Abstract
Objective
To determine whether the first-line treatment using pembrolizumab plus standard chemotherapy of platinum and pemetrexed for patients with metastatic, non-squamous, non–small-cell lung cancer (NSCLC) is cost-effective in China.
Methods
We applied partitional survival analysis to assess the cost-effectiveness of pembrolizumab plus the cytotoxic chemotherapy (cisplatin/carboplatin and pemetrexed) in metastatic NSCLC in China. We took into account direct medical costs according to the data derived from the KEYNOTE-189 trial and literature. Incremental cost-effectiveness ratio (ICER) was assessed as per life-year (LY) and per quality-adjusted life-year (QALY), with 3% per year discounted rate of costs and outcomes. In the performance of sensitivity analysis, cost of disease-management, utility-PFS (progression-free survival), utility-PD (progressive disease) and the discount were considered as variables. In scenario analysis, a philanthropic support programme in China was considered. The threshold was set to be $28 106/QALY (corresponding to three times the GDP in China).
Results
Treatment with pembrolizumab plus platinum and pemetrexed chemotherapy was estimated to increase cost by $139 168 compared with $73 081 (the cost of treatment with chemotherapy alone), leading to ICER of $80 444/LY and $96 644/QALY. Incremental costs/QALY are $90 419, $91 399 and $109 229 for programmed death ligand-1 TPS (tumour proportion scores) ≥50%, 1%–49% and <1% subgroups, respectively. Sensitivity analysis revealed that the price of pembrolizumab and the cost of disease-management in progressive-disease state were major variables.
Conclusion
In patients with metastatic non-squamous NSCLC, pembrolizumab plus standard chemotherapy of platinum and pemetrexed as the first-line treatment is not cost-effective in China, regardless of TPS.
Keywords: pharmacoeconomics, clinical pharmacy, cost-price calculation, health economics, respiratory tract tumours
Introduction
As reported in 2019, lung cancer is the third leading factor of disease-related deaths after stroke and ischaemic heart disease in China.1 The highest proportion of all lung cancers is non–small-cell lung cancer (NSCLC), which accounts for 85% of all types.2 The overall survival rate for 5 years is worldwide estimated to be 10%–15%.3 Thus, the prognosis for these patients is usually poor. Half of the patients have metastatic disease in the early stage of diagnosis.4 As recommended, the standard treatments for metastatic NSCLC contained first-line treatments including platinum-based (carboplatin/cisplatin) chemotherapy and essential maintenance therapy and second-line cytotoxic chemotherapy (docetaxel) used as a sequential treatment after disease progression, with median survival of less than 12 months.5
KEYNOTE-189 was the first double-blind, randomised phase III trial aimed at metastatic non-squamous NSCLC,6 which was designed to evaluate the therapeutic effect of adding Keytruda (pembrolizumab) to the chemotherapy regimen of platinum and pemetrexed, in patients without epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) mutation, irrespective of programmed death ligand-1 (PD-L1) expression status. After a median follow-up of 10.5 months, the KEYNOTE-189 trial illustrated that patients treated with pembrolizumab plus standard chemotherapy had superior estimated rate of overall survival (OS) at 1 year, as compared with those who treated with chemotherapy alone, with a HR for death of 0.49 (95% CI 0.38 to 0.64; p<0.001).6 The patients treated with pembrolizumab plus chemotherapy regimen had longer median progression-free survival (PFS) (8.8 months, 95% CI 7.6 to 9.2) than that of chemotherapy alone (4.9 months, 95% CI 4.7 to 5.5), with a HR for disease progression or death of 0.52 (95% CI 0.43 to 0.64; p<0.001). In May 2019, the analysis of data in the KEYNOTE-189 trial was updated to a longer follow-up time (median 23.1 months), which continued to show substantial benefit of pembrolizumab-combination regimen on OS and PFS.7 In details, pembrolizumab-combination group represented longer OS (HR 0.56; 95% CI 0.45 to 0.70, p<0.00001; median survival: 22.0 months vs 10.7 months) and PFS (HR 0.48; 95% CI 0.40 to 0.58, p<0.00001; median survival: 9.0 months vs 4.9 months), compared with placebo-combination group.7 In April 2019, pembrolizumab had been approved by the National Medical Products Administration (NMPA) of China as a first-line treatment of metastatic NSCLC, coupled with platinum-based drugs for the patients with no EGFR or ALK genomic tumour aberrations, regardless of PD-L1 tumour expression status. This new indication was granted conditional approval based on OS and PFS data from the KEYNOTE-189 phase III trial.
Despite the treatment using pembrolizumab combination for metastatic NSCLC showing possibility of excellent clinical results, the high prices could impose a heavy economic burden on individuals, families and communities in China. The purpose of this economic evaluation was to estimate the cost-effectiveness of pembrolizumab plus standard chemotherapy versus chemotherapy alone within the new approved indication in China.
Methods
Model structure
Using data from the KEYNOTE-189 clinical trial,6 Insinga et al developed partitioned-survival model to estimate costs and outcomes of metastatic NSCLC.8 These patients had three mutually exclusive health states: progressive-disease state (PD), progression-free state (PF) and death. For the transition diagram of this model, see figure 1. In each model cycle, we can read the numbers of patients in three states from the OS and PFS curves. In this model, we followed the definition of progression according to RECIST V1.1 criteria.9 The cycle length of this model was set to 1 week. It suffices to reflect the conditions of treatment management and the transitions among PF, PD and death. Many researchers used this approach to model metastatic cancer.8 10 11 This model was developed in Microsoft Excel 2007.
Figure 1.
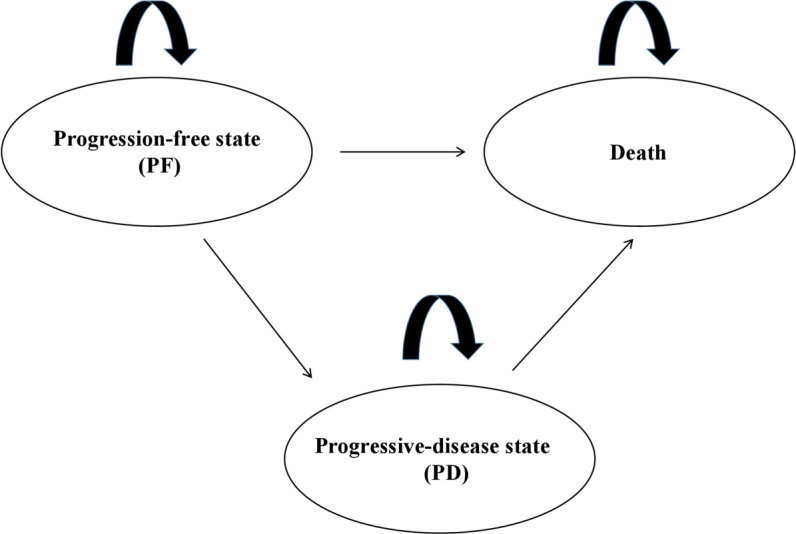
Model structure and transitions.
The incremental cost-effectiveness ratio (ICER) presented as cost per quality-adjusted life-year (QALY) was the major outcome measure. In addition, the incremental cost per life-year (LY) gained was estimated.
Therapeutic regimen was evaluated as ‘cost-effective’ if the ICER was below a threshold of $28 106/QALY, which is three times the 2018 gross domestic product (GDP) per capita in China.
Target population
In this model, the target population was based on the KEYNOTE-189 trial,6 with the following basic characteristics: patients aged at least 18 years (average aged 63 years); diagnosed stage IV non-squamous NSCLC; had no EGFR or ALK mutation; without previous systemic treatment of metastatic NSCLC; performance-status score of Eastern Cooperative Oncology Group ≤1; without symptomatic central nervous system metastases.6 The KEYNOTE-189 median ages are 65 years and 63.5 years for pembrolizumab-combination group and placebo-combination group, respectively. It is consistent with the result that the lung cancer risk is highest in people aged >60 years in epidemiology of lung cancer in China.12 Hence, although the amount of East Asia patients included in KEYNOTE-189 was not enough (10 out of 616), we still consider that KEYNOTE-189 is available for China. KEYNOTE-032 study13 showed that the pharmacokinetic profiles of pembrolizumab in Chinese patients with advanced or metastatic NSCLC were comparable with those observed in international studies. This result was in agreement with a phase I study of pembrolizumab in Japanese patients.14 KEYNOTE-189 did not conduct a China extension study, but the results in KEYNOTE-042 China extension study (NCT03850444)15 and KEYNOTE-407 China extension study (NCT03875092)16 for patients with locally advanced/metastatic NSCLC showed that the clinical outcomes and safety profile are consistent with findings from the global studies.
Interventions
Patients were grouped by a random double-blind approach, to receive either 200 mg of pembrolizumab or placebo, in a ratio of 2:1, every 3 weeks for up to 35 cycles. Cisplatin (75 mg/m2) or carboplatin (area under the concentration–time curve, 5 mg*min/mL) plus pemetrexed (500 mg/m2) were given to them every 3 weeks for four cycles, followed by maintenance pemetrexed. Maintenance treatment was continued until severe toxic effects, progression in radiographic or termination of initial treatment due to patients’ preference.6
Perspective, discount rate and time horizon
The base-case analysis was carried out from the Chinese societal perspective. Following the recommendation given in China Guidelines for the Economic Evaluation of Health Technologies,17 the discount rate for costs and health outcomes was 3% per year. In sensitivity analysis, discount rate had the range from 0% to 5% per year. The median follow-up time available from KEYNOTE-189 was 23.1 months. Extrapolation of survival data was necessary to accommodate patients’ lifetime to ensure important differences in cost-effectiveness analysis. Accordingly, 20 years was chosen as the time horizon for base-case analysis.
Outcomes
Efficacy inputs
The Kaplan-Meier curve was selected to be the appropriate parametric model. Over the model time horizon, the data of OS and PFS extrapolated outcomes. We previously followed a parametric model established by Insinga et al.8 As mentioned in their article, for PFS the Weibull and log-normal functions were the most appropriate for the pembrolizumab-combination group, and the Weibull function was the best fit for the placebo-combination group. For OS, the exponential distribution was selected as the most suitable for the two treatment groups.
Safety inputs
All-cause adverse events (AEs) included in the base-case analysis were of grade ≥3 and frequency ≥5% in KEYNOTE-189. All AEs were calculated at the initial stage of treatment for simplification of this model.
Utility inputs
Utility values were estimated according to EuroQoL-5 Dimensions, 3 Levels (EQ-5D 3L) data gathered from patients enrolled. The time-to-death approach, previously presented by Huang et al 10 and Insinga et al 8 for metastatic NSCLC, reflected the decline in these patients’ quality of life following disease progression. See table 1 for KEYNOTE-189 utility scores classified by time-to-death.
Table 1.
Key input data of the model
| Utility values by time-to-death (pooled treatment groups from KEYNOTE-189) | ||
| Time-to-death (days) | n* | Utilities (95% CI) |
| ≥360 | 184 | 0.834 (0.823 to 0.846) |
| 180 to 360 | 94 | 0.765 (0.743 to 0.786) |
| 30 to 180 | 167 | 0.709 (0.690 to 0.728) |
| <30 | 32 | 0.563 (0.461 to 0.665) |
| Unit cost (2019 China) | ||
| Drug acquisition | ||
| Drugs | Dose | Cost per dose (US$) |
| Carboplatin | 500 mg | 38.50 |
| Cisplatin | 75 mg/m2 | 13.90 |
| Pembrolizumab | 200 mg | 5194.00 |
| Pemetrexed | 500 mg/m2 | 754.60 |
| Drug administration costs | 48.90 | |
| Costs of anti-emetic prophylaxis (ondansetron/tropisetron/palonosetron) | 43.50 | |
| Disease management costs | |||
| Pembrolizumab-combination group | Placebo-combination group | ||
| Weekly cost of disease management in PFS | $136.43 | $175.50 | |
| Weekly cost of disease management in PD | $523.50 | ||
| Terminal care (the last 30 days of life) | $2464.50 | ||
| Average costs of post-discontinuation treatment | |||
| Following pembrolizumab-combination group | $5234.78 | ||
| Following placebo-combination group | $23 642.45 | ||
| Costs and incidence of relevant adverse events (grade 3+) | |||
| Adverse event | Adverse event | Cost (US$) | |
| Pembrolizumab-combination group (%) | Placebo-combination group (%) | ||
| Anaemia | 16.30 | 15.30 | 1380 |
| Asthenia | 6.20 | 3.50 | 141 |
| Diarrhoea | 5.20 | 3.00 | 691 |
| Dyspnoea | 3.70 | 5.40 | 126 |
| Fatigue | 5.70 | 2.50 | 124 |
| Nausea/vomiting | 7.20 | 6.50 | 188 |
| Neutropenia | 15.80 | 11.90 | 1920 |
| Pneumonitis | 2.70 | 2.00 | 2105 |
| Thrombocytopenia | 7.90 | 6.90 | 1208 |
*Number of patients with non-missing EQ-5D 3L index score.
PD, progressive disease; PFS, progression-free survival.
Cost inputs
In this article, the cost inputs taken into account in the cost-effectiveness analysis are summarised in table 1. The prices of drugs, diagnosis and therapies on the list were due to standard fee data from Tianjin Union Medical Center in 2019, thus they were representative in most Chinese hospitals. We translated all the costs to US dollars according to the exchange rate of US$1=6.90 Chinese yuan at June 2019.
Now we considered the drug acquisition costs. Drug consumption was according to the dosing schedule described in KEYNOTE-189. The public hospitals in China implemented a policy that the selling price of drugs was in accordance with the purchasing price of drugs. The available specification of pembrolizumab was 100 mg per vial, and the list price was $2597 per 100 mg vial. The dose of pembrolizumab was 200 mg; therefore, the cost was $5194 per dose. The average dose of carboplatin was estimated to be 500 mg. Dosage of cisplatin and pemetrexed were based on patients’ body surface area. For Asian somatotypes, patients were assumed to be 65 kg weight and 1.64 m height.18 The costs for cisplatin, carboplatin and pemetrexed were evaluated as $13.9, $38.5 and $754.6 per dose, respectively. According to Chinese guidelines, the patients in China administered vitamin B12 and folic acid which were used as prophylaxis for pemetrexed toxicity. The price of vitamin B12 injection is $0.06 per 0.5 mg and the price of folic acid is $1.83 per 0.4 mg*60 tablets, which were not enough to be taken into account.
Drug administration costs included intravenous infusions and pharmacy intravenous admixture services, which are shown in table 1. Costs of anti-emetic prophylaxis for platinum were estimated at $43.5 per cycle.
Disease management costs were incurred in both PFS and PD. The common costs included blood tests, chest X-ray, abdominal CT scan, radiation therapy, home healthcare, nurse, medical specialists and hospital fees. In general, PD was associated with more hospital fees than PFS, especially in emergency department and ICU.
Post-initial trial therapies and outcomes were collected based on updated report (2020) of KEYNOTE-189.7 After progression, 53.9% of placebo-combination patients switched to a PD-1/PD-L1 agent, and 40.8% of placebo-combination patients switched to pembrolizumab-combination regimen. Moreover, 31.2% of patients in the pembrolizumab-combination arm switched to second-line chemotherapy following progression.7 The cumulative costs for subsequent therapies were also estimated.
Incidence and costs of selected AEs (grade ≥3) are listed in table 1, which were estimated within the KEYNOTE-189 trial and update.6 7 Cost per event included medications, outpatient visits and/or hospitalisation. According to incidence of AEs and related costs, the total average cost for each one in AEs management was evaluated as $751 for pembrolizumab combination and $613 for placebo combination.
Variability and uncertainty
Subgroup analyses
The base-case analysis involved the whole trial population regardless of PD-L1 status. However, cost-effectiveness was analysed for subgroups of patients with PD-L1 tumour proportion scores (TPS) ≥50%, 1%–49% and <1%.
Sensitivity analyses
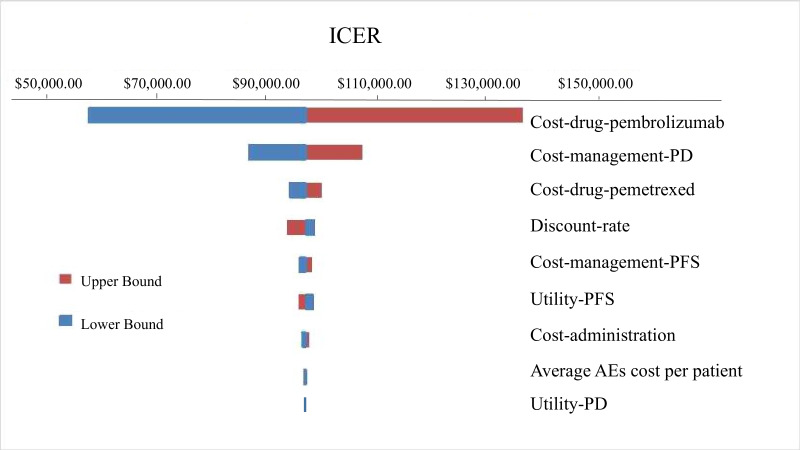
We performed one-way sensitivity analysis in the next section. Some kinds of cost, utility-PFS and utility-PD were varied to explore their influences. These results are shown in figure 2 as a tornado diagram. Scenario analysis examined the effect of philanthropic support programme given by manufacturers on the results.
Figure 2.
Tornado diagram for the ICER per QALY of pembrolizumab plus platinum-based chemotherapy versus chemotherapy alone.
Results
Base-case analysis
In this fundamental analysis, results for the entire population over the time horizon of 20 years are provided in table 2. The total costs of pembrolizumab-combination arm and placebo-combination arm were estimated at $212 249 and $73 081, respectively. The pembrolizumab-combination provided 1.73 LYs and 1.44 QALYs more than placebo combination. Hence, the ICER of pembrolizumab combination versus placebo combination was estimated at $80 444/LY and $96 644/QALY, which exceeds the threshold of $28 106 (three times the GDP in China). Overall, we consider that pembrolizumab plus platinum-based chemotherapy is not a cost-effective scenario for first-line treatment in metastatic NSCLC, compared with chemotherapy alone from the Chinese societal perspective.
Table 2.
Base-case results
| Pembrolizumab-combination group | Placebo-combination group | |
| Life-years | 3.51 | 1.78 |
| Expected time in progression-free state (years) | 1.26 | 0.63 |
| Expected time in progressive state (years) | 2.25 | 1.15 |
| QALYs | 2.84 | 1.4 |
| Costs | $212 249 | $73 081 |
| Drug acquisition cost | $130 974 | $8406 |
| Pre-medication cost | $174 | $174 |
| Drug administration cost | $2543 | $929 |
| Disease management cost | $70 108 | $36 852 |
| Post-discontinuation therapy cost | $5235 | $23 642 |
| Terminal care cost | $2465 | $2465 |
| AEs cost (per patient) | $751 | $613 |
| Incremental cost-effectiveness ratio | ||
| Cost per life-year gained | $80 444 | |
| Cost per QALY gained | $96 644 | |
AE, adverse event; QALY, quality-adjusted life-year.
PD-L1 subgroup analyses
PD-L1 subgroup analyses are shown in table 3. While the ICER was $109 229/QALY for patients with PD-L1 TPS <1%, the ICER was $91 399/QALY and $90 419/QALY for patients with PD-L1 TPS 1%–49% and ≥50%, respectively.
Table 3.
PD-L1 subgroup results
| PD-L1 TPS <1% | 1%≦PD-L1 TPS<49% | PD-L1 TPS≥50% | ||||
| Pembrolizumab-combination group | Placebo-combination group | Pembrolizumab-combination group | Placebo-combination group | Pembrolizumab-combination group | Placebo-combination group | |
| Life-years | 2.37 | 1.82 | 4.26 | 1.86 | 3.98 | 1.74 |
| Time in PFS | 0.71 | 0.56 | 1.93 | 0.88 | 1.81 | 0.58 |
| Time in PD | 1.66 | 1.26 | 2.32 | 0.98 | 2.16 | 1.16 |
| QALYs | 1.89 | 1.44 | 3.47 | 1.47 | 3.24 | 1.37 |
| Costs | $123 989 | $74 835 | $256 949 | $74 151 | $241 379 | $72 296 |
| ICER | ||||||
| Cost per life-year gained | $89 369 | $76 166 | $75 484 | |||
| Cost per QALY gained | $109 229 | $91 399 | $90 419 | |||
ICER, incremental cost-effectiveness ratio; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression-free survival; QALY, quality-adjusted life-year; TPS, tumour proportion scale.
Sensitivity analysis
We varied the cost across a range of ±50%, utility-PFS and utility-PD in 95% CI and the discount at a rate between 0% and 6% in one-way sensitivity analysis. These results are presented in figure 2 as a tornado diagram. The ICER generally ranges from $93 186 to $99 527 with variation in most parameters; the upper bound and the lower bound appear at $56 968 and $136 320, respectively. As shown in figure 2, the price of pembrolizumab was the most influential factor in our study. We could also search the optimal price of pembrolizumab by one-way sensitivity analysis. The results indicated that pembrolizumab combination could be cost-effective if its price was nearly $707 per dose.
Scenario-based sensitivity analysis was also performed. Merck Sharp & Dohme Ltd implemented a philanthropic support programme in China. After patients purchased pembrolizumab, they could receive the same amount of drug by donations. The ICER was $47 419/LY and $56 968/QALY with philanthropic support programme. Overall, the analysis exhibited a similar result to the base-case analysis.
Discussion
This study was based on the scheme of PD-L1 combination with chemotherapy, unlike the previous studies19 20 in China, in which they researched the scheme of PD-L1 monotherapy. In fact, due to a significant number of patients who lack high PD-L1 expression, the schemes of combination therapy are also common. Also, the previous studies19 20 used the Markov model, and we used partitioned-survival model.
The addition of pembrolizumab to current standard chemotherapy was predicted to obtain 1.73 years longer OS and 1.44 years longer PFS than those treated with chemotherapy alone. Over a 20-year time horizon, the ICER of pembrolizumab-combination group versus placebo-combination group was evaluated as $96 644/QALY gained and $80 444/LY gained. At present, there is no consensus on the threshold of the cost-effective ratio in China. Thus, we adopt the following recommendation given by WHO: the threshold of the cost-effectiveness might be three times the GDP of China in 2018, that is, $28 106/QALY. Hence, according to the results in the aforementioned argument, pembrolizumab plus platinum and pemetrexed does not appear to be cost-effective in China. In PD-L1 TPS ≥50%, 1%–49% subgroups, pembrolizumab-combination groups presented obvious clinical benefits and more than doubled QALY as compared with the placebo-combination groups. In PD-L1 TPS <1% subgroup, QALY increased by 0.45 years for patients using pembrolizumab plus chemotherapy as compared with chemotherapy. Within PD-L1 TPS ≥50% group ($90 419/QALY) and 1%–49% group ($91 399/QALY), ICERs are less than those in the full trial population. Also, it is shown that ICER is relatively more for patients with PD-L1 expression <1% ($109 229/QALY). As a result, we did not recognise pembrolizumab plus chemotherapy as a cost-effective choice compared with standard chemotherapy for patients with metastatic NSCLC, regardless of TPS.
This work included one-way sensitivity analyses and scenario-based sensitivity analysis. Sensitivity analyses showed that the results were robust within reasonable ranges of discount rates, utility weights and costs of input included.
The key driver of the increased costs of pembrolizumab-combination group was acquisition cost of pembrolizumab. Through government-led price negotiations and the centralised procurement of medical institutions, the prices of other chemotherapeutic drugs had been substantially reduced. Therefore, the price of pembrolizumab had the most prominent impact on the drug acquisition cost. The second important factor was cost of disease management at the stage of PD. In fact, the cost of disease management reflected clinical practice of metastatic NSCLC in China, but the impact on ICER was also related to the prolongation of LY in PD.
This analysis has a few limitations, mainly owing to data availability and model assumptions. Patients enrolled in the clinical trial met specific inclusion criteria; consequently, the real clinical effects were not clear. Crossover was allowed when primary drugs failed, which may disturb our observation of differences between the two groups in clinical effects. Although the median OS for pembrolizumab-combination group was 22.0 months,7 the average QALY earnings may be heavily influenced by the patients who live longer. The AEs of grade <3 and/or incidence frequency <5% were not included in the model; sensitivity analysis of costs of AEs indicated that ICER was not sensitive to variation in AE costs. However, pembrolizumab can cause immune-related AEs, for example, immune-mediated type 1 diabetes mellitus, which would seriously affect the quality of life for a lifetime. Such immune-mediated AEs were not included in view of the low incidence, but it is worthy of long-term follow-up attention. In order to more accurately reflect survival benefits associated with treatment, real-world research is still needed.
In April 2019, pembrolizumab plus platinum and pemetrexed had been approved by NMPA of China for the first-line treatment of patients with metastatic NSCLC, without EGFR or ALK genetic aberrations. Its clinical application will inevitably increase as a first-line treatment. This study provides a reference for the choice of therapeutic regimen for doctors and also for the establishment of medical insurance policy. Moreover, it will supply experience for introduction and approval of pembrolizumab in other countries which have similar national conditions and economic level to China.
It is interesting to evaluate the cost-effectiveness of pembrolizumab since every country is concerned with this problem. In the USA, based on KEYNOTE-189, Insinga et al 8 gave that ICERs were $104 823/QALY and $87 242/LY in overall trial population. They also gave that ICERs were $103 402/QALY, $66 837/QALY and $183 529/QALY for PD-L1 TPS ≧50%, 1%–49% and <1% groups, respectively. On the basis of the WTP of $180 000/QALY, pembrolizumab in combination with chemotherapy could be a cost-effective treatment in the overall trial population, as well as by PD-L1 subgroups. In France, based on the KEYNOTE-024, Chouaid et al 11 estimated the ICER of pembrolizumab versus platinum-based chemotherapy with pemetrexed at €78 729/QALY. On the basis of the WTP of €100 000/QALY, pembrolizumab appeared cost-effective compared with platinum-based chemotherapy in patients with NSCLC expressing high levels of PD-L1 (TPS≥50%). In the UK, based on the KEYNOTE-024, Hu and Hay21 obtained the ICER of £86 913/QALY, which means pembrolizumab was not cost-effective according to the WTP of £50 000/QALY. Based on the KEYNOTE-024, Liao et al 19 concluded pembrolizumab gained an ICER of $103 128/QALY, which was not a cost-effective first-line treatment due to a WTP threshold of $26 481/QALY in China. Based on the KEYNOTE-042, Zhou et al 20 showed pembrolizumab monotherapy gained ICER of $36 493/QALY, $42 311/QALY and $39 404/QALY in China for patients with TPS ≥50%, ≥20% and ≥1%, respectively. It implies that pembrolizumab monotherapy was not a cost-effective choice compared with standard chemotherapy in China, regardless of TPS.
Conclusions
Pembrolizumab plus platinum and pemetrexed chemotherapy has been confirmed to significantly improve OS and PFS for patients with metastatic NSCLC. However, the results in this paper suggest that from a Chinese societal perspective, this therapeutic regimen seems to be not cost-effective at the current price of pembrolizumab.
What this paper adds.
What is already known on this subject
In April 2019, pembrolizumab has been approved as a first-line treatment of metastatic non–small-cell lung cancer (NSCLC), coupled with platinum-based drugs in China.
From a US healthcare payer perspective, pembrolizumab plus chemotherapy is cost-effective as first-line treatment for eligible patients with metastatic non-squamous NSCLC.
What this study adds
There is a lack of economic evaluation of this new treatment regimen. To our knowledge, we first performed partitional survival analysis to examine the cost-effectiveness of pembrolizumab plus standard chemotherapy versus chemotherapy alone for first-line treatment of metastatic non-squamous NSCLC from a Chinese societal perspective.
This study provides a reference for the choice of therapeutic regimen for doctors and also for the establishment of medical insurance policy. Moreover, it will supply experience for introduction and approval of pembrolizumab in other countries which have similar national conditions and economic level to China.
Footnotes
Contributors: All authors contributed to the design of the study and data analysis. YJ contributed to data collection. XW wrote the manuscript. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3. Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol 2009;471:469–86. 10.1007/978-1-59745-416-2_24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen VW, Ruiz BA, Hsieh M-C, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014;120:3781–92. 10.1002/cncr.29045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abernethy AP, Arunachalam A, Burke T, et al. Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS One 2017;12:e0178420. 10.1371/journal.pone.0178420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 7. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2020;38:1505–17. 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 8. Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ 2018;21:1191–205. 10.1080/13696998.2018.1521416 [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 10. Huang M, Lou Y, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics 2017;35:831–44. 10.1007/s40273-017-0527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer 2019;127:44–52. 10.1016/j.lungcan.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 12. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019;10:3–7. 10.1111/1759-7714.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Y, Fang W, Zhang Y, et al. KEYNOTE-032: a randomized phase I study of pembrolizumab in Chinese patients with advanced non-small-cell lung cancer. Oncologist 2020;25:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimizu T, Seto T, Hirai F, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs 2016;34:347–54. 10.1007/s10637-016-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Y, Zhang L, Fan Y, et al. MA11.02 KEYNOTE-042 China Study: first-line pembrolizumab vs in Chinese patients with NSCLC with PD-L1 TPS ≥1%. J Thorac Oncol 2019;14:S290–1. 10.1016/j.jtho.2019.08.584 [DOI] [Google Scholar]
- 16. Cheng Y, Zhang L, Hu J, et al. Keynote-407 China extension study: pembrolizumab (pembro) plus chemotherapy in Chinese patients with metastatic squamous NSCLC. Ann Oncol 2019;30:ix201–2. 10.1093/annonc/mdz446.019 [DOI] [Google Scholar]
- 17. Liu GE. China guidelines for pharmacoeconomic evaluations and manual. Beijing: Science Press, 2016: 20–6. [Google Scholar]
- 18. Zhang P, Wen F, Fu P, et al. Addition of docetaxel and/or zoledronic acid to standard of care for hormone-naive prostate cancer: a cost-effectiveness analysis. Tumori 2017;103:380–6. 10.5301/tj.5000583 [DOI] [PubMed] [Google Scholar]
- 19. Liao W, Huang J, Hutton D, et al. Cost-effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J Med Econ 2019;22:344–9. 10.1080/13696998.2019.1570221 [DOI] [PubMed] [Google Scholar]
- 20. Zhou K, Jiang C, Li Q. Cost-effectiveness analysis of pembrolizumab monotherapy and chemotherapy in the non-small-cell lung cancer with different PD-L1 tumor proportion scores. Lung Cancer 2019;136:98–101. 10.1016/j.lungcan.2019.08.028 [DOI] [PubMed] [Google Scholar]
- 21. Hu X, Hay JW. First-line pembrolizumab in PD-L1 positive non-small-cell lung cancer: a cost-effectiveness analysis from the UK health care perspective. Lung Cancer 2018;123:166–71. 10.1016/j.lungcan.2018.07.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article.