Abstract
Background:
Coronavirus disease 2019 (COVID-19) has spread globally, and many patients with severe cases have received oxygen therapy through a high-flow nasal cannula (HFNC).
Objectives:
We assessed the efficacy of HFNC for treating patients with COVID-19 and risk factors for HFNC failure.
Methods:
We searched PubMed, Embase, and the Cochrane Central Register of randomized controlled trials (RCTs) and observational studies of HFNC in patients with COVID-19 published in English from January 1st, 2020 to August 15th, 2021. The primary aim was to assess intubation, mortality, and failure rates in COVID-19 patients supported by HFNC. Secondary aims were to compare HFNC success and failure groups and to describe the risk factors for HFNC failure.
Results:
A total of 25 studies fulfilled selection criteria and included 2851 patients. The intubation, mortality, and failure rates were 0.44 (95% confidence interval (CI): 0.38–0.51, I2 = 84%), 0.23 (95% CI: 0.19–0.29, I2 = 88%), and 0.47 (95% CI: 0.42–0.51, I2 = 56%), respectively. Compared to the success group, age, body mass index (BMI), Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE) II score, D-dimer, lactate, heart rate, and respiratory rate were higher and PaO2, PaO2/FiO2, ROX index (the ratio of SpO2/FiO2 to respiratory rate), ROX index after the initiation of HFNC, and duration of HFNC were lower in the failure group (all Ps < 0.05). There were also more smokers and more comorbidities in the failure group (all Ps < 0.05). Pooled odds ratios (ORs) revealed that older age (OR: 1.04, 95% CI: 1.01–1.07, P = 0.02, I2 = 88%), a higher white blood cell (WBC) count (OR: 1.06, 95% CI: 1.01–1.12, P = 0.02, I2 = 0%), a higher heart rate (OR: 1.42, 95% CI: 1.15–1.76, P < 0.01, I2 = 0%), and a lower ROX index(OR: 0.61, 95% CI: 0.39–0.95, P = 0.03, I2 = 93%) after the initiation of HFNC were all significant risk factors for HFNC failure.
Conclusions:
HFNC is an effective way of providing respiratory support in the treatment of COVID-19 patients. Older age, a higher WBC count, a higher heart rate, and a lower ROX index after the initiation of HFNC are associated with an increased risk of HFNC failure.
Keywords: age, COVID-19, high-flow nasal cannula, risk factor, ROX index
Introduction
The SARS-CoV-2 virus, which is responsible for COVID-19, spread rapidly worldwide and created a pandemic in 2019. 1 The high infectivity and exponential spread of SARS-CoV-2, coupled with its potential to develop rapidly into acute respiratory distress syndrome, led to high mortality.2–4 About 15–30% of COVID-19 patients experienced hypoxemia and progress to acute respiratory distress syndrome. 3 Noninvasive respiratory support, including the use of a high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV), is now widely given to these patients.
HFNC is an oxygen supply system capable of delivering up to 100% humidified and heated oxygen at a flow rate of up to 60–100 liters per minute.5,6 It is useful for treating hypoxemic respiratory failure. It may lead to less treatment failure compared to the use of conventional oxygen therapy (COT), 7 and it is better tolerated than NIV. 8
Previous studies, mainly retrospective and with small sample sizes, suggest potential benefits associated with the use of HFNC in treating respiratory failure due to COVID-19.9–11 However, there is a lack of large-sample research on the effectiveness of HFNC for treating COVID-19. Moreover, despite their advantages, the failure rate of noninvasive treatments in patients with COVID-19 is high, and there is a concern that poor patient selection or prolonged trials of HFNC may result in worse clinical outcomes.12,13 Therefore, the main aim of this study was to summarize the characteristics of patients using HFNC and to assess its efficacy. We also compare the characteristics of patients in HFNC success and failure groups and describe risk factors for HFNC failure.
Methods
Search strategy
We searched PubMed, Embase, and the Cochrane Central Register of RCTs and observational studies of HFNC in patients with COVID-19 published in English from January 1st, 2020 to August 15th, 2021. We searched for ( ‘HFNC’ or ‘high-flow nasal cannula’ or ‘high-flow oxygen therapy’ or ‘high-flow nasal oxygen’) and ( ‘COVID-19’ or ‘coronavirus disease 2019’). In addition, we carefully evaluated the reference lists of all primary studies and review articles for other relevant studies.
Study selection
Our inclusion criteria were as follows: i) cohort study, case-control study, or RCT; ii) the inclusion of patients with laboratory-confirmed COVID-19; iii) HFNC used to relieve hypoxemia prior to (invasive or noninvasive) mechanical ventilation; and iv) at least one of the following outcomes: mortality rate, intubation rate, escalation rate (to NIV or invasive mechanical ventilation), and characteristics of patients successfully weaned from HFNC and those not successfully weaned.
HFNC failure was defined as the need for NIV or invasive mechanical ventilation and/or death while on HFNC support. Demographic, clinical, laboratory, management, and outcome data were obtained from medical records.
Exclusion criteria were as follows: i) patients did not meet screening criteria; ii) the study focused on pediatric patients; iii) non-English study, commentary, review, full text not available, and/or duplicate publications from a single study; iv) data could not be extracted by statistical methods or non-targeted outcomes; and v) the study reused patient data.
The ultimate decision to include a study was made following a full-text review of the article by two investigators focusing on publication date, study type, study design, and outcomes. Discrepancies were resolved by consensus.
Quality control
The Newcastle-Ottawa Scale was used to assess the quality of the eligible studies. Each cohort study was assessed on seven items regarding patient selection, trial design, and measurement of outcomes; each case-control study was assessed on seven other items regarding patient selection, comparability, and exposure. One star was awarded for each quality criterion the study satisfied; the highest quality studies were awarded seven stars. A study was considered of good, normal, and poor quality if it was awarded 6 or 7, 3–5, and 0–2 stars, respectively.
Statistical analysis
Data and basic information from each study were independently extracted and cross-checked by two investigators for further analysis. The dates and hospitals of all included studies were checked in detail to avoid duplication. R (version 4.0.2; Comprehensive R Archive Network, 2020) and Review Manager (version 5.3; Nordic Cochrane Center, Cochrane Collaboration, 2014) were used to pool available data.
Baseline data for HFNC success and failure groups were compared with Z tests. P < 0.05 was taken to indicate a significant difference. Outcomes regarding intubation, failure, and mortality rates were measured as proportions with corresponding 95% confidence intervals (CIs) and then pooled and presented in forest plots. Random-effects models were used in cases of obvious heterogeneity. To investigate the risk factors for HFNC failure, we used DerSimonian-Laird random-effects models to pool odds ratios (ORs) and their corresponding 95% CIs. Definitions of the same risk factor should have been similar across all included studies, and the results of multivariate analysis were preferentially adopted. Because of the short follow-up period (usually only 1 month), hazard ratios expressed similar meaning to ORs and were therefore pooled together in this meta-analysis.
In each analysis, the heterogeneity between studies was measured with the I2 statistical index (range: 0–100%), with 25%, 50%, and 75% corresponding to low, moderate, and high heterogeneity, respectively. Random-effects models were used when obvious heterogeneity existed. Funnel plots, Egger’s test, and Begg’s test were used to evaluate publication bias. Qualitative data were compared with Z tests. P < 0.05 was considered to indicate a significant difference.
Results
Search results
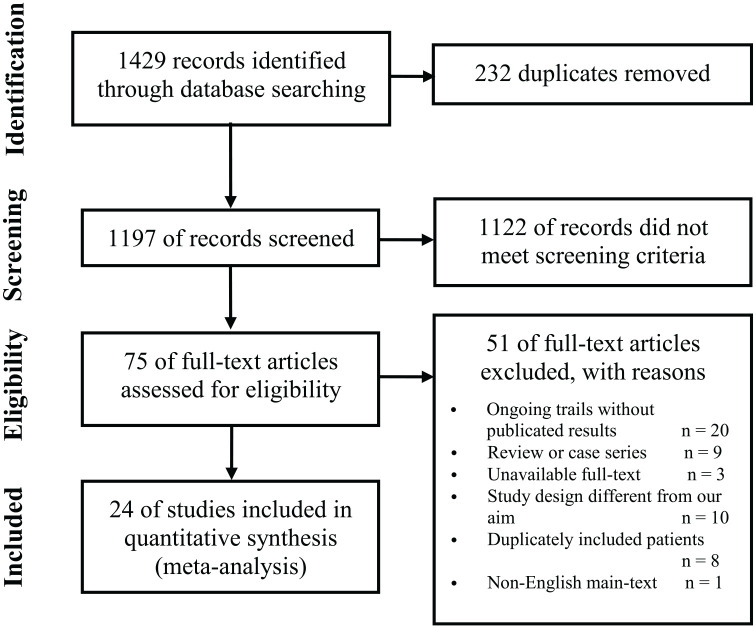
As depicted in Figure 1, a total of 1429 records were identified from the search. After excluding duplicates and evaluating the full texts of articles, we identified 25 eligible studies (1 RCT, 12 case-control studies, and 12 cohort studies).14–38 The search and screening process is described in Figure 1. The main characteristics of the articles included in the meta-analysis are shown in Tables 1, 2, 3 and 4.
Figure 1.
Selection of studies for the meta-analysis (PRISMA).
PRISMA: preferred reporting items for systematic reviews and meta-analyses.
Table 1.
Characteristics of the studies.
| Study | Study design | Location | Setting | Inclusion date | Inclusion criteria |
|---|---|---|---|---|---|
| Beduneau et al. 14 | Single-center Retrospective cohort | France | ICU | 2020.03.13-2020.04.11 | COVID-19 patients with AHRF |
| Blez et al. 15 | Single-center Case-control study | France | ICU | 2020.03.01-2020.04.30 | Severe COVID-19 pneumonia patients |
| Bonnet et al. 16 | Multi-center Retrospective cohort | France | ICU | 2020.03.11-2020.05.03 | Adult COVID-19 patients with AHRF |
| Calligaro et al. 17 | Multi-center Case-control study | South Africa | MD | 2020.04.16-2020.06.30 | Adult COVID-19 patients with severe respiratory failure |
| Celejewska-Wójcik et al. 18 | Single-center Prospective cohort | Poland | Respiratory wards | 2020.03.01-2020.12.31 | Severe COVID-19 patients with AHRF |
| Chandel et al. 19 | Multi-center Case-control study | United States | MD | 2020.03.01-2020.06.09 | Adult COVID-19 patients treated with HFNC ⩾ 2 h |
| Delbove et al. 20 | Single-center Retrospective cohort | France | ICU | 2020.02.26-2020.06.30 | Adult patients with COVID-19 related ARDS |
| Demoule et al. 21 | Multi-center Retrospective cohort | France | ICU | 2020.02.21-2020.04.24 | COVID-19 patients with AHRF |
| Deng et al. 22 | Multi-center Retrospective cohort | China | MD | 2020.01.14-2020.03.05 | Elderly patients with severe COVID-19 |
| Duan et al. 23 | Multi-center Case-control study | China | MD | 2020.01.15-2020.03.31 | Adult COVID-19 patients |
| Franco et al. 24 | Multi-center Prospective cohort | Italy | Respiratory wards | 2020.03.01-2020.05.10 | Adult COVID-19 patients treated with HFNC, CPAP and NIV |
| Garner et al. 25 | Single-center Case-control study | United States | MD | 2020.03.01-2020.04.28 | Adult COVID-19 patients with AHRF |
| Goury et al. 26 | Single-center Case-control study | France | ICU | 2020.03.01-2020.05.23 | AHRF COVID-19 patients treated by HFNC ⩾ 2 h |
| Grieco et al. 27 | Multi-center RCT | Italy | ICU | 2020.10.13-2020.12.13 | Adult COVID-19 patients with AHRF |
| Hernandez-Romieu et al. 28 | Single-center Retrospective cohort | United States | ICU | 2020.03.06-2020.05.07 | Adult COVID-19 patients with severe acute respiratory syndrome |
| Hu et al. 29 | Multi-center Case-control study | China | Respiratory wards | 2020.01.01-2020.03.01 | Adult COVID-19 patients with hypoxemic respiratory failure |
| Katsuno et al. 30 | Single-center Case-control study | Japan | MD | 2020.01.01-2020.09.31 | COVID-19 patients failed on oxygen mask |
| Liu et al. 31 | Multi-center Retrospective cohort | China | MD | 2020.01.01-2020.02.29 | Adult COVID-19 patients with severe AHRF receiving non-invasive respiratory support |
| Mellado-Artigas et al. 32 | Multi-center Prospective cohort | Spain | ICU | 2020.03.12-2020.08.13 | Adult COVID-19 patients with AHRF |
| Panadero et al. 33 | Single-center Case-control study | Spain | Intermediate Respiratory Care Unit | 2020.03.18-2020.04.18 | Adult COVID-19 patients with ARDS |
| Sayan et al. 34 | Single-center Retrospective cohort | Turkey | ICU | 2020.03.15-2020.05.30 | Adult COVID-19 patients with AHRF |
| Vianello et al. 35 | Single-center Case-control study | Italy | Respiratory ICU | 2020.03.13-2020.03.23 | COVID-19 patients with AHRF |
| Xia et al. 36 | Multi-center Case-control study | China | MD | 2020.02.15-2020.03.17 | Adult COVID-19 patients with AHRF |
| Xu et al. 37 | Multi-center Case-control study | China | ICU | 2019.12.29-2020.04.30 | Adult COVID-19 patients |
| Zucman et al. 38 | Single-center Retrospective cohort | France | ICU | 2020.03.08-2020.04.16 | Adult COVID-19 patients with AHRF |
Note: COVID-19: novel coronavirus 19 disease; ICU: Intensive Care Unit; AHRF: acute hypoxemic respiratory failure.
ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula; MD, missing data; NIV, non-invasive ventilation.
Table 2.
Characteristics of the studies.
| Study | Patients on HFNC |
Age | Sex (male%) |
BMI | SOFA | APACHE II | P/F | RR | ROX | Intubated | Failure | Mortality | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beduneau et al. 14 | 43 | 61 ± 7 | 72% | 28 ± 4.4 | 2.7 ± 1.5 | MD | 145 ± 68 | 24 ± 6 | MD | 14 | 14 | 3 | 5 |
| Blez et al. 15 | 30 | 64 ± 7 | 70% | 28.3 ± 4.6 | MD | MD | MD | 31 ± 8 | MD | 16 | MD | MD | 6 |
| Bonnet et al. 16 | 76 | 60 ± 11 | 82% | 29.0 ± 6.0 | MD | MD | MD | 32 ± 6 | MD | 39 | 39 | 12 | 7 |
| Calligaro et al. 17 | 293 | 51 ± 10 | 56% | MD | MD | MD | MD | MD | MD | 111 | 156 | 129 | 7 |
| Celejewska- Wójcik et al. 18 |
116 | 61 ± 14 | 78% | 29.5 ± 5.2 | MD | MD | MD | 23 ± 6 | MD | 51 | 54 | 35 | 6 |
| Chandel et al. 19 | 272 | 57 ± 13 | 66% | 29.1 ± 3.5 | 3.0 ± 3.0 | MD | MD | 30 ± 9 | MD | 108 | MD | 49 | 8 |
| Delbove et al. 20 | 35 | 72 ± 10 | 77% | 27.7 ± 5.1 | 3.5 ± 0.8 | 9.7 ± 3.4 | 237 ± 95 | 31 ± 8 | MD | 20 | 20 | 7 | 6 |
| Demoule et al. 21 | 146 | 60 ± 10 | 79% | 27.3 ± 3.7 | 4.0 ± 1.5 | MD | 134 ± 77 | MD | MD | 82 | MD | 30 | 7 |
| Deng et al. 22 | 110 | 72 ± 8 | 59% | MD | 4.0 ± 2.3 | 12.7 ± 6.8 | 245 ± 42 | MD | MD | 42 | MD | 24 | 6 |
| Duan et al. 23 | 66 | 67 ± 16 | 38% | MD | 3.9 ± 2.0 | MD | 194 ± 111 | 25 ± 6 | 9.0 ± 3.9 | 25 | 29 | 14 | 7 |
| Franco et al. 24 | 163 | 66 ± 15 | 70% | MD | 2.5 ± 0.9 | MD | 166 ± 65 | 25 ± 5 | MD | 47 | 62 | 26 | 6 |
| Garner et al. 25 | 30 | MD | 47% | MD | 5.1 ± 1.8 | MD | MD | MD | MD | 23 | MD | MD | 7 |
| Goury et al. 26 | 42 | 66 ± 10 | 67% | MD | 3.8 ± 1.5 | MD | 68 ± 16 | 24 ± 7 | MD | 20 | 20 | 6 | 6 |
| Grieco et al. 27 | 54 | 62 ± 11 | 85% | 28.0 ± 4.0 | 2.3 ± 0.8 | MD | 102 ± 33 | 28 ± 7 | MD | 28 | MD | 14 | 7 |
| Hernandez- Romieu et al. 28 |
109 | 63 ± 14 | 58% | 32 ± 7.2 | 7.3 ± 3.8 | MD | MD | MD | MD | 78 | MD | 34 | 7 |
| Hu et al. 29 | 105 | 64 ± 11 | 49% | MD | 3.3 ± 0.8 | 8.2 ± 2.6 | 117 ± 23 | MD | MD | 9 | 40 | MD | 8 |
| Katsuno et al. 30 | 15 | 70 ± 11 | 80% | 25.7 ± 4.7 | MD | MD | MD | 25 ± 7 | MD | 6 | 7 | 3 | 6 |
| Liu et al. 31 | 366 | 64 ± 13 | 66% | MD | 3.3 ± 2.2 | 9.7 ± 5.2 | 145 ± 138 | 23 ± 5 | 7.7 ± 6.2 | 130 | MD | 178 | 7 |
| Mellado- Artigas et al. 32 |
259 | 62 ± 11 | 71% | 28.3 ± 5.2 | 4.7 ± 3 | 11.7 ± 6 | 114 ± 51 | 26 ± 6 | 4.6 ± 2.1 | 119 | MD | MD | 6 |
| Panadero et al. 33 | 40 | 59 ± 12 | 70% | 29.5 ± 4.5 | 4.4 ± 0.7 | MD | MD | 29 ± 10 | 3.8 ± 1.2 | 21 | 21 | 9 | 6 |
| Sayan et al. 34 | 24 | 63 ± 12 | 71% | 26.5 ± 2.6 | MD | MD | 171 ± 19 | 33 ± 5 | MD | 13 | MD | 12 | 5 |
| Vianello et al. 35 | 28 | 69 ± 11 | 86% | MD | 2 ± 0.5 | MD | 108 ± 61 | 26 ± 7 | MD | 5 | 9 | 3 | 6 |
| Xia et al. 36 | 43 | 63 ± 10 | 58% | 24.2 ± 2.5 | MD | MD | MD | 24 ± 6 | 8.2 ± 5.5 | 13 | 20 | 13 | 8 |
| Xu et al. 37 | 324 | 63 ± 15 | 68% | MD | 3 ± 1.5 | MD | 115 ± 38 | 26 ± 6 | 4.4 ± 1.6 | 147 | MD | 202 | 8 |
| Zucman et al. 38 | 62 | 58 ± 11 | MD | MD | MD | MD | MD | 26 ± 8 | MD | 39 | 39 | 10 | 5 |
APACHE, acute physiology and chronic health evaluation; BMI, body mass index; HFNC, high flow nasal cannula; MD, missing data; NOS, Newcastle-Ottawa quality assessment Scale; P/F: oxygenation index(PaO2/FiO2); ROX index, ratio of SpO2/FiO2 to respiratory rate); RR, respiratory rate; SOFA, sequential organ failure assessment.
Table 3.
Characteristics of the studies.
| Study | Baseline | characteristics |
|---|---|---|
| Beduneau et al. 14 | (1) (2) (3) (5) (9) (11) (12) (13) (21) (22) (25) | (29) (30) (31) (32) |
| Blez et al. 15 | (1) (2) (3) (9) (11) (20) (21) (24) | (27) (30) |
| Bonnet et al. 16 | (1) (2) (3) (9) (11) (16) (17) (21) (22) | (29) (30) (31) (32) |
| Calligaro et al. 17 | (1) (2) (4) (9) (11) (16) (17) (18) (22) | (27) (30) (31) (32) |
| Celejewska-Wójcik et al. 18 | (1) (2) (3) (4) (9) (10) (11) (12) (13) (17) (18) (19) (21) (22) (23) | (27) (28) (30) (31) (32) |
| Chandel et al. 19 | (1) (2) (3) (5) (9) (10) (11) (12) (13) (14) (15) (17) (18) (19) (20) (21) (22) (24) | (27) (28) (30) (31) |
| Delbove et al. 20 | (1) (2) (3) (5) (6) (7) (8) (9) (11) (12) (13) (16) (19) (21) (24) (25) | (28) (29) (30) (31) (32) |
| Demoule et al. 21 | (1) (2) (3) (5) (9) (11) (15) (16) (18) (19) (25) | (28) (30) (31) |
| Deng et al. 22 | (1) (2) (5) (6) (9) (10) (11) (12) (14) (15) (16) (17) (18) (19) (24) (25) | (29) (30) (31) |
| Duan et al. 23 | (1) (2) (5) (9) (10) (11) (12) (15) (16) (17) (19) (20) (21) (23) (24) (25) (26) | (27) (29) (30) (31) (32) |
| Franco et al. 24 | (1) (2) (4) (5) (9) (11) (12) (13) (14) (21) (25) | (29) (30) (31) (32) |
| Garner et al. 25 | (2) (5) (17) (19) | (30) |
| Goury et al. 26 | (1) (2) (4) (5) (15) (21) (25) | (27) (30) (31) (32) |
| Grieco et al. 27 | (1) (2) (3) (5) (8) (9) (11) (14) (20) (21) (23) (25) | (30) (31) |
| HerMDndez-Romieu et al. 28 | (1) (2) (3) (5) (9) (11) (12) (13) (15) (16) (17) (18) | (30) (31) |
| Hu et al. 29 | (1) (2) (5) (6) (8) (16) (17) (18) (25) | (27) (28) (29) (30) (32) |
| Katsuno et al. 30 | (1) (2) (3) (8) (9) (10) (11) (13) (20) (21) | (28) (30) (31) (32) |
| Liu et al. 31 | (1) (2) (5) (6) (7) (9) (10) (11) (12) (20) (21) (23) (24) (26) | (28) (29) (30) (31) |
| Mellado-Artigas et al. 32 | (1) (2) (3) (5) (6) (7) (9) (10) (11) (12) (13) (14) (15)
(18) (20) (21) (23) (24) (25) (26) |
(30) |
| PaMDdero et al. 33 | (1) (2) (3) (5) (9) (10) (11) (12) (13) (16) (17) (18) (20) (21) (22) (23) (26) | (27) (28) (30) (31) (32) |
| Sayan et al. 34 | (1) (2) (3) (9) (10) (11) (12) (14) (21) (22) (23) (24) (25) | (30) (31) |
| Vianello et al. 35 | (1) (2) (5) (8) (17) (18) (20) (21) (22) (23) (24) (25) | (30) (31) (32) |
| Xia et al. 36 | (1) (2) (3) (8) (9) (11) (15) (16) (19) (20) (21) (22) (23) (24) (26) | (27) (28) (29) (30) (31) (32) |
| Xu et al. 37 | (1) (2) (5) (9) (11) (12) (14) (16) (18) (20) (21) (23) (24) (25) (26) | (27) (28) (30) (31) |
| Zucman et al. 38 | (1) (21) (24) | (27) (30) (31) (32) |
Note: (1) Age (2) Sex (3) BMI (Body Mass Index) (4) Obesity (BMI > 30) (5) SOFA (Sequential Organ Failure Assessment) (6) APACHE II (Acute Physiology and Chronic Health Evaluation II) (7) Glasgow score (8) Smoke (9) Hypertension (10) CoroMDry artery disease (11) Diabetes (12) Chronic pulmoMDry disease (13) Chronic kidney disease (14) MaligMDncy (15) White blood cell (16) Lymphocyte (17) C-reactive protein (18) D-dimer (19) Lactate (20) Heart rate (21) Respiratory rate (22) PaO2 (23) PaCO2 (24) SpO2 (25) PaO2/FiO2 (26) ROX index (defined as the ratio of SpO2/FiO2 to respiratory rate) (27) ROX after HFNC initiation (28) Length of HFNC use (29) Length of stay (30) Intubation (31) Mortality (32) HFNC failure.
Table 4.
Characteristics of the studies.
| Study | FiO2
(%) |
Flow rate (L/min) |
HFNC setting | Anti-spread measures on patients | Medical Staff infection |
|---|---|---|---|---|---|
| Beduneau et al. 14 | 63 ± 23 | 46.7 ± 7.7 | MD | MD | 4 /120 + |
| Blez et al. 15 | MD | MD | MD | MD | MD |
| Bonnet et al. 16 | MD | MD | FiO2 was adjusted to maintain the SpO2 above 92%. Flow rate was limited below 30 L/min before March 27. | Surgical mask for patients | 6/176 |
| Calligaro et al. 17 | MD | MD | Flow was initiated at 50-60 L/min with FiO2
0.8-1.0, titrated to aim for an oxygen saturation
(SpO2) ⩾ 92%. |
MD | MD |
| Celejewska- Wójcik et al. 18 |
78 ± 19 | 56.7 ± 6.5 | FiO2 and flow were titrated to keep SpO2 between 92% and 96% for patients without hypercapnia and between 88% and 92% for those with hypercapnia. | MD | MD |
| Chandel et al. 19 | MD | MD | MD | MD | MD |
| Delbove et al. 20 | 70 ± 13 | MD | MD | MD | 3/148 |
| Demoule et al. 21 | MD | MD | HFNC targeted a flow > 50 L/min, which could be reduced in case of poor tolerance. | MD | MD |
| Deng et al. 22 | MD | MD | HFNC was started from low levels and gradually titrated to 60 L/min for patients without obvious complaint of chest distress or shortness of breath. For patients who were short of breath, the flow rates were commenced at 60 L/min. The goal of oxygen therapy was to maintain the SpO2 at 93%96%. | MD | MD |
| Duan et al. 23 | MD | 41.0 ± 12.7 | Flow and FiO2 were adjusted to maintain SpO2 above 93% and the respiratory rate below 30 breaths/min, while favoring patients’ tolerance. | MD | MD |
| Franco et al. 24 | MD | 50.5 ± 8.0 | MD | No special protection | 42/369 |
| Garner et al. 25 | MD | MD | MD | MD | MD |
| Goury et al. 26 | 68 ± 16 | 60.0 | Initiated with a minimum flow of 50 L/min. FiO2 was titrated targeting an SpO2 above 92%, and flow rate was adjusted up to 60 L/min or according to the maximum tolerated dose. | MD | MD |
| Grieco et al. 27 | MD | MD | Flow was initially set at 60 L/min and eventually decreased in case of intolerance, FiO2 titrated to maintain SpO2 between 92% and 98%, and humidification chamber was set at 37 °C or 34 °C according to the patient’s comfort. | MD | MD |
| Hernandez- Romieu et al. 28 |
MD | MD | MD | MD | 1/148 |
| Hu et al. 29 | MD | MD | Initially set the gas flow rate to 30 L/min and the
FiO2 of 1.0, adjust the flow rate
and FiO2 to maintain the SpO2 at 92%-96%, and dynamically adjust it based on the blood gas analysis result. |
MD | MD |
| Katsuno et al. 30 | MD | MD | With a temperature setting of 37.0ºC and absolute humidity of 44 mg/L. The flow rate and FiO2 were adjusted according to individual clinical physician preferences. | Surgical mask and private negative pressure room
for patients |
No related infection |
| Liu et al. 31 | MD | MD | MD | MD | MD |
| Mellado- Artigas et al. 32 |
MD | MD | MD | MD | MD |
| PaMDdero et al. 33 | MD | MD | Temperature was set between 31ºC and 37ºC according to tolerance, with high flows of 50-60 L/min, and adjusting FiO2 to maintain SpO2 > 92%. | MD | MD |
| Sayan et al. 34 | MD | MD | Temperature was set between 31-37 degrees, the flow rate was 30-60 L/min, and the FiO2 was in the range of 40-90% with target SpO2 range of > 93%. | Surgical mask for patients | MD |
| Vianello et al. 35 | 67 ± 47 | MD | HFNC was initially used at 60 L/min with a FiO2 of 1.0; it was then adjusted to maintain a SpO2 ⩾ 92%. | Surgical mask for patients | No related infection |
| Xia et al. 36 | MD | MD | The initial gas flow rate was set to 40–50 L/min, the FiO2 was set to maintain SpO2 greater than 90%, and the temperature was adjusted according to the patient’s comfort. | Surgical mask for patients | MD |
| Xu et al. 37 | MD | 55.0 ± 7.4 | The temperature was adjusted between 31 and 37ºC; the flow was initiated above 30 L/min, and FiO2 was adjusted to reach SpO2 > 90% or higher. | MD | MD |
| Zucman et al. 38 | MD | MD | MD | MD | MD |
HFNC, high flow nasal cannula; MD, missing data.
Literature quality and bias assessment
Because only one RCT was included in the meta-analysis, and the remainder of the included studies were cohort and case-control studies, the quality of the literature was assessed with the Newcastle-Ottawa Scale. The results are shown in Supplemental Figure S1. All articles were of medium quality (⩾3 stars) or higher; eight articles were considered high-quality studies (⩾6 stars). Symmetry funnel plots of intubation and failure rates indicated no obvious publication bias, a finding corroborated by Egger’s test (P = 0.79 >|t| and P = 0.59 >|t|), whereas plots of the mortality rate did indicate bias (Figure 2).
Figure 2.
Funnel plots of the proportion versus the standard error of intubation (a), mortality (b), and failure rates(c).
Circles indicate studies included in the meta-analysis.
Clinical outcomes
A total of 2851 patients from 25 studies, all COVID-19 patients supported by HFNC, were ultimately included in the study. Baseline characteristics and clinical outcomes for the patients are summarized in Table 2. The mean age was 61 ± 13 years, 1603 patients (66.2%) were male, and the mean body mass index (BMI) was 28.9 ± 5.2. The mean Sequential Organ Failure Assessment (SOFA), APACHE II, and Glasgow Coma Scale scores were 3.7 ± 2.5, 11.1 ± 5.7, and 15.0 ± 0.1, respectively. Many patients had underlying comorbidities, including hypertension (1028, 46.3%), coronary artery disease (83, 9.2%), diabetes (655, 29.5%), chronic pulmonary disease (1589, 10.1%), chronic kidney disease (99, 9.4%), and malignancy (55, 4.6%). Inflammatory marker profiles, D-dimer, and lactate are also presented in Table 2. The mean heart rate, respiratory rate, PaO2, PaCO2, SpO2, PaO2/FiO2, and the ROX index at hospital admission were 87.2 ± 16.9, 26.9 ± 7.2, 75.5 ± 23.1, 35.0 ± 7.5, 90.6 ± 6.4, 141.5 ± 70.3, and 5.1 ± 2.9, respectively.
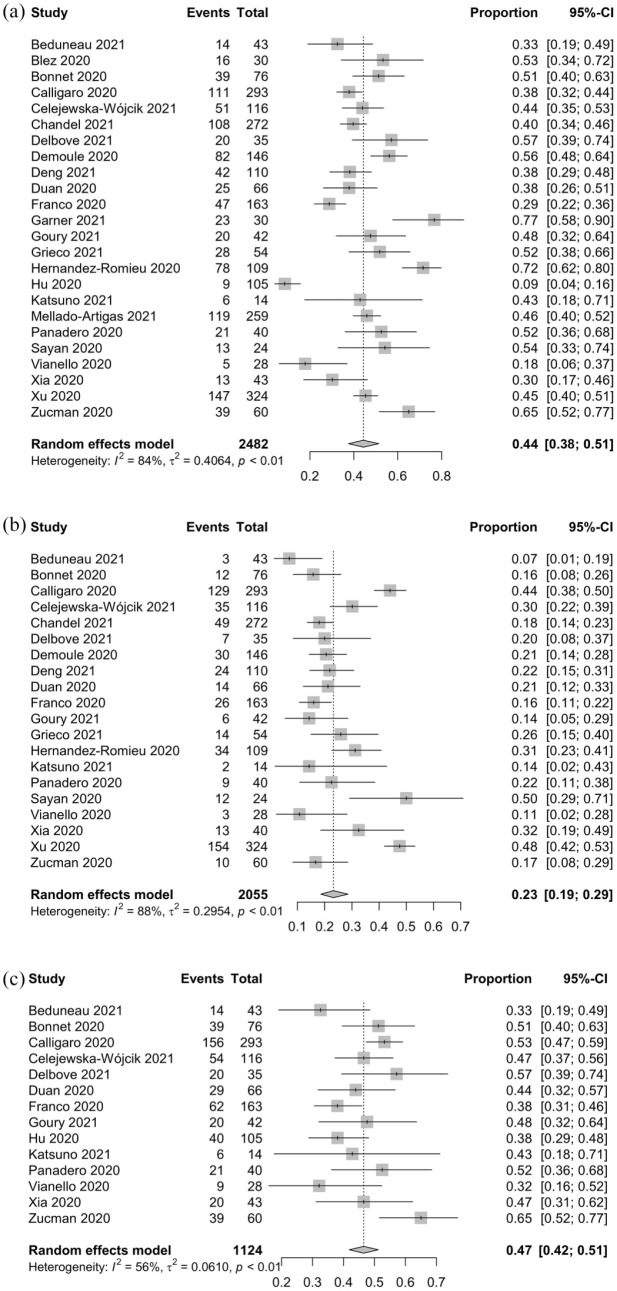
As shown in Table 5, a total of 1076 out of 2482 patients on HFNC from 24 studies14–30,32–38 ultimately received invasive mechanical ventilation, for a pooled intubation rate of 0.44 (95% CI: 0.38–0.51, I2 = 84%; Figure 3(a)). A total of 586 out of 2055 patients on HFNC from 20 studies14,16–24,26–28,30,33–38 ultimately died, for a mortality rate of 0.23 (95% CI: 0.19–0.29, I2 = 88%; Figure 3(b)). A total of 529 out of 1124 patients from 14 studies14,16–18,20,23,24,26,29,30,33,35,36,38 experienced HFNC failure (escalation to NIV or intubation and/or death), for a failure rate of 0.47 (95% CI: 0.42–0.51, I2 = 56%; Figure 3(c)). When reported, the ROX index after the initiation of HFNC was 5.0 ± 2.4, the duration of HFNC was 4.9 ± 5.0 days, and the length of the hospital stay was 19.6 ± 13.6 days.
Table 5.
Main demographic variables, clinical characteristic, and outcomes of the enrolled patients.
| Number of studies(n) | Total (Mean ± SD/n,%) | Number of studies(n) | Success (Mean ± SD/n,%) | Failure (Mea n ± SD/n,%) | P value | Number of studies(n) | OR [95% CI] | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | |||||||||
| Age | 23 | 61.3 ± 13.1 | 13 | 56.6 ± 14.0 | 61.9 ± 13.6 | < 0.01 | 8 | 1.04 [1.01-1.07] | 0.02 |
| Male | 23 | 1603 (66.2%) | 14 | 347 (54.4%) | 346 (57.8%) | 0.23 | 8 | 1.09 [0.66-1.79] | 0.75 |
| BMI | 11 | 28.9 ± 5.2 | 8 | 28.5 ± 5.7 | 29.7 ± 6.1 | < 0.01 | 3 | 0.98 [0.92-1.05] | 0.62 |
| Obesity (BMI > 30) | 4 | 245 (39.9%) | 3 | 105 (57.5%) | 107 (46.5%) | 0.83 | 3 | 0.90 [0.69-1.18] | 0.46 |
| SOFA | 16 | 3.7 ± 2.5 | 8 | 2.7 ± 1.8 | 5.4 ± 3.7 | < 0.01 | 3 | 1.72 [0.95-3.13] | 0.07 |
| APACHE II | 4 | 11.1 ± 5.7 | 2 | 8.0 ± 3.6 | 9.5 ± 2.9 | < 0.01 | 2 | 0.94 [0.87-1.03] | 0.17 |
| GCS | 3 | 15.0 ± 0.1 | 2 | 15.0 ± 0.2 | 15.0 ± 0.0 | 1.00 | 2 | 0.75 [0.50-1.13] | 0.17 |
| Smoke | 6 | 54 (19.3%) | 5 | 16 (12.3%) | 27 (28.1%) | < 0.01 | 1 | 0.92 [0.25-3.37] | 0.90 |
| Hypertension | 20 | 1028 (46.3%) | 10 | 239 (45.5%) | 243 (47.9%) | 0.44 | 3 | 1.00 [0.76-1.31] | 1.00 |
| Coronary artery disease | 8 | 83 (9.2%) | 5 | 17 (5.9%) | 22 (10.0%) | 0.08 | 1 | 3.78 [0.38–37.58] | 0.26 |
| Diabetes | 20 | 655 (29.5%) | 10 | 192 (36.6%) | 220 (43.4%) | 0.03 | 2 | 0.86 [0.46-1.58] | 0.62 |
| Chronic pulmonary disease | 12 | 1589 (10.1%) | 6 | 29 (9.8%) | 44 (16.3%) | 0.02 | 1 | 4.32 [1.03-18.12] | 0.05 |
| Chronic kidney disease | 9 | 99 (9.4%) | 7 | 22 (6.7%) | 43 (14.2%) | < 0.01 | 1 | 1.23 [0.37–4.10] | 0.73 |
| Malignancy | 7 | 55 (4.6%) | 1 | 1 (0.6%) | 6 (5.6%) | 0.01 | 3 | 1.35 [0.15-11.84] | 0.78 |
| Clinical Characteristic | |||||||||
| WBC *109 | 8 | 8.0 ± 4.0 | 5 | 8.4 ± 4.3 | 8.6 ± 4.1 | 0.58 | 3 | 1.06 [1.01-1.12] | 0.02 |
| Lymphocyte *109 | 10 | 0.9 ± 0.5 | 7 | 0.99 ± 0.6 | 0.97 ± 0.48 | 0.63 | 2 | 1.04 [0.90-1.20] | 0.60 |
| CRP mg/L | 8 | 44.5 ± 58.8 | 7 | 42.9 ± 54.1 | 45.8 ± 64.8 | 0.47 | 3 | 1.00 [0.99-1.01] | 0.85 |
| D-dimer μg/ml | 10 | 2.4 ± 5.2 | 7 | 1.4 ± 3.2 | 1.9 ± 2.6 | < 0.01 | 4 | 1.00 [0.99-1.01] | 0.92 |
| Lactate mmol/L | 8 | 1.7 ± 0.9 | 6 | 1.6 ± 0.8 | 1.8 ± 1.1 | 0.02 | 1 | 1.33 [0.96-1.83] | 0.08 |
| Heart rate | 10 | 87.2 ± 16.9 | 6 | 88.8 ± 15.6 | 91.9 ± 16.8 | 0.04 | 2 | 1.42 [1.15, 1.76] | < 0.01 |
| Respiratory rate | 18 | 26.9 ± 7.2 | 10 | 26.4 ± 8.0 | 27.6 ± 7.9 | < 0.05 | 4 | 1.01 [1.00-1.03] | 0.10 |
| PaO2 | 9 | 75.5 ± 23.1 | 6 | 82.2 ± 21.8 | 75.3 ± 19.5 | < 0.01 | 1 | 0.98 [0.95-1.01] | 0.24 |
| PaCO2 | 9 | 35.0 ± 7.5 | 5 | 35.0 ± 7.4 | 36.5 ± 8.5 | 0.11 | 1 | 1.03 [0.87-1.21] | 0.76 |
| SpO2 | 11 | 90.6 ± 6.4 | 5 | 92.9 ± 4.1 | 91.3 ± 4.5 | < 0.01 | 2 | 0.77 [0.44-1.34] | 0.36 |
| PaO2/FiO2 | 13 | 141.5 ± 70.3 | 7 | 124.9 ± 75.3 | 99 ± 69.2 | < 0.01 | 1 | 1.04 [0.97-1.11] | 0.21 |
| ROX index | 5 | 5.1 ± 2.9 | 3 | 8.3 ± 4.2 | 6.3 ± 4.6 | < 0.01 | 1 | 0.53 [0.38-0.72] | < 0.01 |
| Outcomes | |||||||||
| ROX index after HFNC | 9 | 5.0 ± 2.4 | 7 | 6.1 ± 2.7 | 4.9 ± 2.5 | < 0.01 | 3 | 0.61 [0.39-0.95] | 0.03 |
| Length of HFNC use | 9 | 4.9 ± 5.0 | 6 | 5.0 ± 3.6 | 3.1 ± 4.0 | < 0.01 | NA | NA | NA |
| LOS | 8 | 19.6 ± 13.6 | 3 | 18.1 ± 9.7 | 19.3 ± 19.2 | 0.59 | NA | NA | NA |
| Intubation | 24 | 1076 (43.4%) | NA | NA | NA | NA | NA | NA | NA |
| Mortality | 20 | 586 (28.5%) | NA | NA | NA | NA | NA | NA | NA |
| HFNC failure | 14 | 529 (47.1%) | NA | NA | NA | NA | NA | NA | NA |
APACHE, acute physiology and chronic health evaluation; BMI, body mass index; CRP, C-reactive protein; HFNC, high flow nasal cannula; LOS, length of stay; NA, Not applicable.; OR, odd ratio; ROX index, the ratio of SpO2/FiO2 to respiratory rate; SD, standard deviation; SOFA, sequential organ failure assessment; WBC, white blood cell.
Figure 3.
Intubation (a), mortality (b), and failure (c) rates for COVID-19 patients who received HFNC.
COVID-19: coronavirus disease 2019; HFNC: high-flow nasal cannula.
Differences in demographics and clinical characteristics between patients with a successful outcome on HFNC (success group) and those who experienced HFNC failure (failure group) are summarized in Table 5. When reported, compared to the HFNC success group, patients in the HFNC failure group had higher age, BMI, SOFA scores, APACHE II scores, D-dimer, lactate, heart rate, and respiratory rate and lower PaO2, SpO2, PaO2/FiO2, and duration of HFNC (all Ps < 0.05). Numbers of smokers and patients with diabetes, chronic pulmonary disease, chronic kidney disease, and malignancy were also higher in the failure group (all Ps < 0.05). The ROX index at admission and the ROX index after the initiation of HFNC also differed significantly between patients in the success and failure groups: 8.3 ± 4.2 versus 6.3 ± 4.6 (P < 0.01) and 6.1 ± 2.7 versus 4.9 ± 2.5 (P < 0.01), respectively. We did not find any differences in the number of male patients, the number of obese patients (BMI > 30), certain underlying comorbidities (hypertension and coronary artery disease), inflammatory marker profiles at admission, or PaCO2 at admission between the success and failure groups (all Ps > 0.05). Detailed data and corresponding P values are shown in Table 5.
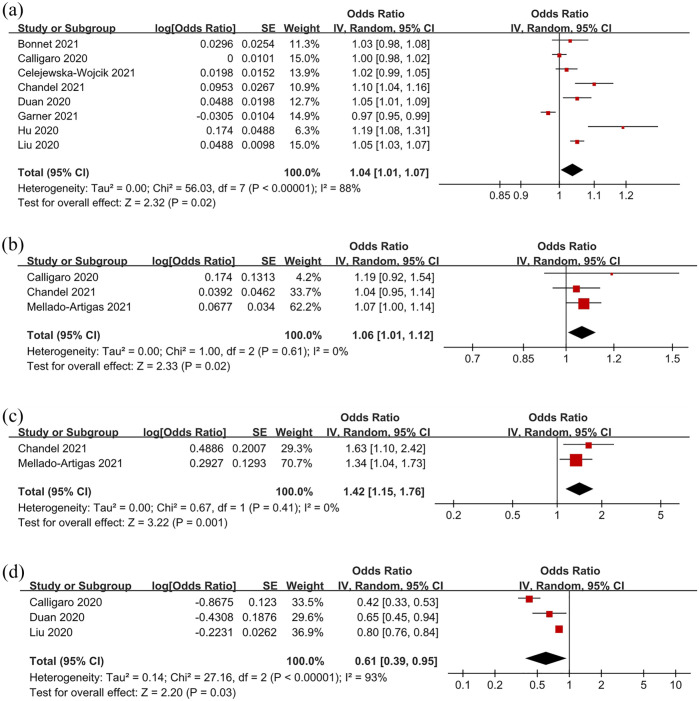
Several risk factors for HFNC failure were weighed in some studies, and pooled results are shown in Table 5 and Figure 4 . The pooled ORs revealed that older age (OR: 1.04, 95% CI: 1.01–1.07, P = 0.02, I2 = 88%), a higher white blood cell (WBC) count (OR: 1.06, 95% CI: 1.01–1.12, P = 0.02, I2 = 0%), and a higher heart rate (OR: 1.42, 95% CI: 1.15–1.76, P < 0.01, I2 = 0%) were all significant risk factors for HFNC failure. In contrast, a higher ROX index after the initiation of HFNC (OR: 0.61, 95% CI: 0.39–0.95, P = 0.03, I2 = 93%) indicated a lower chance of HFNC failure.
Figure 4.
Forest plots of significant risk and protective factors for HFNC failure: age (a), white blood cell count (b), heart rate (c), ROX index (d).
HFNC, high-flow nasal cannula; ROX index, the ratio of SpO2/FiO2 to respiratory rate.
Discussion
In this meta-analysis based on 2851 patients from 25 studies hospitalized for COVID-19, intubation, mortality, and failure rates were 0.44, 0.23, and 0.47, respectively. Compared to the HFNC success group, patients in the HFNC failure group were older; had higher BMI, SOFA and APACHE II scores, CRP, D-dimer, RR, HR, and lactate; and had lower PaO2, SpO2, PaO2/FiO2, baseline ROX index, and ROX index after the initiation of HFNC. There were also more smokers and more comorbidities in the failure group. Pooled ORs showed that older age, a higher WBC count, a higher heart rate, and a lower ROX index after the initiation of HFNC were associated with an increased risk of HFNC failure.
HFNC has several positive physiological and clinical advantages in treating acute respiratory failure. 39 A meta-analysis of nine RCTs of acute hypoxemic respiratory failure in the pre-COVID-19 era found that HFNC resulted in lower intubation rates without affecting survival. 40 Preliminary data, mainly from case reports and small case series, also point to its potential utility in treating COVID-19.9–11 In this study, the failure rate of HFNC in COVID-19 patients was 0.47, and intubation and mortality rates were 0.44 and 0.23, respectively. In one of the largest published studies on the effectiveness of NIV for treating COVID-19, failure and mortality rates were 37.6% and 25%, respectively. 41 One meta-analysis found that 26% of COVID-19 patients experienced NIV failure and required intubation, with intra-hospital mortality of 72%. 42 However, compared to NIV and COT, the effects of HFNC on intubation and mortality are not clear. As few studies in our meta-analysis compared HFNC to COT16,34 or NIV,24,27 we did not compare intubation and mortality rates between HFNC and COT or NIV in patients with COVID-19. Whether HFNC can benefit COVID-19 patients clinically must be studied further.
The use of HFNC to treat COVID-19 is very controversial. A literature review found that HFNC can reduce the need for intubation in patients with COVID-19 and decrease the length of stay in the intensive care unit and complications related to mechanical ventilation. 43 Some guidelines recommend HFNC over COT or NIV to treat hypoxia associated with COVID-19.44–46 World Health Organization suggest a short trial (about 1 h) of HFNC may be used in selected patients with COVID-19 and mild ARDS, and patient should be in a monitored setting and cared for in case the patient acutely deteriorates or does not improve after a the trial. 47 Besides, there is insufficient evidence to classify HFNC as an aerosol-generating procedure that is associated with transmission of COVID-19. 47 In the studies included in this meta-analysis and in other studies, some measures such as negative-pressure rooms, high-energy particulate accumulator filters, adequate personal protective equipment, and surgical masks may be sufficient to protect staff. 13,30,35,48
Patients at greater risk of HFNC failure are older, have a higher BMI, have higher SOFA and APACHE II scores, have more severe hypoxia, are more likely to be smokers, and have more comorbidities than those who experience successful HFNC. SARS-CoV-2 is capable of infecting people of all ages, but older people and people with preexisting medical conditions are predisposed to infection and severe forms of COVID-19.49,50 The list of comorbidities includes obesity, diabetes, hypertension, lung, liver, and kidney disease, compromised immunity (for cancer patients on chemotherapy, transplant recipients), smoking, and chronic use of steroids. 51 Higher SOFA and APACHE II scores have also been associated with increased mortality in critically ill patients with COVID-19. 52 Careful continuous monitoring of hypoxemic COVID-19 patients treated with HFNC is needed to detect early signs of failure and avoid delays in intubation. The World Health Organization has also pointed out that the oxygenation status of COVID-19 patients on HFNC should be closely monitored to enable timely adjustment of respiratory support.
Using objective criteria when observing patients on HFNC can improve the detection of clinical failure and avoid delays in intubation. Our study suggests that, as in other cases of hypoxemic respiratory failure, 53 the ROX index (defined as the ratio of SpO2/FiO2 to respiratory rate) has high sensitivity in identifying HFNC failure in COVID-19 patients. In a previous study, the ROX index was considered a better predictor of successful HFNC than SpO2/FiO2 or respiratory rate alone when measured 2, 6, or 12 h after the initiation of HFNC in patients with severe community- or hospital-acquired pneumonia. A ROX index ⩾ 4.88 measured within 2–12 h of HFNC was associated with an increased likelihood of successful HFNC in treating nonviral pneumonia.53,54 However, because the studies in this meta-analysis measured the ROX index at very different time points, we only used the latest ROX index after the initiation of HFNC in the analysis. As the condition of COVID-19 patients changes quickly, treatment providers should closely monitor changes in the ROX index after the initiation of HFNC. Whether more sensitive indicators such as ROX-HR and mROX 55 are better than ROX for monitoring COVID-19 patients needs to be studied further.
The inflammatory response associated with mortality appears to be dysregulated in response to COVID-19, and this likely drives the high mortality among critically ill patients with COVID-19. 56 Pooled ORs showed that a higher WBC count was associated with an increased risk of HFNC failure. A previous study found that patients with more severe disease had a higher WBC count. 57 However, we found no difference in inflammatory indices between the failure and success groups (Table 5), although pooled ORs showed that an increased WBC count was a risk factor for HFNC failure. This contradictory result may be due to the fact that these results came from different studies. Further study may be needed to verify this.
The present study has several limitations. First, our results were based mostly on cohort and case-control studies, and the quality of the evidence in the studies was low. The lack of RCTs may have reduced accuracy and increased heterogeneity. Second, as the distribution of resources and recommended guidelines differed in different countries during the COVID-19 epidemic, there was no clear protocol for initiating HFNC. Thus, treatment results depended on the judgment of individual physicians and differences in patient selectivity of HFNC, which may have affected our results. Third, data on biomarkers were incomplete, which reduced the power of the multivariate predictive model. Some important indicators were also incomplete, such as the time of failure and the day of illness, are very important for the failure prediction of HFNC, future research focus on these is needed. Some variables were likely skewed and would best be reported as medians with interquartile ranges and compared using a non-parametric statistical test, but this maybe related to the original data provided by the included study. Finally, we did not examine whether HFNC reduced intubation and mortality rates compared to conventional oxygen therapy or NIV; further studies may be needed.
Conclusions
Our meta-analysis found an aggregated failure rate of HFNC in COVID-19 patients of 0.47 and intubation and mortality rates of 0.44 and 0.23, respectively. Pooled ORs showed that older age, a higher WBC count, a higher heart rate, and a lower ROX index after the initiation of HFNC were associated with an increased risk of HFNC failure.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666221091931 for Effectiveness of the use of a high-flow nasal cannula to treat COVID-19 patients and risk factors for failure: a meta-analysis by Dong-yang Xu, Bing Dai, Wei Tan, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease
Footnotes
Author contributions: Dongyang Xu: Formal analysis; Methodology; Resources; Writing – original draft
Bing Dai: Formal analysis; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Wei Tan: Formal analysis; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Hongwen Zhao: Formal analysis; Resources; Supervision; Writing – original draft; Writing – review & editing.
Wei Wang: Formal analysis; Resources; Supervision; Writing – original draft; Writing – review & editing.
Jian Kang: Formal analysis; Resources; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This meta-analysis was funded by the Science and Technology Planning Project, Shenyang (21-172-9-12).
Registration: This review protocol was prospectively registered with PROSPERO (no. CRD42021261541).
ORCID iD: Wei Tan  https://orcid.org/0000-0003-1149-4168
https://orcid.org/0000-0003-1149-4168
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Dong-yang Xu, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Bing Dai, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, No.155, Nanjing North Street, Heping District, Shenyang, 110001 China.
Wei Tan, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, No.155, Nanjing North Street, Heping District, Shenyang, China.
Hong-wen Zhao, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Wei Wang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
Jian Kang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China.
References
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Attaway AH, Scheraga RG, Bhimraj A, et al. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021; 372: n436. [DOI] [PubMed] [Google Scholar]
- 4. Hendrickson KW, Peltan ID, Brown SM. The epidemiology of acute respiratory distress syndrome before and after coronavirus Disease 2019. Crit Care Clin 2021; 37: 703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma S, Danckers M, Sanghavi D, et al. High flow nasal cannula. Treasure Island, FL: StatPearls, 2021. [PubMed] [Google Scholar]
- 6. Chikata Y, Morinishi K, Nishimura M. Humidification in very-high-flow nasal-cannula therapy in an adult lung model. Respir Care 2019; 64: 809–817. [DOI] [PubMed] [Google Scholar]
- 7. Lewis SR, Baker PE, Parker R, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev 2021; 3: CD010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boccatonda A, Groff P. High-flow nasal cannula oxygenation utilization in respiratory failure. Eur J Intern Med 2019; 64: 10–14. [DOI] [PubMed] [Google Scholar]
- 9. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med 2020; 202: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonough G, Khaing P, Treacy T, et al. The use of high-flow nasal oxygen in the ICU as a first-line therapy for acute hypoxemic respiratory failure secondary to coronavirus disease 2019. Crit Care Explor 2020; 2: e0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020; 383: 2451–2460. [DOI] [PubMed] [Google Scholar]
- 12. Bohne LJ, Jansen HJ, Daniel I, et al. Electrical and structural remodeling contribute to atrial fibrillation in type 2 diabetic db/db mice. Heart Rhythm 2021; 18: 118–129. [DOI] [PubMed] [Google Scholar]
- 13. Raoof S, Nava S, Carpati C, et al. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest 2020; 158: 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beduneau G, Boyer D, Guitard PG, et al. Covid-19 severe hypoxemic pneumonia: a clinical experience using high-flow nasal oxygen therapy as first-line management. Respir Med Res 2021; 80: 100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blez D, Soulier A, Bonnet F, et al. Monitoring of high-flow nasal cannula for SARS-CoV-2 severe pneumonia: less is more, better look at respiratory rate. Intensive Care Med 2020; 46: 2094–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonnet N, Martin O, Boubaya M, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care 2021; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. E Clin Med 2020; 28: 100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Celejewska-Wójcik N, Polok K, Górka K, et al. High-flow nasal oxygen therapy in the treatment of acute respiratory failure in severe COVID-19 pneumonia: a prospective observational study. Pol Arch Intern Med 2021; 131: 658–665. [DOI] [PubMed] [Google Scholar]
- 19. Chandel A, Patolia S, Brown AW, et al. High-flow nasal cannula therapy in COVID-19: using the ROX index to predict success. Respir Care 2021; 66: 909–919. [DOI] [PubMed] [Google Scholar]
- 20. Delbove A, Foubert A, Mateos F, et al. High flow nasal cannula oxygenation in COVID-19 related acute respiratory distress syndrome: a safe way to avoid endotracheal intubation? Ther Adv Respir Dis 2021; 15: 17534666211019555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demoule A, Baron AV, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med 2020; 202: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng L, Lei S, Wang X, et al. Course of illness and outcomes in older COVID-19 patients treated with HFNC: a retrospective analysis. Aging (Albany NY) 2021; 13: 15801–15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan J, Zeng J, Deng P, et al. High-flow nasal cannula for COVID-19 patients: a multicenter retrospective study in China. Front Mol Biosci 2021; 8: 639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J 2020; 56: 2002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garner O, Dongarwar D, Salihu HM, et al. Predictors of failure of high flow nasal cannula failure in acute hypoxemic respiratory failure due to COVID-19. Respir Med 2021; 185: 106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goury A, Moussanang JA, Bard M, et al. Predictive factors associated with high-flow nasal cannula success for COVID-19-related acute hypoxemic respiratory failure. Health Sci Rep 2021; 4: e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA 2021; 325: 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med 2020; 48: e1045–e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu M, Zhou Q, Zheng R, et al. Application of high-flow nasal cannula in hypoxemic patients with COVID-19: a retrospective cohort study. BMC Pulm Med 2020; 20: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katsuno T, Suzuki M, Hojo M, et al. Clinical experience with high-flow nasal cannulas for coronavirus disease 2019 patients in Japan. Respir Investig 2021; 59: 569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Health 2021; 3: e166–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mellado-Artigas R, Mujica LE, Ruiz ML, et al. Predictors of failure with high-flow nasal oxygen therapy in COVID-19 patients with acute respiratory failure: a multicenter observational study. J Intensive Care 2021; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panadero C, Abad-Fernández A, Rio-Ramirez MT, et al. High-flow nasal cannula for Acute Respiratory Distress Syndrome (ARDS) due to COVID-19. Multidiscip Respir Med 2020; 15: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sayan İ, Altınay M, Çınar AS, et al. Impact of HFNC application on mortality and intensive care length of stay in acute respiratory failure secondary to COVID-19 pneumonia. Heart Lung 2021; 50: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax 2020; 75: 998–1000. [DOI] [PubMed] [Google Scholar]
- 36. Xia J, Zhang Y, Ni L, et al. High-flow nasal oxygen in coronavirus disease 2019 patients with acute hypoxemic respiratory failure: a multicenter, retrospective cohort study. Crit Care Med 2020; 48: e1079–e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Yang X, Huang C, et al. A novel risk-stratification models of the high-flow nasal cannula therapy in COVID-19 patients with hypoxemic respiratory failure. Front Med (Lausanne) 2020; 7: 607821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zucman N, Mullaert J, Roux D, et al. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med 2020; 46: 1924–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CC, Mankodi D, Shaharyar S, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: a systematic review. Respir Med 2016; 121: 100–108. [DOI] [PubMed] [Google Scholar]
- 40. Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 41. Bellani G, Grasselli G, Cecconi M, et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARd-COVID). Ann Am Thorac Soc 2021; 18: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cammarota G, Esposito T, Azzolina D, et al. Noninvasive respiratory support outside the intensive care unit for acute respiratory failure related to coronavirus-19 disease: a systematic review and meta-analysis. Crit Care 2021; 25: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gürün Kaya A, Öz M, Erol S, et al. High flow nasal cannula in COVID-19: a literature review. Tuberk Toraks 2020; 68: 168–174. [DOI] [PubMed] [Google Scholar]
- 44. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with Coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Australian and New Zealand Intensive Care Society. COVID-19 guidelines, version 1, https://www.anzics.com.au/coronavirus-guidelines/ (2020, accessed 16 April 2020).
- 46. National Institutes of Health. Care of critically ill patients with COVID-19: summary recommendations, https://covid19treatmentguidelines.nih.gov/critical-care/ (2020, accessed 23 April 2020).
- 47. World Health Organization. Living guidance for clinical management of COVID-19, http://apps.who.int/iris/handle/10665/349321 (accessed 23 Novemeber 2021). [PubMed]
- 48. Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J 2020; 55:2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Esteve A, Permanyer I, Boertien D, et al. National age and coresidence patterns shape COVID-19 vulnerability. Proc Natl Acad Sci U S A 2020; 117: 16118–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rod JE, Oviedo-Trespalacios O, Cortes-Ramirez J. A brief-review of the risk factors for covid-19 severity. Rev Saude Publica 2020; 54: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med 2020; 2: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White-Dzuro G, Gibson LE, Zazzeron L, et al. Multisystem effects of COVID-19: a concise review for practitioners. Postgrad Med 2021; 133: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care 2016; 35: 200–205. [DOI] [PubMed] [Google Scholar]
- 54. Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med 2019; 199; 1368–1376. [DOI] [PubMed] [Google Scholar]
- 55. Goh KJ, Chai HZ, Ong TH, et al. Early prediction of high flow nasal cannula therapy outcomes using a modified ROX index incorporating heart rate. J Intensive Care 2020; 8: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taylor EH, Marson EJ, Elhadi M, et al. Factors associated with mortality in patients with COVID-19 admitted to intensive care: a systematic review and meta-analysis. Anaesthesia 2021; 76: 1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iftikhar R, Kamran SM, Mirza ZE, et al. Haematological parameters and outcome in hospitalized patients with covid-19: a developing country experience. J Ayub Med Coll Abbottabad 2021; 33: 416–424. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666221091931 for Effectiveness of the use of a high-flow nasal cannula to treat COVID-19 patients and risk factors for failure: a meta-analysis by Dong-yang Xu, Bing Dai, Wei Tan, Hong-wen Zhao, Wei Wang and Jian Kang in Therapeutic Advances in Respiratory Disease