Abstract
Viral infections emerge in the pathogenesis of subacute thyroiditis. Aside from this, subacute thyroiditis following vaccines utilizing inactivated viruses has been shown on rare occasions. Due to the COVID-19 pandemic, several vaccines have been developed all over the world; mass and unprecedented vaccination has thus been initiated. However, it is known that cases such as subacute thyroiditis have been reported, albeit rarely, after administration of COVID-19 vaccines. In this case report, we present a 59-year-old patient with multiple myeloma developing subacute thyroiditis following BNT162b2 vaccine. Patient had swelling in the neck, and his symptoms were controlled with non-steroidal anti-inflammatory drugs. Subacute thyroiditis following administration of the COVID-19 vaccine is rare; however, it is likely an under-reported condition that is difficult to detect. Clinicians should stay informed and have increased awareness of post-COVID-19 vaccine subacute thyroiditis.
Keywords: Subacute thyroiditis, coronavirus vaccine, COVID-19, SARS-CoV-2
Introduction
Subacute thyroiditis, also known as De Quervain’s thyroiditis, is a benign self-limiting disorder of the thyroid. 1 In clinical presentation, the thyroid gland is usually enlarged with pain radiating to the neck, throat, and jaw being observed. Fever and other symptoms of infection could be seen. 2 Several viral infections have been associated with subacute thyroiditis. 3 Subacute thyroiditis has also been reported after vaccination for viral disease.4–6
The COVID-19 disease caused by the SARS-CoV-2 virus has resulted in a global pandemic. This pandemic has brought an enormous burden upon people and caregivers, and more importantly, caused many deaths. 7 With the progression of the COVID-19 pandemic, the development of many vaccines has accelerated. Thanks to these developments, we have watched many vaccines being approved for emergency use and entered our daily life. Vaccination with Coronavac, which is an inactive virus vaccine, started in Turkey and then continued with the BNT162b2 (Pfizer-BioNTech) vaccine.
In addition, it has been shown that COVID-19 infection is more severe and fatal in patients with hematological malignancy.8,9 Multiple myeloma, which is the second most common hematological malignancy, is characterized by production of malign plasma cells. 10 Due to the nature of the disease, there is an increased risk for infections, and COVID-19 may become more serious in these patients.11,12 Infections are a major cause of morbidity and mortality in patients with multiple myeloma; 13 there is an increased risk for bacterial and viral infections in multiple myeloma patients when compared to healthy controls. 14 Therefore, vaccination is mandatory for multiple myeloma patients as the disease itself affects antibody-producing plasma cells, impairing T cell and B cell functions, thus causing an immunosuppressive state.
In this case report, we aimed to present a 59-year-old male patient with multiple myeloma who developed subacute thyroiditis after vaccination with Pfizer-BioNTech (BNT162b2) messenger RNA (mRNA)-based vaccine.
Case presentation
Anemia and hypercalcemia were present in the evaluation of a 59-year-old male patient who arrived with complaints of fatigue and bone pain. An IgA Lambda Multiple myeloma diagnosis is present with his detailed work-up that includes a bone marrow biopsy. He had no comorbid conditions, and his Eastern Cooperative Oncology Group (ECOG), performance status was 0. He was Stage 1 for International Staging System (ISS) and Stage 1 for revised International Staging System (R-ISS) prognosis classification. 15 The patient is fit for chemotherapy and eligible for autologous hematopoietic stem cell transplant (ASCT). Planned for induction were four cycles of the CyBorD regimen, which is a combination of bortezomib–cyclophosphamide–dexamethasone.16,17 A very good partial response has been achieved at the end of the treatment according to the International Myeloma Working Group (IMWG) consensus criteria for response. 18
Mobilization of stem cells was performed with cyclophosphamide 3 gr/m2, and after that, the patient underwent a successful ASCT. Lenalidomide 10 mg for maintenance therapy was added after the third month of ASCT. Six months after the ASCT, the first dose of mRNA-based BNT162b2 vaccine (Pfizer–BioNTech) was administered to the patient.
He presented with fever, neck pain, swelling in the neck, and fatigue 10 days after receiving the first dose of BNT162b2 vaccine. Upon physical examination, there was swelling and tenderness in his right thyroid lobe. Other bodily functions were normal. In a laboratory work up, a slightly elevated ESR (erythrocyte sedimentation rate) 95 mm and CRP (C-reactive protein) level 158.5 mg/L was observed. His complete blood cell count and other biochemical tests were normal. There was no infiltration on his chest X-rays. His blood and urine cultures were negative. In thyroid function tests, thyrotropin (TSH): 0.02 mU/L (normal = 0.5–4), free T3: 5.14 pg/mL (normal = 2.3–4.2), and free T4: 200 ng/dL (normal = 0.8–1.8) were found. Anti-TPO (thyroid peroxidase) and anti-Tg (thyroglobulin) antibodies were negative.
A thyroid ultrasound revealed an enlarged right lobe of the thyroid and heterogeneous echogenicity. Decreased blood flow, especially in the right lobe, was also found on color Doppler. Technetium-99m (99m Tc)-pertechnetate scintigraphy were performed and demonstrated markedly reduced uptake in the thyroid gland which supports subacute thyroiditis (Figure 1). The patient was evaluated as subacute thyroiditis with clinical, laboratory, and imaging findings. Diclofenac potassium 2 × 50 mg/day oral treatment was started. At the end of the second week, the pain in the neck completely regressed according to all clinical parameters. His laboratory parameters returned to normal, and diclofenac potassium treatment was stopped. (The course of laboratory parameters at the time of diagnosis and during follow-up is summarized in Table 1.) We performed the Naranjo Adverse Drug Reaction Probability Scale and BNT162b2 vaccine was the probable cause for subacute thyroiditis with a score of six. 19 We have also obtained written informed consent/permission to publish the case report from the patient.
Figure 1.
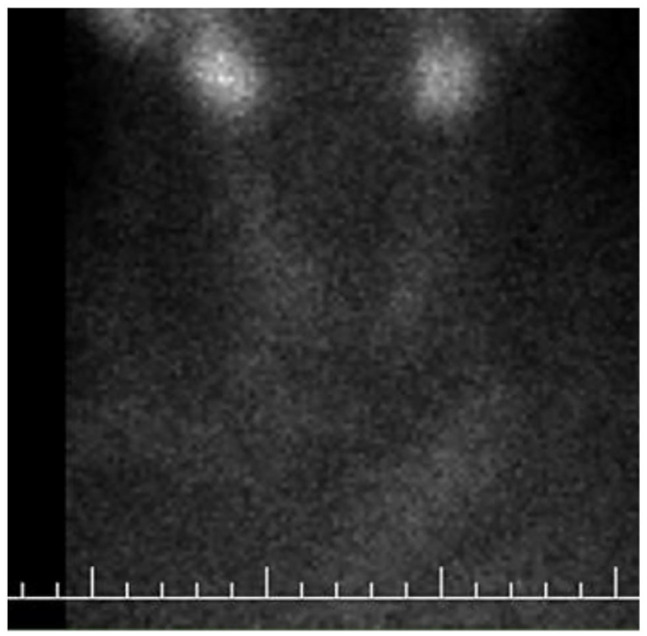
Technetium-99m pertechnetate scan showing decreased and heterogeneous radiotracer uptake in both lobes of thyroid gland.
Table 1.
Laboratory findings during the disease course.
| At diagnosis | Follow-up first week | Follow-up second week | Follow-up fourth week | |
|---|---|---|---|---|
| TSH (mU/L) | 0.02 | 0.03 | 0.05 | 0.56 |
| fT4 (ng/dL) | 2.02 | 2.46 | 1.79 | 0.96 |
| fT3 (pg/mL) | 5.14 | 3.98 | 2.56 | 2.24 |
| ESR (mm/h) | 95 | 37 | 17 | 14 |
| CRP (mg/L) | 158.5 | 68.6 | 15.2 | 3.5 |
| Hemoglobin (g/dL) | 10.5 | 10.9 | 11.6 | 12.4 |
| WBC (×109/L) | 3.9 | 4.2 | 4.8 | 4.5 |
| Thrombocyte (×109/L) | 176 | 211 | 209 | 198 |
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; fT3: free triiodothyronine; fT4: free thyroxine; TSH: thyroid-stimulating hormone; WBC: white blood cell count.
Discussion
Infections are the main causes of death, and one of the major obstacles in care, where multiple myeloma patients are concerned. 20 In addition, infections could also result in early mortality multiple myeloma. 13 Multiple myeloma could, in other words, cause premature mortality, and it has also been observed in a nationwide analysis study. 21 In our routine practice, vaccination of multiple myeloma patients against various viruses and bacterial pathogens is recommended. 22 An intensive vaccination schedule is also advised for patients undergoing ASCT. 22 However, impaired responses to mRNA COVID-19 vaccines in multiple myeloma patients have been reported. 23 Intensive vaccination and even booster doses are expected to continue, as vaccines are our strongest weapon against this deadly enemy.
The exact cause of subacute thyroiditis is unknown, but subacute thyroiditis following several viral infections, as well as 3 subacute thyroiditis following inactivated virus vaccines in healthy people have been described.4,6 In history, it was associated with the mumps virus outbreaks. 24 In the era of COVID-19, subacute thyroiditis developed after COVID-19 disease and after SARS-CoV-2 vaccines were reported.25–34 The SARS-CoV-2 virus, which affects many of our systems, also affects our endocrine system. Thyroid gland is one of the most affected endocrine organs. Direct and indirect effects of the SARS-CoV-2 on the thyroid glands could cause thyroid dysfunction. It can lead to changes in the structure or function of the thyroid gland. 35 Subacute thyroiditis is thought to be a post-inflammatory syndrome of viral infections. 29 It usually appears 2–8 weeks following viral infections. In cases that develop subacute thyroiditis after vaccine administration, the time between the vaccine and subacute thyroiditis is usually 5 days to 2 weeks. In our case, the interval time was 10 days. Subacute thyroiditis after SARS-CoV-2 vaccine is usually has a mild prognosis and could be treated clearly. 36
In recent years, autoimmune/inflammatory syndrome (ASIA) induced by adjuvants which are used for augmenting immunogenicity of vaccines have been suggested.37,38 It has also been proposed that subacute thyroiditis is also a phenomenon of ASIA. It is also known that ACE2 (angiotensin-converting enzyme 2) receptors play a profound role in the pathogenesis of SARS-CoV-2 in penetrating cells. 39 Aside from the lungs, the thyroid gland is another one of the organs that carry ACE2 receptors. 40 In this setting, ACE2 receptors may be a clue to subacute thyroiditis following COVID-19 infections and vaccines. 41 In addition, a possible cross-reaction and antigenic mimicry between thyroid cell antigens and the spike protein of the coronavirus have been suggested. 42 However, the precise mechanism of subacute thyroiditis developing after the COVID-19 vaccine has not been understood now.
Subacute thyroiditis usually starts with a sudden onset of neck pain; on laboratory findings, it starts with a transient thyrotoxic phase, and is usually followed by an also transient hypothyroidic phase, with an eventual recovery with euthyroid state occurring. 2 Treatment is often targeted to alleviate symptoms. Non-steroidal anti-inflammatory drugs (NSAIDs) are given for controlling pain. Prednisone can be started when NSAIDs are not helpful for symptoms. Usually, NSAIDs are the first choice and cause a dramatic response as in our case.
Conclusion
To our knowledge, this is the first report of a multiple myeloma patient developing subacute thyroiditis following SARS-CoV-2 BNT162b2 vaccine. With developing vaccination rates all over the world, and subacute thyroiditis cases reported after both mRNA-based vaccines and inactivated virus vaccines, we can suggest that there may be an increase in cases of subacute thyroiditis, clinicians should stay informed and have increased awareness of post-COVID-19 vaccine subacute thyroiditis. However, we should keep in mind that this is a mild and transient side effect, and this should not discourage from getting vaccinated.
Supplemental Material
Supplemental material, sj-docx-1-sco-10.1177_2050313X221091392 for Subacute thyroiditis after SARS-CoV-2 BNT162b2 vaccine in a multiple myeloma patient by Nihan Alkis and Mehmet Baysal in SAGE Open Medical Case Reports
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Mehmet Baysal  https://orcid.org/0000-0001-7681-4623
https://orcid.org/0000-0001-7681-4623
Supplemental material: Supplemental material for this article is available online.
References
- 1. Shrestha RT, Hennessey J. Acute and subacute, and Riedel’s thyroiditis, 2000, https://www.ncbi.nlm.nih.gov/books/NBK285553/
- 2. Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician 2000; 61(4): 104754–104752. [PubMed] [Google Scholar]
- 3. Singer PA. Thyroiditis. Acute, subacute, and chronic. Med Clin North Am 1991; 75(1): 61–77. [DOI] [PubMed] [Google Scholar]
- 4. Hsiao JY, Hsin SC, Hsieh MC, et al. Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Kaohsiung J Med Sci 2006; 22(6): 297–300. [DOI] [PubMed] [Google Scholar]
- 5. Michas G, Alevetsovitis G, Andrikou I, et al. De Quervain thyroiditis in the course of H1N1 influenza infection. Hippokratia 2014; 18(1): 86–87. [PMC free article] [PubMed] [Google Scholar]
- 6. Altay FA, Guz G, Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother 2016; 12(4): 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer 2020; 1: 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020; 136(25): 2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yigenoglu TN, Ata N, Altuntas F, et al. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol 2021; 93(2): 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J 2020; 10(9): 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teh BW, Harrison SJ, Worth LJ, et al. Risks, severity and timing of infections in patients with multiple myeloma: a longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol 2015; 171(1): 100–108. [DOI] [PubMed] [Google Scholar]
- 12. Martinez-Lopez J, Mateos MV, Encinas C, et al. Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J 2020; 10(10): 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlinski G, Tyczynska A, Malecki B, et al. Risk factors and causes of early mortality in patients with newly diagnosed multiple myeloma in a ‘real-world’ study: experiences of the Polish Myeloma Group. Pol Arch Intern Med 2021; 131(6): 527–534. [DOI] [PubMed] [Google Scholar]
- 14. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica 2015; 100(1): 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15(12): e538–e48. [DOI] [PubMed] [Google Scholar]
- 16. Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 2009; 23(7): 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012; 119(19): 4375–4382. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17(8): e328–e346. [DOI] [PubMed] [Google Scholar]
- 19. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30(2): 239–245. [DOI] [PubMed] [Google Scholar]
- 20. Mai EK, Haas EM, Lucke S, et al. A systematic classification of death causes in multiple myeloma. Blood Cancer J 2018; 8(3): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pham TM, Shen-Tu G, Nguyen KH, et al. Premature mortality due to Hodgkin’s lymphoma, non-Hodgkin lymphoma, multiple myeloma, and leukemia in Canada: a nationwide analysis from 1980 to 2015. Am J +Epidemiol 2021; 190(1): 59–75. [DOI] [PubMed] [Google Scholar]
- 22. Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia 2021; 35(1): 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stampfer SD, Goldwater MS, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021; 35(12): 3534–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martino E, Buratti L, Bartalena L, et al. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest 1987; 10(3): 321–323. [DOI] [PubMed] [Google Scholar]
- 25. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinoli Metab 2020; 105(10): dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aemaz Ur, Rehman M, Farooq H, Ali MM, et al. The association of subacute thyroiditis with COVID-19: a systematic review. SN Compr Clin Med. Epub ahead of print 29 April 2021. DOI: 10.1007/s42399-021-00912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ayhan M, Guner R. A subacute thyroiditis case after SARS-CoV-2 infection: a case report and current literature review. Med Bull Haseki 2021; 59(1): 54–56. [Google Scholar]
- 28. Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med 2021; 8: 737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrin Metabol 2021; 106(9): 2600–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruggeri RM, Campenni A, Siracusa M, et al. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones 2021; 20(1): 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahin Tekin M, Saylisoy S, Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccin Immunother 2021; 17: 4090–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saygili ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep 2021; 14(10): e244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sigstad E, Grøholt KK, Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr Nor Laegeforen 2021; 141: 0554. [DOI] [PubMed] [Google Scholar]
- 34. Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin Case Rep 2021; 9(10): e04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruggeri RM, Campenni A, Deandreis D, et al. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol 2021; 17(7): 737–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ippolito S, Gallo D, Rossini A, et al. SARS-CoV-2 vaccine-associated subacute thyroiditis: insights from a systematic review. J Endocrinol Invest. Epub ahead of print 29 January 2022. DOI: 10.1007/s40618-022-01747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watad A, David P, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol 2016; 7: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest 2022; 45: 465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020; 181(4): 894–904.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology 2020; 161(9): bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest 2021; 44(5): 1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol 2021; 11: 617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sco-10.1177_2050313X221091392 for Subacute thyroiditis after SARS-CoV-2 BNT162b2 vaccine in a multiple myeloma patient by Nihan Alkis and Mehmet Baysal in SAGE Open Medical Case Reports


