Abstract
The pharmacodynamic and pharmacokinetic properties of trovafloxacin were studied in a standardized murine model of established subcutaneous abscesses. Daily dosing regimens of 37.5 to 300 mg/kg every 8 h (q8h) or every 24 h (q24h) were started 3 days after inoculation with mixtures containing either Bacteroides fragilis-Escherichia coli-autoclaved cecal contents (ACC) or B. fragilis–vancomycin-resistant Enterococcus faecium (VREF)–ACC. Treatment was continued for 3 or 5 days. The efficacy of treatment was determined by the decrease in abscess bacterial counts and abscess weights, as well as by the reduction in inflammation (biodistribution of 99mTc-HYNIC immunoglobulin G) compared to saline-treated controls. Trovafloxacin showed a significant dose-response effect on the bacterial counts, weight, and inflammation of B. fragilis-E. coli abscesses after 3 and/or 5 days of treatment. A maximum 3.4 and 3.1 log10 reduction in CFU/abscess in the respective B. fragilis and E. coli bacterial counts was attained after 5 days of treatment with daily doses of 300 mg/kg. The peak serum concentration was more predictive for effect than the area under the concentration-time curve. The Cmax was the pharmacodynamic index most predictive for success, and the efficacy of the q24h regimens was significantly better than the q8h regimens. The antibiotic was ineffective against the VREF in mixed infection with B. fragilis, while the killing of the anaerobe in the same combination was significantly less than in the E. coli combination (P < 0.05). We conclude that this is a useful model for studying the activity of antimicrobials for the treatment of small (<1-cm), undrainable, mixed-infection abscesses. In addition, we have shown for the first time that a decrease in bacterial numbers also leads to a reduction in both abscess weight and inflammation.
The formation of intra-abdominal abscesses (IAA) after peritoneal contamination is the end result of an inflammatory process aimed at containing the spread of infection. However, as a consequence, phagocytosis and the clearance of microorganisms are impaired (8). Once established, IAA are very difficult to treat and continue to be associated with high rates of morbidity and mortality despite improvement in antimicrobial therapy and drainage procedures (17). Furthermore, when abscesses are multiple and/or small, antibiotic treatment is the only option to clear the infection.
IAA are mostly polymicrobial infections resulting from a synergistic association between facultative species and anaerobic species, with Escherichia coli and Bacteroides fragilis being the most frequently isolated strains (2). Enterococci are found in 10 to 20% cases of IAA. Although their pathogenic role in these mixed infections is not fully understood (7, 14), enterococci can play a role after selection by antimicrobial therapy in secondary peritonitis and residual abscesses. Furthermore, in immunocompromised or debilitated patients, there is great concern with regard to resistant strains, especially those resistant to vancomycin, and the subsequent difficulty in treating infections caused by these microorganisms (13).
Dual or even triple therapy effective against all bacterial components of an abscess has been used in the treatment of IAA, since failure to treat either the facultative or the anaerobe strains can lead to disappointing results (12). Other reasons for the failure of conservative treatments are limited penetration of antibiotics into the abscess or reduced activity of certain antibiotics under the environmental conditions present in the abscesses (low redox potential, low pH, high bacterial counts, debris binding antimicrobial agents, and enzymes protecting the bacteria) (12).
Trovafloxacin is a third-generation fluoroquinolone with a broad spectrum of activity against both gram-negative and gram-positive aerobes, as well as against vancomycin-resistant enterococci and anaerobes (19). It is virtually unaffected by changes in the pH, increases in inoculum size, or changes in anaerobic conditions (3) and is active against cultures of nondividing cells (15, 24). All of these properties would indicate that trovafloxacin is an agent of choice for treating IAA. Recent studies (18, 27) have found trovafloxacin to be effective in animal models of mixed infection in protecting animals from lethal infection, preventing IAA formation and inhibiting bacterial growth. However, in these studies, treatment was started early (4 h after inoculation) and therefore before abscesses had been properly established.
The aim of the present study was to determine the efficacy of trovafloxacin in the treatment of small (<1-cm), undrainable, mixed-infection abscesses by employing a murine subcutaneous abscess model. In this model, abscesses were allowed to develop for 3 days and become well established before initiation of therapy. Two different combinations of microorganisms, B. fragilis-E. coli and B. fragilis–vancomycin-resistant Enterococcus faecium (VREF), were employed. The parameters of efficacy were reduction in bacterial counts and reduction in abscess size (weight). In addition, we investigated whether an eventual reduction in abscess weight and/or in bacterial counts by antimicrobial treatment would also lead to a reduction in inflammation in this model.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, abstr. 800, p. 58, 1999.)
MATERIALS AND METHODS
Antibiotic and media.
Trovafloxacin (alatrovafloxacin mesylate, pure powder [CP-116,517-27 lot number 34307-098-04] and Trovan) was supplied by Pfizer Inc. (Groton, Conn.) and Pfizer B.V. (Capelle a/d IJssel, The Netherlands). Wilkens Chalgren (WC) broth, WC agar, eosine methylene blue (EM) agar, brain heart infusion, and diagnostic sensitivity test (DST) agar were all supplied by Unipath, Ltd. (Haarlem, The Netherlands). Columbia blood agar plates were from Becton Dickinson B.V. (Woerden, The Netherlands).
Bacterial strains.
B. fragilis ATCC 23745, E. coli ATCC 25922, and a vancomycin-and amoxicillin-resistant clinical isolate E. faecium BM 4147 (i.e., VREF) were used. All strains were first passaged in BALB/c mice and standardized suspensions were made and frozen at −80°C until required. Overnight cultures were obtained by inoculating 30-ml volumes of WC broth with 0.1 ml of the standardized frozen bacterial suspensions and incubating the mixtures aerobically (E. coli and VREF) or anaerobically (B. fragilis) at 37°C for 18 h.
Animals.
Female specific-pathogen-free (SPF) BALB/c mice (IFFA Credo, l'Arbresle, France), 12 to 18 weeks old and weighing 20 to 25 g, were used throughout the study. The cecal contents from male SPF Swiss mice (Broekman Institute B.V., Someren, The Netherlands) were used for the production of autoclaved cecal contents (ACC) (see below). All animals received water and food ad libitum. The study was approved by the Institutional Animal Care and Use Committee of the Erasmus University, Rotterdam, The Netherlands.
ACC.
ACC were obtained as previously described (11). Briefly, the cecal contents were removed from 100 mice, diluted 1:4 in WC broth, homogenized, and filtered. The suspension was autoclaved in 5-ml volumes at 121°C for 2 h and stored at −80°C. Batches were standardized by measuring the dry weight.
Mouse model.
The model of Joiner et al. (11) was adapted and standardized. Inocula were prepared by diluting overnight cultures of B. fragilis and either E. coli or VREF in WC broth, which were then mixed together with ACC in a ratio 1:1:2. The final inocula contained B. fragilis (107 CFU), E. coli (105 CFU) (or VREF [107 CFU]), and ACC (4 mg, dry weight) in a total volume of 0.25 ml. Mice were anesthetized with a 70-mg/kg dose of pentobarbitol sodium given intraperitoneally (Sanofi Diagnostics Pasteur BV, Maassluis, The Netherlands), and both flanks were shaved and depilated. Groups of three to five BALB/c mice were inoculated subcutaneously on both flanks with the 0.25-ml mixtures of B. fragilis and ACC and either E. coli or VREF. At different times after inoculation (12 h to 8 days), mice were killed by CO2 asphyxiation, and the abscesses were dissected, weighed, and homogenized in 1 ml of phosphate-buffered saline (PBS) for 10 s (Pro 200; B.V. Centraal Magazijn, Abcoude, The Netherlands). Bacterial counts were performed on the resulting suspensions by making duplicate serial 10-fold dilutions in PBS and plating 20 μl of each dilution onto EM agar (E. coli), blood agar (VREF), or WC agar containing 100 mg of gentamicin per liter (B. fragilis). Plates were incubated at 37°C aerobically for 24 h (EM or blood agar) or anaerobically for 48 h (WC agar). Counts were expressed as the log10 CFU/abscess.
Pharmacokinetic studies.
Single-dose pharmacokinetic studies with a 150-mg/kg dose of trovafloxacin were performed on groups of three to six mice 3 days after inoculation with B. fragilis-E. coli. Blood was removed by orbital puncture and collected in serum-gel microtubes (Sarstedt B.V., Etten Leur, The Netherlands). Abscesses were dissected and homogenized in 0.5 ml of PBS and centrifuged at 13,000 × g for 1 min, and the antibiotic concentrations were measured in the resulting supernatants.
Trovafloxacin concentrations were determined in duplicate by the agar diffusion bioassay. Standard concentrations were prepared in mouse serum or abscess homogenate which had been spiked with twofold increasing trovafloxacin concentrations. Abscess homogenates were further centrifuged, and the supernatants were used in the assay. Test samples containing high concentrations of antibiotic were first diluted in serum or abscess homogenate and similarly processed. DST agar plates were inoculated with Bacillus subtilis, and 8.5-mm wells were cut. Each well was filled with 50 μl of standard or test sample, and the plates were incubated overnight at 37°C. The zones of inhibition were read to the nearest 0.05 mm using vernier calipers. Trovafloxacin concentrations were calculated by linear regression. The standard curves were linear within the range 0.2 to 1.6 μg/ml, with an r2 of 0.980 to 0.999 (median, 0.997). The assay variability was determined on two to four replicate measurements of samples containing 0.27, 0.53, or 1.07 μg of trovafloxacin per ml in serum or abscess homogenate, and the test was repeated on a separate day. There was good linear correlation between the observed and expected values (r2 = 0.91). The median within-run coefficient of variation was 9.4% (range, 0 to 23%) and the between-run coefficient was 13.9% (range, 8.8 to 24.4%).
Pharmacokinetic parameters were determined using the MW/Pharm computer program package (Mediware, Groningen, The Netherlands) (22) with a one-compartment open model. The obtained parameters were used to simulate various dosing regimens and determine pharmacokinetic properties of each regimen, such as the Cmax and the area under the concentration-time curve (AUC) during multiple dosing regimens.
Serum protein binding.
The serum protein binding of trovafloxacin was determined by ultrafiltration. Standards (1 ml) at concentrations of 1, 5, and 10 μg/ml in mouse serum were filtered through a Unisep Ultracent-30 (molecular weight cutoff, 30,000) ultrafilter (Bio-Rad, Veenendaal, The Netherlands) according to the manufacturer's instructions. Filters were first rinsed by centrifugation of 1.5 ml of PBS at 2,000 × g for 30 min. Ultrafiltration was similarly performed on the standard trovafloxacin concentrations in mouse serum. Filter binding was determined by comparing the drug concentrations in ultrafiltrates prepared in PBS with those prepared in spiked PBS. Protein binding was adjusted to account for the binding to the filter. Trovafloxacin concentrations were determined by the bioassay described above.
Antibiotic treatment.
All three strains employed in the present study were susceptible to trovafloxacin, with MICs of 0.25, 0.06, and 1 μg/ml for B. fragilis, E. coli, and VREF, respectively. However, there was a 4- to 8-fold (B. fragilis and E. coli) and a >64-fold (VREF) increase in the MICs when the inoculum was increased to 108 CFU/ml (24). Treatment of established abscesses was started 3 days after inoculation with either B. fragilis-E. coli or B. fragilis-VREF to groups of six mice by subcutaneous injections. Total daily doses of trovafloxacin ranged from 37.5 to 300 mg/kg/day administered every 8 h or every 24 h (q8h or q24h) for 3 or 5 days. Control animals were included in each experiment and received subcutaneous injections of pyrogen-free saline. The efficacy of the treatment was determined by measuring the decrease in abscess bacterial counts and abscess weights, as well as the reduction in inflammation, compared to saline-treated controls.
Biodistribution of 99mTc labeled nonspecific human IgG.
The HYNIC immunoglobulin G (IgG) conjugate was prepared and radiolabeled with 99mTc as described previously (5). The labeling efficiency was >95%. To determine the efficacy of trovafloxacin in the reduction of abscess inflammation, groups of six to eight mice were inoculated only on the right flank with B. fragilis-E. coli-ACC. The skin of the left flank was used as control. After 3 days, treatment with trovafloxacin (37.5, 75, or 150 mg/kg/24 h) or pyrogen-free saline was started and continued for 5 days. At 24 h before dissection, mice were injected via the tail vein with 1 MBq of 99mTc-HYNIC IgG. Mice were killed by CO2 asphyxiation, and the complete pelt was removed from each animal. Abscesses (including a section of surrounding skin) were removed using an 18-mm punch, and an identical sized segment of skin was similarly dissected from the uninfected left flank. Abscess and skin samples were weighed, and their activity was counted in a gamma counter (Minaxe 5000 Autogamma Series; Packard). To correct for physical decay and to determine the fraction of injected 99mTc-HYNIC IgG taken up by each sample, aliquots of the injected dose were counted simultaneously. The percentage of injected dose per abscess or per skin section was determined.
Statistics.
Abscess weights are given as the mean ± the standard error of the mean (SEM). All bacterial counts are given as the mean ± the SEM log10 CFU/abscess. The tissue distribution of 99mTc-HYNIC IgG in mice is expressed as the mean ± the standard deviation (SD) abscess/skin ratio. The relationship between trovafloxacin doses and the resulting abscess/skin ratios was analyzed by linear correlation. To determine the pharmacodynamic index explaining most of the effect, a stepwise regression analysis was performed using the REG procedure from the SAS computer program package (23). To determine whether there was a significant difference between dosing regimens q8h and q24h, a multiple regression analysis was performed. A P value (two sided) of <0.05 was considered significant.
RESULTS
Development of abscesses.
Mice inoculated on both flanks developed two separate subcutaneous abscesses at the site of inoculation. Inocula contained an average of 6.9 ± 0.2 log10 CFU (B. fragilis), 4.8 ± 0.1 log10 CFU (E. coli), and 7.0 ± 0 log10 CFU (VREF). Untreated animals retained normal activity during the 8-day observation period, with no mortality. Of all the inoculations, 95% developed successfully into abscesses. In the remaining 5%, the overlying skin became necrotic with external leakage of pus. These inoculations were considered technical failures and therefore excluded from further evaluation before day 3. Similarly, by >8 days after inoculation, some abscesses could partially resolve or drain externally. Consequently, examination of the abscesses was limited to 8 days. In all experiments, each datum point represents the average of at least six abscesses.
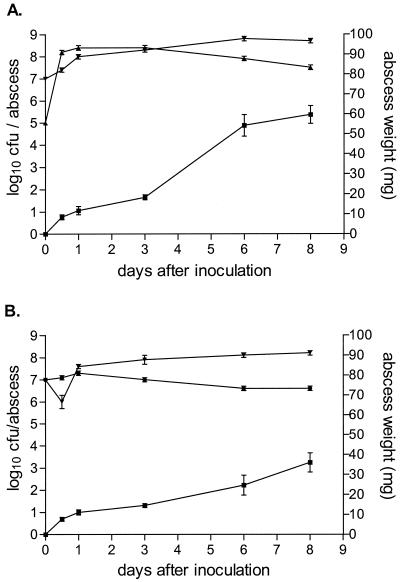
Figure 1 shows the development of B. fragilis-E. coli (Fig. 1A) and B. fragilis-VREF (Fig. 1B) abscesses in BALB/c mice. Histologically confirmed abscesses developed within 24 h of inoculation. Distinct encapsulation was present at day 3. There were no histological differences found between the abscesses of the two different bacterial combinations. Due to variations found in the shape and compartmentalization of different abscesses, abscess weight was chosen as a more accurate and reproducible parameter of abscess size in contrast to the planimetry method employed by Joiner et al. (11). Abscesses continued to increase in size, reaching a diameter of 5 to 6 mm and an average weight of 60 ± 4.5 mg (B. fragilis-E. coli) or 36 ± 4.8 mg (B. fragilis-VREF) 8 days after inoculation. There was a significant difference between the weight of abscesses with the different bacterial combinations on days 3, 6, and 8 (P ≤ 0.03). Bacterial counts in the model with B. fragilis and E. coli increased within the first 24 h to 8.0 and 8.4 log10 CFU/abscess, respectively, and remained relatively stable until day 8, when the respective counts were 8.7 and 7.5 log10 CFU/abscess. In the B. fragilis-VREF combination, an initial decrease in B. fragilis counts to 6.0 log10 CFU/abscess 12 h after inoculation was followed by a steady increase in bacterial counts to 8.2 log10 CFU/abscess on day 8. However, the B. fragilis counts remained significantly lower in the combination with VREF than with the E. coli combination (P = 0.003). Bacterial counts for VREF remained approximately 7.0 log10 CFU/abscess throughout the 8-day experiment.
FIG. 1.
Development of abscesses in BALB/c mice inoculated with B. fragilis ATCC 23745 and either E. coli ATCC 25922 (A) or VREF BM4147 (B). The mean ± the SEM is given for abscess weights (■) and for B. fragilis (▾), E. coli (▴), and VREF (●) bacterial counts.
Pharmacokinetic studies.
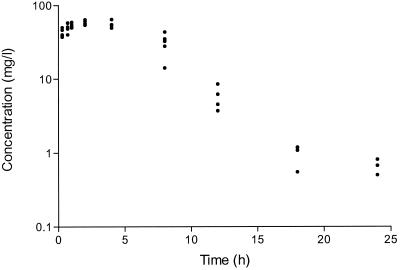
Trovafloxacin concentrations were measured in the serum and abscesses of mice infected with B. fragilis-E. coli and given a single subcutaneous dose of 150 mg/kg. The serum pharmacokinetic data are shown in Fig. 2. A peak trovafloxacin concentration of 67 μg/ml was attained at 1.76 h after administration and the half-life was 2.9 h. The AUC0–24h was 457.7 mg · h/liter. The pharmacokinetic data in abscesses were difficult to interpret due to the extreme variations in the concentrations of trovafloxacin that were measured in the individual abscesses at the same time points (Table 1). This disparity was not only found between the different animals in each group but was also observed between the right and left abscesses within the same mice. There were no significant differences in the weights of the abscesses at each time point. Pharmacokinetic studies performed on mice infected with the B. fragilis-VREF combination produced results similar to those reported above (results not shown).
FIG. 2.
Trovafloxacin concentrations in the serum of mice treated with a single subcutaneous injection of 150 mg/kg 3 days after inoculation with B. fragilis ATCC 23745 and E. coli ATCC 25922. Concentrations were measured in a bioassay with B. subtilis as the test organism.
TABLE 1.
Trovafloxacin concentrations in established subcutaneous abscesses treated with a single subcutaneous injection of 150 mg/kg 3 days after inoculation with B. fragilis ATCC 23745 and E. coli ATCC 25922
| Time (h) | No. of abscesses | Mean abscess wt ± SD (mg) | Trovafloxacin concn (μg/g)a
|
|
|---|---|---|---|---|
| Median | Range | |||
| 0.3 | 5 | 41 ± 6.4 | 30.6 | 2–329 |
| 0.7 | 5 | 36 ± 3.9 | 7.9 | 3–112 |
| 1 | 5 | 38 ± 6.9 | 111.0 | 3–157 |
| 2 | 4 | 29 ± 5.5 | 13.5 | 10–62 |
| 8 | 5 | 34 ± 9.4 | 33.2 | 25–82 |
| 24 | 3 | 32 ± 2.3 | 4.0 | <0.1–4 |
Concentrations were measured in a bioassay with B. subtilis as the test organism.
Serum protein binding.
The in vitro binding values of trovafloxacin to mouse serum proteins were 78.7, 80.6, and 80.0% for the standard concentrations of 1, 5, and 10 μg/ml, respectively.
Effect of trovafloxacin on B. fragilis-E. coli abscess weights.
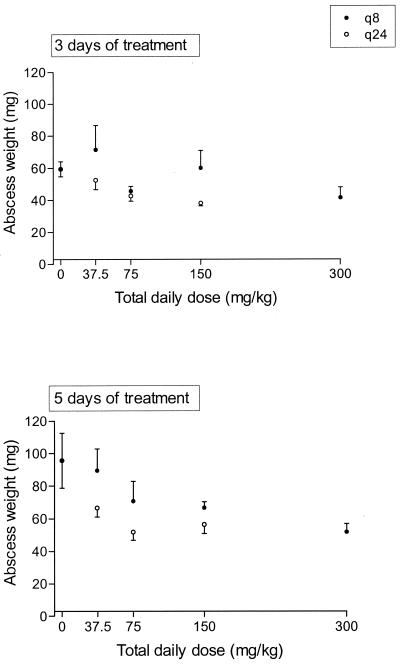
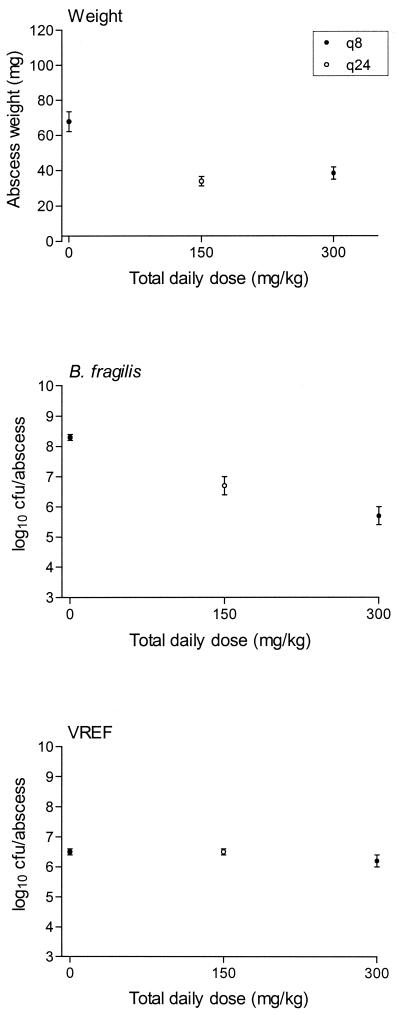
Although large variations in abscess weights were found within the different groups of mice, there was a significant negative correlation between administered total daily doses and abscess weights after both 3 days and 5 days of treatment with trovafloxacin (Fig. 3). There was a difference in effect between dosing regimens q8h and q24h. These differences were significant (P = 0.04 for the 3-day treatment and P < 0.01 for the 5-day treatment).
FIG. 3.
Effect of trovafloxacin on B. fragilis-E. coli abscess weights after 3 and 5 days of treatment with 37.5- to 300-mg/kg q8h or q24h regimens. The mean ± the SEM is given.
Effect of trovafloxacin on B. fragilis-E. coli abscess bacterial counts.
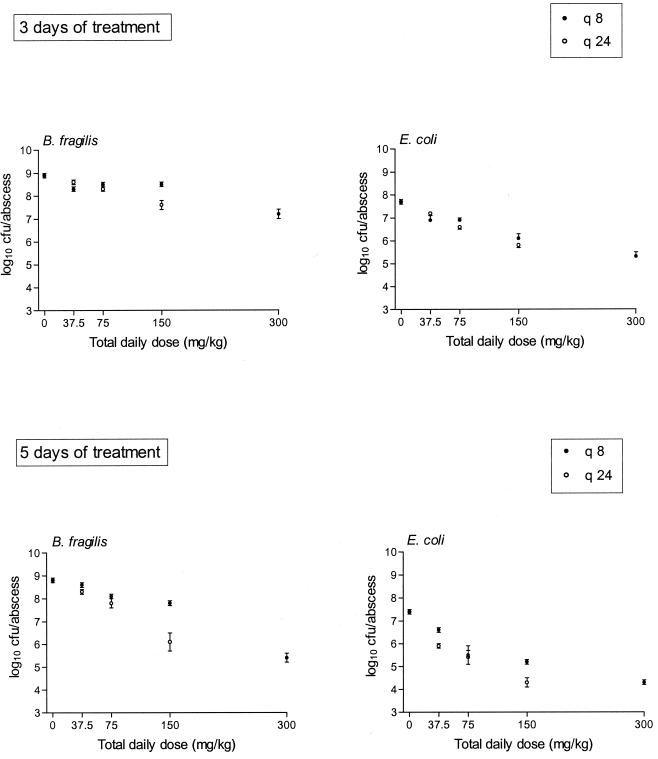
Figure 4 shows the effect of trovafloxacin treatment on bacterial counts of B. fragilis and E. coli abscesses after 3 and 5 days. Similar to the abscess weights, there was a significant negative correlation between effect and total daily dose after both 3 and 5 days of treatment. In addition, there was a significant difference in effect between the dosing regimens q8h and q24h (P < 0.05 for all treatment groups, except for E. coli treated for 3 days [P = 0.06]). Abscess bacterial counts of both strains after 5 days of treatment with trovafloxacin were significantly lower (P < 0.05) than those obtained after 3 days of treatment for all respective regimens except one (37.5 mg/kg q8h).
FIG. 4.
Effect of trovafloxacin on B. fragilis-E. coli abscess bacterial counts after 3 or 5 days of treatment with 37.5- to 300-mg/kg q8h or q24h regimens. The mean ± the SEM is given.
Pharmacodynamic analysis.
To determine which pharmacodynamic index was most important in explaining effect, a regression analysis was carried out (Table 2). The Cmax was the index most predictive for success except for one case. It must be noted that, due to the relatively low MICs, the time above the MIC of the free fraction of the drugs was 100% in the majority of the dosing regimens; therefore, the contribution of this parameter to total effect could not be reliably determined.
TABLE 2.
Main pharmacodynamic parameters explaining effect and coefficients of determination
| Effect measurea | Days of treatment | Pharmacodynamic parameter | r2 | P |
|---|---|---|---|---|
| Wt | 3 | Log (Cmax) | 0.09 | 0.0108 |
| 5 | Log (Cmax) | 0.21 | 0.0005 | |
| ΔCFU B. fragilis | 3 | Log (Cmax) | 0.37 | <0.0001 |
| 5 | Log (Cmax) | 0.61 | <0.0001 | |
| ΔCFU E. coli | 3 | Log (AUC)b | 0.57 | <0.0001 |
| 5 | Log (Cmax) | 0.67 | <0.0001 |
ΔCFU, difference between CFU at the start and at the end of treatment.
For E. coli (3 days of treatment), the log (Cmax) was only slightly lower (r2 = 0.51) and was also highly significant.
Effect of trovafloxacin on B. fragilis-VREF abscesses.
There was a significant negative correlation between total daily dose and both the abscess weights (P = 0.0002) and the B. fragilis bacterial counts (P = 0.0001) after 5 days of treatment with trovafloxacin (Fig. 5). Trovafloxacin, at the highest doses of 100 mg/kg q8h and 150 mg/kg q24h for 5 days, was ineffective in reducing the numbers of VREF. The effect of the 3-day treatment or lower doses on these abscesses was therefore not determined. When the respective log10 CFU reductions in B. fragilis per abscess in the different combinations were compared, there was significantly less killing of the anaerobe (ca. 1-log10 CFU/abscess difference) when it was in a mixed infection with VREF than in the E. coli combination after 5 days of treatment with trovafloxacin (Table 3).
FIG. 5.
Effect of trovafloxacin on B. fragilis-VREF abscess weights and bacterial counts after 5 days of treatment with 150- or 300-mg/kg q8h or q24h regimens. The mean ± the SEM is given.
TABLE 3.
Comparison of the efficacy of trovafloxacin at reducing the bacterial counts of B. fragilis ATCC 23745 in a mixed infection with either E. coli ATCC 25922 or VREF BM4147 in subcutaneous abscesses in mice
| Total daily dose (mg/kg)a | Duration (days)b | Frequency (h) | Mixed-infection abscess | Mean reduction in B. fragilis (log10 CFU/abscess) ± SD |
|---|---|---|---|---|
| 150 | 5 | 24 | B. fragilis-E. coli | 2.7 ± 1.1c |
| B. fragilis-VREF | 1.6 ± 0.8 | |||
| 300 | 5 | 8 | B. fragilis-E. coli | 3.4 ± 0.4d |
| B. fragilis-VREF | 2.5 ± 1.1 |
Treatment was started 3 days after inoculation with a 0.25-ml inoculum of B. fragilis (107 CFU), E. coli (105 CFU; or VREF [107 CFU]), and ACC (4 mg).
Bacterial counts were determined 8 days after inoculation.
The difference between combinations was significant (P = 0.037).
The difference between combinations was significant (P = 0.032).
Biodistribution of 99mTc-HYNIC IgG in B. fragilis-E. coli abscesses.
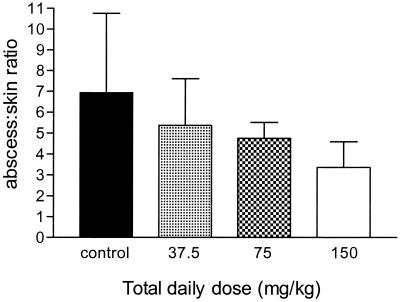
The biodistribution of 99mTc-HYNIC IgG in the abscesses and skin of mice treated with either saline or trovafloxacin (q24h) for 5 days was compared (Fig. 6). In saline-treated mice, the percent injected dose radioactivity detected in abscesses was 5.2% ± 2.0% compared to 0.9% ± 0.4% in uninfected skin, giving a mean abscess/skin ratio of 6.9 ± 3.8. When mice were treated with trovafloxacin, there was a significant negative correlation between antibiotic dose and the abscess/skin ratios (r = −0.471; P = 0.0043).
FIG. 6.
Biodistribution of 99mTc-HYNIC IgG in B. fragilis-E. coli abscesses. Abscess/skin ratios (mean ± the SD) of abscesses treated for 5 days with 37.5, 75, or 150 mg of trovafloxacin per kg.
Toxic effects of trovafloxacin treatment.
Of 12 mice, 4 (33%) treated with the maximum dose of 300 mg/kg died after 2 or 3 days of treatment. A total of 34 (43%) animals in the dosage group of ≥75 mg/kg developed necrotic skin patches at the subcutaneous injection site at the back of the neck. Some animals experienced distinct agitation shortly after the injection of doses of ≥150 mg/kg but resumed normal activity after a few minutes. There was no autopsy or organ pathology performed.
DISCUSSION
The present subcutaneous abscess model permitted the efficacy of trovafloxacin in the treatment of well-established (encapsulated) small mixed infection abscesses to be studied. Antibacterial treatment was difficult in this model, since very high doses (well into the range of murine toxicity) were necessary to obtain more than a 2-log10 CFU/abscess reduction of both E. coli and B. fragilis after 5 days of treatment. Whether prolonged treatment would lead to better results was not investigated due to the limitations imposed by the model. Nevertheless, 5 days of treatment was found to be more effective than the 3-day regimen, which suggests that longer treatment could result in further reductions of bacterial numbers. In other subacute infection models such as endocarditis, it has been shown that, after 4 days of therapy, the efficacy of different antibiotic regimens could be satisfactorily assessed (20). In previous experiments with the same B. fragilis-E. coli combination, imipenem and ceftizoxime were barely effective in this model in reducing bacterial counts even when high-dosage regimens, which allowed for the short half-life in mice, were employed (I. C. Gyssens, L. E. T. Stearne, S. L. C. E. Buijk, J. W. Mouton, I. A. J. M. Bakker-Woudenberg, and H. A. Verbrugh, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-37, 1998; I. C. Gyssens, L. E. T. Stearne, J. W. Mouton, W. H. Goessens, and H. A. Verbrugh, Abstr. 39th ICAAC, abstr. 800, 1999). Similarly, the authors of the original B. fragilis subcutaneous abscess model described a reduced efficacy of older drugs when treatment was started after 24 h, despite abscess penetration of antibiotics at concentrations well above the MIC for most of the drugs studied (4, 10).
Because of the relatively long half-life of trovafloxacin, even in the mouse, the conventional approach (multiple dose-dosing intervals over 24 h to select out the predictive explaining pharmacodynamic parameter [6, 30]) posed a problem. Dividing up the dosing regimen into more than three times daily was meaningless since the fluctuation in peaks and troughs would hardly be significant and since accumulation would start to play a major role. On the other hand, administration less often than once daily was also considered not to be ideal, most certainly because a relationship with dosing regimens in humans would disappear and, if concentrations did fall below the MIC, they would remain below the MIC for too long a time; also, it would entail doses which would be even more toxic than was observed. To determine whether the AUC or peak concentration would be more predictive for the outcome, we therefore compared two dosing regimens in this model, a q8h versus a q24h regimen.
The results show that the peak concentration is more predictive for effect than the AUC. On the one hand, regression analysis showed that the Cmax explained more variation of the effects than did the AUC while, on the other hand, the q24h dosing regimens yielded a significantly better result than the q8h regimens in all but one case. These findings support earlier findings that, although there is a clear relationship between AUC and effect, the peak concentrations are more important for final outcome than the AUC (F. Scaglione, J. W. Mouton, and R. Mattina, Abstr. 39th ICAAC abstr. 20, 1999) (21).
Although a maximum effect could not be determined by an Emax model and, therefore, the dose needed to obtain a maximum effect cannot be readily calculated, the results indicate that a dose of at least 150 mg/kg is needed for a maximum effect in this model. If we take into account the protein binding of trovafloxacin in mice, the AUC/MIC ratio of the free fraction of the drug amounts to 362 mg · h/liter for B. fragilis, a value which is considerably higher than the values described in the literature for a maximum effect for quinolones (21) and which is higher than can be reached with a 200-mg dose given once daily in humans (25). This indicates that the type or severity of infection is important in determining the dose, as has also been found for beta-lactams (16), and that “one size does not fit all” (J. J. Schentag, Editorial, JAMA 279:159–160, 1998).
The efficacy of 40- and 100-mg/kg doses of trovafloxacin given thrice daily against established B. fragilis infections has also been reported in other murine models (9, 26). Girard et al. observed a significantly higher reduction in bacterial counts of both B. fragilis and E. coli with trovafloxacin compared to other antibiotics with similar MICs and similar daily doses. Other studies in rats have shown trovafloxacin to be effective at even lower doses. However, in these models treatment was started early (4 h after inoculation), with the percent mortality and the prevention of abscess formation serving as the parameters of efficacy (18, 27). When treatment was started before inoculation in our model, ceftizoxime and imipenem were also effective in reducing bacterial counts and abscess growth (Gyssens et al., 38th ICAAC; Gyssens et al., 39th ICAAC).
The reduction in bacterial counts of E. coli and B. fragilis in mixed infections by trovafloxacin was also associated with a decrease in abscess size, as measured by weight, and a reduction in inflammation. In the B. fragilis-VREF combination, treatment with trovafloxacin did not reduce the numbers of VREF but nevertheless resulted in a significant decrease in abscess weights. These findings imply that the reduction in B. fragilis numbers was responsible for the decreases in abscess weights. Former studies on abscess pathogenesis found B. fragilis virulence factors such as capsular polysaccharide (28) and succinic acid (1) responsible for inflammatory responses such as abscess formation and growth. Our findings suggest that the severity of the inflammatory response can be diminished when the numbers of E. coli and/or B. fragilis are reduced by antimicrobial treatment. Whether this will further lead to earlier and better resolution of the abscess is yet unproven.
Recently, we have reported on the excellent bactericidal activity of trovafloxacin in vitro against mixed cultures of B. fragilis and E. coli containing high bacterial numbers (108 CFU/ml), such as are found in untreated abscesses (24). In the study, trovafloxacin was markedly less effective at reducing the numbers of VREF, as well as another (vancomycin-susceptible) strain of E. faecium, when in mixed cultures with B. fragilis. The killing of the anaerobe in the mixed culture with VREF required a 23- and 18-fold increase in the concentration of trovafloxacin to produce the same effect as that found in pure cultures and in mixed cultures with E. coli ATCC 25922, respectively. The current in vivo study corroborates these findings. The reason for the reduced killing of B. fragilis when in combination with VREF is unknown (24); nevertheless, the possibility that enterococci could in some way compromise the efficacy of an antibiotic against other members of a mixed infection only compounds the increasing problem of emerging resistant enterococcal strains (13).
In addition to the characteristic shared with other quinolones of maintaining activity against large numbers of static cultures, the relatively high concentrations of trovafloxacin found in the abscesses in the present study and by others (9, 26) could also explain the efficacy of the drug in these difficult-to-treat infections while other antibiotics fail. Extreme variations were found, however, in the concentrations of trovafloxacin measured in the abscesses at each time point. Since the concentration range was greatest in abscesses measured at ≤1 h, the disparate findings could be due to large variations in the diffusion rate of the antibiotic between the serum and abscesses and consequently in the time to equilibration. Variation in the serum-abscess barrier caused by differences in abscess composition would account for this phenomenon. In addition, the high cellular/extracellular ratio of trovafloxacin in granulocytes and monocytes (up to 10-fold) (29), together with the high level of serum protein binding found in this study, could also affect the accumulation of trovafloxacin in the abscesses.
In conclusion, this is a useful model for studying and comparing the efficacy of antimicrobial agents for the treatment of small, undrainable, mixed-infection abscesses. Trovafloxacin was effective at reducing B. fragilis and E. coli bacterial numbers but ineffective against VREF. Treatment with trovafloxacin in this model showed for the first time that a decrease in bacterial numbers also leads to a reduction in both abscess weight and inflammation in established abscesses. Furthermore, we consider that our findings provide valuable information that could be relevant to the activity of other (future) quinolones with a similar broad spectrum.
ACKNOWLEDGMENTS
This study was financially supported by Pfizer B.V., Capelle a/d IJssel, The Netherlands.
We thank W. Eling and T. H. van der Kwast for the histological examination of the abscesses. H. Mattie is gratefully acknowledged for valuable comments on the manuscript.
REFERENCES
- 1.Abdul-Majid K B, Kenny P A, Finlay-Jones J J. The effect of the baterial product, succinic acid, on neutrophil bactericidal activity. FEMS Immunol Med Microbiol. 1997;17:79–86. doi: 10.1111/j.1574-695X.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge K E. The occurrence, virulence and antimicrobial resistance of anaerobes in polymicrobial infections. Am J Surg. 1995;169(Suppl. 5A):2S–7S. [PubMed] [Google Scholar]
- 3.Aldridge K E, Ashcraft D, Bowman K A. Comparative in vitro activities of trovafloxacin (CP-99,219) and other antimicrobials against clinically significant anaerobes. Antimicrob Agents Chemother. 1997;41:484–487. doi: 10.1128/aac.41.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett J G, Dezfulian M, Joiner K. Relative efficacy and critical interval of antimicrobial agents in experimental infections involving Bacteroides fragilis. Arch Surg. 1983;118:181–184. doi: 10.1001/archsurg.1983.01390020037006. [DOI] [PubMed] [Google Scholar]
- 5.Claessens R A M J, Boerman O C, Koenders E B, Oyen W J G, van der Meer J W M, Corstens F H M. Technetium-99m labelled hydrazinonicotinamido human non-specific polyclonal immunoglobulin G for detection of infectious foci: a comparison with two other technetium-labelled immunoglobulin preparations. Eur J Nuclear Med. 1996;23:414–421. doi: 10.1007/BF01247370. [DOI] [PubMed] [Google Scholar]
- 6.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 7.Dupont H, Montravers P, Mohler J, Carbon C. Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infect Immun. 1998;66:2570–2575. doi: 10.1128/iai.66.6.2570-2575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay-Jones J J, Davies K V L, Sturm L P, Kenny P A, Hart P H. Inflammatory processes in a murine model of intra-abdominal abscess formation. J Leukoc Biol. 1999;66:583–587. doi: 10.1002/jlb.66.4.583. [DOI] [PubMed] [Google Scholar]
- 9.Girard A E, Girard D, Gootz T D, Faiella J A, Cimochowski C R. In vivo efficacy of trovafloxacin (CP-99,219), a new quinolone with extended activities against gram-positive pathogens, Streptococcus pneumoniae, and Bacteroides fragilis. Antimicrob Agents Chemother. 1995;39:2210–2216. doi: 10.1128/aac.39.10.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joiner K, Lowe B, Dzink J, Bartlett J G. Comparative efficacy of 10 antimicrobial agents in experimental infections with Bacteroides fragilis. J Infect Dis. 1982;145:561–568. doi: 10.1093/infdis/145.4.561. [DOI] [PubMed] [Google Scholar]
- 11.Joiner K A, Onderdonk A B, Gelfand J A, Bartlett J G, Gorbach S L. A quantitative model for subcutaneous abscess formation in mice. Br J Exp Pathol. 1980;61:97–107. [PMC free article] [PubMed] [Google Scholar]
- 12.McClean K L, Sheehan G J, Harding G K M. Intraabdominal infection: a review. Clin Infect Dis. 1994;19:100–116. doi: 10.1093/clinids/19.1.100. [DOI] [PubMed] [Google Scholar]
- 13.Moellering R C J. Vancomycin-resistant enterococci. Clin Infect Dis. 1998;26:1196–1199. doi: 10.1086/520283. [DOI] [PubMed] [Google Scholar]
- 14.Montravers P, Andremont A, Massias L, Carbon C. Investigation of the potential role of Enterococcus faecalis in the pathogenicity of experimental peritonitis. J Infect Dis. 1994;169:821–830. doi: 10.1093/infdis/169.4.821. [DOI] [PubMed] [Google Scholar]
- 15.Morrissey I. Bactericidal activity of trovafloxacin (CP-99,219) J Antimicrob Chemother. 1996;38:1061–1066. doi: 10.1093/jac/38.6.1061. [DOI] [PubMed] [Google Scholar]
- 16.Mouton J W, Vinks A A. Is continuous infusion of beta-lactam antibiotics worthwhile?—efficacy and pharmacokinetic considerations. J Antimicrob Chemother. 1996;38:5–15. doi: 10.1093/jac/38.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Nichols R L. Surgical infections: prevention and treatment. Am J Surg. 1996;172:68–74. doi: 10.1016/S0002-9610(96)00049-9. [DOI] [PubMed] [Google Scholar]
- 18.Onderdonk A B. Efficacy of trovafloxacin (CP-99,219), a new fluoroquinolone, in an animal model of intraabdominal sepsis. Infect Dis Clin Pract. 1996;5(Suppl. 3):S117–S119. [Google Scholar]
- 19.Onderdonk A B. Pharmacodynamics and microbiology of trovafloxacin in animal models of surgical infection. Am J Surg. 1998;176(Suppl. 6A):39S–45S. doi: 10.1016/s0002-9610(98)00219-0. [DOI] [PubMed] [Google Scholar]
- 20.Pangon B, Joly V, Vallois J M, Abel L, Bure A, Brion N, Contrepois A, Carbon C. Comparative efficacy of cefotiam, cefmenoxime, and ceftriaxone in experimental endocarditis and correlation with pharmacokinetics and in vitro efficacy. Antimicrob Agents Chemother. 1987;31:518–522. doi: 10.1128/aac.31.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 22.Proost, J. M., and D. K. F. Meijer. MW/PHARM, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Mediware, Groningen, The Netherlands. [DOI] [PubMed]
- 23.SAS Institute, Inc. SAS users guide. Cary, N.C: SAS Institute, Inc.; 1990. [Google Scholar]
- 24.Stearne L E T, Kooi C, Goessens W H, Bakker-Woudenberg I A J M, Gyssens I C. In vitro activity of trovafloxacin against Bacteroides fragilis in mixed culture with either Escherichia coli or a vancomycin resistant strain of Enterococcus faecium using an anaerobic time kill technique. Antimicrob Agents Chemother. 2001;45:243–251. doi: 10.1128/AAC.45.1.243-251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,2219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 26.Thadepalli H, Chuah S K, Reddy U, Hanna N, Clark R, Polzer R J, Gollapudi S. Efficacy of trovafloxacin for treatment of experimental Bacteroides infection in young and senescent mice. Antimicrob Agents Chemother. 1997;41:1933–1936. doi: 10.1128/aac.41.9.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thadepalli H, Reddy U, Chuah S K, Thadepalli F, Malilay C, Polzer R J, Hanna N, Esfandiari A, Brown P, Gollapuda S. In vivo efficacy of trovafloxacin (CP-99,217), a new quinolone, in experimental intra-abdominal abscesses caused by Bacteroides fragilis and Escherichia coli. Antimicrob Agents Chemother. 1997;41:583–586. doi: 10.1128/aac.41.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 29.van den Broek P J, Koot T G A, van Strijen E, Mattie H. Intracellular activity of trovafloxacin against Staphylococcus aureus. J Antimicrob Chemother. 1999;44:193–199. doi: 10.1093/jac/44.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Vogelman B, Gudmundsson S, Legget J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]