Abstract
(+)-Calanolide A is a novel, naturally occurring, nonnucleoside inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase first isolated from a tropical tree (Calophyllum lanigerum) in the Malaysian rain forest. Previous studies have demonstrated that (+)-calanolide A has specific activity against the reverse transcriptase of HIV-1 and a favorable safety profile in animals. In addition, (+)-calanolide A exhibits a unique HIV-1 resistance profile in vitro. The safety and pharmacokinetics of (+)-calanolide A was examined in four successive single-dose cohorts (200, 400, 600, and 800 mg) in healthy, HIV-negative volunteers. In this initial phase I study, the toxicity of (+)-calanolide A was minimal in the 47 subjects treated. Dizziness, taste perversion, headache, eructation, and nausea were the most frequently reported adverse events. These events were not all judged to be related to study medication nor were they dose related. While 51% of subjects reported mild and transient dizziness, in many cases this appeared to be temporally related to phlebotomy. Calculation of the terminal-phase half-life (t1/2) was precluded by intrasubject variability in the 200-, 400-, and 600-mg dose cohorts but was approximately 20 h for the 800-mg dose group. (+)-Calanolide A was rapidly absorbed following administration, with time to maximum concentration of drug in plasma (Tmax) values occurring between 2.4 and 5.2 h postdosing depending on the dose. Plasma levels of (+)-calanolide A at all dosing levels were quite variable; however, both the mean concentration in plasma (Cmax), and the area under the plasma concentration-time curve increased proportionately in relation to the dose. Although raw plasma drug levels were higher in women than in men, when doses were normalized for body mass, the pharmacokinetic profiles were virtually identical with those observed for males. In general, levels of (+)-calanolide A in human plasma were higher than would have been predicted from animal studies, yet the safety profile remained benign. In conclusion, this study demonstrated the safety and favorable pharmacokinetic profile of single doses of (+)-calanolide A in healthy, HIV-negative individuals.
(+)-Calanolide A, (+)-[10R,11S,12S]-10,11-trans-dihydro- 12-hydroxy-6,6,10,11-tetramethyl-4-propyl-2H,6H-benzo[1,2-b: 3,4-b′:5,6-b"]tripyran-2-one, is a novel nonnucleoside reverse transcriptase inhibitor (NNRTI) with potent activity against the human immunodeficiency virus type 1 (HIV-1) (7, 10). The compound was first isolated from a tropical tree (Calophyllum lanigerum) in Malaysia (10). Due to low availability of naturally occurring (+)-calanolide A, a total synthesis of this polycyclic coumarin was developed to provide material for preclinical and clinical research (6, 10).
Previous in vitro studies have demonstrated the protective activity of (+)-calanolide A to both established cell lines and primary human cells against a wide variety of HIV-1 isolates including syncytium-inducing (SI) and non-syncytium-inducing (NSI) viruses, T-tropic, and monocyte-macrophage tropic viruses (1, 2, 4, 7, 10). The activity (i.e., the 50% effective concentration) of the compound ranged from 0.02 to 0.5 μM. No activity was detected against HIV-2 or simian immunodeficiency virus. Direct cytotoxicity (i.e., the 50% infective concentration) of (+)-calanolide A was apparent at concentrations approximately 100 to 200 times greater than the anti-HIV-1-activity concentration in all cell lines tested (3, 5, 10). Kinetic analyses indicated that (+)-calanolide A inhibited HIV-1 reverse transcriptase (RT) by a complex mechanism involving two possible binding sites, a property that has not been observed for any other NNRTI (5, 10). Evidence suggests that one of the (+)-calanolide A binding sites is near both the pyrophosphate binding site and the active site of the RT enzyme (5, 10).
In vitro synergy has been demonstrated between (+)-calanolide A and a number of other antiretroviral agents, including NRTI's, NNRTI's, and protease inhibitors (8, 10; R. W. Buckheit, Jr., J. Russell, V. F. Boltz, L. A. Pallansch, Z.-Q. Xu, and M. T. Flavin, Abstr. Conf. Rec. 12th World AIDS Conf., abstr. 12366, p.86, 1998). (+)-Calanolide A remains fully active against virus isolates with zidovudine (AZT) and 3TC resistance-engendering mutations (1, 2, 8, 10). The compound has enhanced activity against virus isolates with the Y181C mutation, which confers resistance to other NNRTIs and against viruses that have both AZT resistance and the Y181C mutation. Even though (+)-calanolide A exhibits reduced activity against HIV-1 with the K103N mutation, it remains fully active against virus isolates that express both the K103N and the Y181C mutations. This resistance profile is a unique feature of the compound, since the Y181C and K103N mutations are two of the most commonly observed mutations in laboratory and clinical virus isolates from patients receiving other NNRTIs, including nevirapine, delavirdine, and efavirenz (9). In vitro, (+)-calanolide A predominantly selects for a unique drug-resistant virus having a mutation at amino acid residue 139 (T139I). This virus remains susceptible to all other anti-HIV agents tested, including other NNRTIs (3).
The toxicity of (+)-calanolide A in a number of animal species, including mice, rats, and dogs, has been studied (P. Frank, M. T. Flavin, J. Roca-Acin, and Z.-Q. Xu, Abstr. 4th Conf. Retrovir. Opportunistic Infect. [CROI], abstr. 225, p. 106, 1997). (+)-Calanolide A was well tolerated at oral doses of up to 150 mg/kg in rats and 100 mg/kg in dogs. Toxicities associated with the oral administration of (+)-calanolide A for up to 28 days in animals were gastric irritation and subsequent gastric hyperplasia and edema in the rat and salivation in the dog. Emesis in the dog was the dose-limiting side effect, but a 50% lethal dose could not be attained. In vitro and in vivo assays for mutagenicity have been negative, and (+)-calanolide A does not produce teratologic effects when administered to rats during gestation (Frank et al., 4th CROI).
In vitro studies indicate that metabolism is qualitatively similar in rats, dogs, monkeys, and humans, with four to seven main metabolites produced (S. D. Patil, A. K. Thilagar, P. Frank, and Z.-Q. Xu, PharmSci Suppl. 1:S-41, abstr. 1125, 1998). CYP3A4 is the primary isoform of P450 that metabolizes (+)-calanolide A, although CYP2C may be involved as a minor isoform (S. D. Patil, A. K. Thilagar, P. Frank, and Z.-Q. Xu, PharmSci Suppl. 1:S-41, abstr. 1126, 1998). Animal studies have shown that compound-related radioactivity distributes into both the brain and the lymph after oral administration, while after intravenous administration the radioactivity accumulates in the brain (Frank et al., 4th CROI). These studies indicate that (+)-calanolide A crosses the blood-brain barrier and may be preferentially distributed in the lymphatic system. (Frank et al., 4th CROI). Indeed, in rat studies, the oral administration of radiolabeled (+)-calanolide A results in a mean ratio of lymph to serum radioactivity of 2.8:1 after 6 h. (+)-Calanolide A binds extensively (>97%) to human and animal plasma proteins and to human α1-acid glycoprotein (Frank et al., 4th CROI).
The favorable safety profile and pharmacokinetics of (+)-calanolide A, coupled with its unique in vitro resistance pattern, has led to further clinical development. The first phase I study, described in this report, was conducted primarily to evaluate the safety and secondarily to evaluate the pharmacokinetics of single escalating doses of (+)-calanolide A in humans. In addition, the effect of dosing in a fasted or fed state was explored in the second and third cohorts in order to obtain information valuable in the design of future clinical trials. Because of a concern in regard to the rapid emergence of viral resistance with many monotherapy antiretroviral therapies in HIV-positive patients, this preliminary study was conducted in healthy, HIV-negative subjects.
MATERIALS AND METHODS
Study population.
Enrollment in the study was limited to healthy, HIV-negative subjects of any race, 18 years of age or older, weighing within 20% of their ideal body weight (Society of Actuaries and Association of Life Insurance Medical Directors of America; also called the “Met-Life Tables”). Subjects were excluded from enrollment if they had a history of hemophilia, sickle cell disease, or other known blood dyscrasias or if they had intractable diarrhea or severe malabsorption. Subjects were also excluded if they had any chemical or hematological findings that were more than 10% outside the laboratory normal range (modified Adult ACTG toxicity grading scale) within 30 days prior to receiving the study dose of (+)-calanolide A. Treatment within 30 days of dosing, or required use during the study, of biological response modifiers, antimetabolites, systemic corticosteroids, antibiotics, or antivirals was prohibited. Subjects previously treated with dexfenfluramine, phentermine, or fenfluramine were excluded from participation. Negative hepatitis panel, serum pregnancy test, and HIV-1 enzyme-linked immunosorbent assay results were required prior to enrollment. Subjects were excluded if pregnant, lactating, or unwilling to employ adequate birth control. Qualified subjects must not have had any history of intolerance to any components of the (+)-calanolide A formulation. Participation in any other clinical trial involving drug therapy within the previous 6 months was prohibited. Written informed consent was obtained from all participants, and the study was both reviewed and approved by the Western Institutional Review Board (Olympia, Wash.).
Study medication and dosing.
(+)-Calanolide A was supplied as translucent, soft gelatin capsules containing 100 mg of (+)-calanolide A formulated in an oil-based vehicle. Study medication was supplied in blister-packs of 10 capsules each.
Subjects in this phase I, single escalating-dose study were enrolled in sequential, gender-balanced cohorts of 8 (cohort 1) or 12 (cohorts 2, 3, and 4) subjects. Cohort sizes of 8 to 12 subjects/group were chosen to limit the effects of intrasubject variability. Within 21 days prior to dosing, subjects underwent a screening evaluation, including a medical history, physical examination, and clinical and laboratory measurements including weight, height, ECG, chest X-ray, hepatitis panel, urinalysis-drug screen, and serum pregnancy test. Eligible subjects in each cohort were admitted to the clinical research unit (Northwest Kinetics, LLC, Tacoma, Wash.) in the evening prior to the day of dosing. An additional negative serum pregnancy test for female subjects was required during this predose evening. All subjects were confined to the unit (24 h postdose for cohorts 1 to 3, 48 h postdose for cohort 4) until after the final pharmacokinetic sample had been obtained. All prescription and over-the-counter medications were withheld for the 24 h prior to and 48 h after dosing. (+)-Calanolide A was administered to eligible subjects in four successive dose cohorts of 200 mg (cohort 1), 400 mg (cohort 2), 600 mg (cohort 3), and 800 mg (cohort 4). Subjects in cohorts 2 (400-mg dose) and 3 (600-mg dose) were randomly assigned to receive the drug with food (eggs, bacon, milk, fruit juice) or fasting (at least 8 h before and 2 h after dosing). All subjects in cohort 1 were given the drug with food. All cohort 4 subjects fasted prior to dosing and for 4 h after dosing; water consumption was allowed ad libitum, with the exception of the period between 1 h before and 2 h after dosing.
Immediately prior to the administration of study drug, weight measurements, vital-sign determinations, urinalysis (dipstick, microscopic), hematology-chemistry panels, urine drug screens, and an ECG exam were performed. All subjects within each cohort were dosed simultaneously. Study personnel administered (+)-calanolide A with 100 to 150 ml of water and recorded the exact time of dosing for each subject. Adverse events were monitored, and vital signs obtained at various time points postdosing.
Pharmacokinetic sampling.
Blood samples for pharmacokinetic analysis were obtained via an indwelling catheter or via direct venipuncture. Patency of the indwelling catheter was maintained by saline flush, and heparin flush was not permitted. Blood samples were collected before dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h postdosing. Cohort 4 subjects had additional samples drawn at 32, 36, and 48 h postdose. The exact times of doses, meals, and pharmacokinetic blood collections were recorded.
Each pharmacokinetic blood sample was collected in a 7-ml lavender top (pyrogen-free, EDTA-containing) tube and processed to provide approximately 3 to 4 ml of plasma. Plasma was stored in two storage cryotubes (equal amounts per tube) at −70°C. Each cryotube was labeled with the date and time of dose, the time of blood draw, the time of last meal prior to the blood draw, the study day, and the subject identification number and initials. One of each pair of stored samples was shipped to Analytic Development Corporation (Colorado Springs, Colo.) for pharmacokinetic analyses. The other sample was temporarily stored at the clinical research site prior to shipment to the study sponsor.
Assay for plasma samples.
Concentrations of (+)-calanolide A in plasma were determined using a validated high-performance liquid chromatographic (HPLC) method with fluorescent detection (11). Briefly, the synthetic intermediate (±)-12-oxocalanolide A prepared in 100% acetonitrile (4 μg/ml) was used as an internal standard, and 50 μl of this internal standard solution was mixed with the plasma sample to be analyzed. The sample mixtures (1.0 ml) were loaded onto a Varian Bond Elut column (Harbor City, Calif.) (6 ml containing 1.0 g of C18 packing material). Each column was preconditioned with 5 ml of acetonitrile followed by 5 ml of HPLC-grade water flowing through the column by gravity. The column was washed with water (acidified with acetic acid) and eluted with 5 ml of acetonitrile under vacuum, and the eluents were collected and dried under N2 at 50 to 60°C. The residue was reconstituted in 300 μl of acetonitrile, mixed by vortexing, sonicated, and filtered (0.2 μm [pore size]; 13-mm PTFE Gelman filter attached to a 1-ml disposable syringe) into an autoinjector vial for HPLC analysis.
The liquid chromatograph (Hewlett-Packard 1050 HPLC) was operated at a flow-rate of 1.3 ml/min, and the autoinjector (Spectra-Physics 8775/3506) was set to deliver 100 μl. The analytical column was a 250-by-4.6-mm Zorbax ODS C18 with a 5-μm particle size at ambient temperature, preceded by a C18 guard column (Brownlee Newguard, 15 by 3.2 mm, 7-μm particle size). The system was also equipped with an in-line filter (Fisher Scientific, 0.5 μm) and a fluorescence detector (Applied Biosystems 980), which was set for excitation at 285 nm with an emission cutoff filter at 418 nm. Mobile-phase A was acetonitrile-water (70:30), and mobile-phase B was acetonitrile. The gradient profile of the mobile phase was as follows: 0- to 2-min hold at 100% A, 2- to 5-min linear increase to 95% B in A, 5- to 10-min hold at 95% B in A, and 10- to 12-min linear return to 100% A.
The ratios of the peak areas for (+)-calanolide A and internal reference (±)-12-oxocalanolide A were plotted against the (+)-calanolide A concentration to check for linearity, and the correlation coefficient was calculated. Curves with a correlation coefficient of >0.98 from the unweighted regression analysis were accepted. (+)-Calanolide A was quantifiable over the assay range of 12.5 to 800 ng/ml, and the assay was determined to be linear over this range. The interday precisions (percent coefficients of variation), as determined using the relative standard deviation, were 15.1% at 12.5 ng/ml, 3.3% at 200 ng/ml, and 5.8% at 800 ng/ml; and the intraday variabilities were 19.3, 4.3, and 8.3%, respectively.
Safety evaluation.
The safety of single escalating doses of (+)-calanolide A was evaluated based on adverse experience reports, measurements of vital signs (heart rate, body temperature, respiratory rate, and blood pressure), clinical laboratory values, and the results of physical examination. Successive cohorts were not dosed until all subjects in the previous cohort had completed their week 1 follow-up visits with no unacceptable toxicities. For this study, toxicity was considered unacceptable if, within any cohort, the following occurred: (i) any subject experienced a life-threatening grade 4 toxicity that was reasonably attributable to study treatment or (ii) any three subjects experienced non-life-threatening grade 3 or 4 toxicities that were reasonably attributable to study medication or did not resolve within a reasonable time after dosing. Periodic clinical trial monitoring was conducted according to protocol and regulatory requirements.
Complete physical exams were conducted on day 0 (dosing), day 1 (postdosing), and week 1 (postdosing) for all subjects; a symptom-directed physical exam was required at week 2. In addition, 12-lead ECG exams were performed at the day 0, day 1, and week 1 time points. Laboratory safety assessments included chemistry studies (ALT, AST, alkaline phosphatase, lactate dehydrogenase, lipase, creatinine, blood urea nitrogen, bilirubin, albumin, total protein, total cholesterol, triglycerides, glucose, CPK, sodium, potassium, bicarbonate), coagulation studies (APTT, PT, and PTT), hematology studies (complete blood count with differential, MCV, platelets), and urinalysis (macroscopic and microscopic). Review of concomitant medications and adverse event assessment were closely monitored at each study time point. Specifically, adverse event assessment required noting the severity, start time, treatment, possible contributing factors, full clinical course, and outcome of the adverse event.
Day 2 assessments, involving cohort 4 only, included weight and vital signs, a symptom-directed physical examination, and completion of the 48-h urine collection. The total volume was recorded before each subject's urine sample was homogenized. After homogenization, two 100 -ml urine aliquots were removed, labeled, and stored at −70°C for future analysis.
Statistical analysis.
The primary outcome measure in the study was the safety of escalating single doses of (+)-calanolide A, as measured by the appearance of adverse signs and/or symptoms. The primary planned comparisons between dosing cohorts evaluated the profile of changes in all safety measures. The study was not designed to detect statistically significant differences in safety measures but rather was designed to identify a safe dosing regimen for use in future studies.
A secondary outcome measure was the pharmacokinetics of single oral doses of (+)-calanolide A when administered to healthy HIV-negative volunteers. (+)-Calanolide A concentrations were determined in plasma samples by a validated HPLC assay with a limit of quantitation at 12.5 ng/ml. Levels of (+)-calanolide A were determined at all specified time points in the pharmacokinetic profile. All plasma sample analyses were conducted at Analytic Development Corporation.
Pharmacokinetic parameters were calculated using noncompartmental analysis by means of a previously validated LOTUS 1-2-3 macro. All mean plasma concentrations and derived pharmacokinetic parameters are presented as the mean ± the standard error of the mean (SEM). The Cmax and corresponding Tmax were obtained by direct inspection of the plasma concentration data. Concentrations in plasma below the limit of quantitation of the assay were set to zero. Area under the plasma concentration time-curve (AUC) was calculated according to the trapezoidal rule, from time zero to the last time at which unchanged drug was detectable in the plasma. Where possible, t1/2 was determined by nonweighted linear regression of at least three nonzero points in the terminal phase. The t1/2 value was reported only if the correlation coefficient for the elimination rate constant was ≥0.98. The apparent clearance from plasma (CL/F) was determined by dividing the dose by the AUC value and normalizing this value to the body weight. Exploratory analyses of pharmacokinetics of (+)-calanolide A focused on relative bioavailability and effect of food on absorption. Similar comparisons of pharmacokinetics by gender were also undertaken to provide information that would be helpful in developing a rational design for future clinical trials in HIV-positive patients.
RESULTS
(+)-Calanolide A was administered to 47 healthy HIV-negative subjects in four successive single-dose cohorts of 200, 400, 600, and 800 mg. All cohorts were balanced by gender, and the demographics are detailed in Table 1. The subjects in cohorts 2 (400-mg dose) and 3 (600-mg dose) were randomly assigned to receive the drug either with food or fasting.
TABLE 1.
Demographics of subjects receiving a single dose of (+)-calanolide A
| Parameter | Dosing cohort
|
All subjects (n = 44) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 mg, alla(n = 8) | 400 mg, fed (n = 6) | 400 mg, fasted (n = 6) | 400 mg, all (n = 12) | 600 mg, fed (n = 6) | 600 mg, fasted (n = 6) | 600 mg, all (n = 12) | 800 mg, allb(n = 12) | ||
| Mean dose (mg/kg)c | 2.6 ± 0.1 | 5.3 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.2 | 8.4 ± 0.3 | 8.9 ± 0.5 | 8.6 ± 0.3 | 10.8 ± 0.6 | |
| Age group (n) | |||||||||
| 18–20 yr | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 21–30 yr | 3 | 0 | 2 | 2 | 3 | 2 | 5 | 5 | 15 |
| 31–40 yr | 3 | 2 | 1 | 3 | 1 | 1 | 2 | 3 | 1 |
| 41–50 yr | 0 | 2 | 3 | 5 | 2 | 1 | 3 | 2 | 10 |
| 51–60 yr | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 |
| 61–70 yr | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 4 |
| Mean age (yr)c | 37.0 ± 4.6 | 47.7 ± 4.9 | 36.2 ± 3.5 | 41.9 ± 3.4 | 36.0 ± 3.9 | 34.7 ± 5.4 | 35.3 ± 3.2 | 36.5 ± 3.8 | 37.8 ± 1.8 |
| Mean baseline wt (kg)c | 78.1 ± 4.0 | 75.8 ± 3.6 | 75.4 ± 4.4 | 75.6 ± 2.7 | 72.0 ± 2.3 | 68.8 ± 3.8 | 70.4 ± 2.2 | 76.2 ± 3.8 | 74.8 ± 1.6 |
| Race or ethnicity (n) | |||||||||
| White, not Hispanic | 6 | 6 | 3 | 9 | 6 | 3 | 9 | 9 | 33 |
| African American | 2 | 0 | 2 | 2 | 0 | 3 | 3 | 2 | 9 |
| Hispanic | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 |
| Gender (n) | |||||||||
| Female | 4 | 3 | 3 | 6 | 3 | 3 | 6 | 6 | 22 |
| Male | 4 | 3 | 3 | 6 | 3 | 3 | 6 | 6 | 22 |
All subjects receiving 200 mg were fed.
All subjects receiving 800 mg were fasted.
Data are reported as the mean ± the SEM.
Safety evaluation.
In the four dosing cohorts, a total of 110 adverse events were reported, 101 of which were considered to be mild (grade 1). Forty-two treated subjects reported at least one adverse event, but only seven subjects experienced any event of greater than grade 1 severity. Table 2 summarizes the most frequent and/or most severe adverse events reported in this study. Of the events listed in Table 2, only dizziness, taste perversion (oily aftertaste), headache, eructation, and dyspepsia were considered to be probably related to study drug.
TABLE 2.
Numbers and percentages of subjects experiencing most frequent adverse events observed
| Adverse event and cohort (n) | Total (n = 47)
|
Severity | ||
|---|---|---|---|---|
| No. | % | |||
| Dizziness | ||||
| 200 mg (8) | 2 | 25.0 | All mild | |
| 400 mg (12) | 6 | 50.0 | Five mild; one moderate | |
| 600 mg (15) | 9 | 60.0 | Eight mild; one moderate | |
| 800 mg (12) | 7 | 58.3 | All mild | |
| Total | 24 | 51.1 | ||
| Taste perversion | ||||
| 200 mg (8) | 8 | 100.0 | All mild | |
| 400 mg (12) | 6 | 50.0 | All mild | |
| 600 mg (15) | 5 | 33.3 | All mild | |
| 800 mg (12) | 5 | 25.0 | All mild | |
| Total | 24 | 51.1 | ||
| Headache | ||||
| 200 mg (8) | 3 | 37.5 | All mild | |
| 400 mg (12) | 2 | 16.7 | All mild | |
| 600 mg (15) | 4 | 26.7 | All mild | |
| 800 mg (12) | 2 | 16.7 | All mild | |
| Total | 11 | 23.4 | ||
| Eructation | ||||
| 200 mg (8) | ||||
| 400 mg (12) | 5 | 41.7 | All mild | |
| 600 mg (15) | 2 | 13.3 | All mild | |
| 800 mg (12) | ||||
| Total | 7 | 14.9 | ||
| Nausea | ||||
| 200 mg (8) | 3 | 37.5 | All mild | |
| 400 mg (12) | 2 | 16.7 | One mild; one moderate | |
| 600 mg (15) | ||||
| 800 mg (12) | 2 | 16.7 | All mild | |
| Total | 7 | 14.9 | ||
| Dyspepsia | ||||
| 200 mg (8) | 4 | 50.0 | All mild | |
| 400 mg (12) | ||||
| 600 mg (15) | ||||
| 800 mg (12) | 1 | 8.3 | Mild | |
| Total | 5 | 10.6 | ||
| Diarrhea | ||||
| 200 mg (8) | 1 | 12.5 | Mild | |
| 400 mg (12) | 1 | 8.3 | Mild | |
| 600 mg (15) | 1 | 6.7 | Mild | |
| 800 mg (12) | 1 | 8.3 | Mild | |
| Total | 4 | 8.5 | ||
| Abdominal pain | ||||
| 200 mg (8) | ||||
| 400 mg (12) | 2 | 16.7 | One mild; one moderate | |
| 600 mg (15) | 1 | 6.7 | Mild | |
| 800 mg (12) | ||||
| Total | 3 | 6.4 | ||
| Pharyngitis | ||||
| 200 mg (8) | ||||
| 400 mg (12) | ||||
| 600 mg (15) | 2 | 13.3 | One mild; one moderate | |
| 800 mg (12) | 1 | 8.3 | Mild | |
| Total | 3 | 6.4 | ||
| Vomiting | ||||
| 200 mg (8) | ||||
| 400 mg (12) | 1 | 8.3 | Moderate | |
| 600 mg (15) | 1 | 6.7 | Mild | |
| 800 mg (12) | ||||
| Total | 2 | 4.3 | ||
Six subjects reported eight adverse events that were of moderate intensity (grade 2). Table 3 summarizes all grade 2 adverse events reported during the study, as well as the dose received (in milligrams/kilogram) for each case. One subject's laboratory results indicated a one-time grade 3 lipase elevation; however, this subject was completely asymptomatic, and no other subject had any lipase value above grade 1. No direct relationship was apparent between dose and severity of adverse event.
TABLE 3.
Pharmacokinetic data for subjects experiencing grade 2 adverse events
| Subject | Dose (mg) | Dose (mg/kg)a | Dose range (mg/kg) for cohort | Grade 2 adverse event | Cmax (ng/ml) | AUC (ng · h/ml) | Mean AUC (ng · h/ml) for cohort | AUC range (ng · h/ml) for cohort |
|---|---|---|---|---|---|---|---|---|
| 206 | 400 | 4.7∗ | 4.3–6.1 | Abdominal painb | 1,186 | 4,305 | 9,570 | 1,761–14,858 |
| 212 | 400 | 6.0† | 4.3–6.1 | Dizziness | 1,117 | 11,173 | 9,570 | 1,761–14,858 |
| 212 | 400 | 6.0† | 4.3–6.1 | Nausea | 1,117 | 11,173 | 9,570 | 1,761–14,858 |
| 212 | 400 | 6.0† | 4.3–6.1 | Vomiting (gagging, no emesis) | 1,117 | 11,173 | 9,570 | 1,761–14,858 |
| 306 | 600 | 9.0† | 7.3–10.8 | Dizziness | 2,308 | 10,445 | 12,973 | 1,840–15,328 |
| 311 | 600 | 7.5∗ | 7.3–10.8 | Arthralgia and arthritisb | 325 | 1,840 | 12,973 | 1,840–15,328 |
| 314 | 600 | 8.5∗ | 7.3–10.8 | Hypertensionb | 1,370 | 8,247 | 12,973 | 1,840–15,328 |
| 315 | 600 | 10.8† | 7.3–10.8 | Pharyngitisb | 3,443 | 14,746 | 12,973 | 1,840–15,328 |
∗, subject was fed prior to dosing; †, subject was fasted prior to dosing.
Considered by investigator to be unrelated to dosing.
Pharmacokinetic evaluation.
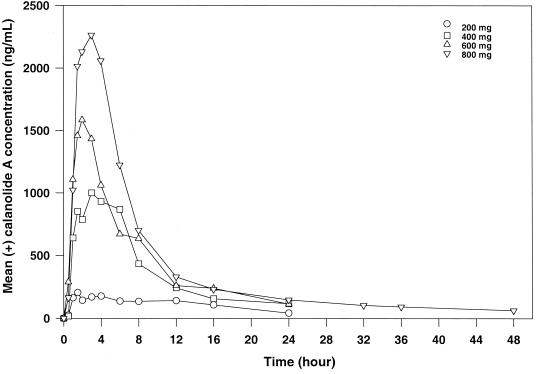
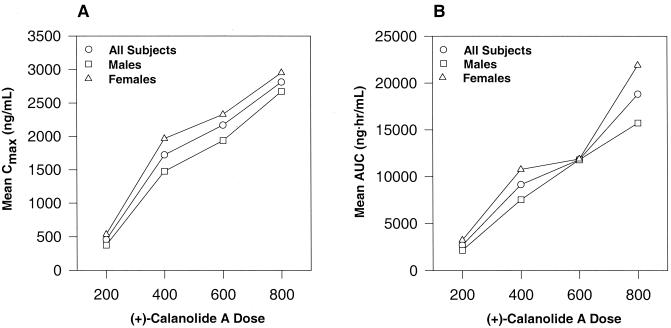
Pharmacokinetic parameters were highly variable among subjects. Table 4 and Fig. 1 and 2 summarize the overall profile. Intrasubject variability and limited elimination phase sampling precluded calculation of the half-life (t1/2) for all subjects in the first three cohorts. The data from cohort 4 (800-mg dose) indicated a t1/2 of about 20 h (Table 4). Concentrations of (+)-calanolide A were measurable beyond 24 h for all but three test subjects in this cohort. Both Cmax and AUC values increased with dose (Fig. 2). An increase in Cmax was accompanied by a respective increase in the AUC. Dose escalation across cohorts increased 100% from cohort 1 to cohort 2, 50% from cohort 2 to cohort 3, and 33% from cohort 3 to cohort 4. As determined from mean AUC values, the drug exposure increased 340, 129, and 159% across the three cohorts, while mean Cmax values increased 379, 126, and 130%.
TABLE 4.
Comparison of pharmacokinetics of single doses of (+)-calanolide A by dose and by subject gender
| Dose cohort (n) | Mean ± SEM
|
|||||
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Cmax (ng/ml) | Tmax (h) | AUC (ng · h/ml) | t1/2 (h) | CL/F (ml/min/kg) | |
| 200 mg | ||||||
| All (8) | 2.6 ± 0.1 | 454 ± 127 | 5.2 ± 1.6 | 2,690 ± 680 | CNCa | 29.8 ± 9.8 |
| Males (4) | 2.4 ± 0.2 | 374 ± 180 | 4.5 ± 2.6 | 2,149 ± 894 | CNC | 38.1 ± 18.4 |
| Females (4) | 2.8 ± 0.1 | 534.5 ± 196.6 | 5.9 ± 2.2 | 3,230 ± 1,079 | CNC | 21.6 ± 8.1 |
| 400 mg | ||||||
| All (12) | 5.4 ± 0.2 | 1,721 ± 222 | 3.5 ± 0.6 | 9,149 ± 1,273 | CNC | 14.3 ± 3.6 |
| Males (6) | 4.9 ± 0.1 | 1,476 ± 366 | 2.5 ± 0.6 | 7,530 ± 2,192 | CNC | 18.9 ± 6.8 |
| Females (6) | 5.8 ± 0.2 | 1,966 ± 243 | 4.4 ± 1.1 | 10,768 ± 1,130 | CNC | 9.7 ± 1.3 |
| 600 mg | ||||||
| All (12) | 8.6 ± 0.3 | 2,168 ± 296 | 4.4 ± 1.2 | 11,808 ± 1,096 | CNC | 16.5 ± 4.8 |
| Males (6) | 8.2 ± 0.3 | 1,936 ± 295 | 4.2 ± 0.9 | 11,739 ± 893 | CNC | 12.2 ± 1.3 |
| Females (6) | 9.0 ± 0.5 | 2,400 ± 526 | 4.7 ± 2.4 | 11,878 ± 2,117 | CNC | 20.9 ± 9.6 |
| 800 mg | ||||||
| All (12) | 10.8 ± 0.6 | 2,813 ± 198 | 2.4 ± 0.4 | 18,821 ± 1,384 | 19.8 ± 1.2 | 10.0 ± 0.8 |
| Males (6) | 9.3 ± 0.4 | 2,673 ± 277 | 2.3 ± 0.4 | 15,729 ± 1,317 | 18.3 ± 2.3 | 10.2 ± 1.1 |
| Females (6) | 12.4 ± 0.7 | 2,952 ± 297 | 2.5 ± 0.8 | 21,912 ± 1,693 | 21.0 ± 1.2 | 9.8 ± 1.2 |
CNC, could not be calculated.
FIG. 1.
Mean (+)-calanolide A concentration-time curve following oral administration of single-escalating doses to healthy, HIV-negative subjects.
FIG. 2.
(A) Examination of mean Cmax versus (+)-calanolide A dose following oral administration. (B) Examination of mean AUC-versus-(+)-calanolide A dose following oral administration.
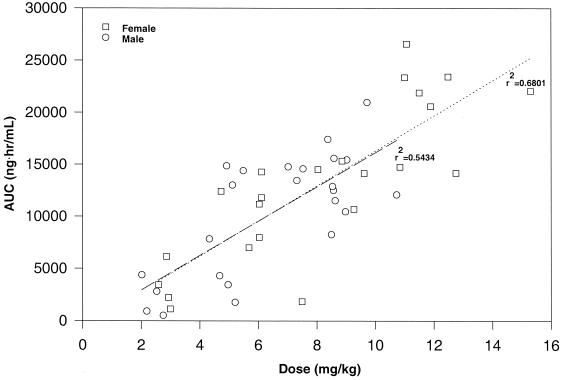
Variability between individual subjects of either gender varied by dosing cohort, i.e., neither males nor females consistently showed an increased intersubject variability. In general, women appeared to have higher levels in plasma, a later Tmax and a longer elimination half-life than men. These differences, however, may be related to the weight differences between the two genders, since female subjects enrolled in the study had a significantly lower average body weight. Indeed, when the dose was expressed as milligrams per kilogram the pharmacokinetic profile was essentially linear, and the profiles in terms of both AUC and Cmax were virtually identical in men and women (Fig. 3).
FIG. 3.
Analysis of mean AUC versus (+)-calanolide A dose for male and female healthy, HIV-negative individuals following single oral doses. The dose represented in the figure is based on the body weight of the individual subject. Linear regression was performed for both male and female subjects with the results of regression analysis indicated in the figure.
In general, taking the drug with food appeared to create even wider variability in pharmacokinetics (Table 5). However, other than increased variability, food did not appear to have any observable effect in the subjects dosed with 400 mg of (+)-calanolide A. In contrast, food did appear to have a considerable effect on the pharmacokinetic parameters of subjects receiving 600 mg of (+)-calanolide A. Patients who took the drug with food demonstrated a 49% decrease in Cmax and a 29% decrease in AUC compared to fasting subjects receiving the same dose.
TABLE 5.
Comparison of pharmacokinetics of single doses of (+)-calanolide A in fasted versus fed subjects
| Dose cohort (n) | Mean ± SEMa
|
||||
|---|---|---|---|---|---|
| Dose (mg/kg) | Cmax (ng/ml) | Tmax (h) | AUC (ng · h/ml) | CL/F (ml/min/kg) | |
| 400 mg | |||||
| All (12) | 5.4 ± 0.2 | 1,721 ± 222 | 3.5 ± 0.6 | 9,149 ± 1,273 | 14.3 ± 3.6 |
| Fasted (6) | 5.4 ± 0.3 | 1,725 ± 279 | 3.3 ± 1.0 | 8,708 ± 1,592 | 12.5 ± 2.7 |
| Fed (6) | 5.3 ± 0.3 | 1,716 ± 373 | 3.7 ± 0.8 | 9,590 ± 2,126 | 16.1 ± 6.9 |
| 600 mg | |||||
| All (12) | 8.6 ± 0.3 | 2,168 ± 296 | 4.4 ± 1.2 | 11,808 ± 1,096 | 16.5 ± 4.8 |
| Fasted (6) | 8.9 ± 0.5 | 2,868 ± 295 | 2.6 ± 0.7 | 13,301 ± 661 | 11.2 ± 0.8 |
| Fed (6) | 8.4 ± 0.3 | 1,468 ± 320 | 6.3 ± 2.2 | 10,315 ± 1,988 | 21.8 ± 9.5 |
t1/2 values could not be calculated.
Interestingly, The Tmax appeared to be the pharmacokinetic parameter most affected by the presence or absence of food. Subjects receiving 200 mg of (+)-calanolide A who received their doses 30 min after eating had a mean Tmax of 5.2 h, whereas subjects who received 800 mg of (+)-calanolide A without food had a mean Tmax of 2.4 h. Subjects receiving 600 mg of (+)-calanolide A with food had a mean Tmax of 6.3 h, while subjects who received their doses while fasting had a mean Tmax of 2.6 h. The food effect on Tmax for subjects receiving 400 mg of (+)-calanolide A was present but not as pronounced (Table 5).
DISCUSSION
This was the first study designed to evaluate the safety and pharmacokinetics of (+)-calanolide A in humans. In order to evaluate multiple doses of (+)-calanolide A, these phase I studies were performed in an escalating dose fashion, with careful monitoring of multiple safety parameters at each dosing increase. During the course of this study, no acute serious or life-threatening adverse experiences were seen. In fact, almost all previously described adverse experiences were of minimal clinical significance and without consequence.
Because the (+)-calanolide A ring structure has some resemblance to anticlotting clotting agents, such as coumarin and warfarin, special attention was paid to hematological findings in both previous animal studies and these human studies. No evidence of a clotting deficiency was observed in rats or dogs after 28 days of administration of (+)-calanolide A (Frank et al., 4th CROI). In this initial phase I human trial, no evidence of aberrations in PT or PTT values was observed.
(+)-Calanolide A produced venous irritation after intravenous administration to rats and dogs. In addition, some signs of gastric mucosal irritation were observed after its oral administration to animals. Consequently, signs and symptoms of nausea, upset stomach, and oral irritation have been closely monitored in this phase I study. No evidence of any serious gastrointestinal intolerance or oral irritation was seen in any of the dosing cohorts.
Single-doses of (+)-calanolide A appeared to be well tolerated in healthy, HIV-negative individuals. With the possible exception of dizziness, no adverse event appeared to be dose related. The dizziness reported by 51% of the subjects was transient and mild; in many cases, it appeared to be temporally related to phlebotomy or to the subjects having taken the drug while fasting. However, since (+)-calanolide A can readily cross the blood-brain barrier in animals, it is not possible to rule out causality in relationship to the observed transient dizziness.
A single serious adverse event occurred during this study. This event involved an unexplained increase in a subject's lipase value 24 h postdose. This male subject's lipase value increased to 1,046 IU/liter, approximately 3.5 times the upper limit of normal. While this event qualified as a grade 3 adverse experience according to a modified Adult ACTG laboratory test toxicity grading scale, the subject was completely asymptomatic, and the event completely resolved within 1 week with no complications.
A great deal of intrasubject variability was seen in the pharmacokinetic parameters examined. While this was seen across all dosing cohorts, certain relative significance and possible trends can be extrapolated from these data. Since the possibility of gender differences in pharmacokinetics had been suggested by animal studies, the study was designed to be gender balanced in order that any differences could be examined.
Elimination profiles of (+)-calanolide A were not fully characterized as a result of inadequate sampling times postdosing. Only three subjects of the 32 examined in the first three cohorts had undetectable (+)-calanolide A levels in plasma at the 24-h postdosing sampling point. This lack of data regarding the complete elimination of (+)-calanolide A precluded the calculation of the elimination rate constant, which resulted in the inability to calculate t1/2 values as well as the AUC0–∞ for most subjects in the first three cohorts. In addition, all 12 subjects receiving 800 mg of (+)-calanolide A had detectable drug concentrations in plasma 48 h postdosing. In future studies, additional time points may help to better assess the elimination phase of the parent drug and aid in calculating a more precise half-life.
Due to the large degree of variability, inadequate sampling duration postdosing, and small sample size, it was impossible to calculate a mean half-life for the 200-, 400-, and 600-mg cohorts. The calculated half-life seen in the 800-mg dosing cohort was approximately 20 h. This rather long half-life may allow for a decreased frequency of dosing. The half-life observed in female subjects was approximately 3 h longer than that seen in male subjects. This may be due to a difference in the metabolic rate of (+)-calanolide A elimination or may simply be a result of the increased dose (in milligrams per kilogram) taken by female subjects due to their decreased weight.
The Cmax and AUC data demonstrate an increase in each parameter with increasing dose (Fig. 2). This increase appears to become more linear at the higher doses of (+)-calanolide A, with a disproportionate increase observed between the 200- and 400-mg dosing regimens. This may indicate a possible saturable metabolism upon dose escalation, or it may simply be the result of increased variability in the detection of the low plasma concentrations seen in subjects receiving the 200-mg dose. In animal studies, this nonlinearity was not observed, and this may have been due to the relatively higher doses (150 mg/kg) of (+)-calanolide A administered to the animals, resulting in plasma levels significantly greater than those seen in these initial human studies. Once again, female subjects across all cohorts demonstrated an increased AUC and increased Cmax compared to male subjects. However, when the dose was expressed as milligrams per kilogram, the AUC and Cmax profiles of (+)-calanolide A were virtually identical in males and females. Males appeared to display increased apparent clearance rates compared with females; however, these differences were less pronounced at higher dosages.
The mean AUC data appears to indicate that in the 600-mg dosing cohort there is little difference in (+)-calanolide A absorption between male and female subjects. This discrepancy can be explained by the existence of a single female study subject whose body weight was considerably higher than the other female subjects. This subject reduced the mean value of the AUC significantly, resulting in what appears to be a reduction in gender difference in this cohort.
The effect of food on the pharmacokinetic profile of (+)-calanolide A was initially examined in this study. Briefly, the food effect seemed to be most evident in the Tmax values obtained. The presence of food appeared to delay the onset of Tmax across all groups examined. In addition, fed subjects appeared to display increased clearance compared to fasting subjects. A general conclusion, however, could not be reached with regard to the Cmax and AUC results obtained. These parameters appeared to be much more variable between fed and fasted subjects. Obviously, the small sample size played a role in this high degree of variability, and more -defined studies will be required to properly delineate the effect of food on the pharmacokinetic profile of (+)-calanolide A.
In general, the levels of (+)-calanolide A in plasma seen in human subjects receiving single doses were higher than would have been predicted from animal studies. The highest AUC observed previously in any animal study was 30,455 ng · h/ml documented in rats receiving a dose of 150 mg/kg. In this first human study, the highest AUC observed was 26,569 ng · h/ml in a subject receiving 800 mg of (+)-calanolide A (11.1 mg/kg). These data support the assertion that humans may achieve significantly higher plasma levels of (+)-calanolide A with a given dose than have been seen in any animal model.
Taken together, the above data demonstrate that the favorable safety profile, long half-life, and increased plasma concentrations may allow for twice-daily dosing of this novel anti-HIV agent. Further clinical development is ongoing for this compound.
REFERENCES
- 1.Boyer P L, Currens M J, McMahon J B, Boyd M R, Hughes S H. Analysis of non-nucleoside drug-resistant variants of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1993;67:2412–2420. doi: 10.1128/jvi.67.4.2412-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckheit R W, Jr, White E L, Fliakas-Boltz V, Russell J, Stup T L, Kinjerski T L, Osterling M C, Welgand A, Bader J P. Unique anti-human immunodeficiency virus activities of the nonnucleoside reverse transcriptase inhibitors calanolide A, costatolide, and dihydrocostatolide. Antimicrob Agents Chemother. 1999;43:1827–1834. doi: 10.1128/aac.43.8.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckheit R W, Jr, Fliakas-Boltz V, Decker W D, Roberson J B, Stup T L, Pyle C A, White E L, McMahon J B, Currens M J, Boyd M R, Bader J P. Comparative anti-HIV evaluation of diverse HIV-1-specific reverse transcriptase inhibitor-resistant virus isolates demonstrates the existence of distinct phenotypic subgroups. Antiviral Res. 1995;26:117–132. doi: 10.1016/0166-3542(94)00069-k. [DOI] [PubMed] [Google Scholar]
- 4.Currens M J, Gulakowski R J, Mariner J M, Moran R A, Buckheit R W, Jr, Gustafson K R, McMahon J B, Boyd M R. Antiviral activity and mechanism of action of (+)-calanolide A against the human immunodeficiency virus type 1. J Pharmacol Exp Ther. 1996;279:646–651. [PubMed] [Google Scholar]
- 5.Currens M J, Mariner J M, McMahon J B, Boyd M R. Kinetic analysis of inhibition of human immunodeficiency virus Type 1 reverse transcriptase by (+)-calanolide A. J Pharmacol Exp Ther. 1996;279:652–661. [PubMed] [Google Scholar]
- 6.Flavin M T, Rizzo J R, Khilevich A, Kucherenko A, Sheinkman A K, Vilaychack V, Lin L, Chen W, Greenwood E M, Pengsuparp T, Pezzuto J M, Hughes S H, Flavin T M, Cibulski M, Boulanger W A, Shone R L, Xu Z-Q. Synthesis, chromatographic resolution, and anti-human immunodeficiency virus activity of (±)-calanolide A and its enantiomers J. Med Chem. 1996;39:1303–1313. doi: 10.1021/jm950797i. [DOI] [PubMed] [Google Scholar]
- 7.Kashman Y, Gustafson K R, Fuller R W, Cardellina J H, McMahon J B, Currens M J, Buckheit R W, Jr, Hughes S H, Cragg G M, Boyd M R. The (+)-calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem. 1992;35:2735–2743. doi: 10.1021/jm00093a004. [DOI] [PubMed] [Google Scholar]
- 8.Quan Y, Motakis D, Buckheit R, Jr, Xu Z-Q, Flavin M T, Parniak M A, Wainberg M A. Sensitivity and resistance to (+)-calanolide A of wild type and mutated forms of HIV-1 reverse transcriptase. Antiviral Ther. 1999;4:203–209. [PubMed] [Google Scholar]
- 9.Vella S. Clinical implications of resistance to antiretroviral drugs. AIDS Clin Care. 1997;9:45–49. [PubMed] [Google Scholar]
- 10.Xu Z-Q, Flavin M T, Jenta T R. Calanolides, the naturally occurring anti-HIV agents. Curr Opin Drug Disc Dev. 2000;3:155–166. [PubMed] [Google Scholar]
- 11.Xu Z-Q, Norris K J, Weinberg D S, Kardatze J, Wertz P, Frank P, Flavin M T. Quantification of (+)-calanolide A, a novel and naturally occurring anti-HIV agent, by high-performance liquid chromatography in plasma from rat, dog and human. J Chromatogr B. 2000;742:267–275. doi: 10.1016/s0378-4347(00)00170-5. [DOI] [PubMed] [Google Scholar]