.07) | <0.001 |
| Male gender | 1.13 (0.81 to 1.59) | 0.471 |
| Arterial pH | 0.01 (0.00 to 0.06) | <0.001 |
| Heart rate, bpm | 1.01 (1.00 to 1.02) | 0.007 |
| Acute kidney injury | 2.00 (1.47 to 2.73) | <0.001 |
| Respiratory compliance, mL/cm H2O | 1.00 (0.99 to 1.01) | 0.821 |
| Use of prone positioning | 0.82 (0.60 to 1.13) | 0.221 |
| Moderate vs Mild ARDSa | 1.30 (0.88 to 1.91) | 0.191 |
| Severe vs Mild ARDSa | 1.72 (1.01 to 2.92) | 0.047 |
| Thrombotic events | 1.05 (0.78 to 1.41) | 0.746 |
All models were mixed-effect models with centres as frailty and considering a binomial distribution. Continuous variables were centered. The hazard ratios show the increase in one SD of the variable.
Abbreviations, LTVV: Low tidal volume ventilation;
Berlin definition: Mild ARDS P/F ratio > 200 mmHg, Moderate ARDS: 101 < P/F ratio < 200 mmHg, Severe ARDS: P/F ratio < 100 mmHg.
3.4. Secondary outcomes
LTVV patients had lower mortality rates in ICU and hospital, and at day 90 (Table 3). LTVV patients had more VFD-28, but this difference did not reach statistical significance. Duration of ventilation, length of stay in hospital and ICU, rate of successful extubation, rate of reintubation, and rate of tracheostomy was not different between the groups.
3.5. Factors associated with LTVV use
In a multivariate analysis, age, height, the initial VT and continuous infusion of NMBA were associated with use of LTVV (Table 5 ).
Table 5.
Logistic regression analysis of factors associated with use of LTVV.
| Univariate logistic regression | Odds ratio (95% CI) | P value |
|---|---|---|
| First set VT, mL/kg PBWa | 0.49 (0.44 to 0.56) | <0.001 |
| Age, years | 0.78 (0.71 to 0.85) | <0.001 |
| Male gender | 1.64 (1.35 to 2.01) | <0.001 |
| Height, cm | 1.81 (1.64 to 1.99) | <0.001 |
| Moderate vs Mild ARDSb | 1.08 (0.88 to 1.32) | 0.459 |
| Severe vs Mild ARDSb | 0.85 (0.53 to 1.37) | 0.503 |
| Use of NMBA | 1.36 (1.13 to 1.63) | <0.001 |
| Use of prone positioning | 1.17 (0.97 to 1.43) | 0.105 |
| Multivariate logistic regression |
Odds ratio (95% CI) |
P value |
| First set VT, mL/kg PBWa | 0.59 (0.52 to 0.67) | <0.001 |
| Age, years | 0.84 (0.76 to 0.93) | <0.001 |
| Male gender | 0.78 (0.60 to 1.02) | 0.068 |
| Height, cm | 1.63 (1.44 to 1.84) | <0.001 |
| Moderate vs Mild ARDSb | 1.19 (0.95 to 1.48) | 0.127 |
| Severe vs Mild ARDSb | 0.98 (0.58 to 1.67) | 0.950 |
| Use of NMBA | 1.31 (1.07 to 1.60) | 0.010 |
| Use of prone positioning | 1.15 (0.93 to 1.43) | 0.204 |
All models were mixed-effect models with centres as random factor and considering a binomial distribution. Continuous variables were centered. The odds ratio show the increase in one SD of the underuse of LTVV variable.
Abbreviations, LTVV: low tidal volume ventilation; NMBA: neuromuscular blocking agents; PBW: predicted bodyweight; VT: tidal volume.
First set tidal volume at the first hour of invasive ventilation.
Berlin definition Mild ARDS: P/F ratio > 200 mmHg, Moderate ARDS: 101 < P/F ratio < 200 mmHg, Severe ARDS: P/F ratio < 100 mmHg.
3.6. Posthoc analyses
Females received LTVV less frequent than males (Supplement eTable 2). Univariate logistic regression showed that the odds of mortality at day 28 increased by 19% (95% CI [1.02 to 1.39]) for a one unit increase in VT per PBW (Supplement eFigure 2).
4. Discussion
The findings of this secondary analysis of a conveniently-sized study in COVID-19 patients that needed invasive ventilation in the first wave of the national outbreak in the Netherlands can be summarized as follows: (1) LTVV was used in approximately a third of all patients; (2) LTVV had an independent association with 28-day mortality; and (3) potentially modifiable factors associated with LTVV use were the initial VT and continuous muscle paralysis.
This study has several strengths. First, we used a dataset with granular ventilation and sufficient data that allowed us to focus our analysis to VT under controlled ventilation and in absence of spontaneous breathing activity. We strictly followed a predefined analysis plan, and the study had a convenient sample size, including nearly a third of all COVID-19 patients that needed invasive ventilation in the first wave [14]. Second, patients were enrolled in various types of hospitals, including university hospitals, and teaching and non-teaching hospitals, all increasing the generalizability of our findings. Third, to deal with effects of potential differences between hospitals we accounted centers as random effect in the statistical analysis.
VT was much lower than in previous cohorts of patients with ARDS due to another cause than COVID-19 [10,17], but in line with VT in cohorts of patients with COVID-19 [[18], [19], [20], [21], [22], [23], [24]]. The lower VT in COVID-19 patients may reflect an improved implementation of lung-protective ventilation over the recent years [25]. Otherwise, the fact that care for invasively ventilated COVID-19 patients had to be provided by hospital personnel that had much less experience or confidence with setting a ventilator may have resulted in a much better compliance with current guidelines for the ventilation of patients with ARDS, which includes measures to prevent lung-injury, i.e., ventilation with a low VT [14]. This might also be the reason that our cohort was homogenous with respect to ventilator mechanics and the use of certain interventions like NMBA use and prone positioning.
The definition for LTVV, however, remains a matter of debate. Previous studies used different cutoffs, e.g., 8 mL/kg PBW [21], 6.5 mL/kg PBW [9], or 6 mL/kg PBW [20] in patients with ARDS due to COVID-19; 8 mL/kg PBW [10] in patients with ARDS not caused by COVID-19; or 6 mL/kg PBW [26], or 8 mL/kg PBW in patients without ARDS [17]. Even guidelines show discrepancies in recommendations [5,27,28]. We chose to use the rather ‘strict’ cutoff based on the landmark ARMA trial [6]. Furthermore, although it may seem that the median VT does not differ greatly, it is important to note that the distribution of VT between these groups is different. In other words, patients in the non-LTVV group received a larger VT. The findings of our posthoc analyses suggest that these differences in VT may have clinical consequences, also in patients with ARDS due to COVID-19. This supports a previous analysis which showed that one SD increase in VT per PBW translated into a 28% increase in 28-day mortality [14].
One novelty of our analysis is that we investigated the association of LTVV in passive patients, i.e., in patients during controlled ventilation and without evidence of spontaneous breathing activity, with outcomes. In a spontaneous breathing patient, a VT > 6 mL/kg PBW could be acceptable for several reasons. An active diaphragm increases the vital capacity of the lungs [29], and distribution of air in the lungs during spontaneous ventilation is more homogeneous than during passive ventilation [30]. Under these conditions an increase in VT size could be suitable. Also, there is much less control over VT size during spontaneous ventilation [31]. In other words, we focused on the effects of a low VT in a scenario wherein it is important, and also possible, to limit the size of a VT.
Our results confirm the findings of recent investigation in Brazil that studied the association of LTVV with outcomes in COVID-19 patients [21]. In that study, LTVV was also associated with a better survival [21]. The association of LTVV with other endpoints in our study further reinforces the idea that LTVV use could be an important target in COVID-19 patients. In absence of randomized clinical trial evidence, the findings of these two studies together could be used as a strong argument to use LTVV, at least in patient that are passive.
Several factors had an association with use of LTVV. One fixed factor that had an association with LTVV use was patient's height. Previous studies showed that short patients are at a higher risk for not receiving LTVV—this was found in critically ill patients that received ventilation in the ICU [25], and also in surgery patients that received intraoperative ventilation in the operating room [32]. VT should be titrated to the PBW, which is a function of height. Of note, measuring patients' height in critically ill patients, i.e., in a supine position, can be challenging [33], and estimates of height are often inaccurate—height is often overestimated in shorter individuals [34,35]. Another non-modifiable factor that had an association with use of LTVV was age. Older age has been identified as a risk factor for underuse of LTVV before [36], but the reason for this remains unexplained.
The initial VT was one of the potentially modifiable factors associated with use of LTVV. This finding is in line with findings from a previous study in patients with ARDS not caused by COVID-19 [37]. The VT often remains unchanged after its initial setting, as was also found in other settings, like in the emergency room [[38], [39], [40], [41], [42], [43]]. This finding emphasizes the importance of correct initial ventilator settings and if needed, corrections later on. One other potentially modifiable factor was continuous muscle paralysis. We are not aware of previous studies that investigated whether use of NMBA has an association with use of LTVV. However, muscle paralysis may reduce oxygen consumption through decreased work of breathing, eventually allowing the use of a lower minute ventilation, and thus a lower VT.
The finding that patients receive LTVV more often with volume-controlled ventilation may be explained by the fact that healthcare providers ‘directly’ set VT with this modus, while with pressure-controlled ventilation VT is set ‘indirectly’, i.e., by adjusting the inspiratory pressure. Our results that female patients receive LTVV less frequent than male patients during invasive ventilation are in accordance with findings from previous studies [25,44]. Furthermore, one of these studies illustrated that this difference is largely driven by the body height using a mediation analysis [44].
This analysis has several limitations. First, the collection of ventilation variables and adjunctive treatments was restricted to the first 4 days of ventilation due to the data available in the PRoVENT-COVID dataset [45]. It is possible that ventilation practice and adjunctive therapies beyond these days have an effect on outcomes. Second, this data was collected during the first 3 months of the pandemic. Ventilation practice may have changed thereafter, and particularly the increased use of high-flow oxygen systems that may have prevented intubation and invasive ventilation may have caused changes in patient case mix—and with that the association of LTVV and outcome may have changed. Third, this study enrolled patients in only one country, which may hamper the generalizability of the findings. Fourth, the centers that participated in the study may have had increased interest in invasive ventilation, in particular use of LTVV, or bias may have been caused by the fact that the healthcare workers were aware that ventilation data were being collected. Fifth, in this study we did not collect data on adjunctive supportive therapies before and during ICU admission, such as antiviral or steroidal drugs. These may have affect outcomes as well. Sixth, we did not analyze how the healthcare providers titrated VT, which could be different with the various modes used, but also be driven by other factors, e.g., airway pressures, respiratory rate, and minute volume, and even changes in lung compliance. Seventh, we did neither collect how height was measured nor how PBW was calculated by the healthcare workers. Eight, the high use of vasopressor in this cohort is challenging to interpret because we collected vasopressor use at any time during the first four calendar days, including the moment close to tracheal intubation.
5. Conclusion
LTVV is used in approximately a third of all patients with ARDS related to COVID-19. Use of LTVV was independently associated with reduced 28-day mortality. Potentially modifiable factors that have an association with use of LTVV are the initial VT and continuous infusion of NMBA.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. The institutional review boards of each participating center approved the study protocol, and need for individual patient informed consent was waived based on the observational nature of the study.
Author contributions
Nijbroek and Serpa Neto had full access to the database of the study and take responsibility for the integrity of the data and the accuracy of the current analysis.
Conceptualization and design:
Nijbroek, Ivanov, Schultz, Paulus and Serpa Neto.
Software:
Castor EDC (2019), a cloud-based clinical data management platform for electronic data capture (available at: https://castoredc.com) and R: A language and environment for statistical computing, R Core Team (2014). R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Acquisition, analysis or interpretation of data:
All authors.
Drafting of the manuscript:
All Authors.
Critical revision of the manuscript for important intellectual content:
All authors.
Statistical analysis
Nijbroek and Serpa Neto.
Obtained funding:
Paulus, Schultz.
Administrative, technical, or material support:
None.
Supervision:
Schultz, Paulus and Serpa Neto.
Funding/Support:
The Amsterdam University Medical Centers, location ‘AMC’ funded this study. It had no a role in the design, analysis, interpretation of data, writing or submission of this study.
Role of the Funder/Sponsor:
The funding sources had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and material:
The dataset will be made available upon request to the corresponding authors one year after the publication of this study. Any request must include a statistical analysis plan.
Code availability:
The code will be made available.
Declaration of Competing Interest
Ary Serpa Neto reports personal fees from Dräger, outside of the submitted work. Marcus Schultz reports personal fees from Hamilton and Xenios/Novalung, outside of the submitted work. Sunny Nijbroek, Liselotte Hol, Dimitri Ivanov, and Frederique Paulus declare no competing interests.
Acknowledgements
PRoVENT-COVID COLLABORATIVE GROUP
Investigators (in alphabetic order) J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; O.L. Baur; P. van de Berg; D.C.J.J. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J.G.H. Bindels; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; L.D.J. Bos; M. Botta; J.S. Breel; H. de Bruin; S. de Bruin; C.L. Bruna; L.A. Buiteman-Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; D.A. Dongelmans; H.S. Franke; M.S. Galek Aldridge; M.J. de Graaff; L.A. Hagens; J.J. Haringman; N.F.L.Heijnen; S.Hiel; S.T. van der Heide; P.L.J. van der Heiden; L.L. Hoeijmakers; L. Hol; M. W. Hollmann; M.E. Hoogendoorn; J. Horn; R. van der Horst; E.L.K. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W.M. Koopman; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; D.M. van Meenen; Ignacio Martin-Loeches, Guido Mazzinari; N. van Mourik; S.G. Nijbroek; M. Onrust; E.A.N. Oostdijk; F. Paulus; C.J. Pennartz; J. Pillay; L. Pisani; I.M. Purmer; T.C.D. Rettig; J.P Roozeman; M.T.U. Schuijt; M.J. Schultz; A. Serpa Neto; M.E. Sleeswijk; M.R. Smit; P.E. Spronk; W. Stilma; A.C. Strang; A. M. Tsonas; P.R Tuinman; C.M.A. Valk; F.L. Veen; A.P.J. Vlaar; L.I. Veldhuis; P. van Velzen; W.H. van der Ven; P. van Vliet; P. van der Voort; H.H. van der Wier; L. van Welie; H.J.F.T. Wesselink; B. van Wijk; T. Winters; W.Y. Wong; A.R.H. van Zanten.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2022.154047.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Velicu M.A., Furlanetti L., Jung J., Ashkan K. Epidemiological trends in COVID-19 pandemic: prospective critical appraisal of observations from six countries in Europe and the USA. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-045782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Domecq J.P., Lal A., Sheldrick C.R., Kumar V.K., Boman K., Bolesta S., et al. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the international viral infection and respiratory illness universal study registry. Crit Care Med. 2021;49(3):437–448. doi: 10.1097/CCM.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim Z.J., Subramaniam A., Ponnapa Reddy M., Blecher G., Kadam U., Afroz A., et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021;203(1):54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 6.Brower R.G., Matthay M.M., Morris A., Schoenfeld D., Taylor Thompson B. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342 doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Schultz M.J., Wolthuis E.K., Moeniralam H.S., Levi M. Struggle for implementation of new strategies in intensive care medicine: anticoagulation, insulin, and lower tidal volumes. J Crit Care. 2005;20(3):199–204. doi: 10.1016/j.jcrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Spece L.J., Mitchell K.H., Caldwell E.S., Gundel S.J., Jolley S.E., Hough C.L. Low tidal volume ventilation use remains low in patients with acute respiratory distress syndrome at a single center. J Crit Care. 2018;44:72–76. doi: 10.1016/j.jcrc.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qadir N., Bartz R.R., Cooter M.L., Hough C.L., Lanspa M.J., Banner-Goodspeed V.M., et al. Variation in early management practices in moderate-to-severe ARDS in the United States: the severe ARDS: generating evidence study. Chest. 2021;160(4):1304–1315. doi: 10.1016/j.chest.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 11.Rubenfeld G.D., Cooper C., Carter G., Thompson B.T., Hudson L.D. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6):1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 12.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 13.Grasselli G., Tonetti T., Protti A., Langer T., Girardis M., Bellani G., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botta M., Tsonas A.M., Pillay J., Boers L.S., Algera A.G., Bos L.D.J., et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serpa Neto A., Barbas C.S.V., Simonis F.D., Artigas-Raventós A., Canet J., Determann R.M., et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med. 2016;4(11):882–893. doi: 10.1016/S2213-2600(16)30305-8. [DOI] [PubMed] [Google Scholar]
- 18.Camporota L., Sanderson B., Dixon A., Vasques F., Jones A., Shankar-Hari M. Outcomes in mechanically ventilated patients with hypoxaemic respiratory failure caused by COVID-19. Br J Anaesth. 2020;125(6):e480–e483. doi: 10.1016/j.bja.2020.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R., Hernández M., Gea A., Arruti E., et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira J.C., Ho Y.L., Besen B., Malbouisson L.M.S., Taniguchi L.U., Mendes P.V., et al. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11(1):92. doi: 10.1186/s13613-021-00882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusina F., Albani F., Bertelli M., Cavallo E., Crisci S., Caserta R., et al. Corrected minute ventilation is associated with mortality in ARDS caused by COVID-19. Respir Care. 2021;66(4):619–625. doi: 10.4187/respcare.08314. [DOI] [PubMed] [Google Scholar]
- 23.COVID ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenck E.J., Hoffman K., Goyal P., Choi J., Torres L., Rajwani K., et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swart P., Deliberato R.O., Johnson A.E.W., Pollard T.J., Bulgarelli L., Pelosi P., et al. Impact of sex on use of low tidal volume ventilation in invasively ventilated ICU patients-a mediation analysis using two observational cohorts. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0253933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serpa Neto A., Simonis F.D., Barbas C.S., Biehl M., Determann R.M., Elmer J., et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med. 2014;40(7):950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 27.Papazian L., Aubron C., Brochard L., Chiche J.D., Combes A., Dreyfuss D., et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths M.J.D., McAuley D.F., Perkins G.D., Barrett N., Blackwood B., Boyle A., et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6(1) doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laroche C.M., Carroll N., Moxham J., Green M. Clinical significance of severe isolated diaphragm weakness. Am Rev Respir Dis. 1988;138(4):862–866. doi: 10.1164/ajrccm/138.4.862. [DOI] [PubMed] [Google Scholar]
- 30.Mauri T., Cambiaghi B., Spinelli E., Langer T., Grasselli G. Spontaneous breathing: a double-edged sword to handle with care. Ann Transl Med. 2017;5(14):292. doi: 10.21037/atm.2017.06.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H.-L., Chen L., Brochard L. Protecting lungs during spontaneous breathing: what can we do? J Thorac Dis. 2017;9(9):2777–2781. doi: 10.21037/jtd.2017.08.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijbroek S.G., Hol L., Swart P., Hemmes S.N.T., Serpa Neto A., Binnekade J.M., et al. Sex difference and intra-operative tidal volume: insights from the LAS VEGAS study. Eur J Anaesthesiol. 2021;38(10):1034–1041. doi: 10.1097/EJA.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien I.D., Shacklock E., Middleditch A., Bigham C. Inaccuracies in calculating predicted body weight and its impact on safe ventilator settings. J Intensive Care Soc. 2016;17(3):191–195. doi: 10.1177/1751143715626163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloomfield R., Steel E., MacLennan G., Noble D.W. Accuracy of weight and height estimation in an intensive care unit: implications for clinical practice and research. Crit Care Med. 2006;34(8):2153–2157. doi: 10.1097/01.CCM.0000229145.04482.93. [DOI] [PubMed] [Google Scholar]
- 35.Determann R.M., Wolthuis E.K., Spronk P.E., Kuiper M.A., Korevaar J.C., Vroom M.B., et al. Reliability of height and weight estimates in patients acutely admitted to intensive care units. Crit Care Nurse. 2007;27(5):48–55. [quiz 6] [PubMed] [Google Scholar]
- 36.Walkey A.J., Wiener R.S. Risk factors for underuse of lung-protective ventilation in acute lung injury. J Crit Care. 2012;27(3):323. doi: 10.1016/j.jcrc.2011.06.015. e1-.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Needham D.M., Yang T., Dinglas V.D., Mendez-Tellez P.A., Shanholtz C., Sevransky J.E., et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison M.G., Scott M.C., Hu K.M., Witting M.D., Winters M.E. High initial tidal volumes in emergency department patients at risk for acute respiratory distress syndrome. J Crit Care. 2015;30(2):341–343. doi: 10.1016/j.jcrc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Foley T.M., Philpot B.A., Davis A.S., Swanson M.B., Harland K.K., Kuhn J.D., et al. Implementation of an ED-based bundled mechanical ventilation protocol improves adherence to lung-protective ventilation. Am J Emerg Med. 2021;43:186–194. doi: 10.1016/j.ajem.2020.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuller B.M., Ferguson I.T., Mohr N.M., Drewry A.M., Palmer C., Wessman B.T., et al. Lung-protective ventilation initiated in the emergency department (LOV-ED): a quasi-experimental, before-after trial. Ann Emerg Med. 2017;70(3):406–418. doi: 10.1016/j.annemergmed.2017.01.013. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller B.M., Mohr N.M., Drewry A.M., Carpenter C.R. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care. 2013;17(1):R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens R.J., Siegler J.E., Fuller B.M. Mechanical ventilation in the prehospital and emergency department environment. Respir Care. 2019;64(5):595. doi: 10.4187/respcare.06888. [DOI] [PubMed] [Google Scholar]
- 43.Stoltze A.J., Wong T.S., Harland K.K., Ahmed A., Fuller B.M., Mohr N.M. Prehospital tidal volume influences hospital tidal volume: a cohort study. J Crit Care. 2015;30(3):495–501. doi: 10.1016/j.jcrc.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swart P., Nijbroek S.G.L.H., Paulus F., Neto A.S., Schultz M.J. Sex differences in use of low tidal volume ventilation in COVID-19—insights from the PRoVENT–COVID study. Front Med. 2022;8(2697) doi: 10.3389/fmed.2021.780005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boers N.S., Botta M., Tsonas A.M., Algera A.G., Pillay J., Dongelmans D.A., et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): rationale and protocol for a national multicenter observational study in the Netherlands. Ann Transl Med. 2020;8(19):1251. doi: 10.21037/atm-20-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.