Key Points
Question
What is the risk of cutaneous squamous cell carcinoma (cSCC) in patients with multiple actinic keratoses (AKs), and which factors contribute to an increased risk?
Findings
In this secondary analysis of a randomized clinical trial of 624 participants that compared 4 field-directed treatments, the 4-year risk of developing cSCC in a previously treated area of AK was 3.7%. In patients with severe AK (Olsen grade III), the risk was 20.9%, and the risk was especially high (33.5%) in patients with severe AK who needed additional treatment.
Meaning
Olsen grade III AK was identified as a marker for an increased risk of cSCC, as was the need for additional treatment.
This secondary analysis of a randomized clinical trial evaluates the risk of invasive cutaneous squamous cell carcinoma after 4 different treatments for patients with multiple actinic keratoses.
Abstract
Importance
Treatment of actinic keratosis (AK) aims to prevent cutaneous squamous cell carcinoma (cSCC). However, whether AK can progress into invasive cSCC is a matter of debate, and little is known about the effect of treatment on preventing cSCC.
Objectives
To evaluate the risk of invasive cSCC and factors that may contribute to increased risk in patients with multiple AKs.
Design, Setting, and Participants
In this secondary analysis of a multicenter randomized clinical trial, 624 patients with a minimum of 5 AKs within an area of 25 to 100 cm2 on the head were recruited from the Department of Dermatology of 4 hospitals in the Netherlands. Long-term follow-up was performed from July 1, 2019, to December 31, 2020.
Interventions
Patients were randomized to treatment with 5% fluorouracil, 5% imiquimod cream, methylaminolevulinate photodynamic therapy, or 0.015% ingenol mebutate gel.
Main Outcomes and Measures
The primary outcome was the proportion of patients with invasive cSCC in the target area during follow-up. Secondary outcomes were the associations between risk of invasive cSCC and a priori defined potential prognostic factors, including type of treatment, severity of AK (Olsen grade), history of nonmelanoma skin cancer, and additional treatment.
Results
Of the 624 patients (558 [89.4%] male; median age, 73 years [range, 48-94 years]) in the study, 26 were diagnosed with a histologically proven invasive cSCC in the target area during follow-up. The total 4-year risk of developing cSCC in a previously treated area of AK was 3.7% (95% CI, 2.4%-5.7%), varying from 2.2% (95% CI, 0.7%-6.6%) in patients treated with fluorouracil to 5.8% (95% CI, 2.9%-11.3%) in patients treated with imiquimod. In patients with severe AK (Olsen grade III), the risk was 20.9% (95% CI, 10.8%-38.1%), and the risk was especially high (33.5%; 95% CI, 18.2%-56.3%) in patients with severe AK who needed additional treatment.
Conclusions and Relevance
In this secondary analysis of a randomized clinical trial, risk of invasive cSCC was highest in patients with Olsen grade III AK and was substantially increased in patients who received additional treatment. These patients should be closely followed up after treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT02281682
Introduction
Actinic keratosis (AK) has an estimated prevalence worldwide that ranges from 11% to 60% and is a disease frequently diagnosed and treated by dermatologists and general practitioners.1,2,3,4,5,6 Several field-directed therapies for AK are available.7,8,9 The most important reason for treatment is the supposed reduction of the risk of developing invasive cutaneous squamous cell carcinoma (cSCC). However, whether AK can really progress to invasive cSCC is a matter of debate, and evidence about good indicators for the lesions at risk is scarce.10 Furthermore, there is limited evidence that treatment of AK decreases the risk of invasive cSCC development. In a recently published randomized clinical trial,11 patients who were supposed to have a high risk of SCC because of substantial sun exposure and a history of at least 2 keratinocyte carcinomas were empirically treated with fluorouracil as a chemoprevention strategy. Topical fluorouracil treatment significantly reduced the risk of cSCC when compared with placebo the first year after treatment: 1% of patients treated with fluorouracil cream developed cSCC on the face or ears compared with 4% in the placebo group.11 However, after 4 years of follow-up, this significant difference disappeared, and the incidence of cSCC was 11% in the fluorouracil group vs 12% in the placebo group. In that trial, only veterans with substantial sun exposure and a history of at least 2 keratinocyte carcinomas were included. In other patients with AK, such as those with no history of nonmelanoma skin cancer (NMSC), the risk of developing cSCC is likely lower.12,13,14,15
The current study aims to evaluate the risk of invasive cSCC and factors that may contribute to increased risk in patients with multiple AKs. Data on the incidence of invasive cSCC during a 4-year follow-up period were collected in patients who had participated in a randomized clinical trial16 that compared 4 field-directed treatments of AK. The results of that trial16 indicated that 1 year after treatment with 5% fluorouracil, the probability of partial (at least 75%) clearance of AK was higher than after treatment with 5% imiquimod cream, methylaminolevulinate photodynamic therapy (PDT), or 0.015% ingenol mebutate gel. The meticulous registration and grading (Olsen grades I-III) of every AK lesion at baseline makes it possible to evaluate the risk of invasive cSCC according to the severity of AK. Moreover, the effect on the risk of other factors, such as allocated treatment, a positive history of NMSC, and need for retreatment, can also be studied.
Methods
Study Design and Study Population
The long-term risk of invasive cSCC was evaluated as a secondary analysis in patients with AK who participated in a single-blinded, multicenter randomized clinical trial with intention to treat that compared the efficacy of 5% fluorouracil cream, 5% imiquimod cream, methylaminolevulinate PDT, and 0.015% ingenol mebutate gel. Patients were recruited from the Department of Dermatology of 4 hospitals in the Netherlands between November 1, 2014, and March 31, 2017. Details on the design and the results from this trial have previously been published,16 and the trial protocol is available in Supplement 1. The study was performed according to the Declaration of Helsinki17 principles with approval of the local medical ethics committee and was reviewed and approved by Maastricht University Medical Center and Ethics Committee. The initial protocol did not include a long-term follow visit. After the study was completed (12-month visit), we obtained approval of the local ethics committee to prolong the study and perform long-term follow-up visits. All participants gave written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients 18 years or older, with Fitzpatrick skin types I to IV, and with a minimum of 5 AK lesions within a treatment area of 25 to 100 cm2 in the head and neck region were eligible for the study. Patients who had skin cancer in the target area at the time of enrollment, used immunosuppressant drugs or retinoids, or received treatment for AK in the target area within 3 months before enrollment were excluded.
Baseline characteristics included age, sex, and prior history of NMSC and severity of AK lesions according to the Olsen classification scheme.16 Olsen grade I reflects slightly palpable AK, more easily felt than seen; grade II reflects moderately thick AK, easy to see and feel; and grade III reflects very thick AK.18 The severity of the target area was determined by the AK lesion with the highest Olsen grade.
Treatment Protocol, Randomization, and Outcomes
Patients were randomly assigned to 1 of 4 different treatments in a 1:1:1:1 ratio. Stratifying factors were participating center and severity of AK. Patients were not blinded to the assigned treatment.
A 5% fluorouracil cream was applied by the patient twice daily for 4 weeks. A 5% imiquimod cream was applied by the patient once daily 3 days a week for 4 weeks. Ingenol mebutate 0.015% gel was applied by patients once daily for 3 days. For treatment with methylaminolevulinate PDT, the methylaminolevulinate cream was applied to the treatment area by a trained nurse and covered with light-blocking foil and occlusive dressing for 3 hours. The area was then illuminated with a light-emitting diode for 7.23 minutes. After the PDT session, the treatment site was covered with an occlusive dressing for 24 hours. In case of insufficient response (<75% lesion response) at the first follow-up visit, the treatment strategy allowed for a subsequent treatment with the same allocated treatment.
In patients who were assigned to 1 of the 3 creams, adherence to the treatment regimen was evaluated using diaries that were completed by patients during treatment. The patients were asked to record the number of times they had applied the cream, the duration of treatment, and reasons for ending the treatment. Moreover, 2 weeks after treatment, patients were contacted by telephone by an investigator (J.P.H.M.K.) who checked how often they had applied the cream.
The primary outcome in this secondary analysis was the 4-year cumulative probability of a histologically proven invasive cSCC in the target area. Secondary outcomes were the associations between risk of developing invasive cSCC and a priori defined potential prognostic factors: type of treatment, the severity of AK lesions, history of NMSC, and additional treatment.
For each patient who had participated in the trial, a study visit was planned for follow-up within a period of 2 to 5 years after the end of treatment. An investigator (S.A.), who was blinded to treatment assignment, evaluated whether a lesion suggestive of cSCC was visible in the treated area using a transparent sheet on which all AK lesions at baseline were drawn. Clinical photographs that were taken at baseline were used as a reference to check the localization of the lesions that were already present at baseline. In case of doubt regarding whether a diagnosed cSCC had arisen at the site of a former AK lesion, an experienced oncodermatologist (K.M., N.W.J.K.-S., A.H.M.M.A., J.P.H.M.K., or M.J.M.d.R.) was consulted as a second observer. If there was any suspicion of malignancy, a punch biopsy was performed. If a patient had been diagnosed with invasive cSCC before the follow-up visit, the exact location and date of diagnosis were retrieved from the electronic patient database of all participating centers. Whether and when patients had received any retreatments for AK until the date of the study visit was also registered.
Statistical Analysis
Categorical variables are presented as numbers (percentages) and continuous variables as means (SDs) or medians (ranges). Time-to-event analysis was used to estimate the cumulative probability of invasive cSCC. Follow-up started at the end of treatment. In patients who developed an invasive cSCC, follow-up ended at the date of diagnosis of cSCC. Observations of patients who did not develop a cSCC were censored at the date of the last follow-up visit. Both intention-to-treat and per protocol analyses were performed.
Univariate and multivariate Cox regression models were used to calculate hazard ratios (HRs) with 95% CIs and P values for each a priori defined potential prognostic factor. Additional treatment that occurred later during follow-up was entered as a time-dependent covariate. Fluorouracil was used as the reference group because it was the treatment with the highest effectiveness in terms of AK lesion reduction.
A 2-sided P < .05 was considered statistically significant. All statistical analyses were performed with SPSS Statistics software, version 23 (IBM Corp), and Stata software, version 14.0 (StataCorp LLC).
Results
Patients
A total of 624 patients (558 [89.4%] male and 66 [10.6%] female; median age, 73 years [range, 48-94 years]) were randomized to treatment with fluorouracil (155 patients), imiquimod (156 patients), methylaminolevulinate PDT (156 patients), or ingenol mebutate (157 patients). Relevant baseline characteristics are given in Table 1.
Table 1. Distribution of Baseline Characteristicsa.
| Characteristic | Total (n = 624) | Fluorouracil (n = 155) | Imiquimod (n = 156) | Methylaminolevulinate PDT (n = 156) | Ingenol mebutate (n = 157) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 558 (89.4) | 136 (87.7) | 143 (91.7) | 140 (89.7) | 139 (88.5) |
| Female | 66 (10.6) | 19 (12.3) | 13 (8.3) | 16 (10.3) | 18 (11.5) |
| Age, median (range), y | 73 (48-94) | 74 (48-90) | 73 (59-89) | 73 (55-90) | 72 (51-94) |
| History of NMSC | 353 (56.6) | 90 (58.1) | 82 (52.6) | 86 (55.1) | 95 (60.5) |
| Target area, median (range), cm2 | 81 (25-100) | 80 (27-100) | 86.5 (25-100) | 81 (25-100) | 78 (25-100) |
| No. of AK lesions, median (range) | 16 (5-48) | 16 (5-48) | 16.5 (5-37) | 16 (5-38) | 15 (5-40) |
| Severity of AK | |||||
| Olsen grade I | 25 (4.0) | 7 (4.5) | 7 (4.5) | 7 (4.5) | 4 (2.5) |
| ≥1 Lesion of Olsen grade II | 550 (88.1) | 137 (88.4) | 136 (87.2) | 137 (87.8) | 140 (89.2) |
| ≥1 Lesion of Olsen grade III | 49 (7.9) | 11 (7.1) | 13 (8.3) | 12 (7.7) | 13 (8.3) |
Abbreviations: AK, actinic keratosis; NMSC, nonmelanoma skin cancer; PDT, photodynamic therapy.
Unless otherwise noted, data are reported as number (percentage) of patients.
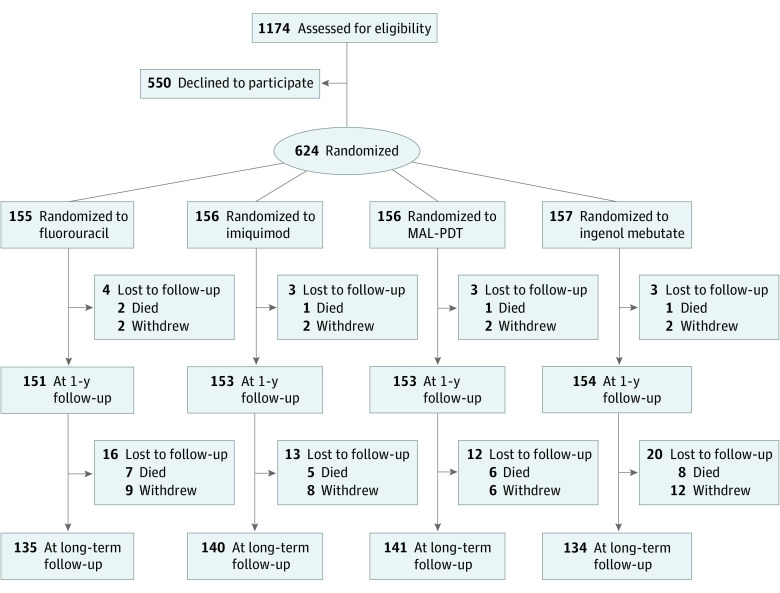
All patients were invited for a follow-up visit between July 1, 2019, and December 31, 2020. The median follow-up was 46 months (IQR, 39-51 months). A total of 74 patients did not attend the follow-up visit mainly because of death from causes unrelated to our study, refusal to attend follow-up visits, inability to visit the hospital, and limited clinical research activities during the COVID-19 pandemic (Figure 1). For these 74 patients, only data for the first 2 years after the end of treatment were available.
Figure 1. Randomization and Follow-up of Study Participants.
MAL-PDT indicates methylaminolevulinate photodynamic therapy.
During follow-up, 227 patients needed additional treatment with field-directed therapy in the target area if they had multiple AK lesions. The decision that there was an indication for additional treatment was at the discretion of the treating dermatologist. The probability of an indication for additional treatment within 4 years after the end of the first treatment was lowest after treatment with fluorouracil (23.3%) and significantly increased to 34.5% in the imiquimod group (P = .04), 42.7% in the methylaminolevulinate PDT group (P < .001), and 53.1% in the ingenol mebutate group (P < .001).
Of the 227 patients with an indication for additional treatment, 218 (96.0%) actually received additional treatment. The most frequently prescribed retreatment was fluorouracil; 30 of 33 patients (90.9%) in the fluorouracil group, 36 of 49 patients (73.5%) in the imiquimod group, 61 of 63 patients (96.8%) in the PDT group, and 61 of 73 patients (83.6%) in the ingenol mebutate group received additional treatment with fluorouracil.
Probability of Invasive cSCC During 4-Year Follow-up
A histologically proven invasive cSCC in the target area was diagnosed in 26 patients. Of these 26 patients, 4 were in the fluorouracil group, 10 in the imiquimod group, 6 in the methylaminolevulinate PDT group, and 6 in the ingenol mebutate group. All of the invasive cSCCs were observed in patients with Olsen grades II and III AK. Four were located in a preexistent grade III AK lesion, 14 in a preexistent grade II AK lesion, and 3 not in a preexistent AK lesion. For 5 cSCCs, whether they were located in a preexistent AK lesion was uncertain. Seven patients had developed cSCC within 12 months after the end of treatment. In the total study population, the 4-year risk of cSCC after treatment was 3.7% (95% CI, 2.4%-5.7%).
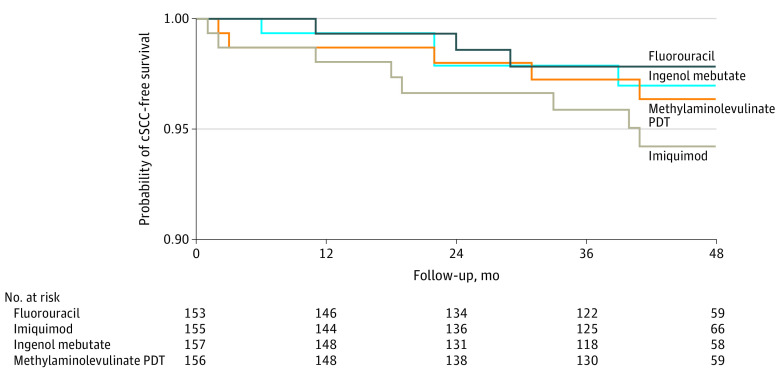
The 1-year and 4-year cumulative risks of developing invasive cSCC within randomized groups are given in the eTable in Supplement 2. The results from the intention-to-treat analysis indicate that the risk of cSCC was lowest in the patient group that was initially treated with fluorouracil (2.2%; 95% CI, 0.7%-6.6%) and was higher in patients treated with imiquimod (5.8%; 95% CI, 2.9%-11.3%), methylaminolevulinate PDT (3.6%; 95% CI, 1.5%-8.6%), or ingenol mebutate (3.0%; 95% CI, 1.1%-7.9%) (Figure 2).
Figure 2. Cumulative Probability of Cutaneous Squamous Cell Carcinoma (cSCC)–Free Survival According to Treatment.
PDT indicates photodynamic therapy.
Risk of Invasive cSCC Associated With Severity of AK and Additional Treatment
Table 2 details the 4-year cumulative risk of developing invasive cSCC according to severity of AK stratified by additional treatment (no vs yes). The risk of invasive cSCC was higher for patients who had received additional treatment (6.4%; 95% CI, 3.7%-10.7%) compared with patients who did not receive additional treatment (2.1%; 95% CI, 1.0%-4.4%) and was especially increased in patients with Olsen grade III AK who received additional treatment (33.5%; 95% CI, 18.2%-56.3%).
Table 2. The 4-Year Cumulative Risk of Invasive cSCC According to the Severity of AK Stratified by Additional Treatment .
| Treatment | No. of patients | Fluorouracil (n = 155) | Imiquimod (n = 156) | Methylaminolevulinate PDT (n = 156) | Ingenol mebutate (n = 157) | Total (n = 624) | 4-Year risk of cSCC, % (95% CI) |
|---|---|---|---|---|---|---|---|
| Retreatment | |||||||
| No | 406 | 1 | 6 | 0 | 1 | 8 | 2.1 (1.0-4.4) |
| Olsen grade I-II AK | 383 | 1 | 5 | 0 | 1 | 7 | 2.2 (1.0-4.6) |
| Olsen grade III AK | 23 | 0 | 1 | 0 | 0 | 1 | 0a |
| Yes | 218 | 3b | 4b | 6b | 5b | 18b | 6.4 (3.7-10.7) |
| Olsen grade I-II AK | 192 | 1 | 2 | 2 | 5 | 10 | 2.7 (1.1-6.4) |
| Olsen grade III AK | 26 | 2 | 2 | 4 | 0 | 8 | 33.5 (18.2-56.3) |
| Total | 624 | 4 | 10 | 6 | 6 | 26 | NA |
| 4-Year risk cSCC, % (95% CI) | NA | 2.2 (0.7-6.6) | 5.8 (2.9-11.3) | 3.6 (1.5-8.6) | 3.0 (1.1-7.9) | 3.7 (2.4-5.7) | NA |
Abbreviations: AK, actinic keratosis; cSCC, cutaneous squamous cell carcinoma; NA, not applicable; PDT, photodynamic therapy.
Cutaneous SCC occurred 4 years after treatment.
Additional treatment was given with fluorouracil.
Table 3 presents the results from multivariate Cox proportional hazards regression analyses. The HRs represent the independent effect of a specific factor after adjustment for the other factors in the model. The relative risk of developing invasive cSCC in patients who received additional treatment was 3.67 (95% CI, 1.52-8.81; P = .004). Olsen grade III AK (vs Olsen grades I-II) was also associated with a significantly higher risk of cSCC (HR, 6.72; 95% CI, 2.94-15.34; P < .001). The adjusted HRs for methylaminolevulinate PDT and ingenol mebutate were close to 1, indicating that after adjustment for other risk factors, the risk of invasive cSCC was comparable with that after fluorouracil treatment. The adjusted HR for imiquimod was 2.09 (95% CI, 0.65-6.74; P = .22).
Table 3. The 4-Year Cumulative Risk of Invasive cSCC According to History of NMSC, Severity of AK, Randomized Treatment, Additional Treatment, and Corresponding HRs.
| Characteristic | No. of patients | Cumulative risk of cSCC, % (95% CI) (n = 26) | Univariate analysis, HR (95% CI) | P value | Multivariate analysis, HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| History of NMSC | ||||||
| Yes | 353 | 4.8 (2.9-7.8) | 1.21 (0.53-2.74) | .65 | 1.39 (0.60-3.19) | .44 |
| No | 271 | 2.2 (0.9-5.2) | 1 [Reference] | |||
| Severity of AK | ||||||
| Olsen grade IIIa | 49 | 20.9 (10.8-38.1) | 7.11 (3.16-15.99) | <.001 | 6.72 (2.94-15.34) | <.001 |
| Olsen grades I-II | 575 | 2.4 (1.4-4.2)b | 1 [Reference] | |||
| Randomized treatment | ||||||
| Imiquimod | 156 | 5.8 (2.9-11.3) | 2.58 (0.81-8.26) | .11 | 2.09 (0.65-6.74) | .22 |
| Methylaminolevulinate PDT | 156 | 3.6 (1.5-8.6) | 1.48 (0.42-5.26) | .54 | 1.00 (0.27-3.62) | >.99 |
| Ingenol mebutate | 157 | 3.0 (1.1-7.9) | 1.35 (0.38-4.79) | .65 | 0.86 (0.24-3.13) | .82 |
| Fluorouracil | 155 | 2.2 (0.7-6.6) | 1 [Reference] | NA | NA | NA |
| Additional treatment after end of study treatment | ||||||
| Yes | 218 | 6.4 (3.7-10.7) | 3.80 (1.61-8.97) | .002 | 3.67 (1.52-8.81) | .004 |
| No | 406 | 2.1 (1.0-4.4) | 1 [Reference] |
Abbreviations: AK, actinic keratosis; cSCC, cutaneous squamous cell carcinoma; HR, hazard ratio; NA, not applicable; NMSC, nonmelanoma skin cancer; PDT, photodynamic therapy.
One or more lesions of Olsen grade III.
No cSCCs were found in patients with Olsen grade I.
Discussion
This secondary analysis of a randomized clinical trial evaluated the incidence of invasive cSCC within the specific area that was treated for multiple AK lesions with 1 of 4 field-directed therapies. A histologically proven cSCC in the target area was diagnosed in only 26 patients. The 4-year risk of developing cSCC in a previously treated area of AK was 3.7% overall and varied from 2.2% in patients treated with fluorouracil to 5.8% in patients treated with imiquimod. In patients with severe AK (Olsen grade III), the risk of cSCC was 20.9%, and the risk was especially high (33.5%) in patients with severe AK who needed additional treatment.
The higher risk of cSCC development in patients who needed additional treatment, specifically those with 1 or more AK grade III lesions, confirms the findings of previous studies19,20 in which rapid recurrence or persistence after therapy, hyperkeratosis, and palpability were found as risk factors for cSCC in AK. This finding could indicate that the need for additional treatment may be an indicator of an underlying condition that did not respond to treatment.
Whether AKs transform into invasive cSCC or whether sun-damaged skin is the underlying reason why patients with AK also have increased risk of invasive cSCC has long been debated.2 In the current study, 18 of the 26 invasive cSCCs were located in preexistent lesions. This finding may indicate that AK lesions can transform into invasive cSCC, but the possibility that these lesions were already invasive cSCC before treatment was started cannot be excluded, although clinical assessment of AK was performed by 2 physicians independently. An important question that remains to be answered is whether treatment resulting in reduction of AK lesions also results in preventing progression of those AK lesions into invasive cSCC.
The effectiveness of fluorouracil as a chemoprevention strategy was demonstrated by Weinstock et al,11 who performed a randomized clinical trial that compared fluorouracil with placebo in veterans with substantial sun exposure and a history of at least 2 keratinocyte carcinomas. After 1 year, the risk of cSCC was significantly lower in the fluorouracil group than in the placebo group (1% vs 4%). This difference disappeared at 4 years of follow-up, when 11% of the patients in the fluorouracil group had developed cSCC vs 12% in the placebo group. The authors concluded that a single 2- to 4-week fluorouracil course is effective in reducing the risk of cSCC for the first year after use, and yearly treatment with fluorouracil is suggested. However, the high-risk population in this study makes these findings less generalizable to other patients with AK.
The current randomized clinical trial allowed for comparison of long-term SCC risk after 4 different field-directed treatments. The HRs using the fluorouracil group as the reference category show a slightly increased risk of cSCC in patients who initially had one of the other treatments. However, none of these HRs were statistically significant, and approximately one-third of patients received additional treatment, mostly with fluorouracil. Therefore, the findings in this study do not permit definite conclusions on whether fluorouracil is the most effective treatment for preventing the development of invasive cSCC.
The finding that imiquimod treatment was associated with an increased risk of cSCC may be attributable to chance. Currently, no studies have found that imiquimod treatment of AK leads to an increased risk of developing cSCC. An alternative explanation may be that in the imiquimod group, only 25% of patients received additional treatment with fluorouracil, whereas in the methylaminolevulinate PDT and ingenol mebutate groups, these percentages were much higher.
Ingenol mebutate has been reported to potentially be linked to increased risk of developing skin cancer.21,22 Consequently, the European Medicines Agency has recommended that the use of ingenol mebutate be suspended until further investigations demonstrate the safety of the product.21 We did not find any evidence of a higher risk of cSCC after ingenol mebutate gel treatment compared with the other treatments.
Limitations
This study has some limitations. The sample size was calculated to compare 4 field-directed treatments of AK with respect to effectiveness on the reduction of AK lesions and not for comparing long-term risk of invasive cSCC. Thus, a limitation is the small number of cSCC events, resulting in low power to detect small but relevant differences in long-term risk of cSCC with significance. Furthermore, the decision to give additional treatment was at the discretion of the treating dermatologist, and there were no predefined criteria for the need for additional treatment. However, misclassification of the need for additional treatment by dermatologists can occur in 2 directions: no additional treatment in patients needing additional treatment and additional treatment in patients not needing additional treatment. Such so-called nondifferential misclassification generally leads to bias of a true effect toward the null value. Therefore, it is likely that the positive association that was found in this study represents a real effect that has been underestimated rather than overestimated.23
Finally, a placebo group was not available: all patients received treatment with an active ingredient. Therefore, more studies are necessary to conclude whether treatment could be omitted in patients with low-risk AK.
Conclusions
In this secondary analysis of a randomized clinical trial, the risk of invasive cSCC was highest in patients with Olsen grade III AK lesions and was substantially increased in patients who received additional treatment. We therefore recommend close follow-up of these patients.
Trial Protocol
eTable. Cumulative Probability of cSCC-Free Survival 1 and 4 Years Post-Treatment and Hazard Ratios for Developing cSCC in the Treated Area
Data Sharing Statement
References
- 1.Flohil SC, van der Leest RJ, Dowlatshahi EA, Hofman A, de Vries E, Nijsten T. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol. 2013;133(8):1971-1978. doi: 10.1038/jid.2013.134 [DOI] [PubMed] [Google Scholar]
- 2.Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177(2):350-358. doi: 10.1111/bjd.14852 [DOI] [PubMed] [Google Scholar]
- 3.Spencer JM, Hazan C, Hsiung SH, Robins P. Therapeutic decision making in the therapy of actinic keratoses. J Drugs Dermatol. 2005;4(3):296-301. [PubMed] [Google Scholar]
- 4.Eder J, Prillinger K, Korn A, Geroldinger A, Trautinger F. Prevalence of actinic keratosis among dermatology outpatients in Austria. Br J Dermatol. 2014;171(6):1415-1421. doi: 10.1111/bjd.13132 [DOI] [PubMed] [Google Scholar]
- 5.Frost C, Williams G, Green A. High incidence and regression rates of solar keratoses in a Queensland community. J Invest Dermatol. 2000;115(2):273-277. doi: 10.1046/j.1523-1747.2000.00048.x [DOI] [PubMed] [Google Scholar]
- 6.Memon AA, Tomenson JA, Bothwell J, Friedmann PS. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol. 2000;142(6):1154-1159. doi: 10.1046/j.1365-2133.2000.03541.x [DOI] [PubMed] [Google Scholar]
- 7.Beljaards RC, Van Der Sande A, Buis P, et al. Update richtlijn actinische keratosen 2017. Ned Tijdschr Dermatol Venereol. 2017;27:190-192. [Google Scholar]
- 8.de Berker D, McGregor JM, Mohd Mustapa MF, Exton LS, Hughes BR. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20-43. doi: 10.1111/bjd.15107 [DOI] [PubMed] [Google Scholar]
- 9.Werner RN, Stockfleth E, Connolly SM, et al. ; International League of Dermatological Societies; European Dermatology Forum . Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—short version. J Eur Acad Dermatol Venereol. 2015;29(11):2069-2079. doi: 10.1111/jdv.13180 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31(suppl 2):5-7. doi: 10.1111/jdv.14151 [DOI] [PubMed] [Google Scholar]
- 11.Weinstock MA, Thwin SS, Siegel JA, et al. ; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group . Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154(2):167-174. doi: 10.1001/jamadermatol.2017.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group . Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523-2530. doi: 10.1002/cncr.24284 [DOI] [PubMed] [Google Scholar]
- 13.Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):23-24. doi: 10.1067/mjd.2000.103339 [DOI] [PubMed] [Google Scholar]
- 14.Lanoue J, Chen C, Goldenberg G. Actinic keratosis as a marker of field cancerization in excision specimens of cutaneous malignancies. Cutis. 2016;97(6):415-420. [PubMed] [Google Scholar]
- 15.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795-797. doi: 10.1016/S0140-6736(88)91658-3 [DOI] [PubMed] [Google Scholar]
- 16.Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935-946. doi: 10.1056/NEJMoa1811850 [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Olsen EA, Abernethy ML, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24(5 pt 1):738-743. doi: 10.1016/0190-9622(91)70113-G [DOI] [PubMed] [Google Scholar]
- 19.Quaedvlieg PJ, Tirsi E, Thissen MR, Krekels GA. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16(4):335-339. [PubMed] [Google Scholar]
- 20.Dréno B, Amici JM, Basset-Seguin N, Cribier B, Claudel JP, Richard MA; AKTeam™ . Management of actinic keratosis: a practical report and treatment algorithm from AKTeam™ expert clinicians. J Eur Acad Dermatol Venereol. 2014;28(9):1141-1149. doi: 10.1111/jdv.12434 [DOI] [PubMed] [Google Scholar]
- 21.EMA suspends Picato as a precaution while review of skin cancer risk continues. 2020. Accessed October 13, 2021. https://www.ema.europa.eu/en/news/ema-suspends-picato-precaution-while-review-skin-cancer-risk-continues
- 22.Risk of squamous cell carcinoma on skin areas treated with ingenol mebutate gel, 0.015% and imiquimod cream, 5%. ClinicalTrials.gov identifier: NCT01926496. Updated August 21, 2020. Accessed October 13, 2021. https://clinicaltrials.gov/ct2/show/NCT01926496
- 23.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Cumulative Probability of cSCC-Free Survival 1 and 4 Years Post-Treatment and Hazard Ratios for Developing cSCC in the Treated Area
Data Sharing Statement